Abstract
Background
Primary biliary cholangitis (PBC) is a rare, chronic autoimmune, cholestatic liver disease affecting approximately 318 per million Canadians. There is limited information regarding the characterization of this patient population in Canada. Consequently, we aim to describe a cohort of PBC patients managed across liver centres serving this type of population.
Methods
A cross-sectional examination of 1,125 PBC patient charts at 15 liver centres across Canada was conducted between January 2016 and September 2017.
Results
Data from 1,125 eligible patients were collected from 7 Canadian provinces. The patient population was largely female (90.2%), had a median overall age of 61.3 years, and a median overall time since diagnosis of 6.4 years. Of the patients included in the study, 89% were on ursodeoxycholic acid (UDCA) therapy at a median dose of 14.0 mg/kg/day and 4.4% were previously treated with UDCA, whereas 6.6% were never treated with UDCA. Of the patients with available data (n = 1067), 289 (27.1%) presented with alkaline phosphatase (ALP) levels ≥200 IU/L and/or total bilirubin levels ≥21 µmol/L. Assessment of UDCA treatment response revealed that 26.6% and 38.3% of patients were inadequate responders according to the Toronto and Paris-II criteria, respectively. Mortality occurred in 1.2% (14) of patients, with liver-related adverse outcomes being more commonly observed in patients who discontinued UDCA compared to those who are currently on treatment (36.3% and 19.6%, respectively).
Conclusion
This study showed that Canadian PBC patients present with demographics and features commonly reported in the literature for this disease. Over one third of PBC patients had inadequate response to UDCA treatment or were not currently being treated with UDCA. Consequently, there is a significant unmet therapeutic need in this Canadian PBC population.
Keywords: autoimmune liver disease, Canada, characterization, chart audit, management, national, primary biliary cholangitis, retrospective, ursodeoxycholic acid
Introduction
Primary biliary cholangitis (PBC) is a chronic, autoimmune, cholestatic liver disease affecting 318 per million Canadians (1). When projected nationally, the prevalence corresponded to 11,290 cases of PBC in Canada in 2015 (1). PBC is thought to arise as a result of environmental and genetic factors, and as such, its prevalence varies geographically (1,2). The highest incidences of PBC are observed in Europe and North America where it is diagnosed primarily in middle-aged and elderly women (2–4). Within Canada, the prevalence of PBC varies across the country with the highest incidence rates in the Atlantic Provinces and the lowest rates in Ontario (1).
The natural history of PBC appears to have changed from a slow, to almost universally progressive disease, with various rates of disease progression in some and clinical remission in others (5). The disease is characterized by the presence of antimitochondrial antibody (AMA) in the serum, a hallmark of PBC. Common symptoms include fatigue and pruritus with most patients being asymptomatic at first presentation (6). Over the past few decades, there have been many changes in the diagnosis and management of PBC, with more patients being recognized with earlier-stage disease and with the use of the recommended first-line pharmacotherapy, ursodeoxycholic acid (UDCA) (6,7). The use of liver stiffness measurement (LSM), using vibration-controlled transient elastography (VCTE), is now routinely performed in PBC (8,9).
UDCA is a choleretic and hydrophilic endogenous bile acid. When initiated at early disease stages, UDCA can delay disease progression and reduce the risk of requiring a liver transplant (10,11). A biochemical response to UDCA treatment, including a reduction in alkaline phosphatase (ALP) and/or bilirubin, has been associated with improved long-term prognosis in risk of liver transplantation or liver-related death in PBC patients (12,13). However, it is estimated that up to 50% of PBC patients have an incomplete biochemical response to UDCA treatment at a dose of 13–15 mg/kg of body weight (11,14–16). PBC still contributes significantly to mortality and morbidity from chronic liver disease and is a major indication for liver transplantation (1). For the subset of incomplete responders to UDCA therapy, Health Canada has approved obeticholic acid (OCA).
OCA is a Farnesoid-X-Receptor (FXR) agonist with anti-cholestatic, anti-inflammatory, and anti-fibrotic effects (17). Its use is recommended in combination with UDCA in PBC patients who have an inadequate response to UDCA, or as a monotherapy in patients who cannot tolerate UDCA treatment (18). A pivotal phase III trial, POISE, and an open-label extension (OLE) study demonstrated that treatment with 5–10 mg of OCA induces significant and sustained improvements in ALP and bilirubin in 46% of patients (17,19,20). In recent years, Bezafibrate, a pan-peroxisome-proliferator-activated receptor (PPAR) agonist, has emerged as another alternative therapeutic approach for PBC patients. In the BEZURSO phase III clinical trial, PBC patients with incomplete response to UDCA monotherapy were treated with 400 mg/day of bezafibrate (21). The primary endpoints of normalization of ALP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, albumin, and prothrombin index were achieved by 31% of patients treated with UDCA and bezafibrate (21). Bezafibrate is currently not approved for the treatment of PBC in Canada and thus used off-label when prescribed to PBC patients. For PBC patients who fail to achieve adequate response with dual therapy, either UDCA and OCA or UDCA and fibrates combinations, triple therapy is currently being investigated (22). Other molecules, including non-steroidal FXR agonists (23–25) as well as other PPAR agonists (26–28) are also in development for the treatment of PBC.
In Canada, there is a lack of data on patient characteristics, treatment management, and disease- related outcomes in PBC. Consequently, we conducted a descriptive analysis of the patient demographics, treatment patterns, and disease severity of PBC across Canada to better characterize this patient population and explore future patterns of patient care.
Methods
Study design and patient population
A retrospective cross-sectional examination of PBC patients who received follow-up care at 15 liver centres across Canada was conducted between January 2016 and September 2017. To be eligible for inclusion in the study, patients must have had a confirmed PBC diagnosis meeting at least 2 of the following criteria: biochemical evidence of cholestasis based on ALP elevation, presence of AMA, and/or histological evidence of nonsuppurative destructive cholangitis and destruction of interlobular bile ducts consistent with PBC. Eligible patients must also have received follow-up care at the participating centre within 24 months of study initiation.
Data collection
A web-based tool was used to collect data between June 3, 2016 and September 15, 2017. All sites, with the exception of one, were trained on the use of the data collection platform prior to initiating the chart review and data entry. One site provided a data extract of the requested information for each eligible patient.
Chart reviews were conducted by designated research associates and/or nurses within each participating institution. Medical records from the most recent visit of each eligible patient were reviewed, and relevant information was collected. Each entry in the data collection tool was assigned a clinic and district identification number in its internal log to facilitate data queries.
Data analysis
Eligible PBC patients were stratified according to UDCA treatment status: patients who have never been on UDCA, those currently on UDCA, and those who were previously on UDCA but have since discontinued. Categorical data was reported as counts and percentages, while continuous data was expressed as a median (25th to 75th percentiles). The following variables were described and compared across each UDCA treatment group: sex, year of diagnosis, AMA-positivity (earliest available measurement), age at last visit, time from diagnosis to last visit, liver biochemistry (ALP, ALT, AST, total bilirubin, gamma-glutamyl transferase [GGT], total bilirubin, platelet count, albumin), PBC-related symptoms, and LSM by VCTE. Differences in categorical variables across the UDCA treatment groups were compared with the Chi-square test, while differences in continuous variables were compared with the Kruskal-Wallis test.
Stage liver biopsy was reported in patients with a liver biopsy, as staged from 0 to 4 based on the Batt-Ludwig grading system, where 0 indicates no disease, 1 is mild disease, 2 is moderate disease, 3 is severe disease, and 4 is cirrhosis (29). In patients that received treatment with UDCA for at least 1 year, response according to Toronto (ALP ≤200 IU/L, total bilirubin ≤21 µmol/L) and Paris-II criteria (ALP ≤180 IU/L, AST ≤60 IU/L, total bilirubin ≤21 µmol/L) was reported. The number of liver-related outcomes (ascites, esophageal varices, encephalopathy, jaundice, and hepatocellular carcinoma [HCC]) were also reported. Transplant-free survival was estimated with a Kaplan-Meier curve from the time of PBC diagnosis. Patients without an event at the last visit were censored.
A p value less than 0.05 was considered statistically significant. All analysis were two-sided and were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA).
Results
Patient population
Data from a total of 1,125 eligible PBC patients from 15 liver centres across Canada were collected from June 3, 2016 to September 15, 2017 (Table 1). Most patients included in the study were from British Columbia (n = 275), followed by Quebec (n = 247), Nova Scotia (n = 176), Alberta (n = 164), Manitoba (n = 126), Ontario (n = 120), and New Brunswick (n = 17) (Supplemental Figure 1 in the Appendix).
Table 1:
Demographics and disease characteristics according to ursodeoxycholic acid use
| Previously on UDCA (n = 50) | Currently on UDCA (n = 1001) | Never on UDCA (n = 74) | |
|---|---|---|---|
|
Sex, no. (%) Female Male |
46 (92) 4 (8.0) |
903 (90.3) 97 (9.7) |
65 (87.8) 9 (12.2) |
| Year of diagnosis, median (25th–75th percentile)* | 2008 (2002–2014) | 2010 (2006–2013) | 2014 (2009–2015) |
| AMA-positive, no. (%)† | 31 (62.0) | 803 (80.4) | 55 (75.3) |
|
Age at last visit, y,‡ median (25th–75th percentile) <40, no. (%) 40–49, no. (%) 50–59, no. (%) 60–69, no. (%) ≥70, no. (%) |
62.5 (56.6–69.4)
2 (4.1) 4 (8.2) 13 (26.5) 19 (38.8) 11 (22.4) |
61.7 (54.7–68.7)
24 (2.5) 105 (10.8) 300 (30.8) 342 (35.1) 202 (20.8) |
58.7 (51.6–66.6)
9 (12.2) 7 (9.5) 25 (33.8) 21 (28.4) 12 (16.2) |
|
Time from diagnosis to last visit, y,§ median (25th–75th percentile) <1, no. (%) 1–4, no. (%) 5–9, no. (%) 10–14, no. (%) 15–19, no. (%) ≥20, no. (%) |
8.8 (4.5–20.4) 3 (7.0) 8 (18.6) 15 (34.9) 5 (11.6) 1 (2.3) 11 (25.6) |
6.5 (2.8–10.4) 93 (10.1) 270 (29.3) 310 (33.7) 138 (15.0) 55 (6.0) 55 (6.0) |
2.6 (0.5–7.7) 24 (36.4) 18 (27.3) 19 (28.8) 4 (6.1) 1 (1.5) 0 (0.0) |
|
Platelet count, x 103,¶ median (25th–75th percentile) <150, no. (%) ≥150, no. (%) |
185 (139–254) 13 (28.9) 32 (71.1) |
232 (174–284) 170 (17.7) 790 (82.3) |
244 (194–288) 12 (17.1) 58 (82.9) |
|
Albumin, g/L,** median (25th–75th percentile) <35, no. (%) ≥35, no. (%) |
40 (35–42) 9 (21.4) 33 (78.6) |
39 (36–42) 172 (19.3) 717 (80.7) |
39 (36–42) 12 (17.9) 55 (82.1) |
|
ALP, IU/L,†† median (25th–75th percentile) <120, no. (%) 120–179, no. (%) 180–189, no. (%) 190–199, no. (%) 200–299, no. (%) 300–399, no. (%) ≥400, no. (%) |
131 (81–240) 20 (41.7) 12 (25.0) 2 (4.2) 1 (2.1) 5 (10.4) 4 (8.3) 4 (8.3) |
133 (99–189) 393 (40.2) 309 (31.6) 32 (3.3) 21 (2.1) 140 (14.3) 42 (4.3) 40 (4.1) |
106 (77–157) 43 (58.9) 16 (21.9) 1 (1.4) 1 (1.4) 6 (8.2) 4 (5.5) 2 (2.7) |
| Total bilirubin, µmol/L, median (25th–75th percentile)‡‡ | 10.4 (6.5–14.7) | 9.0 (6.1–13.0) | 8.0 (5.8–14.0) |
|
Combined ALP (IU/L) and total bilirubin (µmol/L), no. (%)§§ ALP ≥200 + total bilirubin ≤21 ALP <200 + total bilirubin >21 ALP ≥200 + total bilirubin >21 ALP <200 + total bilirubin ≤21 |
7 (15.6) 2 (4.4) 5 (11.1) 31 (68.9) |
176 (18.5) 41 (4.3) 42 (4.4) 693 (72.8) |
9 (12.9) 5 (7.1) 2 (2.9) 54 (77.1) |
| AST, IU/L, median (25th–75th percentile)¶¶ | 31 (21–62) | 30 (23–41) | 28 (22–43) |
| ALT, IU/L, median (25th–75th percentile)*** | 29 (18–54) | 27 (20–40) | 29 (22–50) |
| GGT, IU/L, median (25th–75th percentile)††† | 118 (40–301) | 62 (31–138) | 66 (25–185) |
Notes: UDCA duration for those currently on UDCA (time from UDCA start date to last visit): median 6.1 years (2.6–9.8) – n = 715;
UDCA duration for those previously on UDCA (time from UDCA to stop date) median 2.2 years (0.8–7.1) – n = 29; current UDCA dose for those currently on UDCA (mg/kg/day): Median 14.0 (12.5–16.0) – n = 694
UDCA = Ursodeoxycholic acid; AMA = Antimitochondrial antibody; ALP = Alkaline phosphatase; AST = Aspartate aminotransferase, ALT = Alanine aminotransferase; GGT = Gamma-glutamyl transferase
* UDCA groups: n = 50, n = 999, n = 73;
† UDCA groups: n = 50, n = 969, n = 71;
‡ UDCA groups; n = 49, n = 973, n = 74;
§ UDCA groups; n = 43, n = 921, n = 66;
¶ UDCA groups; n = 45, n = 960, n = 70;
** UDCA groups, n = 42, n = 889, n = 67;
†† UDCA groups, n = 48, n = 977, n = 73;
‡‡ UDCA groups; n = 45, n = 962, n = 70;
§§ UDCA groups; n = 45, n = 952, n = 70;
¶¶ UDCA groups, n = 41, n = 835, n = 68;
*** UDCA groups; n = 48, n = 989, n = 73
††† UDCA groups; n = 33, n = 815, n = 59
As per the typical demographics of this patient population, 90.2% of patients were female (Supplemental Table 1 in the Appendix). The median age of patients was 61.3 years at last visit: 20.5% of patients were above 70 years of age, 34.9% were between 60 and 69 years of age, 30.8% were between 50 and 59 years of age, and 25% were below 49 years of age. Antimitochondrial antibody (AMA) positivity was observed in most patients (p = 0.005) (Table 1 and Supplemental Table 1 in the Appendix). The most common symptoms were fatigue (33.5%) and pruritus (26.4%) (Table 2).
Table 2:
Primary biliary cholangitis-related symptoms and comorbidities
| All Patients (N = 998) | Previously on UDCA (n = 50) | Currently on UDCA (n = 890) | Never on UDCA (n = 58) | p | |
|---|---|---|---|---|---|
| Fatigue | 334 (33.5) | 18 (36.0) | 301 (33.8) | 15 (25.9) | 0.43 |
| Pruritus | 263 (26.4) | 13 (26.0) | 241 (27.1) | 9 (15.5) | 0.15 |
| Sicca* | 209 (21.0) | 5 (10.0) | 197 (22.2) | 7 (12.1) | 0.03 |
| Osteoporosis | 133 (13.3) | 8 (16.0) | 123 (13.8) | 2 (3.4) | 0.07 |
| Hyperlipidemia† | 122 (12.3) | 6 (12.0) | 111 (12.5) | 5 (8.6) | 0.68 |
| Portal hypertension | 82 (8.2) | 12 (24.0) | 68 (7.6) | 2 (3.4) | <0.001 |
* n = 997
† n = 995
UDCA = ursodeoxycholic acid
UDCA treatment
Of the 1,125 patients included in the study, 1,001 (89.0%) were currently on UDCA therapy, 50 (4.4%) were previously treated with UDCA but have since discontinued, and 74 (6.6%) were never treated with UDCA. For those currently on UDCA, the median dose was 14.0 mg/kg/day (12.5–16.0) (Table 1). The median time from diagnosis to last visit for patients currently on UDCA, previously on UDCA, and never treated with UDCA were 6.5 years, 8.8 years, and 2.6 years, respectively (p < 0.001), with a median overall time since diagnosis of 6.4 years. Overall, 6.4% of patients were diagnosed more than 20 years ago. Of the 74 patients (6.6%) who had never been treated with UDCA, 36.4% were diagnosed less than 1 year ago, with only 5 patients (7.6%) diagnosed more than 10 years ago. (Table 1 and Supplemental Figure 1 in the Appendix). The most common reasons for UDCA treatment discontinuation were lack of efficacy, which includes patients who discontinued because they received a liver transplant, intolerance, and others.
Liver biochemistry
The median serum ALP for patients currently on UDCA was 133 IU/L, while the median serum ALP for those previously treated with UDCA or those who had never been treated with UDCA were 131 IU/L and 106 IU/L, respectively (p = 0.003 for all comparisons) (median overall ALP level was 132 IU/L) (Table 1 and Supplemental Table 1 in the Appendix).
The median total bilirubin levels for patients currently on UDCA was 9.0 µmol/L, while the median total bilirubin for those previously treated with UDCA or those who had never been treated with UDCA were 10.4 µmol/L and 8.0 µmol/L, respectively (p = 0.38) (median overall total bilirubin was 9.0 µmol/L) (Table 1 and Supplemental Table 1 in the Appendix).
Overall median values of 18.1% and 19.3% were reported for platelet counts less than 150 x 103, and serum albumin levels less than 35 g/L (Supplemental Table 1 in the Appendix).
A total of 289 (27.1%) patients presented with ALP levels ≥200 IU/L and/or total bilirubin levels ≥21 µmol/L. Of those, 192 (18.0%) had ALP ≥200 IU/L + total bilirubin ≤21 µmol/L, 48 (4.5%) had ALP <200 IU/L + total bilirubin >21 µmol/L, and 49 (4.6%) had ALP ≥200 IU/L + total bilirubin >21 µmol/L (Table 1). Assessment of combined ALP, bilirubin, and platelet measures determined that 11.1% (115) of patients included in the study had ALP <200 IU/L, total bilirubin ≤21µmol/L, and platelet counts <150 × 103 (Supplemental Table 3 in the Appendix).
Liver fibrosis stage
Approximately 42% (438/1027) of patients had liver biopsy at any point. Of those who underwent liver biopsy, 74.2% (325/438) of patients had a fibrosis stage available. The fibrosis stages F0-1, F2, F3, and F4 were 36.3% (118), 25.8% (84), 24.0% (78), and 13.8% (n = 45), respectively (data not shown).
Liver stiffness was assessed based on research work by Corpechot et al (8). Vibration-controlled transient elastography (VCTE) was performed on 50.6% (569) of patients included in the study. Of those with available data, 63.3% (360) had a fibrosis score of F0–1 (≤8.7 kPa), 14.4% (82) were classified as F2 (8.8 to ≤10.6 kPa), 10.2% (58) were classified as F3 (10.7 to ≤16.8 kPa), and 12.1% (69) were classified as F4 (≥16.9 kPa) (p <0.001 for all comparisons) (Table 3).
Table 3:
Liver stiffness measurements by transient elastography
| All patients (N = 569) | Previously on UDCA (n = 16) | Currently on UDCA (n = 515) | Never on UDCA (n = 38) | |
|---|---|---|---|---|
| Liver stiffness, kPa, median TE (25th–75th percentile) | 7.1 (5.3–10.3) | 6.6 (4.5–12.2) | 7.4 (5.5–10.4) | 4.9 (4.3–7.5) |
|
Corpechot et al 2012*, no. (%) F0-1 (≤8.7 kPa) F2 (8.8–10.6 kPa) F3 (10.7–16.8 kPa) F4 (≥16.9 kPa) |
360 (63.3) 82 (14.4) 58 (10.2) 69 (12.1) |
10 (62.5) 2 (12.5) 2 (12.5) 2 (12.5) |
321 (62.3) 73 (14.2) 55 (10.7) 66 (12.8) |
29 (76.3) 7 (18.4) 1 (2.6) 1 (2.6) |
* Liver stiffness measures (LSM) were assessed by transient elastography (TE): fibrosis stage F0-1 is associated with LSM of 8.7 kPa or, F2 with a LSM of 8.8 kPa to 10.6 kPa, F3 with a LSM of 10.7 kPa to 16.8 kPa, and F4 with a LSM of 16.9 kPa or more
UDCA = ursodeoxycholic acid; TE = transient elastography
When stratified by ALP level, the median LSM for patients with ALP <200 IU/L (434) was 6.8 kPa, whereas it was to 9.3 kPa for those with ALP ≥200 IU/L (116) (data not shown). Through analysis of ALP and fibrosis stage, it was determined that among patients with ALP < 200 IU/L, 68.4% were classified as F0–1, 21.9% were classified as F2–3, and 9.7% were classified as F4. Among patients with ALP ≥200 IU/L, 42.2%, 34.5%, and 23.3% were classified as F0–1, F2–3, and F4, respectively. Lower median ALP levels were correlated with a lower LSM (Figure 1a).
Figure 1:
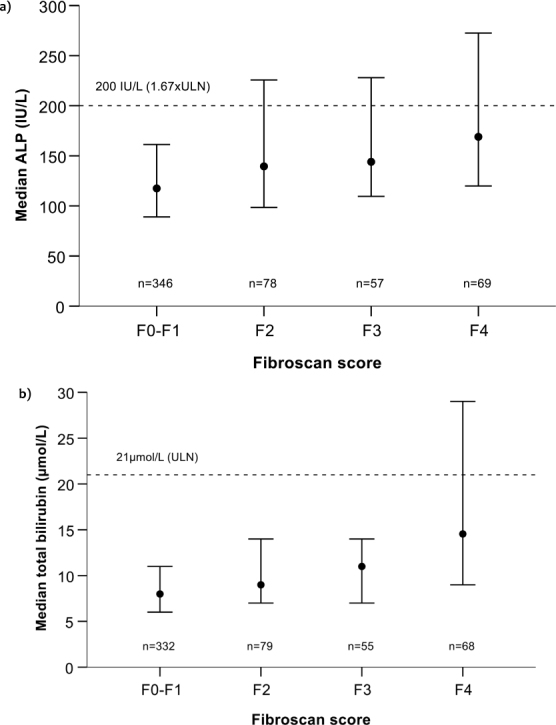
Combined analysis of FibroScan score and alkaline phosphatase (ALP) or total bilirubin. Correlation between median (25th–75th percentile) ALP and FibroScan score (a) and median (25th–75th percentile) total bilirubin and FibroScan score (b)
Assessment of combined total bilirubin and LSM showed that patients with total bilirubin levels ≤21 µmol/L and available measurements (n = 493) had a median LSM of 6.9 kPa, with 65.5%, 24.3%, and 10.1% having fibrosis stages of F0–1, F2–3, and F4, respectively. The median LSM among those with total bilirubin levels >21 µmol/L was 14.3 kPa (41). Within this cohort, 22.0% were classified as F0–1, 34.1% were classified as F2–3, and 43.9% were classified as F4 (Figure 1b).
Liver-related clinical outcomes
Overall, 19.8% (198) liver-related outcome were reported. The most commonly reported outcomes were esophageal varices (7.1%; 71), ascites (4.5%; 45), jaundice (4.0%; n = 40), and hepatic encephalopathy (2.9%; 29) (Table 4). Hepatocellular carcinoma (HCC) was observed in 1.4% (13) of patients. Liver-related mortality occurred in 1.2% (14) of the patients. Compared to patients currently on UDCA, or those who have never been on UDCA treatment, patients who were previously on UDCA had a higher incidence of liver-related outcomes (Table 4).
Table 4:
Liver-related clinical outcomes
| All patients (N = 998) | Previously on UDCA (n = 50) | Currently on UDCA (n = 890) | Never on UDCA (n = 58) | |
|---|---|---|---|---|
| Ascites | 45 (4.5) | 4 (8.0) | 40 (4.5) | 1 (1.7) |
| Esophageal varices | 71 (7.1) | 7 (14.0) | 62 (7.0) | 2 (3.4) |
| Encephalopathy | 29 (2.9) | 2 (4.0) | 27 (3.0) | 0 (0.0) |
| Jaundice | 40 (4.0) | 3 (6.0) | 35 (3.9) | 2 (3.4) |
| HCC* | 13 (1.4) | 2 (4.3) | 10 (1.2) | 1 (1.9) |
* n = 949 (UDCA groups: n = 46, n = 851, n = 52)
Total liver transplantation in cohort (n = 36), total deaths (n = 14)
UDCA = Ursodeoxycholic acid; LT = Liver transplantation; HCC = Hepatocellular carcinoma
Treatment response
Patients who were currently being treated with UDCA were assessed for UDCA treatment response according to the Toronto Criteria (ALP ≤200 IU/L, total bilirubin ≤21μmol/L, and UDCA treatment duration ≥1 year), and Paris-II Criteria (ALP ≤180 IU/L, AST ≤60 IU/L, total bilirubin ≤21 μmol/L, and UDCA treatment duration ≥1 year). Of the 612 patients with data available for assessment according to the Toronto Criteria, 449 (73.4%) were considered responders, and 163 (26.6%) were deemed inadequate responders to UDCA treatment (Figure 2). Similarly, of the 507 patients who had data available for the assessment according to the Paris-II criteria, 313 (61.7%) were classified as responders, and 194 (38.3%) were deemed inadequate responders to UDCA therapy (Figure 2).
Figure 2:
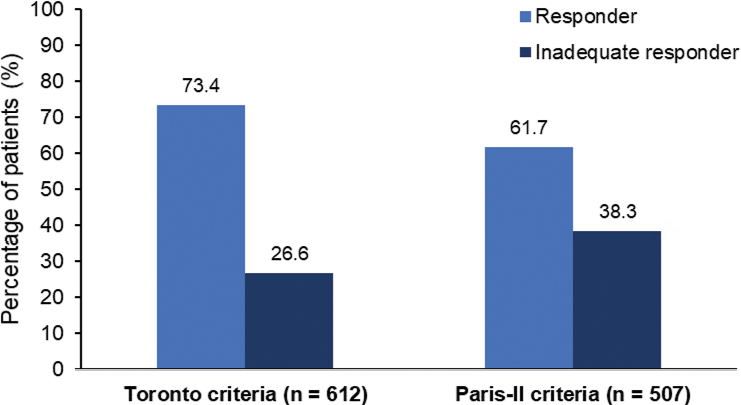
Assessment of UDCA treatment response Proportion of patients categorized as responders and incomplete responders to UDCA therapy according to the Toronto criteria (ALP ≤200 IU/L, total bilirubin ≤21 µmol/L), and Paris-II criteria (ALP ≤180 IU/L, AST ≤60 IU/L, total bilirubin ≤21 µmol/L)
UDCA = Ursodeoxycholic acid; ALP = Alkaline phosphatase; AST = Aspartate aminotransferase
Liver transplantation-free survival
Since the time of PBC diagnosis, 28 patients underwent liver transplant (LT) and 13 patients died. LT-free survival was 96.0% at 10 years. At 20 years since diagnosis, the cumulative LT-free survival rate was 86.8% (Figure 3).
Figure 3:
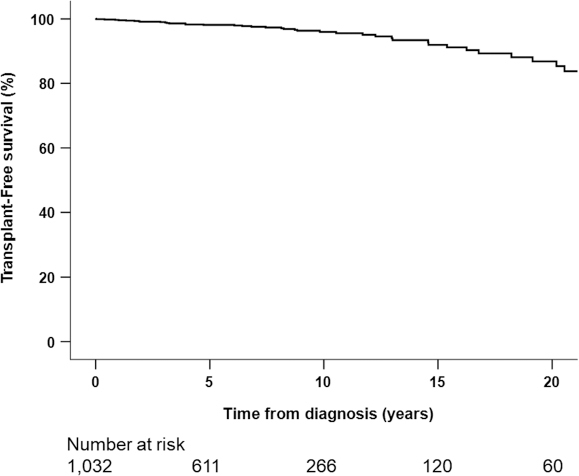
Transplant-free survival from time of primary biliary cholangitis diagnosis
Notes: Transplant-free survival: 5-yr: 98.1%, 10-yr: 96.0%, 15-yr: 91.9, 20-yr: 86.8%; n smaller than the number of participants because some patients had a transplant date before date of diagnosis; total events in this KM (n=41, 28 LT and 13 death); there were only 2 events in the UDCA untreated group
KM = Kaplan-Meier; UDCA = Ursodeoxycholic acid
Discussion
This study examined the characteristics and treatment patterns of 1,125 active patients with PBC from 15 liver centres across Canada. The demographics and overall presentation of this Canadian PBC cohort is typical of patients in Europe and North America. They were mostly white middle-aged women with detectable AMA. When presenting with symptoms, fatigue and pruritus were most common. Most patients (93.4%) were previously treated, or are currently treated, with UDCA, reflecting the strong physician adoption of UDCA as the first-line therapy to actively manage PBC.
Compared to patients currently on UDCA, those who discontinued UDCA treatment had a longer time since diagnosis (6.5 versus 8.8 years), a higher prevalence of liver-related outcomes (19.6% versus 36.3%) and, had higher median bilirubin levels (9.0 versus 10.4 µmol/L) supporting the continuous use of UDCA to optimize the clinical benefits. The most common reason for UDCA treatment discontinuation was lack of efficacy. Recent research has demonstrated that regardless of the observed biochemical response, the use of UDCA improves LT-free survival among patients with PBC and reduces the risk of death (11), thus urging physicians not to discontinue the use of UDCA in inadequate responders.
Patients who never received UDCA were more recently diagnosed with PBC (median time since diagnosis of 2.6 years) than those who had previously been or are currently on UDCA (mean time since diagnosis of 8.8 and 6.5 years, respectively). These patients also had lower levels of fibrosis compared to those currently treated with UDCA (4.9 and 7.4 kPa) suggesting they had earlier stage disease. These patients could benefit from UDCA since the therapy can significantly delay the progression of PBC when initiated at early stages (30).
In this study, 26.6% and 38.3% of patients were classified as inadequate responders using the Toronto criteria and the Paris-II criteria, respectively, consistent with findings from previous studies (11,14–16). Current guidelines recommend that patients showing inadequate biochemical response to UDCA by 12 months after initiation of therapy should be considered for further treatments (6,7). The unmet need in this population of PBC patients may be addressed with appropriate, approved second-line treatments, such as OCA (6,7). Since the characterization of PBC patients in this study occurred before approval and wide-spread availability of OCA in Canada, none of the patients included in the study had been treated with OCA. Therefore, these patients represent the proportion of individuals that may benefit from OCA. Off-label fibrate derivatives may also be considered for the management of those with insufficient benefit from UDCA (31). Combination therapy with UDCA and second-line treatments may potentially provide multiple anticholestatic mechanisms.
Overall, analysis of combined measures identified that lower median ALP levels were correlated with a lower LSM. However, among patients with ALP <200 IU/L, more than 30% had liver fibrosis stages greater than F2. In patients with total bilirubin levels ≤21 µmol/L, 34.4% presented with fibrosis stages F2, F3, and F4. This finding implies that advanced fibrosis can occur in patients with currently accepted ALP and bilirubin treatment target levels. This observation is in line with a previous report that concluded that liver enzymes do not always reliably correlate with fibrosis stage (32). It is important to recognize that patients with advanced liver disease and cirrhosis may present with only subtle changes in laboratory values that fall within the normal ranges (33). The GLOBAL PBC group recently demonstrated that fibrosis is an independent predictor for transplant-free survival in PBC patients. They recommend the assessment of fibrosis stage, either histologically or via non-invasive measures, for PBC risk stratification in addition to biochemical response to treatment (32). Combined biochemical and fibrosis assessment will help identify patients at risk for disease progression and ensure that patients with disease progression are recognized to allow for timely additional medical intervention (33,34).
This study assessed a large, heterogeneous cohort of PBC patients to provide insight into the management of PBC in Canada. Patients from select clinics in British Columbia, Alberta, Manitoba, Ontario, Quebec, Nova Scotia, and New Brunswick were included; however, some provinces and territories were not represented in the analysis. Other limitations of this study include a potential inherent selection bias of the Canadian PBC population since participating clinics were large, experienced liver centres, and community-based clinics were not included. The number of records in each province was not confirmed to be proportional to the national population and we cannot exclude the possibility of selection bias. The inability to verify the status of UDCA treatments (eg, patient prescriptions), patient adherence to therapy, or the proper recording of all patient deaths may confound the stratification and comparisons between patient subgroups. As well, the cross-sectional design of this study does not allow for long-term monitoring of health outcomes due to variations in UDCA treatment pattern and response over time.
In conclusion, assessment of PBC patients between January 2016 and September 2017 demonstrated that the Canadian cohort presents similarly to PBC patients from other countries, with the disease mostly affecting middle-aged women, with a strong female preponderance (9:1). Antimitochondrial antibodies (AMA) were found in the majority of patients and, the most common clinical symptoms were fatigue and pruritus. The majority of PBC patients were treated with UDCA, highlighting the strong adherence of Canadian gastroenterologists and hepatologists to the use of the first-line therapy in the management of the disease.
From this cohort, approximately a third of PBC patients treated with UDCA may benefit from additional therapy. In addition, significant proportion of patients with ALP <200 IU/L and bilirubin ≤21 µmol/L had fibrosis scores of F2 and greater. This confirms the need to move beyond biochemistry and to assess fibrosis as an independent prognostic factor for determining risk of disease progression and need for second-line therapy. As the treatment landscape evolves and more therapy becomes available, it is imperative that all Canadian PBC patients are appropriately monitored so that patients receive the best available care that mitigates their risk of disease progression.
Acknowledgements:
A preliminary summary of this work was presented as a poster at the Liver Meeting of the American Association for the Study of Liver Disease in San Francisco CA, November 2018.
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
Intercept Pharma Canada Inc. funded the medical writing services and statistical analysis to assist in the development of this manuscript.
Disclosures:
EM Yoshida has received an unrestricted research grant from Paladin Laboartories and clinical trials sponsorship from Gilead Sciences, Intercept, Abbvie, Merck, Genfit, Pfizer, Celgene, Allergan, and Madrigal. He has also received honoraria for CME/Ad Board lectures from Intercept, Gilead, and Merck. He is President of the Vancouver Medical Dental Allied Staff Association; C Tsien received grants/contracts from Lupin, and payment or honoraria for lectures, presentations, speaker bureaus, or educational events from Abbvie and Intercept; MG Swain received grants/contracts for research studies from Gilead, Intercept, CymaBay, Genkyotex, GSK, Genfit, Novartis, Pfizer, Celgene, AstraZeneca, AbbVie, and Novo Nordisk, payment or honoraria for lectures and presentations from Gilead, Intercept, and AbbVie, and participated on the advisory boards for Gilead, Novartis, and Intercept; KM Peltekian participated on the advisory board for Intercept and has held a leadership or fiduciary role on the Canadian Liver Foundation board; MA Puglia has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Intercept as well as support from them for attending meetings and/or travel and participating on their advisory board; M Elkhashab has received consulting fees and payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from AbbVie, Gilead, and Merck, as well as participated on the advisory boards for AbbVie, Gilead, and Merck; M O’Brien has received payment or honoraria for lectures, presentations, speaker bureaus, or educational events from Intercept; HH Ko has received payment or honoraria for lectures, presentations, speaker bureaus, or educational events from Intercept and has held a leadership or fiduciary role on the Intercept advisory board; E Tam has received consulting fees from Intercept as well as payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Intercept; A Ramji has received grants/contracts from AbbVie, Allergan, Assembly, Galmed, Gilead, Janssen, Intercept, Novartis, and Merck, as well as payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from AbbVie, Allergan, Amgen, Celgene, Gilead, Intercept, Amgen, Janssen, Novartis, and Merck. He has participated on advisory board for AbbVie, Gilead, Janssen, Intercept, Novartis, Merck, and Novo Nordisk; the other authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
Appendix
This appendix has been provided by the authors to give readers additional information about their work.
Supplement to: Yoshida EM, D Gribic, Swain MG et al. Clinical characterization of Canadian patients with primary biliary cholangitis.
Number of records collected per participating clinic from June 3, 2016 to September 9, 2017.
Supplemental Figure 1:
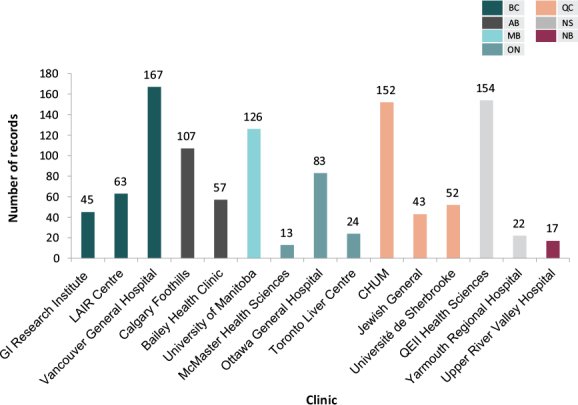
Number of patient records collected from participating centres
Supplemental Table 1:
Demographics and disease characteristics of the participants
| All patients (N = 1125) | |
|---|---|
|
Sex, no. (%) Female Male |
1014 (90.2) 110 (9.8) |
| Year of diagnosis, median (25th–75th percentile)* | 2010 (2006–2014) |
| AMA positive, no. (%)† | 889 (79.2) |
|
UDCA, no. (%) Currently on UDCA Previously on UDCA Never on UDCA |
1001 (89.0) 50 (4.4) 74 (6.6) |
|
Age at last visit, y,‡ mean (SD) <40, no. (%) 40–49, no. (%) 50–59, no. (%) 60–69, no. (%) ≥70, no. (%) |
61.3 (11.2) 35 (3.2) 116 (10.6) 338 (30.8) 382 (34.9) 225 (20.5) |
|
Time from diagnosis to last visit, y,§ median (25th–75th percentile) <1, no. (%) 1–4, no. (%) 5–9, no. (%) 10–14, no. (%) 15–19, no. (%) ≥20, no. (%) |
6.4 (2.7–10.2)
120 (11.7) 296 (28.7) 344 (33.4) 147 (14.3) 57 (5.5) 66 (6.4) |
|
Platelet count, x 103,¶ median (25th–75th percentile) <150, no. (%) ≥150, no. (%) |
231 (173–283)
195 (18.1) 880 (81.9) |
|
Albumin, g/L,** median (25th–75th percentile) <35, no. (%) ≥35, no. (%) |
39 (36–42)
193 (19.3) 805 (80.7) |
|
ALP, IU/L,†† median (25th–75th percentile) <120, no. (%) 120–179, no. (%) 180–189, no. (%) 190–199, no. (%) 200–299, no. (%) 300–399, no. (%) ≥400, no. (%) |
132 (96–188)
456 (41.5) 337 (30.7) 35 (3.2) 23 (2.1) 151 (13.8) 50 (4.6) 46 (4.2) |
| Total bilirubin, µmol/L, median (25th–75th per)‡‡ | 9 (6–13) |
|
Combined ALP (IU/L) and total bilirubin (µmol/L), n (%)§§ ALP ≥200 + total bilirubin ≤21 ALP <200 + total bilirubin >21 ALP ≥200 + total bilirubin >21 ALP <200 + total bilirubin ≤21 |
192 (18.0) 48 (4.5) 49 (4.6) 778 (72.9) |
| AST, IU/L, median (25th–75th percentile)¶¶ | 30 (23–41) |
| ALT, IU/L, median (25th–75th percentile)*** | 27 (20–41) |
| GGT, IU/L, median (25th–75th percentile)††† | 62 (31–141) |
Notes: Significant pairwise comparisons: Platelet count (continuous measurement): UDCA versus previously on UDCA (P = 0.01), never on UDCA versus previously on UDCA (P = 0.02); ALP (continuous measurement): never on UDCA versus UDCA (P = 0.002)
AMA = Antimitochondrial antibody; UDCA = Ursodeoxycholic acid; ALP = Alkaline phosphatase; AST = Aspartate aminotransferase, ALT = Alanine aminotransferase; GGT = Gamma-glutamyl transferase
* n = 1122
† n = 1090
‡ n = 1096
§ n = 1030
¶ n = 1075
** n = 998
†† n = 1098
‡‡ n = 1077
§§ n = 1067
¶¶ n = 944
*** n = 1110
††† n = 907
Supplemental Table 2:
Anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies (ASMA) in the participants
| All patients (N = 1125) | |
|---|---|
| ANA, n = 1113 | 344 (30.9) |
| ASMA, n = 1114 | 77 (6.9) |
Supplemental Table 3:
Combined ALP, total bilirubin, and platelet
| All patients (N = 1036) | Previously on UDCA (n = 43) | Currently on UDCA (n = 925) | Never on UDCA (n = 68) | |
|---|---|---|---|---|
| ALP <200 IU/L + total bilirubin ≤21 µmol/L + platelet <150 x 103, no. (%) | 115 (11.1) | 9 (20.9) | 98 (10.6) | 8 (11.8) |
UDCA = Ursodeoxycholic acid; ALP = Alkaline phosphatase
Supplemental Table 4:
Combined ALP, total bilirubin, and liver fibrosis stage measured by transient elastography
| All patients (N = 526) | Previously on UDCA (n = 13) | Currently on UDCA (n = 479) | Never on UDCA (n = 34) | |
|---|---|---|---|---|
|
Combined ALP (IU/L), total bilirubin (µmol/L), fibrosis score Corpechot et al 2012, no. (%) ALP <200 + total bilirubin ≤21 + F0-1 ALP <200 + total bilirubin ≤21 + F2 ALP <200 + total bilirubin ≤21 + F3 ALP <200 + total bilirubin ≤21 + F4 |
275 (52.3) 51 (9.7) 37 (7.0) 33 (6.3) |
4 (30.8) 2 (15.4) 0 (0.0) 0 (0.0) |
248 (51.8) 45 (9.4) 36 (7.5) 32 (6.7) |
23 (67.6) 4 (11.8) 1 (2.9) 1 (2.9) |
UDCA = Ursodeoxycholic acid; ALP = Alkaline phosphatase
Funding Statement
Intercept Pharma Canada Inc. funded the medical writing services and statistical analysis to assist in the development of this manuscript.
References
- 1.Yoshida EM, Mason A, Peltekian KM, et al. Epidemiology and liver transplantation burden of primary biliary cholangitis: A retrospective cohort study. CMAJ Open 2018;6:E664–70. 10.9778/cmajo.20180029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015;386:1565–75. 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 3.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J Hepatol 2012;56:1181–8. 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Prince M, Chetwynd A, Newman W, Metcalf JV, James OFW. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: Follow-up for up to 28 years. Gastroenterology 2002;123:1044–51. 10.1053/gast.2002.36027. [DOI] [PubMed] [Google Scholar]
- 5.Lee YM, Kaplan MM. The natural history of PBC: Has it changed? Semin Liver Dis 2005; 25:321–326. 10.1055/s-2005-916323. [DOI] [PubMed] [Google Scholar]
- 6.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69:394–419. 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. 10.1016/j.jhep.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;6:198–208. 10.1002/hep.25599. [DOI] [PubMed] [Google Scholar]
- 9.Cristoferi L, Calvaruso V, Overi D, et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in naïve patients with primary biliary cholangitis: A dual cut-off approach. Hepatology 2021; 74:1496–1508. 10.1002/hep.31810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997;113:884–90. 10.1016/S0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 11.Harms MH, van Buuren HR, Corpechot C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol 2019;71:357–65. 10.1016/j.jhep.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014;147:1338–49. 10.1053/j.gastro.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 13.Carbone M, Sharp SJ, Flack S, et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016;63:930–50. 10.1002/hep.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009; 136:1281–7. 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L-N, Shi T-Y, Shi X-H, et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: Results of a 14-year cohort study. Hepatology 2013;58:264–272. 10.1002/hep.26322. [DOI] [PubMed] [Google Scholar]
- 16.Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: Biochemical response to treatment and prediction of long-term outcome. J Hepatol 2011; 55:1361–7. 10.1016/j.jhep.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631–43. 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 18.Intercept Pharmaceuticals. OCALIVA Product Monograph. June 5, 2018. [Google Scholar]
- 19.Trauner M, Nevens F, Shiffman ML, et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol 2019;4:445–53. 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 20.Nevens F, Shiffman ML, Drenth JPH, et al. Durable response in the markers of cholestasis through 5 years of open-label extension study of obeticholic acid in primary biliary cholangitis. Dig Liver Dis 2020;52(Suppl 1):e30. 10.1016/j.dld.2019.12.114. [DOI] [Google Scholar]
- 21.Corpechot C, Chazouillères O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018;378:2171–81. 10.1056/NEJMoa1714519. [DOI] [PubMed] [Google Scholar]
- 22.Soret P-A, Lam L, Carrat F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther 2021; 53:1138–46. [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov. National Library of Medicine (US). NCT03394924, A study to assess the safety, tolerability, pharmacokinetics and efficacy of EDP-305 in subjects with primary biliary cholangitis. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03394924&cntry=&state=&city=&dist= (June 15, 2021).
- 24.ClinicalTrials.gov. National Library of Medicine (US). NCT02943447, Study to evaluate the safety, tolerability, and efficacy of Cilofexor in adults with primary biliary cholangitis without cirrhosis (PBC-Phase 2). Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02943447&cntry=&state=&city=&dist= (June 15, 2021).
- 25.ClinicalTrials.gov. National Library of Medicine (US). NCT02516605, A multi-part, double blind study to assess safety, tolerability and efficacy of Tropifexor (LJN452) in PBC patients. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02943447&cntry=&state=&city=&dist= (June 15, 2021).
- 26.Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol 2017; (10):716–26. 10.1016/S2468-1253(17)30246-7. [DOI] [PubMed] [Google Scholar]
- 27.Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol 2021; 74:1344–54. 10.1016/j.jhep.2021.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Vuppalanchi R, González-Huezo MS, Payan-Olivas R, et al. A multicenter, open-label, single-arm study to evaluate the efficacy and safety of saroglitazar in patients with primary biliary cholangitis. Clin Transl Gastroenterol 2021;12:e00327. 10.14309/ctg.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995;19:1409–17. 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Poupon RE, Lindor KD, Pares A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effects of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol 2003;39:12–6. 10.1016/S0168-8278(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 31.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology 2021: online ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Murillo Perez CF, Harms MH, Lindor KD, et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol 2020; 115:1066–74. 10.14309/ajg.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed Z, Ahmed U, Walayat S, et al. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol 2018;11:301–7. 10.2147/CEG.S160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efe C, Tascilar K, Henriksson I, et al. Validation of risk scoring systems in ursodeoxycholic acid-treated patients with primary biliary cholangitis. Am J Gastroenterol 2019;114:1101–8. 10.14309/ajg.0000000000000290. [DOI] [PubMed] [Google Scholar]


