Background
The incidence of thyroid cancer has risen dramatically in the U.S. over the past four decades, with similar patterns observed internationally. Thyroid cancer currently ranks as the 13th most common cancer diagnosis overall and the 6th most common among women. Since the greatest increases in incidence have been observed for small and localized tumors having the highest rate of survival, the incidence trends have been primarily attributed to overdiagnosis, resulting from more widespread use of diagnostic imaging and more sensitive diagnostic tools. Increasing incidence of large and advanced thyroid cancers, as well as thyroid cancer mortality, suggest that etiological factors may have contributed to rising incidence of the disease, albeit to a lesser extent than overdiagnosis. Until recently, childhood exposure to ionizing radiation was considered the only established modifiable risk factor for thyroid cancer. Obesity has emerged as another important risk factor, although the underlying biological mechanisms remain poorly understood. The potential influence of endocrine disrupting chemicals and thyroid dysfunction on thyroid cancer development has been a focus of recent etiologic studies. Important recent advances in identifying molecular subtypes of thyroid cancer and genetic susceptibility factors provide insights regarding the etiology of this disease.
Tumor classification
Of the major histologic types, about 90% are papillary thyroid carcinomas (PTC), 4% follicular thyroid carcinomas (FTC), 2% Hürthle-cell carcinomas, 2% medullary thyroid carcinomas (MTC), and 1% anaplastic thyroid carcinomas (ATC) (1). MTCs are neuroendocrine tumors arising from calcitonin-producing C-cells (parafollicular cells). Other rare thyroid cancers, with some exceptions (e.g. squamous cell, lymphoma, mesenchymal tumors), arise from follicular cells.
Thyroid cancers are also classified according to genetic drivers. The 2014 Cancer Genome Atlas Research Network landmark publication reported work that led to the identification of driver mutations in over 95% of nearly 500 PTCs (2). Subsequent work using a similar approach has identified Hürthle-cell thyroid cancers as a separate from PTCs and FTCs (3). PTCs can now be distinguished according to those with somatic mutations related to the BRAF mutations in the MAPK pathway (BRAF-like) and those related to RAS mutations (RAS-like). BRAFV600 mutation has been associated with disease recurrence and mortality in patients with PTC, particularly when co-occurring with TERT promotor mutations (4, 5). BRAF and RAS mutations appear to play an important role in the development of poorly differentiated and anaplastic thyroid cancers, with BRAFV600 being an early molecular event and TP53 mutations contributing to progression of PTCs to poorly differentiated and anaplastic types (5). TP53 mutations are present in about 26% of poorly differentiated cancers and 80% of ATCs, but rarely occur in PTCs (5). In general, the TERT promotor mutation and TP53 mutation, especially if co-occurring or co-occurring with other mutations, are strongly linked to higher degrees of dedifferentiation, disease aggressiveness, and poor outcomes (5). RET/PTC rearrangements are another distinct and common molecular event in the development of PTC; these occur more frequently in individuals exposed to radiation in childhood where they act through the MAPK pathway (6) (see Ionizing radiation, below). RET mutations are the most common somatic mutations in MTCs, following by H-RAS and K-RAS (5).
Descriptive Epidemiology
Incidence and Mortality
Thyroid cancer incidence varies substantially by geographic location (Fig. 1a), especially in women (7). In general, the highest incidence is observed in higher-income countries, including the Republic of Korea, Canada, Italy, France, Israel, Croatia, Austria, and the U.S., as well as some middle- to upper-middle-income countries, such as Turkey, Brazil, Costa Rica, and China (7, 8). Incidence is also high in some island nations and territories, including Cyprus, Cabo Verde, French Polynesia, New Caledonia, and Puerto Rico (7). This variation is thought to be mainly attributable to geographic differences in access to care and diagnostic practices, although environmental exposures also may play a role (9). Compared to incidence, thyroid cancer mortality rates tend to be much lower and vary much less geographically (Fig. 1b).
Figure 1a-b.
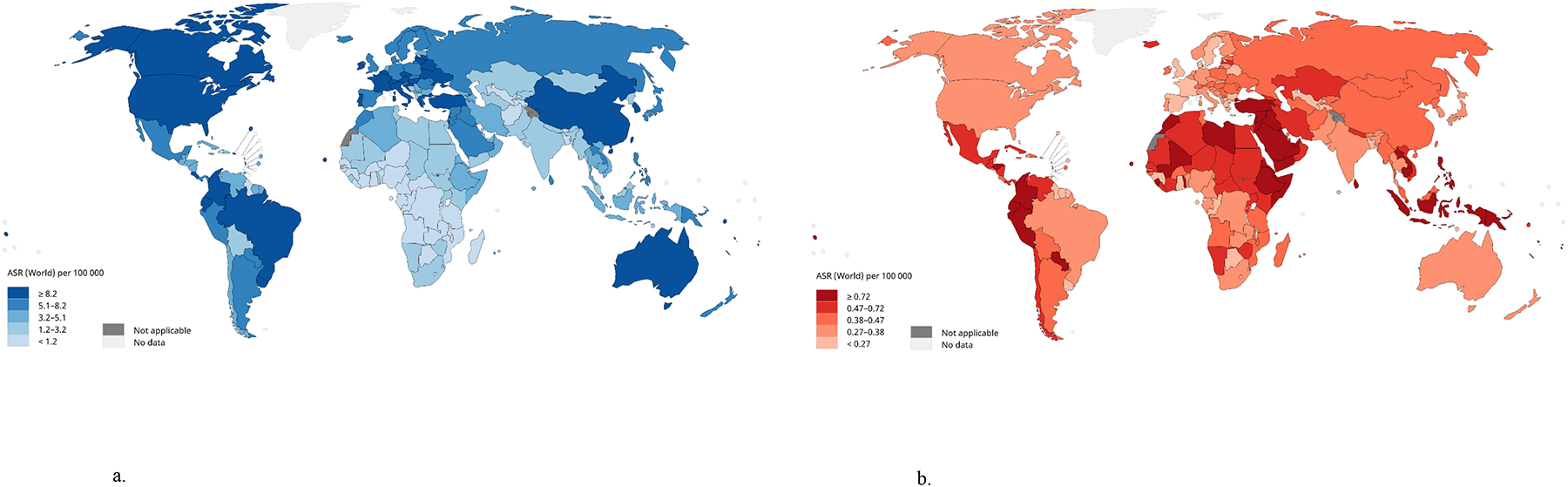
Estimated age-standardized rates (ASR) for thyroid cancer incidence (a) and thyroid cancer mortality (b) worldwide. Data source and graph production: GLOBOCAN 2020, International Agency for Research on Cancer, World Health Organization (7).
In the U.S., thyroid cancer is estimated to be the 13th most commonly diagnosed cancer, accounting for nearly 44,000 new cancer diagnoses in 2022 (2.3% of the total), and the 6th most commonly diagnosed cancer among women (10). Thyroid cancer incidence is approximately three-fold higher in women (22.8 per 100,000 per year in 2014–2018) than in men (8.0 per 100,000 per year) (Fig. 2a) (1). Thyroid cancer incidence increases from adolescence through middle age, peaking around 55 years in women and 65 years in men, and subsequently declining with older age (Fig. 2b) (11). Currently, one in 55 U.S. women and one in 149 U.S. men are expected to be diagnosed with thyroid cancer during their lifetime (10). Thyroid cancer mortality is very low relative to incidence (approximately 0.5 deaths per 100,000 per year) with less evidence of a sex disparity (7).
Figure 2a-b.
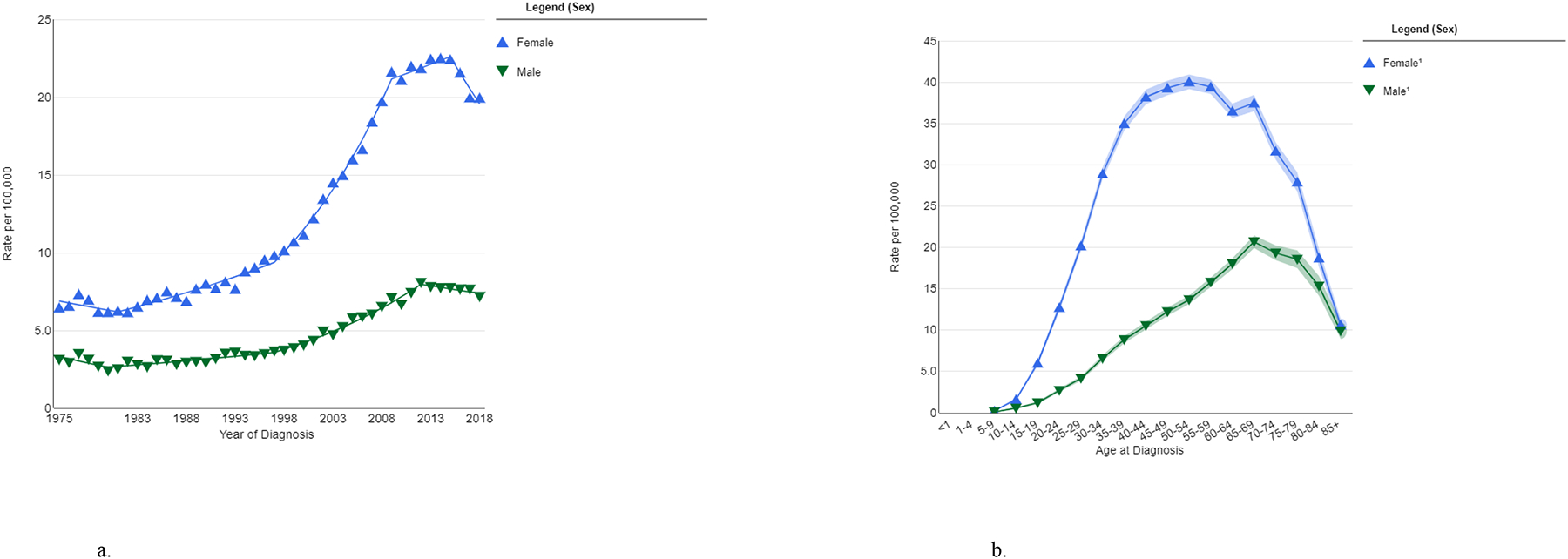
Thyroid cancer incidence in U.S. men and women, by calendar year at diagnosis (SEER-9, 1975–2018) (a) and age at diagnosis (SEER-21, 2014–2018) (b), (all races). Data source and graph production: SEER, Bethesda, MD (11). 1Estimates based on <16 cases are suppressed.
Survival
The prognosis for thyroid cancer is typically excellent, as most cases are PTCs and are localized to the thyroid gland at diagnosis (1). In the U.S., the 5-year relative survival is 98.6% overall, 99.9% for localized, 98.3% for regional, and 54.9% for distant metastatic disease (1). Relative survival rates are highest for PTC and FTC compared to other histologic types owing to the slow-growing nature of these tumors and effective therapies. Most are effectively managed with total or partial removal of the thyroid or, in some cases, even by prospective observation. In cases that are more advanced, surgery is usually followed by radioactive iodine for the destruction of any remaining thyroid cells or tissue. ATCs, poorly differentiated thyroid cancers, and some other uncommon variants are highly aggressive and less amenable to treatment, although individualized medications, kinase inhibitors and others, have been developed (12, 13). During 1974–2013, annually ATC accounted for only 1% of thyroid cancer diagnoses, but 30% of U.S. thyroid cancer deaths (14).
Trends in incidence and the role of overdiagnosis
From the early-1980s to mid-2010s the incidence of thyroid cancer in the U.S. nearly tripled, rising at a faster rate than any other cancer type, before stabilizing and then declining during the mid-to-late-2010s (Fig. 2a) (11, 15, 16). The upward trend was driven primarily by PTC, with the greatest rate of increase observed for small, early-stage PTCs (14). While very modest relative increases in thyroid cancer mortality have been observed during this time (rising, on average, ~1% per year), in absolute terms, mortality has remained very low and stable over time relative to incidence (14). The rising incidence coincided with the introduction and increasingly widespread use since the 1980s-90s of medical imaging techniques, including thyroid ultrasonography, and sensitive diagnostic tools (17, 18), resulting in the incidental detection and diagnosis of cancers that would previously not been detected. For these reasons, the rising incidence of thyroid cancer has been referred to as an “epidemic of overdiagnosis” (19, 20). Overdiagnosis is defined as the diagnosis of a condition that would not have caused harm to the individual over their lifetime if left undetected. Similar trends in incidence have been observed in nearly every region of the world, including some lower-resource countries, without clear corresponding increases in mortality (17). As an extreme example, thyroid cancer incidence increased 15-fold between 1993 and 2011 in the Republic of Korea following the launch of a national campaign to promote cancer screening, while thyroid cancer mortality remained stable (21). Although thyroid cancer incidence subsequently declined after 2014 following major efforts to reverse this trend (22), the country continues to have the highest incidence of thyroid cancer in the world (7).
Clinical practice guidelines around the management of thyroid cancer have been modified over the last 15 years, with the goal of reducing the potential for overdiagnosis and resulting overtreatment. For instance, biopsying small nodules is no longer recommended by the American Thyroid Association (ATA) (23, 24). In 2017, the ATA recommended reclassification of non-invasive encapsulated follicular variant subtype of PTCs (NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features), known for their indolent behavior, from a malignant to an in situ carcinoma (16, 25). These efforts appear to have contributed to the recent reduction in total PTC incidence, driven by declining incidence of small PTCs and the PTC follicular variant (16). On the other hand, the incidence of larger and advanced-stage PTCs continues to increase rapidly (16). Therefore, it is likely that diagnostic practices largely, but not fully, account for the changing trends in thyroid cancer incidence. This raises the question, discussed below, of whether and to what extent lifestyle and environmental risk factors may have played a role (14, 26–28).
Disparities
Thyroid cancer is susceptible to socioeconomic disparities in incidence because it is often detected during routine physical examinations or incidentally during work-up of other conditions. Greater access to and utilization of healthcare increases the likelihood of overdiagnosis and, thus, overdiagnosis and overtreatment. For example, in the U.S., PTC patients with private health insurance are more likely to be diagnosed with early-stage disease compared to uninsured patients or those with Medicare or Medicaid (29). They are also more likely to undergo extensive treatment (total thyroidectomy, lymphadenectomy, and/or radioactive iodine). In contrast, those without private insurance may be susceptible to delayed diagnosis and treatment, potentially leading to worse outcomes.
Sex disparities.
Questions have been raised about whether the higher female-male ratio in thyroid cancer incidence is real or artifactual (30). Autopsy studies have suggested a high prevalence (>10%) of subclinical PTC in the population, with no apparent changes in prevalence over time and no evidence of sex differences (30, 31). This contrasts with registry data showing a four-fold higher incidence of small (≤2 cm), localized PTC in women versus men and two-fold higher incidence of all other types of PTC (30). As mentioned above, no sex disparities are observed for thyroid cancer mortality. Women may have more opportunities for incidental detection of thyroid nodules in clinical settings, via palpation or imaging, and overdiagnosis may be more likely during the reproductive years and around menopause; this may account for the more exaggerated age-at-diagnosis curves in women than men over time (19) (Fig. 2b). On the other hand, the higher incidence in women than men has been observed for decades and consistently across nearly every region of the world, including regions less affected by overdiagnosis (7). In the U.S., a two-fold higher incidence of thyroid cancer in women than men was observed in the 1970s, prior to the introduction of thyroid ultrasonography and fine-needle aspiration biopsy (11). Finally, thyroid screening studies in Chernobyl and Fukushima area residents showed a slightly higher prevalence of thyroid nodules and cancer in females than males, with sex ratios of 1.4 to 1.6 (32–34). Thus, overdiagnosis appears to have inflated the observed sex disparity in thyroid cancer incidence beyond biological reasons for these differences.
Racial/ethnic disparities.
In the U.S., thyroid cancer incidence is highest in non-Hispanic whites and lowest in Blacks and Native American/Alaskan Natives (11) (Fig. 3a). Non-Hispanic whites also have a higher incidence of small, localized PTCs, suggesting more overdiagnosis, while no racial/ethnic differences are observed for large or advanced PTCs (35). Five-year relative survival is similar by race/ethnicity, although survival for metastatic thyroid cancer is slightly lower in Black patients versus other race/ethnic groups (Fig. 3b). A study using California Cancer Registry data showed that Black, Hispanic, and Asian/Pacific Islander patients received care at hospitals with lower quality evaluations for thyroid cancer treatment than white patients after controlling for socioeconomic and insurance status (36). Treatment of thyroid cancer in lower-quality hospitals was associated with worse overall and disease-specific survival (37). Substantial heterogeneity in incidence exists across Asian/Pacific Islander ethnic subgroups. Filipino-Americans have higher rates of PTC than non-Hispanic whites and other Asian/Pacific Islander groups and are more likely to be diagnosed with advanced disease (38), differences which persist after adjustment for sociodemographic factors (39). As higher incidence has been observed in first- versus subsequent-generation Filipinos and not other Asian/Pacific Islander groups (40), environmental exposures are considered to play a role (38) (see Other environmental exposures, below).
Figure 3a-b.
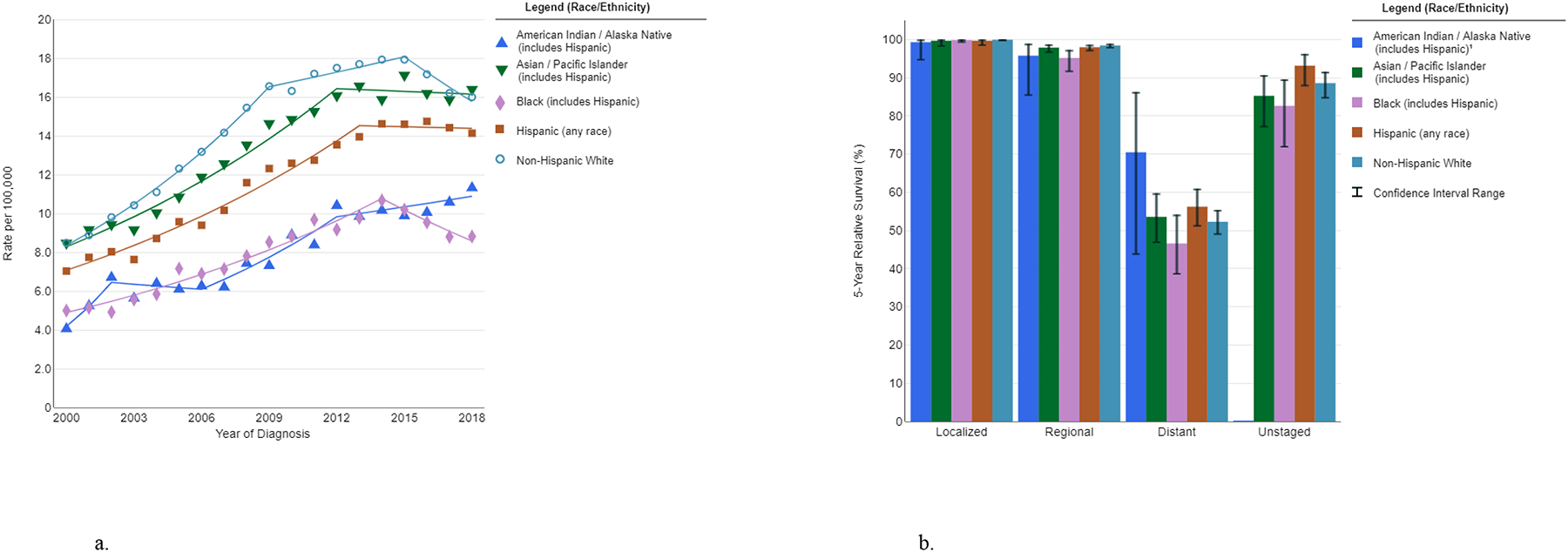
Race/ethnicity differences in thyroid cancer incidence trends (SEER-21, 2000–2018, delay-adjusted) (a) and five-year relative survival by stage at diagnosis (SEER-18, 2011–2017) (b). Data source and graph production: SEER, Bethesda, MD (11). 1Estimates based on <16 cases are suppressed.
Etiology
Identifying true etiologic (causal) risk factors has been a major challenge in thyroid cancer epidemiologic research. A high proportion of these tumors are indolent and detected incidentally, through thyroid screening, imaging for unrelated reasons, or diagnostic work-up of benign thyroid conditions. Thus, the term “risk factor” in the context of thyroid cancer may mean any characteristic or exposure that increases the likelihood of having a thyroid cancer diagnosis. Where possible, we use the term “causal risk factor” or “etiologic factor” to denote any factor that appears to fulfill most or all of the main criteria for causation: temporality, strength of association, consistency, biological gradient, specificity, biological plausibility, and coherence (41). Modifiable risk factors are those that can, in theory, be changed to increase or lower an individual’s risk of the outcome (thyroid cancer). Evidence regarding modifiable (or potentially modifiable) risk factors is summarized in Table 1.
Table 1.
Summary of epidemiologic evidence on risk factors for thyroid cancer
| Association direction | Evidence of consistency across studies and dose-response | Bias potential (epidemiologic studies) | |
|---|---|---|---|
| Ionizing radiation | ↑ Childhood exposure only | Consistent findings; evidence of dose-response | Some; exposure, if known, may be associated with thyroid screening |
| Benign thyroid conditions | ↑ Thyroid nodules ↑ Goiter ↑ Hyperthyroidism − Hypothyroidism |
Consistent findings; some evidence of dose-response for thyroid function parameters | Substantial; conditions likely associated with thyroid screening |
| Dietary factors | ↑ Iodine (papillary thyroid cancer) ↓ Iodine (follicular thyroid cancer) − Vegetables or fruits − Meat − Coffee or tea |
Inconsistent findings; no clear evidence of dose-response (except iodine and follicular thyroid cancer) | Moderate (iodine); confounding likely in ecological studies |
| Metabolic factors | ↑ Obesity/excess adiposity − Metabolic syndrome − Diabetes Type II − Hypertension − Dyslipidemia |
Consistent findings; evidence of dose-response (obesity/excess adiposity) | Some; conditions may be associated with thyroid screening |
| Reproductive factors/sex steroid hormones | − Parity, age at menarche, age at menopause, use of exogenous hormones ↑ Hysterectomy ↑ Infertility |
Inconsistent findings; no clear evidence of dose-response with hormonal exposures | Moderate; reproductive events or hormone use may be associated with thyroid screening |
| Perinatal and in utero exposures | ↑ Birth weight − Maternal or paternal age − Pregnancy characteristics and complications − Maternal smoking ↑ Maternal thyroid conditions ↑ Congenital hypothyroidism |
Consistent findings and evidence of dose-response (birth weight); evidence on maternal and congenital thyroid conditions based on only one study | Moderate; maternal/paternal characteristics and medical conditions may be associated with thyroid screening |
| Cigarette smoking and alcohol consumption | ↓ | Consistent findings; evidence of dose-response | Some; behavior may be associated with thyroid screening |
| Environmental contaminants | − Flame retardants − Pesticides − Volcanic environment/heavy metals |
Inconsistent findings; no clear evidence of dose-response | Some; exposure, if known, may be associated with thyroid screening |
Association with thyroid cancer risk, based on overall evidence: ↑ = positive; ↓ = inverse; − = null
Modifiable (or potentially modifiable) risk factors
Ionizing radiation
Ionizing radiation exposure in childhood is currently the most well-established modifiable risk factor for thyroid cancer. Based on astute clinical observation, Duffy and Fitzgerald were the first to draw attention to the relationship between radiation exposure and thyroid cancer (42). This was confirmed by a groundbreaking study in Rochester, NY which prospectively followed a cohort of people exposed to thymus-directed radiation treatments as children (43). The third landmark occurred in the 1995 publication of a pooled analysis of seven cohort studies showing that the association between external radiation exposure and thyroid cancer risk follows a linear dose-response pattern, and that the association is much stronger for individuals exposed as young children (44).
Perhaps the strongest evidence so far comes from the recent update and expansion of the pooled analysis (45–47). At one time, it was thought that very high radiation exposure to the thyroid, characteristic of cancer therapy, destroys thyroid tissue rendering malignant transformation of cells impossible. However, the updated pooled analysis showed that the risk of thyroid cancer continues to increase until very high doses before curving downward, but never returning to baseline (46). Another long-standing question relates to the existence of a “threshold” effect of radiation exposure on thyroid cancer risk, meaning a level of exposure below which the risk of thyroid cancer is zero. The pooled analysis focused on the dose-response relation at in the lowest dose range, finding that 40 mGy is associated with a statistically elevated relative risk, lower than the 100 mGy found in the earlier pooled analysis (47). The data also support a minimum latency period of 5–10 years; this is the minimum time after an exposure that its effects on an outcome may be clinically observable. Finally, it showed that the risk increases for 30–40 years before decreasing, although not to the baseline. The dose-response estimates from this study can be used as a benchmark for assessing the potential for bias in other epidemiologic studies of radiation-exposed populations (Table 1), particularly in the context of the estimated radiation exposure levels and age and time since exposure (48).
There has been long-standing debate about whether thyroid cancer risk is influenced by internal sources of radiation exposure (i.e., from radiopharmaceuticals, such as 131I targeting the thyroid gland) in the same way and to the same degree as with external sources of exposure. 131I and other iodine isotopes are used in the diagnosis and treatment of some thyroid disorders, such as hyperthyroidism. Data from the Chernobyl accident recently confirmed that the association of childhood exposure to 131I and thyroid cancer risk is linear and compatible with findings from populations exposed to external radiation, at least at low to moderate doses (49). However, other factors, especially iodine deficiency in the region of the Chernobyl accident, could affect this comparison. It remains unclear whether 131I treatment for hyperthyroidism influences thyroid cancer risk, although most patients are treated at older ages, and the very high treatment doses (~100 Gy) used in this therapy may have cell killing effects similar to that of radiotherapy for cancer (45, 50).
Whether ionizing radiation exposure may have contributed to population-level trends in thyroid cancer incidence is unclear. Although unique biomarkers of radiation exposure have not yet been identified (to determine with complete certainty that ionizing radiation was the cause of a specific PTC), RET/PTC chromosomal rearrangements are much more prevalent in radiation-exposed cases, and a recent large-scale integrated genomic landscape analysis of PTCs in individuals exposed in utero or in childhood following the Chernobyl accident showed that these carcinogenic events are linearly associated with radiation doses up to 1 Gy (51, 52). Moreover, the DNA damaging effects of radiation are more apparent for individuals exposed at younger age (52). However, single-institution studies have shown that the proportion of PTC cases with RET/PTC rearrangements appears to decline over time, while the proportion of PTCs with BRAF and RAS point mutations remain stable or increase (53, 54). These observations suggest that exposures capable of inducing BRAF or RAS mutations may have had greater influence on population-level trends in PTC incidence than those causing RET/PTC rearrangements. On the other hand, radiation exposure to the general population has increased in recent decades, especially in the U.S., owing to a dramatic increase in use of diagnostic imaging, particularly computed tomography (CT) (55). In 2016, CT scans constituted 63% of the total radiation exposure to the U.S. population from all medical sources (56). Between 5% and 15% of all CT scans are conducted in children, and a single head or neck CT scan in children can deliver a dose to the thyroid of about 10–20 mGy, although the dose can vary widely depending on the scan type, age of the individual, and other factors (57). Repeated CT scans are common, and each scan contributes to greater cumulative thyroid dose and, thereby, greater risk of thyroid cancer, although it remains uncertain whether the effects of repeated exposures are additive effects or sub-additive due to DNA repair mechanisms. From the pooled analysis, cumulative radiation doses between 50 and 100 mGy were estimated to increase thyroid cancer risk by 50–100% (46). Consistent with this, a large Australian cohort found that a single head CT scan before age 20 was associated with a 33–53% increased risk of thyroid cancer accounting for a one-year exposure lag (58). Thus, it is conceivable that the increased use of CT imaging in children has had at least a small influence on population-level trends in thyroid cancer incidence since the 1980s.
Benign thyroid disease
Thyroid cancer is often preceded by benign thyroid disease (e.g. thyroid nodules, goiter, hyperthyroidism, hypothyroidism, and thyroiditis), but it remains unclear whether benign thyroid diseases cause the development or progression of thyroid cancer. Diagnostic work-up of benign thyroid conditions may lead to the incidental detection of thyroid cancer. The impact of this bias may be reduced by excluding thyroid cancers occurring in the first few months or years after benign disease. Studies that have done this, including a large international pooled analysis of case-control studies (59), typically have shown elevated (albeit attenuated) thyroid cancer risks in relation to goiter and benign thyroid nodules, more modest positive associations for hyperthyroidism (characterized by high thyroid hormone and low thyroid stimulating hormone [TSH] levels) and hypothyroidism (low thyroid hormone and high TSH levels), and weak or null associations for autoimmune thyroiditis (60–62). Nonetheless, it is not fully possible to eliminate detection bias as an explanation for these results, as diagnosis of one or more thyroid conditions may mean many years or even decades of regular thyroid hormone testing or more intense monitoring of the thyroid gland. It has also been difficult to disentangle whether an association for a particular benign disease is due to the disease itself or its treatment (e.g., in the case of 131I- therapy for hyperthyroidism).
Prospective cohort studies incorporating pre-diagnostic measures of thyroid hormones, TSH, and/or thyroid autoantibodies, and with long-term duration of follow-up for thyroid cancer incidence, provide an opportunity to evaluate the relation between thyroid disorders and thyroid cancer risk, while also minimizing the potential for the biases highlighted above. For instance, such studies may exclude individuals with a prior thyroid disease diagnosis, allowing more direct focus on whether normal variation in thyroid function or autoimmunity may influence thyroid cancer risk. Two separate prospective studies of individuals in the normal (euthyroid) range of thyroid function demonstrated an inverse association between pre-diagnostic TSH and differentiated thyroid cancer risk, while no association was observed for thyroid hormones (63, 64). These findings were surprising considering that TSH has been shown in experimental studies to promote growth and proliferation of thyroid cancer cells and has long been hypothesized to play an important role in thyroid cancer etiology (65). On the other hand, the inverse association of TSH is somewhat consistent with the positive association of hyperthyroidism and thyroid cancer risk, described above, and the finding that thyroid cancer risk alleles located near the FOXE1 and NKX2-1 genes are associated with low TSH levels (66). Additional research may be needed to evaluate whether the underlying genetic, autoimmune-related, dietary, or environmental causes of overt or structural thyroid disorders contribute to thyroid cancer development.
Iodine
Iodine is a trace element essential for the formation of thyroid hormones and found primarily in and around coastal areas (67). Iodine deficiency is a major risk factor for several types of benign thyroid diseases, including goiter and hypothyroidism, while iodine excess can induce thyroid dysfunction in patients with certain risk factors, such as preexisting thyroid disease, the elderly, fetuses, and newborns (67). However, it has been difficult to determine whether iodine insufficiency or excess causes thyroid cancer, owing to the lack of data from prospective cohort studies and reliance on self-reported dietary surveys, which provide an unreliable estimate of iodine consumption (68). A comprehensive review of the animal and human data has supported the view that iodine deficiency increases the risk of FTC and possibly ATC (69). Ecological studies have demonstrated that the introduction of iodine supplementation into areas of iodine deficiency increases the ratio of PTC to FTC, raising the question of whether excessive iodine intake is a risk factor for PTC (70–72), although such studies are strongly confounded by changes over time in thyroid imaging and diagnostic practices. Lee et al. performed a meta-analysis of 16 studies to address the role of iodine intake and thyroid cancer risk (73). Rather than settling the question, however, the report highlighted the weaknesses in the available evidence. For example, the two studies with urinary iodine measurements that showed evidence of a positive association between urinary iodine and PTC had low quality scores (74, 75).
Few epidemiologic studies have attempted to address the association between iodine intake and thyroid cancer risk using dietary intake instruments (food frequency questionnaires) to approximate iodine intake or to evaluate associations for individual iodine-rich food items, including fortified foods (bread, dairy, salt), seaweed/kelp, and fish/shellfish. In an international pooled analysis of case-control studies, fish intake was inversely associated with thyroid cancer in endemic goiter areas, and intake of cruciferous vegetables (which contain goitrogens, thyroid function-disrupting substances that result in the stimulation of TSH production) was inversely associated with thyroid cancer in iodine-rich and endemic goiter regions (76, 77). In a Japanese cohort study, seaweed consumption (the primary source of dietary iodine in the population) was positively associated with risk of PTC (HR=1.71, 95% CI 1.01–2.90 for daily consumption versus ≤2 days/week) (78). However, this finding was not confirmed in a separate Japanese cohort (79). In a large U.S. cohort of middle-to-older adults, higher adolescent intakes of canned tuna and mid-life intake of broccoli were positively associated with thyroid cancer risk among males, providing some support for an association of iodine-rich or goitrogen-containing foods, respectively (80); however, errors in recall of diet during adolescence may have been substantial. No association was observed for middle-to-older adulthood consumption of fish or shellfish in the large European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study (81). Further insights about the role of iodine in thyroid cancer development could come from large prospective cohort studies with pre-diagnostic measures of iodine, such as in urine, and the ability to separately assess risks according to thyroid cancer histologic type.
Other dietary factors
Selenium has been hypothesized to play a protective role in thyroid carcinogenesis owing to its antioxidant properties and role in thyroid hormone metabolism, but results from epidemiologic studies have been inconsistent (82). Similarly, the hypothesis that nitrates and nitrites influence thyroid cancer risk owing to their ability to inhibit iodide intake and disrupt thyroid homeostasis has not been consistently supported by results from epidemiologic studies (83–85).
In large cohort studies, positive associations of starch intake and glycemic index with thyroid cancer have been observed in individuals who were overweight or obese (possibly because of their greater insulin resistance), with inverse associations in normal-weight individuals (86). Inverse associations of polyunsaturated fat intake (86) and positive associations of fruit juice intake (87, 88) have been observed. Results from studies evaluating tea consumption and thyroid cancer risk have been mixed (88–90), while coffee intake has not been associated with risk (89, 90). Reported evidence on the relation between polyphenol intake and thyroid cancer risk has been mixed (88, 91, 92). However, foods and beverages rich in polyphenols, including tea, wine, and citrus fruit, also contain other vitamins and nutrients and have other properties that may independently influence thyroid cancer risk. This, as well as the potential for substantial measurement error in dietary assessment from food frequency questionnaires, are some of the challenges faced when attempting to investigate individual dietary risk or protective factors for thyroid cancer.
Obesity
In recent decades, the global prevalence of overweight and obesity, including morbid obesity (a body mass index [BMI] above 40), has increased substantially (93). The impact of these trends may not be fully realized for decades, although it is well-understood that excess body fat leads to development of adverse metabolic conditions, including insulin resistance, hormonal fluctuations, and inflammation and is a cause of diabetes, cardiovascular disease, and several types of cancer (94). The parallel increasing trends in overweight and obesity and PTC incidence since at least the 1980s has led many to question as to whether a direct relationship exists between excess adiposity and thyroid cancer development (95).
Until about a decade ago, most epidemiologic studies on overweight and obesity were cross-sectional or case-control in design. A large international pooled case-control study revealed a positive association between BMI and thyroid cancer confined to women; however, the number of cases in men was small (96). The earliest prospective studies provided some evidence that higher BMI was associated with increased risk of differentiated thyroid cancer, not only in women but also in men (62, 97–99). A pooled analysis of five prospective U.S. cohorts demonstrated that BMI was positively associated with risk of thyroid cancer (95). Overall, study subjects who were obese (BMI ≥30 kg/m2) at study entry had about a 53% greater risk of thyroid cancer than those with normal weight. The positive association was observed for all major histologic types of thyroid cancer, apart from MTC, and appeared to be strongest for ATC. Similar patterns by histologic type were observed in a Norwegian cohort study of over two million individuals (98). An expanded, international pooled analysis of 22 prospective cohort studies confirmed these results and showed positive associations for additional measures of adiposity, including waist circumference (a better measure of central, or visceral, adiposity) and adulthood weight gain (a more accurate measure of excess adiposity than weight or BMI at one point in time) (100). In addition, greater adiposity was more strongly associated with thyroid cancer mortality than incidence, providing further evidence that excess adiposity may directly influence tumor promotion and progression. Similar findings were observed in a recent case-control study in Australia; BMI was positively associated with PTCs characterized by the BRAFV600E mutation, while no association was observed for BRAF-negative PTC (101).
Although some researchers have argued that much, if not all, of the observed association could be explained by greater likelihood of thyroid function testing and imaging in obese, versus, normal-weight individuals (102), the associations were not limited to indolent tumors, as one would expect in the presence of major detection bias. There is also little evidence that method of initial detection of differentiated thyroid cancer (palpation, imaging, incidental) differs by obesity status (103). Associations of childhood BMI and thyroid cancer risk are less likely to be influenced by detection bias than those of adulthood BMI. In a large Danish study, childhood BMI (based on annual measurements of height and weight) was positively associated with risk of adult thyroid cancer (104). Also, the magnitude of the association appeared to be stronger than that for adulthood BMI, based on published results from other studies. Due to the mounting evidence from epidemiologic studies, a panel of experts convening at the International Agency for Research on Cancer in 2016 added thyroid cancer to a growing list cancers having sufficient evidence of a causal relationship with excess adiposity (105).
A provocative question, therefore, has been whether and to what extent overweight and obesity has contributed to rising thyroid cancer incidence trends. Kitahara et al. estimated that the rising prevalence of overweight and obesity in the U.S. was responsible for about 14% of the rise in PTC incidence between 1995 and 2015 and 58% of the rise in large (>4 cm) PTCs (106). Hypothetically, if all of the individuals in the overweight or obese categories been of normal weight, the PTC incidence would have increased by an average of 5.1% per year during 1995–2015, as opposed to 5.9% per year (Fig. 4), and the incidence of the larger PTCs would have increased, on average, 1.9% per year, as opposed to 4.5% per year. Comparable estimates of population-attributable risk were obtained in an Australia-based study using similar methods (107).
Figure 4.
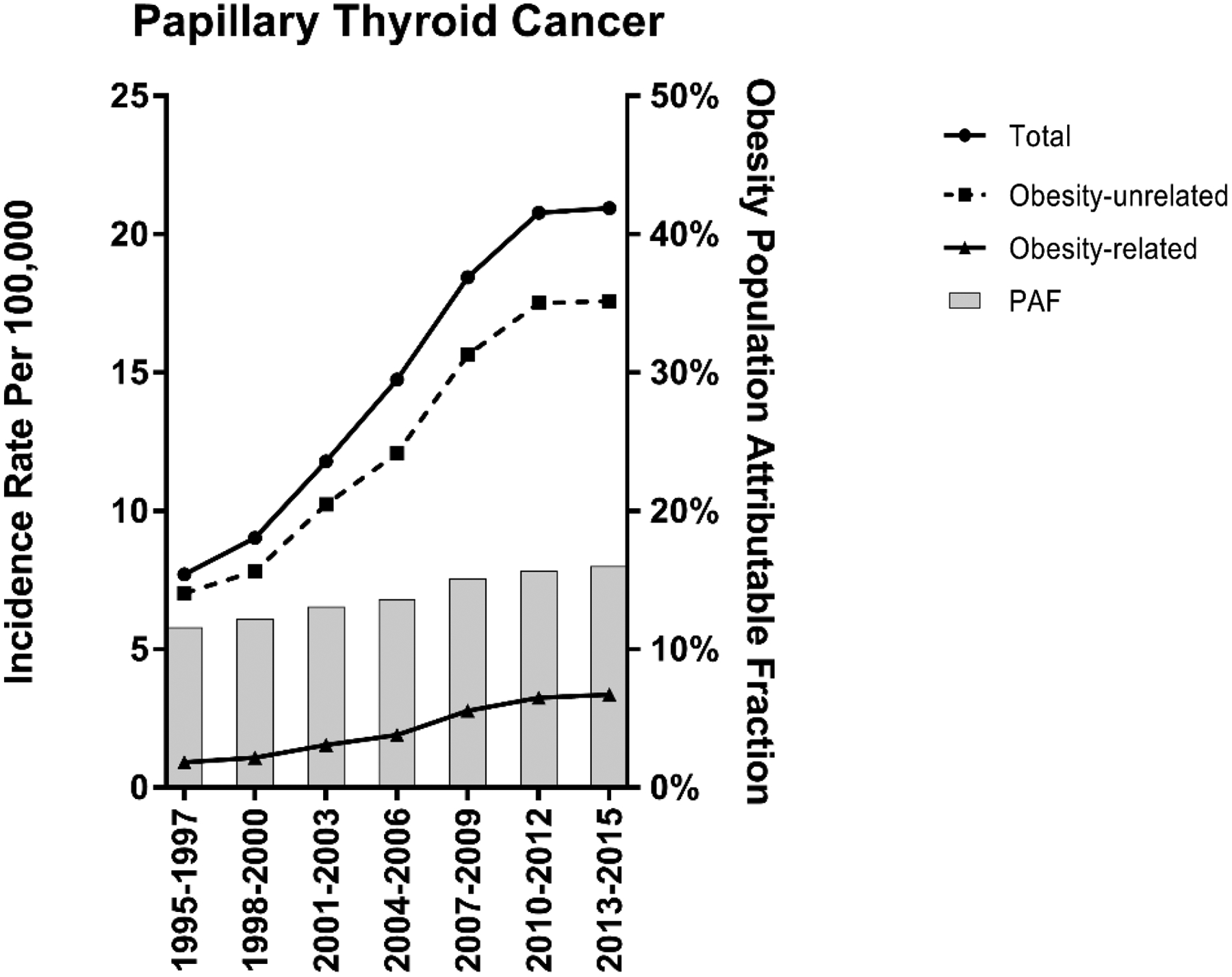
Trends in the incidence of papillary thyroid cancer in the U.S., overall and stratified by overweight and obesity-related and -unrelated cases, 1995–2015. The trend lines represent the incidence in each calendar-year period, and the gray bars represent the population-attributable fraction. Adapted from Kitahara et al., J Natl Cancer Inst 2020 (106).
Biological mechanisms underlying the association between obesity and thyroid cancer have been proposed, partly based on the more developed knowledge of the role of obesity in development of other cancer types, with inflammation and insulin resistance being the most well-studied factors (108). Epidemiologic evidence linking diabetes with thyroid cancer has been mixed, with some studies suggesting a positive association (109), and many others finding no or inconsistent evidence of an association (61, 110–113). Some studies have shown a reduced risk of thyroid cancer in individuals treated with metformin (111, 114). Findings from studies evaluating pre-diagnostic markers of insulin resistance, dyslipidemia, and blood pressure in relation to thyroid cancer risk also have been mixed (115, 116). In the EPIC cohort, some but not all pre-diagnostic markers of inflammation were associated with risk of differentiated thyroid cancer (117). Interestingly however, a doubling in pre-diagnostic concentrations of insulin-like growth factor (IGF)-I was associated with a 48% increase in risk (95% CI: 6% to 108%) (118). A future study investigating IGF-I levels in younger individuals in relation to thyroid cancer risk could provide further insights, considering the separate findings regarding childhood BMI (described above) (104) and larger birth size (119–121) (see also: Perinatal and in utero exposures).
Reproductive and hormonal factors
The higher incidence of thyroid cancer in women than men (7), along with evidence from laboratory-based studies that estrogen stimulates proliferation of thyroid cancer cells (122), suggest a direct role of sex steroid hormones in thyroid cancer development. However, evidence from case-control and cohort studies evaluating traditional “proxies” for lifetime estrogen exposure, including parity, ages at menarche and menopause, and use of oral contraceptives and menopausal hormone therapy, in relation to thyroid cancer risk has been weak and inconsistent (62, 123–131). Several explanations have been proposed for the mixed findings: 1) strong confounding by medical surveillance or access to care inhibits the ability to identify any “true” effects of reproductive or hormonal factors on thyroid cancer risk, 2) the proxy indicators evaluated in those studies do not accurately capture estrogen exposure during the etiologically relevant time windows for thyroid cancer, and/or 3) the higher incidence of thyroid cancer risk in women than men is largely explained by other factors that are more prevalent in women, such as autoimmunity (68, 132). Some of the more consistent epidemiologic findings (i.e., for infertility and use of infertility drugs (125, 133), recent pregnancy (125, 128), irregular menstrual cycles (128), and hysterectomy or surgical menopause (125, 127, 134) could provide etiologic clues, including the importance of specific exposures and exposure time windows, or they may be explained by detection bias (30, 125); it is also possible that both scenarios are true. Detection bias does not seem to be a likely explanation for some observations, such as the modest reduction in risk observed with greater duration of breastfeeding (125, 126); findings such as this warrant further exploration.
Perinatal and in utero exposures
Whether certain perinatal or in-utero exposures influence risk of thyroid cancer in mothers or their offspring is an intriguing question considering the rising incidence of pediatric thyroid cancer (135), the higher incidence of thyroid cancer in women than men starting at young ages (132), and the elevated risk of thyroid cancer shortly after pregnancy (125, 128).
As discussed above, younger age at exposure to ionizing radiation is much more strongly associated with thyroid cancer risk than older age at exposure (46); however, the influence of in utero radiation exposure on thyroid cancer risk remains unclear. Based on just eight cases of thyroid cancer, including one case of a Hürthle cell carcinoma, in utero exposure to 131I at the time of the Chernobyl accident was associated with a non-significant increased odds ratio of 11.66 per Gy (136).
In a large Swedish cohort study, greater birthweight and fetal growth (birthweight standardized by gestational age and infant sex) was associated with higher maternal risk of PTC and FTC (121). These associations were adjusted for maternal age, height, weight, smoking, and sociodemographic factors. The authors hypothesize that IGF-I may underlie these associations due to its pro-carcinogenic properties and association with fetal growth.
Two large registry-based studies in California and the Nordic countries were conducted to evaluate a variety of factors including maternal and paternal characteristics, pregnancy complications, and birth outcomes in relation to thyroid cancer risk in offspring (119, 120). Both studies found positive associations between birthweight and risk of thyroid cancer (particularly PTC), with a 10–20% increase in risk for every 1 kg, and inverse associations for male sex and birth order. In the California study, birth order was inversely associated with PTC and positively associated with FTC (120), while in the Nordic study, associations trended in the inverse direction for across all major histologic types except MTC (119). Birth order serves as a crude proxy for a wide range of childhood exposures, including childhood exposure to infectious diseases and exposure to maternal hormones and environmental contaminants in utero and during breastfeeding (120). Other findings from the California study included positive associations for Hispanic ethnicity (with PTC only) and higher maternal education (120). Other findings from the Nordic study included very strong positive associations for neonatal diagnosis of congenital hypothyroidism (OR=4.55, 95% CI 1.58–13.08) and maternal history of most benign thyroid disorders (with ORs ranging from 11.91 for maternal hyperthyroidism to 67.36 for maternal goiter); the magnitude of these associations was generally stronger for FTC than PTC, suggesting a possible underlying role of iodine deficiency (119). Maternal diabetes diagnosis before pregnancy and postpartum hemorrhage were modestly positively associated with thyroid cancer risk. No associations were observed for other maternal characteristics examined, including marital status, age at birth, smoking, or other comorbid conditions or pregnancy complications apart from those listed above. Paternal age at birth and other birth characteristics (multiple births, preterm birth, Cesarian section birth) also were not associated with offspring thyroid cancer risk. There was no evidence that smoking or pre-pregnancy BMI biased the associations observed in the Nordic study. Detection bias is a plausible explanation for some of these findings. For instance, offspring of mothers of higher socioeconomic standing and those with comorbid conditions, especially benign thyroid diseases, may be more likely to undergo routine thyroid monitoring throughout life leading to more incidental detection of thyroid nodules and cancer. However, the authors found that many of the associations held after restricting the outcome to advanced thyroid cancers, which are more likely to be detected due to symptoms.
Smoking and alcohol
Case-control studies and cohort studies have consistently shown that cigarette smoking and alcohol consumption are inversely associated with risk of thyroid cancer (137–141). Current smokers were found to have a 40–50% reduction in risk of thyroid cancer compared to never smokers (141). The associations appeared to be independent and not modified by other thyroid cancer risk factors, including obesity. Risks associated with both smoking and alcohol consumption appear to be dose-dependent with regard to duration and frequency of use (137–139, 141), although a dose-dependent association of smoking and thyroid cancer was not observed in a Korean cohort of 10 million adults (140). The potential underlying mechanisms remain unclear, although alterations in TSH, thyroid hormones, and thyroid autoantibodies, as well as sex steroid hormones, have been suggested to play a role (137). While it is possible that detection bias could confound these results, as current smokers and heavier drinkers may be less likely to undergo thyroid imaging or screening, a case-control study in Australia found that the inverse association of current smoking and thyroid cancer became stronger after adjusting for detection bias, and smoking was more strongly inversely associated with thyroid cancers harboring BRAFV600E mutations (142).
Other environmental exposures
Interest in environmental contaminants and thyroid cancer arises because some classes of chemicals may influence thyroid homeostasis, although the exact mechanisms of action are likely complex and not entirely understood. Some of these mechanisms include adverse effects on thyroid hormone metabolism and inhibition of iodine uptake by follicular thyroid cells. Also, since many of these chemicals have similar molecular structure to thyroid hormones, they can alter the ability of thyroid hormone to bind to receptors and transport proteins and contribute to the development of subclinical hypothyroidism (143).
Use of flame retardants chemicals in commercial and household items, including furniture, electronics, and construction materials, increased substantially since the 1970s, and household exposure to these chemicals is now ubiquitous. A recent case-control study evaluated whether 12 flame retardants measured from household dust and 27 polybrominated diphenyl ethers (PBDEs) measured in serum were associated with occurrence of PTC (144). Higher concentrations of decabromodiphenyl ether (BDE-209) and tris(2-chloroethyl) phosphate (TCEP) were found in the dust samples of PTC cases compared to controls. TCEP was associated with aggressive tumor features, including extrathyroidal extension, greater size, and nodal metastasis, while BDE-209 was associated only with smaller, low-stage PTCs. However, serum PBDEs were not associated with PTC risk in that study nor in a large, nested case-control study in the U.S. (145).
Evidence linking pesticide exposures with thyroid cancer risk has been mixed (146–148). In a U.S. cohort of male licensed pesticide applicators (mostly farmers), use of the fungicide metalaxyl and the organochlorine insecticide lindane were associated with a two-fold and 74% increased risk of thyroid cancer, respectively, while use of the herbicide chlorimuron-ethyl and insecticide carbaryl were inversely associated with risk (148). Exposure to organophosphate insecticides by the spouses of the cohort participants was associated with increased risks of thyroid, breast, and ovarian cancer (147). Malathion, the most used organophosphate, was positively associated with risk, although the number of exposed cases was small. In a Norwegian nested case-control study, pre-diagnostic concentrations of several polychlorinated biphenyl (PCB) congeners and organochlorine pesticide analytes were inversely associated with thyroid cancer risk (149); positive associations for certain PCBs and chlordane metabolites were observed but only in the most recent birth cohort (1943–1957).
Exposure to trace elements associated with volcanic activity, including vanadium, sulfur, thiocyanates, zinc, and cadmium, has been suggested to contribute to the relatively high thyroid cancer incidence in the Phillipines, Hawaii, Iceland, New Caledonia, French Polynesia, and the Catania providence of Sicily (150–152) (see Disparities, above). However, exposures to these specific trace elements and thyroid cancer have not been investigated in well-controlled epidemiologic studies, and the potential underlying biological mechanisms remain unclear (152).
Other potential modifiable risk factors
Other potential risk (or protective) factors for thyroid cancer have been explored, mainly centered around exposures influencing thyroid hormone synthesis or with estrogen-like or inflammatory properties. Several medical conditions, other than the ones described above, have been linked with an increased risk of thyroid cancer, including end-stage renal and liver disease in solid organ transplant recipients (153), benign breast disease (62, 123, 154), breast cancer (155), uterine fibroids (124), asthma (62), and autoimmune conditions such as lupus and Sjӧgren’s syndrome (156, 157). Positive associations have been observed for artificial light at night (158), and inverse associations have been observed for ultraviolet radiation exposure (159). While several of these findings are novel and compelling, they require replication in other studies before drawing strong conclusions.
Non-modifiable risk factors
Familial clusters and germline mutations
That developing thyroid cancer is affected by genetic factors is supported by at least two types of evidence. First, the importance of genetic risk factors in thyroid cancer is evidenced by studies using national cancer registry data showing a substantial familial risk (160, 161). In some cases, the occurrence of non-syndromic familial clusters of thyroid carcinomas, referred to as “familial non-medullary thyroid carcinoma” (FNMTC), can be clinically identified. However, while the existence of FNMTC has been well documented, not all clusters have a genetic cause, especially in view of the recent increase of incidentally diagnosed cases. Based on a statistical analysis, it is generally accepted that three first degree relatives with thyroid cancer are usually necessary to conclude that a genetic factor is causing a particular family cluster (162). Specific genetic factors have been identified in only a minority of family clusters (163).
Second, many studies have identified germline polymorphisms found in the general population that contribute to the susceptibility of developing thyroid cancer. The first to be identified, initially among people living in Iceland, were variants in the gene FOXE1 (66). This finding has been duplicated in a variety of other populations and remains the strongest risk factor. Several polymorphisms have been identified in this thyroid transcription factor that are related to its role in thyroid carcinogenesis (164). Many other genetic polymorphisms, with weaker effects, have been identified (165). A recent effort combining 10 polymorphisms found that their contribution to genetic predisposition is 8% (166).
Screening
Screening for thyroid cancer can be performed by palpation of the thyroid, as is done in a general physical examination, or by imaging using thyroid ultrasound. Based on epidemiological and clinical observation, the U.S. Preventative Services Task Force and other clinical practice guidelines strongly recommend against thyroid cancer screening in asymptomatic people without demonstrable risk factors (167, 168). The effects of widespread thyroid cancer screening were clearly demonstrated in the example set by the Republic of Korea (21), as discussed above (see Trends in incidence and the role of overdiagnosis). At the other end of the spectrum is screening for genetic syndromes. The clearest example is related to MTC (169). Every patient with MTC should be screened for a genetic basis. For people known to have a syndrome that includes MTC, often identified in relatives of a MTC patient, screening is indicated. In some forms of the syndrome, prophylactic thyroid surgery is recommended (170).
In familial clusters of thyroid cancer, as defined above, some suggest that screening family members should be carried out, but “with caution” (171). However, aside from routine palpation, no guideline recommends screening (172). Screening is suggested for people with genetic syndromes associated with a high risk of thyroid cancer, although it is less clear whether screening should be by palpation or by ultrasound (173).
Whether to screen people exposed to radiation at a young age is unclear, although it is difficult to envision scenarios where the benefits of screening (ideally, reduced morbidity and mortality) outweigh the risks. Screening after the Chernobyl accident found many radiation-related cases and may have improved the outcomes for some individuals (174). However, screening after the Fukushima accident, which yielded much lower radiation doses to the population, identified many thyroid cancers that were highly unlikely to be radiation-related, resulting in unnecessary psychological distress over a cancer diagnosis and subjecting some individuals to unnecessary treatment (48). Childhood and young adult cancer survivors who received therapeutic doses of radiation exposing the thyroid and survived their cancer diagnosis are clearly at increased risk of developing thyroid nodules and second primary thyroid cancer, especially those exposed at very young ages (45). Many papers report the sensitivity of thyroid ultrasound in detecting thyroid cancer in these individuals, and some recommend thyroid ultrasound as routine (175). However, those that take into account the potential for overdiagnosis are cautious about following this recommendation. In fact, the International Late Effects of Childhood Cancer Guideline Harmonization Group found that the available data were insufficient to recommend for or against screening cancer survivors (176).
Primary prevention
As summarized above (see Trends in incidence and the role of overdiagnosis and Disparities), trends and disparities in thyroid cancer are explained largely, but not completely, by diagnostic practices. Greater awareness of the extent and problems associated with thyroid cancer overdiagnosis, clinical recommendations against thyroid cancer screening, introduction of the NIFTP terminology for tumors previously considered malignant, and higher tumor size thresholds for fine-needle aspiration biopsy have been among the most effective measures of reducing the incidence of thyroid cancer (15, 16).
Epidemiologic research on potential modifiable risk factors raises the possibility of identifying other specific targets for primary prevention. Together with clinical efforts to avoid overdiagnosis and overtreatment, further advancement in thyroid cancer epidemiologic research has the potential to substantially reduce the total disease burden in the population. However, few modifiable risk factors have been identified to date apart from ionizing radiation and obesity.
With increasing evidence supporting a linear relationship between ionizing radiation exposure and thyroid cancer risk (except at very high levels) (46), minimizing exposure to the thyroid to the extent possible, especially in children, is likely to be effective for thyroid cancer prevention. A concerted effort to minimize medical radiation exposure to children has been operationalized by the slogan and educational campaign “Image Gently” (177), initiated by the Society of Pediatric Radiology in collaboration with the American Association of Physicists in Medicine, American College of Radiology, and the American Society of Radiologic Technologists, with many other professional organizations subsequently joining the effort. The campaign encourages the use of pediatric-specific imaging protocols, avoidance of multiphase scanning, and consideration of non-radiation imaging procedures whenever possible. While thyroid radiation from dental radiology has decreased over time, and suggestions have been made to decrease this even further (178), radiation exposures from dental radiologic procedures represent a tiny fraction of the total radiation exposure from medical sources (57). Reduction in the use of CT imaging to the head and neck in children would have a far greater impact on thyroid cancer prevention (57).
Minimizing radiation exposure to the thyroid is especially important in young children due to their much higher radiosensitivity compared with adults (46). High-dose radiation therapy for most benign conditions have been long abandoned due to concerns over the late effects (179), and treatment of hyperthyroidism with 131I has been declining in favor of long-term antithyroid drugs (180). Alternatives to radiotherapy are unavailable for several types of cancer, but modalities and collimation have improved over time, reducing off-target effects.
Another potential source of radiation exposure is the release of radioactive iodine isotopes from nuclear power plant accidents, not unlike the one that occurred in Chernobyl. Should a similar accident occur, it is to be expected that the food pathway would be quickly controlled. However, this may not be complete, and people very near the accident would be subject to thyroid exposure through the inhalation pathway. If the age-specific potential dose threshold is exceeded, ingestion of potassium iodide will be advised by public health officials. The ATA has reviewed the issues related to this dose-reduction measure (181). The World Health Organization, the U.S. Food and Drug Administration, and others have published Guidelines for the use of potassium iodide in a radiological emergency (182, 183).
Efforts to promote weight loss in individuals who are overweight or obese and avoidance of excess weight gain throughout adulthood may help to prevent the occurrence of thyroid cancer or the promotion of existing thyroid cancers (106). Considering the immense difficulty that many adults face in achieving or maintaining a healthy body weight, studies that contribute to a better understanding of the biological mechanisms underlying the association between obesity and thyroid cancer may lead to the identification of more specific, and potentially more effective, targets for prevention. On the other hand, widespread interventions to promote healthy body weight or weight loss have the potential for numerous and extensive health benefits, including the prevention and improvement of a spectrum of metabolic diseases apart from thyroid cancer.
Future directions
There remains a critical need for prospective studies with objective pre-diagnostic measures of exposure, which would help to avoid measurement error, minimize detection bias, and allow for assessment of dose-response. Such studies are needed to better understand the biological mechanisms underlying commonly observed associations for benign thyroid conditions, obesity, smoking, alcohol consumption, for instance. More confirmatory studies or in-depth investigations of some of the more novel or compelling findings to date (e.g., associations for benign thyroid diseases, iodine consumption, infertility medications, and endocrine-disrupting chemicals, to name a few) are also needed. Studies that evaluate exposures that are unique or more prevalent among women, including certain cosmetic products, particularly those with endocrine-disrupting properties, and certain medical conditions and their associated therapies (autoimmune conditions, benign thyroid disorders), could help to improve understanding around the higher incidence in women than men. Additional studies investigating environmental and lifestyle-related exposures and screening practices across racial/ethnic subgroups and immigration status may provide further etiologic clues. In general, there is a major need for large case-control and prospective studies with detailed diagnostic information, including markers of tumor aggressiveness and pathways to diagnosis (i.e., how the tumor was initially detected), which would enable researchers to better distinguish incidentally detected thyroid cancers from those considered clinically relevant. There is great potential for advancing current understanding of thyroid cancer epidemiology by designing epidemiologic studies that characterize thyroid cancer cases according to their molecular profiles, as in the recent example of radiation and thyroid cancer in Chernobyl (see Ionizing Radiation, above).
Funding
This work was funded by the Intramural Research Program of the National Cancer Institute (C.M. Kitahara).
Footnotes
Disclosure of Potential Conflicts of Interest
None
References
- 1.Surveillance, Epidemiology, and End Results Program (SEER) Cancer Statistics Review, 1975–2018 [Internet]. Bethesda (MD): National Cancer Institute; 2021. Apr 15 [cited 2021 Nov 24]. Available from: http://seer.cancer.gov/csr/1975_2018. [Google Scholar]
- 2.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, et al. Integrated genomic analysis of Hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell 2018;34:256–70.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romei C, Elisei R. A narrative review of genetic alterations in primary thyroid epithelial cancer. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol 2002;13:3–16. [DOI] [PubMed] [Google Scholar]
- 7.Ferlay J, Lam F, Colombet M, Mery L, Pineros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today [Internet]. Lyon (France): International Agency for Research on Cancer; 2020. Dec [cited 2021 Nov 24]. Available from: https://gco.iarc.fr/today. [Google Scholar]
- 8.Lortet-Tieulent J, Franceschi S, Dal Maso L, Vaccarella S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int J Cancer 2019;144:2082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol 2022;10:264–272. [DOI] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 11.SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Bethesda (MD): Surveillance Research Program, National Cancer Institute; 2021. Sep 27 [cited 2021 Nov 24]. Available from: https://seer.cancer.gov/explorer/. [Google Scholar]
- 12.Baloch Z, LiVolsi VA, Tondon R. Aggressive variants of follicular cell derived thyroid carcinoma; the so called ‘real thyroid carcinomas’. J Clin Pathol 2013;66:733–43. [DOI] [PubMed] [Google Scholar]
- 13.Porter A, Wong DJ. Perspectives on the treatment of advanced thyroid cancer: approved therapies, resistance mechanisms, and future directions. Front Oncol 2020;10:592202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017;317:1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee M, Powers AE, Morris LGT, Marti JL. Reversal in thyroid cancer incidence trends in the United States, 2000–2017. Thyroid 2020;30:1226–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitahara CM, Sosa JA, Shiels MS. Influence of nomenclature changes on trends in papillary thyroid cancer incidence in the United States, 2000 to 2017. J Clin Endocrinol Metab 2020;105:e4823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020;8:468–70. [DOI] [PubMed] [Google Scholar]
- 18.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006;295:2164–7. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614–7. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi S, Vaccarella S. Thyroid cancer: an epidemic of disease or an epidemic of diagnosis? Int J Cancer 2015;136:2738–9. [DOI] [PubMed] [Google Scholar]
- 21.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med 2014;371:1765–7. [DOI] [PubMed] [Google Scholar]
- 22.Ahn HS, Welch HG. South Korea’s thyroid-cancer “epidemic”--turning the tide. N Engl J Med 2015;373:2389–90. [DOI] [PubMed] [Google Scholar]
- 23.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16:109–42. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2:1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol 2016;12:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito Y, Nikiforov YE, Schlumberger M, Vigneri R. Increasing incidence of thyroid cancer: controversies explored. Nat Rev Endocrinol 2013;9:178–84. [DOI] [PubMed] [Google Scholar]
- 29.Ullmann TM, Gray KD, Limberg J, Stefanova D, Moore MD, Buicko J, et al. Insurance status Is associated with extent of treatment for patients with papillary thyroid carcinoma. Thyroid 2019;29:1784–91. [DOI] [PubMed] [Google Scholar]
- 30.LeClair K, Bell KJL, Furuya-Kanamori L, Doi SA, Francis DO, Davies L. Evaluation of gender inequity in thyroid cancer diagnosis: differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med 2021;181:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, Doi SAR. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol 2016;34:3672–9. [DOI] [PubMed] [Google Scholar]
- 32.Bogdanova TI, Zurnadzhy LY, Nikiforov YE, Leeman-Neill RJ, Tronko MD, Chanock S, et al. Histopathological features of papillary thyroid carcinomas detected during four screening examinations of a Ukrainian-American cohort. Br J Cancer 2015;113:1556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zablotska LB, Nadyrov EA, Rozhko AV, Gong Z, Polyanskaya ON, McConnell RJ, et al. Analysis of thyroid malignant pathologic findings identified during 3 rounds of screening (1997–2008) of a cohort of children and adolescents from Belarus exposed to radioiodines after the Chernobyl accident. Cancer 2015;121:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahoon EK, Nadyrov EA, Polyanskaya ON, Yauseyenka VV, Veyalkin IV, Yeudachkova TI, et al. Risk of thyroid nodules in residents of Belarus exposed to Chernobyl fallout as children and adolescents. J Clin Endocrinol Metab 2017;102:2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcadis AR, Davies L, Marti JL, Morris LGT. Racial disparities in cancer presentation and outcomes: the contribution of overdiagnosis. JNCI Cancer Spectr 2020;4:pkaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Megwalu UC, Ma Y. Racial/ethnic disparities in use of high-quality hospitals among thyroid cancer patients. Cancer Invest 2021;39:482–8. [DOI] [PubMed] [Google Scholar]
- 37.Megwalu UC, Ma Y, Hernandez-Boussard T, Divi V, Gomez SL. The impact of hospital quality on thyroid cancer survival. Otolaryngol Head Neck Surg 2020;162:269–76. [DOI] [PubMed] [Google Scholar]
- 38.Lee AW, Mendoza RA, Aman S, Hsu R, Liu L. Thyroid cancer incidence disparities among ethnic Asian American populations, 1990–2014. Ann Epidemiol 2022;66:28–36. [DOI] [PubMed] [Google Scholar]
- 39.Megwalu UC, Ma Y, Osazuwa-Peters N, Orloff LA. Clinical presentation and survival outcomes of well-differentiated thyroid cancer in Filipinos. Cancer Med 2021;10:5964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossing MA, Schwartz SM, Weiss NS. Thyroid cancer incidence in Asian migrants to the United States and their descendants. Cancer Causes Control 1995;6:439–44. [DOI] [PubMed] [Google Scholar]
- 41.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duffy BJ Jr., Fitzgerald PJ. Thyroid cancer in childhood and adolescence; a report on 28 cases. Cancer 1950;3:1018–32. [DOI] [PubMed] [Google Scholar]
- 43.Hempelmann LH. Risk of thyroid neoplasms after irradiation in childhood. Studies of populations exposed to radiation in childhood show a dose response over a wide dose range. Science 1968;160:159–63. [DOI] [PubMed] [Google Scholar]
- 44.Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 1995;141:259–77. [PubMed] [Google Scholar]
- 45.Veiga LH, Lubin JH, Anderson H, de Vathaire F, Tucker M, Bhatti P, et al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res 2012;178:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res 2016;185:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lubin JH, Adams MJ, Shore R, Holmberg E, Schneider AB, Hawkins MM, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab 2017;102:2575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clero E, Ostroumova E, Demoury C, Grosche B, Kesminiene A, Liutsko L, et al. Lessons learned from Chernobyl and Fukushima on thyroid cancer screening and recommendations in case of a future nuclear accident. Environ Int 2021;146:106230. [DOI] [PubMed] [Google Scholar]
- 49.Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 2005;97:724–32. [DOI] [PubMed] [Google Scholar]
- 50.Kitahara CM, Berrington de Gonzalez A, Bouville A, Brill AB, Doody MM, Melo DR, et al. Association of radioactive iodine treatment with cancer mortality in patients with hyperthyroidism. JAMA Intern Med 2019;179:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas GA, Bunnell H, Cook HA, Williams ED, Nerovnya A, Cherstvoy ED, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab 1999;84:4232–8. [DOI] [PubMed] [Google Scholar]
- 52.Morton LM, Karyadi DM, Stewart C, Bogdanova TI, Dawson ET, Steinberg MK, et al. Radiation-related genomic profile of papillary thyroid carcinoma after the Chernobyl accident. Science 2021;372: eabg2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr., Sigurdson AJ, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab 2014;99:E276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kowalska A, Walczyk A, Kowalik A, Palyga I, Trybek T, Kopczynski J, et al. Increase in papillary thyroid cancer incidence is accompanied by changes in the frequency of the BRAF V600E mutation: a single-institution study. Thyroid 2016;26:543–51. [DOI] [PubMed] [Google Scholar]
- 55.National Council on Radiation Protection and Measurements (NCRP). Ionizing radiation exposure of the population of the United States. NCRP Report 160. Bethesda, MD; 2009. p. 235–246. [Google Scholar]
- 56.Mettler FA Jr. Medical radiation exposure in the United States: 2006–2016 trends. Health Phys 2019;116:126–8. [DOI] [PubMed] [Google Scholar]
- 57.Schonfeld SJ, Lee C, Berrington de Gonzalez A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 2011;23:244–50. [DOI] [PubMed] [Google Scholar]
- 58.Mathews JD, Forsythe AV, Brady Z, Butler MW, Goergen SK, Byrnes GB, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franceschi S, Preston-Martin S, Dal Maso L, Negri E, La Vecchia C, Mack WJ, et al. A pooled analysis of case-control studies of thyroid cancer. IV. Benign thyroid diseases. Cancer Causes Control 1999;10:583–95. [DOI] [PubMed] [Google Scholar]
- 60.Kitahara CM, Kormendine Farkas D, Jorgensen JOL, Cronin-Fenton D, Sorensen HT. Benign thyroid diseases and risk of thyroid cancer: a nationwide cohort study. J Clin Endocrinol Metab 2018;103:2216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balasubramaniam S, Ron E, Gridley G, Schneider AB, Brenner AV. Association between benign thyroid and endocrine disorders and subsequent risk of thyroid cancer among 4.5 million U.S. male veterans. J Clin Endocrinol Metab 2012;97:2661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, et al. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol 2010;171:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rinaldi S, Plummer M, Biessy C, Tsilidis KK, Ostergaard JN, Overvad K, et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: the EPIC study. J Natl Cancer Inst. 2014;106:dju097. [DOI] [PubMed] [Google Scholar]
- 64.Huang H, Rusiecki J, Zhao N, Chen Y, Ma S, Yu H, et al. Thyroid-stimulating hormone, thyroid hormones, and risk of papillary thyroid cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2017;26:1209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab 2012;97:2682–92. [DOI] [PubMed] [Google Scholar]
- 66.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nature Genetics 2009;41:460–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014;10:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kitahara CM, Schneider AB, Brenner AV. Thyroid Cancer In: Michael Thun MSL, Cerhan James R., Haiman Christopher A., and Schottenfeld David, editor. Schottenfeld and Fraumeni Cancer Epidemiology and Prevention, 4th Edition. New York, NY: Oxford University Press; 2018. p. 839–860. [Google Scholar]
- 69.Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harach HR, Escalante DA, Day ES. Thyroid cancer and thyroiditis in Salta, Argentina: a 40-yr study in relation to iodine prophylaxis. Endocr Pathol 2002;13:175–81. [DOI] [PubMed] [Google Scholar]
- 71.Lind P, Kumnig G, Heinisch M, Igerc I, Mikosch P, Gallowitsch HJ, et al. Iodine supplementation in Austria: methods and results. Thyroid 2002;12:903–7. [DOI] [PubMed] [Google Scholar]
- 72.Burgess JR, Dwyer T, McArdle K, Tucker P, Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978–1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab 2000;85:1513–7. [DOI] [PubMed] [Google Scholar]
- 73.Lee JH, Hwang Y, Song RY, Yi JW, Yu HW, Kim SJ, et al. Relationship between iodine levels and papillary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2017;39:1711–8. [DOI] [PubMed] [Google Scholar]
- 74.Huang F, Cong W, Xiao J, Zhou Y, Gong M, Sun J, et al. Association between excessive chronic iodine exposure and the occurrence of papillary thyroid carcinoma. Oncol Lett 2020;20:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Fang C, Liu L, Liu X, Fan S, Li J, et al. A case-control study of urinary levels of iodine, perchlorate and thiocyanate and risk of papillary thyroid cancer. Environ Int 2018;120:388–93. [DOI] [PubMed] [Google Scholar]
- 76.Bosetti C, Kolonel L, Negri E, Ron E, Franceschi S, Dal Maso L, et al. A pooled analysis of case-control studies of thyroid cancer. VI. Fish and shellfish consumption. Cancer Causes Control 2001;12:375–82. [DOI] [PubMed] [Google Scholar]
- 77.Bosetti C, Negri E, Kolonel L, Ron E, Franceschi S, Preston-Martin S, et al. A pooled analysis of case-control studies of thyroid cancer. VII. Cruciferous and other vegetables (International). Cancer Causes Control 2002;13:765–75. [DOI] [PubMed] [Google Scholar]
- 78.Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev 2012;21:254–60. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, Yatsuya H, Li Y, Ota A, Tamakoshi K, Fujino Y, et al. Prospective study of seaweed consumption and thyroid cancer incidence in women: the Japan collaborative cohort study. Eur J Cancer Prev 2016;25:239–45. [DOI] [PubMed] [Google Scholar]
- 80.Braganza MZ, Potischman N, Park Y, Thompson FE, Hollenbeck AR, Kitahara CM. Adolescent and mid-life diet and subsequent risk of thyroid cancer in the NIH-AARP diet and health study. Int J Cancer 2015;137:2413–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zamora-Ros R, Castaneda J, Rinaldi S, Cayssials V, Slimani N, Weiderpass E, et al. Consumption of fish is not associated with risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. J Nutr 2017;147:1366–73. [DOI] [PubMed] [Google Scholar]
- 82.O’Grady TJ, Kitahara CM, DiRienzo AG, Gates MA. The association between selenium and other micronutrients and thyroid cancer incidence in the NIH-AARP Diet and Health Study. PloS One 2014;9:e110886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aschebrook-Kilfoy B, Shu XO, Gao YT, Ji BT, Yang G, Li HL, et al. Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai Women’s Health Study. Int J Cancer 2013;132:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21(3):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kilfoy BA, Zhang Y, Park Y, Holford TR, Schatzkin A, Hollenbeck A, et al. Dietary nitrate and nitrite and the risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int J Cancer 2011;129:160–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zamora-Ros R, Rinaldi S, Tsilidis KK, Weiderpass E, Boutron-Ruault MC, Rostgaard-Hansen AL, et al. Energy and macronutrient intake and risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer 2015;138:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamora-Ros R, Beraud V, Franceschi S, Cayssials V, Tsilidis KK, Boutron-Ruault MC, et al. Consumption of fruits, vegetables and fruit juices and differentiated thyroid carcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Int J Cancer 2018;142:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao Q, Park Y, Hollenbeck AR, Kitahara CM. Dietary flavonoid intake and thyroid cancer risk in the NIH-AARP Diet and Health Study. Cancer Epidemiol Biomarkers Prev 2014;23:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michikawa T, Inoue M, Shimazu T, Sasazuki S, Iwasaki M, Sawada N, et al. Green tea and coffee consumption and its association with thyroid cancer risk: a population-based cohort study in Japan. Cancer Causes Control 2011;22:985–93. [DOI] [PubMed] [Google Scholar]
- 90.Zamora-Ros R, Alghamdi MA, Cayssials V, Franceschi S, Almquist M, Hennings J, et al. Coffee and tea drinking in relation to the risk of differentiated thyroid carcinoma: results from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 2019;58:3303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zamora-Ros R, Cayssials V, Franceschi S, Kyro C, Weiderpass E, Hennings J, et al. Polyphenol intake and differentiated thyroid cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer 2020;146:1841–50. [DOI] [PubMed] [Google Scholar]
- 92.Zamora-Ros R, Lujan-Barroso L, Achaintre D, Franceschi S, Kyro C, Overvad K, et al. Blood polyphenol concentrations and differentiated thyroid carcinoma in women from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr 2020;113:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019;92:121–35. [DOI] [PubMed] [Google Scholar]
- 95.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev 2011;20:464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dal Maso L, La Vecchia C, Franceschi S, Preston-Martin S, Ron E, Levi F, et al. A pooled analysis of thyroid cancer studies. V. Anthropometric factors. Cancer Causes Control 2000;11:137–44. [DOI] [PubMed] [Google Scholar]
- 97.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 2004;15:35–43. [DOI] [PubMed] [Google Scholar]
- 98.Engeland A, Tretli S, Akslen LA, Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer 2006;95:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol 2005;23:4742–54. [DOI] [PubMed] [Google Scholar]
- 100.Kitahara CM, McCullough M, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid 2016;26:306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahman ST, Pandeya N, Neale RE, McLeod DSA, Bain CJ, Baade PD, et al. Obesity Is associated with BRAF(V600E)-mutated thyroid cancer. Thyroid 2020;30:1518–27. [DOI] [PubMed] [Google Scholar]
- 102.Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocr Pract 2015;21:686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zagzag J, Malone MK, Lopresti MA, Ogilvie JB, Patel KN, Heller KS. Method of detection of well-differentiated thyroid cancers in obese and non-obese patients. PloS One 2016;11:e0152768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kitahara CM, Gamborg M, Berrington de Gonzalez A, Sorensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Research 2014;74:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitahara CM, Pfeiffer RM, Sosa JA, Shiels MS. Impact of overweight and obesity on US papillary thyroid cancer incidence trends (1995–2015). J Natl Cancer Inst 2020;112:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laaksonen MA, MacInnis RJ, Canfell K, Shaw JE, Magliano DJ, Banks E, et al. Thyroid cancers potentially preventable by reducing overweight and obesity in Australia: A pooled cohort study. Int J Cancer 2022;150:1281–90. [DOI] [PubMed] [Google Scholar]
- 108.Pazaitou-Panayiotou K, Polyzos SA, Mantzoros CS. Obesity and thyroid cancer: epidemiologic associations and underlying mechanisms. Obes Rev 2013;14:1006–22. [DOI] [PubMed] [Google Scholar]
- 109.Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid 2011;21:957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015;38:734–5. [DOI] [PubMed] [Google Scholar]
- 111.Becker C, Jick SS, Meier CR, Bodmer M. No evidence for a decreased risk of thyroid cancer in association with use of metformin or other antidiabetic drugs: a case-control study. BMC Cancer 2015;15:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tseng CH. Thyroid cancer risk is not increased in diabetic patients. PloS One 2012;7:e53096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitahara CM, Platz EA, Beane Freeman LE, Black A, Hsing AW, Linet MS, et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control 2012;23:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tseng CH. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PloS One 2014;9:e109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Almquist M, Johansen D, Bjorge T, Ulmer H, Lindkvist B, Stocks T, et al. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can). Cancer Causes Control 2011;22:743–51. [DOI] [PubMed] [Google Scholar]
- 116.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev 1997;6:863–73. [PubMed] [Google Scholar]
- 117.Dossus L, Franceschi S, Biessy C, Navionis AS, Travis RC, Weiderpass E, et al. Adipokines and inflammation markers and risk of differentiated thyroid carcinoma: The EPIC study. Int J Cancer 2018;142:1332–42. [DOI] [PubMed] [Google Scholar]
- 118.Schmidt JA, Allen NE, Almquist M, Franceschi S, Rinaldi S, Tipper SJ, et al. Insulin-like growth factor-I and risk of differentiated thyroid carcinoma in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2014;23:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kitahara CM, Slettebo Daltveit D, Ekbom A, Engeland A, Gissler M, Glimelius I, et al. Maternal health, in-utero, and perinatal exposures and risk of thyroid cancer in offspring: a Nordic population-based nested case-control study. Lancet Diabetes Endocrinol 2021;9:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deziel NC, Zhang Y, Wang R, Wiemels JL, Morimoto L, Clark CJ, et al. Birth characteristics and risk of pediatric thyroid cancer: a population-based record-linkage study in California. Thyroid 2021;31:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Crump C, Sundquist J, Sieh W, Winkleby MA, Sundquist K. Fetal growth and subsequent maternal risk of thyroid cancer. Int J Cancer 2016;138:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeng Q, Chen GG, Vlantis AC, van Hasselt CA. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Prolif 2007;40:921–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schonfeld SJ, Ron E, Kitahara CM, Brenner A, Park Y, Sigurdson AJ, et al. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol 2011;35:e85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Braganza MZ, de Gonzalez AB, Schonfeld SJ, Wentzensen N, Brenner AV, Kitahara CM. Benign breast and gynecologic conditions, reproductive and hormonal factors, and risk of thyroid cancer. Cancer Prev Res (Phila) 2014;7:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zamora-Ros R, Rinaldi S, Biessy C, Tjonneland A, Halkjaer J, Fournier A, et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer 2015;136:1218–27. [DOI] [PubMed] [Google Scholar]
- 126.Kabat GC, Kim MY, Wactawski-Wende J, Lane D, Wassertheil-Smoller S, Rohan TE. Menstrual and reproductive factors, exogenous hormone use, and risk of thyroid carcinoma in postmenopausal women. Cancer Causes Control 2012;23:2031–40. [DOI] [PubMed] [Google Scholar]
- 127.Shin S, Sawada N, Saito E, Yamaji T, Iwasaki M, Shimazu T, et al. Menstrual and reproductive factors in the risk of thyroid cancer in Japanese women: the Japan Public Health Center-Based Prospective Study. Eur J Cancer Prev 2018;27:361–9. [DOI] [PubMed] [Google Scholar]
- 128.Horn-Ross PL, Canchola AJ, Ma H, Reynolds P, Bernstein L. Hormonal factors and the risk of papillary thyroid cancer in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev 2011;20:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pham TM, Fujino Y, Mikami H, Okamoto N, Hoshiyama Y, Tamakoshi A, et al. Reproductive and menstrual factors and thyroid cancer among Japanese women: the Japan Collaborative Cohort Study. J Womens Health (Larchmt) 2009;18:331–5. [DOI] [PubMed] [Google Scholar]
- 130.La Vecchia C, Ron E, Franceschi S, Dal Maso L, Mark SD, Chatenoud L, et al. A pooled analysis of case-control studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control 1999;10:157–66. [DOI] [PubMed] [Google Scholar]
- 131.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, et al. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control 1999;10:143–55. [DOI] [PubMed] [Google Scholar]
- 132.Troisi R, Bjorge T, Gissler M, Grotmol T, Kitahara CM, Myrtveit Saether SM, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med 2018;283:430–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hannibal CG, Jensen A, Sharif H, Kjaer SK. Risk of thyroid cancer after exposure to fertility drugs: results from a large Danish cohort study. Hum Reprod 2008;23:451–6. [DOI] [PubMed] [Google Scholar]
- 134.Rahman ST, Pandeya N, Neale RE, McLeod DSA, Baade PD, Youl PH, et al. Risk of thyroid cancer following hysterectomy. Cancer Epidemiol 2021;72:101931. [DOI] [PubMed] [Google Scholar]
- 135.Bernier MO, Withrow DR, Berrington de Gonzalez A, Lam CJK, Linet MS, Kitahara CM, et al. Trends in pediatric thyroid cancer incidence in the United States, 1998–2013. Cancer 2019;125:2497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hatch M, Brenner AV, Cahoon EK, Drozdovitch V, Little MP, Bogdanova T, et al. Thyroid cancer and benign nodules after exposure in utero to fallout from Chernobyl. J Clin Endocrinol Metab 2019;104:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitahara CM, Linet MS, Beane Freeman LE, Check DP, Church TR, Park Y, et al. Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control 2012;23:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang H, Zhao N, Chen Y, Deziel N, Dai M, Li N, et al. Alcohol consumption and risk of thyroid cancer: a population based case-control study in Connecticut. Adv Exp Med Biol 2018;1032:1–14. [DOI] [PubMed] [Google Scholar]
- 139.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst 2009;101:296–305. [DOI] [PubMed] [Google Scholar]
- 140.Yeo Y, Shin DW, Han KD, Kim D, Kim TH, Chun S, et al. Smoking, alcohol consumption, and the risk of thyroid cancer: a population-based Korean cohort study of 10 million people. Thyroid 2022; Online ahead of print. doi: 10.1089/thy.2021.0675. [DOI] [PubMed] [Google Scholar]
- 141.Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, et al. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control 2003;14:773–85. [DOI] [PubMed] [Google Scholar]
- 142.Rahman ST, Pandeya N, Neale RE, McLeod DSA, Baade PD, Youl PH, et al. Tobacco smoking and risk of thyroid cancer according to BRAF(V600E) mutational subtypes. Clin Endocrinol (Oxf) 2021;95:891–900. [DOI] [PubMed] [Google Scholar]
- 143.Boas M, Main KM, Feldt-Rasmussen U. Environmental chemicals and thyroid function: an update. Curr Opin Endocrinol Diabetes Obes 2009;16:385–91. [DOI] [PubMed] [Google Scholar]
- 144.Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, et al. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ Int 2017;107:235–42. [DOI] [PubMed] [Google Scholar]
- 145.Aschebrook-Kilfoy B, DellaValle CT, Purdue M, Kim C, Zhang Y, Sjodin A, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the Prostate, Colorectal, Lung, and Ovarian Cancer Screening Trial cohort. Am J Epidemiol 2015;181:883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aschebrook-Kilfoy B, Ward MH, Della Valle CT, Friesen MC. Occupation and thyroid cancer. Occup Environ Med 2014;71:366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lerro CC, Koutros S, Andreotti G, Friesen MC, Alavanja MC, Blair A, et al. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med 2015;72:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lerro CC, Beane Freeman LE, DellaValle CT, Andreotti G, Hofmann JN, Koutros S, et al. Pesticide exposure and incident thyroid cancer among male pesticide applicators in Agricultural Health Study. Environ Int 2021;146:106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lerro CC, Jones RR, Langseth H, Grimsrud TK, Engel LS, Sjodin A, et al. A nested case-control study of polychlorinated biphenyls, organochlorine pesticides, and thyroid cancer in the Janus Serum Bank cohort. Environ Res 2018;165:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pellegriti G, De Vathaire F, Scollo C, Attard M, Giordano C, Arena S, et al. Papillary thyroid cancer incidence in the volcanic area of Sicily. J Natl Cancer Inst 2009;101:1575–83. [DOI] [PubMed] [Google Scholar]
- 151.Duntas LH, Doumas C. The ‘rings of fire’ and thyroid cancer. Hormones (Athens) 2009;8:249–53. [DOI] [PubMed] [Google Scholar]
- 152.Malandrino P, Russo M, Giani F, Pellegriti G, Vigneri P, Belfiore A, et al. Increased thyroid cancer incidence in volcanic areas: a role of increased heavy metals in the environment? Int J Mol Sci 2020;21:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kitahara CM, Yanik EL, Ladenson PW, Hernandez BY, Lynch CF, Pawlish KS, et al. Risk of thyroid cancer among solid organ transplant recipients. Am J Transplant 2017;17:2911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo J, Hendryx M, Nassir R, Cheng TD, Lane D, Margolis KL. Benign breast disease and risk of thyroid cancer. Cancer Causes Control 2017;28:913–20. [DOI] [PubMed] [Google Scholar]
- 155.Bolf EL, Sprague BL, Carr FE. A linkage between thyroid and breast cancer: a common etiology? Cancer Epidemiol Biomarkers Prev 2019;28:643–9. [DOI] [PubMed] [Google Scholar]
- 156.Clarke AE, Pooley N, Marjenberg Z, Langham J, Nicholson L, Langham S, et al. Risk of malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis. Semin Arthritis Rheum 2021;51:1230–41. [DOI] [PubMed] [Google Scholar]
- 157.Kang J, Kim H, Kim J, Choi S, Jung SY, Jang EJ, et al. Risk of malignancy in Korean patients with primary Sjogren’s syndrome. Int J Rheum Dis 2020;23:1240–7. [DOI] [PubMed] [Google Scholar]
- 158.Zhang D, Jones RR, James P, Kitahara CM, Xiao Q. Associations between artificial light at night and risk for thyroid cancer: A large US cohort study. Cancer 2021;127:1448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zamoiski RD, Cahoon EK, Freedman DM, Linet MS, Kitahara CM. Prospective study of ultraviolet radiation exposure and thyroid cancer risk in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hemminki K, Li X. Familial risk of cancer by site and histopathology. Int J Cancer 2003;103:105–9. [DOI] [PubMed] [Google Scholar]
- 161.Hemminki K, Eng C, Chen B. Familial risks for nonmedullary thyroid cancer. J Clin Endocrinol Metab 2005;90:5747–53. [DOI] [PubMed] [Google Scholar]
- 162.Dudbridge F, Brown SJ, Ward L, Wilson SG, Walsh JP. How many cases of disease in a pedigree imply familial disease? Ann Hum Genet 2018;82:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miasaki FY, Fuziwara CS, Carvalho GA, Kimura ET. Genetic mutations and variants in the susceptibility of familial non-medullary thyroid cancer. Genes (Basel) 2020;11:1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen YH, Zhang YQ. Exploration of the association between FOXE1 gene polymorphism and differentiated thyroid cancer: a meta-analysis. BMC Med Genet 2018;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ran R, Tu G, Li H, Wang H, Mou E, Liu C. Genetic variants associated with thyroid cancer risk: comprehensive research synopsis, meta-analysis, and cumulative epidemiological evidence. J Oncol 2021;2021:9967599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liyanarachchi S, Gudmundsson J, Ferkingstad E, He H, Jonasson JG, Tragante V, et al. Assessing thyroid cancer risk using polygenic risk scores. Proc Natl Acad Sci USA 2020;117:5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.PDQ® Screening and Prevention Editorial Board. PDQ Thyroid Cancer Screening [Internet]. Bethesda (MD): National Cancer Institute. 2017. Aug 29 [cited 2021 Nov 24]. Available from: https://www.cancer.gov/types/thyroid/hp/thryoid-screening-pdq. [Google Scholar]
- 168.U.S. Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, et al. Screening for thyroid cancer: U.S. Preventive Services Task Force recommendation statement. JAMA 2017;317:1882–7. [DOI] [PubMed] [Google Scholar]
- 169.Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016;375:2307. [DOI] [PubMed] [Google Scholar]
- 170.Skinner MA, Moley JA, Dilley WG, Owzar K, Debenedetti MK, Wells SA Jr. Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med 2005;353:1105–13. [DOI] [PubMed] [Google Scholar]
- 171.Klubo-Gwiezdzinska J, Yang L, Merkel R, Patel D, Nilubol N, Merino MJ, et al. Results of screening in familial non-medullary thyroid cancer. Thyroid 2017;27:1017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Geurts JL, Strong EA, Wang TS, Evans DB, Clarke CN. Screening guidelines and recommendations for patients at high risk of developing endocrine cancers. J Surg Oncol 2020;121:975–83. [DOI] [PubMed] [Google Scholar]
- 173.Lamartina L, Grani G, Durante C, Filetti S, Cooper DS. Screening for differentiated thyroid cancer in selected populations. Lancet Diabetes Endocrinol 2020;8:81–8. [DOI] [PubMed] [Google Scholar]
- 174.Reiners C, Biko J, Haenscheid H, Hebestreit H, Kirinjuk S, Baranowski O, et al. Twenty-five years after Chernobyl: outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. J Clin Endocrinol Metab 2013;98:3039–48. [DOI] [PubMed] [Google Scholar]
- 175.Brignardello E, Felicetti F, Castiglione A, Gallo M, Maletta F, Isolato G, et al. Ultrasound surveillance for radiation-induced thyroid carcinoma in adult survivors of childhood cancer. Eur J Cancer 2016;55:74–80. [DOI] [PubMed] [Google Scholar]
- 176.Clement SC, Kremer LCM, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, et al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of childhood, adolescent and young adult cancer: Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer Treat Rev 2018;63:28–39. [DOI] [PubMed] [Google Scholar]
- 177.Goske MJ, Frush DP, Brink JA, Kaste SC, Butler PF, Pandharipande PV. Curbing potential radiation-induced cancer risks in oncologic imaging: perspectives from the ‘image gently’ and ‘image wisely’ campaigns. Oncology (Williston Park) 2014;28:232–8, 43. [PubMed] [Google Scholar]
- 178.Schneider AB, Kaplan MM, Mihailescu DV. Thyroid collars in dental radiology: 2021 update. Thyroid 2021;31:1291–6. [DOI] [PubMed] [Google Scholar]
- 179.Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev 2010;31:756–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brito JP, Schilz S, Singh Ospina N, Rodriguez-Gutierrez R, Maraka S, Sangaralingham LR, et al. Antithyroid drugs-the most common treatment for Graves’ disease in the United States: a nationwide population-based study. Thyroid 2016;26:1144–5. [DOI] [PubMed] [Google Scholar]
- 181.Leung AM, Bauer AJ, Benvenga S, Brenner AV, Hennessey JV, Hurley JR, et al. American Thyroid Association scientific statement on the use of potassium iodide ingestion in a nuclear emergency. Thyroid 2017;27:865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.World Health Organization (WHO). Iodine thyroid blocking: guidelines for use in planning for and responding to radiological and nuclear emergencies. WHO guidelines approved by the Guidelines Review Committee. Geneva, Switzerland; 2017. [PubMed] [Google Scholar]
- 183.U.S. Food & Drug Administration. Guidance: Potassium iodide as a thyroid blocking agent in radiation emergencies. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2001. [Google Scholar]


