ABSTRACT
Purpose
We aimed to study variations in strength and power performance during the menstrual cycle (MC) in eumenorrheic young women and during the pill cycle in oral contraceptives (OC) users.
Methods
Forty healthy, normal-weight women between 18 and 35 yr (n = 30 eumenorrheic women; n = 10 OC users) completed this prospective cohort study. Seven to nine times during the MC/pill-cycle, the participants completed a physical performance test series, a questionnaire about psychological well-being, blood sampling, and determination of body mass. The physical tests included isometric handgrip strength, elbow flexor strength, countermovement jump (CMJ) height, and a 10-s Wingate bike test.
Results
No direct correlation was observed between the variations in sex hormones and physical performance parameters. However, positive correlations were observed between physical performance outcomes and self-reported motivation, perception of own physical performance level, pleasure level, and arousal level. CMJ was 6% lower in the late luteal phase (LL) compared with the midluteal phase (ML) (P = 0.04). Wingate peak power was 3% lower in early follicular (EF) compared with the ML (P = 0.04). Furthermore, Wingate average power was 2%–5% lower in LL compared with all other MC phases. In line with these observations, physical pain was higher in EF and LL, and the pleasure level was lower in EF compared with the other MC phases. In OC users, we observed no variation in performance and self-reported parameters between the placebo-pill phase and the OC-pill phase.
Conclusions
Impairments in CMJ and Wingate performance were observed at the end and start of MC compared with other MC phases, which were associated with lower psychological well-being, but not the sex hormone fluctuations.
Key Words: FEMALE ATHLETES, WOMEN, ESTROGEN, PROGESTERONE, PHYSICAL PERFORMANCE, MUSCLE STRENGTH
The primary function of the female sex hormones is to regulate fertility and thereby reproduction. However, research has suggested that the sex hormones, specifically estrogen, positively influence cardiovascular, respiratory, neuromuscular, neurocognitive, and metabolic parameters and thereby physical performance (1,2). In female elite athletes, a minor difference in performance (<5%) related to the menstrual cycle (MC) will be of great importance during competition.
Variation in performance during the MC has been evaluated in a meta-analysis by McNulty et al. (3). This meta-analysis included 78 studies in which 1193 women had their performance tested during the MC. However, 53 studies (68%) were of low or very low quality, and only 6 studies (8%) were classified as being of high quality. Therefore, the authors concluded that current evidence does not permit any strong conclusion concerning variation in physical performance during the MC, and future studies of higher quality are needed. Another recent systematic review by Blagrove et al. (4) came to a similar conclusion. Studies that have investigated the effect of the MC on performance parameters in women have either reported no significant difference in strength performance during the MC (5–12) or greater muscle strength just before ovulation (13–15). The lack of consensus could be due to methodological differences and limitations in the individual studies (16). Particularly, the methods used to identify the MC phases vary between studies, including measurement of body temperature, self-reported calendar counting, use of urinary ovulation tests, and analyses of sex hormone levels in saliva or blood (4). Measurement of sex hormones is essential for confirmation of MC phases, but has not been performed in several of the previous studies (13,14,17). In the majority of studies, the number of participants has been <20 (5–14), and the number of test days completed during an MC has been ≤3 (5–10,12). Furthermore, including trained women as participants may create bias through prior training sessions in the days leading up to the testing confounding the results, as well as increasing the incidence of suppressed female sex hormone levels and irregular MC (18,19). If women presenting with an anovulatory or irregular MC are included in the data analysis, the likelihood of detecting a variation in performance during the MC is reduced. Therefore, even though research performed specifically in trained women is essential in perspective to performance optimization, it is relevant to test untrained women as a starting point because performance in this population may be less confounded by previous exercise training and menstrual dysfunction.
The mechanistic basis for the MC influencing muscular performance is derived from animal studies showing that estrogen is important for the actin–myosin binding and may thereby influence muscle strength (20). Compared with estrogen, the current knowledge surrounding the impact of progesterone on muscle strength is sparse. However, studies have suggested an antagonistic effect of progesterone on estrogen’s actions (21). Furthermore, animal findings indicate that progesterone has a potentially inhibiting effect on neural activity (22) and seems to antagonize the stimulating effect of estrogen on contraction-stimulated glucose uptake during short, high-intensity exercise (23).
Psychological well-being is reported to vary during the MC (24), which may also influence physical performance. Just before the menstrual bleeding and in the beginning of the bleeding period, pain and headaches are commonly reported in female athletes from a range of sport disciplines (25,26) and in nonathletes (27). This may negatively impact physical performance, but to our knowledge, no one has investigated the correlation between physical performance and changes in psychological parameters during the MC.
More than 100 million women use oral contraceptives (OC) worldwide today (28) as birth control, but also to control menstrual bleeding and alleviate pain and discomfort related to the MC (29). A recent systematic review investigated the impact of OC use on physical performance in the OC-pill and placebo-pill phase, and observed no difference between phases (30), which is in line with the use of OC result in a more stable sex hormonal profile. Nevertheless, the latter review also highlighted the need for further high-quality studies because most of the limited research carried out in this field was shown to be of low quality.
The purpose of the present study was to investigate if muscular strength in young eumenorrheic women varies 1) with fluctuations in sex hormones during an MC and 2) between defined MC phases. In addition, we aimed to examine the relationship between physical performance and the participants’ self-reported psychological well-being during an MC. Finally, we aimed to elucidate if muscular strength differed between the OC-pill phase and the placebo-pill phase in OC users.
We hypothesized that eumenorrheic women would perform better around ovulation when the estrogen/progesterone ratio is highest compared with the other MC phases, whereas lower self-reported well-being around the menstrual bleeding period would diminish the physical performance. In addition, we hypothesized that performance would not differ between the OC-pill and placebo-pill phases in OC users. Still, the latter is important to clarify in perspective to future studies including OC users who have their physical performance tested.
MATERIALS AND METHODS
Experimental design
Using a prospective cohort design, we recruited women using OC and eumenorrheic women not using any hormonal contraception. The physical performance of the women was tested, on average, eight times (range, 7–9) during 1 MC (Fig. 1). The test of physical performance was intensified (each second day) from day 10 (or 4 d before predicted ovulation) and until the women experienced a positive ovulation kit test result. This was done to enhance the chance for catching the day where estrogen was peaking. Before the testing period, the women had their body composition determined by a dual-energy x-ray absorptiometry (DXA) scan and performed three familiarization tests to become accustomed to the testing protocols and procedures. The testing procedures were identical in each testing session to exclude variation between testing days, and the participants were block-randomized to start the testing in either the follicular or the luteal phase of their MC. The OC users were randomized to start in the placebo-pill or OC-pill phase.
FIGURE 1.
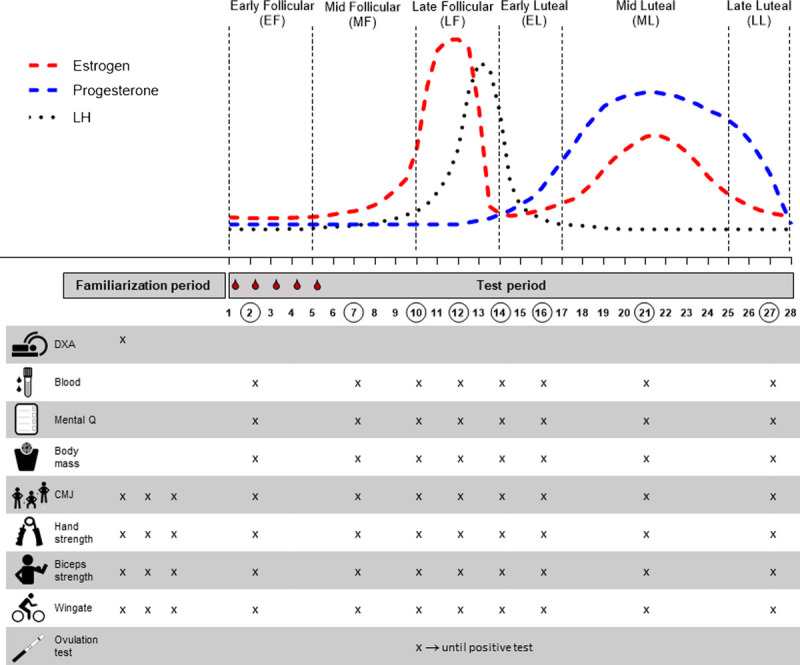
Overview of the study protocol describing days of assessments and the outcomes measured in relation to a standard 28-d MC. The participants first completed a familiarization period including a DXA scan and 3 habituations test days to be accustomed with the physical performance tests. Then the participants were randomized to begin the testing period in either the follicular or the luteal phase. The testing period included an average of eight visits, each conforming with a standardized testing protocol as outlined: collecting a blood sample, completing a Mental Q (questionnaires determining psychological and physical well-being parameters), determining body mass, and performing a CMJ, hand grip and elbow flexor strength tests, and finally a 10-s Wingate test. A urinary ovulation test was performed each morning from day 10 of the MC until a positive test result was experienced.
Participants
The healthy OC users and eumenorrheic women were recruited through social media, flyers, and posters in the local area around Aarhus, Denmark. As part of the screening process, the participants received information about the study and completed a questionnaire regarding the inclusion/exclusion criteria. Moreover, measurements of weight and height were carried out. The inclusion criteria were as follows: 1) regular MC (between 25 and 35 d of length for the last 6 months) OR user of second-generation OC-pill containing ethinyl-estradiol and either levonorgestrel or norethisterone, 2) body mass index (BMI) between 18.5 and 25.0 kg·m−2, 3) age between 18 and 35 yr, and 4) maximum 2 h of planned physical training per week during the last 3 months. Because bicycles are commonly used for transportation in Denmark, a maximum of a total of 70-km cycling for transportation was allowed per week.
Participants were excluded if any of the following criteria applied to them: 1) smoking, 2) diabetes, 3) use of medication, 4) vegetarianism, 5) pregnancy, 6) dieting, 7) unstable body mass defined as a change of more than 5 kg the last 6 months, and 8) injuries that would hinder participation in the physical tests. In addition, the eumenorrheic women were excluded if they had used any kind of hormonal contraception during the past 3 months. OC users should have been using OC for at least 3 months.
The study was carried out in accordance with the Helsinki Declaration, approved by the local ethical committee of the Central Denmark Region (1-10-72-259-18), and registered at Clinical.Trials.gov (ID: NCT03832972). All participants provided written consent before the investigation.
Test protocol
An overview of the testing protocol is shown in Figure 1. On each test day, an initial blood sample was obtained after overnight fasting, and body mass was measured using the same digital scale for all participants (Tanita, Tokyo, Japan). Then, the participants filled out a questionnaire about their psychological well-being, which included marks on visual analog scales (VAS) about discomfort and changes in mood (31) and a mark in the Affect Grid: A Single-Item Scale of Pleasure and Arousal (32). Afterward, the participants performed a 5-min standardized warm-up on a cycle ergometer (Monark, Ergomedic 818E; Monark Exercise AB, Vansbro, Sweden). Thereafter, the participants performed four performance tests in the following order: countermovement jump (CMJ), maximal handgrip strength, maximal isometric elbow flexor strength, and a 10-s Wingate bike test. During the tests, the participants received standardized verbal encouragement from the test personnel.
For the CMJ, handgrip, and elbow flexor strength tests, the participants had three attempts interspersed with a 2-min recovery. The best result for each test was used for further analysis. The CMJ test was performed on a speed-force platform (wireless SpeedMAT; Swift Performance Equipment, Wacol, Australia). Jumping height is calculated based on displacement of center mass (1-cm resolution) using Speedlight for iPad to measure contact time (1-ms resolution) and flight time (1-ms resolution). The participants were instructed to perform a maximal vertical CMJ with their hands placed on their hips. Maximal handgrip strength of the dominant hand was assessed using a North Coast Medical dynamometer (North Coast Medical Inc, Morgan Hill, CA). Maximal isometric elbow flexor strength was measured in a custom-made strain gauge dynamometer at a join angle of 90°. The elbow flexor test rig has a built-in full wheatstone bridge force transducer. The force signal is amplified and sampled with a sampling frequency of 1000 Hz using an NI CompactDAQ Chassie, type cDAQ-9174, with a CompactDAQ module, type NI-9237 (National Instruments, Austin, TX). The NI-9237 module is a combined strain gauge amplifier and AD Converter with a resolution of 24 bit. The software used for sampling of the force signal is custom-made and developed using LabVIEW (National Instruments). The participants were seated on their knees with their dominant arm placed and strapped to the dynamometer. The participants were instructed to keep their torso in a relaxed upright position and, after a 3-s countdown, to pull as hard as possible against the dynamometer arm using their elbow flexors.
Before the Wingate bike test, the participants cycled for 10 min with a self-selected resistance at submaximal intensity (Monark, Ergomedic 818E). The Wingate bike test was performed on a cycle ergometer (Monark, Ergomedic 894E). The participants were instructed to pedal as fast as they could for 10 s against a resistance equal to 7.5% of their body mass. Every participant had two attempts, separated by a 5-min break.
Determination of MC phases
MC length varies from MC to MC within women, as well as between women. However, during a regular normalized 28-d MC, eumenorrheic women will experience fluctuations in the female sex hormones (33), which can be used to categorize the MC into six different defined phases (34). In this study, the first phase of MC (days 1–5) initiated by the start of menstrual bleeding was named the early follicular phase (EF). This was followed by the midfollicular phase (MF; days 6–10), which is characterized by a slow increase in estrogen and a low progesterone level. The following late follicular phase (LF; ovulation phase; days 11–14) is characterized by a rapid increase in estrogen and luteinizing hormone (LH) reaching their peaks just before ovulation. After LF, the early luteal phase (EL; days 15–17) follows, where estrogen initially declines; hereafter, both estrogen and progesterone levels slowly increase. In the midluteal phase (ML; days 18–25), both estrogen and progesterone levels are high. The last part of the MC is the late luteal phase (LL; days 26–28), where progesterone and estrogen decline just before the menstrual bleeding marking the start of a new MC (16). An illustration of the MC divided into the defined phases (EF, MF, LF, EL, ML, and LL phases) is shown in Figure 1.
To verify the MC phases, a combination of calendar-based counting, ovulation tests, and hormone analysis was used as recommended in the literature (16). From day 10 in the MC (or 4 d before expected ovulation), the eumenorrheic women performed a urinary ovulation test in the morning to detect an increase in LH as an indicator of ovulation (Babyplan Ovulation test stick, Copenhagen, Denmark). The participants continued testing each morning until they had a positive test result. The results from the ovulation test combined with the sex hormone levels were used to determine the different phases of the MC for each individual. To be included in the data analysis, a positive ovulation test result and progesterone level greater than 16 mmol·L−1 in the luteal phase had to be present in the eumenorrheic women (16).
Blood sampling and analysis
The blood samples were obtained from the antecubital vein after overnight fasting, centrifuged, and stored at −80°C for later analysis. The samples were analyzed by standard clinical procedures at the Department of Clinical Biochemistry at Aarhus University Hospital, Aarhus, Denmark, accredited according to ISO/IEC 15189. The blood was analyzed for the following parameters: serum (s)-estradiol, s-testosterone, s-sex hormone–binding globulin (SHBG), s-progesterone, s-follicle–stimulating hormone (FSH), and s-LH.
DXA and anthropometrics
A whole-body DXA scan was performed to determine body composition (GE Lunar DXA scan; GE Healthcare, Madison, WI), and the system’s software package (enCORE software v16.0; GE Healthcare) was used to determine fat mass, fat-free mass, and percent body fat.
Psychological measures
Physical pain, motivation, self-perception of performance level (i.e., participant performance evaluation), and affective responses (i.e., pleasure level and arousal level) were assessed by a self-report questionnaire. A VAS (anchors 0–8) (31) were used to assess physical pain, motivation, and perception of performance. The value 0 corresponded to the lowest level, and the value 8 to the highest level. Affective responses (i.e., pleasure level and arousal level) were assessed by the Affect Grid (anchors 1–9 in the two dimensions of displeasure–pleasure and sleepiness–arousal, respectively) developed by Russell et al. (32) and validated by Killgore et al. (35) in a group of college students. The Affect Grid was designed as a quick means of assessing affect along the dimensions of pleasure–displeasure and arousal–sleepiness. Participants were asked to place a single cross (X) within one of the squares on the grid.
Statistical analysis
Sample size was calculated based on a previous study reporting 5% higher muscle strength in the leg muscles around ovulation compared with the beginning of the MC (14). Assuming a similar variation between the highest and lowest muscle strength during the cycle, we calculated that 28 eumenorrheic women would be required to achieve a statistical power of 80% at a significance level of 5%, when assuming an SD of 9%, as previously reported (14). We chose to include 30 eumenorrheic women to take into account potential missing data. Participants, who did not complete the entire testing period were substituted by new participants until 30 eumenorrheic women had completed the study protocol. Only 10 OC users were included because OC use induces stable endogenous sex hormone levels except for small daily fluctuations in the exogenous sex hormones in the hours after the daily pill is ingested (36). No direct comparison between the two groups in physical performance outcomes was performed because the study was not powered to analyze the differences in the outcomes between OC users and eumenorrheic nonusers of OC.
In the statistical analysis, samples that showed s-estradiol and s-progesterone levels below the analytic detection level were set to have a hormone concentration corresponding to the analytic detection level provided by the Department of Clinical Biochemistry at Aarhus University Hospital, Aarhus, Denmark (15 pmol·L−1 and 0.7 nmol·L−1, respectively). In the eumenorrheic women not using OC, we analyzed the relationship between the different performance measures and the circulating sex hormone levels (estradiol, progesterone and testosterone, the estradiol/progesterone ratio, the estradiol/SHBG ratio, the testosterone/SHGB ratio), and the psychological parameters (physical pain, motivation, performance level, pleasure level, and arousal level), respectively, using a random coefficient model (linear regression for repeated measurements). To take into account any potential training effect or effect of test order, all the analyses were adjusted for test day number (1–9).
When analyzing data divided into the six different MC and two OC phases, means were used if a specific phase included more than 1 test day. The analysis of the relationships between the different performance measures and the MC phases was performed using a mixed linear model with group and phases (and the interaction between the two) as fixed effects and participant as a random effect. In the analyses, an unstructured variance–covariance matrix for the repeated measurements was used corresponding to different SDs that were allowed in the different phases, as well as different correlations for different pairs of phases. This was done because observations for the same woman (in different stages) are positively correlated. However, it is conceivable that the correlation between phases close to each other is better than the correlation between phases far from each other. By using this analysis model, we take this into account, together with the circumstance, that SD may also be different in the different phases. The analysis for different performance measures and the OC and non-OC phase was performed using a Student’s paired t-test. Model validation was performed by inspecting plots of standardized residuals against the fitted values and Q-Q plots of the residuals.
STATA version 15.1 (StataCorp, College Station, TX), was used as statistical software, and the statistical significance level was set at P < 0.05. GraphPad Prism 8 (GraphPad Software, San Diego, CA) was used as graphics software.
RESULTS
Forty women (eumenorrheic women, n = 30; OC users, n = 10) completed the study. A flowchart reflecting the recruitment process is shown in Supplemental Figure S1 (Supplemental Digital Content 1, Flow diagram illustrating inclusion of participants, http://links.lww.com/MSS/C623). Participant characteristics are shown in Table 1. No statistically significant difference was apparent between the two groups in the following characteristics at baseline: age, body mass, height, BMI, fat-free mass, and body fat percentage. The two groups differed in that the eumenorrheic women did not presently use OC, and the total life span use of OC was shorter for the eumenorrheic women (P = 0.02; Table 1). The OC brands used were Femicept®(n = 7), Microgyn® (n = 1), Cilest® (n = 1), and Malonetta® (n = 1), all of which provide 30 μg ethinyl-estradiol and 150 μg levonorgestrel each day during the active OC-pill phase.
TABLE 1.
Participant characteristics.
| Measurement | Eumenorrheic Women (n = 28) | OC Users (n = 10) | P |
|---|---|---|---|
| Age (yr) | 23.8 ± 2.7 | 23.7 ± 2.0 | 0.89 |
| Body mass (kg) | 66.5 ± 6.7 | 64.5 ± 8.9 | 0.46 |
| Height (cm) | 170.6 ± 6.2 | 167.2 ± 6.4 | 0.13 |
| BMI (kg·m−2) | 22.9 ± 2.0 | 23.1 ± 2.5 | 0.81 |
| Age at first menstruation (yr) | 12.7 ± 1.5 | 13.2 ± 0.6 | 0.32 |
| Life time using OC (months) | 31.9 ± 44.1 | 64.5 ± 26.2 | 0.02 |
| FFM (kg) | 43.8 ± 4.0 | 42.8 ± 4.6 | 0.47 |
| FFMleg (kg) | 15.2 ± 1.7 | 15.1 ± 2.2 | 0.50 |
| Body fat (%) | 30.2 ± 4.8 | 29.6 ± 6.0 | 0.77 |
| FMleg (%) | 33.2 ± 3.7 | 32.8 ± 5.9 | 0.84 |
Values are presented as mean ± SD. P values less than 0.05 indicating group differences are reported in bold.
FM, fat mass; FFM, fat-free mass.
Data from 38 women were included in the analysis (eumenorrheic women, n = 28; OC users, n = 10; Table 1). Two eumenorrheic women were excluded from the analyses because of their progesterone level failing to rise above 16 mmol·L−1 during the luteal phase (16). The majority of the participants had tracked their MC using an application before inclusion into the study and could thereby confirm a regular MC pattern before inclusion. However, during the testing period, many of the women experienced a prolonged MC compared with their habitual reported MC length. The initial randomization to start the testing period in either the follicular or the luteal phase of MC was based on MC length, but because of the variation in MC length, 17 participants started the testing period in the follicular phase, whereas 11 started in the luteal phase. The statistical analyses confirmed that the test order did not influence the results.
Changes in sex hormones and body mass during the MC
For the eumenorrheic women, the sex hormones fluctuated between the MC phases as expected (Table 2 and Supplemental Fig. S2, Supplemental Digital Content 2, Variation in sex hormone levels during the MC phases, http://links.lww.com/MSS/C624). The individual hormone profiles are shown in Supplemental Figure S3 (Supplemental Digital Content 3, Individual variation in sex hormone levels during the MC phases, http://links.lww.com/MSS/C625). Body mass did not differ significantly between the MC phases (Supplemental Fig. S4, Supplemental Digital Content 4, Bodyweight during the MC and OC cycle phases, http://links.lww.com/MSS/C626).
TABLE 2.
Hormones, estrogen/progesterone ratio, and testosterone/SHBG ratio in MC phases.
| Hormone | EF | MF | LF | EL | ML | LL |
|---|---|---|---|---|---|---|
| Estrogen (pmol·L−1) | 100 (75.9–116)b,c,d,e,f | 181 (149–262) | 730 (539.5–947.3)a,b,d,f | 386 (302–497) | 599 (454–764) | 395 (209.0–636.0) |
| Prog. (nmol·L−1) | 1.6 (0.9–2.2)e | 1.2 (0.9–1.7) | 2 (1.4–2.6) | 11.3 (4.8–16.9) | 42.5 (31.0–50.0)a,b,c,d,f | 18.2 (5.4–38.0) |
| E/P ratio | 64.1 (41.1–97.6) | 157.2 (94.9–223.8) | 334.9 (287.7–523.6)a,b,d,e,f | 34.2 (19.9–258.4) | 14.4 (11.5–17.4) | 28.4 (16.3–36.8) |
| LH (IU·L−1) | 6.8 (5.4–88) | 9.5 (6.2–10.9) | 20.5 (13.4–37.6)a,b,d,e,f | 11.3 (8.3–14.4) | 8.4 (5.6–12.1) | 5.7 (5.1–5.9) |
| FSH (IU·L−1) | 5.6 (5.2–7.7) | 6.4 (5.7–7.7) | 6.5 (5.0–8.5)d,e,f | 4.5 (3.9–5.0) | 3.4 (2.8–4.0) | 2.3 (1.9–3.5) |
| Testo. (nmol·L−1) | 1.1 (0.84–1.3)b,c,d,e,f | 1.4 (1.07–1.6) | 1.5 (1.23–1.7) | 1.7 (1.4–2.2)a,b,c,e,f | 1.3 (1.1–1.5) | 1.2 (0.9–1.5) |
| SHBG (nmol·L−1) | 66.0 (55.0–96.0) | 62.4 (48.0–90.3) | 63.5 (45.8–88.0) | 72.5 (46.0–91.0) | 70.0 (48.0–94.0) | 75.0 (36.0–95.0)a,b,c,d |
| Testo./SHBG ratio | 1.67 (1.15–2.13)b,c,d,e,f | 2.25 (1.59–2.87) | 2.61 (1.78–3.38)a,b,e,f | 2.43 (1.67–2.98) | 1.82 (1.59–2.5) | 1.85 (1.46–2.79) |
Values are presented as medians and interquartile range.
aSignificantly different from EF.
bSignificantly different from MF.
cSignificantly different from LF.
dSignificantly different from EL.
eSignificantly different from ML.
fSignificantly different from LL.
E/P ratio, estrogen/progesterone ratio; Prog., progesterone; Testo., testosterone.
Associations between sex hormones and performance
Performance in the four physical tests (CMJ, handgrip strength, elbow flexor strength, and Wingate bike test) did not correlate significantly with the individual variations in circulating estradiol, progesterone, or testosterone (Table 3). Similarly, individual variations in the estrogen/progesterone ratio, estrogen/SHBG ratio, and testosterone/SHGB ratio did not correlate with any of the performance parameters.
TABLE 3.
Association between hormone levels and performance and psychological parameters in eumenorrheic women.
| Measurement | Estrogen | Progesterone | E/P Ratio | Testosterone | Testosterone/SHBG Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Body mass (kg) | 0.02 | (−0.01 to 0.05) | 0.18 | −0.16 | (−0.65 to 0.34) | 0.52 | 0.00 | (−0.07 to 0.07) | 0.86 | −0.09 | (−0.44 to 0.26) | 0.59 | 0.08 | (−0.13 to 0.30) | 0.42 |
| CMJ (mm) | −0.16 | (−0.71 to 0.39) | 0.56 | 0.06 | (−0.04 to 0.16) | 0.23 | −0.00 | (−0.00 to 0.00) | 0.81 | 0.00 | (−0.00 to 0.01) | 0.58 | −0.01 | (−0.01 to 0.01) | 0.93 |
| CMJ power (W) | 0.35 | (−0.60 to 1.30) | 0.46 | 9.45 | (−5.64 to 24.54) | 0.21 | 0.07 | (−1.86 to 2.00) | 0.92 | 5.69 | (−4.71 to 16.08) | 0.27 | 3.39 | (−3.08 to 9.86) | 0.29 |
| Handgrip (kg) | 0.12 | (−0.34 to 0.58) | 0.81 | 1.11 | (−0.54 to 2.77) | 0.18 | −0.04 | (−0.24 to 0.16) | 0.64 | −0.78 | (−1.81 to 0.25) | 0.13 | −0.47 | (−1.15 to 0.21) | 0.17 |
| Elbow flexor MVC (N·m) | 0.00 | (−0.09 to 0.09) | 0.94 | 1.12 | (−0.58 to 2.81) | 0.18 | 0.01 | (−0.21 to 0.24) | 0.87 | −0.21 | (−1.30 to 0.87) | 0.67 | −0.11 | (−0.78 to 0.55) | 0.72 |
| Wingate PP (W) | 1.05 | (−0.66 to 2.76) | 0.21 | 20.5 | (−9.30 to 50.29) | 0.17 | 1.08 | (−3.72 to 5.88) | 0.50 | 3.78 | (−16.50 to 24.07) | 0.70 | 3.38 | (−8.29 to 15.04) | 0.55 |
| Wingate PP (W·kg−1) | 0.01 | (−0.01 to 0.04) | 0.25 | 0.28 | (−0.17 to 0.72) | 0.21 | 0.02 | (−0.04 to 0.07) | 0.43 | 0.07 | (−0.21 to 0.36) | 0.61 | 0.03 | (−0.14 to 0.20) | 0.73 |
| Wingate AP (W) | 0.53 | (−0.64 to 1.71) | 0.35 | 18.14 | (−2.17 to 38.46) | 0.08 | 0.56 | (−2.22 to 3.35) | 0.59 | −0.81 | (−15.00 to 13.39) | 0.91 | 0.44 | (−7.78 to 8.67) | 0.91 |
| Wingate AP (W·kg−1) | 0.01 | (−0.01 to 0.03) | 0.40 | 0.26 | (−0.05 to 0.56) | 0.09 | 0.01 | (−0.03 to 0.04) | 0.55 | 0.00 | (−0.20 to 0.20) | 0.99 | −0.01 | (−0.13 to 0.11) | 0.89 |
| Physical pain | 0.001 | (−0.05 to 0.05) | 0.97 | −0.43 | (−1.22 to 0.36) | 0.27 | 0.00 | (−0.13 to 0.13) | 0.91 | −0.13 | (−0.57 to 0.32) | 0.57 | 0.14 | (−0.10 to 0.38) | 0.24 |
| Motivation | 0.01 | (−0.05 to 0.07) | 0.64 | 0.07 | (−0.94 to 1.08) | 0.89 | 0.00 | (−0.14 to 0.14) | 0.94 | 0.35 | (−0.26 to 0.95) | 0.24 | 0.07 | (−0.28 to 0.42) | 0.68 |
| Performance level | 0.02 | (−0.04 to 0.08) | 0.45 | −0.03 | (−0.99 to 0.94) | 0.96 | 0.00 | (−0.09 to 0.09) | 0.95 | 0.59 | (−0.04 to 1.21) | 0.07 | 0.13 | (−0.19 to 0.46) | 0.41 |
| Pleasure level | 0.00 | (−0.07 to 0.07) | 0.94 | 0.25 | (−0.99 to 1.47) | 0.68 | 0.02 | (−0.15 to 0.19) | 0.73 | 0.08 | (−0.71 to 0.87) | 0.84 | 0.19 | (−0.15 to 0.52) | 0.26 |
| Arousal level | −0.00 | (−0.08 to 0.08) | 0.99 | 0.65 | (−0.73 to 2.03) | 0.34 | −0.01 | (−0.21 to 0.19) | 0.87 | 0.32 | (−0.41 to 1.05) | 0.36 | 0.09 | (−0.32 to 0.49) | 0.66 |
The number of eumenorrheic women was 28. Change in performance per unit increase in hormone level.
AP, average power in the Wingate bike test; MVC, maximal voluntary contraction; PP, peak power in the Wingate bike test.
Associations between psychological well-being and performance
Both the peak power and the average power in the Wingate bike test positively correlated with motivation and pleasure level, and the participant’s own perception of performance level (Table 4). Moreover, a positive correlation was observed between jumping height and arousal level, as well as jumping power and own perception of performance level. Finally, a positive correlation was observed between elbow flexor strength and arousal level (Table 4).
TABLE 4.
Association between psychological parameters and performance in eumenorrheic women.
| Measurement | Physical Pain | Motivation | Performance Level | Pleasure Level | Arousal Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | |
| CMJ (mm) | −0.15 | (−0.37 to 0.06] | 0.14 | 0.12 | (−0.04 to 2.75) | 0.15 | 0.11 | (−0.04 to 0.27) | 0.15 | 0.06 | (−0.08 to 0.20) | 0.35 | 0.24 | (0.10 to 0.38) | <0.01 |
| CMJ power (W) | −2.50 | (−6.1 to 1.1) | 0.15 | 1.58 | (−1.14 to 4.30) | 0.24 | 2.82 | (0.25 to 5.39) | 0.03 | 2.01 | (−0.50 to 4.51) | 0.11 | 2.24 | (−0.13 to 4.62) | 0.06 |
| Handgrip (kg) | −0.07 | (−0.53 to 0.39) | 0.76 | −0.10 | (−0.35 to 0.15) | 0.42 | −0.09 | (−0.35 to 0.18) | 0.49 | −0.12 | (−0.34 to 0.09) | 0.24 | −0.01 | (−0.22 to 0.19) | 0.88 |
| Elbow flexor MVC (N·m) | −0.12 | (−0.54 to 0.30) | 0.55 | 0.09 | (−0.17 to 0.35) | 0.47 | 0.13 | (−0.15 to 0.41) | 0.34 | 0.06 | (−0.18 to 0.29) | 0.62 | 0.34 | (0.06 to 0.61) | 0.02 |
| Wingate PP (W) | −3.71 | (−11.18 to 3.77) | 0.28 | 5.65 | (0.01 to 11.31) | 0.05 | 5.67 | (−0.36 to 11.71) | 0.06 | 6.94 | (2.50 to 11.40) | <0.01 | 0.88 | (−2.97 to 4.72) | 0.64 |
| Wingate PP (W·kg−1) | −0.05 | (−0.16 to 0.07) | 0.36 | 0.09 | (0.01 to 0.17) | 0.02 | 0.08 | (−0.01 to 0.16) | 0.08 | 0.1 | (0.04 to 0.16) | <0.01 | 0.02 | (−0.04 to 0.08) | 0.54 |
| Wingate AP (W) | −3.32 | (−8.42 to 1.78) | 0.18 | 5.17 | (1.43 to 8.91) | <0.01 | 6.02 | (1.67 to 10.38) | <0.01 | 4.34 | (0.86 to 7.82) | 0.02 | 2.01 | (−1.05 to 5.08) | 0.19 |
| Wingate AP (W·kg−1) | −0.04 | (−0.12 to 0.03) | 0.24 | 0.09 | (0.04 to 0.14) | <0.01 | 0.09 | (0.03 to 0.15) | <0.01 | 0.06 | (0.02 to 0.11) | 0.01 | 0.04 | (−0.01 to 0.08) | 0.09 |
P values less than 0.05 are reported in bold and indicate a significant association between the psychological factor and the performance outcome parameter.
AP, average power in the Wingate bike test; MVC, maximal voluntary contraction; PP, peak power in the Wingate bike test.
Physical performance in the different MC phases
Table 5 and Supplemental Figure S5 (Supplemental Digital Content 5, Physical performance during the MC phases, http://links.lww.com/MSS/C627) show the strength and performance test results in the different MC phases. The handgrip and elbow flexor strength showed no significant differences over the six MC phases. For CMJ, a significantly lower (6%) jump height was observed in LL compared with ML (P = 0.04; estimate, −0.01; 95% confidence interval (CI), −0.02 to −0.00; Supplemental Fig. S5, Supplemental Digital Content 5, Physical performance during the MC phases, http://links.lww.com/MSS/C627).
TABLE 5.
Performance and psychological parameters in eumenorrheic women in the different MC phases.
| Measurement | EF | MF | LF | EL | ML | LL |
|---|---|---|---|---|---|---|
| Body mass (kg) | 66.2 ± 7.1 | 66.2 ± 7.0 | 66.3 ± 7.0 | 66.0 ± 7.5 | 66.1 ± 7.2 | 67.2 ± 8.0 |
| CMJ (m) | 0.228 ± 0.03 | 0.228 ± 0.03 | 0.226 ± 0.03 | 0.229 ± 0.03 | 0.231 ± 0.03 | 0.218 ± 0.03e |
| CMJ power (W) | 681.5 ± 74.7 | 683.2 ± 75.7 | 684.8 ± 78.4 | 682.8 ± 76.1 | 689.3 ± 84.8 | 679.5 ± 92.2 |
| Handgrip (kg) | 32.4 ± 4.2 | 32.0 ± 4.4 | 31.6 ± 4.7 | 32.5 ± 3.8 | 31.6 ± 4.9 | 32.0 ± 3.2 |
| Elbow flexor MVC (N·m) | 67.5 ± 8.4 | 66.5 ± 9.9 | 67.7 ± 8.7 | 66.5 ± 7.8 | 67.8 ± 8.9 | 67.4 ± 9.2 |
| Wingate PP (W) | 607.1 ± 86.4e | 618.3 ± 89.6 | 614.5 ± 91.4 | 634.3 ± 75.0 | 627.0 ± 94.0 | 602.3 ± 65.4 |
| Wingate PP (W·kg−1) | 9.2 ± 1.2e | 9.4 ± 1.2 | 9.3 ± 1.4 | 9.6 ± 1.0 | 9.5 ± 1.1 | 9.1 ± 1.4 |
| Wingate AP (W) | 528.5 ± 70.3 | 533.2 ± 73.9 | 531.5 ± 73.9 | 544.0 ± 67.4 | 543.8 ± 80.9a,b,c | 516.8 ± 48.4a,b,c,d,e |
| Wingate AP (W·kg−1) | 8.0 ± 0.9e | 8.1 ± 0.9 | 8.1 ± 1.1 | 8.3 ± 0.8 | 8.2 ± 1.0c | 7.8 ± 1.1a,b,e |
| Physical pain | 0.6 (0.0–1.3)b,d,e | 0.3 (0.1–0.5) | 0.5 (0.0–1.8) | 0.0 (0.0–0.5) | 0.2 (0.0–0.6) | 1.0 (0.0–1.2)b,e |
| Motivation | 5.2 (3.8–6.0) | 5.1 (4.5–5.8) | 5.0 (4.2–6.1) | 5.2 (4.7–6.1) | 5.5 (4.6–6.4) | 5.3 (5.0–5.8) |
| Performance level | 4.9 (4.0–5.6)d,e | 4.9 (4.5–6.0) | 5.0 4.2–5.7) | 5.2 (4.5–5.7) | 5.3 (4.7–6.1) | 4.7 (3.5–5.7) |
| Pleasure level | 6.0 (4.0–7.0)b,c,e,f | 6.7 (6.0–7.0) | 6.3 (5.5–7.0) | 6.0 (6.0–7.0) | 7.0 (6.0–7.0) | 7.0 (5.0–7.0) |
| Arousal level | 4.0 (3.0–5.0)e | 4.5 (3.3–5.4) | 3.8 (3.0–4.8) | 4.0 (3.0–5.0) | 4.5 (4.0–6.0) | 4.0 (3.5–5.0) |
Values are presented as mean ± SD (n = 28), except for the psychological parameters, which are presented as medians and interquartile range. Physical pain, motivation, and performance level: range, 0–8 on VAS score. Pleasure level and arousal level: range, 1–9 on the affect grid.
aSignificantly different from EF.
bSignificantly different from MF.
cSignificantly different from LF.
dSignificantly different from EL.
eSignificantly different from ML.
fSignificantly different from LL.
AP, average power in the Wingate bike test; MVC, maximal voluntary contraction; PP, peak power in the Wingate bike test.
Wingate peak power and relative peak power (in watts per kilogram) were significantly lower (3%) in EF compared with ML (P = 0.04 (estimate, 17.0; 95% CI, 1.0–33.0) and P = 0.03 (estimate 0.27; 95% CI, 0.03–0.51; Table 5 and Supplemental Fig. S5, Supplemental Digital Content 5, Physical performance during the MC phases, http://links.lww.com/MSS/C627). Average power was significantly higher in ML compared with LL (P = 0.001; estimate, 25.8; 95% CI, 10.3–41.3) and compared with EF (P = 0.03; estimate, 12.6; 95% CI, 1.4–23.9). The same pattern was observed for relative average power (in watts per kilogram) in ML compared with LL (P = 0.003; estimate, 0.41; 95% CI, 0.14–0.68) and compared with EF (P = 0.02; estimate, 0.20; 95% CI, 0.03–0.36; Table 5 and Supplemental Fig. S5, Supplemental Digital Content 5, Physical performance during the MC phases, http://links.lww.com/MSS/C627).
Psychological reporting of well-being in the different MC phases
The psychological well-being changed during the MC in eumenorrheic women. Physical pain was, in general, reported to be low but higher in EF compared with MF (P = 0.02; estimate, 0.6; 95% CI, 0.1–1.1) and ML (P = 0.01; estimate, 0.7; 95% CI, 0.2–1.1; Table 5). Furthermore, the participants’ own perception of their performance level was significantly lower in EF compared with EL (6%; P = 0.03; estimate, 0.6; 95% CI, 0.1–1.6) and ML (8%; P = 0.03; estimate, 0.5; 95% CI, 0.06–0.9). Similarly, the participants reported a lower pleasure level in EF compared with those in MF, LF, ML, and LL (Table 5). Moreover, the women reported that their arousal level was significantly lower in EF compared with ML (P = 0.02; estimate, 0.8; 95% CI, 0.1–1.5; Table 5). No significant difference between the MC phases was observed for motivation, which, in general, was reported to be high (~5.0 to 5.5 out of 8).
Difference in the sex hormone level and performance between the placebo-pill phase and OC-pill phase
No significant difference was found in the hormone levels between the OC-pill and placebo-pill phases in OC users (Table 6). Physical performance (Supplemental Table S1, Supplemental Digital Content 6, Performance and psychological parameters in OC users, http://links.lww.com/MSS/C628), body mass (Supplemental Fig. S4, Supplemental Digital Content 4, Bodyweight during the MC and OC cycle phases, http://links.lww.com/MSS/C626), and the psychological well-being (Supplemental Table S1, Supplemental Digital Content 6, Performance and psychological parameters in OC users, http://links.lww.com/MSS/C628) did not differ between the OC-pill and placebo-pill phases.
TABLE 6.
Hormones in the placebo-pill and OC-pill phases in OC users.
| Hormone | Placebo-Pill Phase | OC-Pill Phase | Estimate | 95% CI Interval Diff | P |
|---|---|---|---|---|---|
| Estrogen (pmol·L−1) | 15.0 (15.0 to 95.2) | 15.0 (15.0 to 18.9) | 5.00 | (−42.1 to 52.0) | 0.83 |
| Progesterone (nmol·L−1) | 1.1 (0.9 to 1.4) | 1.2 (0.8 to 1.4) | −0.07 | (−0.36 to 0.21) | 0.60 |
| LH (IU·L−1) | 3.2 (0.3 to 5.9) | 2.9 (0.3 to 7.2) | −1.23 | (−4.04 to 1.58) | 0.37 |
| FSH (IU·L−1) | 3.8 (0.3 to 6.7) | 3.2 (0.4 to 5.2) | 1.15 | (−0.78 to 3.07) | 0.23 |
| Testosterone (nmol·L−1) | 0.8 (0.74 to 1.0) | 0.8 (0.62 to 0.99) | 0.02 | (−0.28 to 0.31) | 0.91 |
| SHBG (nmol·L−1) | 147.0 (135.0 to 163.0) | 140.5 (93.5 to 170.5) | 2.32 | (−36.1 to 40.8) | 0.90 |
Values are presented as medians and interquartile range.
Diff, difference.
DISCUSSION
The major finding in the present study was that CMJ height and performance in the Wingate bike test were lower at the end and start of the MC (LL and EF) compared with the other MC phases. Coinciding with this observation, physical pain was higher in EF and LL, and the pleasure level was lower in EF compared with the other MC phases. Accordingly, a positive correlation was observed between physical performance outcomes and self-reported motivation, perception of own physical performance level, pleasure level, and arousal level. In contrast to our hypothesis, we observed no correlations between the fluctuations in the circulating sex hormones during the MC and the performance outcomes. Taken together, our data indicate that MC-related changes in psychological and physical well-being are predictors for variations in power performance during MC more than the sex hormone fluctuations per se. Moreover, the data illustrate the variation that exists between eumenorrheic women with a regular MC, both regarding absolute hormone levels and the changes in hormone levels during an MC.
Influence of the MC phases on physical performance
In the present study, power performance in the CMJ test and Wingate bike test was impaired in LL and EF, respectively, whereas we observed no statistical significant variation over the MC in the strength tests (handgrip and isometric elbow flexor strength). The literature reports conflicting results on strength and power performance between specific menstrual phases (5–11,13–15,17,37). Phillips et al. (17) reported a greater adductor pollicis strength (~10%) in the LF compared with the EF, and Sarwar et al. (13), testing 10 women five times during an MC, observed a markedly greater (+11%) quadriceps and handgrip strength at midcycle compared with the EF and luteal phases. Similarly, Bambaeichi et al. (14) tested eight women four times during two MC and reported a greater isometric contraction of knee flexors (+10%) and a greater isokinetic peak torque of the knee flexors (+10%) and extensors (+5%) in the LF compared with the EF. However, in all three studies, the number of participants was low, and verification of MC phases by blood sampling was not performed (13,14) or performed for a limited number of the participants (17). Pallavi et al. (15) tested 100 young (~18 yr old) women three times in each of two MC, with menstrual phases roughly estimated without blood analysis. They observed a remarkably higher handgrip strength (+46%) in the estimated LF phase compared with the EF phase. Even though this is a noteworthy study based on the number of test days (six times for each of the 100 women), it does not seem physiologically plausible that hormone fluctuations could have such a marked impact on strength performance in women in general. Elite athletes, in particular, would notice such a variation in strength performance. In support, when 186 female elite athletes in an online survey were asked whether they felt that their performance was positively influenced by the MC in specific MC phases, only 6% of the eumenorrheic athletes reported that they felt that their physical performance capacity was improved during their MC and it was not related to any specific MC phase (38).
In the current investigation, we found only minor variations in power-related performance tests and the Wingate bike test between MC phases. Still, a small percentage of improvement or impairment in performance can be of great importance to elite athletes (39). The difference in CMJ height corresponded to 1.3 cm (6%) between the ML phase and the LL phase. The mean difference in peak power in the Wingate bike test between the EF and ML phases was 20 W (3%). The lower performance in CMJ and the Wingate bike test in the LL and the EF could potentially be explained by several factors, including psychological and physical well-being and variation in sex hormones, which both vary between MC phases.
Psychological responses during the MC
The eumenorrheic women in this study reported a higher level of physical pain and a lower pleasure level in the EF phase compared with the other phases (Table 3). Moreover, the eumenorrheic women rated their own performance level to be lower in the EF phase. In elite female athletes, psychological responses during the MC can manifest as worry, distraction, agitation, negative mood states, feeling emotional, and having reduced motivation to practice (40,41). Similarly to our findings, Armour et al. (42) reported, that menstrual pain was associated with fatigue and with perceived reductions in performance during or just before the menstrual bleeding. In support, our data showed a lower pleasure level and performance level in EF compared with several of the other phases, and a positive correlation between performance outcomes and motivation, level of pleasure, and the participant’s own perception of physical performance level. This is also in line with the systematic review of McNulty et al. (3), which concluded that the likelihood of an impaired performance was higher in the EF phase relative to other MC phases. This may primarily be related to reduced psychological well-being during EF in eumenorrheic women, as also observed in the current investigation. Even though the changes in the psychological parameters and performance outcome parameters did not directly correlate with the measured sex hormone levels on the days of testing, we regard the impairment in these parameters specifically in the LL and EF phases as consequences of the hormonal fluctuations during the MC. The mechanism behind the changes in psychological parameters during the MC is not fully elucidated, but fluctuations in gonadal hormone levels during the MC have been shown to affect brain processes in regions involved in emotion regulation (43–45).
Influence of hormone variations during the MC on performance
The findings of the present study are based on data collected from young eumenorrheic women, who ovulated during the test period and fulfilled the criteria for a normal sex hormone profile. Despite this, the individual hormone profiles varied markedly between the participants during MC (Supplemental Fig. S3, Supplemental Digital Content 3, Individual variation in sex hormone levels during the MC, http://links.lww.com/MSS/C625), even though the average sex hormone fluctuations corresponded to a normal profile. For instance, the maximal individual estrogen/progesterone ratio ranged from 13 to 675, and the maximal individual concentration of estradiol varied from 100 to 2400 pmol·L−1. Therefore, we found it mechanistically interesting to perform an analysis of variation in physical performance based on the individual fluctuations in sex hormones (estrogen, progesterone, estrogen/progesterone ratio, testosterone, and testosterone/SHBG ratio) instead of only focusing on predefined MC phases.
We hypothesized that strength performance would be greatest when estrogen peaks and progesterone is still low, because estrogen is suggested to have a positive influence on muscle strength (46), and progesterone is suggested to have antagonistic effects to estrogen (21). To investigate this in detail, we increased the number of testing sessions on the days around ovulation, when the hormone variations are most marked and the greatest estrogen/progesterone ratio is expected. However, we did not find that the fluctuations in estrogen, estrogen/SHBG ratio, progesterone, testosterone, testosterone/SHBG ratio, and estrogen/progesterone ratio correlated with the individual variation in physical performance outcome parameters. Thereby, our initial hypothesis was rejected. Nevertheless, the theory that estrogen should have a positive effect on strength performance is based on estrogen-deficient models where ovariectomized rodents (47) or postmenopausal women (48) are supplemented with estrogen and improvements in power and strength are observed (47,48). Eumenorrheic women are not estrogen deficient, and although the sex hormone levels fluctuate during the MC, the estrogen level in the EF is still well above that of ovariectomized animals and postmenopausal women. The latter may explain why we did not observe a direct link between the variation in circulating levels of sex hormones during the MC and the variation in muscle strength and power performance. Furthermore, the reported positive effect of estrogen administration on strength parameters in ovariectomized animals and postmenopausal women may be the result of more long-term effects of estrogen administration on skeletal muscle rather than an acute effect.
Influence of use of OC on psychological responses and performance
We observed no significant difference between the OC phases in the psychological responses related to well-being (Supplemental Table S1, Supplemental Digital Content 6, Performance and psychological parameters in OC users, http://links.lww.com/MSS/C628), which may relate to the fact that OC is often used as a tool to control menstrual pain (38). In support of this suggestion, the questionnaire-survey by Oxfeldt et al. (38) showed that the prevalence of mood swings and feelings of sadness or depression was lower in OC users than non-OC users. Still, both groups reported a reduced motivation in EF (38). Nevertheless, our observation of no significant difference in the majority of the psychological responses may be related to our observation of no difference in physical strength and power performance between the placebo-pill phase and OC-pill phase in OC users. Even though this suggests that OC use stabilizes physical performance over time, there is a need to clarify whether OC use in general lowers or heightens the performance level in athletes compared with those athletes not using OC.
Body mass
A feeling of being bloated is often reported in the last days of the cycle and the first days of the menses phase (38). Nevertheless, several relatively small studies have not detected any significant bodyweight changes between the MC phases (6,8,9,14,49), whereas a 1-yr prospective study including 765 cycles in 62 women observed a peak in fluid retention scores based on self-reports on the first day of menstrual flow (50). In line with this, Watson and Robinson (51) measured bodyweight in 28 women during 68 consecutive days and reported the highest bodyweight in the LL phase and the EF compared with the other MC phases. However, they also observed dissimilarities between subsequent cycles for the same individuals, which make it difficult to interpret these data. Even though we did not observe any significant difference in body mass overall, some of the women experienced a variance in bodyweight up to 2 kg during the MC. A 2-kg increase in bodyweight will likely influence performance in weight-bearing activities, such as the CMJ test. Furthermore, during the Wingate test, we adjusted the resistance equal to 7.5% of the bodyweight on the specific test day. As part of the day-to-day variation in bodyweight was probably due to water retention, it would have been more correct to use the same individual resistance on all the test days. However, because we cannot be certain about the reasons for day-to-day variation in bodyweight, we applied the adjustment to the resistance to account for such random variations.
Methodical considerations
The strengths of the present study are a) verifying the menstrual phases using ovulation kits and blood analyses, b) testing seven to nine times during an MC, and c) including 30 eumenorrheic women, d) who were relatively untrained, such that the test results were not confounded by previous training sessions.
Several of the earlier small studies, which did not find a difference in performance during the MC (5,6,8–11), also used hormone analysis to define the MC phases. This is suggested to be the most valid method to categorize the phases (16,52). In addition, we used a cutoff minimum limit for the progesterone level in the luteal phase (16 pmol·L−1) to reduce the risk of including participants with either luteal deficiency or an anovulatory cycle (16). Only a few studies, other than our current study, have used this exclusion criterion (8,11). In the present study, two participants were excluded because of low progesterone levels in the luteal phase. Because one of these two participants experienced a positive ovulation test, it is important to apply several methods to establish the different phases and thereby ensure that women with irregular ovulatory cycles and hormone profiles are excluded. If women with luteal deficiency or an anovulatory cycle are included in a study designed to elucidate variation in performance between menstrual phases, their results will reduce the possibility for detecting a potential difference as a consequence of MC-related hormone fluctuations.
In the current investigation, we chose to include relatively untrained women because well-trained women more often experience problems with amenorrhea, lower levels of sex hormones, and menstrual disturbances (18,19). Another disadvantage of including trained women is that it is likely that the testing of performance frequently during an MC is biased by variation in performance related to the trained women’s training schedule. In line with this, a majority of studies reporting no significant variation in performance between the MC phases have tested trained women (5–10). We cannot exclude that untrained women may experience a learning or training effect with repeated testing. However, our data did not show a statistically significant effect of repeated testing (order effect). The latter may be explained by the fact that we performed 3 familiarization test days before the MC testing period was initiated.
We only tested the participants during one MC. Several of the participants’ cycle lengths seemed to be affected by participating in the study. Both shorter and longer MC cycles were experienced compared with the participant’s reported MC history over the past 6 months. This underlines the importance of verifying instead of estimating the MC phases when performing studies including premenopausal women and particularly those aiming at determining differences in performance. Nevertheless, it would have been optimal to obtain data for at least two cycles from all participants.
CONCLUSIONS
In conclusion, small but significant impairments in CMJ height and Wingate bike test performance were observed just before and in the beginning of the menstrual bleeding period compared with other MC phases. No significant correlations were observed between the variation in the sex hormones and the performance parameters, whereas physical performance varied with changes in psychological and physical well-being parameters. In OC users, no significant differences were observed between the OC-pill phase and the placebo-pill phase in strength and power performance, as well as psychological and physical well-being parameters.
Supplementary Material
Acknowledgments
The authors thank the participants for their participation and devotion to this study. The authors also thank the students involved in the execution of the investigation and the analysis (Lotte Iversen, Anton Bundgård, Jeppe Michael Bundgaard Westendorp, Esther Thams, Julie Louise Jensen, Camilla Søgaard, and Mikkel Oxfeldt), and the laboratory technicians for their technical assistance during the experimental days and laboratory analysis (Janni Mosgaard Jensen and Gitte Kaiser Hartvigsen).
This work was supported by Team Denmark, the Aarhus University Research Foundation, and the Toyota Foundation, Denmark.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Ethics statement: All participants gave written informed consent in accordance with the Declaration of Helsinki. The Central Denmark Region Committee on Health Research Ethics approved the protocol (journal no.: 1-10-72-259-18).
Authors contributions: T. V. D., C. H. G., V. S., B. M. B., and M. H. conceived and designed the study. T. V. D. carried out the experiments. T. V. D., L. B. D., B. M. B., and M. H. analyzed the samples and data. T. V. D., L. B. D., V. S., B. M. B., X. J. d. J., and M. H. interpreted the results of the experiments. T. V. D., L. B. D., and M. H. prepared the figures. T. V. D., L. B. D., V. S., B. M. B., and M. H. drafted the manuscript. T. V. D., L. B. D., V. S., B. M. B., X. J. d. J., C. H. G., and M. H. edited and revised the manuscript. T. V. D., L. B. D., V. S., B. M. B., X. J. d. J., C. H. G., and M. H. approved the final version of the manuscript.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
TINE VRIST DAM, Email: tinedam@ph.au.dk.
LINE BARNER DALGAARD, Email: lbdalgaard@ph.au.dk.
VASSILIS SEVDALIS, Email: v.sevdalis@ph.au.dk.
BO MARTIN BIBBY, Email: bibby@ph.au.dk.
XANNE JANSE DE JONGE, Email: x.jansedejonge@outlook.com.
CLAUS H. GRAVHOLT, Email: claus.gravholt@clin.au.dk.
REFERENCES
- 1.Ansdell P Brownstein CG Škarabot J, et al. Menstrual cycle–associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol (1985). 2019;126(6):1701–12. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129(3):229–40. [DOI] [PubMed] [Google Scholar]
- 3.McNulty KL Elliott-Sale KJ Dolan E, et al. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blagrove RC, Bruinvels G, Pedlar CR. Variations in strength-related measures during the menstrual cycle in eumenorrheic women: a systematic review and meta-analysis. J Sci Med Sport. 2020;23(12):1220–7. [DOI] [PubMed] [Google Scholar]
- 5.Wiecek M, Szymura J, Maciejczyk M, Cempla J, Szygula Z. Effect of sex and menstrual cycle in women on starting speed, anaerobic endurance and muscle power. Physiol Int. 2016;103(1):127–32. [DOI] [PubMed] [Google Scholar]
- 6.Tsampoukos A, Peckham EA, James R, Nevill ME. Effect of menstrual cycle phase on sprinting performance. Eur J Appl Physiol. 2010;109(4):659–67. [DOI] [PubMed] [Google Scholar]
- 7.Romero-Moraleda B, Coso JD, Gutiérrez-Hellín J, Ruiz-Moreno C, Grgic J, Lara B. The influence of the menstrual cycle on muscle strength and power performance. J Hum Kinet. 2019;68:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Med Sci Sports Exerc. 1995;27(3):437–44. [PubMed] [Google Scholar]
- 9.Giacomoni M, Bernard T, Gavarry O, Altare S, Falgairette G. Influence of the menstrual cycle phase and menstrual symptoms on maximal anaerobic performance. Med Sci Sports Exerc. 2000;32(2):486–92. [DOI] [PubMed] [Google Scholar]
- 10.Fridén C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13(4):238–41. [DOI] [PubMed] [Google Scholar]
- 11.Janse de Jonge XA, Boot CR, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530(Pt 1):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasa MS Kristoffersen M Ersvær E, et al. The female menstrual cycles effect on strength and power parameters in high-level female team athletes. Front Physiol. 2021;12(164):600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(Pt 1):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bambaeichi E, Reilly T, Cable NT, Giacomoni M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21(4–5):645–60. [DOI] [PubMed] [Google Scholar]
- 15.Pallavi LC, Souza UJD, Shivaprakash G. Assessment of musculoskeletal strength and levels of fatigue during different phases of menstrual cycle in young adults. J Clin Diagn Res. 2017;11(2):CC11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janse DE Jonge X, Thompson B, Han A. Methodological recommendations for menstrual cycle research in sports and exercise. Med Sci Sports Exerc. 2019;51(12):2610–7. [DOI] [PubMed] [Google Scholar]
- 17.Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J Physiol. 1996;496(Pt 2):551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JB, Mitchell D, Musey PI, Collins DC. The relationship of exercise to anovulatory cycles in female athletes: hormonal and physical characteristics. Obstet Gynecol. 1984;63(4):452–6. [PubMed] [Google Scholar]
- 19.Pirke KM, Schweiger U, Broocks A, Tuschl RJ, Laessle RG. Luteinizing hormone and follicle stimulating hormone secretion patterns in female athletes with and without menstrual disturbances. Clin Endocrinol (Oxf). 1990;33(3):345–53. [DOI] [PubMed] [Google Scholar]
- 20.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol (1985). 2007;102(4):1387–93. [DOI] [PubMed] [Google Scholar]
- 21.Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010;40(3):207–27. [DOI] [PubMed] [Google Scholar]
- 22.Smith SS, Woodward DJ, Chapin JK. Sex steroids modulate motor-correlated increases in cerebellar discharge. Brain Res. 1989;476(2):307–16. [DOI] [PubMed] [Google Scholar]
- 23.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282(5):E1139–46. [DOI] [PubMed] [Google Scholar]
- 24.Prado RCR, Silveira R, Kilpatrick MW, Pires FO, Asano RY. Menstrual cycle, psychological responses, and adherence to physical exercise: viewpoint of a possible barrier. Front Psychol. 2021;12:525943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambanis M Kofotolis N Kalogeropoulou E, et al. A study of the effects on the ovarian cycle of athletic training in different sports. J Sports Med Phys Fitness. 2003;43(3):398–403. [PubMed] [Google Scholar]
- 26.Kishali NF, Imamoglu O, Katkat D, Atan T, Akyol P. Effects of menstrual cycle on sports performance. Int J Neurosci. 2006;116(12):1549–63. [DOI] [PubMed] [Google Scholar]
- 27.d’Arcangues C, Jackson E, Brache V, Piaggio G. Women’s views and experiences of their vaginal bleeding patterns: an international perspective from Norplant users. Eur J Contracept Reprod Health Care. 2011;16(1):9–17. [DOI] [PubMed] [Google Scholar]
- 28.Christin-Maitre S. History of oral contraceptive drugs and their use worldwide. Best Pract Res Clin Endocrinol Metab. 2013;27(1):3–12. [DOI] [PubMed] [Google Scholar]
- 29.Martin D, Sale C, Cooper SB, Elliott-Sale KJ. Period prevalence and perceived side effects of hormonal contraceptive use and the menstrual cycle in elite athletes. Int J Sports Physiol Perform. 2018;13(7):926–32. [DOI] [PubMed] [Google Scholar]
- 30.Elliott-Sale KJ McNulty KL Ansdell P, et al. The effects of oral contraceptives on exercise performance in women: a systematic review and meta-analysis. Sports Med. 2020;50(10):1785–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis. 1987;40(12):1129–33. [DOI] [PubMed] [Google Scholar]
- 32.Russell J, Weiss A, Mendelsohn G. Affect Grid: A Single-Item Scale of Pleasure and Arousal. J Pers Soc Psychol. 1989;57(3):493–502. [Google Scholar]
- 33.Owen JA, Jr. Physiology of the menstrual cycle. Am J Clin Nutr. 1975;28(4):333–8. [DOI] [PubMed] [Google Scholar]
- 34.Janse de Jonge XA. Effects of the menstrual cycle on exercise performance. Sports Med. 2003;33(11):833–51. [DOI] [PubMed] [Google Scholar]
- 35.Killgore WD. The affect grid: a moderately valid, nonspecific measure of pleasure and arousal. Psychol Rep. 1998;83(2):639–42. [DOI] [PubMed] [Google Scholar]
- 36.Rechichi C, Dawson B, Goodman C. Athletic performance and the oral contraceptive. Int J Sports Physiol Perform. 2009;4(2):151–62. [DOI] [PubMed] [Google Scholar]
- 37.Ekenros L, Hirschberg AL, Heijne A, Fridén C. Oral contraceptives do not affect muscle strength and hop performance in active women. Clin J Sport Med. 2013;23(3):202–7. [DOI] [PubMed] [Google Scholar]
- 38.Oxfeldt M, Dalgaard LB, Jørgensen AA, Hansen M. Hormonal contraceptive use, menstrual dysfunctions, and self-reported side effects in elite athletes in Denmark. Int J Sports Physiol Perform. 2020;15(10):1377–84. [DOI] [PubMed] [Google Scholar]
- 39.Christensen PM, Shirai Y, Ritz C, Nordsborg NB. Caffeine and bicarbonate for speed. A meta-analysis of legal supplements potential for improving intense endurance exercise performance. Front Physiol. 2017;8:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Findlay RJ, Macrae EHR, Whyte IY, Easton C, Forrest Née Whyte LJ. How the menstrual cycle and menstruation affect sporting performance: experiences and perceptions of elite female rugby players. Br J Sports Med. 2020;54(18):1108–13. [DOI] [PubMed] [Google Scholar]
- 41.Brown N, Knight CJ, Forrest Née Whyte LJ. Elite female athletes’ experiences and perceptions of the menstrual cycle on training and sport performance. Scand J Med Sci Sports. 2021;31(1):52–69. [DOI] [PubMed] [Google Scholar]
- 42.Armour M, Parry KA, Steel K, Smith CA. Australian female athlete perceptions of the challenges associated with training and competing when menstrual symptoms are present. Int J Sports Sci Coaching. 2020;15(3):316–23. [Google Scholar]
- 43.Ossewaarde L Hermans EJ van Wingen GA, et al. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. [DOI] [PubMed] [Google Scholar]
- 44.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diekhof EK, Ratnayake M. Menstrual cycle phase modulates reward sensitivity and performance monitoring in young women: preliminary fMRI evidence. Neuropsychologia. 2016;84:70–80. [DOI] [PubMed] [Google Scholar]
- 46.Collins BC, Laakkonen EK, Lowe DA. Aging of the musculoskeletal system: how the loss of estrogen impacts muscle strength. Bone. 2019;123:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms behind estrogen’s beneficial effect on muscle strength in females. Exerc Sport Sci Rev. 2010;38(2):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64(10):1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rael B Romero-Parra N Alfaro-Magallanes VM, et al. Body composition over the menstrual and oral contraceptive cycle in trained females. Int J Sports Physiol Perform. 2021;16(3):375–81. [DOI] [PubMed] [Google Scholar]
- 50.White CP, Hitchcock CL, Vigna YM, Prior JC. Fluid retention over the menstrual cycle: 1-year data from the prospective ovulation cohort. Obstet Gynecol Int. 2011;2011:138451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson PE, Robinson MF. Variations in body-weight of young women during the menstrual cycle. Br J Nutr. 1965;19:237–48. [DOI] [PubMed] [Google Scholar]
- 52.Landgren BM, Undén AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94(1):89–98. [DOI] [PubMed] [Google Scholar]


