Abstract
Introduction
Early detection of Alzheimer's disease and related dementias allows clinicians and patients to prepare for future needs and identify treatment options. Medicare's Annual Wellness Visit (AWV) requires detection of cognitive impairment and may increase dementia diagnosis. We estimated the relationship between AWV receipt and incident dementia.
Methods
Using a retrospective cohort of Medicare Fee‐For‐Service (FFS) beneficiaries enrolled for at least 3 years from 2009 to 2016 and two‐stage least squares, we quantified the relationship between AWV and incident diagnosis of cognitive impairment/dementia, and by race/ethnicity. The county‐level change in percent of beneficiaries receiving AWVs was used as an instrumental variable to account for unobserved factors associated with individuals’ AWV receipt and diagnosis. Sample included 3,333,617 beneficiaries ages 67 years and older, without dementia at the beginning of the study.
Results
Beneficiaries included 2,713,573 White, 251,958 Black, 196,845 Hispanic, 95,719 Asian, 11,727 American Indian/Alaska Native, and 63,795 of other race/ethnicity. Using ordinary least squares, dementia incidence was ‐0.79 percentage points (95% CI ‐0.81 to ‐0.76) lower for persons receiving an AWV compared to no AWV. Using instrumental variables reversed the direction of the effect: AWV receipt increased dementia diagnoses by 0.47 percentage points (95% CI 0.14 to 0.80), 15% over baseline. AWVs increased diagnoses 2.0 percentage points (95% CI 0.05 to 3.94) among Blacks, 0.40 percentage points (95% CI 0.05 to 0.75) among Whites, but est were imprecise for Hispanics and Asians.
Discussion
Increasing AWV take‐up and supporting physicians’ performance of cognitive assessment may further improve dementia detection in the population and among groups at higher risk of undiagnosed dementia.
Keywords: annual wellness visit, cognitive screening, detection, dementia, disparities
1. BACKGROUND
The Patient Protection and Affordable Care Act of 2010 (ACA) provided a new benefit for an annual wellness visit (AWV) to all Medicare Part B beneficiaries starting in 2011. The ACA mandated that an AWV include detection of cognitive impairment annually, along with checks of blood pressure, weight, medical history, and other routine services.
Despite the new benefit, initial take‐up of the AWV was low. Eight percent of Medicare beneficiaries in traditional Medicare had an AWV in 2011. Use increased over time; by 2018 about 32% of beneficiaries had an AWV. 1 Take up of the AWV was lower among non‐white beneficiaries, who are also at higher risk of cognitive impairment and dementia, and varied by region of the United States. 2 , 3 , 4 , 5 Rates of AWVs were particularly low in rural areas, 2 where rates of undiagnosed dementia are likely higher.
The law provided no specific guidance on how to detect cognitive impairment other than using “assessment of an individual's cognitive function by direct observation, with due consideration of information obtained by way of patient report, concerns raised by family members, friends, caretakers or others.” 6 The lack of specific guidance on how to conduct a cognitive screening led to variation in physicians’ approach to assessment. 7 Adding to potential confusion among physicians, there are at least 15 brief screening tools, with variation in assessment time and accuracy, and different tools must be used when ascertaining cognition from informants. 8 A further barrier is likely physicians’ time to invest in learning and implementing techniques for cognitive screening. Among persons reporting ever having an AWV visit by 2019, only one‐quarter reported receipt of a structured cognitive assessment at an AWV visit. 1 Thus, it is unclear whether AWVs improved detection of cognitive impairment.
RESEARCH IN CONTEXT
Systematic Review: The authors used traditional search methods to review the literature on Medicare's annual wellness visit (AWV) use and subsequent outcomes. While the literature demonstrates increasing take‐up of these visits over time, study of their impact on dementia diagnoses is limited.
Interpretation: Our findings indicate regular cognitive screenings, such as those provided at Medicare's AWV, can improve detection of dementia, particularly among groups at high risk of underdiagnosis.
Future Directions: This study suggests improving detection of cognitive impairment may reduce racial/ethnic disparities in dementia diagnosis. However, access to cognitive screening was measured through use of an AWV. Future study is needed to elucidate whether cognitive screenings occur at these visits and/or additional tests are used to identify dementia diagnoses (e.g., MRI). In addition, future research on barriers to use of the AWV and cognitive assessment should be identified and addressed.
Studies analyzing the impact of AWV on preventive service use have come to somewhat conflicting findings, with some finding increases in use in the months after an AWV, and others finding no changes in use. 9 , 10 , 11 , 12 , 13 , 14 , 15 Specific to dementia, one study 16 found diagnostic tests for dementia (e.g., brain imaging and B12 deficiency tests) were higher and dementia diagnosis slightly lower among people who had an AWV visit compared to persons who had a health care, but not an AWV, visit. A second study 17 examined the effect of AWV receipt on new dementia diagnoses using county‐level rates of “Welcome to Medicare” visits as an instrumental variable. The finding of a large increase in dementia diagnoses in the 6 months after the AWV visit is likely an overestimate of the AWV's effect on diagnosis due to use of an instrument that is not exogenous—the county level Welcome to Medicare Visit Rate. Specifically, differences in visit rates across counties likely captures other county‐specific differences that impact dementia diagnosis. For example, visit rates vary systematically by county population size, which may affect dementia diagnoses for reasons other than the Welcome to Medicare visit itself.
We advance understanding of the impact of AWV receipt on diagnosis of Alzheimer's disease and related dementias (ADRD) or cognitive impairment. We use a large, representative sample of older Americans, with sample size large enough to estimate effects in different racial and ethnic subpopulations, and use methods to account for unobservable confounders. We use a plausibly exogenous (to dementia diagnosis) instrumental variable based on area rates of change in AWV visits to estimate the effect of AWV on diagnosis. We restrict analyses to samples of persons in comparably sized geographic areas to assess robustness of estimates.
2. METHODS
2.1. Data source and sample selection
We used a random 20% sample of Medicare beneficiaries aged 67 and older, continuously enrolled in Medicare Fee‐For‐Service Parts A (hospital stay), B (out‐patient) and D (prescription drugs) for at least 3 years between 2009 and 2016. The first year of the AWV was 2011. Thus, we required that beneficiaries enrolled in 2009 and 2010, prior to the introduction of the AWV, were continuously enrolled and dementia free for that period. Thereafter, beneficiaries are dementia free in years t‐1 and t‐2 (see Supplemental Digital Content, Figure S1 for additional information). The year 2016 was used to verify dementia in 2014 and 2015 (see method below) and was not included in our analyses.
Data on enrollment and demographics was obtained from the Beneficiary Summary Files. For socioeconomic information, data on beneficiaries was merged with annual zip code‐level data on percentage of high school graduates and median income from the American Communities Survey. Our analytical sample consists of 3,333,617 unique beneficiaries over the study period – 2,713,573 white, 251,958 Black, 196,845 Hispanic, 95,719 Asian, 11,727 American Indian/Alaska Native, and 63,795 of other/unknown race/ethnicity. Access to the Medicare claims data was provided via a data use agreement with the National Bureau of Economic Research and was accessed remotely using a virtual private network.
2.2. Outcome measure
We used the International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9‐ CM, ICD‐10‐CM) diagnosis codes as defined by the Chronic Conditions Warehouse (CCW) to identify cognitive impairment and Alzheimer's disease and related dementias (ADRD). In addition to diagnosis codes, we used codes to identify mild cognitive impairment (MCI) and symptoms/conditions associated with dementia: amnesia, aphasia, and apraxia and agnosia. We also used Part D claim codes for drugs approved to treat Alzheimer's disease, acetylcholinesterase inhibitors (donepezil, galantamine, rivastigmine) or NMDA receptor antagonists (memantine). 18 A complete list of codes is in Table S1 in Supplemental Digital Content. The primary outcome was incident diagnosis of cognitive impairment/dementia, defined as any occurrence of either an ADRD or MCI diagnosis, a dementia symptom, or Alzheimer's disease drug prescription claim within a calendar year and no prior diagnosis at the beginning of the study period (hereinafter “dementia”). We required at least one claim to be an ADRD or MCI diagnosis, occurring in any order or combination with symptoms or drugs. To avoid counting “rule‐out” diagnoses, we required verification of the diagnosis within 2 years of incident diagnosis. Beneficiaries with incident dementia were dropped from the at‐risk population in the following calendar year (see Supplemental Digital Content, Figure S1 for additional information on dementia and AWV measurement timing).
2.3. Annual wellness visits
We used an indicator of whether a beneficiary received an AWV, defined as a claim with Healthcare Common Procedure Coding System (HCPCS) codes G0428 (first ever AWV) and G0439 (for subsequent AWVs). Because AWVs are only allowed once every 12 months, we measure diagnoses in the 12 months after an AWV. Figure S1 in Supplemental Digital Content depicts the timing of AWV and dementia diagnoses measurement.
2.4. Confounding variables
We controlled for characteristics of the individual and their neighborhood. At the individual level, we used demographic measures: age, sex, and race/ethnicity (white, Black, Hispanic, Asian, American Indian/Alaska Native, and other/unknown). We also included indicators of chronic conditions (hypertension, hyperlipidemia, acute myocardial infarctio, atrial fibrillation, diabetes, stroke, depression) identified using CCW. We included proxy variables for socioeconomic status using indicators of low‐income subsidy and dual beneficiary status, percentage of high school graduates, and median income in beneficiaries’ zip code.
2.5. Analysis
We quantified annual rates of incident dementia and AWV visits. We compared the characteristics of beneficiaries that received an AWV and those that did not in 2015 and tested for differences between the two groups using two‐tailed t‐tests for continuous variables and chi‐squared for categorical variables. We restricted this analysis to 1 year of data to avoid overcounting beneficiaries who appear in more than 1 year of our data. Next, we used linear probability models (OLS) to estimate the relationship between AWV receipt and diagnosis of dementia, adjusting for observed confounders. Robust standard errors were clustered at the beneficiary level to account for having more than one observation from a beneficiary across the sample. We additionally tested the sensitivity of our results to the linearity assumption of OLS using logistic regression.
Given the likelihood of selection into treatment (AWV) for reasons potentially related to dementia risk and unobserved in our data, we used instrumental variables estimation to quantify the relationship between AWVs and dementia diagnosis independent of unobserved (and observed) differences between those who did and did not get AWVs. Instrumental variables (IV) estimation is an advanced statistical method that attempts to isolate exogenous variation in a potentially endogenous predictor, in our case individual AWV receipt. The key reason for using the IV approach is that there are unobserved confounders that intervene in the relationship between the outcome (dementia diagnosis) and the predictor. For an instrument to be valid, it must be related to the outcome only through its relationship with the predictor. See Supplemental Digital Content Figure S2 for a visual representation of these relationships.
Our instrument exploited geographic variation in AWV utilization over time, that is, the county‐level growth rate in visits. We calculated growth rate as the percent of beneficiaries in a county receiving an AWV in the present year (time t) less the percent in the prior year (time t‐1) for each county. We tested the relationship between a beneficiary's receipt of an AWV and county‐level change in AWV (Figure 1) and found a positive correlation between the two variables, Pearson's correlation coefficient 0.117 (p < 0.001). As mentioned above, a key assumption is that, after controlling for observable confounders, the county‐level change in AWV utilization is related to the likelihood of dementia diagnosis only through its impact on AWV use. We analyzed the strength of the instrument using the first‐stage magnitude and significance of the estimate and the first stage F‐statistic. We conducted several sensitivity analyses to further test these assumptions. All analyses were conducted using Stata statistical software version 16.1.
FIGURE 1.
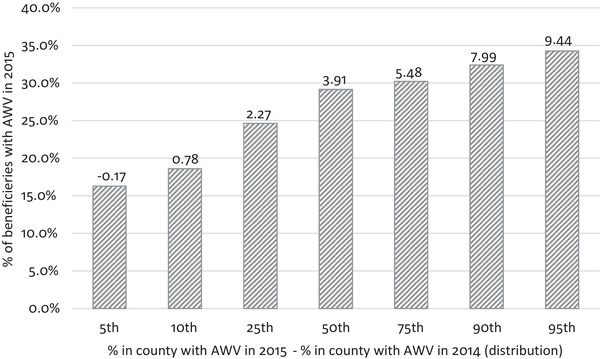
Percent of beneficiaries with an Annual Wellness Visit in 2015 and County‐level Changes in rates of AWV use 2014 to 2015. Notes: Pearson's correlation coefficient 0.117 (p < 0.0001)
3. RESULTS
3.1. Descriptive statistics
Dementia incidence declined slightly during the study period, from 3.5% in 2011 to 3.2% in 2015 (Figure 2). At the same time, the AWV visit rates increased markedly from 7.9% to 21.9% between 2011 and 2015. Beneficiaries who received AWVs and those who did not were different in observable ways. In 2015, dementia incidence was 2.2% among those who received AWVs versus 3.5% among those who did not. There were other demographic, health condition, and socioeconomic differences that are associated with risk of dementia (Table 1). Beneficiaries receiving AWVs were younger (mean age 75.95AWV, 77.11No AWV, p‐value < 0.001) and had fewer co‐morbid conditions including: hypertension (52.7%AWV, 58.0%No AWV, p‐value < 0.001), hyperlipidemia (54.4%AWV, 56.0%No AWV, p‐value < 0.001), acute myocardial infarction (3.0%AWV, 4.7%No AWV, p‐value < 0.001), atrial fibrillation (10.6%AWV, 13.6%No AWV, p‐value < 0.001), diabetes (22.9%AWV, 27.4%No AWV, p‐value < 0.001), stroke (9.5%AWV, 12.6%No AWV, p‐value < 0.001) and depression (18.6%AWV, 22.9%No AWV, p‐value < 0.001). They were less likely to be eligible for low‐income subsidies (2.0%AWV, 3.3%No AWV, p‐value < 0.001) and Medicaid (10.8%AWV, 19.9%No AWV, p‐value < 0.001) and from higher socioeconomic neighborhoods (percent high school graduates 83.5%AWV, 81.2%No AWV, p‐value < 0.001; mean income 62,695AWV, 57,914No AWV, p‐value < 0.001) than persons who did not receive an AWV. In sum, we found healthier, younger beneficiaries were more likely to get an AWV, but they are less likely to have dementia and dementia incidence was decreasing over time. Thus, detecting a relationship between AWV receipt and incident dementia presents an empirical challenge.
FIGURE 2.
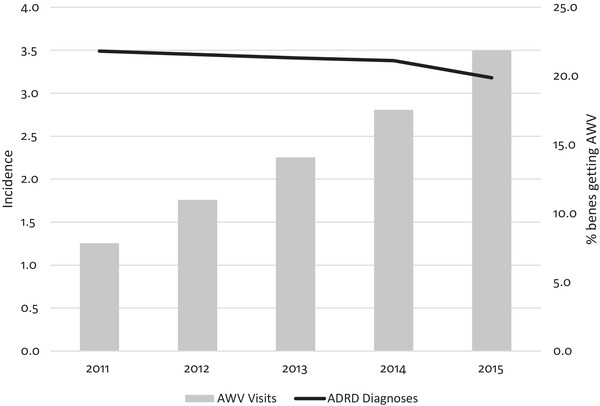
Percent of beneficiaries with incident dementia diagnoses and percent receiving an Annual Wellness Visit (2011 to 2015). Notes: ADRD incidence adjust for beneficiary age
TABLE 1.
Sample characteristics by AWV receipt (2015)
| AWV | No AWV | p‐value | |
|---|---|---|---|
| Age‐adjusted dementia incidence (%) | 2.19 | 3.47 | 0.000 |
| Individual characteristics | |||
| Age (mean) | 75.95 | 77.11 | 0.000 |
| Female (%) | 60.57 | 60.48 | 0.246 |
| Low‐income subsidy (%) | 2.04 | 3.25 | 0.000 |
| Dual eligible (%) | 10.81 | 19.93 | 0.000 |
| Race/ethnicity (%) | |||
| White | 86.65 | 83.01 | 0.000 |
| Black | 4.91 | 7.01 | |
| Other | 1.62 | 1.59 | |
| Asian/PI | 2.62 | 2.50 | |
| Hispanic | 3.42 | 4.97 | |
| American Indian/Alaska Native | 0.09 | 0.40 | |
| Local area | |||
| % HS grads | 83.53 | 81.22 | 0.000 |
| Median income (mean $) | 62,695 | 57,914 | 0.000 |
| Comorbid conditions (%) | |||
| Hypertension | 52.69 | 58.03 | 0.000 |
| Hyperlipidemia | 54.38 | 55.97 | 0.000 |
| AMI | 3.04 | 4.69 | 0.000 |
| A. fibrillation | 10.64 | 13.57 | 0.000 |
| Diabetes | 22.91 | 27.37 | 0.000 |
| Stroke | 9.45 | 12.64 | 0.000 |
| Depression | 18.61 | 22.87 | 0.000 |
| Observations | 520,472 | 1,784,852 |
Notes: continuous and dichotomous variables (age, sex, LIS, dual, % HS grads, median income) tested using two‐tailed t‐tests; categorical variables (race/ethnicity) tested using chi‐2.
Abbreviation: PI, Pacific Islanders.
Figure 1 illustrates the relationship between the instrumental variable and individual level AWV receipt. It displays the distribution of the AWV growth rate (our instrument): the difference in the percent of beneficiaries receiving AWVs by county in 2015 versus 2014. It also shows the percent of beneficiaries receiving an AWV in 2015 (our predictor of interest). At the median, the percent of beneficiaries with an AWV increased by 3.91 percentage points. About 29% of persons in those counties received an AWV in 2015. Beneficiaries in counties with more growth in AWVs were more likely to receive an AWV – of those living in the 5% of counties with the most growth in AWVs (9.44 percentage point increase), 34% received an AWV (p < 0.001).
3.2. Multivariate analyses
Table 2, Model 1, shows ordinary least squares estimates of the association between receiving an AWV and likelihood of incident dementia diagnosis after adjusting for confounders for all beneficiaries, and separately by race/ethnicity. Beneficiaries receiving AWVs were 0.79 percentage points (95% CI ‐0.87 to ‐0.76, p‐value < 0.001) less likely to receive a dementia diagnosis within 12 months after the visit. The magnitude of the estimate was largest for whites (‐0.82, 95% CI ‐0.85 to ‐0.80, p‐value < 0.001) and Blacks (‐0.83, 95% CI ‐0.96 to ‐0.71, p‐value < 0.001) and smallest for Asian/Pacific Islanders (‐0.37, 95% CI ‐0.52 to ‐0.23, p‐value < 0.001) and Hispanics (‐0.29, 95% CI ‐0.41 to ‐0.15, p‐value < 0.001). Results from logistic regression were similar; a comparison is included in the Supplement Table S2. In Model 2, we adjusted for unobservable differences using two‐stage least squares (2SLS) estimation. Based on the 2SLS estimation, individuals with AWVs were 0.47 (95% CI 0.14 to 0.80, p‐value 0.005) percentage points more likely to get an incident dementia diagnosis within 12 months of an AWV visit. Model 2 also shows test of strength of instrument. First stage estimates of annual county AWV growth on the likelihood a beneficiary received an AWV in the year was 1.22 (95% CI 1.21 to 1.23, p‐value < 0.001). Test of strength of instrument is given by the F‐statistic (17,410).
TABLE 2.
Effect of AWV use on detection of cognitive impairment or dementia
| Age‐adjusted difference | Model 1 (OLS) | Model 2 (IV, 2SLS) | |||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | p‐value | 1st stage (95% CI) | F‐statistic | 2nd Stage (95% CI) | p‐value | ||
| All | −1.28 | −0.79 (−0.87, −0.76) | <0.001 | 1.22 (1.21, 1.23) | 17,410 | 0.47 (0.14,0.80) | 0.005 |
| White | −1.38 | −0.82 (−0.85, −0.80) | <0.001 | 1.24 (1.23, 1.25) | 19,206 | 0.40 (0.05, 0.75) | 0.024 |
| Black | −0.91 | −0.83 (−0.96, −0.71) | <0.001 | 0.90 (0.87, 0.94) | 1,253 | 2.00 (0.05, 3.95) | 0.044 |
| Hispanic | 0.13 | −0.29 (−0.41, −0.15) | <0.001 | 1.20 (1.16, 1.24) | 827 | 0.37 (−1.40, 2.13) | 0.684 |
| Asian/PI | −0.37 | −0.37 (−0.52, −0.23) | <0.001 | 1.17 (1.06, 1.25) | 298 | −0.28 (−3.50, 2.94) | 0.864 |
Notes: N (full sample) = 9,911,237; model 1 and 2 estimates multiplied by 100 to reflect percentage points; AWV instrumented with county‐level change in AWV. Model 1 and 2 adjusted for age, low‐income subsidy, dual enrollment status, comorbidities, and zip‐code level percent high school grads and median income; robust standard errors clustered on the beneficiary.
Abbreviation: PI, Pacific Islanders.
The 2SLS estimate for whites (0.40, 95% CI 0.05 to 0.75, p‐value = 0.02) was similar to the overall sample estimate. The AWV had an imprecise effect on dementia diagnoses for Asians/Pacific Islanders (‐0.28, 95% CI ‐3.50 to 2.94, p‐value = 0.86) and Hispanics (1.37, 95% CI ‐1.40 to 2.13, p‐value = 0.68). Blacks who had an AWV were 2.0 percentage points (95% CI 0.05 to 3.95, p‐value = 0.04) more likely to receive an incident dementia diagnosis in the year they had an AWV than Blacks who did not have an AWV.
3.3. Sensitivity analyses
Our 2SLS approach implicitly assumes that, after controlling for observable characteristics, growth in the AWV within a county was unrelated to dementia diagnosis except through take‐up of the AWV itself. If AWV growth rates were correlated with other county level changes in care patterns or health not captured by our covariates and associated with dementia diagnosis, the 2SLS estimates may be biased. This is more likely to occur across counties of different sizes. To test this, we ran separate models for similarly sized urban and rural counties (defined by USDA classification of urban and rural counties). The estimates were similar to the main model, although they were less precise: 0.28 percentage points (95% CI ‐0.18 to 0.74, p‐value 0.235) for those in urban counties and 0.40 percentage points (95% CI ‐0.16 to 0.97 p‐value 0.160) for those in rural counties (Table S3).
It is also possible that AWV receipt and dementia diagnoses were both correlated with overall changes in county level healthcare use. To test for this possibility, we included a measure of healthcare utilization in our instrumental variables model, county level changes in inpatient stays. The main results did not change; the second stage estimate increased from 0.47 to 0.52 percentage points and remained statistically significant (Table S4 for comparison).
Finally, following the prior paper that instrumented for AWVs with county level Welcome to Medicare Visits, 17 we tested the hypothesis that there would likely be little effect in the first or second year of the program, but it would likely grow over time. Indeed, our instrument assumes overall growth in AWVs will influence one's likelihood of receiving an AWV. We found the relationship between (instrumented) AWV and ADRD diagnosis became more positive over time, although it was imprecise prior to 2015 (Table 3).
TABLE 3.
Sensitivity analysis of effect of AWV use on detection of cognitive impairment or dementia, by year
| Year | 1st Stage (95% CI) | 2nd Stage (95% CI) | p‐value |
|---|---|---|---|
| 2012 | 1.28 (1.26, 1.90) | −0.40 (−1.13, 0.33) | 0.281 |
| 2013 | 1.31 (1.29, 1.33) | 0.24 (−0.48, 0.96) | 0.508 |
| 2014 | 1.30 (1.29, 1.32) | 0.25 (−0.35, 0.85) | 0.413 |
| 2015 | 1.09 (1.07, 1.11) | 1.35 (0.74. 1.95) | 0.000 |
Note: Second stage estimates multiplied by 100 to reflect percentage points; AWV instrumented with county‐level change in AWV. Model adjusted for age, low‐income subsidy, dual enrollment status, comorbidities, and zip‐code level percent high school grads and median income; robust standard errors.
4. DISCUSSION
In our retrospective analysis of a large, representative sample of Medicare beneficiaries, we found beneficiaries receiving an AWV were 0.47 percentage points more likely to receive an incident dementia diagnosis in the 12 months following the AWV than beneficiaries who did not receive an AWV. The effect was largest for Blacks, while there was no AWV effect among Asians/Pacific Islanders and Hispanics. AWV use among Blacks increased twice as quickly as all other races (Table S5). Increased detection among this higher‐risk group is an important finding; however, the null finding among Hispanics is also notably important, as they are also at higher risk of dementia than whites. This is particularly notable due to recent findings suggesting both Blacks and Hispanics experience missed or delayed diagnoses. 5 , 19
In contrast to the previous study on AWVs and dementia diagnoses, 17 our estimates suggested a more modest effect of AWV on diagnoses. Nonetheless, the effects found here are sizeable when considered relative to the low rates of dementia diagnosis: AWVs increased dementia diagnoses by 15%, relative to the baseline in 2015. Unlike the prior study, we found no impact of the AWV on dementia diagnoses for Asians or Hispanics, although the estimates for most races/ethnicities were quite imprecise and should be interpreted with caution. One reason for these differences in findings is that the prior study's instrument drew on differences in visits among Hispanics and Asians across large and small geographic areas (e.g., urban compared to rural), and dementia diagnoses at ages 68 to 76 when dementia incidence is low. In contrast, our instrument accounted for changes in rates of county‐level visits, and among similar sized areas. While differences across counties in level of visits in a year may be correlated with individuals’ AWV receipt and dementia diagnoses, for example through generally better (or worse) access to healthcare, changes in AWV visits measure growth or decline from year to year is less likely to be associated with dementia diagnoses, except through an individual's AWV (as illustrated in Figure S2). Furthermore, our sample included beneficiaries of all ages and our validated measure of dementia allowed us to eliminate “rule‐out” diagnoses, which could be more likely to occur at younger ages.
Our results indicate AWVs can improve detection of cognitive impairment and dementia. Diagnosing cognitive impairment and dementia involves a brief initial screening followed by a full cognitive assessment and diagnostic tests (e.g., lab tests and imaging); 8 citing insufficient evidence, the US Preventive Services Task Force currently recommends such screening only for persons exhibiting symptoms. 20 Thus, although we identify a link between AWVs and diagnoses, we have not identified the mechanisms connecting AWVs and detection of cognitive impairment or dementia, such as follow‐up testing and dementia specialist visits. Although one study showed an association between AWV and increased tests for reversible causes of dementia (e.g., thyroid stimulating hormone and B12) and neurobehavioral testing, this pathway needs confirmation with further study. 16 More generally, further study on what happens at and after the AWV is needed.
The effect of AWVs on increasing rates of cognitive assessment and detecting dementia is notable in the current landscape of treatment and care for persons living with dementia. Several pipeline drugs target pre‐symptomatic or early stage Alzheimer's disease. Recently, the FDA approved Aduhelm 21 which may be used to slow cognitive decline for persons with mild cognitive impairment or in early‐stage Alzheimer's disease. However, the Centers for Medicare and Medicaid 22 subsequently announced their decision to only reimburse for this costly treatment for beneficiaries involved in approved “CMS‐approved studies.” Since willingness to seek attention for cognitive impairment may be affected by the availability treatment, the AWV is likely to play an important ongoing roll in the detection of cognitive impairment.
The data for this study came from fee‐for‐service (FFS) beneficiaries, but Medicare Advantage (MA) now accounts for about 40% of all beneficiaries. Beneficiaries in MA plans compared to FFS are more likely to be non‐White, and Blacks, Hispanics and Alaska Native/American Indians populations and at higher risk of dementia. 23 AWV use and cognitive assessment, however, may be different in MA, in part because of a recent change in risk adjustment models that incentivizes dementia detection. 24 In a recent study, we surveyed a representative group of older Americans and found that use of AWVs and receipt of cognitive screening was higher among MA beneficiaries that those in FFS. 1 The higher rates of cognitive assessment in MA compared to FFS suggest that increasing assessment rates is technically feasible.
A strength of our study is the use of a larger sample over a longer period than prior study on AWVs and cognitive impairment detection. Further, our use of a large, representative cohort allowed us to quantify differences in AWV's effect across racial/ethnic groups. A second strength is the use of econometric methods to quantify the relationship between AWVs and incident dementia that reduced bias in the estimates. Significant observed differences between beneficiaries that did and did not receive AWVs suggest a high likelihood of selection bias resulting from unobserved confounders. Instrumental variable estimation allowed us to exploit plausibly exogenous geographic variation in AWVs to identify the effect of AWV receipt on incident dementia diagnoses. As the AWV is intended to detect possible cognitive impairment, not necessarily diagnose dementia, using a broad definition of detection that included dementia symptoms, and drugs to treat symptoms as well as diagnoses of dementia allowed us to identify cases across the disease spectrum.
4.1. Limitations
AWV claims are billed as a single visit and individual components are not identified, so we do not know whether a structured cognitive assessment took place at the visit. Thus, our results reflect incident dementia diagnoses due to increased focus on cognitive detection via an AWV, rather than changes due to structured cognitive assessments.
Although the key assumptions necessary for instrument validity are impossible to prove, we performed sensitivity analyses to test the robustness of our instrument. While results were robust to these tests, instrumental variables estimation may still not account for all possible observed or unobserved confounders. It may be, for example, that increased interaction with the healthcare system, that we did not account for, increased AWVs and the increased likelihood of dementia detection and that this detection would have occurred even absent the AWV. In a recent survey of Medicare beneficiaries, however, we found that only about 27% of fee‐for‐service beneficiaries reported ever having received a structured cognitive assessment, the majority of which occurred at an AWV. 1 Thus, even with increased access to the healthcare system, the likelihood of dementia detection likely remains quite low, except at AWVs.
5. CONCLUSION
The AWV benefit is an important tool for detecting cognitive impairment and dementia among Medicare beneficiaries and provides an opportunity to reduce racial/ethnic disparities in dementia diagnosis. Increasing take‐up of AWV and supporting primary care physicians’ performance of cognitive assessment may further improve detection. Barriers to use of the AWV and cognitive assessment should be identified and addressed.
CONFLICT OF INTEREST
The authors have no relationships/activities/interests related to the content of this submission. Human subjects’ consent was not necessary for this study as it used de‐identified secondary data. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Patricia Ferido for assistance during data analysis and Drs. Geoffrey Joyce and Bryan Tysinger for thoughtful feedback. This work was supported by the National Institute on Aging at the National Institutes of Health [grant numbers R01AG055401, P30AG066589].
Thunell JA, Jacobson M, Joe EB, Zissimopoulos JM. Medicare's Annual Wellness Visit and diagnoses of dementias and cognitive impairment. Alzheimer's Dement. 2022;14:e12357. 10.1002/dad2.12357
REFERENCES
- 1. Jacobson M, Thunell J, Zissimopoulos J. Cognitive assessment at Medicare's Annual Wellness Visit in fee‐for‐service and medicare advantage plans: Study examines the use of Medicare's annual wellness visit and receipt of cognitive assessment among Medicare beneficiaries enrolled in fee‐for‐service Medicare or Medicare Advantage. Health Aff. 2020;39(11):1935‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Trends in use of the US medicare annual wellness visit, 2011‐2014 trends in use of the US medicare annual wellness visit, 2011‐2014 Letters. JAMA. 2017;317(21):2233‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Practices caring for the underserved are less likely to adopt Medicare's Annual Wellness Visit. Health Aff (Project Hope). 2018;37(2):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu J, Jensen GA, Nerenz D, Tarraf W. Medicare's annual wellness visit in a large health care organization: who is using it? Ann Intern Med. 2015;163(7):567‐568. [DOI] [PubMed] [Google Scholar]
- 5. Lin P‐J, Daly AT, Olchanski N, et al. Dementia diagnosis disparities by race and ethnicity. Med Care. 2021;59(8):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Medicare & Medicaid Services H. 42 CFR § 410.15: Annual wellness visits providing personal‐ ized prevention plan services: conditions for limitations on coverage. CMS. Published 2011. Accessed September 10, 2020. https://www.govinfo.gov/content/pkg/CFR‐2012‐title42‐vol2/pdf/CFR‐2012‐title42‐vol2‐sec410‐15.pdf
- 7. Ayarti M. Statement of Dr. Mehrdad Ayati, MD . US Senate Special Committee on Aging; January 24, 2018 2018. [Google Scholar]
- 8. Cordell CB, Borson S, Boustani M, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9(2):141‐150. [DOI] [PubMed] [Google Scholar]
- 9. Shen AK, Warnock R, Kelman JA. Driving immunization through the Medicare Annual Wellness Visit: a growing opportunity. Vaccine. 2017;35(50):6938‐6940. [DOI] [PubMed] [Google Scholar]
- 10. Jiang M, Hughes DR, Wang W. The effect of Medicare's Annual Wellness Visit on preventive care for the elderly. Prev Med. 2018;116:126‐133. [DOI] [PubMed] [Google Scholar]
- 11. Tao G. Utilization pattern of other preventive services during the US Medicare annual wellness visit. Prev Med Rep. 2018;10:210‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen GA, Salloum RG, Hu J, Ferdows NB, Tarraf W. A slow start: Use of preventive services among seniors following the Affordable Care Act's enhancement of Medicare benefits in the U.S. Prev Med. 2015;76:37‐42. [DOI] [PubMed] [Google Scholar]
- 13. Camacho F, Yao N, Anderson R. The effectiveness of medicare wellness visits in accessing preventive screening. J Prim Care Community Health. 2017;8(4):247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganguli I, Souza J, McWilliams JM, Mehrotra A. Association Of Medicare's Annual Wellness Visit with cancer screening, referrals, utilization, and spending. Health Aff. 2019;38(11):1927‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung S, Lesser LI, Lauderdale DS, Johns NE, Palaniappan LP, Luft HS. Medicare annual preventive care visits: use increased among fee‐for‐service patients, but many do not participate. Health Aff. 2015;34(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 16. Fowler NR, Campbell NL, Pohl GM, et al. One‐year effect of the Medicare annual wellness visit on detection of cognitive impairment: a cohort study. J Am Geriatr Soc. 2018;66(5):969‐975. [DOI] [PubMed] [Google Scholar]
- 17. Lind KE, Hildreth K, Lindrooth R, Morrato E, Crane LA, Perraillon MC. The effect of direct cognitive assessment in the Medicare annual wellness visit on dementia diagnosis rates. Health Serv Res. 2021;56(2):193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thunell J, Ferido P, Zissimopoulos J. Measuring Alzheimer's disease and other dementias in diverse populations using medicare claims data. J Alzheimers Dis. 2019;72(1):29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Owens DK, Davidson KW, Krist AH, et al. Screening for cognitive impairment in older adults: US Preventive Services Task Force recommendation statement. Jama. 2020;323(8):757‐763. [DOI] [PubMed] [Google Scholar]
- 21. Mahase E. FDA approves controversial Alzheimer's drug despite uncertainty over effectiveness. In: British Medical Journal Publishing Group. BMJ. 2021;373:n1462. [DOI] [PubMed] [Google Scholar]
- 22. CMS Finalizes Medicare Coverage Policy for Monoclonal Antibodies Directed Againsst Amyloid for the Treatment of Alzheimer's Disease [press release]. Centers for Medicare and Medicaid, April 7, 2022 2022. [Google Scholar]
- 23. Murphy‐Barron C, Pyenson B, Ferro C, Emery M. Comparing the Demographics of Enrollees in Medicare Advantage and Fee‐For‐Service Medicare. Milliman;2020. [Google Scholar]
- 24. Centers for Medicare & Medicaid Services H . Medicare Advantage and Part D rate announcement and final call letter fact sheet. CMS. Published 2019. Accessed October 29, 2020. https://www.cms.gov/newsroom/fact‐sheets/2020‐medicare‐advantage‐and‐part‐d‐rate‐announcement‐and‐final‐call‐letter‐fact‐sheet
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


