Supplemental Digital Content is Available in the Text.
Key Words: varenicline, OC-01 nasal spray, dry eye disease
Purpose:
The purpose of this trial was to evaluate the safety and efficacy of OC-01 (varenicline solution), a nicotinic acetylcholine receptor agonist nasal spray, on signs and symptoms of dry eye disease.
Methods:
A phase 2b, multicenter, randomized, double-masked, vehicle-controlled trial (ONSET-1; NCT03636061) was performed. Patients were aged 22 years or older with a physician's diagnosis of dry eye disease and previous use of artificial tears were randomized 1:1:1:1 to control (vehicle nasal spray twice daily [BID]), OC-01 0.006 mg BID, OC-01 0.03 mg BID, and OC-01 0.06 mg BID. The primary end point was the change in the anesthetized Schirmer test score from baseline to day 28 in the study eye. The secondary end points included the change in the eye dryness score from baseline to day 28.
Results:
One hundred eighty-two patients were randomized. After 28 days, patients who received OC-01 0.03 or 0.06 mg showed a statistically significant improvement in tear film production relative to vehicle, with least squares mean differences from vehicle of 7.7 mm [95% confidence interval, 3.8–11.7; P < 0.001] with OC-01 0.03 mg and 7.5 mm (95% confidence interval, 3.4–11.6; P < 0.001) with OC-01 0.06 mg. Patients receiving OC-01 0.03 mg showed a significant reduction in the eye dryness score by day 28 versus vehicle (P = 0.021); those receiving the OC-01 0.06 mg dose showed a nonsignificant reduction versus vehicle. OC-01 administration was associated with sneezing (62%–84%) and cough (9%–25%); these were transient and predominantly mild in severity.
Conclusions:
OC-01 nasal spray administered BID at 0.03 and 0.06 mg resulted in significant improvements in signs and symptoms of dry eye disease, was well tolerated, and warrants further clinical investigation.
Dry eye disease, a multifactorial, age-related disorder affecting the ocular surface, results in severe pain, visual impairment, tear film hyperosmolarity and instability, inflammation, and corneal damage.1,2 Approximately 14.5% (30 million) of the adult population in the United States experience dry eye disease, with many people affected worldwide.2,3
Our understanding of the complexity of the tear film itself, as well as the etiology and pathogenesis of dry eye disease, has improved markedly over the past several decades.4,5 Normal tear film—a complex, dynamic solution responsible for keeping the ocular surface healthy—is maintained by the lacrimal functional unit, which includes the ocular surface (tear film and corneal and conjunctival epithelia with mucin-producing goblet cells), meibomian glands, the main and accessory lacrimal glands, and their interconnecting innervation.1 Dry eye disease is associated with a reduction of at least 1 of the 3 major layers of the tear film (mucin, aqueous, or lipid), resulting in loss of tear film homeostasis.2,6 The natural tear film contains numerous proteins, growth factors, immune modulators, lysozymes, and antibodies and has both antimicrobial and antiinflammatory properties.6 In patients with dry eye disease and corneal damage, a feedback loop has been shown that upregulates production of beneficial proteins in the lacrimal gland that have a supportive role in promoting tissue maintenance.7,8
A reduction in the volume and disruption of homeostasis in numerous animal models have elucidated the beneficial effects of tear film and the consequences of insufficient tear film production. One such animal model of dry eye disease injects botulinum toxin into the lacrimal gland to block the release of acetylcholine at cholinergic nerves to inhibit both fusion of the neurotransmitter at the presynaptic junction and contraction of myoepithelial cells of the lacrimal gland.9 This model allows investigation of the effects of a decrease in tear film production that lasts for weeks but is reversible once the effects of the toxin wear off. Because lacrimal gland tear film production decreases after toxin injection, corneal fluorescein staining and levels of inflammatory biomarkers on the ocular surface correspondingly increase.9,10 Interestingly, when the botulinum toxin wears off and tear film production returns to normal levels, corneal fluorescein staining disappears and inflammatory markers return to normal levels. This model highlights the importance of tear film homeostasis on the ocular surface in reducing corneal staining and inflammation. The available clinical evidence suggests that stimulation of naturally produced tear film is a viable treatment approach to restore tear film homeostasis, increase the concentration of numerous important tear components on the ocular surface, reduce inflammatory mediators, and produce endogenous antiinflammatory compounds, such as lactoferrin and lacritin.11–13
Stimulation of tear film production is driven by sensory afferent nerves of the cornea and conjunctiva, as well as efferent parasympathetic nerves that innervate the lacrimal gland (acinar seromucous secretory and myoepithelial cells), meibomian glands, and goblet cells.14 This parasympathetic nerve pathway, known as the trigeminal parasympathetic pathway (TPP), can be accessed through the central nervous system or peripherally through the nasal cavity.14,15 Accessing the TPP through the nasal cavity represents a local, noninvasive approach that bypasses the ocular surface, which is especially important when treating eyes with corneal pathology. The TPP, which plays a key part in maintaining a healthy tear film,1 accounts for approximately 34% of basal tear production.1,16 Stimulation of the TPP with an electrical neurostimulation device has been shown to be beneficial and to stimulate production of all 3 layers of the tear film.17–22 Thus, activating the TPP can upregulate the body's production of a natural 3-layer tear film.1,23
OC-01 (varenicline solution) is a preservative-free, highly selective nicotinic acetylcholine receptor (nAChR) agonist nasal spray (OC-01 VNS) in clinical development for treating the signs and symptoms of dry eye disease. The active ingredient of OC-01, varenicline, has been approved as a medication to aid smoking cessation since 2006 in the United States under the tradename CHANTIX (Pfizer Inc, Mission, KS; also known as CHAMPIX in some countries). OC-01 VNS activates the TPP, thereby upregulating natural tear production. OC-01 has full agonist activity at the α7 receptor and partial agonist activity at the α3β4, α3α5β4, α4β2, and α4α6β2 receptors.24 nAChRs are present on the trigeminal nerve within the nasal cavity throughout the nasal mucosa.15
Published reports have shown that nAChRs can mediate afferent signals in the trigeminal nerve in response to nasal stimuli.15 These signals form the basis for the role of the TPP in tear production. OC-01, a cholinergic agonist, promotes the activation of the nasolacrimal reflex, promoting natural tear production in individuals with dry eye disease. This phase 2b trial evaluated the safety and efficacy of OC-01 VNS (TYRVAYA [varenicline solution] 0.03 mg; Oyster Point Pharma Inc., Princeton, NJ, USA) on the signs and symptoms of dry eye disease.
METHODS
Study Design and Population
The ONSET-1 clinical trial (clinicaltrials.gov; NCT03636061) was a phase 2b, multicenter, randomized, double-masked, vehicle-controlled trial conducted between August 15, 2018, and September 26, 2018 (Fig. 1). At screening, patients (aged ≥22 years, based on the US Food and Drug Administration definition of an adult population) were eligible if they had a physician's diagnosis of dry eye disease and had used or expressed the desire to use artificial tears for symptoms within 6 months of study start. Eligible patients had an Ocular Surface Disease Index score of at least 23 with up to 3 responses of “not applicable” at screening. Patients also were required to have all of the following in the study eye at screening: corneal fluorescein staining score [National Eye Institute Fluorescein Staining Scale (grade 0, 1, 2, and 3)] of at least 2 in at least 1 corneal region or at least 4 for all corneal regions, baseline Schirmer test score (STS; with topical anesthesia) 10 mm/5 minutes or lower, and STS at least 7 mm larger in the same eye after nasal stimulation (using bilateral intranasal cotton swab stimulation). Performing an anesthetized Schirmer test has been shown to be more objective and reliable in patients with dry eye disease than performing the test without anesthesia, and it measures basal tear film secretion.25 Best-corrected visual acuity (BCVA) less than 0.7 logarithm of the minimum angle of resolution was required in each eye.
FIGURE 1.
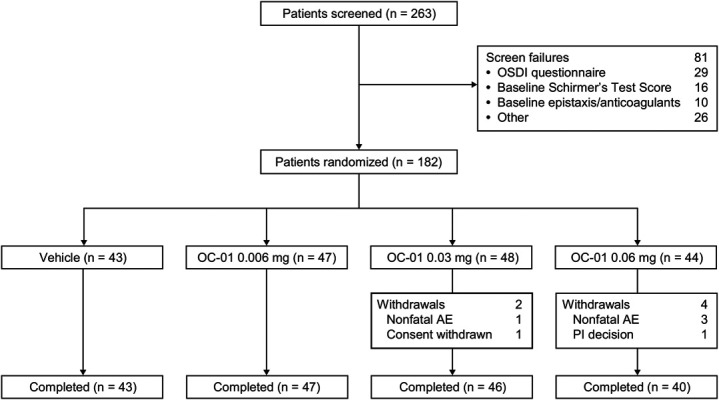
CONSORT flow diagram. AE, adverse event; OSDI, Ocular Surface Disease Index; PI, principal investigator.
The study eye was defined as the eye meeting the inclusion criteria. If both eyes qualified, the study eye was the eye with the greatest increase in tear production with a cotton swab stimulation at the screening visit. If there was no difference in stimulated tear production, the eye with the lower basal STS at screening was chosen as the study eye. If neither measure differed, the right eye was used as the study eye.
The key exclusion criteria included chronic or recurrent epistaxis, coagulation disorders, or other conditions that might lead to a clinically significant risk of increased bleeding; a history of seizures; current use of nasal continuous positive airway pressure therapy; nasal or sinus surgery or significant trauma to these areas; a corneal transplant; contact lens use within 7 days of the first visit or anticipated contact lens use during the study period; and any form of punctal or intracanalicular occlusion. Patients also were excluded if they had any systemic, ocular, or nasal disease or any condition that would interfere with patient safety or data interpretation. Patients who had used snuff, chewing tobacco, e-cigarettes, or cigarettes/cigars within 30 days of study start or during the study were excluded. Patients were also excluded if they used any nAChR agonist [nicotine (NICODERM; GSK, Warren, NJ; NICORETTE; GSK; and NICOTROL NS; Pharmacia & Upjohn, Co, Division of Pfizer, Inc, New York, NY), cytisine (TABEX; Sopharma, Sofia, Bulgaria, and DESMOXAN; Aflofarm, Pabianice, Poland), and varenicline (CHANTIX, Pfizer Inc)] within 30 days of study start. Patients with an ongoing ocular condition, such as infection or clinically significant corneal condition, were excluded, although blepharitis not requiring treatment and/or mild meibomian gland disease was allowed. Institutional review board/ethics committee approval was obtained. The study was conducted in compliance with the ethical principles of the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice. All patients provided written informed consent before participation.
Procedures
Eligible patients were randomized 1:1:1:1 to 1 of 4 treatment groups: 1) vehicle [a proprietary formulation consisting primarily of phosphate-buffered saline, twice daily (BID)], 2) OC-01 0.006 mg BID (low dose), 3) OC-01 0.03 mg BID (medium dose), and 4) OC-01 0.06 mg BID (high dose). Randomization was performed electronically; there was no stratification by baseline factors. Each patient received a study kit labeled with a kit number that corresponded with the system-assigned number. The study sponsor, investigators, statisticians, and staff were masked to treatment assignment during randomization and throughout the duration of the study. Patients were instructed to self-administer the study drug, except on days when STS was assessed, when the first daily dose of the study drug was given in the clinic. The STS collection procedure was standardized across all investigational centers to be collected at 5 minutes after study drug administration.
Demographics and Baseline Characteristics
Demographic and baseline characteristics were summarized for age, sex, ethnicity, race, and ocular history. Quantitative variables were summarized using the number of patients, mean, SD, median, 25th and 75th percentiles, and minimum and maximum. Qualitative variables were summarized using counts and percentages.
Efficacy Assessments
The primary end point of the study was the change in anesthetized STS from baseline to day 28 in the study eye. As a post hoc exploratory adjunct to this primary outcome, the percentages of patients gaining at least 10 mm in STS were also evaluated.
The study had 2 secondary end points: change in eye dryness score (EDS) from baseline to day 28 and change in EDS from baseline to day 21 (5 minutes posttreatment) in the controlled adverse environment chamber. To assess EDS, which is frequently used in dry eye disease studies,26,27 patients were asked to rate their ocular symptoms due to eye dryness on a 100-mm visual analog scale, where 0 indicated “no discomfort” and 100 indicated “maximal discomfort” (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/ICO/B329). The controlled adverse environment chamber simulates everyday situations that individuals with dry eye disease encounter.28,29 Importantly, the controlled adverse environment standardizes these environmental conditions by regulating humidity, temperature, airflow, lighting, and visual tasking.28,29 Patients with dry eye disease are exposed to the controlled adverse environment for 2 hours, during which EDS can be evaluated every 5 minutes before, during, and after exposure to the study drug. Patients were tested using the EDS and Ora Calibra Ocular Discomfort Scale (ODS; 0–4 points; 0 = none and 4 = severe) before entering the controlled adverse environment chamber and then every 5 minutes until the end of the 2-hour period. Treatment with the study drug or vehicle was administered if a patient reported an ODS of ≥ 3 at 2 or more consecutive time points in at least 1 eye during controlled adverse environment exposure (patients with an ODS of ≥ 3 at time = 0 in the controlled adverse environment chamber for an eye must have reported an ODS of 4 for 2 consecutive measurements for that eye to receive treatment).
The change in corneal fluorescein staining from baseline to day 28 was an exploratory end point; the study was not powered to detect expected differences in this measurement. Changes in corneal fluorescein staining were based on the National Eye Institute's scale that measures staining in 5 distinct regions of the cornea—central, superior, inferior, nasal, and temporal—as well as a cumulative score across all regions.
Safety Assessments
Safety end points included treatment-emergent adverse events (TEAEs) and change in BCVA, slitlamp biomicroscopy findings, and intranasal examination. Adverse events, which were coded using the Medical Dictionary for Regulatory Activities version 20.1, were categorized as ocular or nonocular and by System Organ Class and Preferred Term. The investigator graded TEAE severity as mild, moderate, or severe and recorded the relationship to the study drug as definite, probable, possible, not related, or unclassified. The investigator defined expectedness (ie, expected, unexpected, or not applicable) using existing safety information about the study drug. A TEAE was considered to be a serious adverse event (SAE) if it was life-threatening, resulted in hospitalization admission or prolonged an existing hospitalization, caused a persistent or significant incapacity or substantial disruption in the ability to conduct normal life functions, caused a congenital anomaly or birth defect, or resulted in death. The intranasal examination, which was conducted to monitor nasal mucosal integrity, was performed through a nasal speculum examination at the screening visit and at day 28 or on early termination.
Statistical Analyses
Determination of Sample Size
The primary hypothesis of the study was that either the medium or high dose, or both, would be superior to vehicle regarding the change in STS from baseline to day 28. Because there was no formal hypothesis for the low-dose group, it was not considered in the power calculations. Data from previous studies led to the assumption that a mean difference of 6 mm and a SD of 10 units between the medium or high doses of OC-01 nasal spray and vehicle would provide 80% power to detect a statistically significant difference for at least 1 of the 2 comparisons (ie, medium dose vs. vehicle or high dose vs. vehicle). Two-sided t tests at a Dunnett-corrected 5% significance level were used to test the primary hypotheses.
Analysis Populations
The statistical analyses of baseline and efficacy data were performed using the intent-to-treat population, which was defined as all randomized patients, with evaluations based on the treatment group to which patients were randomized. Furthermore, post hoc last observation carried forward (LOCF) analyses were performed for mean change from baseline to day 28 in STS and in EDS.
Statistical analysis of safety data was performed using the safety population, which was defined as all randomized patients who received at least 1 dose of study drug; evaluations were based on the treatment patients received.
Primary Efficacy Analyses
The primary end point of the study was the change in anesthetized STS from baseline to day 28 in the study eye. The differences in the means in the study eye of the medium-dose and high-dose groups versus vehicle were analyzed using an analysis of covariance (ANCOVA) model, with treatment, site, baseline STS, and STS with a cotton swab stimulation as covariates. Data were presented as least squares (LS) mean changes with 95% confidence intervals (CIs).
Secondary Efficacy Analyses
The study had 2 prespecified secondary end points: 1) the reduction in EDS from baseline to day 28 and 2) the change in EDS from baseline to day 21 (5 minutes posttreatment) in the controlled adverse environment. Both secondary end points were analyzed using ANCOVA models, with treatment, site, and baseline EDS as covariates.
Exploratory Analysis
The percentages of patients gaining at least 10 mm in STS in the medium-dose and high-dose groups versus vehicle in the study eye and the fellow eye were analyzed, post hoc, using the Pearson χ2 test. The Cochran–Mantel–Haenszel test was used for the LOCF analysis for imputation of missing data. The change in corneal fluorescein staining from baseline to day 28 was analyzed using an ANCOVA model with baseline values and study site as covariates.
RESULTS
Patient Disposition and Baseline Characteristics
In total, 182 patients were randomized (1:1:1:1) to receive vehicle (vehicle nasal spray) (n = 43), OC-01 0.006 mg nasal spray (low dose; n = 47), OC-01 0.03 mg nasal spray (medium dose; n = 48), or OC-01 0.06 mg nasal spray (high dose; n = 44). Six (3%) patients discontinued from the study; 4 discontinued because of nonfatal adverse events (discussed below), including 1 in the medium-dose group and 3 in the high-dose group. The investigator withdrew 1 patient in the OC-01 0.06 mg treatment group because of a protocol violation (prohibited concomitant medication), and 1 patient in the OC-01 0.03 mg treatment group withdrew by choice (Fig. 1).
The study population demographics were representative of the geographic distribution of study sites. Overall, the mean (SD) age of the population was 65.5 (10.8) years; 86% of the patients were White, and 75% were female (Table 1). Baseline clinical characteristics were generally similar across all treatment groups, with the exception of lower mean disease severity in the high-dose group owing to a higher mean STS, lower mean EDS, and lower mean Ocular Surface Disease Index score compared with the other treatment groups (Table 1). These differences indicate a potentially less severe dry eye disease population in the high-dose group by chance.
TABLE 1.
Baseline Characteristics
| Vehicle (n = 43) | OC-01, 0.006 mg (n = 47) | OC-01, 0.03 mg (n = 48) | OC-01, 0.06 mg (n = 44) | Total (N = 182) | |
| Patient characteristic | |||||
| Age (yrs) | 64.0 (10.3) | 64.2 (12.7) | 66.5 (9.4) | 67.4 (10.6) | 65.5 (10.8) |
| Male, no. (%) | 11 (26) | 11 (23) | 14 (29) | 9 (20) | 45 (25) |
| Race, no. (%) | |||||
| White | 40 (93) | 42 (89) | 39 (81) | 36 (82) | 157 (86) |
| Black/African American | 2 (5) | 2 (4) | 4 (8) | 6 (14) | 14 (8) |
| Asian | 1 (2) | 3 (6) | 4 (8) | 0 (0) | 8 (4) |
| Other* | 0 | 0 (0) | 1 (2) | 2 (5) | 3 (2) |
| Clinical characteristic | |||||
| STS (mm) | 4.5 (2.9) | 5.2 (3.1) | 4.8 (2.7) | 5.5 (3.0) | 5.0 (2.9) |
| Cotton swab STS (mm) | 25.9 (7.0) | 28.2 (7.3) | 29.2 (7.8) | 29.6 (7.5) | 28.3 (7.5) |
| EDS (mm)† | 65.2 (17.7) | 65.6 (20.1) | 63.7 (18.4) | 53.5 (22.4) | 62.1 (20.2) |
| Ora Calibra Ocular Discomfort Scale (grade) | 2.7 (0.9) | 2.8 (0.9) | 2.7 (0.9) | 2.5 (1.0) | 2.7 (0.9) |
| Visual acuity (logMAR) | 0.09 (0.12) | 0.12 (0.13) | 0.11 (0.16) | 0.13 (0.17) | 0.11 (0.15) |
| Ocular Surface Disease Index (grade)† | 51.7 (16.6) | 53.8 (17.0) | 49.7 (15.7) | 45.5 (15.0) | 50.2 (16.2) |
| Corneal fluorescein staining (grade) | 6.7 (2.4) | 5.9 (1.6) | 6.7 (2.1) | 6.9 (2.4) | 6.6 (2.2) |
Data are means and SDs unless otherwise specified.
Other includes American Indian or Alaska Native, and Native Hawaiian or other Pacific Islander.
Assessment relates to both eyes.
logMAR, logarithm of the minimum angle of resolution.
A larger percentage of those in the high-dose group reported any ocular medical history compared with the other treatment groups (Table 1). There was variability in the incidence of several individual ocular medical histories across treatment groups, although no consistent pattern was observed. The most common ocular medical histories were the presence of cataract and previous cataract surgery.
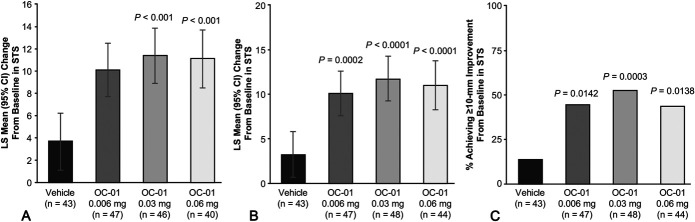
Primary Efficacy End Point
Using the change in anesthetized STS from baseline to day 28, patients who received the OC-01 0.03 or 0.06 mg dose showed statistically significantly greater improvements in tear film production compared with vehicle (Fig. 2A). The LS mean difference between the OC-01 0.03 mg dose and vehicle was 7.7 mm (95% CI, 3.8–11.7; P < 0.001) and was 7.5 mm (95% CI, 3.4–11.6; P < 0.001) with the OC-01 0.06 mg dose (Table 2). The LS mean change in STS after the initial first exposure from baseline for OC-01 0.03 mg, 0.06 mg, and vehicle was 11.4 mm (95% CI, 8.9–13.9), 11.1 mm (95% CI, 8.5–13.7), and 3.7 mm (95% CI, 1.1–6.2), respectively (Table 2).
FIGURE 2.
A, LS mean change from baseline to day 28 in STS. B, The LS mean change from baseline to day 28 in STS with missing data imputed using last available data. C, The percentage of patients with at least a 10-mm change in STS at day 28 versus baseline (intent-to-treat population) with missing data imputed using last available data. All comparisons made with the control group. No formal statistical comparisons were performed for the 0.006 mg dose versus vehicle; P values for this dose were assessed post hoc and represent nominal values. Error bars indicate CIs. The results in A are derived from a prespecified analysis and B and C are from a post hoc analysis.
TABLE 2.
STS in the Study Eye at Day 28 (Primary End Point)
| OC-01 | ||||
| 0.006 mg (n = 47) | 0.03 mg (n = 48) | 0.06 mg (n = 44) | Vehicle (n = 43) | |
| Mean change from baseline (mm) | ||||
| No. | 47 | 46 | 40 | 43 |
| Mean (SD) | 10.0 (9.5) | 11.8 (8.9) | 11.4 (9.3) | 3.2 (5.6) |
| Range (min to max) | −5 to 34 | −2 to 29 | −3 to 31 | −4 to 26 |
| Quartiles (25th, median, 75th) | 3, 7, 15 | 5, 10, 15 | 5, 8, 19 | 0, 2, 5 |
| LS mean change from baseline | ||||
| LS mean (SE) [95% CI] | 10.1 (1.2) [7.7–12.5] | 11.4 (1.3) [8.9–13.9] | 11.1 (1.3) [8.5–13.7] | 3.7 (1.3) [1.1–6.2] |
| Treatment comparisons | ||||
| LS mean difference (SE) [95% CI]* | 6.4 (1.8) | 7.7 (1.8) [3.8–11.7] | 7.5 (1.9) [3.4–11.6] | — |
| P† | <0.001 | <0.001 | — | |
Dunnett-corrected 95% CIs.
ANCOVA P value was calculated using a model with baseline STS (study eye), baseline STS with a cotton swab nasal stimulation (study eye), and study sites as covariates.
max, maximum; min, minimum; SE, standard error.
Secondary Efficacy End Points
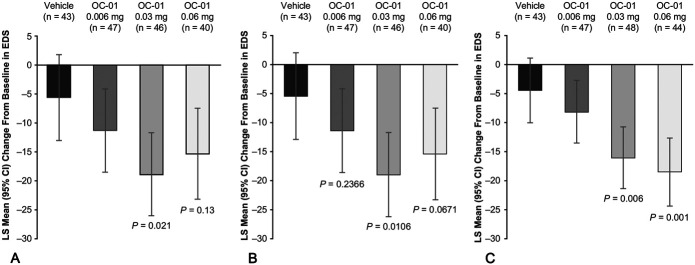
Patients who received the OC-01 0.03 mg dose showed a statistically significantly greater mean reduction in EDS from baseline to day 28 compared with those who received vehicle. The LS mean difference in reduction from baseline between the OC-01 0.03 mg dose and vehicle was −13.3 (95% CI, −25.0 to −1.7; P = 0.021), whereas among patients who received the OC-01 0.06 mg dose, the LS mean difference was not statistically significantly different from vehicle (−9.8; 95% CI, −21.8 to 2.2; P = 0.13). The LS mean change from baseline for OC-01 0.03 mg, 0.06 mg, and vehicle was −19.0 mm (95% CI, −26.2 to −11.7), −15.4 mm (95% CI, −23.3 to −7.5), and −5.6 (−13.1 to 1.8; Fig. 3A).
FIGURE 3.
A, Mean reduction in EDS from baseline to day 28. B, The LS mean reduction from baseline to day 28 with missing data imputed using last available data. C, The LS mean reduction in the controlled adverse environment at day 21. All comparisons made with the control group. No formal statistical comparisons were performed for the 0.006 mg dose versus vehicle; P values for this dose were assessed post hoc and represent nominal values. Error bars indicate CIs. The results in A are derived from a prespecified analysis and B and C are from post hoc analyses.
Patients who received the OC-01 0.03 or 0.06 mg dose showed statistically significantly greater mean reductions in EDS from baseline to day 21 (5 minutes posttreatment) within the controlled adverse environment compared with those patients treated with vehicle. The LS mean differences compared with vehicle were −11.6 (95% CI, −20.1 to −3.0; P = 0.006) for the OC-01 0.03 mg dose and −14.0 (95% CI, −22.9 to −5.1; nominal P value of 0.001) for the OC-01 0.06 mg dose. The LS mean change from baseline for OC-01 0.03 mg, 0.06 mg, and vehicle was −16.0 (95% CI, −21.3 to −10.6), −18.4 (95% CI, −24.3 to −12.5), and −4.4 mg/mL (−9.9 to 1.1) (Fig. 3C).
Exploratory End Points
Post hoc analyses were conducted on the primary end point using a LOCF approach to account for missing data. Patients who received OC-01 0.03 or 0.06 mg nasal spray showed statistically significant improvements in tear film production compared with vehicle, with LS mean changes from baseline in STS of 11.7 mm (95% CI, 9.24–14.26; n = 48; P < 0.0001) with the 0.03 mg dose and 11.0 mm with the 0.06 mg dose (95% CI, 8.24–13.74; n = 44; P < 0.0001; Fig. 2B). In a post hoc exploratory adjunct to the primary end point, the percentage of patients with at least a 10-mm change in STS from baseline to day 28 was significantly greater than in the vehicle group with the 0.03 mg dose [52% (n = 25/48); 95% CI, 20.6–55.7; P = 0.0003] and the 0.06 mg dose [43% (n = 19/44); 95% CI, 11.3 to 47.2; P = 0.0138; Fig. 2C]. Utilizing LOCF for the EDS analysis, the LS mean reduction from baseline in EDS was −18.9 (95% CI, −26.1 to −11.7; P = 0.0106) with the 0.03 mg dose, but among patients who received the 0.06 mg dose, the mean reduction in EDS from baseline to day 28 was not statistically significant (−15.6; 95% CI, −23.5 to −7.7; P = 0.0671; Fig. 3B).
At day 28, in the 0.03 mg dose group, the change in total corneal fluorescein staining (−1.5; 95% CI, −2.9 to −0.2; P = 0.020), nasal corneal fluorescein staining (−0.4; 95% CI, −0.8 to −0.0; P = 0.026), and inferior corneal fluorescein staining (−0.5; 95% CI, −0.8 to −0.1; P = 0.006) from baseline showed a nominally statistically significant beneficial treatment difference compared with vehicle (Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/ICO/B330). There was a directional, but not statistically significant, beneficial treatment difference in the 0.03 mg dose group favoring OC-01 in central (−0.2; 95% CI, −0.6 to 0.1), superior (−0.1; 95% CI, −0.4 to 0.2), and temporal (−0.3; 95% CI, −0.7 to 0.1) staining compared with vehicle. There were no statistically significant differences in change in corneal fluorescein staining from baseline in the 0.06 mg dose group compared with vehicle; however, OC-01 showed a directional benefit relative to vehicle in total, central, temporal, inferior, and nasal staining (Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/ICO/B330).
Safety
Substantially more patients who received OC-01 experienced at least 1 TEAE (70%–93%) compared with those who received vehicle (26%; Table 3). One patient in the OC-01 0.03 mg group experienced an SAE of severe anemia, which resolved and was not suspected to be related to the study drug. As noted above, 1 patient in the OC-01 0.03 mg group experienced a TEAE leading to study withdrawal: dizziness after 1 day of treatment. In the OC-01 0.06 mg group, 1 patient withdrew because of sneezing and throat irritation after 1 day of treatment; 1 patient withdrew because of nasopharyngitis after 2 days of treatment; and 1 patient withdrew because of tinnitus, headache, and eyelid edema after 2 days of treatment.
TABLE 3.
Summary of Adverse Events, including all Ocular Adverse Events and Nonocular Adverse Events Occurring in ≥5% of Patients in Any Treatment Group
| Vehicle, no. (%), (n = 43) | OC-01, 0.006 mg, no. (%), (n = 47) | OC-01, 0.03 mg, no. (%), (n = 48) | OC-01, 0.06 mg, no. (%), (n = 44) | |
| Patients with at least 1 TEAE | 11 (26) | 33 (70) | 44 (92) | 41 (93) |
| Patients with at least 1 SAE | 0 | 0 | 1 (2) | 0 |
| Patients with at least 1 treatment-related SAE | 0 | 0 | 0 | 0 |
| Patients with TEAEs leading to withdrawal | 0 | 0 | 1 (2) | 3 (7) |
| Patients with fatal TEAEs | 0 | 0 | 0 | 0 |
| Patients with at least 1 ocular TEAE | 7 (16) | 1 (2) | 2 (4) | 1 (2) |
| Summary of ocular TEAEs occurring in at least 5% of patients | ||||
| Visual acuity reduced | 3 (7) | 1 (2) | 1 (2) | 0 |
| Patients with at least 1 nonocular TEAE | 5 (12) | 33 (70) | 44 (92) | 41 (93) |
| Summary of nonocular TEAEs occurring in at least 5% of patients | ||||
| Sneezing | 0 | 29 (62) | 38 (79) | 37 (84) |
| Cough | 0 | 4 (9) | 6 (13) | 11 (25) |
| Instillation site irritation | 0 | 3 (6) | 8 (17) | 8 (18) |
| Throat irritation | 0 | 0 | 7 (15) | 9 (20) |
| Dysesthesia pharynx | 0 | 5 (11) | 4 (8) | 3 (7) |
| Nasal dryness | 2 (5) | 1 (2) | 0 | 0 |
| Headache | 1 (2) | 0 | 0 | 2 (5) |
One patient each in the low-dose and high-dose groups, 2 patients in the medium-dose group, and 7 vehicle patients reported at least 1 ocular TEAE; all other TEAEs were classified as nonocular. The distribution of ocular TEAEs showed no obvious pattern. Sneezing, the most common nonocular TEAE in all treatment groups, was associated with study drug administration and resolved soon thereafter (Table 3). Dose-dependent increases in cough and throat irritation occurred with OC-01 (Table 3). Higher levels of instillation site irritation occurred with the 0.03 and 0.06 mg doses compared with the 0.006 mg dose (Table 3). All events were mild (82%) or moderate (18%) in severity; all were transient and resolved by the next visit. No patient reported problems with ocular burning/stinging, taste, or smell.
No clinically significant changes from baseline occurred in BCVA, slitlamp biomicroscopy findings, or intranasal examination at any visit.
DISCUSSION
This phase 2b study investigated the safety and efficacy of 3 doses of OC-01 VNS delivered BID over a 4-week period for the treatment of the signs and symptoms of dry eye disease. It demonstrated that OC-01 0.03 or 0.06 mg improved the signs of dry eye disease, as shown by significant changes in anesthetized STS compared with vehicle, and the symptoms of dry eye disease, as shown by improvements in the EDS in the normal clinic environment and the controlled adverse environment. OC-01 VNS was well tolerated overall, with mild, transient sneezing and cough being the main TEAEs.
OC-01 VNS stimulated tear production in patients with dry eye disease. Patients who received OC-01 0.03 or 0.06 mg showed a statistically significant improvement in tear production versus vehicle, as assessed by changes in mean anesthetized STS from baseline. We found that both the medium and high doses of OC-01 increased STS from ∼5.0 mm at baseline by ∼11 mm (ie, to ∼16 mm) after 28 days. STS increased from a baseline of 4.5 to 8.2 mm after 28 days treatment with vehicle—a change of 3.7 mm. Thus, patients treated with vehicle would still be classified as having moderate dry eye disease.2 Therefore, the difference in mean STS change from baseline with OC-01 treatment compared with vehicle of ∼7.5 mm is large enough for patients to move from having moderate–severe dry eye disease to “normal” tear production.2 This difference may represent a clinically relevant change in the management of dry eye disease.
OC-01 VNS also improved dry eye disease symptoms relative to vehicle over 4 weeks as measured using the EDS in both normal clinic environment and controlled adverse environment, although the mean difference with the 0.06 mg dose at day 28 was not statistically significant. The EDS has been used for many years to measure dry eye disease symptoms, but the minimal clinically important difference has not been defined until recently. Pattar et al30 recently extrapolated changes on the EDS visual analog scale with changes in pain severity visual analog scales and judged that changes of 9 to 13 would be clinically meaningful. We found treatment differences with the higher OC-01 doses of −9.8 to −14.0 compared with vehicle in EDS tests conducted in the normal clinic environment at 28 days and in the controlled adverse environment at 21 days. Therefore, this suggests that treatment with OC-01 0.03 or 0.06 mg improves the symptoms of dry eye disease to a clinically meaningful degree compared with vehicle.
Although the study was not designed with adequate power to allow a formal evaluation of the results of corneal fluorescein staining, we observed an overall benefit in corneal staining with OC-01 (particularly the 0.03 mg dose) compared with vehicle from baseline to day 28. In addition, owing to the repeated use of topical anesthesia and exposure to the controlled adverse environment, the study design may confound the interpretation of corneal fluorescein staining results. These results suggest that OC-01 VNS treatment could mitigate corneal damage due to dry eye disease compared with vehicle but warrant further investigation in adequately designed and powered studies.
Overall, OC-01 VNS was well tolerated; however, it was associated with substantially more TEAEs compared with vehicle, and there was a dose-dependent increase in nonocular TEAEs, such as sneezing and cough. Most sneezing and cough TEAEs were mild and transient, occurring immediately after instillation and led to only 2 discontinuations in the OC-01 0.06 mg group. Sneeze reflex from stimulation of the trigeminal nerve is well documented.31–33 As an ocular surface–sparing nasal spray, OC-01 did not result in many ocular TEAEs, and no case of ocular burning or stinging occurred. Moreover, OC-01 did not affect taste or smell. One SAE of anemia occurred in the OC-01 0.03 mg group and was considered to be unrelated to the study drug. As previously mentioned, the active ingredient of OC-01, varenicline, has been approved as a smoking cessation aid since 2006 in the United States under the tradename CHANTIX (Pfizer Inc; also known as CHAMPIX in some countries). An estimated 20 million individuals worldwide have been prescribed oral varenicline at maintenance doses (eg, 1 mg BID) that are 10 times greater than the highest dose delivered in this study on a per-milligram basis.21,34
Normal tear film production, which is maintained by the lacrimal functional unit, is critical for keeping the ocular surface healthy. Dry eye disease is often associated with reduced tear film production; stimulating the cells and glands responsible for natural tear film may represent a viable treatment approach for dry eye disease and a broad range of ocular surface disorders. The TPP plays a key role in maintaining tear film homeostasis. Activation of this pathway upregulates all 3 layers of the tear film.17–22 Stimulation of the pathway with OC-01 nasal spray resulted in increased tear production, as early as 5 minutes after first administration, and as demonstrated with the first-exposure STS results compared with baseline (before treatment). Typically, tear production increases were observed in the study within 10 to 30 seconds after OC-01 nasal administration. A faster onset to tear production increase may be a desired treatment attribute because OC-01 may have the potential to increase lubrication of the ocular surface, establish a more stable tear film architecture, and provide immediate symptom reduction.35 The nAChR class of receptors exhibits a sustained phenomenon termed “smoldering activation,” in which both activated and desensitized nAChRs are present in equilibrium across a range of agonist concentrations.36 This may result in prolonged underlying activation of the pathway from many minutes to hours after drug delivery.
OC-01 VNS activates the TPP by administration of a preservative-free, low-volume (50 μL), aqueous nasal spray.37 The route of administration of OC-01 VNS offers a number of potential advantages over current topical therapies. First and foremost, nasal delivery offers a route that avoids delivery to the ocular surface. This is especially important with patients who are already experiencing pain and discomfort on the ocular surface or with those who have trouble delivering topical drops. Second, this pathway represents a novel way to stimulate tear film production by bypassing the afferent pathways of the cornea and conjunctiva, a pathway that can often be damaged in eyes with disease and/or pathology.1 Finally, nasal delivery is a common drug delivery method that many patients will have previous experience with and can administer accurately, even with limited dexterity.37 Because the trigeminal nerve endings can be found within the inferior turbinate mucosa,38 the OC-01 VNS can be delivered to the anterior portion of the nasal mucosa as a low-volume spray, thus minimizing the side-effect profile of common nasal sprays targeted to the deep sinus cavity.
The OC-01 formulation does not contain preservatives. This is an important attribute because many preservative-containing solutions have been shown to decrease nasal ciliary beat frequency.39 Common intranasally delivered products are often used to treat episodic diseases such as allergic rhinitis or sinusitis. In the case of dry eye disease where chronic delivery of therapy is warranted, measures to ensure a healthy nasal mucosa are important.
A limitation of this study was that tear production was assessed at the time of OC-01 administration, but the duration of increased tear production after treatment was not assessed because of the potential confounding of data from procedural serial anesthetized Schirmer testing and toxicity associated with exposure to repeated anesthetic procedural Schirmer testing.
In conclusion, the ONSET-1 phase 2b study showed OC-01 VNS to be safe, well tolerated, and efficacious in the treatment of dry eye disease at the 0.03 and 0.06 mg concentrations. Patients treated with OC-01 VNS in ONSET-1 demonstrated significant improvements in both the signs and symptoms of dry eye disease after 28 days of BID administration, at magnitudes in change from baseline that are likely to be clinically meaningful. OC-01 VNS may represent a novel candidate to treat the signs and symptoms of dry eye disease; further clinical investigation is warranted.
ACKNOWLEDGMENTS
The authors acknowledge Fiona Nitsche, PhD, CMPP, of Envision Pharma Group for medical editing support, which was funded by Oyster Point Pharma, Inc.
Footnotes
Funded by Oyster Point Pharma, Inc.
D. Wirta and G. L. Torkildsen have received research grant support from Oyster Point Pharma, Inc. D. A. Hollander is an employee of Ora, Inc. E. Bendert was an employee at Statistics Collaborative, Inc at the time the study was conducted. L. Zeng is a current employee at Statistics Collaborative, Inc. M. Ackermann is a board member at Oyster Point Pharma, Inc. J. Nau is an employee at and shareholder of Oyster Point Pharma, Inc. The remaining author has no conflicts of interest to disclose.
Presented in part at the Ophthalmology Innovation Summit (OIS) at the American Academy of Ophthalmology (OIS@AAO); October 25, 2018; Chicago, IL; OIS at the American Academy of Cataract and Refractive Surgery (OIS@ASCRS); May 2, 2019; San Diego, CA; OIS at the Southeastern Congress of Optometry (OIS@SECO); February 21, 2019; New Orleans, LA; and OIS@AAO; October 10, 2019; San Francisco, CA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
The data sets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Contributor Information
Gail L. Torkildsen, Email: mdlasik@comcast.net.
Blair Boehmer, Email: bboehmermd@midwesteye.com.
David A. Hollander, Email: david.a.hollander.md@gmail.com.
Edward Bendert, Email: ebendert3@gmail.com.
Lijuan Zeng, Email: lijuan.zeng@statcollab.com.
Michael Ackermann, Email: d.michael.ackermann@gmail.com.
Jeffrey Nau, Email: jnau@oysterpointrx.com.
REFERENCES
- 1.Friedman NJ, Butron K, Robledo N, et al. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. 2016;10:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. [DOI] [PubMed] [Google Scholar]
- 3.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar AS, Bhavsar SG, Jain SM. A review on recent advances in dry eye: pathogenesis and management. Oman J Ophthalmol. 2011;4:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350–356. [DOI] [PubMed] [Google Scholar]
- 6.Willcox MDP, Argüeso P, Georgiev GA, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15:366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schicht M, Garreis F, Hartjen N, et al. SFTA3—a novel surfactant protein of the ocular surface and its role in corneal wound healing and tear film surface tension. Sci Rep. 2018;8:9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf. 2007;5:228–239. [DOI] [PubMed] [Google Scholar]
- 9.Suwan-apichon O, Rizen M, Rangsin R, et al. Botulinum toxin B-induced mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2006;47:133–139. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Shen J, Zhang C, et al. Inflammatory cytokine expression on the ocular surface in the botulium toxin B induced murine dry eye model. Mol Vis. 2009;15:250–258. [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91:35–43. [DOI] [PubMed] [Google Scholar]
- 12.McKown RL, Wang N, Raab RW, et al. Lacritin and other new proteins of the lacrimal functional unit. Exp Eye Res. 2009;88:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wang N, Xie J, et al. Restricted epithelial proliferation by lacritin via PKCα-dependent NFAT and mTOR pathways. J Cell Biol. 2006;174:689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25:61–66. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Heigle T, Pflugfelder SC. Nasolacrimal stimulation of aqueous tear production. Cornea. 1997;16:645–648. [PubMed] [Google Scholar]
- 17.Dieckmann G, Jamali A, Pondelis N, et al. In vivo confocal microscopy demonstrates intranasal neurostimulation-induced goblet cell alterations in patients with dry eye disease. Invest Ophthalmol Vis Sci. 2017;58:2694. [Google Scholar]
- 18.Green KB, Kamat M, Franke M, et al. Tear total lipid concentration in patients with dry eye following intranasal neurostimulation. Invest Ophthalmol Vis Sci. 2017;58:2693. [Google Scholar]
- 19.Gumus K, Schuetzle KL, Pflugfelder SC. Randomized controlled crossover trial comparing the impact of sham or intranasal tear neurostimulation on conjunctival goblet cell degranulation. Am J Ophthalmol. 2017;177:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pondelis NJ, Dieckmann G, Kataguiri P, et al. Intranasal neurostimulator induces morphological changes in meibomian glands in patients with dry eye disease. Invest Ophthalmol Vis Sci. 2017;58:2235. [Google Scholar]
- 21.Sheppard JD, Torkildsen GL, Geffin JA, et al. Characterization of tear production in subjects with dry eye disease during intranasal tear neurostimulation: results from two pivotal clinical trials. Ocul Surf. 2019;17:142–150. [DOI] [PubMed] [Google Scholar]
- 22.Woodward A, Senchyna M, Franke M, et al. Effect of intranasal neurostimulation on tear protein content in patient with dry eye. Invest Ophthalmol Vis Sci. 2017;58:2673. [Google Scholar]
- 23.O'Neil EC, Henderson M, Massaro-Giordano M, et al. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Deng X-G, He MF. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int J Ophthalmol. 2012;5:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121:475–483. [DOI] [PubMed] [Google Scholar]
- 27.Tauber J, Karpecki P, Latkany R, et al. ; OPUS-2 Investigators. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122:2423–2431. [DOI] [PubMed] [Google Scholar]
- 28.Ousler GW, Gomes PJ, Welch D, et al. Methodologies for the study of ocular surface disease. Ocul Surf. 2005;3:143–154. [DOI] [PubMed] [Google Scholar]
- 29.Ousler GW, III, Rimmer D, Smith LM, et al. Use of the controlled adverse environment (CAE) in clinical research: a review. Ophthalmol Ther. 2017;6:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pattar GR, Jerkins G, Evans DG, et al. Symptom improvement in dry eye subjects following intranasal tear neurostimulation: results of two studies utilizing a controlled adverse environment. Ocul Surf. 2020;18:249–257. [DOI] [PubMed] [Google Scholar]
- 31.Batsel HL, Lines AJ. Neural mechanisms of sneeze. Am J Physiol. 1975;229:770–776. [DOI] [PubMed] [Google Scholar]
- 32.Songu M, Cingi C. Sneeze reflex: facts and fiction. Ther Adv Respir Dis. 2009;3:131–141. [DOI] [PubMed] [Google Scholar]
- 33.Hydén D, Arlinger S. On the sneeze-reflex and its control. Rhinology. 2007;45:218–219. [PubMed] [Google Scholar]
- 34.FDA Advisory Committees Recommend to Remove Boxed Warning in Labeling for Pfizer's Smoking Cessation Therapy, CHANTIX® (Varenicline). New York, NY: Pfizer; 2016. Available at: https://www.pfizer.com/news/press-release/press-release-detail/fda_advisory_committees_recommend_to_remove_boxed_warning_in_labeling_for_pfizer_s_smoking_cessation_therapy_chantix_varenicline. Accessed February 26, 2020. [Google Scholar]
- 35.Torkildsen G, Brujic M, Cooper MS, et al. Evaluation of a new artificial tear formulation for the management of tear film stability and visual function in patients with dry eye. Clin Ophthalmol. 2017;11:1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campling BG, Kuryatov A, Lindstrom J. Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One. 2013;8:e79653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghori MU, Mahdi MH, Smith AM, et al. Nasal drug delivery systems: an overview. Am J Pharmacol Sci. 2015;3:110–119. [Google Scholar]
- 38.Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N Y Acad Sci. 2009;1170:202–205. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann T, Gugatschga M, Koidl B, et al. Influence of preservatives and topical steroids on ciliary beat frequency in vitro. Arch Otolaryngol Head Neck Surg. 2004;130:440–445. [DOI] [PubMed] [Google Scholar]