Abstract
Background
Neurofilament light chain (NfL) in cerebrospinal fluid (CSF) is a biomarker of multiple sclerosis (MS). However, CSF sampling is invasive and has limited the clinical application. With the development of highly sensitive single-molecule assay, the accurate quantification of the very low NfL levels in blood become feasible. As evidence being accumulated, we performed a meta-analysis to evaluate the diagnostic and predictive value of blood NfL in MS patients.
Methods
We performed literature search on PubMed, EMBASE, Web of Science and Cochrane Library from inception to May 31, 2022. The blood NfL differences between MS vs. controls, MS vs. clinically isolated syndrome (CIS), progressive MS (PMS) vs. relapsing-remitting MS (RRMS), and MS in relapse vs. MS in remission were estimated by standard mean difference (SMD) and corresponding 95% confidence interval (CI). Pooled hazard ratio (HR) and 95%CI were calculated to predict time to reach Expanded Disability Status Scale (EDSS) score≥4.0 and to relapse.
Results
A total of 28 studies comprising 6545 MS patients and 2477 controls were eligible for meta-analysis of diagnosis value, and 5 studies with 4444 patients were synthesized in analysis of predictive value. Blood NfL levels were significantly higher in MS patients vs. age-matched controls (SMD = 0.64, 95%CI 0.44–0.85, P<0.001), vs. non-matched controls (SMD = 0.76, 95%CI 0.56–0.96, P<0.001) and vs. CIS patients (SMD = 0.30, 95%CI 0.18–0.42, P<0.001), in PMS vs. RRMS (SMD = 0.56, 95%CI 0.27–0.85, P<0.001), and in relapsed patients vs. remitted patients (SMD = 0.54, 95%CI 0.16–0.92, P = 0.005). Patients with high blood NfL levels had shorter time to reach EDSS score≥4.0 (HR = 2.36, 95%CI 1.32–4.21, P = 0.004) but similar time to relapse (HR = 1.32, 95%CI 0.90–1.93, P = 0.155) compared to those with low NfL levels.
Conclusion
As far as we know, this is the first meta-analysis evaluating the diagnosis and predictive value of blood NfL in MS. The present study indicates blood NfL may be a useful biomarker in diagnosing MS, distinguishing MS subtypes and predicting disease worsening in the future.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disease affecting over two million people around the world [1]. The clinical courses and manifestations of MS are highly variable encompassing mild or benign forms that may not need treatment and progressive stage that develops irreversible clinical and cognitive deficits with limited response to standard treatment [2]. Highly effective treatments have been developed and become widely available in recent years [3]. Reliable markers for disease detection, staging and prognosis prediction are warranted for the decision-making of best therapy to improve prognosis.
Neurofilament light chain (NfL) in cerebrospinal fluid (CSF) is an emerging biomarker for MS. NfL is a subunit of neurofilaments constituting neuronal and axonal cytoskeleton in central nervous system (CNS) as well as part of the peripheral nervous system, which is released to CSF and blood when neuronal and axonal damage occur [4]. It directly reflects the neuroaxonal injury in many inflammatory, neurodegenerative, traumatic and ischemic diseases of CNS [5, 6]. Previous studies have found more abundant CSF NfL in MS patients than in sex- and age-matched controls and suggested that CSF NfL may help distinguish MS subtypes [7]. It has also reported as a biomarker for frontotemporal dementia (FTD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and atypical parkinsonian disorder (APD) [8]. However, CSF acquisition is a relatively invasive procedure that limits the clinical application, especially longitudinal and repetitive sampling for disease monitoring, of CSF NfL.
In patients with neurological disorders, NfL is released in a large amount to CSF when neural cells are damaged and eventually into the bloodstream [9]. Previous studies mostly focused on CSF levels since the conventional detection methods, such as enzyme‐linked immunosorbent assay (ELISA) and electrochemiluminescence (ECL)‐based assay, had low sensitivity in quantifying the low blood levels [10, 11]. Recently, the development of highly sensitive single-molecule assay (SIMOA) has allowed the accurate quantification of low blood concentrations of NfL and now been widely used [12]. The blood levels of NfL by SIMOA are nearly 40-fold lower than CSF levels but highly correlated with CSF levels, magnetic resonance imaging (MRI) lesions and clinical symptoms [13, 14]. Serum NfL is now widely accepted to monitor disease activity and response to disease-modifying therapy (DMT) [14, 15], and becomes more and more refined as a biomarker in MS [16].
With the increasing evidence of blood NfL measurements in MS patients, we performed the present systematic review and meta-analysis to evaluate the value of blood NfL in diagnosing MS, distinguishing MS subtypes and severity, and predicting disease worsening.
Methods
Literature search strategy
The present systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [17]. Candidate articles investigating the diagnostic or predictive value of blood NfL levels in MS were systematically searched in electronic literature databases including PubMed, EMBASE, Web of Science and Cochrane Library from inception to May 31, 2022. The following keywords were used for literature search: (“neurofilament light chain” OR “neurofilament-light chain” OR “neurofilament” OR NfL OR sNfL OR pNfL) AND “multiple sclerosis”. Additional relevant articles were obtained by manually searching the reference lists of eligible studies.
Inclusion and exclusion criteria
All eligible studies should meet the following criteria: (1) measured serum or plasma NfL concentrations in adult MS patients; (2) investigated the diagnostic or predictive value of blood NfL levels; (3) provided sufficient data for meta-analysis. MS was diagnosed according to Poser [18] or McDonald criteria [19–21]. NfL was measured by SIMOA, electrochemiluminescence method (ECL) or enzyme linked immunosorbent assay (ELISA). In details, for diagnostic value, the blood NfL levels were compared between MS vs. controls which included healthy control (HC) and non-inflammatory neurological disease control (NINDC), MS vs. clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS) vs. progressive MS (PMS), and MS in relapse vs. MS in remission. The mean value and standard deviation (SD) of blood NfL levels, or the other statistics that can be converted to mean and SD, in both groups should be provided. For predictive value, hazard ratio (HR) estimate and corresponding 95%CI for high blood NfL levels predicting the time to Expanded Disability Status Scale (EDSS) score ≥4.0 or relapse should be provided. Cases series, meeting abstracts, reviews, meta-analyses and studies with pediatrics patients were excluded. For articles with overlapped samples, only the one with largest sample size was included.
Quality assessment
For studies comparing the blood NfL levels in two group, the quality was assessed by using Newcastle-Ottawa scale (NOS) for case-control studies, which comprised selection, comparability and exposure domains. For studies investigating the predictive value, the quality was assessed by using NOS for cohort studies, which contained selection, comparability and outcome domains. The total stars assigned to all items were 9. Studies with 5 or 6 stars were considered as moderate-quality studies and those with 7 or more stars were of high quality.
Data extraction
We extracted the following information from all eligible studies: first author, year of publication, country, diagnostic criteria of MS, sample source (serum or plasma), method of blood NfL measurement, baseline characteristics (sample size, age, gender, EDSS score, disease duration, DMT use). For diagnostic value, the mean value and SD of NfL levels in both groups were extracted. If the studies only provided median value with interquartile (IQR) or range of NfL levels, we converted these values to mean and SD statistics by using methods introduced by Wan et al [22] and Luo et al [23]. Similarly, the median with IQR or range of baseline age, EDSS score and disease duration were converted to mean with SD when we performed meta-regression analysis. For predictive value, the cutoffs of high NfL levels and the HR estimates for EDSS score≥4.0 or relapse were extracted.
The literature search and selection, quality assessment and data extraction were performed by two independent researchers. Discrepancies were resolved by further discussion of these two researchers.
Statistical analysis
Between-study heterogeneity was evaluated by I2 statistic and Q test. I2 <25%, between 25% and 50%, and >50% indicated low, medium and high levels of heterogeneity, respectively. For meta-analysis with I2<50% and P value of Q test>0.10, the fixed-effect model was used; otherwise, the random-effect model was applied. The effect sizes were estimated by standard mean difference (SMD) and 95%CI with Cohen’s d [24] for diagnostic value and calculated by HR and 95%CI for predictive value. We considered the SMD of ≤0.2, between 0.2 and 0.8, and ≥0.8 as small, moderate and large effect size, respectively [24]. For MS vs. Control, subgroup analyses regarding control type (HC, NINDC), sample source (serum, plasma), NfL detection method (SIMOA, ECL or ELISA) and DMT use (no, mixed or missing)were performed. Specifically, only if the authors declared enrollment of age-matched controls, the study was classified as age-matched; otherwise it was not age-matched, even though there was no statistical difference by baseline age comparison. Since studies have shown blood NfL was highly correlated with age, we analyzed age-matched studies and non-age-matched studies separately. Meta-regression analyses for mean age, percent of female, mean disease duration, mean EDSS score and sample size were also performed to identify potential source of heterogeneity for meta-analyses including 10 or more eligible studies. Sensitivity analysis was also performed with Leave-One-Out method, i.e. omitting one study and recalculating the pooled effect size each time. Publication bias was assessed by viewing the symmetry of funnel plot and by Egger’s test. All analyses were performed by using STATA 16 (StataCorp, TX, USA).
Results
Baseline characteristics of eligible studies
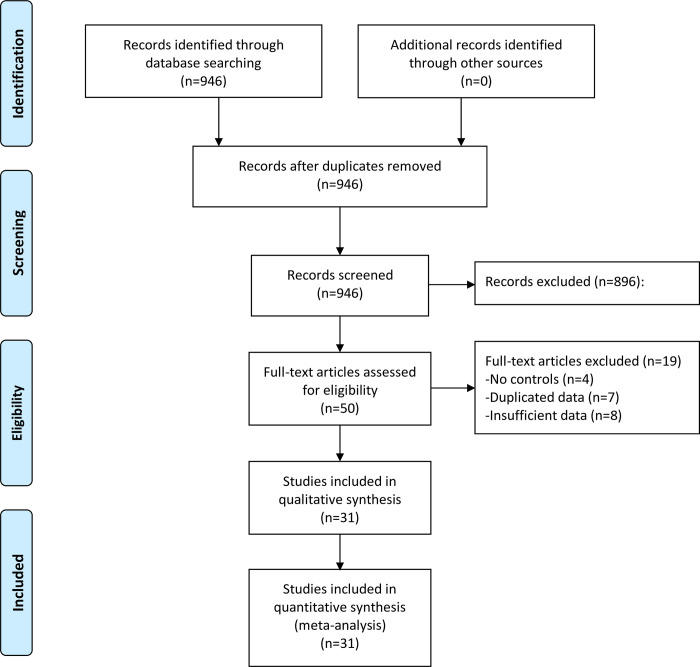
A total of 31 studies fulfilling the inclusion and exclusion criteria were finally included in quantitative analysis (Fig 1) [10, 11, 13, 14, 25–51]. Among them, 28 studies comprising 6545 MS patients and 2477 controls were eligible for meta-analysis of diagnosis value (Table 1), and 5 studies with 4444 MS patients were synthesized in meta-analysis of predictive value (S1 Table).
Fig 1. Flowchart of literature search.
Table 1. Characteristics of studies included in meta-analysis for diagnosis value of blood NfL concentrations.
| Author | Year | Country | Patient group | Control group | Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | N | Age, y | %Female | Disease duration, y | EDSS score | DMT use (%) | Condition | N | Age, y | %Female | ||||
| Disanto | 2015 | Various | MS | 100 | 31.2 | 67 | NA | 2.18 | NA | HC | 92 | 36.4 | 63 | MS vs. HC, MS vs. CIS |
| Kuhle | 2016 | Switzerland | MS | 31 | 31.6 | 64.5 | 1.32 | 2 | 0 | HC | 18 | 30.8 | 55.6 | MS vs. HC, Relapse vs. Remission |
| Disanto | 2017 | Switzerland | MS, SMSC cohort | 246 | 42.4 | 65.9 | 8.21 | 2.82 | 50.8 | HC | 254 | 44.4 | 68.1 | MS vs. HC |
| MS, LUGANO cohort | 142 | 38.5 | 64.9 | NA | NA | NA | ||||||||
| Piehl | 2017 | Sweden | MS | 39 | 39.6 | 61.5 | NA | 2.4 | NA | NINDC | 27 | 35.2 | 55.6 | MS vs. NINDC |
| Novakova | 2017 | Sweden | PMS | 82 | 48 | 54.9 | NA | 5.4 | NA | HC | 42 | 28 | 40.5 | PMS vs. RRMS, Relapse vs. Remission |
| RRMS | 204 | 40.2 | 70.1 | NA | 2.6 | NA | ||||||||
| Barro | 2018 | Switzerland | MS | 257 | 44.5 | 69.6 | 11.05 | 3 | 64.6 | HC | 258 | 44.3 | 68.6 | MS vs. HC, PMS vs. RRMS |
| Hakansson | 2018 | Sweden | MS | 41 | 30.29 | 78 | 11.8 | 1.68 | 0 | HC | 22 | 33.1 | 77.3 | MS vs. HC |
| Abdelhak | 2018 | Germany | MS in relapse | 18 | 31.8 | NA | 0.62 | 1.82 | 11.1 | NA | NA | NA | NA | Relapse vs. Remission |
| MS in remission | 24 | 37.4 | NA | 4.19 | 2.88 | 16.7 | ||||||||
| Hogel | 2018 | Finland | MS | 79 | 50.2 | 70.9 | 15.48 | 3.7 | 64.6 | HC | 13 | 47 | 69.2 | MS vs. HC, PMS vs. RRMS |
| Ferraro | 2019 | Italy | PMS | 70 | 58.9 | 30 | 20 | 6.32 | 0 | HC | 10 | 56.9 | 40 | PMS vs. RRMS |
| RRMS | 21 | 42.9 | 28.6 | 9.56 | 1.32 | 0 | ||||||||
| Watanabe | 2019 | Japan | MS | 49 | 39 | 73.5 | 8.16 | 4.03 | 55.1 | HC | 49 | 46.2 | 85.7 | MS vs. HC, PMS vs. RRMS |
| Thebault | 2019 | Canada | MS | 23 | 27 | 51.9 | 7.42 | 4.82 | 100 | NINDC | 33 | 37.5 | 72.7 | MS vs. NINDC |
| Jakimovski | 2019 | US | MS | 127 | 48.4 | 70.1 | 16.3 | 3.2 | 78.7 | HC | 52 | 43.8 | 86.8 | MS vs. HC, MS vs. CIS, PMS vs. RRMS |
| Sejbaek | 2019 | Denmark | MS | 52 | 34.1 | 86.5 | NA | 1.77 | 0 | HC | 23 | 38.2 | 87 | MS vs. HC |
| Baldassari | 2019 | US | MS | 22 | 46.4 | 68.2 | 12.4 | 5.5 | 0 | HC | 10 | 47.1 | 60 | MS vs. HC |
| Manouchehrinia | 2020 | Sweden | MS | 3092 | 38.4 | 70.3 | 4.23 | NA | NA | HC | 1026 | 39.8 | 73.2 | MS vs. HC |
| Bittner | 2020 | Germany | MS | 445 | 32.4 | 67.2 | 2 | 1.5 | 0 | NA | NA | NA | NA | MS vs. CIS |
| CIS | 369 | 33.4 | 69.4 | 0.14 | 1.5 | 0 | ||||||||
| Thebault | 2020 | Canada | MS | 67 | 38 | 70.1 | NA | 1.5 | 3.0 | NINDC | 37 | 38 | 81.1 | MS vs. NINDC, Relapse vs. Remission |
| Ayrignac | 2020 | France | PMS | 18 | 50.8 | 77.8 | 3.5 | 3.86 | 0 | NA | NA | NA | NA | PMS vs. RRMS, Relapse vs. Remission |
| RRMS | 111 | 39.9 | 74.8 | 7.17 | 1.35 | 48.7 | ||||||||
| Huss | 2020 | Germany | PMS | 39 | 53 | 53.8 | NA | 5.65 | 7.7 | NA | NA | NA | NA | PMS vs. RRMS |
| RRMS | 47 | 36.1 | 61.7 | NA | 2.53 | 14.9 | ||||||||
| Olsson | 2020 | Denmark | MS, cohort 1 | 49 | 36.1 | 65.3 | 2.94 | 1.68 | 0 | HC | 58 | 38.1 | 48.3 | MS vs. HC |
| MS, cohort 2 | 68 | 35.3 | 76.5 | 1.18 | 2 | 0 | HC | 50 | 33 | 68 | MS vs. HC | |||
| Bridel | 2020 | Netherlands | MS | 89 | 45.1 | 71.9 | NA | NA | 23.6 | HC | 88 | 44.5 | 44.3 | MS vs. HC, PMS vs. RRMS |
| Saraste | 2020 | Finland | MS | 79 | 48.1 | 75.9 | 14.27 | 2.91 | 68.4 | HC | 10 | 48.3 | 70 | MS vs. HC, PMS vs. RRMS |
| Szilasiova | 2021 | Slovak | MS | 159 | 40.4 | 64.8 | 7.54 | 3.93 | 100 | HC | 66 | 42.5 | 68.2 | MS vs. HC |
| Liu | 2021 | China | MS | 98 | 32.1 | 67.3 | 5.35 | 2.18 | 77.6 | HC | 84 | 29.4 | 64.3 | MS vs. HC, Relapse vs. Remission |
| Cruz-Gomez | 2021 | Spain | MS | 35 | 38.4 | 57.1 | 3.13 | 1 | 94.3 | HC | 23 | 35.4 | 56.5 | MS vs. HC |
| Niiranen | 2021 | Finland | MS | 63 | 49.7 | 73 | 21.12 | 2.06 | 74.6 | HC | 14 | 47.2 | 50 | MS vs. HC |
| Harp | 2022 | America | MS | 90 | 37.0 | 67.8 | NA | NA | 16.7 | HC | 118 | 42.5 | 60.2 | MS vs. HC |
NfL: neurofilament light chain; MS: multiple sclerosis; PMS: progressive MS; RRMS: relapsing-remitting MS; CIS: clinically isolated syndrome; HC: healthy control; NINDC: non-inflammatory neurological disease control; EDSS: Expanded Disability Status Scale; DMT: disease-modifying therapy; NA: not available.
For diagnosis value analysis, 4 studies detected plasma NfL (pNfL) concentrations [26, 34, 36, 43] and the others measured serum NfL (sNfL) levels. Two studies applied ECL method [10, 11], one used ELISA [40], and the others adopted the highly sensitive SIMOA mothed for NfL measurements in blood. Ten studies enrolled age-matched controls with MS patients [13, 28, 32, 34–37, 40, 44, 45] and 7 recruited sex-matched controls [28, 32, 34–36, 40, 43]. The other 18 studies that did not declare whether controls were age-matched were then considered as not age-matched studies, even though there was no statistical difference of age at baseline comparison in some studies. As to DMT use, 7 recruited treatment-naïve patients [10, 28, 32, 35, 36, 42, 46], while the other studies reported a proportion of patients treated with DMT or missing information of DMT use. Quality assessment using NOS for case-control studies identified 19 moderate-quality studies that had 5 or 6 stars and 9 high-quality studies with 7–9 stars (S2 Table). The characteristics of the included studies were summarized in Table 1.
Among studies exploring the predictive value of blood NfL concentrations, 3 measured sNfL and 2 detected pNfL [32, 34, 41, 47, 48]. The cutoffs for high NfL levels were 80th percentile of age-corrected reference values in two studies but differed in the other studies. Two studies investigated the association of high blood NfL level with time to relapse and 3 with time to reaching ESS score≥4.0. All studies were awarded with 7 stars according to NOS for cohort studies (S3 Table). The characteristics of these studies were summarized in S1 Table.
MS vs. control
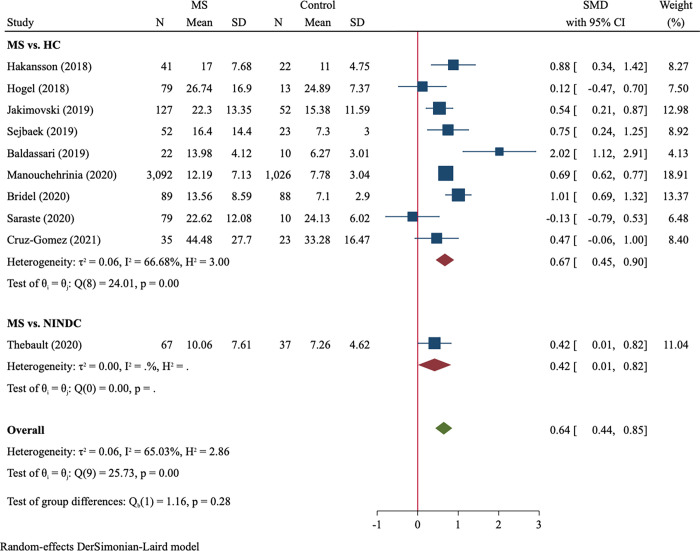
Twenty-three studies compared blood NfL between MS patients and controls. Age-matched and non-age-matched studies were analyzed in separate. In analysis of age-matched studies, 3683 MS patients and 1304 age-matched healthy controls were included (Table 2). There was obvious between-study heterogeneity (I2 = 65.0%) and the random-effect model was used. The blood NfL levels in MS were significantly higher than those in age-matched controls with a moderate effect size (SMD = 0.64, 95%CI 0.44–0.85, P<0.001, Fig 2). We observed large effect size in studies recruiting treatment-naïve MS patients (SMD = 0.91, 95%CI 0.39–1.43) and moderate effect size in studies with mixed use or missing data of DMT (SMD = 0.56, 95%CI 0.32–0.80; between-subgroup comparison P = 0.236). Blood NfL difference between MS and non-matched controls was analyzed in 14 studies comprising 1414 MS patients and 1375 controls (Table 2). MS patients had significantly higher NfL levels than non-matched controls (SMD = 0.76, 95%CI 0.56–0.96, P<0.001, S1 Fig). Between-subgroup comparison showed a significantly larger effect size of treatment-naïve subgroup than treatment subgroup (SMD = 1.20 vs. 0.65, P = 0.007).
Table 2. Summary of meta-analysis for diagnosis value of blood NfL concentrations.
| Analysis | No. of studies | No. of participants | Pooled effect size | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| SMD | 95%CI | P | I2, % | P | |||
| MS vs. Control, age-matched | 10 | 3683/1304 | 0.64 | 0.44–0.85 | <0.001 | 65.0 | 0.002 |
| Control type | |||||||
| HC | 9 | 3616/1267 | 0.67 | 0.45–0.90 | <0.001 | 66.7 | 0.002 |
| NINDC | 1 | 67/37 | 0.42 | 0.01–0.82 | 0.044 | - | - |
| Sample source | |||||||
| Serum | 8 | 539/255 | 0.62 | 0.30–0.95 | <0.001 | 72.4 | <0.001 |
| Plasma | 2 | 3144/1049 | 0.69 | 0.62–0.77 | <0.001 | 0 | 0.830 |
| NfL detection method | |||||||
| SIMOA | 9 | 3648/1281 | 0.66 | 0.44–0.88 | <0.001 | 68.1 | 0.002 |
| ECL or ELISA | 1 | 35/23 | 0.47 | -0.07, 1.00 | 0.085 | - | - |
| DMT use | |||||||
| No | 4 | 182/92 | 0.91 | 0.39–1.42 | <0.001 | 71.4 | 0.015 |
| Mixed or missing data | 6 | 3501/1212 | 0.56 | 0.32–0.80 | <0.001 | 66.6 | 0.010 |
| MS vs. Control, not matched | 14 | 1414/1375 | 0.76 | 0.56–0.96 | <0.001 | 81.5 | <0.001 |
| Control type | |||||||
| HC | 12 | 1352/1315 | 0.74 | 0.53–0.95 | <0.001 | 83.5 | <0.001 |
| NINDC | 2 | 62/60 | 0.94 | 0.37–1.51 | 0.001 | 55.4 | 0.135 |
| Sample source | |||||||
| Serum | 13 | 1255/1309 | 0.78 | 0.57–0.99 | <0.001 | 82.6 | <0.001 |
| Plasma | 1 | 159/66 | 0.54 | 0.25–0.83 | <0.001 | - | - |
| NfL detection method | |||||||
| SIMOA | 12 | 1283/1265 | 0.75 | 0.53–0.97 | <0.001 | 83.8 | <0.001 |
| ECL or ELISA | 2 | 131/110 | 0.87 | 0.61–1.14 | <0.001 | 0 | 0.565 |
| DMT use | |||||||
| No | 3 | 197/152 | 1.20 | 0.85–1.55 | <0.001 | 50.5 | 0.133 |
| Mixed or missing data | 11 | 1217/1223 | 0.65 | 0.46–0.84 | <0.001 | 76.5 | <0.001 |
| RRMS vs. HC | 16 | 1239/858 | 0.58 | 0.36–0.80 | <0.001 | 79.0 | <0.001 |
| PMS vs. HC | 8 | 362/522 | 1.01 | 0.65–1.36 | <0.001 | 76.1 | <0.001 |
| MS vs. CIS | 3 | 672/487 | 0.30 | 0.18–0.42 | <0.001 | 0 | 0.519 |
| PMS vs. RRMS | 10 | 842/419 | 0.56 | 0.27–0.85 | <0.001 | 79.8 | <0.001 |
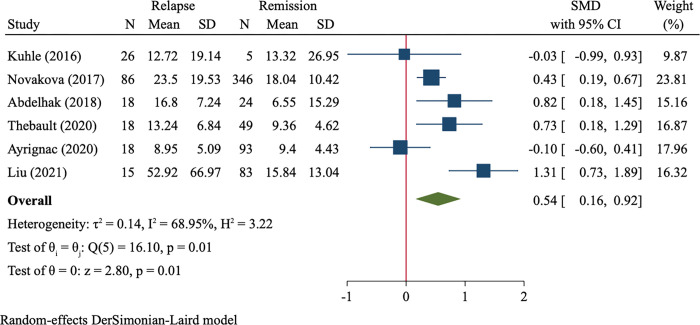
| Relapse vs. Remission | 6 | 181/600 | 0.54 | 0.16–0.92 | 0.005 | 69.0 | 0.007 |
SIMOA: single molecular array; SMD: standard mean difference
Fig 2. Forest plot of blood NfL concentrations between MS patients vs. age-matched controls.
NfL: neurofilament light chain; MS: multiple sclerosis; HC: healthy control; NINDC: non-inflammatory neurological disease control; SMD: standard mean difference.
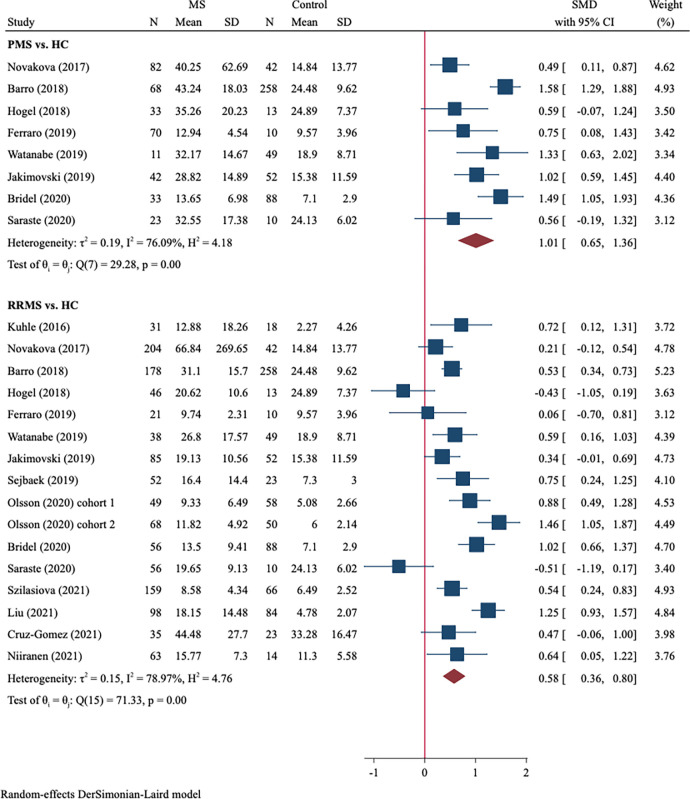
We further compared the blood NfL levels in patients at different MS stages (RRMS and PMS) with those in HC. A total of 1239 RRMS vs. 858 HC from 16 studies and 362 PMS vs. 522 HC from 8 studies were included. RRMS patients had significantly higher levels of blood NfL (SMD = 0.58, 95%CI 0.36–0.80, P<0001, Fig 3) compared with HC, which showed a moderate effect size. Moreover, a large effect size of the blood NfL difference between PMS patients and HC was observed (SMD = 1.01, 95%CI 0.65–1.36, P<0.001, Fig 3).
Fig 3. Forest plot of blood NfL concentrations between PMS vs. HC and RRMS vs. HC.
PMS: progressive MS; RRMS: relapsing-remitting MS.
MS vs. CIS
Three studies involving 672 MS and 487 CIS compared blood NfL levels between both groups. Among them, Disanto et al defined CIS according to the criteria proposed by Miller et al [52], and the other two according to 2010 revised McDonald criteria [20]. There was no between-study heterogeneity. Meta-analysis using the fixed-effect model was used showed significantly higher blood NfL levels in MS than in CIS (SMD = 0.30, 95%CI 0.18–0.42, P<0.001, S2 Fig).
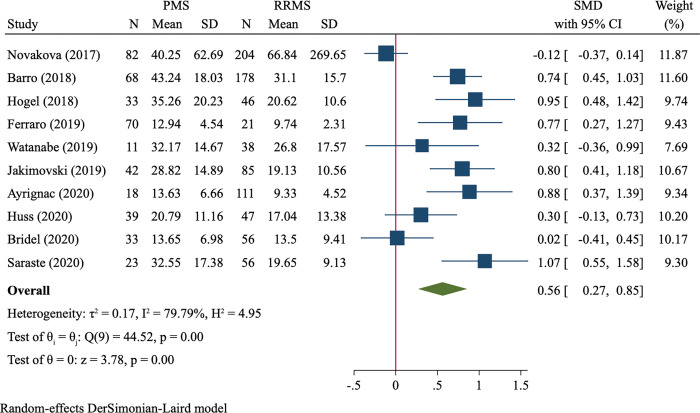
PMS vs. RRMS
A total of 842 PMS and 419 RRMS were included, and the random-effect model was used due to substantial heterogeneity (I2 = 79.8%). We found that PMS patients had significantly higher levels of blood NfL than RRMS patients (SMD = 0.56, 95%CI 0.27–0.85, P<0.001, Fig 4).
Fig 4. Forest plot of blood NfL levels between PMS vs. RRMS.
MS in relapse vs. MS in remission
Six studies compared blood NfL levels of MS in relapse vs. MS in remission (181 cases vs. 600 cases) and were included in synthesis analysis. Random-effect model analysis demonstrated higher NfL levels in relapsed patients than in remitted patients (SMD = 0.54, 95%CI 0.16–0.92, P = 0.005, Fig 5).
Fig 5. Forest plot of blood NfL levels between MS in relapse vs. MS in remission.
Predictive value of high blood NfL level
We investigated whether high blood NfL level at baseline could predict the hazard of reaching EDSS score≥4.0 and relapse. Patients with higher blood NfL levels were earlier to reach EDSS score≥4.0 compared with those with lower levels (HR = 2.36, 95%CI 1.32–4.21, P = 0.004, S3 Fig). However, no difference of time to relapse was observed between both groups (HR = 1.32, 95%CI 0.90–1.93, P = 0.155, S4 Fig).
Meta-regression analysis, sensitivity analysis and publication bias
We explored the potential source of heterogeneity by meta-regression analysis in “MS vs. Control” comparison (Table 3). Mean age was significantly correlated with SMD estimates in not-age-matched subgroup (P = 0.021, S5 Fig), indicating that mean age could partly explain the source of heterogeneity. However, the correlation was not found in age-matched subgroup (P = 0.488, S6 Fig). The association of SMD with percent of female, mean EDSS score, mean disease duration and sample size were not evident according to meta-regression analysis.
Table 3. Results of meta-regression for blood NfL difference between MS and controls.
| Covariate | Coefficient | SE | t | P |
|---|---|---|---|---|
| Age-matched | ||||
| Mean age | -0.014 | 0.02 | -0.65 | 0.488 |
| Percent of female | -1.01 | 1.28 | -0.79 | 0.430 |
| Mean disease duration | -0.015 | 0.035 | -0.43 | 0.670 |
| Mean EDSS score | 0.148 | 0.131 | 1.13 | 0.259 |
| Sample size# | -0.0013 | 0.0032 | -0.39 | 0.696 |
| Not age-matched | ||||
| Mean age | -0.041 | 0.018 | -2.30 | 0.021 |
| Percent of female | 1.25 | 1.85 | 0.68 | 0.498 |
| Mean disease duration | -0.033 | 0.02 | -1.63 | 0.103 |
| Mean EDSS score | -0.090 | 0.098 | -0.92 | 0.358 |
| Sample size | -0.0006 | 0.0007 | -0.86 | 0.387 |
# Excluding Manouchehrinia et al’s study that had a very large sample size.
Sensitivity analysis using Leave-One-Out method demonstrated that omitting one single study did not significantly influence the pooled effect size of the rest of studies. There was no obvious asymmetry in funnel plots of meta-analyses, and Egger’s test indicated no evident publication bias (S4 Table).
Discussion
As far as we know, this is the first meta-analysis investigating the diagnostic and predictive value of blood NfL concentrations in MS patients. In line with previous meta-analyses finding elevated CSF NfL concentration in MS patients [7, 53–55], the present study demonstrates NfL levels in blood, which are strongly correlated with those in CSF, are also significantly higher in MS patients compared with controls. Our study indicates that blood NfL may serve as a biomarker for MS diagnosis.
However, some influential factors, such as age, BMI and quantification process, should be noted upon the clinical utility of blood NfL [56]. Blood NfL levels are highly age-dependent. Among healthy controls, young individuals have low and relatively stable sNfL concentrations while people older than 60 years have annually increased sNfL levels associated with age-related neurodegeneration [14, 57]. Besides, sNfL decreases with BMI in age stratified subgroups [58, 59]. Therefore, age and BMI are confounding factors for sNfL as a biomarker, which may influence the clinical implementation. The comparison between MS and unmatched controls may introduce some bias to the meta-analysis. This is supported by our meta-regression analysis revealing a negative correlation between mean age and blood NfL difference in not-age-matched studies (P = 0.021). On the contrary, among studies recruiting age-matched controls, mean age was not associated with blood NfL difference (P = 0.488). These results indicate that age-specific reference of blood NfL should be established. Recently, several studies have tried to construct an age- and/or BMI-adjusted model for sNfL [16, 51]. Using multiple large datasets, Benkert et al established an age- and BMI-corrected reference database of sNfL values, and further showed the merit of sNfL percentiles and Z scores in predicting disease course and response to DMT [16]. Thus, age-corrected sNfl value or a composite index may be more reliable and can be used in future researches.
Besides of age, DMT use is another influential factor of blood NfL. DMT-treated patients had significantly lower sNfL levels in untreated patients, and the treatment effect was independent of all the other baseline variables as suggested by multivariate analysis [14]. In our meta-analysis, several studies only recruited patients who had not previsouly been treated with DMT. Subgroup analyses, in both age-matched and non-matched studies, demonstrated a larger SMD effect size in treatment-naïve subgroup than treatment subgroup, suggesting a potential role of DMT in reducing blood Nfl. Follow-up of DMT-treated patients showed significantly reduced sNfL levels than baseline, which were not observed in untreated patients [30]. These observations also suggest that longitudinal sampling of blood NfL may help monitor DMT treatment effect in MS patients. However, the impact of DMT on blood NfL may vary among disease subtypes. Teriflunomide reduced sNfL in relapsing MS patients [60] and dimethyl fumarate decreased blood NfL in RRMS patients [36]. Whereas, no significant changes were observed in PMS patients treated with ibudilast [61] and SPMS patients with simvastatin treatment [62].
NfL is not a biomarker specific to MS. It reflects neuro-axonal damage and can be detected in elevated levels in the other inflammatory neurologic disorders. Despite higher blood NfL levels in MS than in NINDCs, no significant difference is observed between MS and inflammatory neurological disease controls (INDCs) [25, 30]. This phenomenon is also found in CSF measurements [8]. Both CSF and blood NfL cannot replace conventional MRI for differential diagnosis between MS and the other inflammatory neurologic disorders.
Apart from disease diagnosis, blood NfL may help differentiate MS from CIS and distinguish MS subtypes. CSF NfL can be used to distinguish CIS from healthy controls with high accuracy [63], whereas a recent meta-analysis showed no significant difference of CSF NfL levels between MS and CIS [7]. In present study, we found blood NfL levels were significantly higher in MS patients than in CIS patients. Bittner et al validated the application of sNfL in reclassifying CIS under McDonald diagnostic criteria 2010 (i.e. CIS[2010]) as CIS or RRMS under McDonald diagnostic criteria 2017 (i.e. CIS[2017] and RRMS[2017]), and found the inclusion of sNfL to McDonald diagnostic criteria significantly increased the area under the curve [33]. Blood NfL may be a useful biomarker for differential diagnosis between CIS and MS.
We observed higher blood NfL concentrations in PMS patients than in RRMS patients with moderate effect size. This may be attributed to greater inflammatory activity in this group of patients, especially in secondary PMS (SPMS) [56], as well as older age of PMS patients than RRMS patients. Several included studies comparing PMS and RRMS showed significantly older age and higher NfL levels of PMS patients [26, 37, 49, 50]. After correction for age, PMS still had higher sNfL levels than RRMS patients [29]. Several studies revealed that RRMS patients with higher serum NfL levels had greater risk of conversion to SPMS [32, 34, 64]. However, there is no such difference in CSF samples, and even opposite results were observed in some meta-analyses [7, 54]. In addition, we found blood NfL levels were higher in relapsed MS patients than in remitted patients, which was similar to what has been found in CSF samples [7, 54].
Blood NfL is associated with future disease activity and progression [65]. Patients with baseline higher sNfL levels had higher risk of experiencing relapse, accelerated brain and spinal cord volume loss, and EDSS worsening post blood sampling [14, 29]. Upper tertile of longitudinal measures of sNfL predicted higher risk of EDSS worsening in a long term as far as 15 years [66]. We further assessed whether blood NfL could predict time to relapse and EDSS worsening through meta-analysis. Patients with high NfL levels were earlier to reach EDSS score≥4.0 but had comparable time to relapse compared with those with low NfL levels. Thus, blood NfL can be used to predict disease progression of MS patients.
Several limitations in our study should be noted. Firstly, there was substantial between-study heterogeneity, which may be caused by cofounders such as age, gender, disease activity, and DMT usage. Secondly, the sample size of some subgroups, including PMS vs. RRMS, MS in relapse vs. MS in remission and the predictive value, was small. Thirdly, NfL levels in most studies were not in normal distribution and shown as median with IQR or range. We had to convert them into mean with SD, which did not accurately reflect the difference. Patient-level data may be warranted.
In conclusion, the present meta-analysis demonstrates that blood NfL is a potential biomarker for MS diagnosis, MS subtype differentiation, and the prediction of disease worsening.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
CIS: clinically isolated syndrome.
(TIF)
EDSS: Expanded Disability Status Scale; HR: hazard ratio.
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Group GBDNDC. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877–97. doi: 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. [DOI] [PubMed] [Google Scholar]
- 3.Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–88. doi: 10.1212/WNL.0000000000005347 [DOI] [PubMed] [Google Scholar]
- 4.Ferreira-Atuesta C, Reyes S, Giovanonni G, Gnanapavan S. The Evolution of Neurofilament Light Chain in Multiple Sclerosis. Front Neurosci. 2021;15:642384. doi: 10.3389/fnins.2021.642384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, et al. NfL (Neurofilament Light Chain) Levels as a Predictive Marker for Long-Term Outcome After Ischemic Stroke. Stroke. 2019;50(11):3077–84. doi: 10.1161/STROKEAHA.119.026410 [DOI] [PubMed] [Google Scholar]
- 6.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–89. doi: 10.1038/s41582-018-0058-z [DOI] [PubMed] [Google Scholar]
- 7.Momtazmanesh S, Shobeiri P, Saghazadeh A, Teunissen CE, Burman J, Szalardy L, et al. Neuronal and glial CSF biomarkers in multiple sclerosis: a systematic review and meta-analysis. Rev Neurosci. 2021;32(6):573–95. [DOI] [PubMed] [Google Scholar]
- 8.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870–81. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 9.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34(2):131–44. doi: 10.1111/j.1365-2990.2007.00926.x [DOI] [PubMed] [Google Scholar]
- 10.Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler. 2016;22(12):1550–9. doi: 10.1177/1352458515623365 [DOI] [PubMed] [Google Scholar]
- 11.Disanto G, Adiutori R, Dobson R, Martinelli V, Dalla Costa G, Runia T, et al. Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. J Neurol Neurosurg Psychiatry. 2016;87(2):126–9. doi: 10.1136/jnnp-2014-309690 [DOI] [PubMed] [Google Scholar]
- 12.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. 2016;54(10):1655–61. doi: 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 13.Hogel H, Rissanen E, Barro C, Matilainen M, Nylund M, Kuhle J, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler. 2020;26(2):210–9. doi: 10.1177/1352458518819380 [DOI] [PubMed] [Google Scholar]
- 14.Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–70. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhle J, Kropshofer H, Haering DA, Kundu U, Meinert R, Barro C, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007–e15. doi: 10.1212/WNL.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli O, Maceski A, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246–57. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227–31. doi: 10.1002/ana.410130302 [DOI] [PubMed] [Google Scholar]
- 19.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7. doi: 10.1002/ana.1032 [DOI] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. doi: 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. Stat Power Anal Behav Sci. 1988;2:567. [Google Scholar]
- 25.Watanabe M, Nakamura Y, Michalak Z, Isobe N, Barro C, Leppert D, et al. Serum GFAP and neurofilament light as biomarkers of disease activity and disability in NMOSD. Neurology. 2019;93(13):e1299–e311. doi: 10.1212/WNL.0000000000008160 [DOI] [PubMed] [Google Scholar]
- 26.Ferraro D, Guicciardi C, De Biasi S, Pinti M, Bedin R, Camera V, et al. Plasma neurofilaments correlate with disability in progressive multiple sclerosis patients. Acta Neurol Scand. 2020;141(1):16–21. doi: 10.1111/ane.13152 [DOI] [PubMed] [Google Scholar]
- 27.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):14798. Epub 2018/10/06. doi: 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, et al. Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflammation. 2018;15(1):209. doi: 10.1186/s12974-018-1249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barro C, Benkert P, Disanto G, Tsagkas C, Amann M, Naegelin Y, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382–91. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 30.Novakova L, Zetterberg H, Sundstrom P, Axelsson M, Khademi M, Gunnarsson M, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230–7. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler. 2018;24(8):1046–54. doi: 10.1177/1352458517715132 [DOI] [PubMed] [Google Scholar]
- 32.Thebault S, Abdoli M, Fereshtehnejad SM, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. doi: 10.1038/s41598-020-67504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bittner S, Steffen F, Uphaus T, Muthuraman M, Fleischer V, Salmen A, et al. Clinical implications of serum neurofilament in newly diagnosed MS patients: A longitudinal multicentre cohort study. EBioMedicine. 2020;56:102807. doi: 10.1016/j.ebiom.2020.102807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manouchehrinia A, Stridh P, Khademi M, Leppert D, Barro C, Michalak Z, et al. Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457–e67. doi: 10.1212/WNL.0000000000009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldassari LE, Planchon SM, Bermel RA, Nakamura K, Fisher E, Feng J, et al. Serum neurofilament light chain concentration in a phase 1/2 trial of autologous mesenchymal stem cell transplantation. Mult Scler J Exp Transl Clin. 2019;5(4):2055217319887198. doi: 10.1177/2055217319887198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sejbaek T, Nielsen HH, Penner N, Plavina T, Mendoza JP, Martin NA, et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naive relapsing MS patients. J Neurol Neurosurg Psychiatry. 2019;90(12):1324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakimovski D, Zivadinov R, Ramanthan M, Hagemeier J, Weinstock-Guttman B, Tomic D, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: A longitudinal retrospective 5-year study. Mult Scler. 2020;26(13):1670–81. doi: 10.1177/1352458519881428 [DOI] [PubMed] [Google Scholar]
- 38.Thebault S, D RT, Lee H, Bowman M, Bar-Or A, Arnold DL, et al. High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e598. doi: 10.1212/NXI.0000000000000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niiranen M, Kontkanen A, Jaaskelainen O, Tertsunen HM, Selander T, Hartikainen P, et al. Serum GFAP and NfL levels in benign relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2021;56:103280. doi: 10.1016/j.msard.2021.103280 [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Gomez AJ, Forero L, Lozano-Soto E, Cano-Cano F, Sanmartino F, Rashid-Lopez R, et al. Cortical Thickness and Serum NfL Explain Cognitive Dysfunction in Newly Diagnosed Patients With Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1074 doi: 10.1212/NXI.0000000000001074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin TY, Vitkova V, Asseyer S, Martorell Serra I, Motamedi S, Chien C, et al. Increased Serum Neurofilament Light and Thin Ganglion Cell-Inner Plexiform Layer Are Additive Risk Factors for Disease Activity in Early Multiple Sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1051. doi: 10.1212/NXI.0000000000001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C, Lu Y, Wang J, Chang Y, Wang Y, Chen C, et al. Serum neurofilament light chain and glial fibrillary acidic protein in AQP4-IgG-seropositive neuromyelitis optica spectrum disorders and multiple sclerosis: A cohort study. J Neurochem. 2021;159(5):913–22. doi: 10.1111/jnc.15478 [DOI] [PubMed] [Google Scholar]
- 43.Szilasiova J, Rosenberger J, Fedicova M, Mikula P, Urban P, Gdovinova Z, et al. Neurofilament Light Chain Levels Are Associated with Disease Activity Determined by No Evident Disease Activity in Multiple Sclerosis Patients. Eur Neurol. 2021;84(4):272–9. doi: 10.1159/000515806 [DOI] [PubMed] [Google Scholar]
- 44.Saraste M, Bezukladova S, Matilainen M, Tuisku J, Rissanen E, Sucksdorff M, et al. High serum neurofilament associates with diffuse white matter damage in MS. Neurol Neuroimmunol Neuroinflamm. 2021;8(1):e926. doi: 10.1212/NXI.0000000000000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bridel C, Verberk IMW, Heijst JJA, Killestein J, Teunissen CE. Variations in consecutive serum neurofilament light levels in healthy controls and multiple sclerosis patients. Mult Scler Relat Disord. 2021;47:102666. doi: 10.1016/j.msard.2020.102666 [DOI] [PubMed] [Google Scholar]
- 46.Olsson A, Gustavsen S, Hasselbalch IC, Langkilde AR, Sellebjerg F, Oturai AB, et al. Biomarkers of inflammation and epithelial barrier function in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102520. doi: 10.1016/j.msard.2020.102520 [DOI] [PubMed] [Google Scholar]
- 47.Haring DA, Kropshofer H, Kappos L, Cohen JA, Shah A, Meinert R, et al. Long-term prognostic value of longitudinal measurements of blood neurofilament levels. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e856. doi: 10.1212/NXI.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson V, Bentley E, Loveless S, Bianchi L, Harding KE, Wynford-Thomas RA, et al. Serum neurofilament-light concentration and real-world outcome in MS. J Neurol Sci. 2020;417:117079. doi: 10.1016/j.jns.2020.117079 [DOI] [PubMed] [Google Scholar]
- 49.Huss A, Otto M, Senel M, Ludolph AC, Abdelhak A, Tumani H. A Score Based on NfL and Glial Markers May Differentiate Between Relapsing-Remitting and Progressive MS Course. Front Neurol. 2020;11:608. doi: 10.3389/fneur.2020.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayrignac X, Le Bars E, Duflos C, Hirtz C, Maleska Maceski A, Carra-Dalliere C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. doi: 10.1038/s41598-020-67934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harp C, Thanei GA, Jia X, Kuhle J, Leppert D, Schaedelin S, et al. Development of an age-adjusted model for blood neurofilament light chain. Ann Clin Transl Neurol. 2022;9(4):444–53. doi: 10.1002/acn3.51524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. 2008;14(9):1157–74. doi: 10.1177/1352458508096878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, and the NFLG, et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(9):1035–48. doi: 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin SJ, McGlasson S, Hunt D, Overell J. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: a meta-analysis of case-control studies. J Neurol Neurosurg Psychiatry. 2019;90(9):1059–67. doi: 10.1136/jnnp-2018-319190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai L, Huang J. Neurofilament light chain as a biological marker for multiple sclerosis: a meta-analysis study. Neuropsychiatr Dis Treat. 2018;14:2241–54. doi: 10.2147/NDT.S173280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jakimovski D, Dwyer MG, Bergsland N, Weinstock-Guttman B, Zivadinov R. Disease biomarkers in multiple sclerosis: current serum neurofilament light chain perspectives. Neurodegener Dis Manag. 2021;11(4):329–40. doi: 10.2217/nmt-2020-0058 [DOI] [PubMed] [Google Scholar]
- 57.Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. doi: 10.1038/s41467-020-14612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koini M, Pirpamer L, Hofer E, Buchmann A, Pinter D, Ropele S, et al. Factors influencing serum neurofilament light chain levels in normal aging. Aging (Albany NY). 2021;13(24):25729–38. doi: 10.18632/aging.203790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manouchehrinia A, Piehl F, Hillert J, Kuhle J, Alfredsson L, Olsson T, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol. 2020;7(1):139–43. doi: 10.1002/acn3.50972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou R, Li H, Yang H, Jiang F, Cai H, Li J, et al. Serological markers exploration and real-world effectiveness and safety of teriflunomide in south Chinese patients with multiple sclerosis. Mult Scler Relat Disord. 2022;58:103446. doi: 10.1016/j.msard.2021.103446 [DOI] [PubMed] [Google Scholar]
- 61.Fox RJ, Raska P, Barro C, Karafa M, Konig V, Bermel RA, et al. Neurofilament light chain in a phase 2 clinical trial of ibudilast in progressive multiple sclerosis. Mult Scler. 2021;27(13):2014–22. doi: 10.1177/1352458520986956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams TE, Holdsworth KP, Nicholas JM, Eshaghi A, Katsanouli T, Wellington H, et al. Assessing Neurofilaments as Biomarkers of Neuroprotection in Progressive Multiple Sclerosis: From the MS-STAT Randomized Controlled Trial. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1130. doi: 10.1212/NXI.0000000000001130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuhle J, Plattner K, Bestwick JP, Lindberg RL, Ramagopalan SV, Norgren N, et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler. 2013;19(12):1597–603. doi: 10.1177/1352458513482374 [DOI] [PubMed] [Google Scholar]
- 64.Jakimovski D, Kuhle J, Ramanathan M, Barro C, Tomic D, Hagemeier J, et al. Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol. 2019;6(9):1757–70. doi: 10.1002/acn3.50872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kouchaki E, Dashti F, Mirazimi SMA, Alirezaei Z, Jafari SH, Hamblin MR, et al. Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J. 2021;20:1308–25. doi: 10.17179/excli2021-3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhle J, Plavina T, Barro C, Disanto G, Sangurdekar D, Singh CM, et al. Neurofilament light levels are associated with long-term outcomes in multiple sclerosis. Mult Scler. 2020;26(13):1691–9. doi: 10.1177/1352458519885613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(TIF)
CIS: clinically isolated syndrome.
(TIF)
EDSS: Expanded Disability Status Scale; HR: hazard ratio.
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.