Abstract
Introduction
African Americans (AA)s have worse inflammation, worse sleep, and a greater incidence of Alzheimer's disease (AD) compared to whites; however, no studies have examined associations between biomarkers, sleep, and cognition, and differences by race.
Methods
Seventy‐six cognitively normal, middle aged (45–65 years) adults with a parental history of AD were included in this study. Associations between biomarkers (tumor necrosis factor‐α [TNF‐α], interleukin‐10 [IL‐10], intercellular adhesion molecule‐1 [ICAM‐1],, and C‐reactive protein [CRP]) and self‐reported sleep or cognition measures, were assessed.
Results
Average sleep duration was significantly lower for AA versus whites (average[SD]) in hours: 6.02(1.18) versus 7.23(0.91), P = .000004). We found a statistically significant association between plasma IL‐10 and sleep duration (Spearman's ρ = 0.26, P = .04) and CSF ICAM‐1 and sleep quality (Spearman's ρ = 0.30, P = .03).
Discussion
Longer sleep duration is positively associated with plasma IL‐10 levels irrespective of race. Sleep quality was positively associated with CSF ICAM‐1 only in African Americans.
Keywords: Alzheimer's disease, biomarker, cerebrospinal fluid, cognition, inflammation, parental history, race, sleep
1. INTRODUCTION
Alzheimer's disease (AD) is a leading public health problem impacting an estimated 24 million people worldwide. 1 Aging is coincident with chronic inflammation, sleep disruption, and increased AD risk. 2 Inflammation is a risk factor for neurodegenerative disorders as well as cognitive changes associated with aging, 3 and modest associations between inflammatory biomarkers and neuropsychological associations (i.e., visual organization, executive functioning, and reading performance) have previously been noted. 4 Furthermore, inflammation may represent an early event in AD pathogenesis, 5 preceding Aβ plaque deposition and facilitating pathogenesis. 6 Although, data on changes in inflammatory cytokines such as interleukin‐6 (IL‐6) and tumor necrosis‐factor‐ α (TNF‐α) as mild cognitive impairment (MCI) progresses to AD are inconsistent, concentrations of pro‐inflammatory cytokines in plasma and CSF increase as AD progresses. 7 High TNF‐α levels can increase the rate of cognitive decline 8 and clinical studies suggest that systemic inflammation, even if unrelated to the central nervous system (CNS), accelerates cognitive decline.
The mechanisms through which altered sleep duration affects health are not fully established; however, experimental device suggests altered sleep may impact cytokines regulating inflammation. Sleep loss increases low‐grade inflammation and microglial activation in a pathogen‐independent mechanism, with deficits in cellular immunity, increased levels of pro‐inflammatory mediators such as such as TNF‐𝛼, IL‐6, and C‐reactive protein (CRP) and vascular alterations 9 (for an in depth review see, 10 ). How habitual sleep disruption influences inflammatory cytokines is less clear. 11 As sleep disturbance has robust effects on inflammatory biology, inflammation may be a pathway linking sleep disturbance and increased risk of AD.
The rate of AD for African Americans (AA) is approximately 64% higher than for non‐Hispanic White (NHW) Americans, 12 and our group has found that AAs with a family history of AD have worse cognition than whites. 13 AAs have the highest risk for, and prevalence of, poor sleep patterns compared to any other racial/ethnic group. 14 , 15 Furthermore, peripheral markers of inflammation, specifically CRP, have been found to be higher in AAs. 16
Our objective was to evaluate self‐reported sleep duration and quality in a cognitively normal cohort, and determine associations with understudied cytokine markers of inflammation and cognition. We examine inflammatory (TNF‐α, CRP, ICAM‐1) and anti‐inflammatory (IL‐10) biomarkers, whether these associations differ by race. We examined this question in a previously described cohort recruited for the Association between Cardiovascular Risk and Preclinical Alzheimer's Disease Pathology (ASCEND) Study. 13 Using baseline data from the ASCEND study, we tested whether, in this cohort of healthy middle‐aged individuals at risk for AD by virtue of family history: (1) sleep duration and cognition are associated with biomarkers, and (2) whether these associations differ by race. Understanding how sleep and race may modify the impact of risk factors associated with cognitive decline may yield new insights into race‐dependent biological mechanisms and inform targeted therapies.
2. METHODS
2.1. ASCEND study design
Eighty middle‐aged (45 years or older) adult children of persons with AD were enrolled in the ASCEND Study. 13 Parental AD diagnosis was either autopsy‐confirmed or probable AD as defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINDS‐ADRDA) criteria, and verified using the validated Dementia Questionnaire (DQ) 17 and medical records when available. Inclusion and exclusion criteria and participant characteristics have been previously reported. 13 Preliminary cohort size after exclusion of individuals with missing sleep or demographic information was 76, except where otherwise noted. Informed consent was obtained from all participants.
RESEARCH IN CONTEXT
Systematic Review: Authors reviewed literature using PubMed, finding no studies having examined race differences in the associations between biomarkers, sleep, and cognition. Given that Black Americans experience significantly higher incidence of AD, poorer sleep, and more inflammation than White Americans, further exploration is warranted.
Interpretation: Our study found shorter sleep duration among B/AA compared to NHWs. Longer sleep duration is positively associated with plasma IL‐10 levels irrespective of race. Mental rotation scores were inversely associated with plasma IL‐10 and TNF‐α; these relationships were not modified by race. However, sleep quality was positively associated with CSF ICAM‐1 only in B/AAs.
Future Directions: These results add to the evidence linking sleep quality, AD neuropathology, and inflammation. Given the evidence that disrupted sleep has a bidirectional relationship with AD pathology, longitudinal studies are warranted to establish how sleep alterations increase the risk or progression of dementia.
2.2. CSF collection
CSF collection procedures have been described previously. 13 Briefly, CSF samples were collected via lumbar puncture (LP) after an 8‐hour overnight fast and according to guidelines put forth in the “Biospecimens Best Practice Guidelines for the ADCs.” 18 Approximately, 22 ml of CSF was collected using sterile polypropylene collection tubes. Samples underwent a light spin and aliquoted into polypropylene cryovials and stored at −80°C.
2.3. Blood collection
Plasma was collected in 10 ml K2 EDTA tubes (BD Vacutainer) with overnight fasting and refrigerated immediately (4°C) before transporting to a central site on ice for centrifugation (2000 g × 15 minutes at 4°C) separation into plasma and cellular components within 4 hours of collection. Plasma aliquots (0.5 ml) were prepared, bar‐coded, and then stored in polypropylene vials at −80°C until analysis. Quality control samples to determine coefficients of variation (CV) included duplicate plasma samples from three control subjects analyzed at the same time as the remaining Penn subjects, and an average intra‐assay CV was obtained for each analyte of interest.
2.4. Biomarker quantification
Analytes were quantified using multiplex antibody‐based assays employing Luminex technology. TNF‐α and IL10 were quantified as part of a 9‐plex Human Cytokine/Chemokine Magnetic Bead Panel (Millipore Sigma, HCYTOMAG‐60K), as per manufacturer's instructions. CRP and ICAM‐1 were quantified using Human Cardiovascular Disease (Acute Phase) Magnetic Bead Panel 3—Cardiovascular Disease Multiplex Assay (Millipore Sigma, HCVD3MAG‐67K) Human Neurodegenerative Disease Magnetic Bead Panel 3—Neuroscience Multiplex Assay (Millipore Sigma, HNDG3MAG‐36K), respectively, according to manufacturer's instructions. Samples were assayed by experienced laboratory technicians. Quality control samples to determine CV included duplicate plasma samples from three control subjects analyzed at the same time as the remaining participants, and an average intra‐assay CV was obtained for each analyte of interest. Intra‐assay coefficients of variation were below 10% for all analytes. Cytokine data were log transformed in order to reduce skewness from the original data.
2.5. Neuropsychological testing
Neuropsychological testing lasted 1 hour and included tasks selectively chosen to be used in a cognitively normal but high‐risk sample. The battery assessed cognitive domains of memory, executive function, visuospatial ability, language, and included the Montreal Cognitive Assessment (MoCA), 19 Trail making test B, 20 Forwards and Backwards digit span, 21 Mental Rotation Test, 22 Benson complex figure recall, 23 Buschke memory test, 24 and the Multilingual Naming Test (MINT). 25
Participants completed a sleep questionnaire self‐reporting hour of sleep (sleep duration), daytime sleepiness, and sleep quality. Sleep quality rates were measured on a scale of 1–5: 1, excellent, 5, poor. Sleep Adequacy, Somnolence, Sleep Problems Index, and Somnolence Problems Index scores were calculated as a sum of contributing questions adapted from similar work. 28
2.6. Statistical methods
Preliminary analysis used summary statistics (mean [SD], median [range], or frequency [percent]) to describe the sample, including the sleep patterns. Biomarkers were log transformed to normalize values as commonly done in biomarker studies and standardized Z‐scores were computed for all cognitive tests. Abnormal levels of CSF biomarkers were defined as Aβ42/40 < 0.068 26 P‐tau > 51 ng/ml, and T‐tau > 100 ng/ml. 27 Differences in the sleep variables across racial groups were assessed using Independent t‐Tests or Fisher's Exact Test. Adequate sleep was encoded as yes if average hours of sleep fell within 15% of self‐reported optimal hours of sleep for a participant. Based on sleep surveys, Sleep Adequacy, Somnolence, Sleep Problems Index, and Somnolence Problems Index scores were calculated as a sum of contributing questions adapted from similar work by Ref. 28 (Table S1). Spearman rank correlation coefficients were computed to test for pairwise associations between the sleep variables and inflammatory markers. Among those significant in the bivariate analysis, we tested if the associations differed by race in a multivariable model. We were also interested in determining whether an interaction between inflammatory biomarkers and race impacts sleep. Analysis of variance was also used to test the interaction effect between inflammatory biomarkers and race on sleep variables after adjusting for age, sex, body mass index (BMI), presence of at least one Average p‐tau was normal at 34.18 ± 15.59, although average t‐tau was high at 276.24 ± 130.03; 1 AA (5.3%) and 7 white (15.9%) participants have p‐tau > 51, and 18 (94.7%) AA and 43 white (100%) participants had t‐tau > 100. APOE4 allele, sleep apnea, diabetes, high cholesterol, hypertension, and history of smoking. Similar analyses were performed to test for associations between standardized cognitive test scores and inflammatory biomarkers, with additional correction for education. All tests were two‐sided and a P ≤ .05 was considered statistically significant. All statistical analyses were performed in IBM SPSS 27.
3. RESULTS
3.1. Participant demographics
Seventy‐six participants were included in the study (Table 1). Average age of the participants was 58.86 ± 6.91 years, 63.3% were female and 35.4% AAs; 14.5% graduated high‐school/graduate equivalency degree (GED) while 38.2% and 47.4% had a college or post‐graduate degree, respectively. The average BMI was 27.30 ± 5.65 kg/m2. Total 18.4% reported sleep apnea, 2.6% diabetes, 59.2% high cholesterol, and 42.1% hypertension; as these elements may influence sleep, multivariable models corrected for these confounders. Total 47.4% of participants with genetics available had at least one APOE4 allele. Although, AD‐associated biomarkers were not available for all participants, for those participants with available biomarker data, AB42/40 ratio was on average normal (0.08 ± 0.02), with 78% of participants AB42/40 > 0.068 26 ; 3 AA participants and 14 white participants had abnormal AB42/40. For a detailed description of the sample characteristics please refer to Kumar et al. 13 Participants with missing demographic or sleep data were excluded, and missing at random was assumed for all analyses.
TABLE 1.
Participant demographics
| Overall | AA | White | |
|---|---|---|---|
| N = 76 | N = 28 | N = 48 | |
| Age a | 58.86 (6.91) | 59.75 (8.06) | 58.33 (6.17) |
| Sex (female) a | 50 (63.3%) | 24 (85.7) = %) | 26 (54.2) |
| Race (African American) a | 28 (35.4%) | 28 (100%) | 0 (0%) |
| BMI a | 27.30 (5.65) | 9.96 (6.17) | 25.75 (4.73) |
| Education b | |||
| High school/GED | 11 (14.5%) | 3 (6.3%) | 8 (16.7%) |
| College graduate | 29 (38.2%) | 11 (22.9%) | 18 (37.5%) |
| Post‐graduate | 36 (47.4%) | 14 (29.2%) | 22 (45.8%) |
| Income b | |||
| $19,000 or less | 2 (2.6%) | 2 (4.2%) | 0 (0.0%) |
| $20,000–39,000 | 10 (13.2%) | 5 (10.4%) | 5 (10.4%) |
| $40,000–59,000 | 11 (14.5%) | 9 (18.8%) | 2 (4.2%) |
| $60,000–79,000 | 13 (17.1%) | 5 (14.4%) | 8 (16.7%) |
| $80,000 or more | 40 (52.6%) | 7 (14.6%) | 33 (68.8%) |
| Physical activity (yes) b | 62 (81.6%) | 22 (78.6%) | 40 (83.3%) |
| History of smoking (yes) b | 21 (27.6%) (n = 74) | 8 (30.8%) | 13 (27.1%) |
| Sleep apnea (yes) b | 14 (18.4%) | 5 (17.9%) | 9 (18.8%) |
| Diabetes (yes) b | 2 (2.6%) | 2 (7.1%) | 0 (0.0%) |
| High cholesterol (yes) b | 45 (59.2%) | 17 (60.7%) | 28 (58.3%) |
| Hypertension (yes) b | 32 (42.1%) | 16 (57.1%) | 16 (33.3%) |
| APOE status (at least one E4 allele) b | 36 (47.4%) (n = 70) | 14 (53.8%) (n = 26) | 22 (50%) (n = 44) |
| Ab42/Ab40 a | 0.08 (0.02) (n = 63) | 0.08 (0.02) (n = 19) | 0.08 (0.02) (n = 44) |
| t‐tau a | 276.24 (130.03) (n = 62) | 205.95 (90.54) (n = 19) | 307.30 (133.49) (n = 43) |
| p‐tau a | 34.18 (15.59) (n = 63) | 27.87 (11.94) (n = 19) | 36.91 (16.29) (n = 44) |
| Plasma TNF‐α a | 7.64 (5.53) | 7.79 (3.95) | 7.56 (6.23) |
| Plasma IL‐10 a | 11.87 (8.35) | 11.63 (10.59) | 12.00 (7.04) |
| Plasma ICAM‐1 a | 561.24 (266.58) | 515.63 (111.56) | 585.04 (317.68) |
| Plasma CRP a | 7.86 (12.22) | 14.04 (18.85) | 4.64 (4.06) |
| CSF TNF‐α a | 1.18 (0.86) | 1.28 (0.99) | 1.13 (0.81) |
| CSF IL‐10 a | 5.71 (2.53) | 5.74 (3.17) | 5.69 (2.24) |
| CSF ICAM‐1 a | 300.97 (160.76) | 332.19 (177.61) | 287.49 (153.10) |
Note: Mean ± SD or count (percent) of all participants. BMI, body mass index values presented as amean (SD) or bn (percent). Cytokine values in pg/ml, n = 46 for white participants and 19 for AA participants.
3.2. African American participants have lower sleep duration
Participants completed a sleep questionnaire self‐reporting hours of sleep (sleep duration), daytime sleepiness, and sleep quality (Table 2). Participants reported a mean of 6.62 ± 1.28 and a median of 7 hours of sleep during the workday; on weekends, participants reported a mean 7.19 ± 1.31 and a median of 7 hours of sleep. 52.6% of participants reported more than 7 hours of sleep on average, although only 22.4% self‐reported adequate sleep. Self‐reported sleep quality was 2.82 ± 1.04. Based on sleep surveys, four subscores were calculated as a sum of contributing questions adapted from similar work by Ref. 28 (Table S1).
TABLE 2.
Sleep patterns by race
| Overall | AA | White | ||
|---|---|---|---|---|
| Sleep variables | N = 76 | N = 28 | N = 48 | P‐value |
| Workday hours of sleep c | 6.62 (1.28) | 5.82 (1.26) | 7.09 (1.04) | .00001 |
| Weekend hours of sleep c | 7.19 (1.31) | 6.52 (1.65) | 7.59 (0.86) | .003 |
| Average hours of sleep c | 6.79 (1.17) | 6.02 (1.18) | 7.23 (0.91) | .000004 |
| Average hours of sleep > = 7 hours (yes) d | 40 (52.6) | 5 (17.86) | 35 (72.92) | .003 |
| Sleep quality rate c | 2.82 (1.04) | 3.11 (1.17) | 2.65 (0.93) | .062 |
| Adequate Sleep (yes) d (n = 74) | 17 (22.4) | 12 (42.86) | 5 (10.87) b | .003 |
| Sleep adequacy a,c | 4.66 (1.72) | 4.64 (1.95) | 4.67 (1.60) | .954 |
| Somnolence a,c | 3.80 (1.14) | 3.46 (0.84) | 4.00 (1.26) | .048 |
| Sleep Problems Index a,c (n = 58) | 18.74 (5.45) | 17.72 (5.78) | 19.20 (5.31) | .344 |
| Somnolence Problems Index a,c (n = 69) | 11.58 (2.54) | 11.85 (2.76) | 11.40 (2.40) | .479 |
Note: Participants completed a detailed sleep questionnaire. Comparisons between groups using asymp. sig (two‐tailed) calculated with Independent T‐tests after testing for equal variance or Fisher's exact test. Bolded P‐values indicate significance. Sleep quality rates on a scale of 1‐5: 1, excellent; 5, poor. Adequate sleep considered as average hours of sleep within 15% of self‐reported optimal hours of sleep. aScoring adapted from Ref., 28 with greater values representing worse sleep. bn = 46; values presented as cmean (SD) or dn (percent).
Workday hours of sleep, weekend hours of sleep, and average sleep hours differed by race. AA participants had lower sleep duration compared to white participants, sleeping 5.82 ± 1.26 hours on workdays, compared to 7.09 ± 1.04 hours (P = .00001). AAs likewise slept fewer hours on weekends, sleeping 6.52 ± 1.31 hours compared to 7.59 ± 0.86 hours (P = .003). Additionally, AA participants slept an average of 6.02 ± 1.18 hours in comparison to an average sleep of 7.23 ± 0.91 hours for NHWs (P = .000004). Overall, only 17.86% of B/AAs reported at least 7 hours of sleep on average, compared to 72.92% of NHWs (P = .003). Despite reporting reduced hours of sleep compared to NHWs, AA reported greater adequate sleep when calculated as average hours of sleep within 15% of self‐reported optimal hours of sleep (P = .003). Additionally, AA participants reported reduced somnolence compared to NHWs (P = .048). Calculated scores for sleep adequacy, sleep problems index, somnolence problems index, and sleep quality rate, did not differ by race.
3.3. Plasma IL‐10 and CSF ICAM‐1 are associated with sleep duration and quality
To determine associations between sleep patterns and AD biomarkers, 29 , 30 , 31 partial correlation analysis was performed correcting for sleep apnea, history of smoking, diabetes, hypertension, and hypercholesterolemia (Table 3). Positive associations between workday hours of sleep with plasma IL‐10 (Spearman's ρ = 0.26, P = .04), sleep quality rate with CSF ICAM‐1 (Spearman's ρ = 0.30, P = .03), and presence of at least 7 hours of sleep on average with plasma IL‐10 (Spearman's ρ = 0.27, P = .03) were observed. Statistically significant negative associations were observed between sleep problems index and plasma CRP (Spearman's ρ = ‐0.32, P = .03).
TABLE 3.
Preliminary analysis of correlations between sleep patterns and biomarkers
| Plasma TNF‐α | Plasma IL‐10 | Plasma ICAM‐1 | Plasma CRP | CSF TNF‐α | CSF IL‐10 | CSF ICAM‐1 | |
|---|---|---|---|---|---|---|---|
| Workday hours of sleep | 0.19 (0.14) | 0.26 (0.04) | 0.07 (0.57) | −0.05 (0.72) | 0.09 (0.51) | 0.01 (0.97) | −0.17 (0.21) |
| Weekend hours of sleep | −0.07 (0.57) | −0.07 (0.59) | 0.09 (0.47) | −0.16 (0.22) | 0 (0.99) | 0.02 (0.86) | −0.07 (0.59) |
| Average hours of sleep | 0.12 (0.34) | 0.18 (0.16) | 0.09 (0.5) | −0.09 (0.5) | 0.07 (0.61) | 0.01 (0.93) | −0.16 (0.24) |
| Average hours of sleep > = 7 hours | 0.15 (0.24) | 0.27 (0.03) | 0.15 (0.24) | −0.09 (0.49) | 0.06 (0.65) | −0.13 (0.35) | −0.04 (0.77) |
| Sleep quality rate (scale of 1‐5: 1, excellent; 5, poor) | −0.19 (0.13) | −0.16 (0.22) | −0.07 (0.61) | −0.07 (0.61) | −0.09 (0.5) | 0.02 (0.9) | 0.3 (0.03) |
| Adequate sleep | −0.17 (0.18) | −0.14 (0.28) | −0.17 (0.18) | −0.09 (0.47) | 0.07 (0.61) | 0.05 (0.69) | 0.24 (0.07) |
| Sleep adequacy | −0.07 (0.59) | −0.06 (0.64) | −0.04 (0.74) | −0.01 (0.95) | 0.01 (0.93) | 0.02 (0.9) | 0.12 (0.39) |
| Somnolence | 0.12 (0.34) | 0.11 (0.39) | 0.07 (0.58) | −0.12 (0.37) | −0.1 (0.45) | −0.06 (0.65) | −0.02 (0.91) |
| Sleep Problems Index | −0.13 (0.38) | −0.09 (0.56) | −0.15 (0.3) | ‐0.32 (0.03) | 0.02 (0.88) | 0.05 (0.74) | 0 (0.98) |
| Somnolence Problems Index | 0.06 (0.66) | −0.16 (0.23) | 0.04 (0.76) | −0.14 (0.31) | 0.14 (0.32) | −0.11 (0.44) | 0.1 (0.49) |
Note: Pairwise correlations (P‐value) between plasma or CSF inflammatory biomarkers and participant sleep patterns were calculated for all participants, after correction for sleep apnea, history of smoking, diabetes, high blood pressure, and high cholesterol. Sleep quality rates on a scale of 1–5: 1, excellent; 5, poor. Correlations reported are Spearman rank correlation coefficients. Bolded P‐values indicate significance. For all blood analytes, n = 70; for all CSF analytes, n = 63. For sleep characteristics, n as in Table 2.
Correlations were further explored using regression analysis. After accounting for baseline covariates including age, sex, and BMI, we observed positive, although not statistically significant relationship between workday sleep duration and plasma IL‐10 (P = .059, Figure 1A); however, race does not moderate the relationship (P = .512). After covariate adjustment, the presence or absence of at least 7 hours of sleep was no longer associated with plasma IL‐10 (P = .267, Figure 1B); negative correlation between sleep problems index and plasma CRP similarly was not significant in the adjusted analysis (P = .298, Figure 1C). In contrast, a positive association between sleep quality and CSF ICAM‐1 (P = .037, Figure 1D) was maintained after correction. However, this positive association only applied to AA participants; the interaction of race and CSF ICAM‐1 was statistically significant in our model (β = ‐1.169, SE = 0.58, P = .049).
FIGURE 1.
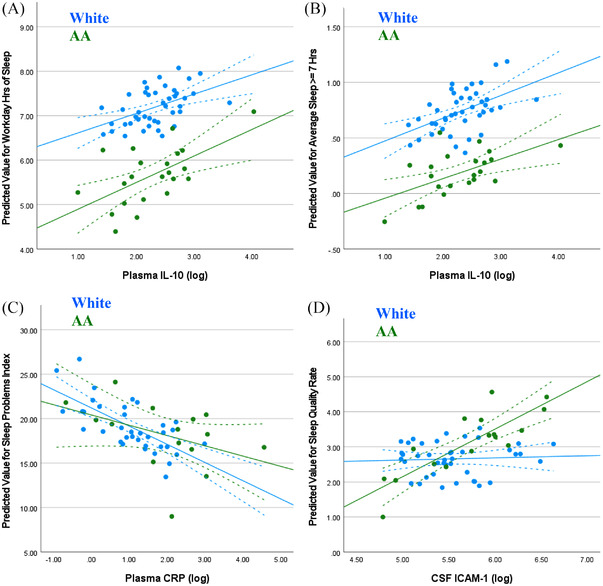
Association between predicted sleep metric and biomarkers varies by participant race. Regression analysis and analysis of variance models correcting for participant age, sex, BMI, presence of at least one APOE4 allele, and relevant medical history exploring. (A) Plasma IL‐10 versus predicted average sleep duration (in hours), (B) plasma IL‐10 versus predicted presence or absence at least 7 hours of sleep, (C) plasma CRP versus predicted sleep problems index, and (D) CSF ICAM‐1 versus predicted Sleep Quality Rate with trend lines ± 95% confidence interval.
For comparison, we also study associations of sleep with CSF AD biomarkers, specifically ratio of AB42/40, p‐tau, and t‐tau and observed no correlations between sleep parameters and AD biomarkers (data not shown).
3.4. Associations between cognition and biomarkers do not vary by race
To determine if weekday sleep duration influences cognitions score, participants were grouped by below or above median hours of weekday sleep, and cognition compared between groups. There were no differences in raw test scores among participants with below median (<7 hours) duration of sleep compared to those above median (≥7 hours).
To determine associations between cognitive test scores and biomarkers implicated in AD pathogenesis, 29 , 30 , 31 bivariate correlation analysis was performed with correction for education and presence or absence of at least one APOE4 allele (Table 4). Several plasma inflammatory biomarkers were correlated with standardized cognition test scores: Mental Rotation Test scores were negatively associated with plasma TNF‐α (Spearman's ρ = ‐0.4, P = .001), plasma IL‐10 (Spearman's ρ = ‐0.25, P = .047), and plasma ICAM‐1 (Spearman's ρ = ‐0.25, P = .047); Buschke Delay scores were positively associated with plasma IL‐10 (Spearman's ρ = 0.35, P = .006), respectively.
TABLE 4.
Preliminary analysis of correlations between cognitive testing and biomarkers
| PlasmaTNF‐α | PlasmaIL‐10 | PlasmaICAM‐1 | PlasmaCRP | CSFTNF‐α | CSFIL‐10 | CSFICAM‐1 | |
|---|---|---|---|---|---|---|---|
| MoCA | −0.07 (0.58) | 0.13 (0.32) | 0.02 (0.88) | −0.18 (0.16) | −0.05 (0.69) | 0.06 (0.68) | 0.03 (0.81) |
| Trails B | 0.11 (0.38) | −0.11 (0.36) | 0.17 (0.18) | −0.01 (0.91) | 0.18 (0.18) | −0.01 (0.97) | 0.11 (0.39) |
| Fwd Digit Span | −0.13 (0.29) | 0.07 (0.59) | −0.16 (0.21) | −0.2 (0.11) | −0.06 (0.66) | 0.14 (0.31) | −0.14 (0.28) |
| Bwd Digit Span | −0.04 (0.73) | 0.08 (0.5) | 0.06 (0.64) | −0.04 (0.74) | 0.03 (0.82) | 0.02 (0.9) | −0.09 (0.49) |
| Mental Rotation | ‐0.4 (0.001) | ‐0.25 (0.047) | ‐0.25 (0.047) | −0.07 (0.61) | −0.05 (0.7) | −0.09 (0.49) | −0.04 (0.76) |
| Benson Delay | −0.14 (0.28) | 0.03 (0.8) | 0.07 (0.59) | 0.06 (0.64) | −0.22 (0.11) | −0.1 (0.44) | −0.01 (0.95) |
| Buschke Delay | 0.22 (0.07) | 0.35 (0.005) | −0.04 (0.73) | −0.12 (0.34) | −0.19 (0.15) | −0.19 (0.15) | −0.1 (0.45) |
| MINT | 0.11 (0.37) | 0.21 (0.09) | 0.02 (0.86) | −0.07 (0.57) | −0.17 (0.21) | −0.18 (0.17) | 0.09 (0.51) |
Note: Pairwise correlations (P‐value) between plasma or CSF inflammatory biomarkers and participant Z‐score transformed cognition were calculated for all participants with available cognitive data (n = 63), corrected for presence or absence of at least one APOE4 allele and education. Correlations reported are Spearman rank correlation coefficients. Bolded P‐values indicate significance. For all blood analytes, n = 70; for all CSF analytes, n = 63. For tests of cognition, n as in Table S2.
Regression with correction for additional covariates was used to further explore these relationships, and to determine if they differed by race. Mental Rotation Test scores remained significantly associated with plasma TNF‐ α (P=.017) and IL‐10 (P=.045, Figure 2A and B), but race did not moderate the relationship (P = .645, P = .553, respectively). Similarly, the associations between Mental Rotation Test scores and plasma ICAM‐1 (P = .141) and Buschke Delay scores with plasma IL‐10 (P = .114, Figure 2C and D)), were not statistically significant in the covariate adjusted analyses.
FIGURE 2.
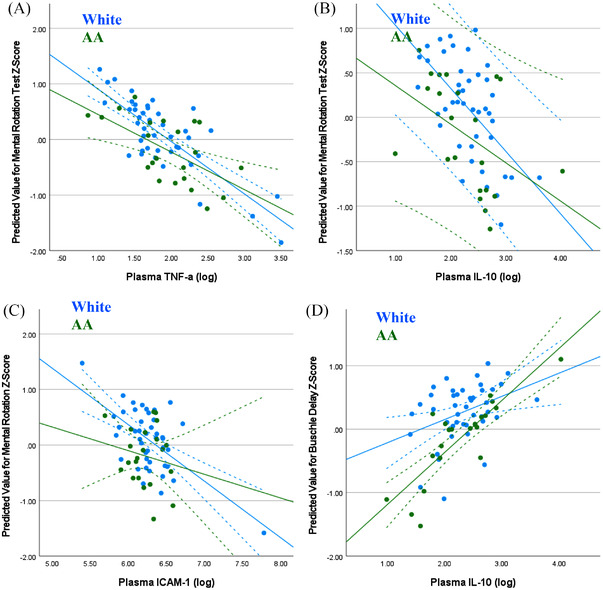
Associations between cognition and biomarkers do not vary by participant race. Regression analysis and analysis of variance models correcting for participant age, sex, BMI, presence of at least one APOE4 allele, education, and relevant medical history exploring. (A) TNF‐α versus mental rotation, (B) plasma IL‐10 versus Mental Rotation, (C) plasma ICAM‐1 versus Mental Rotation; and (D) plasma IL‐10 versus Buschke Delay rate with trend lines ± 95% confidence interval.
4. DISCUSSION
We show that sleep duration (hours per night on workdays) was significantly lower for AAs versus NHW participants. We found positive associations between plasma IL‐10 and sleep duration, and CSF ICAM‐1 and sleep quality. Furthermore, the relationship between CSF ICAM‐1 and sleep quality differed by race in a multivariable model. Cognition measures—Forward Digit Span, Mental Rotation, and Buschke Delay—were significantly associated with plasma biomarkers CRP, TNF‐α, and IL‐10; none of these associations differed by race in the covariate adjusted models.
Longer sleep duration is positively associated with plasma IL‐10 levels irrespective of race. In contrast, sleep quality was positively associated with CSF ICAM‐1 only in AAs. Mental rotation scores were inversely associated with plasma IL‐10 and TNF‐α; these relationships were not modified by race. Some biomarker relationships demonstrated race‐based patterns as we have previously shown. 32 , 33 For comparison, we also study associations of sleep with less studied CSF biomarkers implicated in AD, and observed no correlations contrary to previous studies; however, it is noteworthy that our study examines habitual sleep in a cohort at risk for AD, while most studies evaluate biomarkers after acute sleep disturbance or in AD cohorts. 34 Taken together with previous studies examining traditional and nontraditional AD biomarkers, these findings support the interaction of inflammation, sleep, and increased risk of cognitive decline.
Most studies focus on evaluating Aβ and tau, central hallmarks of AD, in sleep related studies; however, less is known about alternative AD biomarkers including plasma and CSF markers of inflammation. In contrast to the extensive investigation of mechanisms underlying cognitive symptoms and memory loss, few studies have addressed molecular mechanisms underlying non‐cognitive symptoms of AD, despite potential to inform on pathogenesis and potential treatments. Moreover, no studies have investigated associations of self‐reported sleep with cytokine mediators implicated in AD pathogenesis in diverse cohorts at risk for AD by virtue of family history. Here, we observe average sleep duration was significantly lower for B/AA versus NHW participants (average[SD]) in hours: 6.02[1.18] vs. 7.23[0.91], P = .000004) which is consistent with current literature, 14 , 15 and may represent a biological viable mechanism (i.e., reduced sleep in B/AA vs. White) for the increased incidence and prevalence of AD in B/AAs. 12
We found a significant association between plasma IL‐10 and weekday sleep duration (Spearman's ρ = 0.26, P = .04). Interestingly, the literature on IL‐10 and sleep parameters in general is discordant; reduced levels of serum IL‐10 have been found to be associated with poor sleep quality, 35 studies evaluating venous blood for IL‐10 have also found no association with sleep duration. 36 Furthermore, CSF IL‐10 appears increased in person's with AD, 7 and therefore our finding of increased IL‐10 with increased sleep duration may be confounded by early, preclinical alterations in cytokines.
Next, we identified that CSF ICAM‐1 is positively associated with sleep quality (Spearman's ρ = 0.30, P = .03), although only among B/AAs. Short sleep duration or experiencing poor sleep is associated with higher plasma ICAM‐1 levels 37 ; thus, our results appear consistent with the existing literature suggesting increased ICAM‐1 is associated with poorer sleep. Indeed, plasma ICAM‐1 has been proposed as the mediator between poor sleep and higher cardiovascular risk. 38 As AAs are at higher risk for hypertension, 39 which is an independent risk factor for AD, 40 ICAM‐1 may serve as a race‐specific marker for multimodal AD risk, which is worth exploring further in future studies.
Cognition measures—Mental Rotation and Buschke Delay—were significantly associated with plasma biomarkers CRP, TNF‐α, and IL‐10, and none of these associations differed by race in the covariate adjusted models. There is a lack of literature directly comparing levels of these inflammatory plasma biomarkers and specific cognition measures such as Mental Rotation and Buschke Delay in order to compare our work. However, previous work has demonstrated that inflammatory biomarkers are associated with other cognitive changes (such as recall pattern) and decline. 3 , 4 Although, data on changes in inflammatory cytokines such as IL‐6 and TNF‐α as MCI progresses to AD are inconsistent, concentrations of pro‐inflammatory cytokines in plasma and CSF increase as AD progresses. 7 High TNF‐α can increase the rate of cognitive decline. 8 Furthermore, inflammatory cytokines may be impacted by sleep, as meta‐analyses substantiate short sleep duration influencing CRP levels. 41
4.1. Study strengths and novelties
The novelties of this study include evaluating nontraditional cytokine biomarkers potentially associated with sleep disturbance and/or AD pathogenesis and inclusion of gender‐balanced, ethnically diverse, cognitively normal patients at risk for AD by virtue of family history. All the participants underwent a comprehensive protocol evaluating global cognition, memory function, and CSF AD biomarkers, as well as a nonintrusive questionnaire evaluating sleep. Furthermore, despite the evidence that sex, age, and other demographic factor may influence individual response to sleep deprivation, 9 most studies of inflammatory changes in response to sleep deprivation have been conducted in men. 42 Studies evaluating sex differences demonstrate differential responses, with women more responsive to inflammatory or AD biomarker changes following sleep deprivation. 43 Some studies suggest that race may impact increases in inflammation due to short sleep duration, with AAs demonstrating increased inflammation. 44 Our study is the first to link sleep duration and cognitive changes in the context of racial differences in persons with a parental history of AD. Our strengths include a well characterized, middle age cohort at risk for AD by virtue of parental history, inclusion of multiple blood and CSF biomarkers, and detailed data on various confounders.
4.2. Study limitations
Results are based on a small sample size, and focus on cross‐sectionally measured biomarkers implicated in sleep disturbances, inflammation, and AD. It is important to note that inflammatory markers are not specific for AD and there are individual differences in basal levels of inflammation associated with a range of factors, even in healthy individuals. However, we performed fasting blood draws in the morning, controlled for relevant confounders, and excluded individuals with inflammatory diseases or taking immune‐modulating agents.
Importantly, we measured sleep with self‐reported questionnaires; simple questionnaires such as that used in this study represent an easily integratable data point in clinical care, without the need for additional technologies. Thus, correlating to more objective measures of sleep (actigraphy, polysomnography) will be important in future studies.
The analysis of a single time‐point (vs. longitudinal) at middle‐age may subject our study to reverse causation bias due to the long preclinical period associated with AD, 45 such that our analytes have already been affected by the disease process prior to cognitive changes.
4.3. Future directions
Taken together with previous findings, these results add to the growing body of evidence linking sleep quality, AD neuropathology, and inflammation. Given the long onset period for AD and the accumulating evidence that disrupted sleep has a bidirectional relationship with AD pathology, longitudinal studies are warranted to establish how chronic sleep alterations or sleep alterations early in AD increase the risk or progression of dementia, to identify biomarkers associated with altered sleep patterns, and to evaluate the impact of improved sleep or sleep‐based therapies. While we were able to note differences in race between the groups, future studies should also consider “cultural” differences which may help to explain the difference between groups.
Application of progression‐related biomarkers is especially pertinent during the prodromal phase of AD, whether significant therapeutic intervention may still be possible. Indeed, as subtle changes to neuronal connectivity, metabolism, and inflammation may represent early disruptions of neuronal functional prior to accumulation of Aβ and tau, and AD pathology develops years prior to initial clinical symptoms, 46 identification and validation of early diagnostic markers beyond Aβ and tau are of paramount importance. No single biomarker in blood, CSF, imaging, or cognition is capable of predicting AD onset and course, highlighting the importance of a combination of multimodal biomarkers for reliable prediction. 47 , 48 Particularly needed are biomarkers that inform disease risk and progression but do not require the costly and invasive methods of CSF analysis, PET imaging, or genetics, the uses of which are generally limited in routine clinical diagnosis.
As sleep, cognition and CSF AD biomarker levels appear to be mutually related throughout the stages of AD, 49 sleep is a potential therapeutic target for disease‐modifying strategies. Furthermore, the temporal and anatomical relationship between disturbed sleep and AD pathogenesis indicate that improving sleep may slow disease progression. 50 Unlike other pathological consequences of AD including brain atrophy, sleep is a modifiable factor and thus a treatable target. Sleep restoration may minimize cognitive decline through two non‐mutually exclusive mechanisms: (1) increased clearance of Aβ and/or (2) enhancing long‐term memory consolidation. However, it is important to know that it is not currently clear whether local sleep deficits are a biomarker of AD progression, or active contributed to AD pathophysiology; therefore, the utility of sleep interventions remains unknown.
4.4. Conclusion
Detecting pathological components of AD, such as inflammation or sleep disruption, and translating these to CSF or blood‐based biomarkers may correlate with progression of AD, or identify patients who will develop AD pathology. An improved understanding of sleep disturbance and biomarkers of inflammation will aid in identifying targets for prevention. Furthermore, understanding how factors including race and sex modify the impact of risk factors associated with cognitive decline may yield new insights into biological mechanisms and inform targeted therapies.
CONFLICTS OF INTEREST
All authors report having no COI. Author disclosures are available in the supporting information.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the manuscript.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
This project was supported by the National Institute on Aging (K01AG042498 and 1RF1AG051514‐ 01), and the Emory Alzheimer's Disease Research Center (NIH‐NIA 5 P50 AG025688). We thank the ASCEND research participants for their willingness to devote their time to research, and the staff members who work tirelessly to make the research possible.
Pak VM, Paul S, Swieboda D, Balthazar MS, Wharton W. Sleep duration and biomarkers of inflammation in African American and white participants with a parental history of Alzheimer's disease. Alzheimer's Dement. 2022;8:e12332. 10.1002/trc2.12332
REFERENCES
- 1. Ballard C, Gauthier S, Corbett A, et al. Alzheimer's disease. Lancet. 2011;377(9770):1019‐1031. [DOI] [PubMed] [Google Scholar]
- 2. Ingiosi AM, Opp MR, Krueger JM. Sleep and immune function: glial contributions and consequences of aging. Curr Opin Neurobiol. 2013;23(5):806‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein FC, Zhao L, Steenland K, Levey AI. Inflammation and cognitive functioning in African Americans and Caucasians. Int J Geriatr Psychiatry. 2015;30(9):934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jefferson AL, Massaro JM, Beiser AS et al. Inflammatory markers and neuropsychological functioning: the Framingham Heart Study. Neuroepidemiology. 2011;37(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358‐372. [DOI] [PubMed] [Google Scholar]
- 6. Krstic D, Madhusudan A, Doehner J, et al. Systemic immune challenges trigger and drive Alzheimer‐like neuropathology in mice. J Neuroinflammation. 2012;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer's disease: a comparative overview. Mol Neurobiol. 2014;50(2):534‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004. 89(5):2119‐2126. [DOI] [PubMed] [Google Scholar]
- 10. Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol. 2019;18(3):296‐306. [DOI] [PubMed] [Google Scholar]
- 11. Taheri S, Austin D, Lin L, et al. Correlates of serum C‐reactive protein (CRP) ‐ No association with sleep duration or sleep disordered breathing. Sleep. 2007;30(8):991‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steenland K, Goldstein FC, Levey A, Wharton W. A meta‐analysis of Alzheimer's disease incidence and prevalence comparing African‐Americans and Caucasians. J Alzheimers Dis. 2016;50(1):71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar VV, Huang H, Zhao L, et al. Baseline results: the association between cardiovascular risk and preclinical Alzheimer's disease pathology (ASCEND) study. J Alzheimers Dis. 2020;75(1):109‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the multi‐ethnic study of atherosclerosis (MESA). Sleep. 2015;38(6):877‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep, 2014;37(3):601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khera A, Mcguire DK, Murphy SA, et al. Race and gender differences in C‐reactive protein levels. J Am Coll Cardiol. 2005;46(3):464‐469. [DOI] [PubMed] [Google Scholar]
- 17. Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation‐study of the dementia questionnaire. Arch Neurol. 1994;51(9):901‐906. [DOI] [PubMed] [Google Scholar]
- 18. Biospecimen best practice guidelines for the Alzheimer's disease centers. Tatiana Foroud TJM, ed. National Institute of Aging; 2014. [Google Scholar]
- 19. Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 20. Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nat Protoc. 2006;1(5):2277‐2281. [DOI] [PubMed] [Google Scholar]
- 21. Wechsler D. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS‐IV) [Database record]. APA PsycTests. 2008; 10.1037/t15169-000 [DOI] [Google Scholar]
- 22. Vandenberg SG, Kuse AR. Mental rotations, a group test of 3‐dimensional spatial visualization. Percept Mot Skills. 1978;47(2):599‐604. [DOI] [PubMed] [Google Scholar]
- 23. Rey A. Psychological examination in cases of traumatic encephalopathy. Archives De Psychologie. 1941;28(112):286‐340. [Google Scholar]
- 24. Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav. 1973;12(5):543‐550. [Google Scholar]
- 25. Ivanova I, Salmon DP, Gollan TH. The multilingual naming test in Alzheimer's Disease: clues to the origin of naming impairments. J Int Neuropsychol Soc. 2013;19(3):272‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldeiras I, Santana I, Leitão MJ, et al. Addition of the Aβ42/40 ratio to the cerebrospinal fluid biomarker profile increases the predictive value for underlying Alzheimer's disease dementia in mild cognitive impairment. Alzheimers Res Ther. 2018;10(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hertze J, Minthon L, Zetterberg H, et al. Evaluation of CSF biomarkers as predictors of Alzheimer's disease: a clinical follow‐up study of 4.7 years. J Alzheimers Dis. 2010;21(4):1119‐1128. [DOI] [PubMed] [Google Scholar]
- 28. Sprecher KE, Koscik RL, Carlsson CM, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology. 2017;89(5):445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen X‐N, Niu L‐D, Wang Y‐J, et al. Inflammatory markers in Alzheimer's disease and mild cognitive impairment: a meta‐analysis and systematic review of 170 studies. J Neurol Neurosurg Psychiatry. 2019;90(5):590‐598. [DOI] [PubMed] [Google Scholar]
- 30. Park J‐C, Han S‐H, Mook‐Jung I. Peripheral inflammatory biomarkers in Alzheimer's disease: a brief review. Bmb Reports. 2020;53(1):10‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zuena AR, Casolini P, Lattanzi R, Maftei D. Chemokines in Alzheimer's disease: new insights into prokineticins, chemokine‐like proteins. Front Pharmacol. 2019;10:622. 10.3389/fphar.2019.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wharton W, Kollhoff AL, Gangishetti U, et al. Interleukin 9 alterations linked to Alzheimer disease in African Americans. Ann Neurol. 2019;86(3):407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howell JC, Watts KD, Parker MW, et al. Race modifies the relationship between cognition and Alzheimer's disease cerebrospinal fluid biomarkers. Alzheimers Res Ther. 2017;9(1):88. 10.1186/s13195-017-0315-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mander BA. Local sleep and Alzheimer's disease pathophysiology. Front Neurosci. 2020;14:525970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taraz M, Khatami MR, Hajiseyedjavadi M, et al. Association between antiinflammatory cytokine, IL‐10, and sleep quality in patients on maintenance hemodialysis. Hemodial Int. 2013;17(3):382‐390. [DOI] [PubMed] [Google Scholar]
- 36. Patel SR, Zhu X, Storfer‐Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng LL, Tang Y‐Z, Ni C‐L, et al. Impact of inflammatory markers on the relationship between sleep quality and incident cardiovascular events in type 2 diabetes. J Diabetes Complications. 2015;29(7):882‐886. [DOI] [PubMed] [Google Scholar]
- 38. Hurtado‐Alvarado G, Domínguez‐Salazar E, Pavon L, Velázquez‐Moctezuma J, Gómez‐González B. Blood‐brain barrier disruption induced by chronic sleep loss: low‐grade inflammation may be the link. J Immunol Res. 2016;2016:4576012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393‐e423. [DOI] [PubMed] [Google Scholar]
- 40. Kehoe PG. The coming of age of the angiotensin hypothesis in Alzheimer's disease: progress toward disease prevention and treatment? J Alzheimers Dis. 2018;62(3):1443‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta‐analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sauvet F, Drogou C, Bougard C, et al. Vascular response to 1 week of sleep restriction in healthy subjects. A metabolic response? Int J Cardiol. 2015;190:246‐255. [DOI] [PubMed] [Google Scholar]
- 43. Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. Gender differences in the prospective associations of self‐reported sleep quality with biomarkers of systemic inflammation and coagulation: findings from the Heart and Soul Study. J Psychiatr Res. 2013;47(9):1228‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grandner MA, Buxton OM, Jackson N, et al. Extreme sleep durations and increased C‐reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36(5):769‐779e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jack CR, Jr. , Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jack CR, Jr. , Bennett DA, Blennow K, et al. NIA‐AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pedrero‐Prieto CM, García‐Carpintero S, Frontiñán‐Rubio J, et al. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer's disease. Clin Proteomics. 2020;17(1):21. 10.1186/s12014-020-09276-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mila‐Aloma M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer's continuum. Alzheimers Dement. 2020;16(10):1358‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liguori C, Placidi F, Izzi F, et al. Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer's disease course. Alzheimers Res Ther. 2020;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDade E, Bateman RJ. Stop Alzheimer's before it starts. Nature. 2017;547(7662):153‐155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


