Summary:
Current COVID-19 mRNA vaccines induce robust SARS-CoV-2-specific humoral and cellular responses in PLWH. However, the rate of decay of effector immune responses has not been studied in these individuals. Here we report a significant waning of antibody responses but persistent T cell responses six months post vaccination in virally suppressed PLWH with high CD4+ T cell counts. These responses are comparable to those seen in healthy donors.
Current COVID-19 vaccines induce robust humoral and cellular immune responses in healthy vaccine recipients (1) and people living with HIV (PLWH) (2–7). However, while vaccine induced antibody responses wane over time in healthy donors (HDs) (8), it is not known whether PLWH have similar rates of decay of immune responses. Greater declines in antibody titers might explain higher rates of breakthrough infections in PLWH observed in some studies (9) and could explain the increased risk of severe COVID-19 observed in PLWH (10). These studies highlight the importance of understanding vaccine induced immunity in PLWH compared to HDs. We recently showed that PLWH mount similar antibody and T cell responses following two doses of COVID-19 mRNA vaccinations (7). However, the rate of decay of immune responses in PLWH has not been studied. In this study, we analyzed humoral and cellular immunity at six months post vaccination in PLWH and HDs.
The study was approved by the Johns Hopkins Medicine Institutional Review Boards and informed consent was obtained from all study participants. Blood was drawn from 8 PLWH and 25 HDs two weeks and six months after the second dose of COVID-19 vaccinations. 24 HDs received the Pfizer-BioNTech (BNT162b2) vaccine and 1 received Moderna (mRNA-1273) vaccine, while all 8 PLWH received the BNT162b2) vaccine. Age of HDs ranged from 21–60 (7 were 21–30, 5 were 31–40, 5 were 41–50, and 8 were 51–60) and age of PLWH ranged from 41–60 (2 were 41–50, and 6 were 51–60). All PLWH were on suppressive antiretroviral therapy (ART) and had a median CD4+ T cell count of 1044 cells/uL (range of 468 to 1420 cells/uL). Two PLWH had low level viremia (49 and 52 HIV RNA copies /ml respectively), whereas the other six maintained undetectable viral loads (<20 HIV RNA copies /ml).
The quantity SARS-CoV-2 spike binding antibodies was determined using Euroimmun Anti-SARS-CoV-2 immunoglobulin G (IgG) ELISA (Mountain Lakes, New Jersey, USA, 11). PLWH had high levels of binding antibodies at two weeks post second dose that were comparable to peak responses shown in HDs as described in a prior study (7). PLWH had a significant decline in binding antibody responses that was nearly identical to the decline seen in HDs at the six-month time point (Figure 1A).
Figure 1: Antibody and T cell responses in PLWH and healthy controls at peak and six-months following COVID-19 mRNA vaccinations.
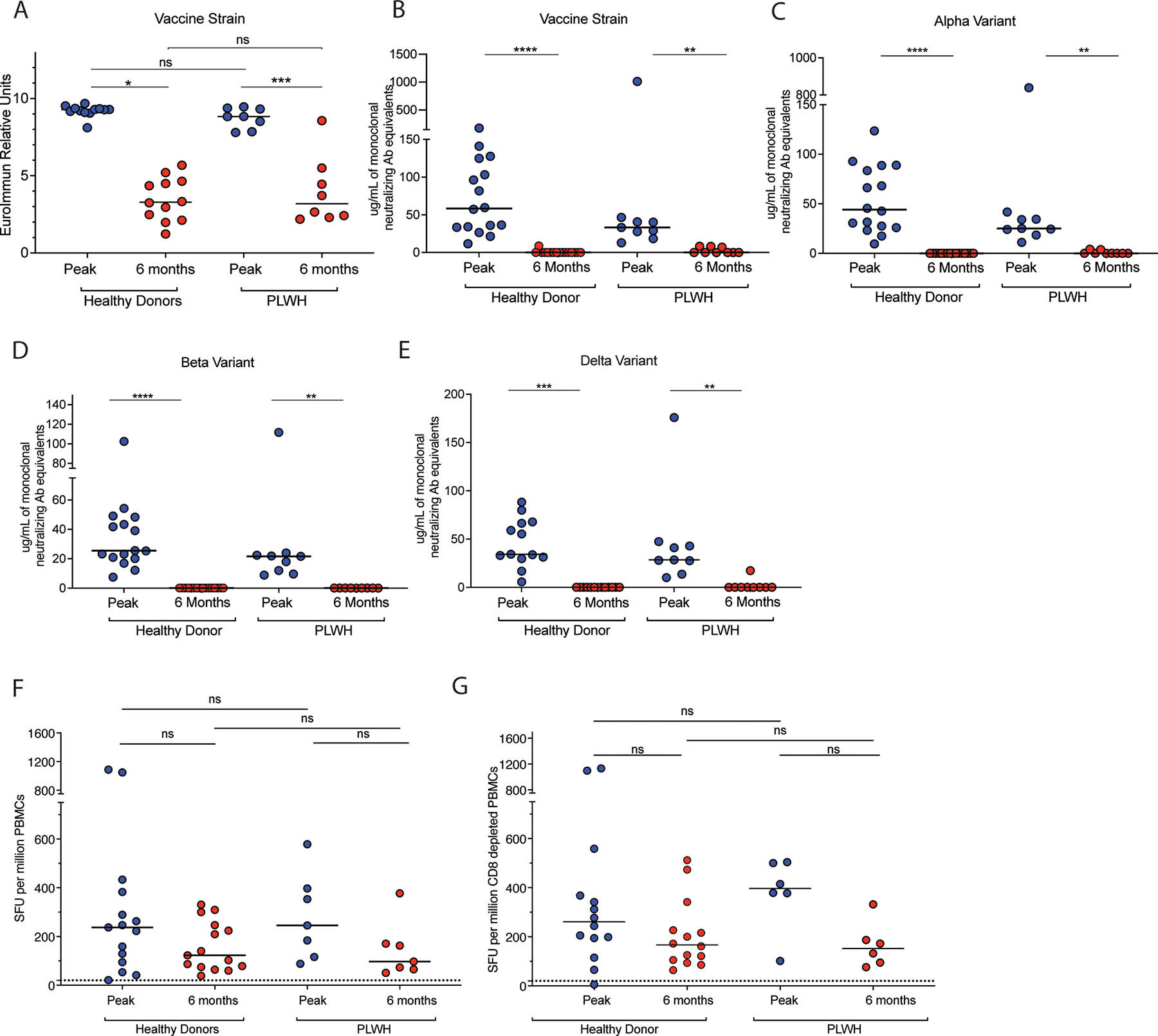
PLWH and HD spike binding antibody responses (A) and levels of antibodies that inhibit ACE2 binding to spike proteins of vaccine strain (B), Alpha (C), Beta (D) and Delta (E) viruses. IFN-y ELISpot responses to stimulation with spike protein peptide pools in unfractionated PBMCs (F) and CD8 depleted PBMCs (G) in PLWH and HDs. The dotted line in F and G represents the limit of detection of the assay. Statistical comparisons for T cell responses were done with Kruskal-Wallis test with Dunn’s multiple comparison or Friedman test with Dunn’s multiple comparison. Statistical comparisons for Antibody responses were done using Wilcoxon matched-pairs signed rank test or Mann-Whitney test. Abbreviations: HD, healthy donors; PLWH, people living with human immunodeficiency virus; PBMC, peripheral blood mononuclear cells; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot-forming units.
We next compared the level of antibodies in plasma that block ACE2 binding to SARS-CoV-2 spike proteins with the V-PLEX SARS-CoV-2 Panel 13 kit from Meso Scale Diagnostics (Rockville, Maryland, USA) (12). As we have previously reported, at the peak time point, PLWH had a slightly lower but comparable level of inhibiting antibodies to HDs (7). However, at the six-month time point, PLWH had a significantly lower level of ACE2 inhibiting antibodies to the vaccine strain and to the variants of concern (VOC) spike proteins (Figure B–E). The decline in anti-spike antibodies at six months was comparable in PLWH to the decline seen in HDs, for antibodies directed at the spike protein of the vaccine strain (Figure 1B) or at the Alpha (Figure 1C), the Beta (Figure 1D) and Delta (Figure 1E) VOC.
We next determined cellular immunity to the SARS-CoV-2 spike protein by performing an interferon-gamma (IFN-γ) ELIspot assay with unfractionated PBMCs that were stimulated with a pool of overlapping SARS-CoV-2 spike peptides (Figure 1F). We previously reported that PLWH had robust T cell responses at the peak time point comparable to responses in HDs, (7). Here we show a slight but insignificant decline of these responses at the six-month time point (Figure 1F). We then performed the assay using CD8 + T cell depleted PBMCs to determine the relative contribution of CD4 + and CD8 + T cells to the cellular immune response (Figure 1G). CD8+ T cell depletion did not decrease the magnitude of the response, suggesting that most of the T cell responses result from CD4+ T cell responses (Figure 1G). We again saw a non-significant decline in CD4+ T cell responses. Collectively, our data suggest that the rate of decay of immune responses in PLWH is similar to healthy donors. Specifically, although antibody responses decline at six months, especially to VOCs spike, T cell responses persist at the six-month time point in PLWH as in HDs (13).
In this study, we show that PLWH who are virally suppressed and have high CD4+ T cell counts have rates of decay of SARS-CoV-2-specific immune responses that are comparable to those of HDs post mRNA SARS-CoV-2 vaccinations. Though our data is limited by the relatively low number of patients in our cohort and further studies will be needed to determine the impact of lower CD4 T cell counts on the vaccine response, we demonstrate that virally suppressed PLWH with high CD4 counts generate robust humoral and cellular immune responses. Our results are consistent with a recent report that showed similar rates of decay of immune responses in PLWH and HD following ChAdOx1 nCoV-19 vaccinations (14).
Our data shows that similar to HDs, virally suppressed PLWH with high CD4+ T cell counts have persistent SARS-CoV-2 specific T cell responses at six months post-vaccination despite a modest decline from the peak levels measured shortly after vaccination. These residual memory T cell responses may partially explain protection from severe disease in breakthrough infections (15, 16). Our data show that similar to HDs, PLWH have a significant decline in SARS-CoV-2-specific antibody responses six months following vaccination. These results suggest that PLWH would benefit from an additional booster dose to increase plasma antibody concentrations that could protect against COVID-19. However, recent reports have shown that memory B cell responses persist six months after two doses of vaccination in HDs (15), potentially mitigating the effects of the dramatic decline of antibody responses and consistent with data showing that mRNA vaccines continue to protect against hospitalization six months following vaccination (16). If SARS-CoV-2 specific memory B cells also persist in PLWH, then a similar level of protection from severe disease driven by memory B cell and effector T cell responses may be seen six months following vaccination.
Acknowledgements:
This work was supported by the Johns Hopkins COVID-19 Vaccine-related Research Fund, the Johns Hopkins Center for AIDS Research, the National Cancer Institute (grant number U54CA260491), and the National Institute Allergy and Infectious Diseases (K08AI156021). Additional support was provided by the Division of Intramural Research, National Institute Allergy and Infectious Diseases.
References:
- 1.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586:594–9. [DOI] [PubMed] [Google Scholar]
- 2.Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. The Lancet HIV. 2021;8(9):e568–e580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clinical Microbiology and Infection. 2021;27(12):1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. The Lancet HIV. 2021;8(8):e474–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lombardi A, Butta GM, Donnici L, et al. Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. The Lancet Regional Health – Europe. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. eClinicalMedicine. 2021;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin Infect Dis. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. The Lancet HIV. 2021;8(1):e24–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coburn SB, Humes E, Lang R, et al. COVID-19 infections post-vaccination by HIV status in the United States. medRxiv. 2021:2021.12.02.21267182 [Google Scholar]
- 11.Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol 2021;59:e02257–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2021. Dec 24. doi: 10.1111/ajt.16933. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woldemeskel BA, Garliss CC, Blankson JN. mRNA Vaccine-Elicited Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Specific T Cells Persist at 6 Months and Recognize the Delta Variant. Clin Infect Dis. 2021:ciab915. [DOI] [PubMed] [Google Scholar]
- 14.Ogbe A, Pace M, Bittaye M, et al. Durability of ChAdOx1 nCov-19 vaccination in people living with HIV. JCI Insight. 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572):abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. The Lancet. 2021;398(10309):1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]


