Abstract
Background
Although various solutions have been recommended for cleansing wounds, normal saline is favoured as it is an isotonic solution and is not thought to interfere with the normal healing process. Tap water is commonly used in community settings for cleansing wounds because it is easily accessible, efficient and cost‐effective; however, there is an unresolved debate about its use.
Objectives
To assess the effects of water for wound cleansing.
Search methods
For this fifth update, in May 2021 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included all randomised controlled trials (RCTs) that assessed wound cleansing using different types of water (e.g. tap water, distilled, boiled) compared with no cleansing or with other solutions (e.g. normal saline). For this update, we excluded quasi‐RCTs, thereby removing some studies which had been included in the previous version of the review.
Data collection and analysis
Two review authors independently carried out trial selection, data extraction and GRADE assessment of the certainty of evidence.
Main results
We included 13 trials in this update including a total of 2504 participants ranging in age from two to 95 years. Participants in the trials experienced open fractures, surgical wounds, traumatic wounds, anal fissures and chronic wounds. The trials were conducted in six different countries with the majority conducted in India and the USA. Three trials involving 148 participants compared cleansing with tap water with no cleansing. Eight trials involving 2204 participants assessed cleansing with tap water compared with cleansing with normal saline. Two trials involving 152 participants assessed cleansing with distilled water compared with cleansing with normal saline. One trial involving 51 participants also assessed cleansing with cooled boiled water compared with cleansing with normal saline, and cleansing with distilled water compared with cleansing with cooled boiled water.
Wound infection: no trials reported on wound infection for the comparison cleansing with tap water versus no cleansing. For all wounds, eight trials found the effect of cleansing with tap water compared with normal saline is uncertain (risk ratio (RR) 0.84, 95% confidence interval (CI) 0.59 to 1.19); very low‐certainty evidence. Two trials comparing the use of distilled water with normal saline for cleansing open fractures found that the effect on the number of fractures that were infected is uncertain (RR 0.70, 95% CI 0.45 to 1.09); very low‐certainty evidence. One trial compared the use of cooled boiled water with normal saline for cleansing open fractures and found that the effect on the number of fractures infected is uncertain (RR 0.83, 95% CI 0.37 to 1.87); very low‐certainty evidence. This trial also compared the use of distilled water with cooled boiled water and found that the effect on the number of fractures infected is uncertain (RR 0.59, 95% CI 0.24 to 1.47); very low‐certainty evidence.
Wound healing: results from three trials comparing the use of tap water with no wound cleansing demonstrated there may be little or no difference in the number of wounds that did not heal between the groups (RR 1.04, 95% CI 0.95 to 1.14); low‐certainty evidence. The effect of tap water compared with normal saline is uncertain; two trials were pooled (RR 0.57, 95% CI 0.30 to 1.07) but the certainty of the evidence is very low. Results from one study comparing the use of distilled water with normal saline for cleansing open fractures found that there may be little or no difference in the number of fractures that healed (RR could not be estimated, all wounds healed); the certainty of the evidence is low.
Reduction in wound size: the effect of cleansing with tap water compared with normal saline on wound size reduction is uncertain (RR 0.97, 95% CI 0.56 to 1.68); the certainty of the evidence is very low.
Rate of wound healing: the effect of cleansing with tap water compared with normal saline on wound healing rate is uncertain (mean difference (MD) ‐3.06, 95% CI ‐6.70 to 0.58); the certainty of the evidence is very low.
Costs: two trials reported cost analyses but the cost‐effectiveness of tap water compared with the use of normal saline is uncertain; the certainty of the evidence is very low.
Pain: results from one study comparing the use of tap water with no cleansing for acute and chronic wounds showed that there may be little or no difference in pain scores. The certainty of the evidence is low.
Patient satisfaction: results from one study comparing the use of tap water with no cleansing for acute and chronic wounds showed that there may be little or no difference in patient satisfaction. The certainty of evidence is low. The effect of cleansing with tap water compared with normal saline is uncertain as the certainty of the evidence is very low.
Authors' conclusions
All the evidence identified in the review was low or very low certainty. Cleansing with tap water may make little or no difference to wound healing compared with no cleansing; there are no data relating to the impact on wound infection. The effects of cleansing with tap water, cooled boiled water or distilled water compared with cleansing with saline are uncertain, as is the effect of distilled water compared with cooled boiled water. Data for other outcomes are limited across all the comparisons considered and are either uncertain or suggest that there may be little or no difference in the outcome.
Keywords: Adolescent; Adult; Aged; Aged, 80 and over; Child; Child, Preschool; Humans; Middle Aged; Young Adult; Drinking Water; Fractures, Open; Pain; Pain/drug therapy; Saline Solution; Sodium Chloride; Sodium Chloride/therapeutic use; Therapeutic Irrigation; Therapeutic Irrigation/methods; Wound Infection; Wound Infection/prevention & control
Plain language summary
The effects of water compared with other solutions for wound cleansing
Background
Infection can interfere with the normal wound‐healing process. In order to reduce the risk of infection, wounds are routinely cleansed to remove dirt, contamination or impurities. In this review, a wound is defined as a break in the skin.
What is the aim of this review?
The aim of this review was to investigate the effects of wound cleansing using different types of water (e.g. tap water, distilled, boiled) compared with no cleansing or with other solutions (e.g. normal saline). We measured effectiveness by looking at wound‐related infection rate and wound healing.
Researchers from Cochrane searched for all randomised controlled trials (RCTs) relating to this question and found 13 relevant studies. RCTs are studies where people are chosen at random to receive different treatments. Allocating participants in this way provides the most reliable evidence about possible relationships between the treatment used and any reported health outcomes.
Key messages
We compared wound cleansing with tap water, distilled water, cooled boiled water or saline with each other or with no cleansing. It is unclear if any of these interventions have an effect on the number of wounds which become infected. It is also unclear if they have an effect on healing (number of wounds healed; change in wound size; and rate of wound healing); costs; pain; or patient satisfaction.
What was studied in the review?
Wounds are commonly cleansed to prevent infection. The cleansing solution can be tap water, distilled water, cooled boiled water or saline. Tap water is commonly used in the community because it is easily accessible, efficient and cost‐effective; however, there is an unresolved debate about its use. We compared the effects of cleansing wounds with water with other types of water, normal saline and no cleansing.
We included all RCTs that compared wound cleansing using different types of water (e.g. tap water, distilled, boiled) compared with no cleansing or with other solutions (e.g. normal saline). Participants were from any age group and any setting e.g. hospital, community, nursing homes, general practice, wound clinics. We excluded trials that compared solutions for dental procedures or for patients with burns.
What are the main results of the review?
We included results from 13 RCTs in this review, with a combined total of 2504 participants. The participants were adults or children with a range of different types of wounds who were treated in the community, emergency departments or hospital wards. Eight trials assessed cleansing with tap water compared with cleansing with normal saline. Three trials compared cleansing with tap water with no cleansing. Two trials assessed cleansing with distilled water compared with cleansing with normal saline, one trial also assessed cleansing with cooled boiled water with cleansing with normal saline and cleansing with distilled water compared with cleansing with cooled boiled water.
We compared wound cleansing with tap water, distilled water, cooled boiled water or saline with each other or with no cleansing. It is unclear if any of these interventions have an effect on the number of wounds which become infected. It is also unclear if they have an effect on healing (number of wounds healed; change in wound size; and rate of wound healing); costs; pain; or patient satisfaction.
We are unsure if the interventions have an effect because not enough participants received each intervention to reliably assess their effects. The way that the studies were designed and conducted also means that the results may not reliably reflect the effects of the interventions. This is partly due to uncertainty over how participants were assigned to the treatments. It is also possible that many participants and healthcare professionals were aware of which treatments were being used.
How up to date is this review?
We searched for studies that had been published up to 20 May 2021.
Summary of findings
Summary of findings 1. Tap water compared with no wound cleansing.
| Tap water compared with no wound cleansing | ||||||
| Patient or population: people with anal fissures Setting: community Intervention: tap water Comparison: no wound cleansing | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no wound cleansing | Risk with Tap water | |||||
| Infection | Infection was not assessed for this comparison. | |||||
| Wound healing | Study population | RR 1.04 (0.95 to 1.14) | 148 (3 RCTs) | ⊕⊕⊝⊝ Low 1 | There may be little or no difference in wound healing between cleansing with tap water and no wound cleansing. | |
| 890 per 1,000 | 926 per 1,000 (846 to 1,000) | |||||
| Reduction in wound size | Reduction in wound size was not assessed for this comparison. | |||||
| Healing rate | Healing rate was not assessed for this comparison. | |||||
| Cost analysis | Cost analysis was not assessed for this comparison. | |||||
| Pain assessed with: VAS 0‐100, 0 = no pain; 100 = worst imaginable pain Scale from: 0 to 100 follow‐up: 4 weeks | The mean pain was 2 (SD 0.6) | The mean pain was 0 (SD 0) | ‐ | 52 (1 RCT) | ⊕⊕⊝⊝ Low 1 | No mean difference could be estimated for this trial. Two other trials did not provide data for the different intervention groups but reported P values. There may be little or no difference in pain between people whose wounds are treated with tap water and those whose wounds are not cleansed. |
| Patient satisfaction | Patient satisfaction was could not be estimated. | ‐ | 102 (2 RCTs) | ⊕⊕⊝⊝ Low 1 | Neither trial reported data that allowed the calculation of a mean difference and the studies could not be pooled. One study found that there may be no difference between the groups in patient satisfaction, the other that satisfaction may be higher in the tap water group. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; VAS: visual analogue score. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded two levels due to very serious risk of imprecision due to low numbers of participants and non‐reporting of data including measures of variance.
Summary of findings 2. Tap water compared with normal saline for wound cleansing.
| Tap water compared with normal saline for wound cleansing | ||||||
| Patient or population: people with acute and chronic wounds Setting: hospital and community Intervention: tap water Comparison: normal saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal saline | Risk with Tap water | |||||
| Infection | Study population | RR 0.84 (0.59 to 1.19) | 2204 (8 RCTs) | ⊕⊝⊝⊝ Very low 1 | The effect of cleansing with tap water compared with normal saline on infection is uncertain. | |
| 62 per 1,000 | 52 per 1,000 (37 to 74) | |||||
| Wounds healed | Study population | RR 0.57 (0.30 to 1.07) | 79 (2 RCTs) | ⊕⊝⊝⊝ Very low2 | The effect of cleansing with tap water compared with normal saline on wound healing is uncertain. | |
| 400 per 1,000 | 228 per 1,000 (120 to 428) | |||||
| Reduction in wound size | Study population | RR 0.97 (0.56 to 1.68) | 30 (1 RCT) | ⊕⊝⊝⊝ Very low 3 | The effect of cleansing with tap water compared with normal saline on reduction in wound size is uncertain. | |
| 643 per 1,000 | 624 per 1,000 (360 to 1,000) | |||||
| Healing rate | The mean healing rate was 0 | MD 3.06 lower (6.7 lower to 0.58 higher) | ‐ | 61 (1 RCT) | ⊕⊝⊝⊝ Very low2 | The effect of cleansing with tap water compared with normal saline on healing rate is uncertain. |
| Cost analysis | In the first study, excluding the cost of the dressing, the estimated cost of wound cleansing using normal saline was AUD$1.43 compared with AUD$1.16 using tap water. In the second study, results demonstrated an adjusted annual saving of US$65,600,000 if wounds were irrigated using tap water. | ‐ | 760 (2 RCTs) | ⊕⊝⊝⊝ Very low 4 | The effect of cleansing with tap water compared with normal saline on costs is uncertain. | |
| Pain | Pain was not assessed for this comparison. | |||||
| Patient satisfaction | Participants in one study were reported to prefer showering their wounds to irrigation with normal saline, but no effect estimate could be calculated and the trial is affected by risk of bias and imprecision. | ‐ | 49 (1 RCT) | ⊕⊝⊝⊝ Very low2 | The effect of cleansing with tap water compared with normal saline on patient satisfaction is uncertain. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded due to risk of bias (three levels) ‐ at high or unclear risk of selection bias; method of randomisation and allocation concealment not stated. At high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken and serious risk of imprecision.
2 Downgraded once due to serious risk of bias (high or unclear risk of selection bias; method of allocation not stated) and twice for very serious risk of imprecision.
3 Downgraded once due to serious risk of bias ‐ at high or unclear risk of selection bias; and twice for very serious risk of imprecision.
4 Downgraded twice due to risk of bias (high or unclear risk of selection bias; method of randomization and allocation concealment not stated; high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken in one trial) and once due to inconsistency between the estimates of effect.
Summary of findings 3. Distilled water compared with normal saline for wound cleansing.
| Distilled water compared with normal saline for wound cleansing | ||||||
| Patient or population: people with fractures Setting: hospital Intervention: distilled water Comparison: normal saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal saline | Risk with Distilled water | |||||
| Infection | Study population | RR 0.70 (0.45 to 1.09) | 152 (2 RCTs) | ⊕⊝⊝⊝ Very low 1 | The effect of distilled water compared with normal saline on infection is uncertain. | |
| 414 per 1,000 | 290 per 1,000 (186 to 452) | |||||
| Wounds healed | Could not be estimated ‐ all wounds had healed. | ‐ | 97 (1 RCT) | ⊕⊕⊝⊝ Low 2 | There may be little or no difference in wound healing for wounds treated with distilled water compared to normal saline. | |
| Reduction in wound size | Reduction in wound size was not assessed for this comparison. | |||||
| Healing rate | Healing rate was not assessed for this comparison. | |||||
| Cost analysis | Cost analysis was not assessed for this comparison. | |||||
| Pain | Pain was not assessed for this comparison. | |||||
| Patient satisfaction | Patient satisfaction was not assessed for this comparison. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded twice due to risk of bias (high or unclear risk of selection bias; method of randomisation and allocation concealment not stated; high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken) and once due to serious risk of imprecision.
2 Downgraded twice due to risk of bias (high or unclear risk of selection bias; method of randomisation and allocation concealment not stated; high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken).
Summary of findings 4. Cooled boiled water compared with normal saline for wound cleansing.
| Cooled boiled water compared with normal saline for wound cleansing | ||||||
| Patient or population: people with fractures Setting: hospital Intervention: cooled boiled water Comparison: normal saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with normal saline | Risk with Cooled boiled water | |||||
| Infection | Study population | RR 0.83 (0.37 to 1.87) | 51 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The effect of cooled boiled water compared with normal saline on infection is uncertain. | |
| 350 per 1,000 | 291 per 1,000 (130 to 655) | |||||
| Wound healing | Wound healing was not assessed for this comparison. | |||||
| Reduction in wound size | Reduction in wound size was not assessed for this comparison. | |||||
| Healing rate | Healing rate was not assessed for this comparison. | |||||
| Cost analysis | Cost analysis was not assessed for this comparison. | |||||
| Pain | Pain was not assessed for this comparison. | |||||
| Patient satisfaction | Patient satisfaction was not assessed for this comparison. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded twice due to risk of bias and imprecision (high or unclear risk of selection bias; method of randomization and allocation concealment not stated; high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken) and twice for very serious risk of imprecision.
Summary of findings 5. Distilled water compared with cooled boiled water for wound cleansing.
| Distilled water compared with cooled boiled water for wound cleansing | ||||||
| Patient or population: people with fractures Setting: hospital Intervention: distilled water Comparison: cooled boiled water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with cooled boiled water | Risk with Distilled water | |||||
| Infection | Study population | RR 0.59 (0.24 to 1.47) | 66 (1 RCT) | ⊕⊝⊝⊝ Very low 1 | The effect of distilled water compared with cooled boiled water on infection is uncertain. | |
| 290 per 1,000 | 171 per 1,000 (70 to 427) | |||||
| Wound healing | Wound healing was not assessed for this comparison. | |||||
| Reduction in wound size | Reduction in wound size was not assessed for this comparison. | |||||
| Healing rate | Healing rate was not assessed for this comparison. | |||||
| Cost analysis | Cost analysis was not assessed for this comparison. | |||||
| Pain | Pain was not assessed for this comparison. | |||||
| Patient satisfaction | Patient satisfaction was not assessed for this comparison. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded twice due to risk of bias and imprecision (high or unclear risk of selection bias; method of randomisation and allocation concealment not stated; high or unclear risk of performance bias; blinding of patients and outcomes assessors was not undertaken. Downgraded twice for very serious risk of imprecision.
Background
Description of the condition
A wound is a disruption of the normal anatomic structure of tissue, comprising a break in integrity of the epithelial layer of the skin (Korting 2011). Wounds are generally classified into three groups, superficial‐thickness, partial‐thickness and full‐thickness, and are defined as either acute or chronic (Korting 2011). Types of acute wounds can include lacerations, abrasions, cuts and acute surgical wounds/incisions (Korting 2011). Common types of chronic wounds can include venous leg ulcers, pressure ulcers and diabetic foot ulcers (James 2008; Stadelmann 1998). These wounds are caused by venous hypertension, damage to skin and underlying tissues from shearing, friction or pressure, and as a frequent complication of diabetes due to a loss of sensation (Posnett 2008). The level of thickness affects the timing and characteristics of healing but typically, acute wounds are generally expected to heal within three to six weeks (Korting 2011; Lee 2009) and chronic wounds take more than six weeks to heal (Korting 2011; Lee 2009).
The healing of either acute or chronic wounds is a complex process involving three overlapping phases including: inflammation, proliferation and remodelling (Harding 2002). However, acute wound healing differs from chronic wound healing in that these phases are generally followed in orderly progression and healing occurs by the approximation of the edges of the wound (primary intention) (Cullum 2016; Korting 2011; Stadelmann 1998). In chronic wounds, healing occurs by the formation of new tissue (secondary intention), however, the inflammatory and/or the proliferative phases may be interrupted and the process of healing delayed (Korting 2011; McCaughan 2018). Interruption of this process is frequently due to underlying illness, the presence of micro‐organisms or build‐up of necrotic tissue, and increased protease activity (Korting 2011). Other factors such as the anatomic location of the wound, aetiology, and surgical techniques used also affect successful wound healing and infection (Lee 2009). Therefore appropriate care and treatment of acute wounds is essential to prevent the formation of chronic wounds.
Infection of wounds occurs when microorganisms have proliferated "to a level that invokes a local and/or systemic response in the host" (Swanson 2016). The problem with infection is that it causes local tissue damage and interferes with wound healing (Lee 2009; Swanson 2016).
Description of the intervention
Management of chronic and acute wounds has changed significantly in the last decade; however, minimal attention has been focused on the types of solutions used for wound cleansing. The process of wound cleansing involves the application of a non‐toxic fluid to remove debris, necrotic tissue, exudate and metabolic wastes from the wound to create an optimal environment for wound healing (Mohamed 2015; Spear 2011; Watret 2009). The technique of cleansing can include irrigation using a syringe, soaking, bathing or swabbing (Fernandez 2004) depending on the classification and specific needs of the wound. Clinicians and manufacturers have recommended various cleansing agents including normal saline (0.9%), tap water and distilled water for their supposed therapeutic value, however the effectiveness of different solutions is unclear.
How the intervention might work
Normal saline (0.9%) is the favoured wound cleansing solution because it is an isotonic solution and it has been suggested that this does not interfere with the normal healing process, damage tissue, cause sensitisation or allergies or alter the normal bacterial flora of the skin (which would allow the growth of more virulent organisms) (Fellows 2006; Resende 2016; Salami 2006). Tap water is also recommended and has the advantages of being efficient, cost‐effective and accessible (Mohamed 2015; Watret 2009; Weiss 2013). However, clinicians have been cautioned against using tap water to cleanse wounds that have exposed bone or tendon, in which case normal saline is recommended (Stashak 2006).
There has been much debate in clinical circles about the potential advantages and disadvantages of cleaning exudate from the wound, as the exudate itself may contain growth factors and chemokines which contribute to wound healing (Falabella 2006). However, the literature also suggests that large amounts of bacteria may inhibit wound healing because of the proteases secreted by the organisms (Brown 2018).
Why it is important to do this review
The ever‐increasing number of wounds, both chronic and acute, place a significant burden on the provision of health care and healthcare resources in terms of the personnel and consumables to perform wound care (Guest 2017; Harding 2002; Nussbaum 2018). Furthermore, wounds cause considerable cost to individuals in terms of morbidity and mortality (Guest 2017; Nussbaum 2018). The purpose of this systematic review is to investigate the effectiveness of water for cleansing wounds in clinical practice.
Objectives
To assess the effects of water for wound cleansing.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) that compared the effect of tap, distilled or boiled water for wound cleansing with each other, with other solutions or with no cleansing on infection rate and wound healing eligible for inclusion in this review. Previous versions of this review included quasi‐RCTs, i.e. those in which randomisation of participants is not strictly random (e.g. randomisation by alternation, date of birth, or medical record number). We have updated our methods and excluded quasi‐RCTs from this update, as allocation of participants by quasi‐randomisation introduces risk of selection bias. We included trials undertaken in any country, irrespective of the tap water quality, and there was no restriction on the basis of the language in which the trial reports were written.
Types of participants
Trials involving people of all ages with a wound of any aetiology, in any setting (hospital, community, nursing homes, general practice, wound clinics). For the purpose of the review a wound was defined as a break in the skin.
We excluded trials if they compared solutions for dental procedures or for patients with burns.
Types of interventions
We considered trials eligible for inclusion if the solutions compared were used specifically for wound cleansing. For the purpose of this review, wound cleansing is defined as: "the use of fluids to remove loosely adherent debris and necrotic tissue from the wound surface" (Hellewell 1997).
We considered all trials evaluating the following comparisons eligible for inclusion in the review:
tap water compared with no cleansing;
tap water compared with normal saline;
distilled water compared with normal saline;
cooled boiled water compared with normal saline;
distilled water compared with cooled boiled water;
tap water compared with cooled boiled water;
tap water compared with distilled water;
cooled boiled water compared with no cleansing;
distilled water compared with no cleansing.
We excluded trials that:
1. utilised solutions for preoperative skin cleansing to prevent postoperative infections;
2. assessed the effectiveness of solutions as part of the operative procedure (for example lavage with povidone‐iodine or normal saline after fascia closure);
3. compared dressings for patients with wounds;
4. used an antiseptic solution, for example povidone‐iodine as a prophylactic treatment;
For this update, we also excluded trials that used tap water or distilled water along with an additive (e.g. H202, olive oil) and trials that did not include the use of any type of water as either a control or intervention.
Types of outcome measures
The following primary and secondary outcomes were of interest.
Primary outcomes
The primary outcome of interest was wound infection, as measured using either clinical signs and symptoms or wound cultures (Lakshmi 2011). The clinical signs and symptoms included abscess, cellulitis, wound discharge, discolouration, delayed healing, friable granulation tissue, unexpected pain and tenderness, pocketing at the base of the wound, epithelial bridging, abnormal smell, wound breakdown (Cutting 2005) fever (Weiss 2013), surgical debridement or early removal of sutures (Moscati 2007)(Appendix 1).
Secondary outcomes
The secondary outcomes of interest were:
wound healing (number of wounds in each group that healed at the completion of the trial period);
reduction in wound size (absolute or percentage change in wound area or volume over time);
cost analysis (cost relating to resources for wound cleansing);
pain (measured by any valid pain assessment instrument);
patient satisfaction (measured objectively or subjectively).
Search methods for identification of studies
Electronic searches
For this fifth update we searched the following databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 20 May 2021);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 4) in the Cochrane Library (searched 20 May 2021);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 20 May 2021);
Ovid Embase (1974 to 20 May 2021);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 20 May 2021).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2021). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2021).We combined the CINAHL Plus search with the trial filter developed by (Glanville 2019). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched 25 May 2021);
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/clinical-trials-registry-platform) (searched 25 May 2021).
Search strategies for clinical trial registries can be found in Appendix 2.
Searching other resources
We scrutinised the reference lists of relevant reviews and trials to identify additional studies.
Data collection and analysis
Selection of studies
Two review authors (RF and RA) independently assessed the references and abstracts of the trials identified by the above search against the eligibility criteria, and obtained the full text of potentially relevant trials. We entered references identified from the search of electronic databases and other literature into Covidence. The same authors jointly made the decision to include or exclude a study against the eligibility criteria. Any disagreements were resolved through discussion or in consultation a third author (LE).
Data extraction and management
We extracted the following data for each trial:
author; title; source; date of study; geographical location of study;
care setting;
type of wound;
inclusion/exclusion criteria;
sample size;
patient characteristics (by treatment group);
design details; study type;
intervention details; outcome measures;
analysis details and outcome data.
We included trials with multiple publications only once, but extracted maximum data from each publication. Two review authors (RF and LE) independently extracted and summarised data from included trials using a data extraction sheet developed and piloted by the review team. We resolved differences in opinion between the authors by discussion.
Assessment of risk of bias in included studies
Two review authors (RF and LE) independently assessed included trials using the Cochrane tool for assessing risk of bias as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved disagreements through discussion or by consulting a third review author. The tool addresses specific domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias and detection bas), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other sources of bias (other bias) (Appendix 3). For studies published after 1st July 2005, the WHO ICTRP (apps.who.int/trialsearch/Default.aspx) and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/) were screened for the a priori trial protocol. However, as no protocols were identified we were unable to compare trial protocols against published reports to assess outcome reporting bias.
Measures of treatment effect
The results of each included trial were plotted as effect estimates, that is risk ratio (RR) with corresponding 95% confidence interval (CI) for dichotomous outcomes (number of infections and number of wounds healed); mean difference (MD) and 95% CI for continuous outcomes (e.g. healing rate). We did not have to compare any continuous outcomes across trials and as such did not calculate standardised mean differences (SMD)(Deeks 2020). For results where no effect estimate could be calculated the results have been described within the narrative text in the review, and in the Characteristics of included studies table.
Unit of analysis issues
Trials in which more than one wound was treated per participant
We expected that the participant would be the unit of randomisation in most of the studies. We planned to include trials in which more than one wound was treated per participant. Where studies included some participants with more than one wound and reported outcome data at the wound level only, we used this wound‐level data. Given the limited number of studies in the review and the fact that these studies were in the same subgroup, we did not perform sensitivity analysis to investigate this decision.
Trials with multiple arms
Where multiple trial arms were reported in a single trial, we combined all relevant experimental intervention groups of the study into a single group (Higgins 2022).
Cluster‐randomised trials
We did not include any cluster‐randomised trials as there were no studies of this type that met the inclusion criteria for this review.
Cross‐over trials
We did not include any cross‐over trials as there were no studies of this type that met the inclusion criteria for this review.
Dealing with missing data
For dichotomous outcomes, we used the number of participants randomised as the denominator, assuming that any missing participants at the end of treatment did not have a positive outcome (e.g. for the outcome of infection, we assumed any missing participants had infection; for the outcome of healing, we assumed missing participants did not heal). For continuous outcomes (e.g. healing rate), we calculated the MD based on the number of participants analysed at that time point. There were no missing standard deviations (SDs) that required us to calculate from standard errors, confidence intervals, or P values.
Assessment of heterogeneity
We assessed clinical and methodological diversity in terms of participants, wound type, interventions, outcomes, and study characteristics for the included studies to determine whether a meta‐analysis was appropriate by observing data from the data extraction tables. We were able to conduct a meta‐analysis for at least one outcome for four comparisons (see Effects of interventions). We assessed statistical heterogeneity between the trials that examined the same intervention and outcome by visual inspection of a forest plot. We used the I² measure to quantify the possible magnitude of and the Chi² statistic to assess the statistical significance of heterogeneity. As recommended in the Cochrane Handbook (Deeks 2020) we considered an I² value of 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% to represent considerable heterogeneity. We interpreted the Chi² statistic such that a P value ≤ 0.10 indicates evidence of statistical heterogeneity. In one meta‐analysis conducted which had considerable heterogeneity, the cause of the heterogeneity was investigated.
Assessment of reporting biases
To explore the possible presence of publication bias we planned to create funnel plots, however, due to the small number of trials this was not possible (Higgins 2017).
Data synthesis
Data were structured according to the type of comparator and then by outcomes and are presented separately for acute and chronic wounds. We considered clinical and methodological heterogeneity and undertook pooling when studies appeared appropriately similar in terms of wound type, intervention type, and outcome type. We were unable to pre‐specify the amount of clinical, methodological and statistical heterogeneity in the included studies. Thus, we used a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. Chi‐squared and I‐squared were used to quantify heterogeneity but were not used to guide choice of model for meta‐analysis. We would have exercised caution when meta‐analysed data were at risk of small‐study effects because use of a random‐effects model may be unsuitable here. We presented data using forest plots where possible. For dichotomous outcomes we presented the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured, we presented a mean difference (MD) with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. We obtained pooled estimates of treatment effect from the available data using RevMan 5 software (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
Only one subgroup analysis by type of wound (acute or chronic) was planned. There were sufficient data to perform this subgroup analysis to determine whether wound infection and wound healing were influenced by type of wound. The formal test for subgroup interactions in Review Manager 5 was used (Review Manager 2020).
Sensitivity analysis
Sensitivity analysis was not performed due to insufficient data. We had planned to assess the robustness of effect estimates for wound infection and wound healing based on selection bias; detection bias; and attrition bias. However, because of the limited data available and the presence of high or unclear risk of bias in these key domains for the majority of trials in analyses, we could not undertake this sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
The main results are presented in the summary of findings tables, which reflect the main comparisons in the review, these are: tap water versus no cleansing Table 1, tap water versus normal saline Table 2, distilled water versus normal saline Table 3, cooled boiled water versus normal saline Table 4, and distilled water versus cooled boiled water Table 5.
Key information regarding the certainty of the evidence, magnitude of the effects of the interventions examined, and sum of available data for the main outcomes were recorded (Schünemann 2020). Two review authors independently assessed the certainty of the evidence contributing to each outcome using the five GRADE considerations (study limitations, inconsistency of results, imprecision, indirectness of evidence, and publication bias), employing GRADEpro software (GRADEpro GDT). All decisions to downgrade the certainty of the evidence have been recorded in the footnotes.
In cases where we judged studies to be at high risk of bias for any of the domains we downgraded the certainty of the evidence by one level. We further downgraded the certainty of the evidence by one level if we assessed an unclear risk in more than two domains. We followed standard methods for downgrading across other domains.
We present the following outcomes in the summary of findings tables:
wound infection;
wound healing;
reduction in wound size;
healing rate;
cost analysis;
pain;
patient satisfaction.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search of the Cochrane Wounds Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL yielded 2235 titles and abstracts after duplicates were removed, a further one record was identified through handsearching. The searches identified eight new trials for this fifth update.
We identified 13 trials (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013) that were eligible for inclusion in this review.
We completed a PRISMA flowchart (Figure 1) to summarise this process (Liberati 2009).
1.
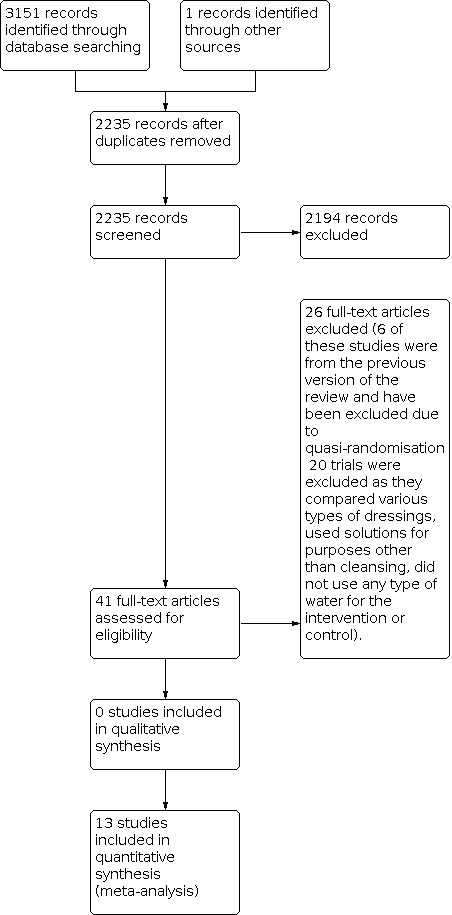
Included studies
The included studies were conducted in the USA (Bansal 2002; Godinez 2002; Moscati 2007; Weiss 2013), India (Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011), Australia (Griffiths 2001), Hong Kong (Chan 2016) Iran (Mirshamsi 2007), Nigeria (Olufemi 2017), and Tanzania (Museru 1989).
All but two (Griffiths 2001; Mirshamsi 2007) of the 13 trials were conducted in single centres. All trials utilised a parallel group design and Museru 1989 had three comparison arms. The total number of participants in the included trials was 2504. The age of the patients ranged from two to 95 years. Two trials were undertaken in children (Bansal 2002; Weiss 2013). In all trials the treatment groups in each individual trial were comparable at baseline. Of the included trials, four trials involved people with lacerations (Bansal 2002; Godinez 2002; Moscati 2007; Weiss 2013); two trials involved people with open fractures (Museru 1989; Olufemi 2017), three trials involved people with chronic wounds (Chan 2016; Griffiths 2001; Lakshmi 2011), two with surgical wounds (Gupta 2007; Gupta 2008), and one trial each involved people with anal fissures (Gupta 2006) and traumatic wounds (Mirshamsi 2007).
Nine of the 13 trials evaluated patients in the hospital emergency departments and ward settings (Bansal 2002; Chan 2016; Godinez 2002; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013) and four trials (Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008) were undertaken in the community. The cleansing process was undertaken by the medical or nursing staff (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013), or by the person themselves (Gupta 2006; Gupta 2007; Gupta 2008). Standard instructions were given to the patients or the health professionals about the cleansing process. Six trials (Godinez 2002; Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011; Moscati 2007) specified the duration of the cleansing process and only seven trials reported on the volume of the cleansing fluid used (Chan 2016; Griffiths 2001; Lakshmi 2011; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013). The solutions used for wound cleansing included tap water (Bansal 2002; Godinez 2002; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Weiss 2013), cooled boiled water (Museru 1989), distilled water (Museru 1989; Olufemi 2017), and normal saline (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013). The duration of follow‐up ranged from two days (Bansal 2002) to six weeks(Chan 2016; Griffiths 2001; Lakshmi 2011). The method used to contain the solution was reported in 10 trials and included bowls (Godinez 2002; Weiss 2013 ), clean washed bottles (Griffiths 2001), sterile bottles or basins (Bansal 2002; Chan 2016; Museru 1989), and tubs (Gupta 2006; Gupta 2007; Gupta 2008). The method for cleansing included irrigation (Bansal 2002; Godinez 2002; Griffiths 2001; Lakshmi 2011; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013), soaking (Gupta 2006; Gupta 2007; Gupta 2008), swabbing (Chan 2016) and washing (Mirshamsi 2007).
Eleven trials (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Gupta 2006; Gupta 2007; Lakshmi 2011; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013) provided a clear description of the inclusion/exclusion criteria. The baseline characteristics (including gender) for each treatment group were given in all but one trial (Mirshamsi 2007). The distribution of males and females was even in 12 trials (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013). Comparability between types of wounds was reported in all but two trials (Godinez 2002; Mirshamsi 2007). Participants were followed up for a maximum of six weeks after therapy (Chan 2016; Griffiths 2001; Lakshmi 2011) thus it is difficult to determine the long‐term effects of tap water on the wounds that had not healed. Sample sizes ranged between 22 and 715 patients (median 60). Six trials described a priori sample size calculation (Gupta 2006; Gupta 2007; Gupta 2008; Mirshamsi 2007; Moscati 2007; Weiss 2013).
Excluded studies
We excluded 20 trials that either compared various types of dressings, used solutions for purposes other than cleansing (e.g. povidone‐iodine for infection prophylaxis) or did not use any type of water for the intervention or control. We also removed six quasi‐RCTs (Angeras 1992; Goldberg 1981; Neues 2000; Riederer 1997; Tay 1999; Valente 2003) that had been included in a previous version of this review in accordance with updates to review methodology. We have listed these trials in the Characteristics of excluded studies, with reasons for their exclusion.
Ongoing studies
Two trials (NCT01846598; NCT02820272) were ongoing at the time of this update.
Studies awaiting classification
Two trials (Cherry 2003; Saw 2006) were awaiting classification at the time of this update. The trials did not provide enough information to establish whether they met the review inclusion criteria.
Risk of bias in included studies
We used the seven‐point Cochrane tool (Higgins 2017) to assess the risk of bias in the included studies. Results of the assessment are presented in the risk of bias tables (Characteristics of included studies), and summarised for each study in Figure 2 and overall in Figure 3. Overall, three trials (Chan 2016; Griffiths 2001; Weiss 2013) were assessed as being at low risk for all domains. One trial (Museru 1989) was at unclear risk for all domains except other bias which was low risk. One trial (Moscati 2007) had low risk in six domains, two trials (Bansal 2002; Gupta 2007) had low risk in five domains, two trials (Gupta 2006; Gupta 2008) had low risk in four domains, and one trial (Olufemi 2017) had low risk for four domains. Two trials (Godinez 2002; Mirshamsi 2007) had unclear risk in five domains, and one trial (Lakshmi 2011) had unclear risk in four domains.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of randomisation sequence and allocation concealment
All trials stated that allocation to treatment was random; random number tables or schedules were used in nine trials (Bansal 2002; Chan 2016; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Lakshmi 2011; Moscati 2007; Weiss 2013), and ballot in one trial (Olufemi 2017). Method of randomisation was not stated in three trials; (Godinez 2002; Mirshamsi 2007; Museru 1989), and these were subsequently rated as unclear.
Blinding
Blinding participants and personnel
Four trials (Bansal 2002; Chan 2016; Griffiths 2001; Weiss 2013) provided sufficient information on whether the patients were blinded to the intervention. In three trials (Gupta 2006; Gupta 2007; Gupta 2008) blinding of participants was not possible due to the nature of the intervention. Seven trials (Bansal 2002; Chan 2016; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Weiss 2013) provided evidence on whether the person performing the intervention was blinded. The remaining trials (Godinez 2002; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017) either offered insufficient evidence or no evidence to suggest that intervention was blinded appropriately.
Blinding outcome assessment
Six trials (Chan 2016; Griffiths 2001; Gupta 2007; Gupta 2008; Moscati 2007; Weiss 2013) provided sufficient information on whether the outcome assessment was blinded, and were subsequently awarded a low risk judgment for this criterion. The remaining studies did not offer sufficient evidence to suggest that the outcome assessment was blinded and were all subsequently awarded an unclear risk judgement.
Incomplete outcome data
In relation to loss to follow‐up and risk of attrition bias, nine trials were awarded a low risk judgement (Bansal 2002; Chan 2016; Griffiths 2001; Gupta 2006; Gupta 2007; Gupta 2008; Moscati 2007; Olufemi 2017; Weiss 2013), due to full reporting of data, low rates of dropout and full reporting of reasons given for dropout. Three trials (Lakshmi 2011; Mirshamsi 2007; Museru 1989) were judged to be unclear, this included one trial where information regarding loss to follow‐up and numbers fully completing the trial was not forthcoming (Museru 1989). One trial (Godinez 2002) was awarded a high‐risk judgement, due to high rates of unexplained dropout or unexplained exclusions.
Selective reporting
There were no trials where selective outcome reporting was identified. The majority of the trials reported on the outcomes outlined on the methodology and were subsequently awarded a low‐risk judgement. We judged the risk of bias for this domain to be unclear in two trials (Godinez 2002; Museru 1989) due to the reporting of outcomes being unclear.
Other potential sources of bias
There were no clearly identified other sources of bias in 11 of the trials. We judged the risk of other sources of bias as unclear in two trials (Gupta 2006; Gupta 2008) due to potential sources of bias with the interventions, as it was unclear if participants adhered to the regimen as they were not observed doing so.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
We identified 13 trials involving 2504 participants that met the inclusion criteria. Three trials involving 148 participants (Gupta 2006; Gupta 2007; Gupta 2008) compared wounds cleansed using tap water with those not cleansed and 10 trials involving 2356 participants (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Museru 1989; Olufemi 2017; Weiss 2013) compared wound cleansing with water and other solutions. There was significant heterogeneity in the types of the wounds, the cleansing solution used and the outcomes measures used in the trials. All but one trial (Lakshmi 2011) used subjective measures to assess wound infection.
Comparison 1: Tap water versus no cleansing (3 RCTs, 148 participants)
See Table 1.
We identified three randomised controlled trials (RCTs) (Gupta 2006; Gupta 2007; Gupta 2008) that compared healing rates in patients with surgical wounds who were randomised to bathe their wounds with tap water with those who were not. The trials did not allow patients assigned to either group to use any cleansing agents.
Primary outcome (infection)
There were no trials that reported on wound infection.
Secondary outcomes
(i) Wound healing
Three trials with 148 participants reported on wound healing (Gupta 2006; Gupta 2007; Gupta 2008). Only two trials reported complete healing and wound epithelialisation as a measure of wound healing (Gupta 2007; Gupta 2008). Pooled data demonstrated that there may be little or no difference in the number of wounds that did not heal between the groups (risk ratio (RR) 1.04, 95% confidence interval (C)I 0.95 to 1.14) (Analysis 1.1). Using the GRADE approach, we judged the certainty of evidence for this result to be low (downgraded twice due to very serious risk of imprecision).
1.1. Analysis.

Comparison 1: Tap water versus no cleansing, Outcome 1: Wounds healed
(ii) Reduction in wound size
There were no trials that reported on reduction in wound size.
(iii) Wound healing rate
There were no trials that reported on wound healing rate.
(iv) Cost
There were no trials that reported on cost.
(v) Pain
Three trials reported on pain (Gupta 2006; Gupta 2007; Gupta 2008). Only one trial (Gupta 2006) involving 52 participants provided means and standard deviations (SDs) for pain scores hence, therefore data could not be pooled in a meta‐analysis. In the trial by (Gupta 2006) the mean pain score for the tap water group was 0 (SD 0) compared with 2 (SD 0.6) for the no cleansing group. The mean difference (MD) and confidence interval (CI) could not be estimated.
Reported means in the remaining two studies (Gupta 2007; Gupta 2008) indicated no difference between the tap water and no cleansing groups (6.95 versus 7.60; P = 0.284) (Gupta 2007); (5.1 versus 5.4; P = 0.12) (Gupta 2008). All three trials reported results which indicated that there may be no clear difference in pain scores between the groups. Using the GRADE approach, we judged the certainty of evidence for this result to be low (downgraded two levels due to very serious risk of imprecision).
(vi) Patient satisfaction
Two trials involving 102 participants reported on patient satisfaction (Gupta 2006; Gupta 2008) however the data could not be pooled into a meta‐analysis as means and SDs were not provided. One trial (Gupta 2006) reported that patients in the sitz bath group expressed better satisfaction than the patients who did not take sitz baths (P < 0.01). In contrast, the second trial (Gupta 2008), reported that there was no difference in patient satisfaction scores between the two groups (P = 0.29). Using the GRADE approach, we judged the certainty of evidence for this result to be low (downgraded two levels due to very serious risk of imprecision).
Comparison 2: Tap water versus normal saline (8 RCTs, 2204 participants)
See Table 2.
Eight trials (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Weiss 2013) compared infection and healing rates in acute and chronic wounds irrigated with either tap water or normal saline.
Primary outcome (infection)
All wounds
Eight trials (Bansal 2002; Chan 2016; Godinez 2002; Griffiths 2001; Lakshmi 2011; Mirshamsi 2007; Moscati 2007; Weiss 2013) involving 2204 participants compared infection rates in acute and chronic wounds.The effect of cleansing with tap water compared with normal saline on infection is uncertain (pooled RR 0.84, 95% CI 0.59 to 1.19). Results of the subgroup analysis by wound type showed similar effects to the overall analysis; the great majority of participants had acute wounds (Analysis 2.1). Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded twice due to risk of bias and once due to imprecision).
2.1. Analysis.

Comparison 2: Tap water versus normal saline, Outcome 1: Infection
Secondary outcomes
(i) Wound healing
Two trials involving 79 participants reported on wound healing (Griffiths 2001; Chan 2016). The effect of cleansing with tap water compared with normal saline on the number of wounds that healed after cleansing with either tap water or normal saline is uncertain (pooled RR 0.57, 95% CI 0.30 to 1.07) (Analysis 2.2). Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded once due to risk of bias and twice due to imprecision).
2.2. Analysis.

Comparison 2: Tap water versus normal saline, Outcome 2: Wounds healed
(ii) Reduction in wound size
One trial involving 30 participants reported on reduction in wound size (Chan 2016). The effect of cleansing with tap water compared with normal saline on the number of wounds that reduced in size after cleansing with either tap water or normal saline is uncertain (RR 0.97, 95% CI 0.56 to 1.68) (Chan 2016) (Analysis 2.3). Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded once due to risk of bias and twice for imprecision).
2.3. Analysis.

Comparison 2: Tap water versus normal saline, Outcome 3: Reduction in wound size
(iii) Wound healing rate
One trial involving 61 participants reported on wound healing rate (Lakshmi 2011). The effect of cleansing with tap water compared with normal saline on the wound healing rate is uncertain (MD ‐3.06, 95% CI ‐6.70 to 0.58) (Analysis 2.4). Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded once due to high or unclear risk of selection bias, method of allocation concealment not stated and twice for imprecision).
2.4. Analysis.

Comparison 2: Tap water versus normal saline, Outcome 4: Healing rate
(iv) Cost analysis
Two trials (Griffiths 2001; Moscati 2007) involving 760 participants reported a cost analysis and demonstrated that the use of tap water may be inexpensive compared with the use of normal saline. In Griffiths 2001, excluding the cost of the dressing, the estimated cost of wound cleansing using normal saline was AUD$1.43 compared with AUD$1.16 using tap water. Costs for wound cleansing using normal saline included staff time, materials and equipment used for the dressings.
In the second trial (Moscati 2007), costs were calculated to include supplies, saline and antibiotics if required. The costs were extrapolated to the eight million lacerations that occur in the USA each year. The results demonstrated an adjusted annual saving of US$65,600,000 if wounds were irrigated using tap water.
Using the GRADE approach, we judged the certainty of evidence to be very low (downgraded twice for risk of bias and once for inconsistency).
(v) Pain
There were no trials that reported on pain.
(vi) Patient satisfaction
One trial (Griffiths 2001) involving 49 participants reported that participants who had showered their wounds preferred that method to irrigation with normal saline; the certainty of the evidence is very low (downgraded three levels due to high or unclear risk of selection and very serious risk of imprecision).
Comparison 3: Distilled water versus normal saline (2 RCTs, 152 participants)
See Table 3.
Two trials (Olufemi 2017; Museru 1989) compared distilled water with normal saline.
Primary outcome (infection)
Pooled data (Olufemi 2017; Museru 1989) involving 152 participants indicated that the effect of using distilled water compared to normal saline on infection is uncertain (RR 0.70, 95% CI 0.45 to 1.09) Analysis 3.1. Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded twice for high risk of bias; and once for imprecision).
3.1. Analysis.

Comparison 3: Distilled water versus normal saline, Outcome 1: Infection
Secondary outcomes
(i) Wound healing
One trial (Olufemi 2017) involving 97 participants compared distilled water with normal saline and reported on wound healing. All wounds in both groups had healed at the eight‐week follow‐up (RR could not be estimated) Analysis 3.2. Using the GRADE approach, we judged the certainty of evidence for this result to be low (downgraded two levels due to high risk of bias).
3.2. Analysis.

Comparison 3: Distilled water versus normal saline, Outcome 2: Wounds healed
There were no trials which reported on any of the other secondary outcomes (reduction in wound size, wound healing rate, cost, pain, or patient satisfaction).
Comparison 4: Cooled boiled water versus normal saline (1 RCT, 51 participants)
See Table 4.
One trial (Museru 1989) involving 51 participants compared cooled boiled water with normal saline.
Primary outcome (infection)
Results of the one study (Museru 1989) involving 51 participants indicated that the effect of cleansing with cooled boiled water compared to normal saline on infection rate is uncertain (RR 0.83, 95% CI 0.37 to 1.87) Analysis 4.1. Using the GRADE approach, we judged the certainty of evidence for this result to be very low (downgraded twice for risk of bias and twice for imprecision).
4.1. Analysis.

Comparison 4: Cooled boiled water versus normal saline, Outcome 1: Infection
Secondary outcomes
There were no trials which reported on any of the secondary outcomes (wound healing, reduction in wound size, wound healing rate, cost, pain, or patient satisfaction).
Comparison 5: Distilled water versus cooled boiled water (1 RCT, 66 participants)
See Table 5.
One trial (Museru 1989) involving 66 participants compared distilled water with cooled boiled water.
Primary outcome (infection)
Six out of 35 participants (17.1%) in the distilled water group and nine out of 31 (29%) in the cooled boiled water group developed a wound infection (RR 0.59, 95% CI 0.24 to 1.47) Analysis 5.1. The small number of wounds cleansed using distilled water (n = 35) and cooled boiled water (n = 31) means that the study lacked power to detect clinically important differences (Museru 1989). Using the GRADE approach, we consider the certainty of the evidence to be very low (downgraded twice for risk of bias, and twice for imprecision) and the effect of distilled water compared with cooled boiled water on infection is uncertain.
5.1. Analysis.

Comparison 5: Distilled water versus cooled boiled water, Outcome 1: Infection
Secondary outcomes
There were no trials which reported on any of the secondary outcomes (wound healing, reduction in wound size, wound healing rate, cost, pain, or patient satisfaction).
Comparison 6: Tap water versus cooled boiled water
No trials were identified that compared tap water with cooled boiled water.
Comparison 7: Tap water compared with distilled water
No trials were identified that compared tap water with distilled water.
Comparison 8: Cooled boiled water compared with no cleansing
No trials were identified that compared boiled water with no cleansing.
Comparison 9: Distilled water compared with no cleansing
No trials were identified that compared distilled water with no cleansing.
Discussion
Summary of main results
This systematic review of the effectiveness of water for wound cleansing has summarised the best available evidence at the time of the report. Following an extensive literature search, we identified 13 trials (eight new) that met the inclusion criteria and we have presented them in this review. Overall, there was no evidence of a benefit of cleansing, nor of any particular type of cleansing solution.
Tap water versus no cleansing
No trials that compared the effect of tap water with no cleansing on wound infection were identified. The evidence obtained from three trials showed there may be little or no difference between cleansing with tap water and not cleansing wounds on wound healing (low‐certainty evidence). There was low‐certainty evidence on the effect on pain or patient satisfaction.
Tap water versus normal saline
There is very low certainty evidence as to whether cleansing with tap water compared with normal saline alters the number of wounds infected or the number of wounds healed. No trials assessed pain and evidence for all of the other review outcomes is very low certainty. This means that we are uncertain about the effect of using one method or the other on any of the outcomes assessed.
Distilled water versus normal saline
We identified only low‐ or very low‐certainty evidence for this comparison, thus we are uncertain whether there is a difference between cleansing with distilled water compared with normal saline in the number of wounds infected. In these trials, all wounds in both groups had healed and there may be little or no difference in healing between the cleansing methods.
Cooled boiled water versus normal saline
There is very low‐certainty evidence as to whether cleansing with cooled boiled water compared with normal saline alters the number of wounds infected; no other outcomes were reported.
Distilled water versus cooled boiled water
There is very low‐certainty evidence as to whether cleansing with distilled water compared with cooled boiled water alters the number of wounds infected; no other outcomes were reported.
We did not identify any evidence for the following comparisons: tap water versus cooled boiled water; tap water compared with distilled water; boiled water compared with no cleansing; distilled water compared with no cleansing.
Overall completeness and applicability of evidence
The included studies have clinical applicability as they were undertaken in either a hospital or community setting for the management of patients with wounds. There were a limited number of trials and data in the included trials and hence subgroup analysis could only be undertaken for the comparison relating to tap water versus normal saline on acute and chronic wounds.
It is essential that the eligibility criteria are well‐defined in order to understand the type of population treated. The eligibility criteria should also define the severity of the wounds of participants. For example the description of the type of wound should accord with a standard criteria. This allows the findings and recommendations to be generalised to other clinical settings. The participant groups in all studies in this review were clearly reported, hence the evidence can be applied to any participants with acute or chronic wounds (but not all comparisons had both). All trials clearly reported the inclusion and exclusion criteria. The outcomes of wound infection and healing are clinically applicable and play an important role in the management of acute and chronic wounds.
However, the completeness of evidence was limited as some trials failed to report important outcomes. In the comparison tap water versus no cleansing there were no trials that reported on the number of wounds that were infected which would have been an important finding for clinicians and health services. The current practice globally in wound management is to cleanse the wound while showering the patient, and in many instances these patients include those who are bedfast (Boga 2019; Wounds Australia 2016). Although all trials in this review used some type of water, only one of the trials used showering as a method to cleanse wounds. Despite the evidence obtained from this review, practitioners and health service managers should therefore interpret the findings with caution. The availability and cost of resources may also determine which solution is used for cleansing wounds in different settings but there were limited findings for this outcome. Results from one trial demonstrated that in countries with limited resources, distilled or boiled water can be used for wound cleansing without complications.
Wound healing was only reported in the comparisons relating to tap water versus no cleansing, tap water versus normal saline, and distilled water versus normal saline. Pain was reported in only one comparison relating to tap water versus no cleansing. Patient satisfaction was reported in only two comparisons relating to tap water versus no cleansing, and tap water versus normal saline. Cost‐effectiveness was only reported in the comparison relating to tap water versus normal saline.
Quality of the evidence
Only low‐ or very low‐certainty evidence was available for the outcomes reported in this review. Much of the evidence was downgraded due to risk of selection, and performance bias and imprecision. There was no indirectness and the evidence was not downgraded for publication bias as there were a small number of trials included in the meta‐analysis. The evidence base could be strengthened with the conduct of further, large, well‐designed randomised controlled trials (RCTs). It could be postulated that the lack of studies comparing tap water with no cleansing could be due to the fact that there is an increased emphasis in the clinical setting on the use of some type of solution for wound cleansing in particular, normal saline.
Potential biases in the review process
For this review, strict adherence to Cochrane methods helped in minimising bias where possible.
The use of a broad literature search increases our confidence that all relevant literature on this topic has been identified. However, it could be possible that some trials may have been missed and we would like to be notified of any RCTs, published or unpublished, that meet the selection criteria. In addition, two review authors independently assessed the trials, extracted data, assessed risk of bias and graded the evidence in order to minimise bias. There may have been bias arising from differences between protocol and review stages and these have been reported in the differences between protocol and review section.
Agreements and disagreements with other studies or reviews
There were no other reviews identified on the topic and the conclusions of this fifth update are consistent with the previous four reviews. This version has no included trials for the outcome of infection in the comparison tap water versus no cleansing.
Authors' conclusions
Implications for practice.
Tap water is a wound cleansing agent commonly used in the community setting and hospitals. Based on the randomised trials undertaken to date, using tap water compared with no cleansing may make little or no difference to wound healing, while there are no data on wound infection. The impact of using tap water compared with saline for wound cleansing on both infection and wound healing is uncertain. Evidence for comparisons of distilled or cooled boiled water with each other or with saline is uncertain. Data for other outcomes are limited across all the comparisons considered and are either uncertain or suggest that there may be little or no difference in the outcome. Given the uncertainty of the evidence base, people with wounds and health professionals caring for them should take into consideration factors such as patient preference and availability of treatments in choosing a method of wound cleansing.
Implications for research.
Properly designed multicentre trials are needed to compare the clinical benefits and cost‐effectiveness of different solutions for wound cleansing in different groups of patients, different types of wounds and in a wide range of settings.
Trials comparing cleansing with no cleansing are required to determine the extent to which cleansing contributes to the healing and infection of acute and chronic wounds. Evidence from these trials may impact on decisions made in the clinical setting to use some form of solution for wound cleansing.
The strongest evidence for whether tap water is an effective wound cleansing solution is likely to be provided by trials in which the volume and the temperature of the comparison solution are the same as the tap water.
Future research should have well‐defined inclusion and exclusion criteria, adequate sample size, methods to ensure baseline comparability of the groups, use of true randomisation with allocation concealment, use of an objective outcome measurement of wound infection and healing (e.g. percentage and absolute change in wound area), blinded outcome assessment, adequate follow‐up period and appropriate statistical analysis.
Given the purchasing costs of equipment, economic evaluations should be undertaken in future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 28 July 2022 | New citation required but conclusions have not changed | Updated, no change to conclusions. |
| 28 July 2022 | New search has been performed | Fifth update, new search with eight new studies added. GRADE assessment of the certainty of evidence included. Six quasi‐randomised trials which were included in previous versions of the review have now been excluded. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 11 January 2013 | Feedback has been incorporated | Feedback received 10 January 2013. Data entry errors have been corrected and the conclusions of the review have been amended. |
| 5 January 2012 | New search has been performed | We carried out new searches in November 2011. We identified no new studies for inclusion. |
| 5 January 2012 | New citation required but conclusions have not changed | Fourth update. |
| 18 March 2010 | New search has been performed | For this third update we carried out new searches in February 2010. We identified no new studies for inclusion. We assigned four studies in awaiting assessment as either duplicate publications of an included trial or as excluded from the review. |
| 13 May 2009 | Amended | Contact details updated. |
| 18 June 2008 | Amended | Converted to new review format. |
| 18 June 2008 | Feedback has been incorporated | Feedback queries received and answered |
| 2 November 2007 | New citation required and conclusions have changed | Substantive amendment. For this second update, new searches were carried out in November 2007. Four studies were identified, of which 2 (Godinez 2002; Moscati 2007a) were included and two studies were excluded. |
| 18 June 2004 | New search has been performed | For the first update new searches were carried out in June 2004. Five studies were identified, of which 3 (Bansal 2002; Goldberg 1981; Valente 2003) were included and 2 were excluded. |
Acknowledgements
We are grateful to the Nursing Director of the South Western Sydney Area Health Service (Australia) for funding this review. In addition we would like to acknowledge the assistance of the librarians and library staff of the Liverpool Health Service library for their assistance with the development of the search strategy and the timely retrieval of articles for the first version of this review; Rachel Langdon and Soufiane Bofous for their assistance with the statistical analysis; Venita Devi for secretarial support; Jeff Rowland, Brenda Ramstadius and Annette Hodgkinson for reading drafts and commenting on the clinical and policy perspective of the review. Thanks to Associate Professor Rosemary Chester for her support throughout the project and to Adrian Bauman, Bin Jalaludin, Maureen McIlwrath, Claire Matthews and Jenny Morris who peer reviewed the review for methodological rigour, readability and clinical relevance. We also acknowledge the assistance of Heidi Otten and Dr Hashi with interpretation of studies in German. The authors would like to thank Ahmad Sofi Mahmudi for assistance with translation. We would like to thank Cheryl Ussia, a community nurse who identified the need for a systematic review as a basis for the development of evidence‐based practice guidelines for community nurses. Cheryl was seconded to work with the review team as a clinical expert in wound care and was responsible for organising the retrieval of papers and obtaining data on unpublished studies for the initial version of this review. Thanks also to Joanne Cummings who assisted with retrieving the publications for the third update and Mathew Fortnam who contributed to an early version of this update.
The authors would like to thank Cochrane Wounds Group referees: Anne‐Marie Bagnall (for providing feedback on this update); Brian Gilchrist, Carol Dealey, Fuijan Song, Ruth Lewis, Mary Harrison, Raj Mani, Seokyung Hahn (for their feedback on previous versions of this review). In addition the authors would like to thank Sally Stapley for support during the third update. The authors would also like to thank Nancy Owens for copy editing a previous version of the review and Heather Maxwell for copy editing this updated version.
Appendices
Appendix 1. Defintion of terms
Definition of terms
Abscess
A swollen area within body tissue, containing an accumulation of pus.
Cellulitis
Inflammation of subcutaneous connective tissue.
Debridement
The removal of foreign material and dead or damaged tissue from a wound.
Dehiscence
Separation of layers of a surgical wound, which may be superficial, partial or complete. Complete dehiscence may lead to evisceration.
Epithelial bridging
Incomplete granulation tissue across the wound bed.
Friable
Tissue that tears, sloughs, and bleeds more easily when touched.
Granulation tissue
Delicate tissue composed mainly of tiny blood vessels and fibres, formed at the site of a wound or infection as part of the healing process.
Pocketing at the base of the wound
When a wound that was assessed as healing starts to develop strips of granulation tissue in the base as opposed to a uniform spread of granulation tissue across the whole of the wound bed.
Purulent discharge
A thick and milky discharge from a wound which is often a sign of infection.
Wound discharge
The result of dilation of the blood vessels during the early inflammatory stage of healing, possibly caused by the presence of certain bacteria. In an attempt to heal the wound, the body creates and maintains an optimal moist wound environment.
Appendix 2. Search strategies for the fifth update
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Wounds and Injuries EXPLODE ALL AND INREGISTER
2 MESH DESCRIPTOR Skin Ulcer EXPLODE ALL AND INREGISTER
3 MESH DESCRIPTOR Diabetic Foot EXPLODE ALL AND INREGISTER
4 (wound or wounds or ulcer or ulcers or ulceration OR ulcerated or bite or bites or abrasion or abrasions or laceration or lacerations or (diabetic NEXT foot) or (diabetic NEXT feet)) AND INREGISTER
5 1 OR 2 OR 3 OR 4 AND INREGISTER
6 MESH DESCRIPTOR Water EXPLODE ALL AND INREGISTER
7 water AND INREGISTER
8 #6 OR #7 AND INREGISTER
9 (clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*) AND INREGISTER
10 #8 AND #9 AND INREGISTER
11 #5 AND #10 AND INREGISTER
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Wounds and Injuries] explode all trees
#2 MeSH descriptor: [Skin Ulcer] explode all trees
#3 MeSH descriptor: [Diabetic Foot] explode all trees
#4 ("wound" or "wounds" or "ulcer" or "ulcers" or "ulceration" or "ulcerated" or "bite" or "bites" or "abrasion" or "abrasions" or "laceration" or "lacerations" or "diabetic foot" or "diabetic feet"):ti,ab,kw
#5 #1 or #2 or #3 or #4
#6 MeSH descriptor: [Water] explode all trees
#7 "water":ti,ab,kw
#8 #6 or #7
#9 (clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*):ti,ab,kw
#10 #8 and #9
#11 #5 and #10
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search via Cochrane Register of Studies
1 MESH DESCRIPTOR Wounds and Injuries EXPLODE ALL AND CENTRAL:TARGET
2 MESH DESCRIPTOR Skin Ulcer EXPLODE ALL AND CENTRAL:TARGET
3 MESH DESCRIPTOR Diabetic Foot EXPLODE ALL AND CENTRAL:TARGET
4 (wound or wounds or ulcer or ulcers or ulceration OR ulcerated or bite or bites or abrasion or abrasions or laceration or lacerations or (diabetic NEXT foot) or (diabetic NEXT feet)) AND CENTRAL:TARGET
5 #1 OR #2 OR #3 OR #4 AND CENTRAL:TARGET
6 MESH DESCRIPTOR Water EXPLODE ALL AND CENTRAL:TARGET
7 water AND CENTRAL:TARGET
8 #6 OR #7 AND CENTRAL:TARGET
9 (clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*) AND CENTRAL:TARGET
10 #8 AND #9 AND CENTRAL:TARGET
11 #5 AND #10 AND CENTRAL:TARGET
12 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET
13 http*:SO AND CENTRAL:TARGET
14 #12 OR #13
15 #11 AND #14
Ovid MEDLINE
1 exp "Wounds and Injuries"/
2 exp Skin Ulcer/
3 (wound*1 or ulcer* or laceration* or bite*1 or abrasion* or tear*1 or diabetic foot or diabetic feet).tw.
4 or/1‐3
5 exp Water/
6 water.ti,ab,hw.
7 or/5‐6
8 (clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*).tw.
9 7 and 8
10 4 and 9
11 randomized controlled trial.pt.
12 controlled clinical trial.pt.
13 randomi?ed.ab.
14 placebo.ab.
15 clinical trials as topic.sh.
16 randomly.ab.
17 trial.ti.
18 or/11‐17
19 exp animals/ not humans.sh.
20 18 not 19
21 10 and 20
Ovid Embase
1 exp Wound/
2 exp Skin Ulcer/
3 (wound*1 or ulcer* or laceration* or bite*1 or abrasion* or tear*1 or diabetic foot or diabetic feet).tw.
4 or/1‐3
5 exp Water/
6 water.ti,ab,hw.
7 or/5‐6 1193314
8 (clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*).tw.
9 7 and 8
10 4 and 9
11 Randomized controlled trial/
12 Controlled clinical study/
13 Random$.ti,ab.
14 randomization/
15 intermethod comparison/
16 placebo.ti,ab.
17 (compare or compared or comparison).ti.
18 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab.
19 (open adj label).ti,ab.
20 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab.
21 double blind procedure/
22 parallel group$1.ti,ab.
23 (crossover or cross over).ti,ab.
24 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 orintervention$1 or patient$1 or subject$1 or participant$1)).ti,ab.
25 (assigned or allocated).ti,ab.
26 (controlled adj7 (study or design or trial)).ti,ab.
27 (volunteer or volunteers).ti,ab.
28 human experiment/
29 trial.ti.
30 or/11‐29
31 (random$ adj sampl$ adj7 (cross section$ or questionnaire$1 or survey$ or database$1)).ti,ab. not (comparative study/ or controlled study/ or randomi?ed controlled.ti,ab. or randomly assigned.ti,ab.)
32 Cross‐sectional study/ not (randomized controlled trial/ or controlled clinical study/ or controlled study/ or randomi?ed controlled.ti,ab. or control group$1.ti,ab.)
33 (((case adj control$) and random$) not randomi?ed controlled).ti,ab.
34 (Systematic review not (trial or study)).ti.
35 (nonrandom$ not random$).ti,ab.
36 Random field$.ti,ab.
37 (random cluster adj3 sampl$).ti,ab.
38 (review.ab. and review.pt.) not trial.ti.
39 we searched.ab. and (review.ti. or review.pt.)
40 update review.ab.
41 (databases adj4 searched).ab.
42 (rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/
43 Animal experiment/ not (human experiment/ or human/)
44 or/31‐43
45 30 not 44
46 10 and 45
EBSCO CINAHL Plus
S34 S10 AND S33
S33 S32 NOT S31
S32 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25
S31 S29 NOT S30
S30 MH (human)
S29 S26 OR S27 OR S28
S28 TI (animal model*)
S27 MH (animal studies)
S26 MH animals+
S25 AB (CLUSTER W3 RCT)
S24 MH (crossover design) OR MH (comparative studies)
S23 AB (control W5 group)
S22 PT (randomized controlled trial)
S21 MH (placebos)
S20 MH (sample size) AND AB (assigned OR allocated OR control)
S19 TI (trial)
S18 AB (random*)
S17 TI (randomised OR randomized)
S16 MH cluster sample
S15 MH pretest‐posttest design
S14 MH random assignment
S13 MH single‐blind studies
S12 MH double‐blind studies
S11 MH randomized controlled trials
S10 S4 AND S9
S9 S7 AND S8
S8 TI ( clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*) OR AB ( clean* or wash* or irrigat* or shower* or bath* or rins* or lavage*)
S7 S5 OR S6
S6 TI water OR AB water
S5 (MH "Water+")
S4 S1 OR S2 OR S3
S3 TI ( wound* or ulcer* or laceration* or bite* or abrasion* or tear* or diabetic foot or diabetic feet ) OR AB ( wound* or ulcer* or laceration* or bite* or abrasion* or tear* or diabetic foot or diabetic feet )
S2 (MH "Skin Ulcer+")
S1 (MH "Wounds and Injuries+")
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov)
(water AND ( rinse OR wash OR cleanse OR irrigate OR lavage) ) | Wounds
World Health Organization International Clinical Trials Registry Platform
Wound [condition] AND water [intervention]
wound or ulcer or diabetic foot [Title] AND water [Intervention]
Water AND wound [title]
Appendix 3. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process is provided to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to permit a definitive judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
· Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
· Either participants or some key study personnel were not blinded, but outcome assessment was blinded, and the non‐blinding of others was unlikely to introduce bias.
High risk of bias
Any one of the following.
· No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
· Blinding of key study participants and personnel attempted, but it is likely that the blinding could have been broken.
· Either participants or some key study personnel were not blinded, and the non‐blinding of others was likely to introduce bias.
Unclear
Either of the following.
· Insufficient information is provided to permit judgement of low or high risk of bias.
· The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
· No missing outcome data.
· Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
· For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
· For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on observed effect size.
· Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
· Reason for missing outcome data is likely to be related to true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
· For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is enough to induce clinically relevant bias in intervention effect estimate.
· For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce clinically relevant bias in observed effect size.
· ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
· Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
· Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
· The study did not address this outcome.
5. Are reports of the study free of the suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
· The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
· The study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
· Not all of the study’s prespecified primary outcomes have been reported.
· One or more primary outcomes are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
· One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
· One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
· The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information is provided to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
· had a potential source of bias related to the specific study design used;
· had extreme baseline imbalance;
· has been claimed to have been fraudulent; or
· had some other problem.
Unclear
There may be a risk of bias, but there is either:
· insufficient information to assess whether an important risk of bias exists; or
· insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Tap water versus no cleansing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Wounds healed | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.95, 1.14] |
| 1.2 Pain | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
1.2. Analysis.

Comparison 1: Tap water versus no cleansing, Outcome 2: Pain
Comparison 2. Tap water versus normal saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Infection | 8 | 2204 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.59, 1.19] |
| 2.1.1 Acute wounds only | 5 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.59, 1.22] |
| 2.1.2 Chronic wounds only | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.15, 1.94] |
| 2.1.3 Acute and chronic wounds (denominator N = wounds) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.41 [0.23, 84.79] |
| 2.2 Wounds healed | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.30, 1.07] |
| 2.3 Reduction in wound size | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.56, 1.68] |
| 2.4 Healing rate | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐3.06 [‐6.70, 0.58] |
Comparison 3. Distilled water versus normal saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Infection | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.45, 1.09] |
| 3.2 Wounds healed | 1 | 97 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
Comparison 4. Cooled boiled water versus normal saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Infection | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.37, 1.87] |
Comparison 5. Distilled water versus cooled boiled water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Infection | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.24, 1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bansal 2002.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation using randomisation schedule Setting: urban paediatric hospital in the USA Follow‐up undertaken after 48 hours |
|
| Participants | 46 children aged 2‐16 years (age range 4‐15 years tap water group, 2‐13 years normal saline group); 46 patients were consented, 44 returned for follow‐up. The remaining 2 patients were contacted by telephone. However baseline data have been reported for only 45 children. Inclusion criteria: patients aged between 1 and 18 years of age who presented within 8 hours of a traumatic laceration to the extremities. Exclusion criteria: patients were excluded if they were immunocompromised, had hand lacerations, the laceration was sustained by a dog bite, or if the patient was on antibiotics at the time of repair. |
|
| Interventions | 1) Tap water group (n = 21): wound irrigation with 500 mL of tap water in a sterile basin prepared by a non‐investigator staff member. The wound was irrigated with a 35 mL syringe attached to an irrigation shield to achieve a pressure of 25‐40 psi. 2) Normal saline group (n = 24): wound irrigation with 500 mL of normal saline in a sterile basin prepared by a non‐investigator staff member. The wound was irrigated with a 35 mL syringe attached to an irrigation shield to achieve a pressure of 25‐40 psi. | |
| Outcomes |
Primary outcome: wound infection (defined as one or more of the following; 1. Cellulitis or erythema of the wound margin of more than 4 mm with tenderness, 2. Purulent discharge from the wound, 3. Ascending lymphangitis, or 4. Dehiscence of the wound with wound separation of > 2 mm). 1) Tap water group: 2/21 (21 randomised) 2) Normal saline group: 2/24 (24 randomised) |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised to receive either sterile normal saline or tap water wound irrigation" Comment: evidence there was a randomisation process, sequence generated using a randomisation schedule. |
| Allocation concealment (selection bias) | High risk | Comment: allocation concealment not provided. |
| Blinding (performance bias and detection bias) participants and personnel | Low risk | Quote: "using a randomization schedule, a non‐investigator staff member then prepared 500ml of the tap water or normal saline solution in a sterile basin and gave it to the investigator to perform wound irrigation. The investigator was blinded to the solution used" Comment: enough evidence to suggest sufficient double blinding achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quotes: "After irrigation was completed, the wound again was cultured. Both the pre‐irrigation and post‐irrigation cultures were submitted to the laboratory for qualitative and quantitative bacterial cultures"…."The patients were asked to return in 48 hours for a wound check. Wound complications consisted of one or more of the following: (1) cellulitis or erythema of the wound margin of more than 4 mm with tenderness, (2) purulent discharge from the wound, (3) ascending lymphangitis, (4) dehiscence of the wound with wound separation of >2mm" Comment: no evidence given as to whether those performing cultures, performing laboratory testing or performing wound checks were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During the study period, a total of 46 patients were consented and enrolled in the study and 44 returned for follow‐up. The remaining 2 patients were contacted by telephone" Comment: adequate evidence to award a low‐risk judgement. It should be noted that the baseline data were presented for only 45 patients and all children were followed up. |
| Selective reporting (reporting bias) | Low risk | No direct quotes, although no discrepancy found between methodology outcomes and reported outcomes. |
| Other bias | Low risk | No other sources of bias identified. |
Chan 2016.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation using computer‐generated random numbers Setting: community nursing service (CNS) of a local hospital in Hong Kong Follow‐up undertaken at the end of 6 weeks |
|
| Participants | 23 adults (mean age of 76.77 years) with 31 acute or chronic wounds living in the community (mean age: 75.69 years tap water group, 78 years normal saline group); 23 adults were consented, 22 were analysed. One patient was excluded because of extreme wound size. Inclusion criteria: aged 18 years or more and receiving either chronic or acute wound care treatment from the CNS. Exclusion criteria: women receiving postnatal care, immunosuppressed persons, patients with acute or chronic leukaemia, malignant lymphoma, solid tumours, long‐term corticosteroid therapy, autologous stem cell transplantation, solid organ transplantation, an infected wound or receiving antibiotics, stage I or IV pressure ulcers, or leg ulcers or leg wounds involving tendon or bone. Participants with wound cleansing under specific wound‐cleansing protocols, such as using silver dressing material, were also excluded from participation. |
|
| Interventions | 1) Tap water group (n = 11 patients with 16 wounds): 100 mL of tap water from the CNS water tap was aseptically collected into 100‐mL sterile bottles a day before wound cleansing by a researcher. Wounds were cleansed daily by the CNS nurses who were blinded to the solution type using a swabbing method. 2) Saline group (n = 11 patients with 14 wounds): 100 mL of sterile saline was poured into 100‐mL sterile bottles a day before wound cleansing by a researcher. Wounds were cleansed daily by the CNS nurses who were blinded to the solution type using a swabbing method. | |
| Outcomes |
Primary outcome: wound infection (defined as 1. Clinical signs and symptoms, 2. Presence of high volumes of exudates and odour, or 3. Sensation of increased pain) 1) Tap water group: 2/16 2) Normal saline group: 0/14. One patient was excluded because of extreme wound size. Secondary outcome: wound healing (measured using 1. 1cm flexible wound grid and, 2. Two‐dimensional measurements in the form of surface area were done by measuring its linear dimension) 1) Tap water group: 0/16 2) Normal saline group: 0/14 . |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to cleansing with tap water (experimental group) or sterile normal saline (control group) by computer‐generated random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "double‐blind, randomised controlled trial" Comment: although quote states double‐blind, no indication how allocation was concealed. |
| Blinding (performance bias and detection bias) participants and personnel | Low risk | Quote: "The CNS nurses followed the same standard protocol to perform wound cleansing using a swabbing method for each patient; only the agent used to cleanse the wound (tap water or sterile saline) varied between the groups" Quote 2: "Since this was a double‐blinded study, 100 mL of tap water and 100 mL of sterile normal saline were prepared using the same kind of sterile bottles by the main researcher who was the only person to know the result of group assignment. Therefore, the randomization procedure was blinded to subjects and CNS nurses who performed wound cleansing" Comment: the CNS nurse was blinded to the solution used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Wound infection and healing were assessed each time when the wound was cleansed by the CNS nurse" Comment: the CNS nurse was blinded to the solution used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Random allocation resulted in 11 subjects (6 female and 5 male) with 16 wounds in the tap water (experimental) group, and 11 subjects (3 female and 8 male) with 14 wounds in the sterile normal saline (control) group" Comment: 13 patients in each group were randomised and 2 patients in each group dropped out of the study due to fever. |
| Selective reporting (reporting bias) | Low risk | Quote: "Two wounds (12.50%) in the experimental group versus no wound in the control group were found to be inflamed, neither exhibited severe pain, high‐volume exudate, or malodor. In addition, 3 wounds (18.75%) within the experimental group and none (0.00%) in the control group had newly developed epithelialization and granulation." |
| Other bias | Low risk | No other sources of bias identified. |
Godinez 2002.
| Study characteristics | ||
| Methods | Randomised controlled trial Method of allocation not stated Baseline comparability not stated | |
| Participants | 94 participants with minor extremity lacerations | |
| Interventions | 1) Irrigation with tap water (n = 36) 2) Irrigation with saline (n = 41) | |
| Outcomes |
Primary outcome: wound infection 1) Tap water group: 0/36 2) Normal saline group: 3/41 |
|
| Notes | Wounds were irrigated with tap water at a flow rate of 7 litres/minute. Saline was poured in a basin and aspirated using a syringe and irrigation was done using a pulsatile motion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised to irrigation with sterile saline using a standard method vs. holding the extremity under a faucet of tap water at a flow rate of 7 litres per minute for one minute" Comment: no indication how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "patients were randomised to irrigation with sterile saline using a standard method vs. holding the extremity under a faucet of tap water at a flow rate of 7 litres per minute for one minute" Comment: no indication how allocation was concealed. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | Quote: "The standardized irrigation method involved pre‐trained ED personnel using a 35mL or 60mL syringe with a splash guard to flush the wound length" Comment: likely that personnel were unblinded to the procedure. Unclear to extent participants were blinded to procedure. Therefore overall judgement is unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The infection rate for the sterile saline group was 7% (3/41) and 0% for the tap water group" Comment: no indication whether infection screen was done by blinded personnel. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "of the 94 subjects consented to the study, data were missing from 17, leaving 77 patients for analysis, 41 in the sterile saline group and 36 in the tap water group" Comment: no information given as to why the loss to follow‐up was sizeable, nor information offered as to why patients were not contacted if failed to attend for wound check. |
| Selective reporting (reporting bias) | Unclear risk | Quote: " The infection rate for the sterile saline group was 7% (3/41), and 0% for the tap water group" Comment: the methods of assessing wounds for infection are not clear. |
| Other bias | Low risk | No other sources of bias identified |
Griffiths 2001.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation was by a list of random numbers nominated by person not entering participants into the trial (closed list). Setting: 2 metropolitan community health centres in Australia Follow‐up undertaken at the end of 6 weeks |
|
| Participants | 43 adults (mean age 78.9 years, range 40‐100 years) with 60 acute or chronic wounds (mean age: 76.63 years tap water group, 81.16 years normal saline group); 8 patients with 11 wounds did not complete follow‐up and were not included in the analysis.
Inclusion criteria: patients with acute or chronic, non‐sutured wounds, grade 2 or 3 Exclusion criteria: grade 1 and 4 wounds, patients receiving antibiotics or who were immunosuppressed due to therapy, and wounds with a sinus where the base was not visible. |
|
| Interventions | 1) Tap water group (n = 20 participants randomised and 16 participants with 23 wounds analysed): empty 100 mL normal saline bottles were cleaned each morning with soap and warm water, and refilled with 100 mL tepid water from a designated faucet by a person not involved in the study. The tap was left to run for 15 seconds before the first bottle was filled. 2) Saline group (n = 23 participants randomised and 19 participants with 26 wounds analysed): 100 mL bottles of sterile normal saline were used and the seal broken by an independent person as there was no mechanism to seal the tap water bottles. | |
| Outcomes |
Primary outcome: wound infection (defined as presence of pus, discolouration, friable granulation tissue, pain tenderness, pocketing or bridging at base of the wound, abnormal smell and wound breakdown). 1) Tap water group: 0/23 2) Normal saline group: 3/26 Four participants from each group were excluded from the analysis. Secondary outcome: wound healing (healing was defined as the presence of epithelial tissue on the wound bed and, wounds were graphed using a 1cm flexible wound grid, the graph was transcribed onto a 1 mm grid graph paper, photocopied at 200% so that the surface area of the wound could be accurately determined). 1) Tap water group: 8/23 2) Normal saline group: 16/26 Secondary outcome:cost‐effectiveness (calculated based on the cost of the solution, dressing packs, syringe and the cannula) Normal saline group: cost of wound cleansing is AUD $1.16 per wound. Secondary outcome: patient satisfaction Participants who had showered their wounds prior to participating in the trial preferred that method to irrigation with normal saline. |
|
| Notes | Quality of tap water reported to meet Australian National Health and Medical Research Council requirements. Funding: the Nursing Directors of the Liverpool and Fairfield Community Health Service funded this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised to one of two groups using a random numbers table. The wounds of participants in the control group were irrigated with normal saline (0.9%) and those in the experimental group for a six‐week period" Comment: adequate evidence of sufficient random sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "the solutions were issued in identical containers...and refilled with tepid water from a designated faucet by someone otherwise not involved in the study...the seals on the bottles of sterile normal saline were broken before allocation" Comments: sufficient evidence of allocation concealment |
| Blinding (performance bias and detection bias) participants and personnel | Low risk | Quote: "To maintain the double‐blind design, the solutions were issued in identical containers to the community nurse. Empty 100ml normal saline bottles were filled with tap water for the experimental group. These bottles were cleaned each morning with soap and warm and refilled with tepid water from a designated faucet by someone otherwise not involved in the study" Comment: adequate evidence of effective blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The project manager, who was blinded to the cleansing solution used, undertook a wound assessment at enrolment and after six weeks of treatment" Comment: although assessor not independent, evidence to suggest they are blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty‐three patients with 60 wounds were eligible for inclusion into the study. However, eight (18.6%) patients, four from each group, were withdrawn because they stopped participating, were admitted to hospital or did not adhere to treatment" Comment: attrition and exclusions were clearly reported by the authors |
| Selective reporting (reporting bias) | Low risk | Comment: no direct quotes, however all outcomes detailed in methodology fully accounted for within results. |
| Other bias | Low risk | No other sources of bias identified |
Gupta 2006.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation using sealed envelopes Setting: hospital in India Follow‐up undertaken after 4 weeks |
|
| Participants | 58 adults (age of participants not stated) with anal fissures. 58 participants were recruited, 2 participants developed perianal rash during the treatment and 4 participants did not report back at the end of 4 weeks and were excluded from the trial. Inclusion criteria: all participants presenting with anal fissure of less than 2 months duration were considered for inclusion in the trial. The diagnosis of anal fissure was made in the presence of (i) visible anal fissure; and (ii) painful defecation with or without rectal bleeding. Participants between 18 and 60 years of age with voluntary, informed consent to participate in the study were included. Exclusion criteria: a history of recurrent fissure, presence of chronicity of anal fissures (sentinel tag, visible fibres of internal sphincter, suppuration etc.), pregnant women or patients operated on for any anorectal pathology in the past. |
|
| Interventions | 1) Sitz bath group (n = 27): participants were instructed to sit in a tub containing lukewarm water (the prescribed temperature of the water was to be equal to what they would have preferred for the whole‐body bath). This was to be carried out once in the morning after defecation and another just before bedtime. Participants were advised not to add anything in the water used for the sitz bath. 2) No sitz bath group (n = 25) |
|
| Outcomes |
Secondary outcome: wound healing (assessed at 4 weeks‐ criteria for healing not defined) 1) Sitz bath group: 23/27 2) No sitz bath group: 21/25 Secondary outcome: pain (assessed using the VAS 0‐100; 0 = no pain, 100 = the worst imaginable pain) 1) Sitz bath group: 0±0 2) No sitz bath group: 2±0.6 Secondary outcome: patient satisfaction (described as excellent, satisfactory, fair or unsatisfactory) Participants in the sitz bath group expressed better satisfaction than the participants who did not take sitz baths (P < 0.01). |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was carried out through simple randomization using a table of random numbers, which were then sealed in envelopes and opened just before commencement of therapy" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was carried out through simple randomization using a table of random numbers, which were then sealed in envelopes and opened just before commencement of therapy" |
| Blinding (performance bias and detection bias) participants and personnel | High risk | Quote: "patients were called weekly to the office and interviewed by an independent observer who ensured the sitz bath was taken correctly and carried out assessments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "patients were called weekly to the office and interviewed by an independent observer who ensured the sitz bath was taken correctly and carried out assessments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients developed perianal rash during the treatment and were excluded from the study. Another four patients did not report back at the end of 4 week and were excluded from the trial" Comment: attrition and exclusions were clearly reported by the authors |
| Selective reporting (reporting bias) | Low risk | Quote: "In all, the fissure had healed in 23/27 (85.1%) patients in sitz bath group and 21/25 (84%) in control group at 4‐week review" |
| Other bias | Unclear risk | Potential source of bias with the intervention ‐ unclear as to whether the participants adhered to the recommended intervention correctly i.e. sitz bath was required twice daily, specifically in the morning after defecation and before bedtime. Participants were only observed doing this weekly during the trial. |
Gupta 2007.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, participants randomly assigned by computer‐based sequential method Setting: hospital in India Follow‐up undertaken after 4 weeks |
|
| Participants | 46 adults (age range 18‐44 years, mean age 33 years sitz bath group and 32 years in the no‐sitz bath group) with chronic idiopathic fissure in ano (defined as anal fissure with > 8 weeks symptom duration). No participants were lost to follow‐up. Inclusion criteria: all participants with chronic idiopathic fissure in ano (defined as anal fissure with > 8 weeks symptom duration) in whom conservative treatment had failed and who were suitable for lateral sphincterotomy were considered for inclusion in this study. Exclusion criteria: previous sphincterotomy or anal dilatation, fissure associated with inflammatory bowel disease, suspicion of malignant fissure or ulcer, and concomitant procedure to be performed at the time of sphincterotomy (excision of skin tag was permitted). Further exclusion criteria were participant's inability to understand the end points of the study and to complete the forms for data recording. |
|
| Interventions | 1) Sitz bath group (n = 23): participants were instructed to sit in a tub containing lukewarm water (the prescribed temperature of the water was to be equal to what they would have preferred for the whole‐body bath). This was to be carried out once in the morning after defecation and another just before bedtime. Participants were advised not to add anything in the water used for the sitz bath. 2) No sitz bath group (n = 23) |
|
| Outcomes |
Secondary outcome: wound healing (assessed at 4 weeks ‐ defined as complete healing and full epithelialisation) 1) Sitz bath group: 22/23 2) No sitz bath group: 21/23 Secondary outcome: post‐operative pain (assessed on a scale of 0 to 3, number of items not stated) 1) Sitz bath group: 6.95 2) No sitz bath group: 7.60 P < 0.284 Secondary outcome: anal burning (described as a burning sensation in the anus immediately after defecation, assessed on a scale of 0 to 3, number of items not stated) 1) Sitz bath group: 3.75 2) No sitz bath group: 8.91 P < 0.00001 |
|
| Notes | Funding: NR Wound infection not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study population was then randomly assigned by computer‐based sequential method to post‐sphincterotomy analgesic therapy plus no sitz bath (control group, n = 23) or identical analgesic therapy plus sitz bath (sitz bath group, n = 23)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding (performance bias and detection bias) participants and personnel | High risk | Quote: "An independent observer...ensured that the sitz bath had been taken as advised, i.e., twice each day in the manner prescribed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent observer, blinded to the postoperative prescription, collected and assessed the data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was excluded for violation of the treatment protocol; nor were any patients lost to follow‐up, and data collection was complete" |
| Selective reporting (reporting bias) | Low risk | Quote: "No significant difference in mean pain score between groups was noticed after one, two and four weeks....However, the patients from the control group experienced significant anal burning compared with patients from sitz bath group" |
| Other bias | Low risk | No other sources of bias identified |
Gupta 2008.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation using computer‐based sequential method Setting: hospital in India Follow‐up undertaken after 4 weeks |
|
| Participants | 50 adults (mean age 45.6 years sitz bath group, 44.3 years no sitz bath group) with an indication for haemorrhoidectomy. No participants were lost to follow‐up. Inclusion criteria: adult patients with symptomatic and prolapsing haemorrhoids grades 3 and 4 with an indication for haemorrhoidectomy and who were able to provide informed consent were eligible for the study. Exclusion criteria: patients having associated fistula or fissure‐ in‐ano, inflammatory bowel disease, dermatitis or proctitis and an inability to understand the end‐points of the study and to complete the forms for data recording were excluded from the study. |
|
| Interventions | 1) Sitz bath group (n = 25): participants were instructed to sit in a tub containing lukewarm water (the prescribed temperature of the water was to be equal to what they would have preferred for the whole‐body bath). This was to be carried out once in the morning after defecation and another just before bedtime. Participants were advised not to add anything in the water used for the sitz bath. 2) No sitz bath group (n = 25) |
|
| Outcomes |
Secondary outcome: wound healing (defined as complete epithelial covering as observed by physical examination (per‐rectal examination and anoscopy)) 1) Sitz bath group: 24/25 2) No sitz bath group: 23/25 Secondary outcome: post‐operative pain after 1 week (evaluated using a visual analogue scale (VAS) 0 to 10, which was recorded by the participants) 1) Sitz bath group: mean score 5.1 2) No sitz bath group: mean score 5.4 Secondary outcome: patient satisfaction score (evaluated using a visual analogue scale (VAS) 0 to 10, which was recorded by the participants) 1) Sitz bath group: mean score 9.7 2) No sitz bath group: mean score 9.4 P = 0.29 |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study group was then randomly assigned by computer‐based sequential method" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding (performance bias and detection bias) participants and personnel | High risk | Quote: "Patients were evaluated at 2 and 4 weeks in the office and were interviewed by an independent observer, who ensured that the sitz bath was correctly taken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At the end of 4 weeks postoperatively, doctors independent of the study group examined the healing of patients’ wounds" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was withdrawn from the study because of adverse events. None of the patients were lost to follow up and data collection was complete" |
| Selective reporting (reporting bias) | Low risk | Quote: "at the end of four weeks the wounds were healed in 23 of the 25 patients from the control group and 24 out of 25 patients from the sitz bath group" |
| Other bias | Unclear risk | Potential bias with the intervention as patients were asked not to add anything to the sitz, however it is unclear if patients adhered to this regimen. |
Lakshmi 2011.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial. Randomisation using computer‐generated table of random numbers.
Setting: hospital in India Follow‐up undertaken after 5 to 6 weeks |
|
| Participants | 61 adults (mean age 46.03 years tap water group and 46.63 years in the saline group) with chronic wounds. No participants were lost to follow‐up. Inclusion criteria: chronic wound in any part of the body which is not healed for 3 weeks, immunised for tetanus within last 5 years. Exclusion criteria: serious medical disorder that impairs healing, malnutrition (BMI <15), extreme obesity (BMI >36), immuno‐compromised patients, pressure ulcer –grade and diabetic foot ulcers with osteomyelitis. |
|
| Interventions | 1) Tap water group (n = 31): wounds irrigated with tap water with the help of a PVC pipe for minimum of 10 minutes. Running the tap water for 5 minutes prior to use had been considered to clear any standing water from the system. 2) Normal saline group (n = 30): wounds irrigated with sterile normal saline by using 50 mL syringe with 18 gauge needle (expected pressure 8 pounds per square inch). |
|
| Outcomes |
Primary outcome: wound infection (assessed using clinical signs and positive wound culture) 1) Tap water group: 3/31 2) Normal saline group: 4/30 Secondary outcome: healing rate (wound dimension was measured by tracing the wound with acetate paper by using 0.5 mm microtip permanent marker, rate was assessed by percentage decrease in area at 2‐weekly intervals) 1) Tap water group: 5.36 ± 7.89 2) Normal saline group: 8.42 ± 6.57 |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisation using computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | Quote: "Group A has been irrigated with sterile normal saline by using 50 ml syringe with18 gauge needle (expected pressure 8 pounds per square inch). Group B has been irrigated with tap water with the help of a PVC pipe for minimum of 10 minutes." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcome assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: " A total of 43 patients in tap water group, and 39 patients in the normal saline group were enrolled in the study. In that, 31 subjects in tap water group, and 30 subjects in the normal saline group" |
| Selective reporting (reporting bias) | Low risk | Quote: "At the end of the 5‐6 weeks follow up the percentage decrease in saline group was 45.34% (mean size: 8.42±6.57) compared to 40.58 % (mean size of 5.36±7.89) in tap water group" |
| Other bias | Low risk | No other sources of bias identified |
Mirshamsi 2007.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial Setting: hospital in Iran Follow‐up undertaken after 5 days |
|
| Participants | 600 adults (age range not stated) with fresh contaminated traumatic wounds. Loss to follow‐up not stated. Inclusion criteria: fresh contaminated traumatic wounds. Exclusion criteria: not stated. |
|
| Interventions | 1) Tap water group (n = 300) 2) Normal saline group (n = 300) Methods of wound cleansing not stated. |
|
| Outcomes |
Primary outcome: wound infection (measured by clinical signs including erythema, swelling, warmth, purulent and bloody drainage and crepitation) 1) Tap water group: 25/300 2) Normal saline group: 26/300 |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | Comment: blinding not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of outcomes assessment not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: withdrawals and loss to follow‐up not stated. |
| Selective reporting (reporting bias) | Low risk | Quote: "In this study wound infection progressively increased by the days after wound management in both groups and finally 8.3% of wounds washed with tap water and 8.6% of wounds washed with Normal saline showed one or more clinical sign of infection" |
| Other bias | Low risk | No other sources of bias identified |
Moscati 2007.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation using computer‐based random numbers generator and sealed envelopes
Setting: community hospital in the USA Follow‐up undertaken after 14 days |
|
| Participants | 715 adults with uncomplicated skin lacerations requiring staple or suture repair. 713 participants were randomised, 77 participants were excluded from the trial due to lost to follow‐up or discontinued intervention. Inclusion criteria: patients presenting to the participating ED who were older than 17 years and had uncomplicated skin lacerations requiring staple or suture repair. Exclusion criteria: included the following: puncture wounds; bite wounds; self‐inflicted wounds; wounds more than 8 hours old; wounds involving tendon, joint, or bone; wounds with gross contamination requiring scrubbing or surgical debridement; patients taking antibiotics; diabetic patients; patients with significant peripheral vascular disease; patients with human immunodeficiency virus or other immunocompromised conditions; patients on corticosteroids; prisoners; patients unable to give consent; or pregnant patients. |
|
| Interventions | 1) Tap water group (n = 339): the area to be irrigated was held beneath an unmodified tap in a steel sink. For other wound locations, the provider attached a 3‐ft length of clear plastic tubing to a tapered tap outlet to facilitate irrigation. The tubing was not sterile, but it was used only once and then discarded. Irrigation with tap water was undertaken by the participant. There were no maximum times or volumes of irrigation. 2) Normal saline group (n = 372): the lacerations of participants were irrigated by the provider using a minimum of 200 mL of sterile saline administered with a sterile 35‐mL syringe with a splash shield. There were no maximum times or volumes of irrigation (2 participants did not receive the intervention due to tendon involvement). | |
| Outcomes |
Primary outcome: wound infection (defined as wounds that required a significant change in their course of treatment such as surgical debridement, antibiotics or early removal of sutures). 1) Tap water group: 12/339 2) Normal saline group: 11/372 Secondary outcome: costs (extrapolation of costs calculated for lacerations per year in the USA based on the worst case scenario of the upper limit of the CI for the percent difference in infection rates of 3.64%, resulting in potentially greater number of participants who would need antibiotic therapy in the tap water group. Included the cost of the sterile saline, 35ml syringe, splash shield, tap water use and plastic extension tubing and antibiotic treatment for infections). 1) Tap water group: USD $7,280,000 2) Normal saline group: USD $72,880,000 |
|
| Notes | Funding: funded in part by a grant from the Federal Highway Administration. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After obtaining consent, subjects were randomised to SS or TW irrigation by opening the next numbered study envelope for that institution. Envelopes were pre‐randomized at each institution using a computer‐based random number generator" Comment: adequate evidence of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "After obtaining consent, subjects were randomised to SS or TW irrigation by opening the next numbered study envelope for that institution. Envelopes were pre‐randomized at each institution using a computer‐based random number generator" Comment: adequate evidence that allocation was concealed. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | Quote: "This study was a multicenter, prospective, randomised, unblinded trial"…."The provider instructed subjects in the TW group with wounds to the upper extremities on how to irrigate their wound under water tap for a minimum of 2 minutes….the lacerations of subjects in the saline group were irrigated by the provider using a minimum of 200mL of SS (Baxter Health Corp., Deerfield, IL), administered with a sterile 35‐mL syringe (Tyco Healthcare Group, Mansfield, MD) with a splash shield (Combiguard II; Ethox Corp., Buffalo, NY). Comment: enough evidence to suggest neither personnel or participant were sufficiently blinded to the treatment given, therefore judged as unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All subjects were instructed to return to the ED in 5‐14 days, depending on the location or the wound, for removal of staples or sutures and wound follow‐up. Providers in the ED removing staples or staples of sutures were blinded to the subject's allocation and judges the presence of the wound infection. Subjects who did not return to the ED were contacted by telephone and questioned about the possible presence of wound infection using the same criteria. Callers were also blinded to allocation" Comment: enough evidence of adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 715 subjects enrolled in the study…eighty‐one subjects were not included in the analysis, with 71 of these due to lack of follow‐up. Those who could not be reached for follow‐up included 35 subjects in the SS [sterile saline] group and 36 subjects in the TW [tap water] group" Comment: adequate intention to treat analysis provided with equal numbers of drop out within each intervention arm at an acceptable level. |
| Selective reporting (reporting bias) | Low risk | All outcomes successfully reported in the results section. |
| Other bias | Low risk | No other sources of bias identified |
Museru 1989.
| Study characteristics | ||
| Methods | 3‐arm randomised controlled trial, no information on the method of randomisation
Setting: medical centre in Tanzania Follow‐up period not stated |
|
| Participants | 86 participants (age not stated) with open fractures. No other inclusion criteria stated. No exclusion criteria stated. |
|
| Interventions | 1) Distilled water group (n = 35): no methods stated. 2) Cooled boiled water group (n = 31): water was first filtered and then put in 500 mL or litre sized bottles. These were placed in an oven containing water, which was then boiled. The prepared water was used the same day. | |
| Outcomes |
Primary outcome: wound infection (method of measurement not described) 1) Distilled water group: 6/35 2) Cooled boiled water group: 9/31 |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomised study was carried out on 86 patients with first, secondary and third degree open fractures in order to compare the effect of isotonic saline, distilled water and boiled water as irrigating fluids" Comment: no clear indication as to how the 86 participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomised study was carried out on 86 patients with first, secondary and third degree open fractures in order to compare the effect of isotonic saline, distilled water and boiled water as irrigating fluids" Comment: no clear indication as to whether allocation to intervention was concealed at all. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | No direct quotes, however no evidence given of blinding to intervention given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In the 86 patients with open fractures, isotonic saline, distilled and boiled water was used for irrigation in 20, 35 and 31 cases respectively. Eleven had grade 1 open fractures, 52 grade 2 and 23 grade 3. Twenty‐two patients developed superficial wound infection and 5 eventually [developed] chronic osteomyelitis" Comment: no information given as to whether those assessing wounds were blinded to assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The only information given as to whether loss to follow‐up occurred was that "one patient died, and this was the only death in the series. None of these complications were related to the type of fluid used" Comment: as no good evidence to suggest no loss to follow‐up then judged as unclear. |
| Selective reporting (reporting bias) | Unclear risk | No direct quotes, but outcomes measured not clear within methodology. |
| Other bias | Low risk | No other sources of bias identified |
Olufemi 2017.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, randomised using simple ballot technique Setting: orthopaedic hospital in Nigeria Follow‐up undertaken 5 days post operation |
|
| Participants | 120 participants (mean age 37.1 years normal saline group, 34.6 years distilled water group) with open lower limb fractures. 120 participants were recruited and 23 were lost to follow‐up. Inclusion criteria: patients of all ages with Gustilo‐Anderson I‐IIIa open fractures of the lower extremities presenting within 24 hours who consented were included in the study. Exclusion criteria: patients with potentially life‐threatening injuries that required emergency interventions were excluded from the study. |
|
| Interventions | 1) Distilled water group (n = 47): wounds were irrigated with at least 3L of distilled water according to guidelines. The wound was irrigated using a 20 mL piston syringe. 2) Normal saline group (n = 50): wounds were irrigated with at least 3L of normal saline according to guidelines. The wound was irrigated using a 20 mL piston syringe. |
|
| Outcomes |
Primary outcome: wound infection (measured using the Cutting and Harding criteria including: abscess, cellulitis, wound discharge, discolouration, delayed healing, friable granulation tissue, unexpected pain and tenderness, pocketing at the base of the wound, epithelial bridging, abnormal smell and wound breakdown) 1) Distilled water group: 16/47 2) Normal saline group: 22/50 Secondary outcome: wound healing at the end of 8 weeks (defined, following wound inspection, as the presence of epithelial tissue covering the wound) 1) Distilled water group: 47/47 2) Normal saline group: 50/50 |
|
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: using a ballot technique |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding (performance bias and detection bias) participants and personnel | Unclear risk | Comment: no evidence of blinding of those delivering treatment or participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no evidence of blinding of outcomes assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twelve patients were lost to follow‐up, while 97 patients were available until conclusion of the study" |
| Selective reporting (reporting bias) | Low risk | Quote: "The wound infection rate was 34% in the distilled water group and 44% in the isotonic saline group" |
| Other bias | Low risk | No other sources of bias identified |
Weiss 2013.
| Study characteristics | ||
| Methods | 2‐arm randomised controlled trial, allocation by computer‐generated randomisation Setting: Follow‐up undertaken after 30 days |
|
| Participants | 663 participants (older than 1 year of age) who presented to the ED with an uncomplicated soft tissue laceration requiring repair. 631 participants were randomised and 6 participants were lost to follow‐up. Inclusion criteria: consecutive patients older than 1 year of age, who presented to the ED with an uncomplicated soft tissue laceration requiring repair. Participants had to provide a telephone number for follow‐up in order to be enrolled in the study. Exclusion criteria: included any underlying immunocompromising illness (e.g. diabetes mellitus, chronic alcoholism, asplenism, primary immune disorder, steroid use or chemotherapy), current use of antibiotics, puncture or bite wounds, underlying tendon or bone involvement, or wounds more than 9 hours old. |
|
| Interventions | 1) Tap water group (n = 318): the tap water was obtained from a single designated faucet in the ED into a sterile bowl by the ED technician. The water ran for 5 seconds prior to being collected. 2) Normal saline group (n = 313): sterile normal saline was poured into a sterile bowl by the ED technician. |
|
| Outcomes |
Primary outcome: wound infection (based on the following criteria: (0) no evidence of infection, (1) simple stitch abscess, (2) surrounding erythema less than 1 cm, (3) surrounding erythema greater than 1 cm or lymphangitis, (4) gross exudate, (5) fever greater than or equal to 38°C and (6) others. A wound was classified as infected if it received a rating of 1 or higher). 1) Tap water group: 11/318 2) Normal saline group: 20/313 |
|
| Notes | Funding: none. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Wound irrigation solution type was computer randomised and allocation was done on a sequential basis" |
| Allocation concealment (selection bias) | Low risk | Quote: "Wound irrigation solution type was computer randomised and allocation was done on a sequential basis" |
| Blinding (performance bias and detection bias) participants and personnel | Low risk | Comment: all wounds were treated the same (except for the intervention) according to a standardised protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patient, the treating physician and the physician checking the wound for infection were all blind regarding solution type" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six patients were lost to follow‐up when they did not return for evaluation of the wound within 5 days of treatment" |
| Selective reporting (reporting bias) | Low risk | Quote: "There were 20 infections 6.4% (95% CI 9.1% to 3.7%) in the SS group compared with 11 infections 3.5% (95% CI 5.5% to 1.5%) in the TW group, a difference of 2.9% (95% CI ‐0.4% to 5.7%)" |
| Other bias | Low risk | No other sources of bias identified |
BMI: body mass index; CNS: community nursing service; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angeras 1992 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Bansal 1993 | This study compared the effects of topical phenytoin powder and normal saline on the healing of trophic leprosy ulcers. |
| Behmanesh 2013 | This study compared the effects of tap water and olive oil to distilled water. |
| Bulstrode 1988 | This study compared the addition of dilute and concentrated amino acids to saline on the rate of healing of chronic leg ulcers. |
| Burke 1998 | Study was excluded because the intervention was combined with saline dressings and whirlpool therapy (water). It is therefore not possible to attribute any effect to whirlpool therapy (water). |
| Chisholm 1992 | This study compared two devices used for irrigation of wounds. Irrigating solution used with both devices was normal saline. |
| Fraser 1976 | The purpose of the trial was not to assess the cleansing of the wound. |
| Goldberg 1981 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Greenway 1999 | Study excluded because it evaluates the effect of insulin and normal saline on the healing rate of wounds. |
| Johnson 1985 | Study excluded because it compares irrigation of perineal wounds with either 1% povidone‐iodine or normal saline. |
| King 1984 | Wound cleansing in this study was part of the operative procedure. |
| Manhold 1976 | The study compared normal saline and glycoside for irrigation during dental procedures. |
| Medves 1997 | The study evaluates solution used to cleanse umbilical cord. A systematic review focusing on umbilical cord care has been undertaken. |
| Neues 2000 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Patterson 2005 | This study used antibacterial soap along with water for cleansing which could influence the findings. |
| Riederer 1997 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Ruhle 2017 | This is a protocol for an RCT to be conducted. |
| Scondotto 1999 | This study evaluates the efficacy of sulodexide compared to cleansing with physiological solution and the application of elastic compression on the healing of venous ulcers. |
| Selim 2000 | Review. |
| Selim 2001 | No data reported. |
| Svedman 1983 | Compares two different methods of wound irrigation. Isotonic saline was the irrigant used in both groups. |
| Sweet 1976 | Not relevant to the review. This study compares two different devices for the irrigation of third molar surgical sites with high volumes of normal saline. |
| Tay 1999 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Valente 2003 | This is a quasi‐RCT, included in a previous version of the review but now removed in accordance with updates to methodology. |
| Voorhees 1982 | The purpose of the trial was not to assess the cleansing of the wound. |
| Weiss 2007 | Abstract only, however, Weiss 2013 paper that is included in this review is the full paper. The authors were contacted to clarify but did not respond. |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Cherry 2003.
| Methods | Quote: "Randomised comparison" |
| Participants | Participants with chronic venous leg ulcers. |
| Interventions | Forced circulation of tap water in a hydrobath (n = 20) and use of Sterilox bacterial disinfectant (n = 20). |
| Outcomes | Total healing of the ulcers within 24 weeks of treatment, confirmed by photography. |
| Notes | Method of randomisation unclear. The trials did not provide enough information to endorse its conformity with the eligibility criteria. |
Saw 2006.
| Methods | Cohort study ‐ unclear whether randomised. |
| Participants | 60 patients undergoing application of external fixation. |
| Interventions | Patients and/or carers taught how to do pin‐site dressing using normal saline or drinking water as a cleansing solution on a daily basis. |
| Outcomes | Pin‐tract infection. |
| Notes | More information required to determine whether this is a randomised study. The trials did not provide enough information to endorse its conformity with the eligibility criteria. |
Characteristics of ongoing studies [ordered by study ID]
NCT01846598.
| Study name | 48 hours after surgery shower patient's wound infection rate, pain score, patient satisfaction and cost |
| Methods | Parallell group randomised controlled trial; randomisation by table of random computer‐generated numbers. |
| Participants | Adults over 20 years of age, 48 hours after thoracic, general, thyroid and orthopaedic surgery. |
| Interventions | Shower (n = 222) versus no shower (n = 222). |
| Outcomes | Wound infection; pain; patient satisfaction; care costs (all at 48 hours postoperative). |
| Starting date | May 2013 |
| Contact information | Principal Investigator: Hsieh Pei‐Yin, MSD, National Taiwan University Hospital, Taipei, Taiwan. |
| Notes | Other study ID number: 201301038RIND |
NCT02820272.
| Study name | Water for reducing pain in negative pressure wound therapy |
| Methods | Three‐arm randomised controlled trial. |
| Participants | Adults aged 18 to 70 years with an open wound and treated with negative pressure wound therapy. |
| Interventions | Negative pressure wound therapy (NPWT) with; 1) cold water at 4 °C injected into NPWT sponge 10 minutes before dressing change; 2) normal saline at room temperature injected into NPWT sponge 10 minutes before dressing change; 3) without other intervention. |
| Outcomes | Pain reduction during dressing changes assessed by Visual Analogue Scale at 3 day time point. |
| Starting date | October 2012 |
| Contact information | Principal Investigator: Apichai Angspatt, Chulalongkorn University. |
| Notes | Completed; record last updated 01 July 2016. |
Differences between protocol and review
1.The published protocol was titled: 'Normal saline vs tap water for wound cleansing'. This was changed at the review stage to: 'Water for wound cleansing' to reflect the different types of water used in the studies.
2. The published protocol objective was to evaluate the infection and healing rates in acute and chronic wounds cleaned with various cleansing solutions (e.g. tap water, sterile normal saline, cooled boiled water), the objective has been changed to assess the effects of water for wound cleansing.
3. The published protocol planned to include RCTs and quasi‐RCTs. This is the first update of this review to exclude quasi‐RCTs. Any subsequent updates of this review will also exclude trials which clearly state the use of quasi‐randomisation.
4. The published protocol included people with wounds of any aetiology. This update excluded trials if they compared solutions for dental procedures or for patients with burns.
5. For this update, the comparison of cooled boiled water with distilled water has been included.
6. In previous versions of this review we included studies that compared normal saline plus additives (e.g. hydrogen peroxide) with water on wound infection and healing. We have now rectified this and excluded such types of interventions as there is a high risk of confounding due to the unknown effects of the additives.
7. For this update, staff satisfaction has been removed.
8. We updated our search strategy to include the term lavage.
Contributions of authors
Ritin Fernandez: conceived the review; designed the review update; coordinated the review update; developed the search strategy; searched the literature; screened search results; retrieved papers; appraised trial quality; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review update; contributed to writing and editing the review update; advised on the review update; secured funding; performed previous work that was the foundation of the current review update; wrote to study authors/experts/companies; provided data; approved final review update prior to submission; is guarantor of the review update.
Heidi L Green: designed the review update; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review update; contributed to writing or editing the review update; advised on the review update; wrote to study authors/experts/companies; provided data; approved final review update prior to submission.
Rhonda Griffiths: conceived the review; designed the review update; developed the search strategy; searched the literature; screened search results; retrieved papers; appraised trial quality; checked quality of data extraction; analysed or interpreted data; checked quality assessment; checked quality of statistical analysis; produced the first draft of the review update; contributed to writing or editing the review update; advised on the review update; secured funding; performed previous work that was the foundation of the current review update; approved final review update prior to submission.
Ross A Atkinson: designed the review update; coordinated the review update; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; checked quality of statistical analysis; produced the first draft of the review update; contributed to writing or editing the review update; advised on the review update; wrote to study authors/experts/companies; provided data; approved final review update prior to submission.
Laura J Ellwood: designed the review update; extracted data; checked quality of data extraction; analysed or interpreted data; undertook quality assessment; checked quality assessment; performed statistical analysis; checked quality of statistical analysis; produced the first draft of the review update; contributed to writing or editing the review update; advised on the review update; wrote to study authors/experts/companies; provided data; approved final review update prior to submission.
Contributions of editorial base
Nicky Cullum (Coordinating Editor): edited previous versions of the review, advised on methodology, interpretation and review content. Approved the previous review updates prior to submission.
Gill Norman (Editor): edited this version of the review, advised on methodology, interpretation and review content. Approved the final review prior to submission.
Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content and edited this version of the review.
Sophie Bishop (Information Specialist): updated the search and edited the search methods section for this version of the review.
Tom Patterson (Editorial Assistant): drafted the Plain Language Summary and edited the reference sections for this version of the review.
Sources of support
Internal sources
-
Centre for Research in Nursing and Health, St George Hospital, Australia
The hospital has provided in‐kind support relative to time and overheads.
-
University of Wollongong, School of Nursing, Australia
The School of Nursing has provided in‐kind support relative to time and overheads.
-
University of Western Sydney Macarthur, Australia
The School of Nursing and Midwifery had provided in‐kind support relative to time and overheads for the first version of the review.
-
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK
The Division of Nursing, Midwifery and Social Work has provided in‐kind support relative to time and overheads.
-
South Western Sydney Area Health Service, Australia
The health service had provided in‐kind support relative to time and overheads for the first version of the review.
External sources
-
The National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
Ritin Fernandez: none known.
Heidi Green: none known.
Rhonda Griffiths: none known.
Ross Atkinson: none known.
Laura Ellwood: works as a health professional.
Ritin Fernandez and Rhonda Griffiths conducted one of the trials included in the review, however the authors did not receive from any commercial entity any payments or pecuniary, in‐kind or other professional or personal benefits that were related in any way to the subject of the work. The authors of this trial were also not involved in the data extraction or RoB assessment for this included trial. This trial was also subject to the same rigorous quality assessment as other trials included in the review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bansal 2002 {published data only}
- Bansal BC, Wiebe RA, Perkins SD, Abramo TJ. Tap water for irrigation of lacerations. American Journal of Emergency Medicine 2002;20(5):469-72. [DOI] [PubMed] [Google Scholar]
Chan 2016 {published data only}
- Chan MC, Cheung K, Leung P. Tap water versus sterile normal saline in wound swabbing. Journal of Wound, Ostomy and Continence Nursing 2016;43(2):140-7. [DOI] [PubMed] [Google Scholar]
Godinez 2002 {published data only}
- Godinez FS, Grant-Levy TR, McGuirk TD, Letterle S, Eich M, O'Malley GF. Comparison of normal saline vs tap water for irrigation of minor lacerations in the emergency department. Academic Emergency Medicine 2002;19(5):396-7. [Google Scholar]
Griffiths 2001 {published data only}
- Griffiths RD, Fernandez RS, Ussia CA. Is tap water a safe alternative to normal saline for wound irrigation in the community setting? Journal of Wound Care 2001;10(10):407-11. [DOI] [PubMed] [Google Scholar]
Gupta 2006 {published data only}
- Gupta PJ. Randomized, controlled study comparing sitz‐bath and no‐sitz‐bath treatments in patients with acute anal fissures. ANZ Journal of Surgery 2006;76(8):718-21. [DOI] [PubMed] [Google Scholar]
Gupta 2007 {published data only}
- Gupta PJ. Effects of warm water sitz bath on symptoms in post-anal sphincterotomy in chronic anal fissure—a randomized and controlled study. World Journal of Surgery 2007;31(7):1480-4. [DOI] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta PJ. Warm sitz bath does not reduce symptoms in posthaemorrhoidectomy period: a randomized, controlled study. ANZ Journal of Surgery 2008;78(5):398-401. [DOI] [PubMed] [Google Scholar]
Lakshmi 2011 {published data only}
- Lakshmi R, Andrews R, Chumber S. A study to compare the effectiveness of normal saline vs tap water in irrigation of chronic wounds. International Journal of Nursing Education 2011;3(1):19-21. [Google Scholar]
Mirshamsi 2007 {published data only}
- Mirshamsi MH, Ayatollahi J, Dashti-R MH. A comparison between traumatic wound infections after irrigating them with tap water and normal saline. World Journal of Medical Sciences 2007;2(1):58-61. [Google Scholar]
Moscati 2007 {published data only}
- Moscati RM, Mayrose J, Reardon RF, Janicke DM, Jehle DV. A multicentre comparison of tap water versus sterile saline for wound irrigation. Academic Emergency Medicine 2007;14:404-10. [DOI] [PubMed] [Google Scholar]
Museru 1989 {published data only}
- Museru LM, Kumar A, Ickler P. Comparison of isotonic saline, distilled water and boiled water in irrigation of open fractures. International Orthopaedics 1989;13(3):179-80. [DOI] [PubMed] [Google Scholar]
Olufemi 2017 {published data only}
- Olufemi OT, Adeyeye AI. Irrigation solutions in open fractures of the lower extremities: evaluation of isotonic saline and distilled water. Société Internationale de Chirurgie Orthopédique et de Traumatologie (SICOT-J) 2017;3(7):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2013 {published data only}
- Weiss EA, Oldham G, Lin M, Foster T, Quinn JV. Water is a safe and effective alternative to sterile normal saline for wound irrigation prior to suturing: a prospective, double-blind, randomised, controlled clinical trial. BMJ Open 2013;3(1):e001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Angeras 1992 {published data only}
- Angeras MH, Brandberg A, Falk A, Seeman T. Comparison between sterile saline and tap water for the cleaning of acute traumatic soft tissue wounds. European Journal of Surgery 1992;158(6-7):347-50. [PubMed] [Google Scholar]
- Falk A, Brandberg Å, Hall-Angerås M, Seeman T. Irrigation of acute traumatic soft tissue wounds - a comparison between sterile saline and tap water. Annales Chirurgiae et Gynaecologiae 1989;78(suppl 204):48. [Google Scholar]
Bansal 1993 {published data only}
- Bansal NK, Mukul MD. Comparison of topical phenytoin with normal saline in the treatment of chronic trophic ulcers in leprosy. International Journal of Dermatology 1993;32(3):210-3. [DOI] [PubMed] [Google Scholar]
Behmanesh 2013 {published data only}
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, Khafri S. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh 2013;14(3):309-15. [Google Scholar]
Bulstrode 1988 {published data only}
- Bulstrode CJ, Goode AW, Scott PJ. A prospective controlled trial of topical irrigation in the treatment of delayed cutaneous healing in human leg ulcers. Clinical Science 1988;75(6):637-40. [DOI] [PubMed] [Google Scholar]
Burke 1998 {published data only}
- Burke DT, Ho CH, Saucier MA, Stewart G. Effects of hydrotherapy on pressure ulcer healing. American Journal of Physical Medicine and Rehabilitation 1998;77(5):394-8. [DOI] [PubMed] [Google Scholar]
Chisholm 1992 {published data only}
- Chisholm CD, Cordel WH, Rogers K, Woods JR. Comparsion of a new pressurized saline canister versus syringe irrigation for laceration cleansing in the emergency department. Annals of Emergency Medicine 1992;21(11):1364-7. [DOI] [PubMed] [Google Scholar]
Fraser 1976 {published data only}
- Fraser I, Askew A, Biles J, Pinchin J. Prospective randomised controlled trial of early postoperative bathing. BMJ 1976;1(6024):1506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 1981 {published data only}
- Goldberg H, Rosenthal S, Nemetz J. Effect of washing closed head and neck wounds on wound healing and infection. American Journal of Surgery 1981;141:358-9. [DOI] [PubMed] [Google Scholar]
Greenway 1999 {published data only}
- Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomised, double-blind, placebo-controlled trial. Journal of Wound Care 1999;8(10):526-8. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson JN, Croton RS, McGlinchey JJ, McLoughlin GA. The effect of povidone-iodine irrigation on perineal wound healing following proctectomy for carcinoma. Journal of Hospital Infection 1985;6(Suppl A):81-6. [DOI] [PubMed] [Google Scholar]
King 1984 {published data only}
- King J, Bulstrode C, Revell P. Irrigating fluid in arthroscopy. Lancet 1984;84(8369):159-60. [DOI] [PubMed] [Google Scholar]
Manhold 1976 {published data only}
- Manhold JH, Manhold EA, Singh S. Animal experimental procedure used in human subjects to compare healing effectiveness of saline, placebo, and a type of commercial oxygenating agent. Journal of Dental Research 1976;55(48):B73. [Google Scholar]
Medves 1997 {published data only}
- Medves JM, O'Brien BA. Cleaning solutions and bacterial colonization in promoting healing and early separation of the umbilical cord in healthy newborns. Canadian Journal of Public Health 1997;88(6):380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neues 2000 {published data only}
- Neues C, Haas P. Modification of postoperative wound healing by showering [[Beeinflussung der postoperativen wundheilung durch duschen]]. Der Chirurg 2000;71(2):234-6. [DOI] [PubMed] [Google Scholar]
Patterson 2005 {published data only}
- Patterson MM. Multicenter pin care study. Orthopaedic Nursing 2005;24(5):349-50. [DOI] [PubMed] [Google Scholar]
Riederer 1997 {published data only}
- Riederer SR, Inderbitzi R. Does a shower put postoperative healing at risk? Der Chirurg 1997;68(7):715-7. [DOI] [PubMed] [Google Scholar]
Ruhle 2017 {published data only}
- Rühle A, Oehme F, Börnert K, Fourie L, Babst R, Link BC, et al. Simple wound irrigation in the postoperative treatment for surgically drained spontaneous soft tissue abscesses: study protocol for a prospective, single-blinded, randomized controlled trial. JMIR research protocols 2017;6(5):e77 p.1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scondotto 1999 {published data only}
- Scondotto G, Aloisi D, Ferrari P, Martini L. Treatment of venous leg ulcers with sulodexide. Angiology 1999;50(11):883-9. [DOI] [PubMed] [Google Scholar]
Selim 2000 {published data only}
- Selim P. Will water do? Cleansing of leg ulcers in the community. ACCNS Journal for Community Nurses 2000;5:11-3. [Google Scholar]
Selim 2001 {published data only}
- Selim P, Bashford C, Grossman C. Evidence-based practice: tap water cleansing of leg ulcers in the community. Journal of Clinical Nursing 2001;10:372-9. [DOI] [PubMed] [Google Scholar]
Svedman 1983 {published data only}
- Svedman P. Irrigation treatment of leg ulcers. Lancet 1983;2(8349):532-4. [DOI] [PubMed] [Google Scholar]
Sweet 1976 {published data only}
- Sweet JB, Butler DP, Drager JL. Effects of lavage techniques with third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology 1976;41(2):152-8. [DOI] [PubMed] [Google Scholar]
Tay 1999 {published data only}
- Tay SK. Is routine procaine spirit application necessary in the care of episiotomy wound? Singapore Medical Journal 1999;40(9):581-3. [PubMed] [Google Scholar]
Valente 2003 {published data only}
- Valente JH, Forti RJ, Freundlich LF, Zandieh SO, Crain EF. Wound irrigation: tap water or saline? In: Emergency Conference. 2001. [DOI] [PubMed]
- Valente JH, Forti RJ, Freundlich LF, Zandieh SO, Crain EF. Wound irrigation: tap water or saline? Pediatric Research 2001;49(4):78A. [Google Scholar]
- Valente JH, Forti RJ, Freundlich LF, Zandieh SO, Crain EF. Wound irrigation in children: saline solution or tap water? Academic Emergency Medicine 2001;8(5):539. [DOI] [PubMed] [Google Scholar]
- Valente JH, Forti RJ, Freundlich LF, Zandieh SO, Crain EF. Wound irrigation in children: saline solution or tap water? Annals of Emergency Medicine 2003;41:609-16. [DOI] [PubMed] [Google Scholar]
Voorhees 1982 {published data only}
- Voorhees E, Rosenthal D. Early postoperative showering. Military Medicine 1982;147(11):967-8. [PubMed] [Google Scholar]
Weiss 2007 {published data only}
- Weiss E, Lin M, Oldham G. Tap water is equally safe and effective as sterile normal saline for wound irrigation; a double blind, randomized, controlled, prospective clinical trial. In: Society for Academic Emergency Medicine 2007 Annual Meeting; 2007 May 16-19; Chicago (IL). 2007:S146-7.
References to studies awaiting assessment
Cherry 2003 {published data only}
- Cherry GW. Comparison of Syerilox with tap water in the treatment of chronic venous ulcers of the leg. National Research Register 2003.
Saw 2006 {published data only}
- Saw A, Chan CK, Penafort R, Sengupta S. A simple practice protocol for care of metal-skin interface of external fixation. Medical Journal of Malaysia 2006;61(Suppl A):61-5. [PubMed] [Google Scholar]
References to ongoing studies
NCT01846598 {published data only}
- NCT01846598. 48 hours after surgery shower patient's wound infection rate, pain score, patient satisfaction and cost. clinicaltrials.gov/ct2/show/nct01846598 (accessed November 2019).
NCT02820272 {published data only}
- NCT02820272. Water for reducing pain in negative pressure wound therapy. clinicaltrials.gov/ct2/show/NCT02820272 (accessed November 2019).
Additional references
Boga 2019
- Boga SM. Nursing practices in the prevention of post-operative wound infection in accordance with evidence-based approach. International Journal of Caring Sciences 2019;12(2):1228. [Google Scholar]
Brown 2018
- Brown A. Dispelling some myths and misconceptions in wound care. Journal of Community Nursing 2018;32(6):24-33. [Google Scholar]
Cullum 2016
- Cullum N, Buckley HL, Dumville J, Hall J, Lamb K, Madden MT, et al. Wounds research for patient benefit: a 5 year programme of research. Health Technology Assessment 2016;1:1-334. [PubMed] [Google Scholar]
Cutting 2005
- Cutting KF, White RJ. Criteria for identifying wound infection—revisited. Ostomy Wound Manage 2005;51(1):28-34. [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Falabella 2006
- Falabella AF. Debridement and wound bed preparation. Dermatologic Therapy 2006;19(6):317-25. [DOI] [PubMed] [Google Scholar]
Fellows 2006
- Fellows J, Crestodina L. Home-prepared saline: a safe, cost-effective alternative for wound cleansing in home care. Journal of Wound Ostomy & Continence Nursing 2006;33(6):606-9. [DOI] [PubMed] [Google Scholar]
Fernandez 2004
- Fernandez R, Griffiths R, Ussia C. Effectiveness of solutions, techniques and pressure in wound cleansing. JBI Reports 2004;2(7):231-70. [DOI] [PubMed] [Google Scholar]
Glanville 2019
- Glanville J, Dooley G, Wisniewski S, Foxlee R, Noel-Storr A. Development of a search filter to identify reports of controlled clinical trials within CINAHL Plus. Health Information and Libraries Journal 2019;36(1):73-90. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org (accessed October 2020).
Guest 2017
- Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, et al. Health economic burden that different wound types impose on the UK's National Health Service. International Wound Journal 2017;14(2):322-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harding 2002
- Harding KG, Morris HL, Patel GK. Healing chronic wounds. BMJ 2002;324(7330):160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hellewell 1997
- Hellewell TB, Major DA, Foresman PA, Rodeheaver GT. A cytotoxicity evaluation of antimicrobial and non antimicrobial wound cleansers. Wounds 1997;9(1):15-20. [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2022
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
James 2008
- James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair and Regeneration 2008;16(1):37-44. [DOI] [PubMed] [Google Scholar]
Korting 2011
- Korting HC, Schöllmann C, White RJ. Management of minor acute cutaneous wounds: importance of wound healing in a moist environment. Journal of the European Academy of Dermatology and Venereology 2011;25(2):130-7. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee CK, Hansen SL. Management of acute wounds. Surgical Clinics of North America 2009;89(3):659-76. [DOI] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Medicine 2006;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCaughan 2018
- McCaughan D, Sheard L, Cullum N, Dumville J, Chetter I. Patients’ perceptions and experiences of living with a surgical wound healing by secondary intention: a qualitative study. International Journal of Nursing Studies 2018;1(77):29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2015
- Mohamed MA, Sharaf AY, Ibrahim MH. Cooled boiled tap water versus sterile normal saline for traumatic wound cleansing: a comparative clinical trial. World Journal of Nursing Sciences 2015;1(3):100-9. [Google Scholar]
Nussbaum 2018
- Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, Nusgart M, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value in Health 2018;21(1):27-32. [DOI] [PubMed] [Google Scholar]
Posnett 2008
- Posnett J, Franks P. The burden of chronic wounds in the UK. Diabetic Med 2008;14(5):S7-85. [PubMed] [Google Scholar]
Resende 2016
- Resende MM, Rocha CA, Corrêa NF, Veiga RR, Passos SJ, Novo NF, et al. Tap water versus sterile saline solution in the colonisation of skin wounds. International Wound Journal 2016;13(4):526-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Salami 2006
- Salami AA, Imosemi IO, Owoeye OO. A comparison of the effect of chlorhexidine, tap water and normal saline on healing wounds. International Journal of Morphology 2006;24(4):673-6. [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Available from www.training.cochrane.org/handbook.
Spear 2011
- Spear M. Wound cleansing: solutions and techniques. Plastic Surgical Nursing 2011;31(1):29-31. [DOI] [PubMed] [Google Scholar]
Stadelmann 1998
- Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. American Journal of Surgery 1998;176(2):26S-38S. [DOI] [PubMed] [Google Scholar]
Stashak 2006
- Stashak TS. Wound infection: contributing factors and selected techniques for prevention. Injury 2006;9(12):13. [Google Scholar]
Swanson 2016
- Swanson T, Angel D, Sussman G, Cooper R, Haesler E, Ousey K, et al. Wound infection in clinical practice: principles of best practice. Wounds International, Internation Consensus Update 2016 2016:1-30.
Watret 2009
- Watret L, McClean A. Cleansing diabetic foot wounds: tap water or saline? Diabetic Foot 2009;12(3):134-9. [Google Scholar]
Wounds Australia 2016
- Wounds Australia. Standards for wound prevention and management. Wounds Australia 2016.
References to other published versions of this review
Fernandez 2002
- Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database of Systematic Reviews 2002, Issue 4. Art. No: CD003861. [DOI: 10.1002/14651858.CD003861] [DOI] [PubMed] [Google Scholar]
Fernandez 2008
- Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD003861. [DOI: 10.1002/14651858.CD003861.pub2] [DOI] [PubMed] [Google Scholar]
Fernandez 2012
- Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD003861. [DOI: 10.1002/14651858.CD003861.pub3] [DOI] [PubMed] [Google Scholar]


