Abstract
Understanding how cholesterol binds to mammalian cells offers critical insights into the waxy substance’s role in protein modulation and cell function.
Cholesterol is an enigma in mammalian biology, posing a fundamental mystery of life. This waxy substance can threaten human life. When plaques composed mostly of cholesterol break apart, they can clog blood flow to the heart and brain, causing heart attacks and strokes. But cholesterol is also essential for mammalian cell function.
Mammalian cells need cholesterol for growth, but the interplay between cells and cholesterol is a delicate balance. Even small changes in the chemical structure of cholesterol can render it incapable of supporting mammalian cell growth. Creating cholesterol is an energetically expensive process, requiring over 30 biochemical steps. Given its importance, exactly how does cholesterol perform its essential role?
In the mid-1970s, biochemist and Nobel laureate Konrad Bloch suggested that cholesterol plays a special role in cell function but did not define the role. He suggested that the pathway from lanosterol to cholesterol was a response to evolutionary pressure and produced a sterol with a chemical structure conforming to specific roles in biology (1). Since the 1970s, multiple hypotheses have surfaced, suggesting specific ways in which cholesterol affects cell growth.
In one hypothesis, cholesterol is specifically required as a modulator of crucial membrane protein activity in mammalian cells (2). In this theory, cholesterol modulates protein activity by binding to specific site(s) on membrane proteins similar to an activator (or inhibitor) of a soluble enzyme. These binding sites are specific for the structure of the cholesterol molecule. Sterols with different chemical structures do not bind with similar affinity to the site and do not modulate the protein function.
One example of this activity is the mammalian sodium- and potassium-dependent adenosine triphosphatase (Na+,K+-ATPase), a molecular pump responsible for active counter transport of Na+ and K+ ions across plasma membranes. At low levels of plasma membrane cholesterol, the Na+,K+-ATPase expresses little or no activity. Increases in membrane cholesterol content lead to increases in Na+,K+-ATPase activity in a manner resembling a binding phenomenon. Other sterols cannot support the Na+,K+-ATPase activity as well as cholesterol. Activation is specific for the structure of cholesterol (3, 4). These and other data suggest that cholesterol binds to a specific site on the Na+,K+-ATPase. Crystal structures of the Na+,K+-ATPase reveal cholesterol binding sites in portion of the protein located within the membrane (5). These data support the hypothesis for cholesterol modulation of critical plasma membrane proteins.
Four recent papers in Science Advances offer evidence that cholesterol binds to specific sites in the protein located within cells. These data add to a growing body of support that cholesterol binding modulates protein function. They also show that this mechanism is one of the essential roles for cholesterol in mammalian cells. The findings also provide potential pathways to target when developing therapies for a range of conditions.
Modulation of Smoothened by cholesterol offers one example. Smoothened is a G protein–coupled receptor that is part of the Sonic Hedgehog signaling pathway involved in cell development and cancer. The regulatory protein, Patched 1, inhibits Smoothened signaling by reducing cholesterol binding to Smoothened. This inhibition is relieved when Sonic Hedgehog binds to Patched 1.
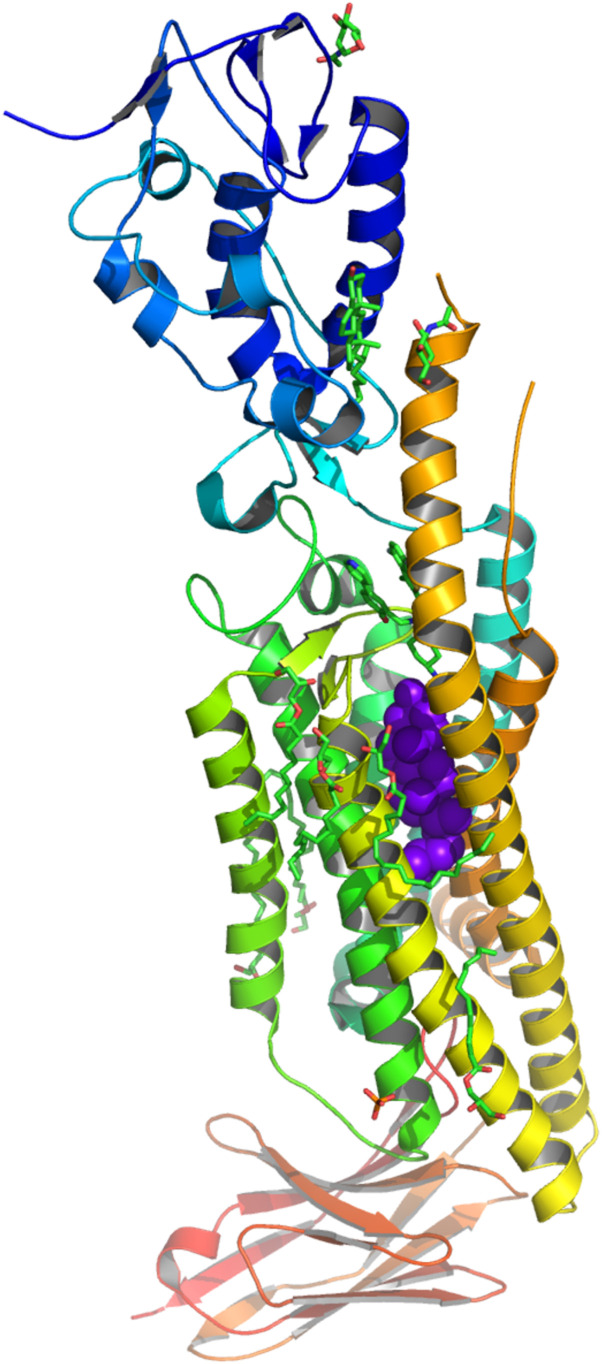
X-ray crystal structures revealed that cholesterol binds to Smoothened (Fig. 1) (6). Cholesterol binds in the cysteine-rich domain outside of the receptor. Structure-directed mutations in that binding site suggest that cholesterol occupancy supports receptor function. A second binding site was found in the transmembrane domain of a putative active form of the receptor.
Fig. 1. One of two cholesterol (purple) binding sites on Smoothened in x-ray crystal structure (PDB: 6O3C), drawn with MacPyMol v1.7.6.4.
Credit: Philip Yeagle.
Studies such as those in (6) raise the question whether cholesterol occupancy of one or both sites modulates the function of Smoothened? Kinnebrew et al. (7) focused on the mechanism of cholesterol activation of the Smoothened receptor. The authors found two effects of cholesterol on the receptor. Occupancy of the binding site in the transmembrane domain of the receptor led to increased Smoothened signaling in both the presence and absence of Sonic Hedgehog. Occupancy by cholesterol of the binding site in the cysteine-rich domain outside the receptor supports the specific Sonic Hedgehog–stimulated increase in receptor signaling. This latter site is likely a key regulatory site by which cholesterol modulates Smoothened function.
Cholesterol necessary for human cells is synthesized endogenously and absorbed from the diet at the intestinal brush border membranes in the small intestine. The protein Niemann-Pick C1-like 1 (NPC1L1) is located in those membranes and is involved in the absorption of dietary cholesterol from the lumen of the intestine. Studies have revealed that NPC1L1 binds cholesterol. Does that bound cholesterol have a physiological role to play in cholesterol absorption by NPC1L1?
Two recent cryo–electron microscopy studies show the presence of multiple cholesterol binding sites in the three-dimensional structure of human NPC1L1 (8, 9). The authors suggest that some of the sites are analogous to cholesterol binding sites on Patched 1. An increase in cholesterol binding can be induced by increasing the level of cholesterol in the preparation and as many as two additional cholesterol binding sites are observed, in addition to the sites evident at low cholesterol content. The increase in bound cholesterol stabilizes the NPC1L1 structure and supports its function. The drug ezetimibe destabilizes the cholesterol cluster on the NPC1L1 and inhibits the function of the protein in absorption of dietary cholesterol. There may be a “channel,” comprising more than one of the cholesterol binding sites, and ezetimibe blocks that channel inhibiting NPC1L1. The physiological effect of ezetimibe is to reduce circulating LDL, which incorporates into plaques that cause cardiovascular disease.
Programmed-death ligand (PD-L1) is a small transmembrane protein associated with the plasma membrane of some cancer cells. The sequence of PD-L1 contains two CRAC motifs, sequences of amino acids that often constitute cholesterol binding sites on proteins. In a study by Wang et al. (10), the authors found that enrichment of the plasma membrane of RKO cells with cholesterol increased PD-L1, whereas cholesterol depletion decreased the level of PD-L1. The structure of PD-L1 in the absence and presence of cholesterol was determined by NMR. The transmembrane domain of PD-L1 is predominantly a helix in the absence of cholesterol. Titration with cholesterol of the two-dimensional NMR spectrum of PD-L1 revealed resonance shifts for the residues in the CRAC sequence. These and other data led to the conclusion that cholesterol bound to PD-L1 at the two CRAC sequences in the transmembrane domain of PD-L1.
The membrane protein studies by Kinnebrew et al., Long et al., Hu et al., and Wang et al. present interesting examples of cholesterol binding to membrane proteins at specific sites and modulating the function of these proteins. These studies add to the body of evidence providing at least a partial answer to the question raised by Bloch and to the enigma of cholesterol and human health.
REFERENCES
- 1.K. Bloch, “On the Evolution of a Biosynthetic Pathway” in Reflections in Biochemistry (Pergamon, 1976), pp. 143–150. [Google Scholar]
- 2.Yeagle P. L., Modulation of membrane function by cholesterol. Biochem. 73, 1303–1310 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Giraud F., Claret M., Garay R., Interactions of cholesterol with the Na pump in red blood cells. Nature 264, 646–648 (1976). [DOI] [PubMed] [Google Scholar]
- 4.Yeagle P. L., Rice D., Young J., Effects of cholesterol on sodium-potassium ATPase ATP hydrolyzing activity in bovine kidney. Biochemistry 27, 6449–6452 (1988). [DOI] [PubMed] [Google Scholar]
- 5.Laursen M., Yatime L., Nissen P., Fedosova N. U., Crystal structure of the high-affinity Na+,K+-ATPase–ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl. Acad. Sci. U.S.A. 110, 10958–10963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X., Zhao F., Wu Y., Yang J., Han G. W., Zhao S., Ishchenko A., Ye L., Lin X., Ding K., Dharmarajan V., Griffin P. R., Gati C., Nelson G., Hunter M. S., Hanson M. A., Cherezov V., Stevens R. C., Tan W., Tao H., Xu F., Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nature Commun. 8, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinnebrew M., Woolley R. E., Bertie Ansell T., Byrne E. F. X., Frigui S., Luchetti G., Sircar R., Nachtergaele S., Grane L. M.-M., Krishnan K., Newstead S., Sansom M. S. P., Covey D. F., Siebold C., Rohatgi R., Patched 1 regulates Smoothened by controlling sterol binding to its extracellular cysteine-rich domain. Sci. Adv. 8, eabm5563 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long T., Liu Y., Qin Y., De Bose-Boyd R. A., Li X., Structures of dimeric human NPC1L1 provide insight into mechanisms for cholesterol absorption. Sci. Adv. 7, eabh3997 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu M., Yang F., Huang Y., You X., Liu D., Sun S., Sui S.-F., Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake. Sci. Adv. 7, eabg3188 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Cao Y., Shen L., Xiao T., Cao R., Wei S., Tang M., Du L., Wu H., Wu B., Yu Y., Wang S., Wen M., Yang B. O., Regulation of PD-L1 through direct binding of cholesterol to CRAC motifs. Sci. Adv. 8, eabq4722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]