Background:
Racial differences in metabolomic profiles may reflect underlying differences in social determinants of health by self-reported race and may be related to racial disparities in coronary heart disease (CHD) among women in the United States. However, the magnitude of differences in metabolomic profiles between Black and White women in the United States has not been well-described. It also remains unknown whether such differences are related to differences in CHD risk.
Methods:
Plasma metabolomic profiles were analyzed using liquid chromatography-tandem mass spectrometry in the WHI-OS (Women’s Health Initiative-Observational Study; 138 Black and 696 White women), WHI-HT trials (WHI-Hormone Therapy; 156 Black and 1138 White women), MESA (Multi-Ethnic Study of Atherosclerosis; 114 Black and 219 White women), JHS (Jackson Heart Study; 1465 Black women with 107 incident CHD cases), and NHS (Nurses’ Health Study; 2506 White women with 136 incident CHD cases). First, linear regression models were used to estimate associations between self-reported race and 472 metabolites in WHI-OS (discovery); findings were replicated in WHI-HT and validated in MESA. Second, we used elastic net regression to construct a racial difference metabolomic pattern (RDMP) representing differences in the metabolomic patterns between Black and White women in the WHI-OS; the RDMP was validated in the WHI-HT and MESA. Third, using conditional logistic regressions in the WHI (717 CHD cases and 719 matched controls), we examined associations of metabolites with large differences in levels by race and the RDMP with risk of CHD, and the results were replicated in Black women from the JHS and White women from the NHS.
Results:
Of the 472 tested metabolites, levels of 259 (54.9%) metabolites, mostly lipid metabolites and amino acids, significantly differed between Black and White women in both WHI-OS and WHI-HT after adjusting for baseline characteristics, socioeconomic status, lifestyle factors, baseline health conditions, and medication use (false discovery rate <0.05); similar trends were observed in MESA. The RDMP, composed of 152 metabolites, was identified in the WHI-OS and showed significantly different distributions between Black and White women in the WHI-HT and MESA. Higher RDMP quartiles were associated with an increased risk of incident CHD (odds ratio=1.51 [0.97–2.37] for the highest quartile comparing to the lowest; Ptrend=0.02), independent of self-reported race and known CHD risk factors. In race-stratified analyses, the RDMP-CHD associations were more pronounced in White women. Similar patterns were observed in Black women from the JHS and White women from the NHS.
Conclusions:
Metabolomic profiles significantly and substantially differ between Black and White women and may be associated with CHD risk and racial disparities in US women.
Keywords: heart diseases, health status disparities, metabolomics, plasma, race, women
Novelty and Significance.
What Is Known?
In the United States, Black women have higher prevalence of coronary heart disease (CHD) than White women, and such differences cannot be fully explained by racial disparities in education, income, and known CHD risk factors.
Metabolomic profiles are influenced by many external factors (eg, diet, lifestyle, environmental pollution, and social exposures) and have been associated with risk of CHD.
Limited data have shown some differences in metabolomic profiles between Black and White individuals in the United States, but the magnitude of such differences has not been well-described.
What New Information Does This Article Contribute?
Levels of several metabolites, especially lipid metabolites and amino acids, significantly differed between Black and White women.
We identified a racial difference metabolomic pattern composed of 152 metabolites that had significantly different distributions between Black and White women.
The racial difference metabolomic pattern and a few lipid metabolites with large differences (>1 SD) by race were significantly associated with incident CHD, independent of self-reported race and known risk factors for CHD.
Differences in CHD risk between Black and White women in the United States have been well-recognized and cannot be fully explained by racial differences in socioeconomic status and known CHD risk factors. Metabolomic profiles reflect the status of human metabolism, which is influenced by many external factors including social exposures. Alterations in metabolomic profiles have been associated with CHD risk in women. However, it remains unknown the magnitude of differences in metabolomic profiles between Black and White women and their roles in racial differences in CHD risk. In this study, we observed substantial and significant differences in metabolomic profiles between Black and White women. We identified and validated a racial difference metabolomic pattern that characterizes differences in the metabolomic patterns between Black and White women. We also found that a few metabolites with large differences (>1 SD) by race and the racial difference metabolomic pattern were significantly associated with risk of incident CHD, independent of self-reported race and known cardiovascular risk factors. Our findings indicate that metabolomics may act as a tool that sums the impact of cumulative exposures to differences in the lived and social experiences between Black and White women on racial disparities in cardiovascular diseases.
In this Issue, see p 559
Meet the First Author, see p 560
Editorial, see p 616
The prevalence and mortality rates of coronary heart disease (CHD) in the United States has been declining over the past several decades; however, racial disparities in CHD have remained.1,2 Compared with non-Hispanic White women, Black/African American women have higher CHD morbidity and mortality as well as significantly and consistently poorer cardiovascular health.2,3 Classifications of race are social and cultural constructs,4 which both create and reflect racial differences in lifetime exposure to environmental (eg, physical environment), social (eg, socioeconomic status, cultural factors, and structural racism), and individual (eg, lifestyle and behavioral factors) factors contributing to health.5,6 However, racial disparities in CHD between Black and White women cannot be fully explained by racial differences in socioeconomic status and other known risk factors for CHD, which are mostly individual level factors.7–9 Racial disparities in health are also caused by adverse social factor exposures at the neighborhood level, which are usually hard to quantify.6,9 Given the difficulties in measuring the impact of social exposures on health outcomes, there is a critical need to identify novel measures of the cumulative impact of the social experience of race on cardiovascular health disparities.6
Recent advances in metabolomic profiling enable the assessment of a wide array of small-molecule metabolites that reflect human metabolic status. Human metabolism is partially determined by the genome10 but is influenced by a large variety of exogenous factors, including dietary and lifestyle factors, environmental exposures, and social exposures.11–14 Recently, metabolomic alterations have been associated with incident CHD.15–17 Limited data on differences in the metabolomic profiles between Black and White individuals in the United States are available, with small sample sizes in populations with high prevalence of specific conditions, such as overweight/obesity (n=500),18 high blood pressure (n=52),19 or bladder cancer (n=73).20 Thus, the magnitude of differences in the metabolomic profiles by race in the United States has not been well-described. It remains unknown whether differences in metabolomic profiles between racial groups might contribute to racial disparities in CHD.
In this study, we determined whether the metabolomic profiles of Black women differ from those of White women, and then investigated whether differences in the metabolomic patterns between Black and White women might be associated with CHD risk, based on data from the Women’s Health Initiative (WHI).15 We performed discovery analyses to (1) estimate differences in metabolite levels between Black and White women and (2) established the racial difference metabolomic pattern (RDMP) representing differences in the metabolomic pattern by race in the WHI-OS (WHI-Observational Study), and these findings were internally validated in the WHI-HT (WHI-Hormone Therapy) trials and externally replicated in the MESA (Multi-Ethnic Study of Atherosclerosis) to assess the generalizability. To estimate the impact of racial differences in metabolomic patterns on racial disparities in CHD risk, we estimated associations of metabolites showing large race differences and the RDMP with incident CHD risk in the WHI and estimated race-specific associations. Findings were then replicated in Black women from the JHS (Jackson Heart Study) and White women from the NHS (Nurses’ Health Study).
Methods
Data Availability
WHI and NHS metabolomics data used in this study are available from the corresponding author upon reasonable request and compliance with the data request processes associated with each cohort. Data access for the MESA was approved by the TOPMed Publications and Presentations Steering Committees with data access provided by an approved project (#10106). The JHS metabolomics data used in this study have been submitted to the JHS Data Coordinating Center and to dbGaP; until posted in dbGaP, all JHS data are available from the JHS Data Coordinating Center on request.
Study Population
In the WHI, MESA, and NHS, participants were asked to self-report their race and ethnicity. Black indicates self-reported Black or African American (not of Hispanic origin), and White indicates self-reported White (not of Hispanic origin). We sought to examine differences in metabolomic profiles between Black and White women, male participants (from MESA and JHS) and participants who reported other races and ethnicities were excluded. The JHS only recruited participants who self-identified as Black. All participants included in this study were free of known clinical cardiovascular disease (CVD) at baseline. The study protocols were approved by the Institutional Review Board of Mass General Brigham/Brigham and Women’s Hospital, the MESA Metabolomics Working Group, and the Beth Israel Deaconess Medical Center. A detailed description of the study cohorts is included in the Supplemental Methods. A flow chart of the analysis approach and the role of each of the cohorts is included in Figure S1.
Women’s Health Initiative
The WHI-OS enrolled 93 676 postmenopausal women across the United States, between 1994 and 1998, who were ineligible or unwilling to participate in the WHI hormone or dietary trials. In the WHI-HT, one of the trials randomly assigned 16 608 postmenopausal women with an intact uterus to estrogen-plus-progestin or placebo, whereas in the other trial 10 739 women with prior hysterectomy were randomly assigned to estrogen or placebo. All participants provided written informed consent. Women included in this study were drawn from a prior nested case-control study of the metabolomics of CHD and were free of CVDs at the study baseline.15 CHD was defined as myocardial infarction or death attributable to CHD. Each CHD case was matched to a control on baseline age (5-year range), self-reported race, hysterectomy status, and enrollment groups (2-year range).15
All WHI participants included in this study had baseline metabolomics data and had no missing covariate data. In analyses examining racial differences in metabolomic profiles, the discovery/training set included 834 women (Black/White: 138/696) from the WHI-OS, and the validation/testing set included 1294 women (Black/White: 156/1138) from the WHI-HT.
In analyses examining associations of metabolites with large differences by race and the RDMP with CHD risk, the discovery set combined women from both WHI-OS and WHI-HT placebo arms to maximize the statistical power. We excluded women from the WHI-HT intervention arms from the CHD analysis because (1) active hormone therapy (HT) use was found to substantially change metabolomic profiles from baseline21 and (2) estrogen-plus-progestin use was associated with increased risk of CHD in the WHI while estrogen-alone use was not.22,23 After exclusions, 717 incident CHD cases (Black/White: 109/608), with a median time to event of 4.8 years, and 719 matched controls (Black/White: 108/611) were included in the analysis.
Multi-Ethnic Study of Atherosclerosis
As a replication set in analyses examining racial differences in metabolomic profiles, 333 women (Black/White: 114/219) from the MESA who had available baseline metabolomics data (blood samples were collected between 2000 and 2002) were included. All participants in the MESA cohort provided written informed consent for participation. Women included in this study were drawn from a multi-omics pilot study in which participants were randomly selected.
Jackson Heart Study
As a replication set in analyses examining associations of metabolites with large differences by race and the RDMP with CHD risk in Black women, we included 1465 Black women from a previous study of the metabolomics of CHD in the JHS, who were free of CVD at baseline and had available baseline metabolomics data (blood samples were collected between 2000 and 2004).16 CHD was defined as definite fatal CHD, definite or probable myocardial infarction, silent myocardial infarction between examinations (as determined by electrocardiography), or coronary revascularization. During a median follow-up of 11.7 years, 107 incident CHD cases were documented.16
Nurses’ Health Study
As a replication set in analyses examining associations of metabolites with large differences by race and the RDMP with CHD risk in White women, this study included 2506 White women from the NHS, who were free of CVD and cancers at the time of blood collection (between 1989 and 1990) and had both metabolomic profiles and blood lipid data measured previously.24 Metabolomic data were available from 10 prior substudies (nested case-control studies) in the NHS that were originally designed for different outcomes25 (see details in the Supplemental Methods). CHD was defined as fatal or nonfatal myocardial infarction or coronary death. During a median follow-up of 24.3 years, 136 incident CHD cases were documented.
Metabolomics Profiling
Metabolomic profiling in baseline plasma samples of participants from the WHI was performed using liquid chromatography-tandem mass spectrometry at the Broad Institute,15 and the same methods were used for MESA, JHS, and NHS samples. A detailed description of metabolomics profiling methods for each cohort is included in the Supplemental Methods. Metabolites with >20% missing values were excluded from the analysis. After quality control, 472 named metabolites were used in the WHI analyses. Of these 472 metabolites, 322 were available in the MESA, 101 were available in the JHS, and 169 were available in the NHS. Differences in the number of available metabolites between cohorts were mainly due to differences in liquid chromatography-tandem mass spectrometry panels used and the number of metabolites annotated. For the metabolites included in the analyses, missing values were imputed to one-half the minimum observed value in the WHI, MESA, and JHS. In the NHS, the imputation was performed within each sub-study (see details in the Supplemental Methods).
Covariates
Information on age, lifestyle factors (smoking, alcohol consumption, and physical activity), body mass index (BMI), education, family income, female-specific variables (hysterectomy, menopausal status, and HT use), baseline health conditions (diabetes, hypertension, and depression), medication use (aspirin, lipid-lowing, antihyperglycemic, antihypertensive, and antidepressants), dietary factors (macronutrients intake, total calorie intake, and Healthy Eating Index-200526), and psychological indicators (emotional well-being, hostility, general health, optimism, social support, social functioning, social strain, and sleep disturbance) was collected at study baseline in the WHI, MESA, and JHS. In the NHS, the above information was collected from biennial questionnaires preceding blood collections and a separate questionnaire completed at the time of blood draw. Total and HDL (high-density lipoprotein) cholesterol levels were measured in plasma samples for all cohorts included in this study. Fasting glucose levels were available in a subset of participants in the WHI-OS (n=217) and the WHI-HT (n=1289).
Statistical Analyses
Metabolite levels were converted to standard normal distributions using inverse normal transformation. Statistical analyses were carried out using R. The statistical analysis approaches are illustrated in Figure S1.
Metabolome-Wide Association Analysis of Race
Discovery
In WHI-OS, we used linear regression to estimate associations between levels of each metabolite (continuous; dependent variables) and self-reported race (binary; Black versus White), adjusting for CHD case-control status, matching factors (age, hysterectomy, and enrollment window), HT use status (never/past/current users), BMI, smoking status, alcohol consumption, education, family income, physical activity, baseline health conditions (diabetes, hypertension, and depression), and medication use (aspirin, lipid-lowing, antihyperglycemic, antihypertensive, and antidepressants). The false discovery rate (FDR) was calculated using the Benjamini-Hochberg procedure to account for multiple comparison, and an FDR<0.05 was considered significant. Sensitivity analyses were performed with additional adjustment for total and HDL-cholesterol, dietary factors (Healthy Eating Index-2005 and intake of proteins, total carbohydrates, total fat, and total calories), fasting glucose levels (in a subset of participants), or psychological characteristics (emotional well-being, hostility, general health, optimism, social support, social functioning, social strain, and sleep disturbance).
Validation
Metabolites with an FDR<0.05 in the WHI-OS were then tested in the WHI-HT using linear regression models adjusting for all above covariates plus HT trial type (estrogen-plus-progestin or estrogen-alone trial) and randomized treatment arms (intervention or placebo). Metabolites with an FDR<0.05 in both WHI-OS and WHI-HT were considered to be validated and, therefore, significantly differ between Black and White women.
Replication
All validated metabolites were tested as available in the MESA; linear regression models were adjusted for age, BMI, HT use, smoking, alcohol consumption, education, family income, physical activity, baseline health conditions (diabetes, hypertension, and depression), and medication use (aspirin, lipid-lowing, antihyperglycemic, antihypertensive, and antidepressants). Sensitivity analyses were performed with additional adjustment for total and HDL-cholesterol levels.
We calculated Pearson correlation coefficients to estimate the concordance of association coefficients estimated in the WHI-OS and those in the WHI-HT and MESA.
Estimation and Validation of the RDMP
Training
Elastic net regression was used in the WHI-OS to select a sparse metabolite set associated with self-reported race and to estimate a corresponding metabolite score of racial differences (the RDMP score). The elastic net regression is a regularized regression that shrinks regression coefficients without indiscriminate elimination of correlated predictors.27 The R package glmnet was used to fit the elastic net regression model with adjustment for age.28 The optimal model was chosen using 10-fold cross-validation. We included all 472 metabolites in the elastic net model. Regression coefficients of each selected metabolite and age in the optimal model were used to calculate the RDMP.
Testing
For internal testing, we calculated the RDMP and compared its distribution between Black and White women in the WHI-HT. For external testing, the RDMP was calculated in the MESA, and the distribution between races was compared. To avoid overfitting, the RDMP was calculated in the training set (WHI-OS) using leave-one-out cross-validation. This approach has been used previously to estimate a metabolic signature that robustly reflects the adherence and metabolic response to a Mediterranean diet.24 We also calculated the RDMP in the JHS and NHS. Additionally, since not all the metabolites used to calculate the RDMP were available in MESA, JHS, and NHS, 3 restricted RDMP scores (rRDMP) were calculated using overlapping metabolites in the WHI-HT (ie, rRDMP1 for overlapping metabolites with the MESA, rRDMP2 for overlapping metabolites with the JHS, and rRDMP3 for overlapping metabolites with the NHS). Finally, to estimate the performance of the RDMP, we calculated the area under the receiver operating characteristic curves for self-reported race in both WHI and MESA.
Associations of Metabolites That Differed by Race and the RDMP With CHD Risk
Because CHD cases were matched to controls in the WHI (combining women from the WHI-OS and placebo arms of the WHI-HT), we applied conditional logistic regression models to estimate associations of selected metabolites (whose levels significantly [FDR<0.05] differed by >1-SD and 0.5-1 SD between Black and White women; continuous) and the RDMP (Z score and in quartiles) with incident CHD, adjusting for substudies (OS/estrogen-alone trial/estrogen-plus-progestin trial), BMI, HT use status, smoking status, diabetes, antihyperglycemic medication use, systolic blood pressure, antihypertensive medication use, aspirin use, lipid-lowing medication use, and total and HDL-cholesterol. We selected 0.5-SD and 1-SD as the cut points because such differences were considered large in magnitude that might potentially be clinically significant. Finally, sensitivity analyses were performed with additional adjustment for education, family income, diet quality, and psychosocial characteristics.
In stratified analyses, we repeated the above analyses separately in Black and White women in the WHI. Because almost all the Black women were distributed in the highest quartile of the RDMP (Figure S2), we calculated race-specific RDMP quartiles and estimated their associations with CHD risk in Black and White women separately, adjusting for the same set of covariates. As a replication, we examined the associations of metabolites with large differences by race (differed >0.5-SD in WHI-HT) and the RDMP with incident CHD risk in Black women from the JHS and White women from the NHS. Cox proportional-hazards models were used to estimate associations of metabolites (continuous) and RDMP (Z score and in quartiles) with incident CHD, adjusting for batch (JHS only), age, BMI, menopausal status, HT use status, smoking status, diabetes, antihyperglycemic medication use, systolic blood pressure, antihypertensive medication use, aspirin use, lipid-lowing medication use, and total and HDL-cholesterol. Similarly, sensitivity analyses were performed with additional adjustment for education (WHI and JHS), family income (WHI and JHS), diet quality (WHI and NHS), and psychosocial characteristics (WHI only).
Results
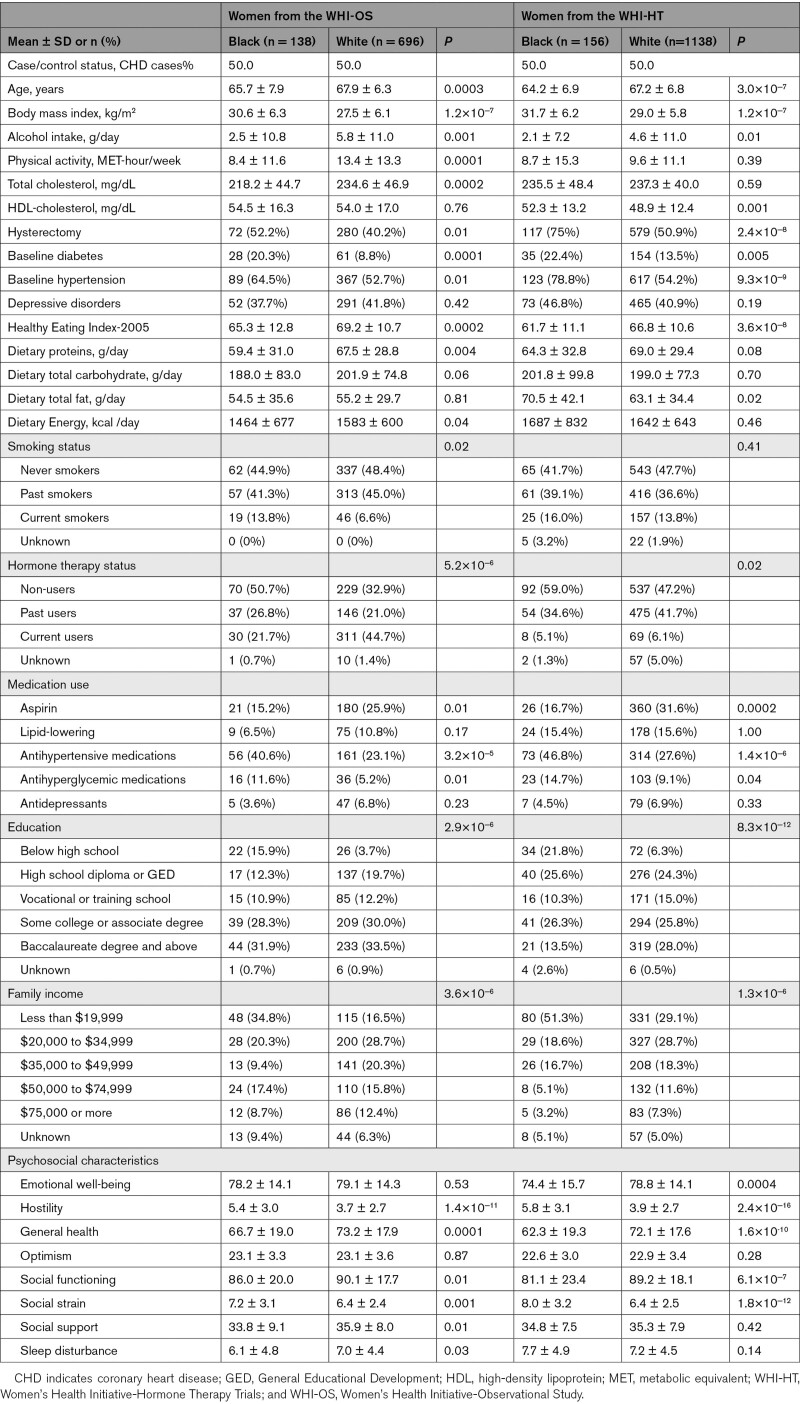
In the WHI, Black women were relatively younger, had a higher BMI and HDL-cholesterol, and had lower physical activity, alcohol intake, dietary quality, education, family income, and total cholesterol, compared with White women. Black women were more likely to be current smokers and had higher prevalence of diabetes and hypertension (Table 1). Baseline characteristics of MESA and WHI participants were mostly similar but women in MESA were younger than the WHI (Table S1). MESA participants had higher total physical activity because both leisure and nonleisure physical activity were assessed, whereas only leisure physical activity was assessed in the WHI. Women from the JHS and NHS were also younger than those from the WHI, but their characteristics were mostly similar to Black and White women in the WHI, respectively (Table S2).
Table 1.
Baseline Characteristics of WHI Participants by Race
Differences in Individual Metabolites Between Black and White Individuals
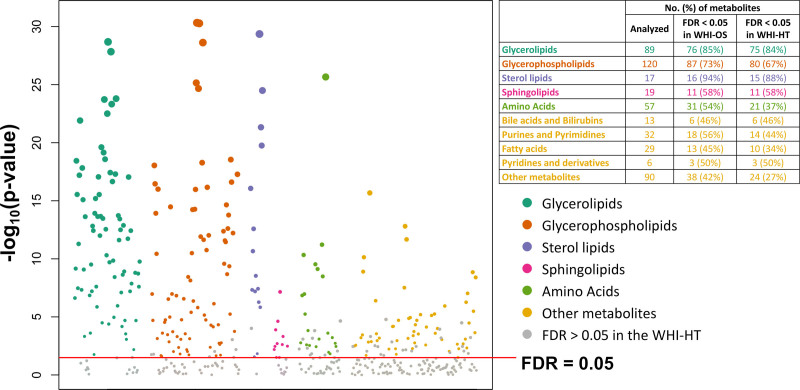
Of 472 tested metabolites, 259 (54.9%) significantly differed between Black and White women (FDR<0.05) in both WHI-OS and WHI-HT in the fully adjusted model (Figure 1 and Table S3). A majority of these metabolites were lipids (n=181; including 75 glycerolipids, 80 glycerophospholipids, 15 sterol lipids, and 11 sphingolipids), followed by amino acids (n=21), purines and pyrimidines (n=14), fatty acids (n=10), and other metabolites across different categories (n=33).
Figure 1.
Metabolites that significantly differed between Black and White women from the Women’s Health Initiative (WHI). The Manhattan plot shows the distributions of P values for regression coefficients of each metabolite in the discovery set (WHI-OS [WHI-Observational Study]). Linear regression models were adjusted for coronary heart disease (CHD) case-control status, matching factors (age, hysterectomy status, and enrollment window), hormone therapy use status, body mass index, smoking status, alcohol consumption, education, family income, physical activity, baseline health conditions (diabetes, hypertension, and depression), and medication use (aspirin, lipid-lowing, antihyperglycemic, antihypertensive, and antidepressants). The table shows the numbers of analyzed metabolites in each category, as well as the numbers and percentages of metabolites that significantly differed by race in each category. False discovery rate (FDR) was estimated for 472 comparisons in the WHI-OS (ie, the number of all metabolites included in the analysis) and 299 comparisons in the WHI-HT (WHI-Hormone Therapy; ie, the number of metabolites with an FDR<0.05 in the WHI-OS).
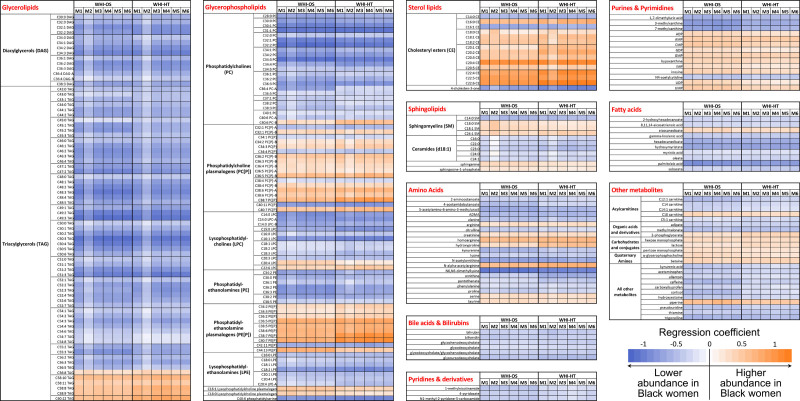
Figure 2 shows the direction and magnitude of the association between self-reported race and each metabolite. In the fully adjusted model (model 1), most of the lipid metabolites had lower abundance in Black than White women, except for several long-chain polyunsaturated triacylglycerols (C56-C60 with 8-12 double-bonds), phosphatidylcholine plasmalogens, phosphatidylethanolamine plasmalogens, cholesteryl esters, and sphingomyelins. Additional adjustment for total and HDL-cholesterol slightly reduced the number of lipid metabolites and amino acids that differed by race but not metabolites from other metabolite classes. Results from sensitivity analyses with additional adjustment for dietary factors, fasting glucose levels, or psychological characteristics were consistent with the main findings (Figure 2 and Table S3).
Figure 2.
Differences in metabolite levels between Black and White women in the Women’s Health Initiative (WHI). The heatmap shows regression coefficients for each metabolite in each category. Model 1 (M1; fully adjusted) is adjusted for coronary heart disease (CHD) case-control status, matching factors (age, hysterectomy, and enrollment window), hormone therapy use status, body mass index, smoking status, alcohol consumption, education, family income, physical activity, baseline health conditions (diabetes, hypertension, and depression), and medication use (aspirin, lipid-lowing, antihyperglycemic, antihypertensive, and antidepressants). Model 2 (M2; lipid-adjusted): M1 plus total and high-density lipoprotein cholesterol. Model 3 (M3; diet quality-adjusted): M1 plus Healthy Eating Index-2005. Model 4 (M4; macronutrient-adjusted): M1 plus dietary intake of proteins, total carbohydrates, and total fat. Model 5 (M5; calorie-adjusted): M1 plus total calorie intake. Model 6 (psychological factors-adjusted): M1 plus psychological indicators (emotional well-being, hostility, general health, optimism, social support, social functioning, social strain, and sleep disturbance). WHI-HT indicates WHI-Hormone Therapy; and WHI-OS, WHI-Observational Study.
The regression coefficients for each metabolite were highly correlated in the WHI-OS and WHI-HT (r=0.98), and regression coefficients from the MESA were concordant with those from the WHI-OS (r=0.52; Figure S3). Although regression coefficients observed in MESA were largely nonsignificant, possibly because of the small sample size, 5 metabolites (C36:3 phosphatidylethanolamine, C20:5 cholesteryl ester, C18 carnitine, proline, and hypoxanthine) significantly differed by race (raw P<0.05), with the regression coefficients in the same direction as observed in the WHI-HT (Table S4).
Associations Between Metabolites With Large Differences by Race and CHD Risk
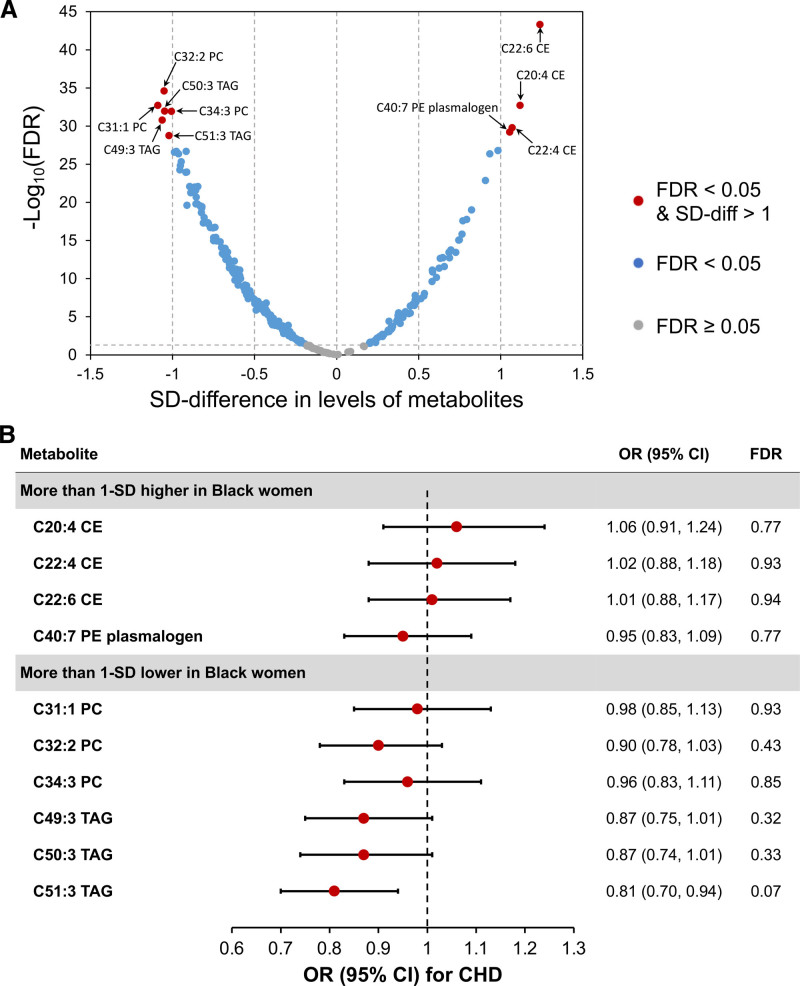
The direction and magnitude of differences in metabolite levels (SD units) in the WHI-HT is displayed in Figure 3A. Ten lipid metabolites differed in abundance by >1-SD between Black and White women and were tested for their associations with CHD in the WHI. For the 4 metabolites that were >1-SD higher in Black women, we did not observe significant associations with CHD risk (FDR>0.5); however, for the remaining 6 metabolites that were >1-SD lower in Black women, higher levels of C51:3 triacylglycerol were marginally significantly associated with a decreased risk of CHD (odds ratio [OR]=0.81 [95% CI, 0.70–0.94]) after accounting for multiple comparisons (FDR<0.1 for 472 comparisons; Figure 3B). In stratified analysis by race, similar results were observed in White women, but the associations in Black women were nonsignificant (Figure S4 and Table S5). Additionally, for the 25 metabolites that were 0.5-1 SD higher in Black women, 3 lipid metabolites were marginally significantly associated with higher CHD risk (FDR<0.1), with one metabolite (C36:2 phosphatidylcholine plasmalogen) showing a significant association (OR=1.18 [95% CI, 1.09–1.49]; FDR=0.03). For the 89 metabolites that were 0.5-1 SD lower in Black women, 8 lipid metabolites (5 triacylglycerols and 3 diacylglycerols) were marginally significantly associated with decreased risk of CHD (FDR<0.1), with 2 metabolites (C52:6 triacylglycerol and C36:4 diacylglycerol) showing significant associations (FDR=0.03). When stratified by self-reported race, similar results were observed in Black and White women in the WHI. In replication analyses in Black women from the JHS and White women from the NHS, findings were similar to race-specific results from the WHI for metabolites showing marginally significant associations with CHD risk (Table S5).
Figure 3.
Magnitude of difference in metabolites between Black and White women, and their associations with coronary heart disease (CHD) risk in the Women’s Health Initiative (WHI). A, The volcano plot highlights 12 metabolites whose levels differed by >1-SD between Black and White women in the WHI-HT (WHI-Hormone Therapy). The x-axis is SD-difference in levels of each metabolite; the y-axis is −log10(false discovery rate [FDR]) for each metabolite. Metabolites with SD-difference ≤1 were highlighted using gray (FDR≥0.05) or blue (FDR<0.05) dots. Red dots highlight metabolites with SD-difference >1 and FDR<0.05. Models were adjusted for age, CHD case-control status, hysterectomy, hormone therapy use, enrollment window, body mass index, smoking, alcohol consumption, education, family income, physical activity, baseline health conditions, and medication use. B, Associations between metabolites with >1-SD difference and CHD risk in the combined dataset of WHI-OS (WHI-Observational Study) and placebo arms of WHI-HT. CHD cases were matched to controls on age, self-reported race, hysterectomy, and enrollment window. Conditional logistic regression models were adjusted for substudies (OS/estrogen-alone trial/estrogen-plus-progestin trial), body mass index, hormone therapy use status, smoking, diabetes, antihyperglycemic medication use, systolic blood pressure, antihypertensive medication use, aspirin use, lipid-lowing medication use, and total and high-density lipoprotein cholesterol. FDR for associations between metabolites and CHD risk were estimated for 472 comparisons to test the statistical significance in the whole metabolomics profile in the WHI. CE indicates cholesteryl ester; OR, odds ratio; PC, phosphatidylcholine; PE, phosphatidylethanolamine; and TAG, triacylglycerol.
Estimation and Validation of the RDMP
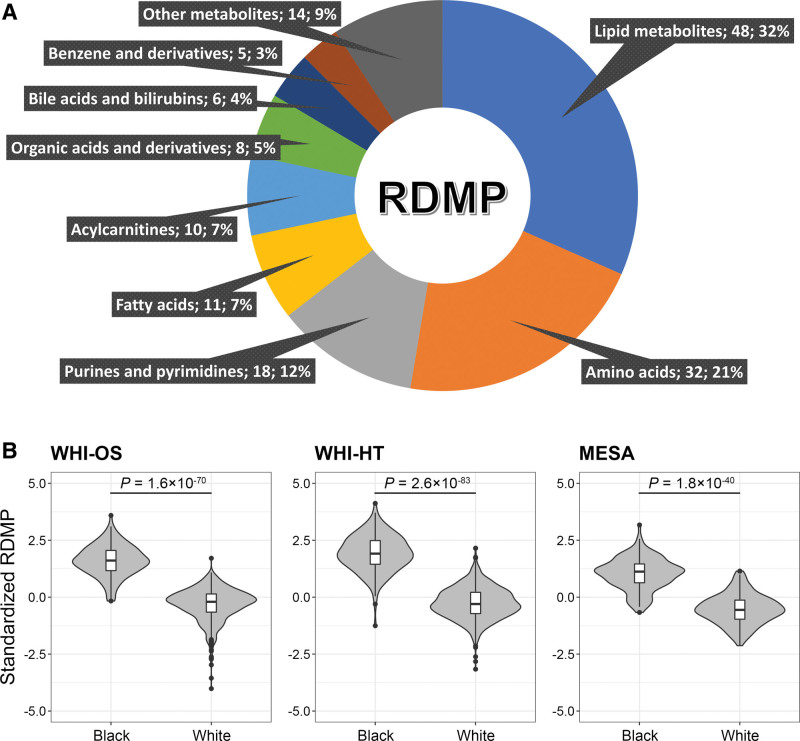
To create a composite score that reflects the cumulative differences in metabolomic profiles between Black and White women, we established the RDMP using elastic net regression. The RDMP consists of 152 metabolites, including 48 lipid metabolites, 32 amino acids, 18 purines and pyrimidines, 11 fatty acids, 11 acylcarnitines, and 32 metabolites from other classes (Figure 4A and Table S6). As expected, the RDMP had high performance in both WHI (area under the receiver operating characteristic curves >0.97) and MESA (area under the receiver operating characteristic curves=0.95; Figure S5). Significant differences in distributions of the RDMP between Black and White women were observed in WHI-OS (P=1.6×10−70), WHI-HT (P=2.6×10−83), and MESA (P=1.8×10−40; Figure 4B). Notably, 210 (96.8%) of all 217 Black women included in this analysis from the WHI were distributed in the highest RDMP quartile (Figure S2). Because not all studies measured the exact same set of metabolites as the WHI, to further test the generalizability of the identified RDMP, we calculated rRDMP1 (using the 93 metabolites available in the MESA), rRDMP2 (using the 79 metabolites available in the JHS), and rRDMP3 (using the 98 metabolites available in the NHS) in the WHI-HT and found significant differences in distributions between Black and White women from the WHI-HT for all 3 rRDMP scores (Figure S6).
Figure 4.
Constituent metabolites of the racial difference metabolomic pattern (RDMP), and the median RDMP in Black and White individuals from Women’s Health Initiative (WHI) and MESA (Multi-Ethnic Study of Atherosclerosis). A, Constituent metabolites of the RDMP in each category. For a full list of metabolites, please see Table S5. B, Violin plots were used to compare the distributions of RDMP for Black and White individuals from the WHI and MESA, and P values were calculated using Wilcoxon signed-rank test.
Associations Between the RDMP and CHD Risk
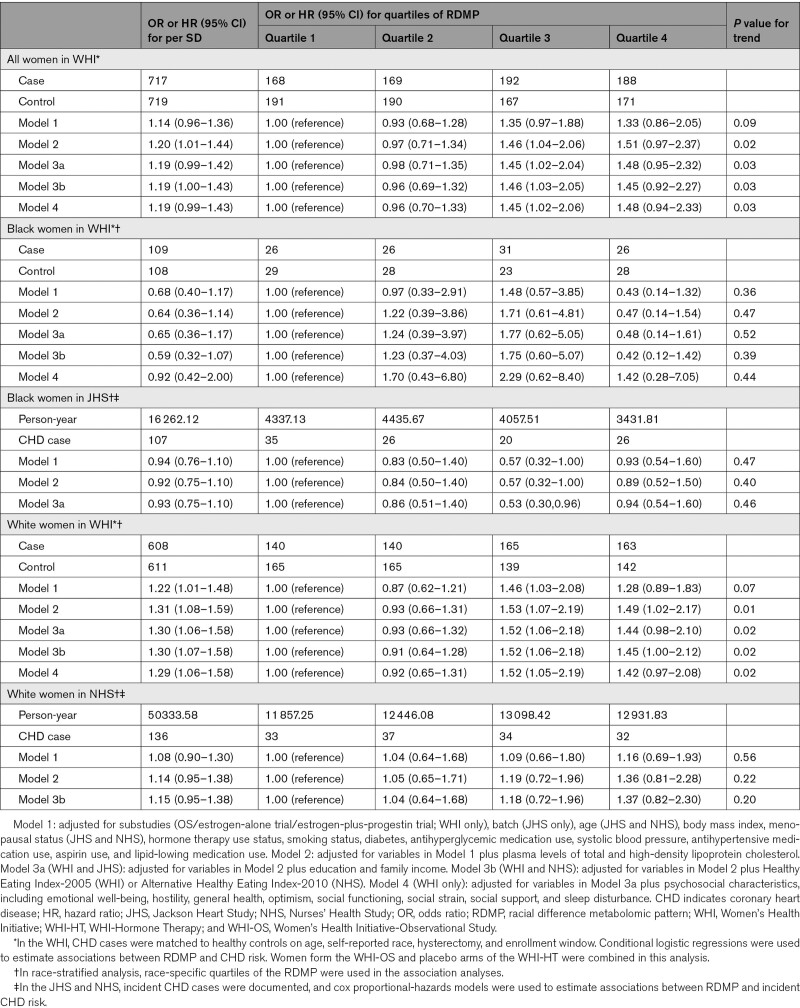
To determine whether metabolite differences by race, as summarized by the RDMP, are associated with risk of incident CHD, we combined women from the WHI-OS and the placebo arms of the WHI-HT to increase the statistical power, where 717 CHD cases were matched to 719 controls on self-reported race and other factors. After adjusting for baseline characteristics and known CHD risk factors (Table 2; model 2), women in the highest RDMP quartile had higher risk of CHD (OR=1.51 [95% CI, 0.97–2.37]; Ptrend=0.02), compared with women in the lowest quartile. When stratified by race and using race-specific RDMP quartiles, higher RDMP was not associated with risk of CHD in Black women in the WHI (Ptrend=0.47). Similar findings were observed among Black women from the JHS (Ptrend=0.40). However, among White women in the WHI, scoring in the highest RDMP quartile, compared with the lowest, was significantly associated with increased risk of CHD (OR=1.49 [95% CI, 1.02–2.17]; Ptrend=0.01). Similar results were observed in White women from the NHS, although not statistically significant (ORQ4_vs_Q1=1.36 [95% CI, 0.81–2.28]; Ptrend=0.22).
Table 2.
Association Between Quartiles of the RDMP and CHD Risk in the WHI, JHS, and NHS
Discussion
Using data from the WHI and MESA, we observed substantial differences in a large number of metabolites, mostly lipid metabolites and amino acids, between Black and White women after adjusting for baseline characteristics, socioeconomic status, lifestyle factors, baseline health conditions, and medication use. The observed differences did not change substantially after additional adjustment for blood lipids, dietary factors, and psychological characteristics. Several lipid metabolites that demonstrated large differences by race were associated with CHD risk in the WHI, independent of self-reported race and known CHD risk factors. Moreover, to estimate the impact of race on metabolomic profiles, we identified and validated an RDMP composed of 152 metabolites, which could reflect the impact of the cumulative exposure to both known and unmeasured differences in the lived experiences between Black and White women in the United States on metabolism. Additionally, higher RDMP was associated with increased risk of CHD in the WHI, independent of self-reported race and CHD risk factors, suggesting that metabolite differences between Black and White women might partially explain differences in CHD risk.
In the United States, racial disparities in CHD have persisted for decades.1,2 Contributors to racial disparities in CHD include differences in structural factors, socioeconomic status, individual level CHD risk factors, neighborhood factors, and unequal access to the medical care system and biases in treatment.6,9,29 These contribute to differences by race in educational attainment, wealth, smoking, physical activity, obesity, hypertension, diabetes, and blood lipids.6,7,29 In this study, the observed differences between Black and White women in baseline characteristics in the WHI were in line with these previous publications. However, many other factors may influence how race and related exposures and environments impact health, including environmental factors,30,31 racial discrimination, and lifestyle factors, but these factors have often been difficult to measure and quantify.
The human metabolome reflects an individual’s metabolic status in response to the interaction of many factors that could impact human metabolism, including environmental exposures,13 dietary factors,12,24 and health status (eg, obesity and diabetes).11,14 In our study participants, Black women had a higher prevalence of obesity and diabetes than White women at the study baseline, which could have a cumulative impact on metabolism and contribute to the observed differences in metabolomic profiles between Black and White women. Moreover, as a social and cultural construct, race has an impact on many individual exposures and other social/environmental determinants of health due to the exposure to inequitable systems,1,4,5 which could influence the human metabolome through a variety of mechanisms and pathways. Thus, metabolomics may be a tool to sum the potential impact of factors related to the embodiment of the experience of race in the United States, including factors which are difficult to measure. However, only a few studies have reported racial differences in metabolites in US adults.18–20 Using metabolomics data from 2 national cohorts in the United States (the WHI and MESA), we observed significant and substantial differences in metabolomic profiles between Black and White women; such differences in metabolomic profiles may reflect the cumulative impact of racial differences in a variety of endogenous and exogenous factors on metabolism, which could play a mechanistic role in the development of CVD and other health outcomes.
Black women had lower levels of total cholesterol and higher levels of HDL-cholesterol than White women in the WHI, which is consistent with prior data from the Million Veteran Program32 and the National Health and Nutrition Examination Survey.2,29 In this study, more than half of the metabolites included in the analysis were lipid metabolites, which also presented significant and substantial differences between Black and White women. Existing evidence indicates that Black individuals have lower total triacylglycerol levels than White individuals.33 Consistently, in this study, most glycerolipids were lower in Black women; however, several long-chain polyunsaturated (C56-C60 with 8-12 double-bonds) triacylglycerols were higher in Black women. Of note, the observed racial differences in lipid metabolite levels persisted after adjusting for blood lipids as well as multiple baseline characteristics, including lifestyle factors, dietary intake, and lipid-lowing medication use. Further studies are needed to reveal factors that influence racial differences in lipidomics, as well as the impact in explaining racial differences in CVDs and other health outcomes.
In addition to lipid metabolites, metabolites in several other categories also differed significantly between Black and White women, including amino acids, purines and pyrimidines, fatty acids, bile acids and bilirubins and acylcarnitines. Our findings are consistent with a recent study of 73 Black and White bladder cancer patients in the United States that reported significant racial differences in the abundance of 53 of 300 analyzed metabolites, most of which were related to the metabolism of amino acids, lipids, and nucleotides.20 In our external validation analysis, the observed associations between self-reported race and metabolite levels in MESA were generally nonsignificant, possibly due to a small sample size. However, the magnitude of the regression coefficients had good concordance with those observed in the WHI-OS.
Levels of several metabolites have been associated with CHD risk in the WHI15 and other populations.17 However, no study has examined whether differences in the metabolomic profiles by race could partially explain the observed racial disparities in CHD. In this study, we observed that C53:1 triacylglycerol has a lower abundance in Black women, and higher levels of C53:1 triacylglycerol were associated with a decreased risk of CHD, indicating that C53:1 triacylglycerol may be involved in a pathway that were associated with higher risk of CHD in Black women. Moreover, the RDMP, which was validated in 2 separate cohorts, may act as a composite score that represents racial differences in the metabolome that are related to cumulative differences in exogenous factors. As such, the RDMP could reflect the impact of cumulative exposure to known and unmeasured differences in the lived experiences between Black and White women on metabolism, and further act as an innovative tool to assess to what degree the differences in social experiences between races contribute to racial disparities in health outcomes.
Higher RDMP was associated with an increased risk of CHD, independent of self-reported race and known CHD risk factors, and such associations were more pronounced in White women but were nonsignificant in Black women (using race-specific RDMP quartiles). Similar results were found in Black women from the JHS and White women from the NHS. These findings suggest that metabolomic patterns that were related to the social and lived experience of Black women may be related to higher risk of CHD and may thus help explain racial disparities in CVD in US women. We could not directly estimate the mediation effects of individual metabolites and the RDMP on racial differences in the risk of CHD, nor determine the causality of the observed associations, because of the matched nested case-control design in the WHI (race was one of the matching factors) and the small number of documented incident CHD cases in the MESA. However, the RDMP could act as a surrogate, rather than a mediator, that reflects the impact of the social and lived experience of Black women on metabolism, and further contribute to racial disparities in cardiovascular outcomes in US women—a hypothesis that warrant further investigations.
This study has several strengths. First, women from the WHI were derived from a nested case-control study of CHD; thus, all women were free of CVD at the study baseline. Second, the statistical methods were robust; racial difference in metabolomic profiles observed in the WHI-OS were validated and replicated in independent population internally (WHI-HT) and externally (MESA). Third, the same well-validated metabolomic profiling method was used in the WHI and all replication cohorts (MESA, JHS, and NHS). Finally, detailed information on covariates was collected in all of the cohorts, and CHD end points were carefully adjudicated in the WHI, JHS, and NHS. All these strengths ensured the accuracy of the information used in this study and the robustness of our findings.
Our study has some limitations. First, the number of Black women in this study was relatively small. However, this study is the largest reported comparison, and we observed significant and substantial differences in the metabolomic profiles between Black and White women. Second, we did not have data on community-level exposures (eg, neighborhood characteristics and environmental exposures) nor on individual experience of racial discrimination or related exposures. Future studies are needed to investigate the degree to which these factors are associated with racial differences in the metabolome. Third, although we validated the associations between the RDMP and incident CHD risk that were discovered in the WHI in Black women (from the JHS) and White women (from the NHS) separately, we did not have another cohort in which the associations with CHD could be directly compared between races. Future cohort studies of larger sample sizes and consortium studies are needed to replicate our findings and to examine differences in metabolomic profiles between other racial and ethnic groups (ie, Asian and Hispanic/Latino women) and to estimate the impact of racial differences in the metabolome on racial disparities in CHD and other health outcomes. CHD cases in Black women may be under-diagnosed in the community, leading to diagnostic bias in CHD detection; however, once reported, all of the cases were carefully adjudicated, and our nested case-control design in the WHI, with self-reported race as one of the matching factors, should help ensure that the observed associations were valid. Finally, potential residual confounding is possible, which includes unmeasured exogenous factors that differ by race (eg, environmental exposures and medication use) and variations in metabolites that were related to baseline characteristics, disease status, or the single time-point measurement of metabolomics profiles. Future studies with repeated measurements of exposures and metabolomics data are needed to examine the contribution of racial differences in these factors to racial differences in metabolites.
Conclusions
Metabolomic profiles significantly and substantially differed between Black and White women. Several metabolites, especially lipid metabolites and amino acids, significantly differed by race. We identified and validated a composite metabolomic score (RDMP) that characterizes differences in metabolomic profiles between Black and White women, which might reflect the cumulative influence of racial differences in a variety of exogenous factors on individual metabolic processes. Furthermore, the RDMP, which may reflect the impact of differences in the social and lived experiences between Black and White women, was associated with incident CHD risk, independent of self-reported race and known risk factors for CHD, suggesting that differences in metabolomic patterns between Black and White women may partially explain racial disparities in CHD in the United States. Further studies are needed to replicate our findings and to estimate the impact of the cumulative exposure to the social experience of race on health disparities in the United States.
Article Information
Acknowledgments
We thank the participants of the Women’s Health Initiative, the Multi-Ethnic Study of Atherosclerosis, the Jackson Heart Study, and the Nurses’ Health Study, as well as investigators and staff who contributed efforts to these cohorts. We would like to thank Dr Alexis C. Wood from the Department of Pediatrics, USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, Houston, Texas, for her help and expertise during the revision stage.
Source of Funding
J. Hu is supported by the Women’s Brain Initiative Research Fellowship Program from Brigham and Women’s Hospital Program for Interdisciplinary Neuroscience. Metabolomic analysis in the Women’s Health Initiative (WHI) was funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, US Department of Health and Human Services through contract HHSN268201300008C. The WHI program is funded by NHLBI, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. A list of WHI investigators is available online at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf. The TOPMed MESA (Multi-Ethnic Study of Atherosclerosis) Multi-Omics project was conducted by the University of Washington and LABioMed (HHSN2682015000031/HHSN26800004). Centralized read mapping and genotype calling, along with variant quality metrics and filtering were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Phenotype harmonization, data management, sample-identity QC, and general study coordination were provided by the TOPMed Data Coordinating Center (R01HL-120393; U01HL-120393; contract HHSN268201800001I). We gratefully acknowledge the studies and participants who provided biological samples and data for TOPMed. MESA project is conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. The JHS (Jackson Heart Study) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the NHLBI and the National Institute for Minority Health and Health Disparities (NIMHD). JHS disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services. The Nurses’ Health Study is supported by NIH grants UM1 CA186107, R01 CA49449, and R01 HL034594.
Disclosures
None.
Supplemental Materials
Supplemental Methods
Tables S1–S6
Figures S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BMI
- body mass index
- CHD
- coronary heart disease
- CVD
- cardiovascular disease
- FDR
- false discovery rate
- HDL
- high-density lipoprotein
- JHS
- Jackson Heart Study
- MESA
- Multi-Ethnic Study of Atherosclerosis
- NHS
- Nurses’ Health Study
- OR
- odds ratio
- RDMP
- racial difference metabolomic pattern
- WHI-HT
- WHI-Hormone Therapy
- WHI-OS
- Women’s Health Initiative-Observational Study
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.121.320134.
For Sources of Funding and Disclosures, see page 614.
References
- 1.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366–380. doi: 10.1161/CIRCRESAHA.116.309115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 3.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999-2012. J Am Heart Assoc. 2017;6:e006027. doi: 10.1161/JAHA.117.006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, Mountain JL, Pérez-Stable EJ, Sheppard D, Risch N. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–1175. doi: 10.1056/NEJMsb025007 [DOI] [PubMed] [Google Scholar]
- 5.Mensah GA, Jaquish C, Srinivas P, Papanicolaou GJ, Wei GS, Redmond N, Roberts MC, Nelson C, Aviles-Santa L, Puggal M, et al. Emerging concepts in precision medicine and cardiovascular diseases in racial and ethnic minority populations. Circ Res. 2019;125:7–13. doi: 10.1161/CIRCRESAHA.119.314970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. ; American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 7.Bell CN, Thorpe RJ, Jr, Bowie JV, LaVeist TA. Race disparities in cardiovascular disease risk factors within socioeconomic status strata. Ann Epidemiol. 2018;28:147–152. doi: 10.1016/j.annepidem.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 8.Ski CF, King-Shier KM, Thompson DR. Gender, socioeconomic and ethnic/racial disparities in cardiovascular disease: a time for change. Int J Cardiol. 2014;170:255–257. doi: 10.1016/j.ijcard.2013.10.082 [DOI] [PubMed] [Google Scholar]
- 9.Shah NS, Ning H, Petito LC, Kershaw KN, Bancks MP, Reis JP, Rana JS, Sidney S, Jacobs DR, Jr., Kiefe CI, et al. Associations of clinical and social risk factors with racial differences in premature cardiovascular disease. Circulation. 2022;146:201–210. doi: 10.1161/CIRCULATIONAHA.121.058311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49:568–578. doi: 10.1038/ng.3809 [DOI] [PubMed] [Google Scholar]
- 11.Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, Kirkness EF, Spector TD, Caskey CT, Thorens B, et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. 2019;29:488–500.e2. doi: 10.1016/j.cmet.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esko T, Hirschhorn JN, Feldman HA, Hsu YH, Deik AA, Clish CB, Ebbeling CB, Ludwig DS. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr. 2017;105:547–554. doi: 10.3945/ajcn.116.144428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley SL, Walker DI, Calafat AM, Chen A, Papandonatos GD, Xu Y, Jones DP, Lanphear BP, Pennell KD, Braun JM. Metabolomics of childhood exposure to perfluoroalkyl substances: a cross-sectional study. Metabolomics. 2019;15:95. doi: 10.1007/s11306-019-1560-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newgard CB. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 2017;25:43–56. doi: 10.1016/j.cmet.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137:841–853. doi: 10.1161/CIRCULATIONAHA.117.029468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz DE, Tahir UA, Hu J, Ngo D, Chen ZZ, Robbins JM, Katz D, Balasubramanian R, Peterson B, Deng S, et al. Metabolomic analysis of coronary heart disease in an African American Cohort From the Jackson Heart Study. JAMA Cardiol. 2022;7:184–194. doi: 10.1001/jamacardio.2021.4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Canela M, Hruby A, Clish CB, Liang L, Martínez-González MA, Hu FB. Comprehensive metabolomic profiling and incident cardiovascular disease: a systematic review. J Am Heart Assoc. 2017;6:e005705. doi: 10.1161/JAHA.117.005705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel MJ, Batch BC, Svetkey LP, Bain JR, Turer CB, Haynes C, Muehlbauer MJ, Stevens RD, Newgard CB, Shah SH. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. OMICS. 2013;17:627–635. doi: 10.1089/omi.2013.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walejko JM, Kim S, Goel R, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. Int J Cardiol. 2018;271:336–339. doi: 10.1016/j.ijcard.2018.04.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vantaku V, Donepudi SR, Piyarathna DWB, Amara CS, Ambati CR, Tang W, Putluri V, Chandrashekar DS, Varambally S, Terris MK, et al. Large-scale profiling of serum metabolites in African American and European American patients with bladder cancer reveals metabolic pathways associated with patient survival. Cancer. 2019;125:921–932. doi: 10.1002/cncr.31890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramanian R, Demler O, Guasch-Ferré M, Paynter NP, Sheehan R, Liu S, Manson JE, Salas-Salvadó J, Martínez-Gonzalez MÁ, Hu FB, et al. Metabolomic effects of hormone therapy and associations with coronary heart disease among postmenopausal women. Circ Genom Precis Med. 2020;13:e002977. doi: 10.1161/CIRCGEN.119.002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, et al. ; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701 [DOI] [PubMed] [Google Scholar]
- 23.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. ; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Guasch-Ferré M, Chung W, Ruiz-Canela M, Toledo E, Corella D, Bhupathiraju SN, Tobias DK, Tabung FK, Hu J, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41:2645–2656. doi: 10.1093/eurheartj/ehaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, Tworoger SS, Wolpin BM. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013;59:1657–1667. doi: 10.1373/clinchem.2012.199133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 27.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B (Stat Methodol). 2005;67:301–320. doi: 10.1111/j.1467-9868.2005.00503 [Google Scholar]
- 28.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01 [PMC free article] [PubMed] [Google Scholar]
- 29.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- 30.Clark LP, Millet DB, Marshall JD. Changes in transportation-related air pollution exposures by race-ethnicity and socioeconomic status: outdoor nitrogen dioxide in the United States in 2000 and 2010. Environ Health Perspect. 2017;125:097012. doi: 10.1289/EHP959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120:1699–1704. doi: 10.1289/ehp.1205201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, Gagnon DR, DuVall SL, Li J, Peloso GM, et al. ; Global Lipids Genetics Consortium; Myocardial Infarction Genetics (MIGen) Consortium; Geisinger-Regeneron DiscovEHR Collaboration; VA Million Veteran Program. Genetics of blood lipids among ~300,000 multi-ethnic participants of the million veteran program. Nat Genet. 2018;50:1514–1523. doi: 10.1038/s41588-018-0222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank AT, Zhao B, Jose PO, Azar KM, Fortmann SP, Palaniappan LP. Racial/ethnic differences in dyslipidemia patterns. Circulation. 2014;129:570–579. doi: 10.1161/CIRCULATIONAHA.113.005757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
WHI and NHS metabolomics data used in this study are available from the corresponding author upon reasonable request and compliance with the data request processes associated with each cohort. Data access for the MESA was approved by the TOPMed Publications and Presentations Steering Committees with data access provided by an approved project (#10106). The JHS metabolomics data used in this study have been submitted to the JHS Data Coordinating Center and to dbGaP; until posted in dbGaP, all JHS data are available from the JHS Data Coordinating Center on request.