Abstract
Replication of the broad-host-range, IncPα plasmid RK2 requires two plasmid loci: trfA, the replication initiator gene, and oriV, the origin of replication. While these determinants are sufficient for replication in a wide variety of bacteria, they do not confer the stable maintenance of parental RK2 observed in its hosts. The product of the incC gene has been proposed to function in the stable maintenance of RK2 because of its relatedness to the ParA family of ATPases, some of which are known to be involved in the active partition of plasmid and chromosomal DNA. Here we show that IncC has the properties expected of a component of an active partition system. The smaller polypeptide product of incC (IncC2) exhibits a strong, replicon-independent incompatibility phenotype with RK2. This incompatibility phenotype requires the global transcriptional repressor, KorB, and the target for incC-mediated incompatibility is a KorB-binding site (OB). We found that KorB and IncC interact in vivo by using the yeast two-hybrid system and in vitro by using partially purified proteins. Elevated expression of the incC and korB genes individually has no obvious effect on Escherichia coli cell growth, but their simultaneous overexpression is toxic, indicating a possible interaction of IncC-KorB complexes with a vital host target. A region of RK2 bearing incC, korB, and multiple KorB-binding sites is able to stabilize an unstable, heterologous plasmid in an incC-dependent manner. Finally, elevated levels of IncC2 cause RK2 to aggregate, indicating a possible role for IncC in plasmid pairing. These findings demonstrate that IncC, KorB, and at least one KorB-binding site are components of an active partition system for the promiscuous plasmid RK2.
The self-transmissible plasmids of incompatibility group P (IncP) are known for their remarkably broad host range. They are capable of promoting conjugative transfer to diverse organisms, including gram-negative and gram-positive bacteria and even some yeast species (14, 31, 35, 36, 67, 87). In addition, IncP plasmids are maintained as stable, autonomously replicating elements in a wide variety of gram-negative hosts (79, 83). The identical IncPα plasmids RK2, RP1, RP4, and R68 (67), as well as the related IncPβ plasmid R751 (86), have been intensively studied to understand the basis for the remarkable replicative promiscuity and segregational stability observed in the various bacterial hosts.
RK2 is a 60,099-bp, self-transmissible IncPα plasmid originally isolated from an antibiotic-resistant Klebsiella aerogenes strain cultured from a burn wound (40, 67). Conjugative transfer of RK2 requires at least 19 genes involved in mating pair formation and DNA processing (67). In contrast, replication of RK2 requires a single plasmid-encoded gene, trfA, which is necessary (6) and sufficient (77) for replication initiation at the plasmid origin of replication, oriV, in all hosts tested (68, 80). Control of initiation is mediated largely through coupled complexes of TrfA and oriV (48), and the plasmid is maintained at the moderate copy number of 5 to 10 plasmids per chromosome (24, 94). However, the minimal oriV-trfA replicon is not sufficient for the remarkable stability observed of RK2 in its various hosts (77), indicating the existence of additional determinants that act to maintain the plasmid in a growing bacterial population.
Deletion studies of otherwise intact RK2 have shown that both the kilE and par loci are involved in the stable maintenance of RK2 in different hosts. The kilE locus, which contains two operons encoding the kleABCDEF genes, is required for the stable inheritance of RK2 in Pseudomonas aeruginosa but not in Escherichia coli (50, 94). The predicted products of the kle genes are not similar to any known or predicted proteins, and the mechanism of stabilization imparted by kilE is not known. The par locus encodes two plasmid maintenance functions (30, 33, 70). The parDE operon specifies a plasmid addiction system that is toxic to plasmidless segregants that emerge after cell division (46, 71). The adjacent and divergently transcribed parCBA operon expresses a multimer resolution system (20, 21). Both par operons contribute to the stability of RK2, although the relative importance of each operon varies from host to host (79).
Some low-copy plasmids, like P1, F, and R1, contain active partition systems to ensure that a copy of the plasmid segregates to each daughter cell at cell division (37, 44, 64, 93). These systems share common features: an autoregulated operon of two genes and a nearby cis-acting sequence that has the properties of a centromere-like element. One of the proteins (e.g., ParA of P1, SopA of F, and ParM of R1) has, or is predicted to have, ATPase activity (18, 19, 44, 62, 90). The second gene of the operon encodes a DNA-binding protein (ParB of P1, SopB of F, and ParR of R1), whose target is the nearby cis-acting centromere-like element (17, 27, 44, 61). The genetic properties of these plasmid stability loci, most notably their incompatibility phenotypes, led to a model for active partition that involves plasmid pairing through proteins bound to the cis element, proper cellular localization of plasmid pairs at cell division, and active separation of the plasmid pairs into the newly forming daughter cells (3, 4, 65). Recent elegant studies using fluorescence microscopy to visualize F and P1 plasmids in the cell have provided dramatic evidence in support of this model (32, 63). Similar studies have also confirmed the active segregation of bacterial chromosomes (32, 60, 91) and revealed chromosomal determinants closely related to the active partition systems of plasmids. Nevertheless, the composition of the host machinery and the mechanism of active partition for plasmids and chromosomes have remained elusive.
An active partition system has been proposed for plasmid RK2 (62). Meyer and Hinds (59) first identified an incompatibility determinant (IncP1-II) in the region that encodes the global transcriptional repressors KorA and KorB. Sequence analysis subsequently revealed a third gene (designated incC) overlapping korA in a different reading frame and extending to the beginning of korB (84) (Fig. 1). Two polypeptides are expressed from incC: the full-length IncC1 protein (38.1 kDa) and a shorter IncC2 protein (27.5 kDa) that is initiated from an internal translational start site (51, 84). The sequences of both IncC polypeptides show significant relatedness with the partition proteins ParA and SopA of P1 and F, respectively (62). This similarity led to the proposal that IncC is a component of an active partition system of RK2. Recent studies from the Thomas laboratory provide strong evidence that incC is involved in plasmid stabilization (9, 92).
FIG. 1.
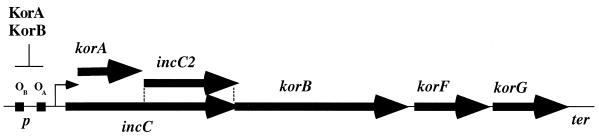
The korA operon of plasmid RK2. The korA, incC, and korB genes (boldface arrows) are described in the text. The korA gene is within the incC coding sequence but in a different reading frame. incC2 is the coding region for the small IncC2 polypeptide product that results from an internal translation initiation site in incC. korF and korG code for small basic proteins of unknown function. p indicates the promoter; OA and OB indicate the operators for the KorA and KorB repressors, respectively; the angled arrow indicates the transcriptional start site. ter indicates a putative transcriptional terminator.
We have undertaken a systematic analysis of the properties of the incC region with respect to incompatibility phenotypes, plasmid stabilization, cis-acting elements, and protein-protein interactions to test for an active partition system on RK2. Our results demonstrate that IncC, KorB, and a KorB-binding site are components of an active partition complex.
MATERIALS AND METHODS
Bacteria and plasmids.
E. coli strains were BL21(DE3, pLysS) {F− hsdS gal dcm ompT [λD69 φ(lacUV5p-T7 gene 1)]} (81); BR2943 {hsdR17 thi-1 relA1 supE44 endA1 gyrA96 recA1 [λDKC266(P1 repA+)]} (from D. Chattoraj); DH5α [supE44 Δ(laclZYA-argF)U169 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 deoR (φ80dlac lacZΔM15)] (34); EKA335 (previously EKA340.2) [thr-1 leu-6 lacY1 thi-1 tonA21 supE44 rfbD1 ΔtrpE5 Δ(argF-lac)U169 deoC1::Tn10(Tcs) srl::Tn10 recA] (79); EKA13 (hsdR lacY leuB6 ΔtrpE5 recA1 gyrA), a spontaneous nalidixic acid-resistant mutant of JA221 (from C. Yanofsky); LS1443 [pcnB80 zad::Tn10 hsdR2 mcrB1 araD139 Δ(ara-leu)7696 ΔlacX74 galU galK rpsL thi-1] (from H. Shuman); and M15(pREP4) (Qiagen, Valencia, Calif.). Saccharomyces cerevisiae stains were L40 (MATa trp1 leu2 his3 URA3::lexA-lacZ LYS2::lexA HIS3) (88) and L41 (MATα trp1 leu2 his3 URA3::lexA-lacZ LYS2::lexA HIS3) (from D. Shore).
The plasmids used in these experiments are described in Table 1 and Fig. 2. The following unpublished plasmids were constructed as indicated: pDB6, by spontaneous Aps deletion of pACYC177 (12); pRK21261, by ligation of a HindIII fragment encoding spectinomycin resistance with HindIII-cleaved pRK2108 (25); pRK21484, by PCR amplification of the korB coding region from pRK2108 using the oligonucleotide primers korBpp1 (5′-GCGGATCCATCGAGGGTAGAATGACTGCGGCTCAAGCCAAGAC-3′) and korBpp2 (5′-CGAGCCAAGCTTGCTCCTTGTAGCGGAACCGTTGTC-3′) followed by end filling of the product using the Klenow fragment of DNA polymerase I, digestion with BamHI and HindIII, and ligation to BamHI- and HindIII-digested pQE-8 (Qiagen); pRK21665, by PCR amplification of the korB coding region from pRK2108 using the oligonucleotide primers korB-1 (5′-GGCTCAAGCCAAGACCACCAAG-3′) and korBpp2, followed by ligation of the amplification product to pCRII (InVitrogen, Carlsbad, Calif.); pRK21673, by ligation of the EcoRI fragment containing the korB coding region from pRK21665 to EcoRI-digested pGAD10; pRK21674, by ligation of the EcoRI fragment containing the korB coding region from pRK21665 to EcoRI-digested pBTM116; pRK21841, by PCR amplification of incC from pRK2108 using the oligonucleotide primers IncCUP (5′-GGGTGTTATCCATGAAGAAA-3′) and IncCLPS1 (5′-GTCGACAGTCATTGGGAAATCTCCA-3′) followed by ligation of the amplification product to pCRII; pRK21842, by ligation of the EcoRI fragment containing the incC coding region from pRK21841 to an EcoRI digest of pGAD10; pRK21845, by ligation of the incC-containing EcoRI fragment from pRK21841 to EcoRI-digested pET-17b (Novagen, Madison, Wis.); pRK21984, by PCR amplification of IncC from pRK2526 using the oligonucleotide primers IncC2_upstream (5′-CGCCAAGAAAAAACAGGAAACCAAACG-3′) and IncC2_dnstream (5′-CTTGAGCCGCAGTCATTGGGAAATCTC-3′) followed by ligation of the amplification product to pCR2.1 (InVitrogen); pRK21985, by digestion of pRK21984 with HindIII and XbaI and ligation to HindIII- and XbaI-digested pJAK16, a derivative of pMMB67 (28) (from J. Kornacki); pRK22323, by PCR amplification of OB3 from pRK2101 (24) using the oligonucleotide primers OB3up (5′-CTGAAATCGGGAAGTGCGAAAAGCATCACCT-3′) and OB3dn (5′-CCCTGCTTCGCAGCCTGGTATTCAGGCTCG-3′), followed by ligation of the amplification product into pCR2.1; pRK22324, by digestion of pRK2362 with BssHII, followed by religation to form an in-frame deletion within incC; pRK22327, by digestion of pRK22323 with EcoRI and ligation to an EcoRI digest of pZeRO (InVitrogen); pRK22329, by digestion of pRK2362 with EcoRV and HindIII, followed by ligation to EcoRV- and HindIII-digested pRK2101; pRK22330, by digestion of pRK22324 with EcoRV and HindIII, followed by ligation to EcoRV- and HindIII-digested pRK2101; and pTR3, by digestion and religation of pZeRO with the compatible end-generating enzymes SpeI and XbaI.
TABLE 1.
Plasmids used in this study
| Plasmid | Marker(s) | Relevant genotype | Description | Reference or source |
|---|---|---|---|---|
| pBR322 | Apr Tcr | pMB1 replicon; vector control | 74 | |
| pBTM116 | Apr TRP+ | lexA(DB) | Cloning vector for lexA(DB) fusion in yeast two-hybrid system | 88 |
| pDB6 | Kmr | P15A replicon; vector control | Bechhofer and Figurskia | |
| pGAD10 | Apr LEU+ | GAL4(AD) | Cloning vector for GAL4(AD) fusion in yeast two-hybrid system | 22 |
| pJAK16 | Cmr | lacIqtacp | IncQ replicon; expression vector | Kornackib |
| pKJ1 | Apr | P15A replicon; vector control | 69 | |
| pRK353 | Trp+ | R6K replicon; vector control | 49 | |
| pRK2013 | Kmr | ΔkilA ΔkilE ΔkilC Δpar ΔoriV | ColE1 replicon; Tra+ (Fig. 2) | 23 |
| pRK2101 | Apr | korA+ incC+ korB+ korF+ korG+ kfrA+ upf54.8+ OB1+ OB2+ OB3+ | pMB1 replicon (Fig. 2) | 24 |
| pRK2178 | Kmr | incC+ korB+ OB1− | P15A replicon; has korA promoter but lacks OB1 (Fig. 2) | 8 |
| pRK2300 | Kmr | ΔincC korB+ OB1− | pRK2178 with 465-bp in-frame deletion within incC (Fig. 2) | 8 |
| pRK2362 | Apr | incC+ korB+ OB1+ | P15A replicon (Fig. 2) | 8 |
| pRK2366 | Apr | incC+ korB− OB1+ | pRK2362 with 14-bp deletion at 3′ end of korB (Fig. 2) | 8 |
| pRK2526 | Apr Kmr Tcs Lac+ | tetA::lacZYA | RK2 with lac operon insertion in tetA | 79 |
| pRK21261 | Spr Trp+ | korA+ incC+ korB+ korF+ korG+ kfrA+ upf54.8+ OB1+ OB2+ OB3+ | pSM1 replicon (Fig. 2) | This study |
| pRK21382 | Apr KmrTcs Lac+ Spr | tetA::lacZYA Δpar1 | pRK2526 with par deleted and replaced with Spr | 79 |
| pRK21408 | Trp+ | lacIq φ[trcp-korB] | pRK353 with korB expressed from trcp | 85 |
| pRK21484 | Apr | φ[T7 φ10p-6xhis-korB] | pQE-8 derivative for expression of his-korB | This study |
| pRK21591 | Apr Kmr Tcs Lac+ Tpr | tetA::lacZYA ΔtrfA::P1ori | pRK2526 with trfA deleted and replaced with the P1 ori and a trimethoprim resistance marker | Sia and Figurskic |
| pRK21673 | Apr LEU+ | φ[GAL4(AD)-korB] | pGAD10 with korB fusion for yeast two-hybrid assay | This study |
| pRK21674 | Apr TRP+ | φ[lexA(DB)-korB] | pBTM116 with korB fusion for yeast two-hybrid assay | This study |
| pRK21842 | Apr TRP+ | φ[GAL4(AD)-incC] | pGAD10 with incC fusion for yeast two-hybrid assay | This study |
| pRK21845 | Apr | φ[T7 φ10p-T7 · TAG-incC] | pET-17b derivative for expression of T7-incC | This study |
| pRK21985 | Cmr | lacIq φ[tacp-incC2] | pJAK16 with incC2 coding region expressed from tacp | This study |
| pRK22324 | Apr | ΔincC korB+ OB1+ | pRK2362 with 465-bp in-frame deletion within incC (Fig. 2) | This study |
| pRK22327 | Zeor | OB3+ | pZeRO with OB3 | This study |
| pRK22329 | Apr | korA+ incC+ korB+ korF+ korG+ kfrA+ upf54.8+ OB1+ OB2+ OB3+ | P15A replicon (Fig. 2) | This study |
| pRK22330 | Apr | korA+ ΔincC korB+ korF+ korG+ kfrA+ upf54.8+ OB1+ OB2+ OB3+ | pRK22329 with 465-bp in-frame deletion within incC (Fig. 2) | This study |
| pRR10 | Apr | trfA+ oriV+ | Mini-RK2 | 70 |
| pTR3 | Zeor | pZeRO with ccdB out of frame; vector control | This study |
D. H. Bechhofer and D. H. Figurski, unpublished results.
J. A. Kornacki, unpublished results.
E. A. Sia and D. H. Figurski, unpublished results.
FIG. 2.
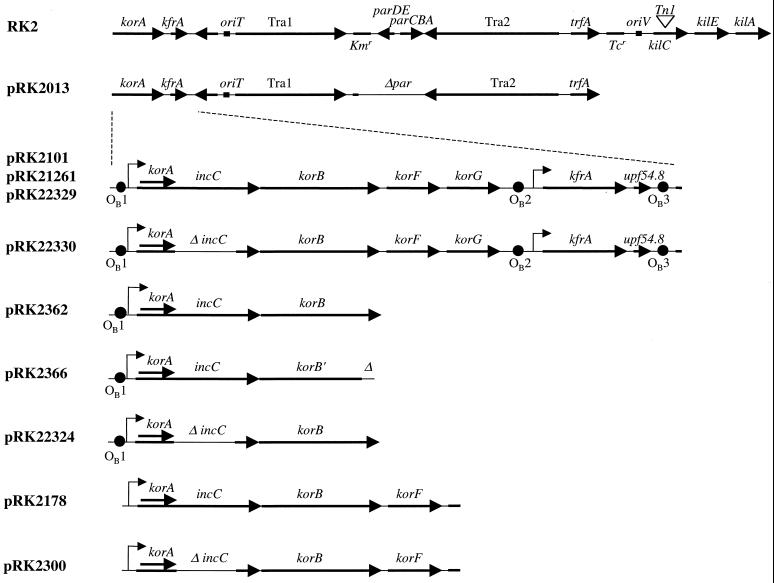
Plasmids used to study the activities of incC. A linear schematic of the RK2 map is shown on the top line with landmark genetic determinants for reference (67). Shown below are the cloned RK2 segments in different plasmid derivatives. Boldface arrows indicate the direction of transcription, angled arrows indicate transcription start sites, and ▵ indicates a deletion. Tra1 and Tra2 are regions for conjugative transfer; oriT is the origin of transfer. Tn1 is a transposon that encodes resistance to ampicillin; Kmr and Tcr indicate genes for resistance to kanamycin and tetracycline, respectively. kfrA is a gene for a DNA-binding protein of unknown function; upf54.8 is a putative gene of unknown function. The specific KorB-binding sites (OB1, OB2, and OB3) are indicated by filled circles.
Media.
Media for growth of bacteria were Luria-Bertani (LB) broth and M9-CAA medium (56). M9-CAA medium was supplemented with tryptophan (50 μg/ml) when necessary. The following antibiotics were used at the indicated concentrations: ampicillin, 50 μg/ml; penicillin, 150 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 50 μg/ml; and zeocin, 50 μg/ml. To induce expression of proteins from tacp or trcp promoters, the medium was supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). To detect Lac+ colonies, solid medium contained 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml. Medium for growth of yeast was yeast extract-peptone-dextrose (YEPD) and synthetic complete (SC) medium (5) lacking histidine, tryptophan, and/or leucine.
DNA procedures.
Preparation of DNA from E. coli was done by the alkaline lysis protocol (5). Preparation of DNA from S. cerevisiae was done according to a glass bead protocol (39). Agarose gel electrophoresis and polyacrylamide gel electrophoresis (PAGE) have been described previously (74). DNA manipulations with restriction endonucleases, T4 DNA ligase, and the Klenow fragment of DNA polymerase I were done according to the manufacturers' recommendations. Amplification of DNA by PCR was done with Taq DNA polymerase (73). All cloned PCR products were confirmed by nucleotide sequencing. Transformation of bacteria was done by the method of Cohen et al. (13). Transformation of yeast was done by the method of high-efficiency Li acetate transformation (75).
Incompatibility assays.
Strains were grown overnight at 37°C in broth with selection for both the tacp-incC2 plasmid and the test plasmid. Dilutions of the cultures were then plated on medium selecting for the tacp plasmid alone (single selection) with and without IPTG, as well as medium selecting for both plasmids (double selection) with and without IPTG. Single-selection medium was supplemented with X-Gal if the test plasmid was Lac+. The presence of the test plasmid was screened for either by taking the ratio of blue to white colonies or by picking individual colonies from single-selection plates and moving them onto medium with the appropriate antibiotic. For each assay, the viable cell count (CFU) of the single selection without IPTG was normalized to an efficiency of plating (EOP) of 1.0. The CFU of other sets were then obtained and the relative EOPs were calculated.
Plasmid stability assays.
Short-term stability assays (0 to 15 generations) were done as follows. Approximately 108 cells were scraped from a fresh selection plate and resuspended in 0.5 ml of LB broth. This sample was diluted 104- or 105-fold into fresh, nonselective medium and then grown at 37°C. Samples were taken every 2 h to obtain time points every few generations, and then they were plated on both selective and nonselective media to determine the number of generations of growth and percent plasmid retention. For longer-term assays (>15 generations), strains were grown overnight at 37°C in broth with selection for resident plasmids. The cultures were diluted 106-fold into nonselective medium, grown to stationary phase, and then diluted as described above into fresh nonselective medium. At each time point, dilutions were plated on nonselective medium, and plasmid retention was measured by picking individual colonies and plating them onto selective medium.
Yeast two-hybrid assay.
Derivatives of pBTM116 (vector for LexA DNA-binding domain fusions) were introduced into S. cerevisiae haploid strain L41 by transformation and selection for growth on SC medium lacking tryptophan. Derivatives of pGAD10 (vector for GAL4 activation domain fusions) were introduced into S. cerevisiae haploid strain L40 with selection for growth on SC medium lacking leucine. To test for interactions between pBTM166 and pGAD10 derivatives, diploid strains were constructed by spotting 5 μl of the L41 and L40 strains together on a YEPD plate. Spots were incubated overnight at 30°C, transferred to sterile velvet, and replica-plated to SC medium lacking tryptophan and leucine to select for the L40/41 a/α strain. Diploid strains containing both the pBTM116 and pGAD10 derivatives were then tested for interaction of fusion products. Broth cultures were grown overnight and β-galactosidase activity was measured as previously described (5).
Purification of His-KorB.
The korB coding region, beginning with the second codon, was fused in-frame with a 12-codon open reading frame which includes an N-terminal six-His tag downstream of an inducible promoter to generate pRK21484 (described above). The korB gene fusion was induced in strain M15(pREP4). One hundred milliliters of cells were grown in LB broth to an optical density at 600 nm of 0.6 and then were induced by adding IPTG to a final concentration of 1 mM. The culture was incubated at 37°C for 2.5 h, and the cells were collected by centrifugation at 4,000 × g for 10 min. The pellet was resuspended in 300 μl of sonication buffer A (50 mM NaH2PO4, 300 mM NaCl [pH 8.0]), supplemented with lysozyme (1 mg/ml). The cells were maintained on ice for 5 min; 0.33 ml of 3 M NaCl was added, and the mixture was maintained on ice an additional 5 min. The cells were then sonicated on ice with four 30-s pulses. The sonicate was passed over a Ni-nitrilotriacetic acid-agarose column (Qiagen), and the His-KorB fusion protein was eluted with 40 mM imidazole. The final concentration was approximately 110 μg/ml. His-KorB is competent for binding its DNA target OB as determined by electrophoretic mobility shift analysis (data not shown). Histidine-tagged glutathione-S-transferase (GST-His), for use as a control for binding specificity, was prepared similarly using the GST-His expression plasmid pALEX (gift of S. J. Silverstein).
Preparation of T7-IncC extracts.
The incC coding region, beginning with the second codon, was fused in-frame to a 34-codon open reading frame whose transcriptional and translational initiation signals were provided by the bacteriophage T7 gene φ10 in the plasmid vector pET-17b (Novagen). This region includes the 12 codons for the leader peptide (T7 · TAG epitope) of T7 gene 10 product. The incC fusion protein was designated T7-IncC, and the resulting plasmid was pRK21845 (described above). T7-incC was induced in strain BL21(DE3, pLysS), which contains an IPTG-inducible T7 RNA polymerase gene (81). For induction, cells were grown in LB broth to an optical density at 600 nm of 0.6, and T7 polymerase was induced by adding IPTG to a final concentration of 1 mM. Incubation continued at 37°C for 3 h; cells were then collected by centrifugation at 4,000 × g for 10 min; and the pellet was stored at −70°C. The pellet was thawed and resuspended in 300 μl of sonication buffer B (100 mM NaCl, 50 mM NaH2PO4, 20 mM Tris-HCl [pH 8.0]) with 1 mM phenylmethylsulfonyl flouride. The suspension was sonicated on ice with two 15-s pulses. PAGE showed that T7-IncC constituted about 50% of the total protein, or approximately 140 μg/ml. The resulting lysate was probed by Western immunoblot analysis for T7-IncC with the chemiluminescence Amersham Life Sciences (Arlington Heights, Ill.) ECL kit and rainbow molecular weight markers. Anti-T7 · TAG monoclonal antibody directed against the T7 gene 10 leader peptide was obtained from Novagen. The remaining lysate was stored at −70°C for future use. For controls, isogenic vector (pET-17b) extracts were made at the same time.
Immuno-affinity assay.
To clear the lysates of proteins that interact nonspecifically with the Sepharose beads, 10 μl each of the T7-IncC and vector control extracts were preadsorbed to 10 μl of protein A-Sepharose beads (Pharmacia, Piscataway, N.J.) in 180 μl of adsorption buffer (20 mM NaHPO4, pH 7.0) at room temperature for 1.5 h. The beads were centrifuged, and the supernatant was used as the source of T7-IncC (or vector extract) for the assay. The anti-T7 · TAG monoclonal antibody was bound to protein A by adding 2 μl of antibody to 10 μl of protein A-Sepharose beads, 180 μl of adsorption buffer, and 10 μl of 10-mg/ml bovine serum albumin. The antibody-bead mixture was incubated on a rocker at 4°C for 1.5 h and washed twice in 200 μl of adsorption buffer. The precleared T7-IncC and vector extracts were each added to the antibody-bead complex and incubated on a rocker at 4°C for 1.5 h and then washed 4 times in 200 μl of adsorption buffer. The precipitated bead complex was resuspended in 180 μl of binding buffer (20 mM HEPES, pH 8.0), and increasing amounts (5 to 50 μl) of purified His-KorB were added. The complex was incubated on a rocker at 4°C for 1.5 h and washed four times in 200 μl of binding buffer. The complex was resuspended in 50 μl of sodium dodecyl sulfate (SDS)-PAGE sample buffer, boiled for 7 min, and then analyzed by Western blotting using the Amersham Life Sciences ECL detection kit and rabbit polyclonal anti-KorB antisera (Cocalico Biologicals, Reamstown, Pa.).
Oligohistidine affinity assay.
His-KorB was added to 25 μl of TALON metal affinity resin (Clontech, Palo Alto, Calif.) along with 10 μl of 10-mg/ml bovine serum albumin (as a nonspecific competitor) and adsorption buffer (20 mM NaHPO4, pH 7.0) to give a final volume of 200 μl. The His-KorB-TALON complex was incubated on a rocker at 4°C for 25 min and washed twice in 200 μl of adsorption buffer. Increasing amounts of T7-IncC extract (5 to 50 μl) were added to the complex; the suspension was then incubated on a rocker at 4°C for 25 min and washed four times in 200 μl of adsorption buffer. The resulting complex was resuspended in 50 μl of SDS-PAGE sample buffer, boiled for 7 min, and then analyzed by Western blotting using the Amersham Life Sciences ECL detection kit and the anti-T7 · TAG monoclonal antibody (Novagen).
RESULTS
Elevated expression of IncC2 causes strong incompatibility with RK2.
Previous studies have demonstrated that plasmids carrying a region of RK2 encoding incC can destabilize RK2 derivatives present in the same cell (59). To determine if incC alone is sufficient to confer this incompatibility phenotype, the portion of incC that encodes the IncC2 polypeptide (incC2) (Fig. 1) was amplified by PCR and cloned downstream of the IPTG-inducible tacp promoter. We confirmed that induction of incC2 in the absence of any other plasmid in the cell has no obvious effect on the growth of the culture or colony formation. We then tested the effect of incC2 induction on the RK2lac plasmid pRK2526, an otherwise wild-type RK2 plasmid with lacZYA inserted into the gene for tetracycline resistance. We have previously shown that RK2lac is stably maintained in E. coli in the absence of selection (79). However, induction of incC2 in trans to RK2lac gave evidence of strong incompatibility (Table 2). The EOP of the culture was reduced >105-fold when selection was maintained for both plasmids, indicating that the plasmids could not coexist in the same cell under inducing conditions. This was confirmed by plating the cells on IPTG- and X-Gal-containing medium in the absence of selection for RK2lac. The colonies that arose were white (Lac−), indicating that the RK2lac plasmid was no longer present. However, even in the absence of selection for RK2lac, the EOP was reduced 10-fold. This result might be expected if loss of RK2lac triggers the parDE plasmid addiction system, which is toxic to plasmidless segregants. The effect of incC2 induction was therefore tested on the RK2lac Δpar derivative pRK21382. In this case, the EOP was not reduced and all colonies were Lac−. Essentially the same incompatibility phenotypes were observed for the tacp promoter fused to a derivative of the complete incC coding region that lacks the translational start site for the korA gene and includes the IncC2 translational start site (data not shown). These results show that elevated expression of IncC causes severe destabilization of RK2.
TABLE 2.
IncC-mediated incompatibility is replicon- independent
| Test plasmid | Induced plasmid | Single selectiona
|
Double selection
|
Incompatibility | ||||
|---|---|---|---|---|---|---|---|---|
| −IPTG
|
+IPTG
|
EOPc
|
||||||
| EOP | Fraction plasmid [+]b | EOPc | Fraction plasmid [+]b | −IPTG | +IPTG | |||
| RK2lac | Vector | 1.0 | 0.99 | 1.2 | 1.0 | 0.7 | 0.9 | − |
| tacp-incC2+ | 1.0 | 0.96 | 0.1 | <10−4 | 0.7 | 2.8 × 10−6 | + | |
| RK2lacΔpar | Vector | 1.0 | 0.99 | 1.3 | 1.0 | 0.8 | 0.8 | − |
| tacp-incC2+ | 1.0 | 0.42 | 1.3 | <10−4 | 0.5 | 3.9 × 10−6 | + | |
| RK2ΔtrfA::P1 orid | Vector | 1.0 | 1.0 | 1.0 | 1.0e | 1.3 | 1.0 | − |
| tacp-incC2+ | 1.0 | 1.0 | 0.4 | <10−2e | 1.2 | 1.6 × 10−5 | + | |
| Mini-RK2 | Vector | 1.0 | 1.0 | 1.0 | 0.93e | 0.5 | 1.1 | − |
| tacp-incC2+ | 1.0 | 0.94 | 1.0 | 0.98e | 1.0 | 1.4 | − | |
Single selection for induced plasmid, without (−) or with (+) IPTG.
Fraction of colonies that retain the test plasmid.
EOP normalized to single selection without IPTG.
BR2943 was the host strain for this combination; EKA335 was the host for all the other combinations.
Based on screening 100 colonies.
IncC-mediated incompatibility is replicon-independent.
We next determined if IncC-mediated incompatibility results from interference with RK2 replication. The RK2ΔtrfA plasmid pRK21591 has an inactive RK2 replicon, and it replicates using the plasmid P1 replication system, which is insensitive to incC2 induction (data not shown). Just as with wild-type RK2, induction of incC2 caused a dramatic loss of RK2ΔtrfA (Table 2), indicating that IncC-mediated incompatibility is not caused by interference with RK2 replication. As before, the reduction in EOP on single selection is probably due to the parDE plasmid addiction system. Conversely, we tested the effect of incC2 induction on the maintenance of the mini-RK2 plasmid pRR10, which consists entirely of the minimum replication determinants, the trfA gene and oriV, along with an Apr marker. In contrast to the results with wild-type RK2, induction of incC2 had no effect on the mini-replicon (Table 2). These results show that the target for IncC-mediated incompatibility lies outside the RK2 replication determinants.
IncC-mediated incompatibility requires KorB.
Replicon-independent incompatibility is consistent with a role for IncC in active partition. We therefore used the strong incompatibility phenotype to identify other factors that function with IncC. To identify the target for IncC-mediated incompatibility, we examined a variety of plasmids carrying different portions of RK2 (Table 3). Plasmids with large segments of RK2, such as pRK2013 and pRK21261, are sensitive to the IncC-mediated incompatibility. The smallest RK2 segment to confer sensitivity is present on plasmid pRK2362, which carries korA, incC, and korB (Fig. 2). In contrast, plasmid pRK2366, an isogenic korB mutant derivative of pRK2362 that has a small deletion at the 3′-end of korB, is not affected by induction of incC2. This result shows that korB is required for sensitivity to IncC-mediated incompatibility and indicates a new function for KorB beyond its role in transcriptional regulation.
TABLE 3.
Sensitivity of RK2 derivatives to IncC-mediated incompatibility
| Test plasmid | Relevant properties | Induced plasmid | Relative EOPa | Incompatibilityb |
|---|---|---|---|---|
| pRK2013 | RK2ΔkilA ΔkilE ΔkilC Δpar | Vector | 1.3 | − |
| Eight OB sites | tacp-incC2+ | 2.4 × 10−5 | + | |
| pRK21261 | incC+ korABFG+ kfrA+ upf54.8+ OB1+ OB2+ OB3+ | Vector | 1.0 | − |
| tacp-incC2+ | 2.2 × 10−5 | + | ||
| pRK2362 | korA+ incC+ korB+ OB1+ | Vector | 0.8 | − |
| tacp-incC2+ | 1.0 × 1.0−3 | + | ||
| pRK2366 | korA+ incC+ korB′ OB1+ | Vector | 1.0 | − |
| tacp-incC2+ | 0.7 | − | ||
| pRK2178 | korA+ incC+ korB+ korF+OB1− | Vector | 0.8 | − |
| tacp-incC2+ | 0.9 | − |
EKA335 was the host strain. Cells were plated on medium selecting both plasmids and containing or lacking IPTG. The relative EOP was calculated as follows: CFU on medium with IPTG/CFU on medium without IPTG.
−, compatible; +, incompatible.
The KorB-binding site (OB) confers sensitivity to IncC-mediated incompatibility.
Because KorB is a DNA-binding protein, we tested the possibility that the presence of its binding site (OB) is required for sensitivity to IncC-mediated incompatibility. KorB binds to a 13-bp palindromic DNA sequence that is present on RK2 in 12 nearly identical, well-distributed copies (OB1-12), 6 of which are involved in transcriptional regulation (67). All the IncC-sensitive plasmids we tested to this point contained at least one KorB-binding site, including the smallest (pRK2362), which has a single site (OB1) in the korA promoter. We therefore tested plasmid pRK2178, which is comparable to pRK2362, except that it lacks the KorB-binding site in the korA promoter (Fig. 2). The results (Table 3) showed that pRK2178 is insensitive to induction of incC2 in trans, indicating that the KorB-binding site may be required for sensitivity to IncC-mediated incompatibility.
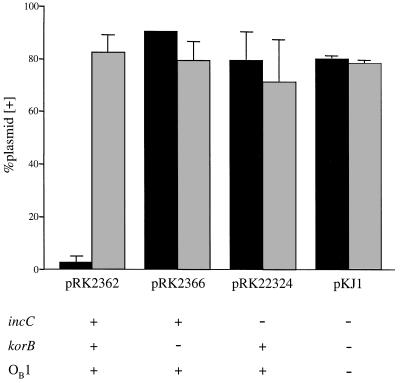
To confirm that a KorB-binding site is the target for IncC-mediated incompatibility, we inserted a single site (OB3) into a plasmid vector (pRK22327) and tested its sensitivity to IncC-mediated incompatibility by placing it in trans to the incC+ korB+ OB1+ plasmid pRK2362. Growth of the cells under selection for pRK2362 resulted in rapid loss of the OB+ plasmid from the population (Fig. 3). The vector control (pTR3) was not destabilized, indicating that the presence of the KorB-binding site on the plasmid caused it to become incompatible with pRK2362. Isogenic derivatives of pRK2362 lacking korB (pRK2366) or incC (pRK22324) failed to destabilize the OB+ plasmid (Fig. 3). Thus, the destabilization of the OB+ plasmid is dependent on incC and korB, and the KorB-binding site is the target of IncC-mediated incompatibility.
FIG. 3.
The KorB-binding site is the target for IncC-mediated incompatibility. The OB+ plasmid pRK22327 (black bars) and the vector control plasmid pTR3 (shaded bars) were tested for sensitivity to IncC-mediated incompatibility in E. coli LS1443 containing pRK2362, pRK2366, pRK22324, or pKJ1 (vector). The presence (+) or absence (−) of incC, korB, and OB1 on these plasmids is indicated at the bottom. After overnight growth under selection, 100% of cells contained both plasmids. Strains were then grown for 20 to 22 generations without selection for pRK22327 or pTR3, as described in Materials and Methods. Shown are the percentages of plasmid-containing cells after unselected growth, as determined by picking colonies and testing for the ampicillin resistance marker on pRK22327 and pTR3. Plotted are the averages of two experiments (error bars, standard deviations).
To determine if an OB site is necessary on both plasmids, we attempted to test the OB+ plasmid in the presence of the incC+ korB+ OB− plasmid pRK2178 but were unable to construct the strain. This incompatibility is independent of incC but dependent on korB, since it occurred with the isogenic incC− korB+ OB− plasmid pRK2300, but not the vector control. This mode of destabilization is distinct from IncC-mediated incompatibility. Since KorB is expected to be expressed at high levels from pRK2178 and pRK2300 (see below), this phenotype is similar to the ParB- and SopB-induced silencing of parS- and sopC-containing plasmids, respectively (55, 72). This phenotype is the subject of another study.
IncC-dependent stabilization of a heterologous plasmid.
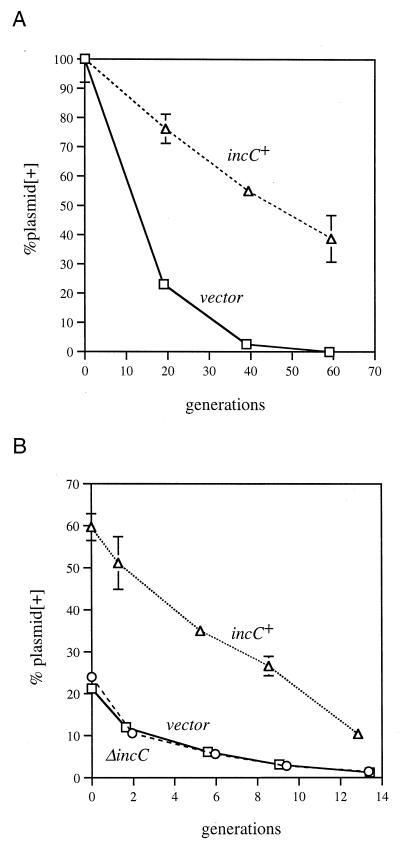
The IncC-mediated incompatibility phenotypes are consistent with a role for IncC, KorB, and the KorB-binding site in the active partition of RK2. We next tested the prediction that these elements should stabilize an unstable plasmid. The copy number of the ColE1-related replicon pMB1 is reduced to 10% of normal in pcnB mutants of E. coli (54). As a result, pMB1-derived plasmid vectors, like pBR322, are lost at a significant rate from pcnB cells during unselected growth (Fig. 4A). We tested the stability of pRK2101, a pMB1 replicon-containing plasmid carrying the incC region on a 6-kb RK2 segment (Fig. 2), and found that it was significantly more stable than pBR322 (Fig. 4A). The stable maintenance of pRK2101 did not result from a significant increase in copy number relative to pBR322. We used a unit copy F plasmid replicon as an internal control in these strains to determine the relative copy numbers of pBR322 and pRK2101. Agarose gel electrophoresis of plasmid DNA showed (i) that both plasmids have copy numbers comparable to that of the F replicon control plasmid and (ii) that the copy number of pRK2101 was at most 50% higher than pBR322 (data not shown). Thus, the lower rate of loss of pRK2101 relative to that of pBR322 suggested that the incC region of RK2 can stabilize a heterologous plasmid in a replication-independent manner.
FIG. 4.
IncC-dependent stabilization of an unstable plasmid. (A) The pMB1 replicon-based plasmids pRK2101 (incC+) (▵) and pBR322 (vector) (□) were tested for their maintenance in the pcnB strain LS1443. Plasmid loss was assayed by growing cells in the absence of selection for several generations. Cells were plated at regular intervals on medium containing ampicillin to determine plasmid-containing cells and on nonselective medium to determine total cells. (B) The P15A replicon-based plasmids pRK22329 (incC+) (▵), pRK22330 (ΔincC) (○), and pKJ1 (vector) (□) were tested for their maintenance in LS1443, as described for panel A. The P15A replicon is highly unstable in the pcnB strain, and even the zero time point shows a high proportion of plasmidless cells. Plotted are averages of three experiments (error bars, standard deviations). The results are highly reproducible, and error bars are visible only for some of the points.
The P15A replicon of vector pKJ1 also exhibits a reduced copy number in a pcnB host, even lower than that of a coresident F replicon control plasmid (data not shown). It is more unstable than pBR322 in this host and colonies of pKJ1-containing cells contain a significant fraction of plasmidless cells even on ampicillin selection (Fig. 4B [t = 0]). Some plasmidless cells are likely to survive within a colony on ampicillin medium because the plasmid-containing cells produce an ampicillin-degrading β-lactamase. To determine if an incC-containing region smaller than 6 kb can stabilize a heterologous P15A plasmid, we examined the stability of the P15A derivative pRK2362, which is incC+ korB+ OB1+, but we were unable to detect stabilization relative to the P15A vector control (data not shown). Plasmid pRK22329 is a P15A derivative that encodes all the determinants present on pRK2101. It is isogenic with pRK2362 but contains the additional downstream genes korF, korG, kfrA, and upf54.8, as well as two additional KorB-binding sites (OB2 and OB3) (Fig. 2). We found that plasmid pRK22329 was stabilized relative to the P15A vector control (Fig. 4B). An isogenic incC derivative (pRK22330), which has an in-frame deletion within incC (Fig. 2), was not stabilized (Fig. 4B), indicating that the stabilization was dependent on incC. Relative copy numbers were again determined using an F plasmid replicon as an internal control, as was done above for the pMB1 plasmids. Both the incC+ and ΔincC plasmids displayed copy numbers comparable to that of the coresident F replicon (data not shown). Thus, incC does not appear to stabilize by increasing plasmid copy number. The stabilization of pRK2101 and pRK22329, while significant and highly reproducible, was not complete, and we discuss possible reasons below. Nevertheless, the results are consistent with a role for incC in the active partition of RK2.
Interaction of IncC and KorB in vivo and in vitro.
Current models for active partition hold that the ATP-hydrolyzing protein and the DNA-binding protein interact to facilitate the formation of plasmid pairs or the positioning of the plasmids in the cell. There is good evidence for the interactions of these proteins in the P1, F, and R1 plasmid systems (10, 18, 38, 44, 47, 95). If IncC and KorB have similar functions in partition, they may be expected to interact.
To test for the possible interaction of IncC and KorB, we first used the yeast two-hybrid system (22). The korB coding region was fused to the coding region for the LexA DNA-binding [LexA(DB)] domain in plasmid pBTM116, and incC was fused to the coding region for the GAL4 transcriptional activation domain [GAL4(AD)] in plasmid pGAD10. As a control, a fusion of GAL4(AD) with korB was also made. The fusion proteins were expressed in different combinations with each other and vector controls in a yeast strain containing a lacZ reporter gene with LexA-binding sites at the promoter. Quantitative β-galactosidase assays revealed significant lacZ expression when both the lexA(DB)-korB fusion and the GAL4(AD)-korB fusion were expressed together in the cell, but not when either fusion was expressed alone (Table 4). This result was expected, because KorB is known to form dimers (7). Significant lacZ expression also occurred when both the lexA(DB)-korB fusion and the GAL4(AD)-incC fusion were expressed together in the cell, but not with either fusion alone (Table 4). These results indicate an interaction between the IncC and KorB moieties of the fusion proteins.
TABLE 4.
Interaction of IncC and KorB in the yeast two-hybrid systema
| Hybrid
|
β-Gal (U) | |
|---|---|---|
| LexA(DB) | GAL4(AD) | |
| —b | —c | 5 ± 1 |
| —b | KorBd | 7 ± 2 |
| KorBe | —c | 8 ± 2 |
| —b | IncCf | 8 ± 2 |
| KorBe | KorBd | 394 ± 33 |
| KorBe | IncCf | 324 ± 25 |
As described in Materials and Methods.
pBTM116.
pGAD10.
pRK21673.
pRK21674.
pRK21842.
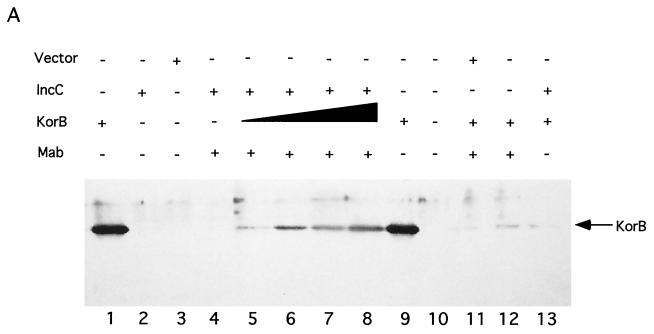
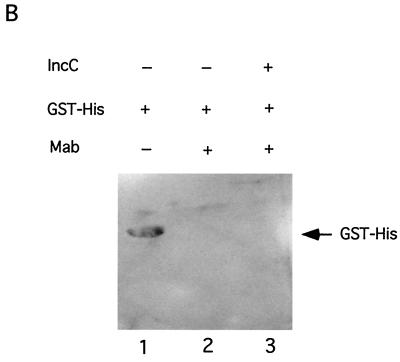
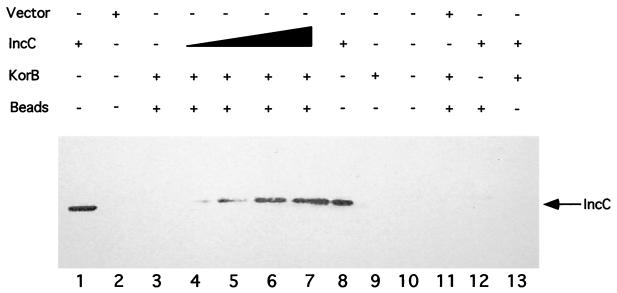
Next we tested for the direct interaction of IncC and KorB proteins in vitro with affinity binding assays. An epitope-tagged version of IncC (T7-IncC) was expressed from a gene fusion, in which the coding region for the T7 · TAG epitope was fused to the second codon at the 5′ end of the incC coding region. Likewise, an amino-terminal six-histidine-tagged version of KorB (His-KorB) was expressed from a fusion constructed by cloning korB from its second codon into vector pQE-8. In the first experiment, the T7-IncC fusion protein was captured from E. coli extracts using protein A-coated Sepharose beads bound to a monoclonal antibody specific for the T7 · TAG epitope. Purified His-KorB was then added to the coated beads. After unbound protein was removed by washing, the proteins on the beads were separated by SDS-PAGE, and Western blot analysis was done using anti-KorB polyclonal antiserum to detect the presence of KorB. His-KorB was found to bind to T7-IncC-coated beads and binding was dependent on the presence of T7-IncC on the beads (Fig. 5A). A purified GST-His fusion did not show detectable binding to the beads (Fig. 5B), indicating that the T7-IncC–His-KorB interaction observed is specific. Cell extracts with wild-type KorB showed results similar to those of His-KorB (data not shown). We did the converse experiment to confirm the interaction. Purified His-KorB was bound to a histidine-affinity resin (TALON), and increasing amounts of a T7-IncC-containing sonicate were added. The complexes were washed to remove unbound protein, and the bound proteins were separated by SDS-PAGE. Western blot analysis was done using the anti-T7 · TAG monoclonal antibody to detect the presence of T7-IncC. T7-IncC was found to bind to His-KorB-coated beads, and binding was dependent on the presence of His-KorB (Fig. 6). Taken together, these data show that IncC and KorB proteins physically interact.
FIG. 5.
Binding of KorB to IncC on a solid matrix. (A) T7-IncC was bound to Protein A-Sepharose containing the anti-T7 · TAG monoclonal antibody (Mab), as described in Materials and Methods. Increasing amounts of partially purified His-KorB were added, the beads were washed, and the proteins bound to beads were separated by SDS-PAGE. Western blot analysis was used to assay the presence of His-KorB (indicated by the arrow). Vector, extract made with the T7 · TAG vector as a control; IncC, extract with T7-IncC; KorB, His-KorB; Mab, protein A-Sepharose beads coated with anti-T7 · TAG monoclonal antibody. Control lanes 1, 9, and 11 to 13 contained 50 μl of His-KorB solution (110 μg/ml); experimental lanes 4, 5, 6, 7, and 8 contained 0, 5, 10, 25, and 50 μl, respectively. The His-KorB in lanes 1 and 9 was applied directly to the gel. (B) Control for binding specificity using purified GST-His. Samples were prepared as for panel A, except that approximately 5 μg of GST-His was used in place of His-KorB. The GST-His in lane 1 (1.25 μg) was applied directly to the gel.
FIG. 6.
Binding of IncC to KorB on a solid matrix. His-KorB was bound to TALON beads, as described in Materials and Methods. Increasing amounts of extracts containing T7-IncC were added, the beads were washed, and the proteins were separated by SDS-PAGE. Western blot analysis was used to assay the presence of T7-IncC (indicated by the arrow). Vector, extract made with the T7 · TAG vector as a control; IncC, extract with T7-IncC; KorB, His-KorB; beads, TALON beads. Control lanes 1, 2, and 8 contain 1 μl of the relevant extract; control lanes 11, 12, and 13 contain 50 μl of the relevant extract; experimental lanes 3, 4, 5, 6, and 7 contain 0, 5, 10, 25, and 50 μl of T7-IncC extract (containing approximately 140 μg of T7-IncC per ml), respectively. The T7-IncC in lanes 1 and 8 was applied directly to the gel.
Cooverexpression of IncC2 and KorB is toxic to E. coli cells.
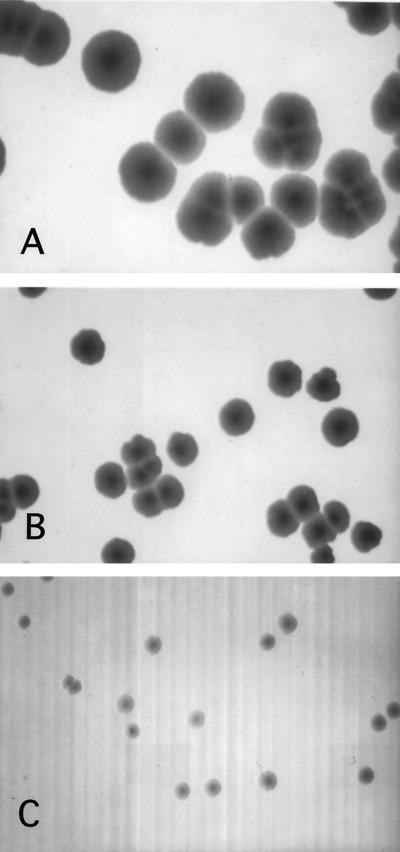
The results above showed that induction of incC2 is not deleterious to the growth of E. coli host cells. Plasmid pRK2300 is a multicopy P15A derivative that contains korA, korB, and korF but lacks incC (Table 1; Fig. 2). If the in-frame incC deletion is nonpolar, the remaining genes are predicted to be expressed at higher-than-normal levels because the korA promoter in this plasmid lacks the operator OB1 for KorB repression. This plasmid has no effect on the growth of E. coli. However, cells containing both pRK2300 and the tacp-incC2 plasmid pRK21985 have markedly reduced EOP on IPTG-containing medium, even in the absence of selection (Table 5). Thus, the plasmids are toxic to E. coli when incC2 is induced. To determine if incC and korB are sufficient for the toxicity, we examined the effect of coinduction of tacp-incC2 (on pRK21985) and trcp-korB (on the compatible plasmid pRK21408) (Fig. 7). Coinduction resulted in a marked reduction in E. coli colony size, whereas induction of either gene alone had no significant effect. After two days, the colonies are largely nonviable, except for IPTG-insensitive variants (data not shown). Thus, simultaneous overexpression of incC2 and korB is toxic to cell growth.
TABLE 5.
incC2 induction is toxic to E. coli in the presence of a korABF+ plasmid
| Plasmid 1 | Relevant property(ies) | Plasmid 2 | Relevant property(ies) | Relative EOPa
|
||
|---|---|---|---|---|---|---|
| Double selection | Single selection for the indicated plasmid
|
|||||
| incC+b | korB+c | |||||
| pDB6 | Vector | pJAK16 | Vector | 1.0 | 1.5 | 1.3 |
| pDB6 | Vector | pRK21985 | tacp-incC2+ | 0.8 | 1.1 | 1.5 |
| pRK2300 | korA+ korB+ korF+ | pJAK16 | Vector | 1.2 | 1.2 | 1.6 |
| pRK2300 | korA+ korB+ korF+ | pRK21985 | tacp-incC2+ | 4.7 × 10−4 | 6.2 × 10−2d | 1.2 × 10−2d |
EKA13 was used as the host strain. The relative EOP was calculated as follows: CFU on medium with IPTG/CFU on medium without IPTG.
Chloramphenicol.
Kanamycin.
Large majority of colonies have lost the unselected plasmid.
FIG. 7.
Simultaneous overexpression of incC2 and korB is toxic to E. coli. E. coli EKA13 strains contained the following combinations of incC and korB plasmids and vector controls: pRK21985 (tacp-incC2) and pRK21408 (trcp-korB), pJAK16 (incC2 vector control) and pRK353 (korB vector control), pRK21985 and pRK353, and pJAK16 and pRK21408. Strains were grown overnight at 37°C with selection for both plasmids and then were plated on medium containing 1 mM IPTG or medium lacking IPTG. Shown are colonies from cells containing pJAK16 and pRK353 on IPTG-containing medium (A) and pRK21985 and pRK21408 on medium lacking IPTG (B) and containing IPTG (C). Magnification is the same for all frames. Results from the other combinations were essentially equivalent to those in panel A, with the exception that the strain with pJAK16 and pRK21408 produced slightly smaller colonies on IPTG.
High levels of IncC2 cause oligocopy RK2 to segregate with low-copy kinetics.
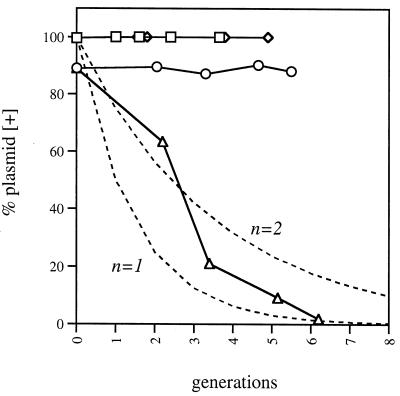
We have shown that induction of incC2 causes the loss of RK2 under nonselective growth conditions. RK2 is present in the cell at 5 to 10 copies per chromosome (24, 94), and we were surprised by how easily RK2 was lost upon induction of incC2. We therefore examined the kinetics of RK2 loss from the population after induction. The RK2lac Δpar plasmid pRK21382 was used to prevent the triggering of toxicity by the parDE plasmid addiction system. Upon induction of incC2, RK2lac Δpar was rapidly lost from the population (Fig. 8). The observed plasmid loss curve was similar to the theoretical curves for a nonreplicating plasmid with a copy number of one or two. Massive plasmid degradation was not triggered by induction of incC2, because RK2lac Δpar-containing cells were present after overnight growth in IPTG-containing broth (data not shown). These results indicate that high levels of IncC2 cause the multiple copies of RK2 in the cell to form an aggregate, which then segregates as a unit to generate a plasmidless daughter cell at cell division.
FIG. 8.
Elevated IncC2 causes RK2 to segregate as a unit. E. coli EKA335 strains contained either pJAK16 (vector) or pRK21985 (tacp-incC) in addition to pRK21382 (RK2lac Δpar). Strains were grown overnight in LB broth with selection for pRK21985 and pRK21382 and then were diluted 1:50 into prewarmed LB broth with selection only for pRK21985 and grown to a cell density of approximately 2 × 108 cells/ml to allow the cells to exit lag phase. At time zero a 10−3 dilution of each culture was inoculated into prewarmed medium with or without 1 mM IPTG and with chloramphenicol to select pRK21895 only. At various times, samples were plated on medium with selection for the tacp-incC2 plasmid and X-Gal to assay RK2lac Δpar retention. Plasmids RK2lac Δpar plus pJAK16: □, no IPTG; ◊, with IPTG. Plasmids RK2lac Δpar plus pRK21985: ○, no IPTG; ▵, with IPTG. The theoretical curves for loss of a nonreplicating plasmid of copy numbers 1 and 2 (n = 1 and n = 2, respectively) are shown.
DISCUSSION
We have undertaken a systematic analysis of the incC determinant to understand its role in the stable maintenance of the promiscuous plasmid RK2. First we showed that the IncC2 product of the incC gene is sufficient to exert replicon-independent incompatibility, a property indicative of plasmid maintenance determinants (3, 65). We then exploited this phenotype to identify other factors that function with IncC. One of these factors was found to be KorB, a protein known previously only as a transcriptional repressor that acts on several operons of the RK2 kor regulon (25, 67). Our studies revealed that KorB is required for IncC-mediated incompatibility and that the KorB and IncC proteins physically interact. A second factor is the cis-acting DNA site (OB) for binding of KorB. A role for OB was revealed by the finding that a plasmid containing a single copy of OB is destabilized by IncC-mediated incompatibility. We also showed that the incC region of RK2 is able to stabilize an unstable, heterologous plasmid in cis in an incC-dependent manner. These results lead us to conclude that IncC, KorB, and OB comprise at least part of an active partition system on plasmid RK2.
In the well-studied active partition systems of plasmids P1 and F, the ATPase (ParA and SopA, respectively), the DNA-binding protein (ParB and SopB, respectively) and the cis-acting element (parS and sopC, respectively) are sufficient to stabilize an unstable plasmid (1, 2, 37, 64, 66). Similar results have been observed for the analogous components of the related partition systems of R1 and NR1 (29, 44, 82). In contrast, a region of RK2 containing incC, korB, and OB1 of RK2 was not sufficient to stabilize an unstable plasmid in E. coli, and an OB3-containing plasmid was not stabilized with incC2 and korB in trans (data not shown). However, a larger RK2 region that includes incC, korB, and OB1 plus additional downstream genes (korF, korG, kfrA, and upf54.8) and two other copies of the KorB-binding site (OB2 and OB3) showed significant stabilization activity that is dependent on the presence of an intact incC gene (Fig. 4). It is possible that one or more of the additional genes or OB sites is required for stabilization activity. Indeed, Williams et al. (92) have presented intriguing evidence suggesting that OB1, OB2, and OB3 sites are not equivalent and that OB3 is the preferred site for an incC-dependent stabilization activity. However, it is difficult to rule out the effects of structural changes, as different fragments of RK2 in test vectors can affect plasmid maintenance independent of partition functions (76). We are currently seeking to establish a well-defined, manipulable system in which a plasmid containing the required OB site(s) is stabilized by the controlled expression of the appropriate genes in trans.
The observed stabilization of the pMB1 and P15A replicons in a pcnB host by the larger incC-korB-OB region (pRK2101 and pRK22329, respectively) was significant relative to the vector and highly reproducible (Fig. 4). Nevertheless, these plasmids were still lost at a significant rate. One explanation is that the replicons occasionally fail to replicate in the pcnB host, thus leading to plasmidless segregants regardless of a stabilization mechanism. It is also possible that the copy number is too low in this host for adequate expression of the partition system components or that E. coli is not the most suitable host for the RK2 partition system. Another possibility is that other genes may be required to enhance the efficiency of the basic incC-korB-OB partition system. In support of this idea, Bignell et al. (9) have recently shown that a larger region of RK2 is even better able to stabilize an unstable plasmid.
The IncC protein was predicted to be involved in partition on the basis of sequence similarity to regions of the ParA and SopA partition proteins of plasmids P1 and F, respectively (62). Both the ParA and SopA proteins have been shown to interact with the cognate DNA-binding proteins ParB and SopB (10, 15, 18, 38, 47, 61, 95). The ParM protein of plasmid R1, which has no sequence relationship with ParA or SopA, but is thought to have a similar function in partition, interacts with the DNA-binding protein ParR (44). Thus, if IncC is involved in active partition, it is predicted to interact with a DNA-binding protein. Our finding that KorB and its binding site OB are required for IncC-mediated incompatibility suggested that KorB is the interacting protein. Recent studies have shown that IncC can affect the binding of KorB to its target site, suggestive of a physical interaction (43, 52). We show here, both by yeast two-hybrid analysis and by in vitro studies with partially purified proteins, that IncC and KorB directly interact.
The discovery that KorB functions both as an active partition protein with IncC and a global transcriptional repressor is a remarkable finding that distinguishes the RK2 system from any other plasmid partition system. KorB is involved in the control of multiple operons of the kor regulon, a feature unique to IncP promiscuous plasmids (25, 67). The regulated operons include genes for replication initiation, conjugative transfer, and stable maintenance in P. aeruginosa (67, 94). In addition, KorB is an autorepressor of the incC-korB operon along with KorA (Fig. 1). An early clue that KorB might have a function other than that of a transcriptional regulator was that both RK2 and the related, but distinct, IncPβ plasmid R751 have multiple KorB-binding sites distributed around their genomes (67, 86). Only some of these sites are involved in transcriptional regulation. Others are conserved in their location but occur downstream of or within genes and have no obvious function. KorB binds as a dimer to a 13-bp palindromic sequence, and there is evidence that KorB can form tetramers (7) that may be able to couple separated OB sites. These properties of the IncC-KorB system resemble the Soj-SpoOJ system of Bacillus subtilis. SpoOJ is involved in chromosome segregation in vegetative and sporulating cells (11, 41, 78). SpoOJ binds to eight sites on the B. subtilis chromosome (53), and recently Soj has been shown to organize the SpoOJ-bound sites into a condensed structure (57). The multiple OB sites on IncP plasmids may likewise be involved in the production of a specialized, KorB- and IncC-mediated, nucleoprotein structure required for efficient partition.
A prediction of the current plasmid partition model is that plasmid copies pair prior to their segregation into daughter cells (2, 3). Electron microscopic studies have implicated partition proteins of plasmid R1 in the pairing of DNA fragments containing the cis element in vitro (45). Remarkable genetic studies on the incompatibility properties of the plasmid P1 parS site and smaller, yet functional, derivatives have demonstrated a requirement for equivalent nucleoprotein structures, a result that can best be explained by the pairing model (16, 17, 58). In addition, recent results of fluorescence microscopy on the cellular locations of plasmids P1 and F indicate that the plasmids localize to the division plane of the cell and then segregate to the 1/4 and 3/4 positions prior to cell division (32, 63), a finding consistent with active partition of plasmid pairs. In this study, we found that elevated levels of IncC cause RK2 to be lost at a rate equivalent to that of a plasmid with a copy number of 1 to 2, even though there are at least 10 to 15 copies of RK2 in the cell at cell division. This result indicates that elevated levels of IncC can cause the copies of RK2 to aggregate and therefore segregate as a unit. Aggregation could result from overpairing caused by intermolecular interactions of the multiple KorB sites to form an interlocked plasmid aggregate. We suggest that these results indicate a role for IncC in the pairing of RK2 molecules prior to segregation.
Remarkably little is known about the basic mechanism for directed DNA movement into daughter cells. Because models for partition require an interaction with an as yet unidentified host cell apparatus (3, 26, 42, 89, 93), the broad host range of IncP plasmids makes them particularly interesting. Has the IncC-KorB partition system evolved to exploit universal properties of host cell DNA segregation machinery such that it can function in a wide variety of bacterial hosts, or is it specific only for certain hosts? We have shown here that high levels of IncC and KorB together are toxic to cell growth, and it is reasonable to suggest that they interact with and perturb the machinery for chromosome segregation. It will be interesting to determine if toxicity occurs in other hosts and if it reflects the host range of incC-dependent stabilization. The toxicity phenotype may also provide a genetic tool for the identification of components of a host segregation apparatus that interacts with the IncC-KorB partition system. Since the discovery of the kor regulon on promiscuous IncP plasmids (25), the genes of the kilA, kilC, and kilE loci have been suggested to encode host-specific functions for stable plasmid maintenance, and studies with kilE support this model (94). Given the strong evidence that IncC, KorB, and OB constitute the basis for a partition system on IncP plasmids, we are investigating the possibility that the gene products of the kil loci function through this basic system.
ACKNOWLEDGMENTS
This research was supported by NIH grant R01-GM29085 to D.H.F. and Cancer Center support grant CA13696 to Columbia University. T.M.R. and M.H.L. were partially supported by NIH training grant AI07161.
REFERENCES
- 1.Austin S, Abeles A. Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J Mol Biol. 1983;169:353–372. doi: 10.1016/s0022-2836(83)80055-2. [DOI] [PubMed] [Google Scholar]
- 2.Austin S, Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983;169:373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- 3.Austin S, Nordström K. Partition-mediated incompatibility of bacterial plasmids. Cell. 1990;60:351–354. doi: 10.1016/0092-8674(90)90584-2. [DOI] [PubMed] [Google Scholar]
- 4.Austin S J. Plasmid partition. Plasmid. 1988;20:1–9. doi: 10.1016/0147-619x(88)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. J. New York, N.Y: Wiley & Sons; 1989. [Google Scholar]
- 6.Ayres E K, Thomson V J, Merino G, Balderes D, Figurski D H. Precise deletions in large prokaryotic genomes by vector-mediated excision (VEX): the trfA gene of promiscuous plasmid RK2 is essential for replication in several gram-negative hosts. J Mol Biol. 1993;230:174–185. doi: 10.1006/jmbi.1993.1134. [DOI] [PubMed] [Google Scholar]
- 7.Balzer D, Ziegelin G, Pansegrau W, Kruft V, Lanka E. KorB protein of promiscuous plasmid RP4 recognizes inverted sequence repetitions in regions essential for conjugative plasmid transfer. Nucleic Acids Res. 1992;20:1851–1858. doi: 10.1093/nar/20.8.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechhofer D H, Kornacki J A, Firshein W, Figurski D H. Gene control in broad host-range plasmid RK2: expression, polypeptide product, and multiple regulatory functions of korB. Proc Natl Acad Sci USA. 1986;83:394–398. doi: 10.1073/pnas.83.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bignell C R, Haines A S, Khare D, Thomas C M. Effect of growth rate and incC mutation on symmetric plasmid distribution by the IncP-1 partitioning apparatus. Mol Microbiol. 1999;34:205–216. doi: 10.1046/j.1365-2958.1999.01565.x. [DOI] [PubMed] [Google Scholar]
- 10.Bouet J Y, Funnell B E. P1 ParA interacts with the P1 partition complex at parS and an ATP-ADP switch controls ParA activities. EMBO J. 1999;18:1415–1424. doi: 10.1093/emboj/18.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervin M A, Spiegelman G B, Raether B, Ohlsen K, Perego M, Hoch J A. A negative regulator linking chromosome segregation to developmental transcription in Bacillus subtilis. Mol Microbiol. 1998;29:85–95. doi: 10.1046/j.1365-2958.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen S N, Chang A C Y, Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA. 1972;69:2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta N, Hedges R. Host ranges of R factors. J Gen Microbiol. 1972;70:453–460. doi: 10.1099/00221287-70-3-453. [DOI] [PubMed] [Google Scholar]
- 15.Davey M J, Funnell B E. Modulation of the P1 plasmid partition protein ParA by ATP, ADP, and P1 ParB. J Biol Chem. 1997;272:15286–15292. doi: 10.1074/jbc.272.24.15286. [DOI] [PubMed] [Google Scholar]
- 16.Davis M A, Austin S J. Recognition of the P1 plasmid centromere analog involves binding of the ParB protein and is modified by a specific host factor. EMBO J. 1988;7:1881–1888. doi: 10.1002/j.1460-2075.1988.tb03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis M A, Martin K A, Austin S J. Specificity switching of the P1 plasmid centromere-like site. EMBO J. 1990;9:991–998. doi: 10.1002/j.1460-2075.1990.tb08201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M A, Martin K A, Austin S J. Biochemical activities of the ParA partition protein of the P1 plasmid. Mol Microbiol. 1992;6:1141–1147. doi: 10.1111/j.1365-2958.1992.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 19.Davis M A, Radnedge L, Martin K A, Hayes F, Youngren B, Austin S J. The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol Microbiol. 1996;21:1029–1036. doi: 10.1046/j.1365-2958.1996.721423.x. [DOI] [PubMed] [Google Scholar]
- 20.Easter C L, Schwab H, Helinski D R. Role of the parCBA operon of the broad-host-range plasmid RK2 in stable plasmid maintenance. J Bacteriol. 1998;180:6023–6030. doi: 10.1128/jb.180.22.6023-6030.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberl L, Kristensen C S, Givskov M, Grohmann E, Gerlitz M, Schwab H. Analysis of the multimer resolution system encoded by the parCBA operon of broad-host-range plasmid RP4. Mol Microbiol. 1994;12:131–141. doi: 10.1111/j.1365-2958.1994.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 22.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 23.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figurski D H, Meyer R J, Helinski D R. Suppression of ColE1 replication properties by the IncP-1 plasmid RK2 in hybrid plasmids constructed in vitro. J Mol Biol. 1979;133:295–318. doi: 10.1016/0022-2836(79)90395-4. [DOI] [PubMed] [Google Scholar]
- 25.Figurski D H, Pohlman R F, Bechhofer D H, Prince A S, Kelton C A. Broad host range plasmid RK2 encodes multiple kil genes potentially lethal to Escherichia coli host cells. Proc Natl Acad Sci USA. 1982;79:1935–1939. doi: 10.1073/pnas.79.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firshein W, Kim P. Plasmid replication and partition in Escherichia coli: is the cell membrane the key? Mol Microbiol. 1997;23:1–10. doi: 10.1046/j.1365-2958.1997.2061569.x. [DOI] [PubMed] [Google Scholar]
- 27.Funnell B E. Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J Bacteriol. 1988;170:954–960. doi: 10.1128/jb.170.2.954-960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fürste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 29.Gerdes K, Molin S. Partitioning of plasmid R1. Structural and functional analysis of the parA locus. J Mol Biol. 1986;190:269–279. doi: 10.1016/0022-2836(86)90001-x. [DOI] [PubMed] [Google Scholar]
- 30.Gerlitz M, Hrabak O, Schwab H. Partitioning of broad-host-range plasmid RP4 is a complex system involving site-specific recombination. J Bacteriol. 1990;172:6194–6203. doi: 10.1128/jb.172.11.6194-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giebelhaus L A, Frost L, Lanka E, Gormley E P, Davies J E, Leskiw B. The Tra2 core of the IncPα plasmid RP4 is required for intergeneric mating between Escherichia coli and Streptomyces lividans. J Bacteriol. 1996;178:6378–6381. doi: 10.1128/jb.178.21.6378-6381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon G S, Sitnikov D, Webb C D, Teleman A, Straight A, Losick R, Murray A W, Wright A. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- 33.Grinter N J, Brewster G, Barth P T. Two mechanisms for the stable inheritance of plasmid RP4. Plasmid. 1989;22:203–214. doi: 10.1016/0147-619x(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 35.Hayman G T, Bolen P L. Movement of shuttle plasmids from Escherichia coli into yeasts other than Saccharomyces cerevisiae using trans-kingdom conjugation. Plasmid. 1993;30:251–257. doi: 10.1006/plas.1993.1056. [DOI] [PubMed] [Google Scholar]
- 36.Heinemann J A, Sprague G. Bacterial conjugative plasmids mobilize DNA transfer between bacteria and yeast. Nature. 1989;340:205–209. doi: 10.1038/340205a0. [DOI] [PubMed] [Google Scholar]
- 37.Hiraga S. Chromosome and plasmid partition in Escherichia coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- 38.Hirano M, Mori H, Onogi T, Yamazoe M, Niki H, Ogura T, Hiraga S. Autoregulation of the partition genes of the mini-F plasmid and the intracellular localization of their products in Escherichia coli. Mol Gen Genet. 1998;257:392–403. doi: 10.1007/s004380050663. [DOI] [PubMed] [Google Scholar]
- 39.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 40.Ingram L C, Richmond M H, Sykes R B. Molecular characterization of the R factors implicated in the carbenicillin resistance of a sequence of Pseudomonas aeruginosa strains isolated from burns. Antimicrob Agents Chemother. 1973;3:279–288. doi: 10.1128/aac.3.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ireton K, Gunther N W, Grossman A D. spoOJ is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacob F, Brenner S, Cuzin F. On the regulation of DNA replication in bacteria. Cold Spring Harbor Symp Quant Biol. 1963;28:329–348. [Google Scholar]
- 43.Jagura-Burdzy G, Kostelidou K, Pole J, Khare D, Jones A, Williams D R, Thomas C M. IncC of broad-host-range plasmid RK2 modulates KorB transcriptional repressor activity in vivo and operator binding in vitro. J Bacteriol. 1999;181:2807–2815. doi: 10.1128/jb.181.9.2807-2815.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen R B, Gerdes K. Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR-parC complex. J Mol Biol. 1997;269:505–513. doi: 10.1006/jmbi.1997.1061. [DOI] [PubMed] [Google Scholar]
- 45.Jensen R B, Lurz R, Gerdes K. Mechanism of DNA segregation in prokaryotes: replicon pairing by parC of plasmid R1. Proc Natl Acad Sci USA. 1998;95:8550–8555. doi: 10.1073/pnas.95.15.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jovanovic O S, Ayres E K, Figurski D H. Host-inhibitory functions encoded by promiscuous plasmids: transient arrest of Escherichia coli segregants that fail to inherit plasmid RK2. J Mol Biol. 1994;237:52–64. doi: 10.1006/jmbi.1994.1208. [DOI] [PubMed] [Google Scholar]
- 47.Kim S K, Shim J. Interaction between F plasmid partition proteins SopA and SopB. Biochem Biophys Res Commun. 1999;263:113–117. doi: 10.1006/bbrc.1999.1317. [DOI] [PubMed] [Google Scholar]
- 48.Kittell B L, Helinski D R. Iteron inhibition of plasmid RK2 replication in vitro: evidence for intermolecular coupling of replication origins as a mechanism for RK2 replication control. Proc Natl Acad Sci USA. 1991;88:1389–1393. doi: 10.1073/pnas.88.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolter R, Helinski D R. Construction of plasmid R6K derivatives in vitro: characterization of the R6K replication region. Plasmid. 1978;1:571–580. doi: 10.1016/0147-619x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- 50.Kornacki J A, Chang C-H, Figurski D H. The kil-kor regulon of promiscuous plasmid RK2: structure, products, and regulation of two operons that constitute the kilE locus. J Bacteriol. 1993;175:5078–5090. doi: 10.1128/jb.175.16.5078-5090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kornacki J A, West A H, Firshein W. Proteins encoded by the trans-acting replication and maintenance regions of broad host range plasmid RK2. Plasmid. 1984;11:48–57. doi: 10.1016/0147-619x(84)90006-4. [DOI] [PubMed] [Google Scholar]
- 52.Kostelidou K, Thomas C M. The hierarchy of KorB binding at its 12 binding sites on the broad-host-range plasmid RK2 and modulation of this binding by IncC1 protein. J Mol Biol. 2000;295:411–422. doi: 10.1006/jmbi.1999.3359. [DOI] [PubMed] [Google Scholar]
- 53.Lin D C, Grossman A D. Identification and characterization of a bacterial chromosome partitioning site. Cell. 1998;92:675–685. doi: 10.1016/s0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 54.Lopilato J, Bortner S, Beckwith J. Mutations in a new chromosomal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol Gen Genet. 1986;205:285–290. doi: 10.1007/BF00430440. [DOI] [PubMed] [Google Scholar]
- 55.Lynch A S, Wang J C. SopB protein-mediated silencing of genes linked to the sopC locus of Escherichia coli F plasmid. Proc Natl Acad Sci USA. 1995;92:1896–1900. doi: 10.1073/pnas.92.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 57.Marston A L, Errington J. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol Cell. 1999;4:673–682. doi: 10.1016/s1097-2765(00)80378-0. [DOI] [PubMed] [Google Scholar]
- 58.Martin K A, Friedman S A, Austin S J. Partition site of the P1 plasmid. Proc Natl Acad Sci USA. 1987;84:8544–8547. doi: 10.1073/pnas.84.23.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer R, Hinds M. Multiple mechanisms for expression of incompatibility by broad-host-range plasmid RK2. J Bacteriol. 1982;152:1078–1090. doi: 10.1128/jb.152.3.1078-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohl D A, Gober J W. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88:675–684. doi: 10.1016/s0092-8674(00)81910-8. [DOI] [PubMed] [Google Scholar]
- 61.Mori H, Mori Y, Ichinose C, Niki H, Ogura T, Kato A, Hiraga S. Purification and characterization of SopA and SopB proteins essential for F plasmid partitioning. J Biol Chem. 1989;264:15535–15541. [PubMed] [Google Scholar]
- 62.Motallebi-Veshareh M, Rouch D A, Thomas C M. A family of ATPases involved in active partitioning of diverse bacterial plasmids. Mol Microbiol. 1990;4:1455–1463. doi: 10.1111/j.1365-2958.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 63.Niki H, Hiraga S. Subcellular distribution of actively partitioning F plasmid during the cell division cycle in E. coli. Cell. 1997;90:951–957. doi: 10.1016/s0092-8674(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 64.Nordström K, Austin S J. Mechanisms that contribute to the stable segregation of plasmids. Annu Rev Genet. 1989;23:37–69. doi: 10.1146/annurev.ge.23.120189.000345. [DOI] [PubMed] [Google Scholar]
- 65.Novick R P. Plasmid incompatibility. Microbiol Rev. 1987;51:381–395. doi: 10.1128/mr.51.4.381-395.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogura T, Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983;32:351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- 67.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPα plasmids: compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 68.Perri S, Helinski D R, Toukdarian A. Interaction of plasmid-encoded replication initiation proteins with the origin of DNA replication in broad host range plasmid RK2. J Biol Chem. 1991;266:12536–12543. [PubMed] [Google Scholar]
- 69.Pohlman R F, Figurski D H. Essential genes of plasmid RK2 in Escherichia coli: trfB region controls a kil gene near trfA. J Bacteriol. 1983;156:584–591. doi: 10.1128/jb.156.2.584-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roberts R C, Burioni R, Helinski D R. Genetic characterization of the stabilizing functions of a region of broad-host-range plasmid RK2. J Bacteriol. 1990;172:6204–6216. doi: 10.1128/jb.172.11.6204-6216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roberts R C, Ström A, Helinski D R. The parDE operon of broad-host-range plasmid RK2 specifies growth inhibition associated with plasmid loss. J Mol Biol. 1994;237:35–51. doi: 10.1006/jmbi.1994.1207. [DOI] [PubMed] [Google Scholar]
- 72.Rodionov O, Lobocka M, Yarmolinsky M. Silencing of genes flanking the P1 plasmid centromere. Science. 1999;283:546–549. doi: 10.1126/science.283.5401.546. [DOI] [PubMed] [Google Scholar]
- 73.Saiki R K, Gelfand D H, Stoffel S, Scharf S I, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 74.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 75.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single-stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 76.Schmidhauser T J, Bechhofer D H, Figurski D H, Helinski D R. Host-specific effects of the korA-korB operon and oriT region on the maintenance of miniplasmid derivatives of broad host-range plasmid RK2. Plasmid. 1989;21:99–112. doi: 10.1016/0147-619x(89)90053-x. [DOI] [PubMed] [Google Scholar]
- 77.Schmidhauser T J, Helinski D R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985;164:446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sharpe M E, Errington J. The Bacillus subtilis soj-spoOJ locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol Microbiol. 1996;21:501–509. doi: 10.1111/j.1365-2958.1996.tb02559.x. [DOI] [PubMed] [Google Scholar]
- 79.Sia E A, Roberts R C, Easter C, Helinski D R, Figurski D H. Different relative importances of the par operons and the effect of conjugal transfer on the maintenance of intact promiscuous plasmid RK2. J Bacteriol. 1995;177:2789–2797. doi: 10.1128/jb.177.10.2789-2797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith C A, Thomas C M. Nucleotide sequence of the trfA gene of broad host-range plasmid RK2. J Mol Biol. 1984;175:251–262. doi: 10.1016/0022-2836(84)90347-4. [DOI] [PubMed] [Google Scholar]
- 81.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 82.Tabuchi A, Min Y N, Kim C K, Fan Y L, Womble D D, Rownd R H. Genetic organization and nucleotide sequence of the stability locus of IncFII plasmid NR1. J Mol Biol. 1988;202:511–525. doi: 10.1016/0022-2836(88)90282-3. [DOI] [PubMed] [Google Scholar]
- 83.Thomas C M, Helinski D R. Vegetative replication and stable inheritance of IncP plasmids. In: Thomas C M, editor. Promiscuous plasmids of gram-negative bacteria. San Diego, Calif: Academic Press Ltd.; 1989. pp. 1–25. [Google Scholar]
- 84.Thomas C M, Smith C A. The trfB region of broad host range plasmid RK2: the nucleotide sequence reveals incC and key regulatory gene trfB/korA/korD as overlapping genes. Nucleic Acids Res. 1986;14:4453–4469. doi: 10.1093/nar/14.11.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomson V J, Jovanovic O S, Pohlman R F, Chang C-H, Figurski D H. Structure, function, and regulation of the kilB locus of promiscuous plasmid RK2. J Bacteriol. 1993;175:2423–2435. doi: 10.1128/jb.175.8.2423-2435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorsted P B, Macartney D P, Akhtar P, Haines A S, Ali N, Davidson P, Stafford T, Pocklington M J, Pansegrau W, Wilkins B M, Lanka E, Thomas C M. Complete sequence of the IncPβ plasmid R751: implications for evolution and organisation of the IncP backbone. J Mol Biol. 1998;282:969–990. doi: 10.1006/jmbi.1998.2060. [DOI] [PubMed] [Google Scholar]
- 87.Trieu-Cuot P, Carlier C, Martin M, Courvalin P. Plasmid transfer by conjugation from Escherichia coli to gram-positive bacteria. FEMS Microbiol Lett. 1987;48:289–294. [Google Scholar]
- 88.Votjek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 89.Wake R G, Errington J. Chromosome partitioning in bacteria. Annu Rev Genet. 1995;29:41–67. doi: 10.1146/annurev.ge.29.120195.000353. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe E, Wachi M, Yamasaki M, Nagai K. ATPase activity of SopA, a protein essential for active partitioning of F plasmid. Mol Gen Genet. 1992;234:346–352. doi: 10.1007/BF00538693. [DOI] [PubMed] [Google Scholar]
- 91.Webb C D, Teleman A, Gordon S, Straight A, Belmont A, Lin D C, Grossman A D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- 92.Williams D R, Macartney D P, Thomas C M. The partitioning activity of the RK2 central control region requires only incC, korB and KorB-binding site OB3 but other KorB-binding sites form destabilizing complexes in the absence of OB3. Microbiol. 1998;144:3369–3378. doi: 10.1099/00221287-144-12-3369. [DOI] [PubMed] [Google Scholar]
- 93.Williams D R, Thomas C M. Active partitioning of bacterial plasmids. J Gen Microbiol. 1992;138:1–16. doi: 10.1099/00221287-138-1-1. [DOI] [PubMed] [Google Scholar]
- 94.Wilson J W, Sia E A, Figurski D H. The kilE locus of promiscuous IncPα plasmid RK2 is required for stable maintenance in Pseudomonas aeruginosa. J Bacteriol. 1997;179:2339–2347. doi: 10.1128/jb.179.7.2339-2347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Youngren B, Austin S. Altered ParA partition proteins of plasmid P1 act via the partition site to block plasmid propagation. Mol Microbiol. 1997;25:1023–1030. doi: 10.1046/j.1365-2958.1997.4761842.x. [DOI] [PubMed] [Google Scholar]