Abstract
Background
Loss of glenoid fixation is a key factor affecting the survivorship of primary total shoulder arthroplasty (TSA). It is not known whether the lower revision rates associated with crosslinked polyethylene (XLPE) compared with those of non-XLPE identified in hip and knee arthroplasty apply to shoulder arthroplasty.
Questions/purposes
We used data from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) to compare the revision rates of primary stemmed anatomic TSA using XLPE to procedures using non-XLPE. In patients receiving a primary stemmed anatomic TSA for osteoarthritis, we asked: (1) Does the rate of revision or reason for revision vary between XLPE and non-XLPE all-polyethylene glenoid components? (2) Is there any difference in the revision rate when XLPE is compared with non-XLPE across varying head sizes? (3) Is there any difference in survival among prosthesis combinations with all-polyethylene glenoid components when they are used with XLPE compared with non-XLPE?
Methods
Data were extracted from the AOANJRR from April 16, 2004, to December 31, 2020. The AOANJRR collects data on more than 97% of joint replacements performed in Australia. The study population included all primary, stemmed, anatomic TSA procedures performed for osteoarthritis using all-polyethylene glenoid components. Procedures were grouped into XLPE and non-XLPE bearing surfaces for comparison. Of the 10,102 primary stemmed anatomic TSAs in the analysis, 39% (3942 of 10,102) used XLPE and 61% (6160 of 10,102) used non-XLPE. There were no differences in age, gender, or follow-up between groups. Revision rates were determined using Kaplan-Meier estimates of survivorship to describe the time to the first revision, with censoring at the time of death or closure of the database at the time of analysis. Revision was defined as removal, replacement, or addition of any component of a joint replacement. The unadjusted cumulative percent revision after the primary arthroplasty (with 95% confidence intervals [CIs]) was calculated and compared using Cox proportional hazard models adjusted for age, gender, fixation, and surgeon volume. Further analyses were performed stratifying according to humeral head size, and a prosthesis-specific analysis adjusted for age and gender was also performed. This analysis was restricted to prosthesis combinations that were used at least 150 times, accounted for at least four revisions, had XLPE and non-XLPE options available, and had a minimum of 3 years of follow-up.
Results
Non-XLPE had a higher risk of revision than XLPE after 1.5 years (HR 2.3 [95% CI 1.6 to 3.1]; p < 0.001). The cumulative percent revision at 12 years was 5% (95% CI 4% to 6%) for XLPE and 9% (95% CI 8% to 10%) for non-XLPE. There was no difference in the rate of revision for head sizes smaller than 44 mm. Non-XLPE had a higher rate of revision than XLPE for head sizes 44 to 50 mm after 2 years (HR 2.3 [95% CI 1.5 to 3.6]; p < 0.001) and for heads larger than 50 mm for the entire period (HR 2.2 [95% CI 1.4 to 3.6]; p < 0.001). Two prosthesis combinations fulfilled the inclusion criteria for the prosthesis-specific analysis. One had a higher risk of revision when used with non-XLPE compared with XLPE after 1.5 years (HR 3.7 [95% CI 2.2 to 6.3]; p < 0.001). For the second prosthesis combination, no difference was found in the rate of revision between the two groups.
Conclusion
These AOANJRR data demonstrate that noncrosslinked, all-polyethylene glenoid components have a higher revision rate compared with crosslinked, all-polyethylene glenoid components when used in stemmed anatomic TSA for osteoarthritis. As polyethylene type is likely an important determinant of revision risk, crosslinked polyethylene should be used when available, particularly for head sizes larger than 44 mm. Further studies will need to be undertaken after larger numbers of shoulder arthroplasties have been performed to determine whether this reduction in revision risk associated with XLPE bears true for all TSA designs.
Level of Evidence
Level III, therapeutic study.
Introduction
Shoulder arthroplasty is the fastest-growing type of joint replacement internationally [3, 17]. In 2019, 7735 shoulder arthroplasty procedures were performed in Australia, an increase of 5% compared with 2018 and an increase of 187% since 2008 [2, 3]. Although shoulder arthroplasty improves pain, function, and quality of life, revision surgery is performed in 6% to 30% of patients [2, 8, 9, 12, 21, 22]. The reasons for revision vary depending on the type of primary arthroplasty. For primary total shoulder arthroplasty (TSA) for osteoarthritis (OA), the most common reasons for revision are rotator cuff insufficiency, instability or dislocation, glenoid loosening or lysis, as well as infection [2, 3]. Data from THA and TKA indicate that the bearing surface material is an important determinant of revision risk. Bearing surface particle-induced lysis and loosening is the main cause of hip and knee revisions when conventional noncrosslinked polyethylene (non-XLPE) is used [2, 11]. XLPE was developed to reduce bearing surface wear and consequent wear-related loosening [13, 14, 16]. XLPE has been associated with a lower cumulative percent revision (CPR) than non-XLPE almost universally in THA and with a lower or comparative CPR in TKA, depending on the prosthesis [6, 7, 19].
In TSA, the loss of glenoid fixation is a key factor impacting survivorship, with cemented polyethylene glenoid components having a lower revision rate than cementless polyethylene glenoid components [3, 17, 18]. The relationship between polyethylene type and revision rate, however, is not well understood. It is not known whether the lower revision rates associated with XLPE in THA and TKA also apply in TSA, which has major differences in design, function, and kinematics compared with THA and TKA. Shoulder ROM differs from that of the hip and knee in that it includes a greater arc of motion but only a small degree of physiologic translation in all directions. Stability and ROM are largely controlled by the rotator cuff, and cuff-deficient shoulders have increased wear [5]. Three shoulder simulator studies comparing non-XLPE and XLPE have demonstrated reduced wear for XLPE [1, 20, 23]. However, to our knowledge, there have been no studies with large numbers and sufficient follow-up to determine whether there is a clinical difference in survivorship between these bearing surfaces.
Using data from the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), we analyzed primary stemmed anatomic TSA to compare XLPE and non-XLPE bearing surfaces and asked: (1) Does the rate of revision or reason for revision vary between XLPE and non-XLPE all-polyethylene glenoid components? (2) Is there a difference in the revision rate when XLPE is compared with non-XLPE across varying head sizes? (3) Is there any difference in survival among prosthesis combinations with all-polyethylene glenoid components when they are used with XLPE compared with non-XLPE?
Patients and Methods
Study Design and Setting
We used data from the AOANJRR from April 16, 2004, to December 31, 2020. The AOANJRR began data collection on September 1, 1999, and includes data on more than 97% of the hip and knee arthroplasty procedures performed in Australia since 2002 [4]. Data collection was expanded in April 2004 to include shoulder arthroplasty procedures and has documented almost all shoulder arthroplasty procedures Australia-wide since November 2007. These data are externally validated against patient-level data provided by all Australian state and territory health departments. A sequential, multilevel matching process is used to identify any missing data, which are subsequently obtained by follow-up with the relevant hospital. Each month, in addition to internal validation and data quality checks, all primary procedures are linked to any subsequent revision involving the same patient, joint, and side. Data are also matched biannually to the Australian National Death Index data to identify patients who have died [15].
Participants
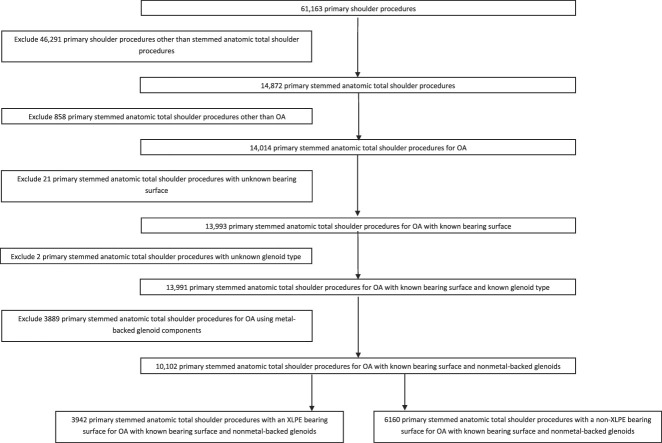
The study population included all primary, stemmed, anatomic TSAs performed for OA with all-polyethylene glenoid components. Procedures other than stemmed anatomic total shoulders (76% [46,291 of 61,163]), with a primary diagnosis other than OA (1.4% [858 of 61,163]), an unknown bearing surface (0.03% [21 of 61,163]), procedures with an unknown glenoid type (0.003% [2 of 61,163]), and procedures using metal-backed glenoid components (6% [3889 of 61,163]) were excluded. This left 17% (10,102 of 61,163) of procedures available for analysis. We excluded metal-backed glenoid components to remove this as a confounding factor because they were disproportionately used with non-XLPE and they have previously been reported to have higher rates of revision unrelated to particle-induced osteolysis [2]. Then, we grouped procedures into XLPE and non-XLPE bearing surface for comparison. Results were adjusted for age, gender, fixation, and surgeon volume, and the different causes of revision were identified. We performed an additional analysis, stratifying humeral head sizes smaller than 44 mm, 44 to 50 mm, and larger than 50 mm.
We also performed a prosthesis-specific analysis adjusted for age and gender. This analysis was restricted to prosthesis combinations that were used at least 150 times, accounted for at least four revisions, with XLPE and non-XLPE options available, and a minimum of 3 years of follow-up. Two prostheses met the inclusion criteria. These prostheses included the Global AP® humeral prosthesis/Global® glenoid (DePuy Synthes) and the Global Advantage® humeral prosthesis/Global® glenoid (DePuy Synthes). The majority of both prosthesis combinations (96% [4352 of 4535]) were pegged design.
Descriptive Data
There were 10,102 primary conventional stemmed anatomic TSAs included in the analysis; 39% (3942 of 10,102) used XLPE and 61% (6160 of 10,102) used non-XLPE (Fig. 1). The first reported use of XLPE for shoulder arthroplasty in Australia was in 2006. The proportion of TSAs using XLPE peaked in 2015 at 52% (475) and in 2020 declined to 45% (200).
Fig. 1.
This STROBE diagram shows the procedures included in this study.
The mean age of patients was 69 years in the XLPE group and 70 years in the non-XLPE group, and most patients in both the XLPE (56% [2223 of 3942]) and non-XLPE groups (59% [3610 of 6160]) were women. The maximum follow-up for the XLPE group was 14 years, and it was 16 years for the non-XLPE group (Table 1).
Table 1.
Summary of primary total stemmed shoulder replacements (primary diagnosis OA)
| Parameter | Non-XLPE (n = 6160) |
XLPE (n = 3942) |
| Follow-up in years | 7 ± 4 | 6 ± 3 |
| Age in years | 70 ± 9 | 69 ± 9 |
| Age group | ||
| < 55 | 4 (260) | 5 (191) |
| 55-64 | 22 (1338) | 23 (895) |
| 65-74 | 45 (2764) | 46 (1808) |
| ≥ 75 | 29 (1798) | 27 (1048) |
| Women | 59 (3610) | 56 (2223) |
| ASA classa | ||
| 1 | 6 (181) | 8 (208) |
| 2 | 55 (1626) | 57 (1422) |
| 3 | 38 (1137) | 34 (865) |
| 4 | 1 (37) | 0.9 (22) |
| 5 | 0 (1) | 0 (1) |
| BMI in kg/m2b | ||
| Underweight: < 18.5 | 0.4 (8) | 0.3 (5) |
| Normal: 8.5-24.9 | 13 (272) | 14 (245) |
| Preobese: 25.0-29.9 | 31 (646) | 33 (583) |
| Obese class 1: 30.0-34.9 | 31 (633) | 31 (546) |
| Obese class 2: 35.0-39.9 | 16 (322) | 13 (232) |
| Obese class 3: ≥ 40.0 | 9 (179) | 8 (135) |
| Surgeon volume by procedures per yearc | ||
| < 10 | 64 (3490) | 76 (2755) |
| ≥ 10 | 36 (1983) | 24 (847) |
| Glenoid morphologyd | ||
| A1 | 42 (492) | 32 (241) |
| A2 | 22 (256) | 28 (217) |
| B1 | 19 (219) | 22 (168) |
| B2 | 14 (160) | 15 (116) |
| B3 | 0.3 (3) | 0 (0) |
| C | 3 (40) | 3 (21) |
| Fixation | ||
| Cemented | 11 (662) | 10 (377) |
| Cementless | 0.6 (38) | 0.1 (4) |
| Hybrid (glenoid cemented) | 89 (5460) | 90 (3561) |
| Head sizee | ||
| < 44 mm | 20 (1202) | 6 (251) |
| 44-50 mm | 57 (3519) | 66 (2587) |
| > 50 mm | 23 (1437) | 28 (1104) |
Data are presented as mean ± SD or % (n). Percentages are derived from the number of procedures with data available for each parameter.
Excludes 4602 procedures with unknown American Society of Anesthesiologists (ASA) score.
Excludes 6296 procedures with unknown BMI (kg/m2).
Excludes 1027 procedures with unknown surgeon volume.
Excludes 8169 procedures with unknown glenoid morphology.
Excludes 2 procedures with unknown head size.
Ethical Approval
The AOANJRR has been approved by the Commonwealth of Australia as a federal quality assurance activity (QAA 3/2017) under section 124X of the Health Insurance Act, 1973. All AOANJRR studies are conducted in accordance with ethical principles of research (the Helsinki Declaration II).
Primary and Secondary Study Outcomes
Our primary study goal was to determine whether XLPE usage in primary, stemmed, anatomic TSA reduces the revision rate compared with non-XLPE, where a revision was defined as any removal, exchange, or addition of a prosthesis component. To achieve this, we compared the revision rates of TSAs using XLPE and non-XLPE all-polyethylene glenoid components. Any difference in reason for revision was also examined.
Our secondary goals were to determine whether any difference in revision rate between the XLPE and non-XLPE primary stemmed anatomic TSAs held true when various head sizes and different prosthesis combinations were compared.
Statistical Analysis
Kaplan-Meier estimates of survivorship were used to report the time to revision, with censoring at the time of death and closure of the dataset at the end of December 2020. We calculated the unadjusted CPR with 95% confidence intervals (CIs) using unadjusted pointwise Greenwood estimates. Age, gender, fixation, and surgeon volume adjusted hazard ratios were calculated from Cox proportional hazard models to compare the revision rate between groups. We checked the assumption of proportional hazards analytically for each model. If the interaction between the predictor and the log of time was statistically significant in the standard Cox model, then a time-varying model was estimated. Timepoints were selected based on the greatest change in hazard, weighted by a function of events. Timepoints were iteratively chosen until the assumption of proportionality was met, and HRs were calculated for each selected period. For the current study, if no period was specified, we calculated the HR for the entire follow-up period. We compared the cumulative percent survival for the two groups and there was no difference. All tests were two-tailed at 5% levels of significance. The statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc).
Results
All-cause Revision Surgery
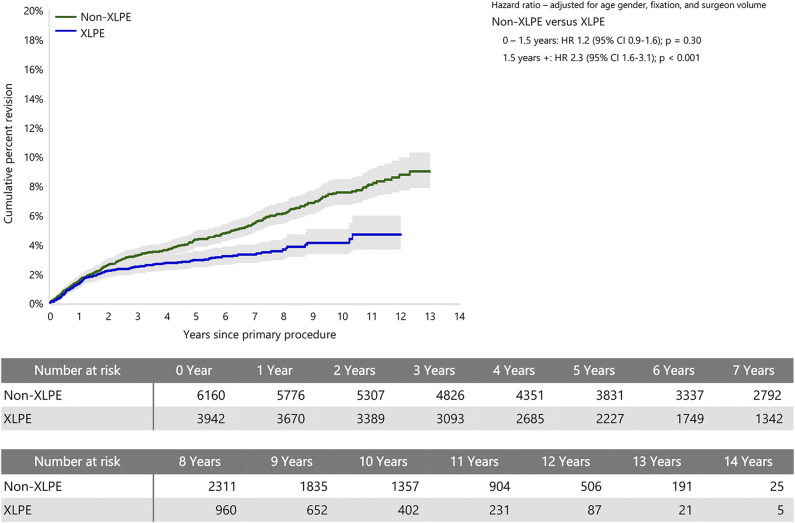
Non-XLPE had a higher risk of revision rate than XLPE after 1.5 years (HR 2.3 [95% CI 1.6 to 3.1]; p < 0.001), with no difference before that time (Fig. 2). The CPR at 12 years was 5% (95% CI 4% to 6%) for XLPE and 9% (95% CI 8% to 10%) for non-XLPE.
Fig. 2.
This graph shows the cumulative percent revision of primary stemmed anatomic TSA using all-polyethylene glenoids by polyethylene type.
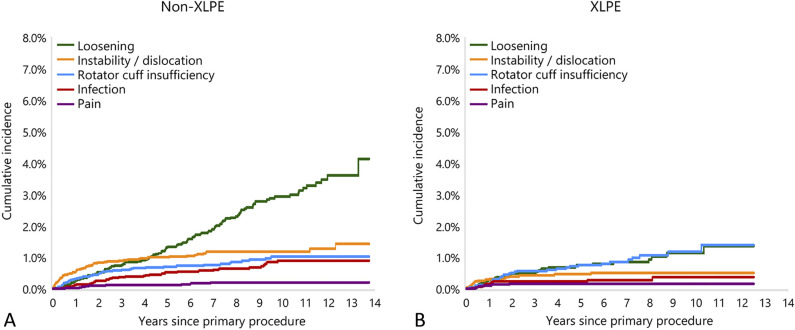
The major reason for the difference was a higher rate of revision for glenoid aseptic loosening in the non-XLPE group (39% of all revisions, 2% primaries revised) than in the XLPE group (26% of all revisions, 0.8% primaries revised) (Fig. 3). Other reasons for revision in both groups were instability/dislocation (non-XLPE: 20% of all revisions, 1% primaries revised; XLPE: 15% of all revisions, 0.5% primaries revised), rotator cuff insufficiency (non-XLPE: 15% of all revisions, 0.8% primaries revised; XLPE: 27% of all revisions, 0.8% primaries revised), and infection (non-XLPE: 11% of all revisions, 0.6% primaries revised; XLPE: 9% of all revisions, 0.3% primaries revised).
Fig. 3.
These graphs show the cumulative incidence revision diagnosis for primary stemmed anatomic TSA using all-polyethylene glenoids for (A) non-XLPE and (B) XLPE.
Head Size Analysis
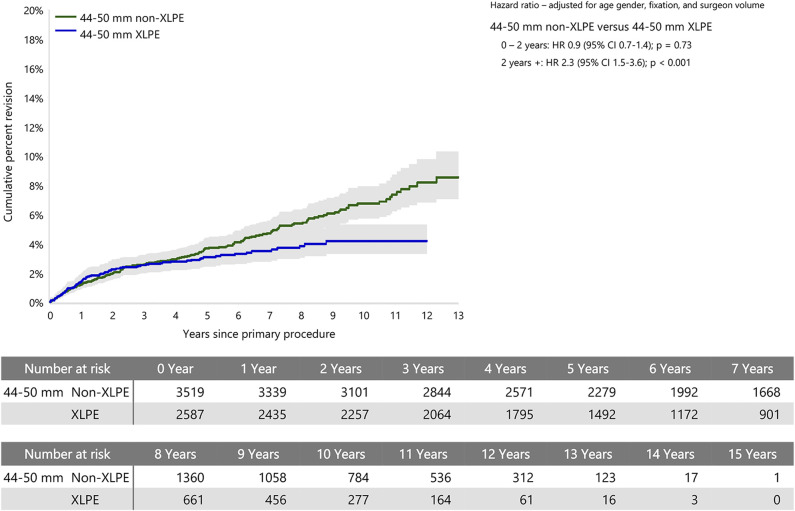
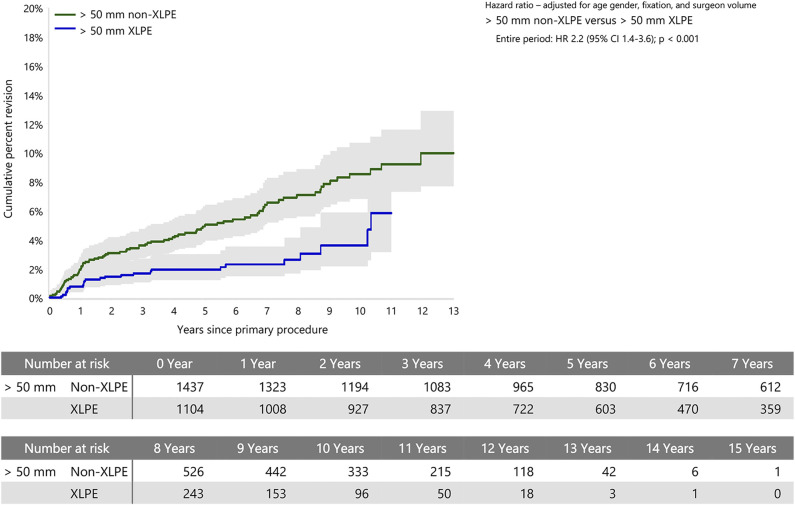
When stratified by head size, there was no difference in the rate of revision between XLPE and non-XLPE for head sizes smaller than 44 mm. Head sizes 44 to 50 mm with non-XLPE had a higher rate of revision than with XLPE after 2 years (HR 2.3 [95% CI 1.5 to 3.6]; p < 0.001), with no difference before that time (Fig. 4). Head sizes larger than 50 mm with non-XLPE also had a higher rate of revision than with XLPE (HR 2.2 [95% CI 1.4 to 3.6]; p < 0.001) (Fig. 5).
Fig. 4.
This graph shows the cumulative percent revision of primary stemmed anatomic TSA using all-polyethylene glenoids for humeral heads sizes 44 to 50 mm by polyethylene type.
Fig. 5.
This graph shows the cumulative percent revision of primary stemmed anatomic TSA using all-polyethylene glenoids for humeral heads sizes larger than 50 mm by polyethylene type.
Prosthesis-specific Analysis
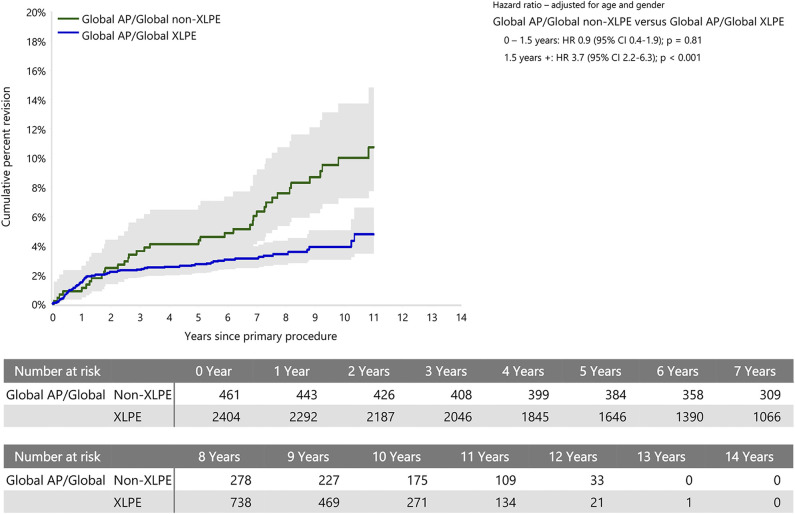
Two prosthesis combinations fit the inclusion criteria for the prosthesis-specific analysis. These were the Global Advantage and Global AP humeral prostheses (DePuy Synthes), both combined with the Global all-polyethylene glenoid. The Global AP with non-XLPE had a higher risk of revision than with XLPE only after 1.5 years (HR 3.7 [95% CI 2.2 to 6.3]; p < 0.001), with no difference between the two groups before this time (Fig. 6). When the Global Advantage was assessed with non-XLPE and XLPE, there was no difference in the revision rate between the two groups.
Fig. 6.
This graph shows the cumulative percent revision of primary stemmed anatomic TSA using the Global AP/Global prosthesis combination and all-polyethylene glenoids by polyethylene type.
Discussion
Loss of glenoid fixation is a key factor influencing the survivorship of primary TSA. It is not known whether the lower revision rates associated with XLPE compared with those of non-XLPE identified in hip and knee arthroplasty apply to shoulder arthroplasty. This study compared the revision rates and reasons for revision between XLPE and non-XLPE used in primary stemmed anatomic TSA to treat OA. XLPE was associated with a reduced revision rate compared with non-XLPE after 1.5 years. Loosening was the main reason for difference in revision risk, suggesting that—like hip and knee arthroplasty—XLPE reduces bearing surface particle generation and resultant osteolysis. Why this difference is evident earlier in the shoulder compared with hip and knee arthroplasty, however, is yet to be investigated. The reduction in revision rate with XLPE was seen in head sizes 44 to 50 mm and in those larger than 50 mm, suggesting that in patients with larger head sizes, XLPE usage should be considered. Although more data are needed to determine whether the difference in revision rate between polyethylene types is seen across more prosthesis combinations, the difference in revision risk when all-polyethylene prostheses were included should encourage surgeons to select XLPE over non-XLPE when available and companies to offer XLPE as a bearing surface option for all TSA implants.
Limitations
This study has several limitations. There are many potentially confounding variables, and it was not possible to control for all of them in this study. The AOANJRR collects limited data, and factors such as surgical approach and correction of version, which may affect revision risk, are not available. Rotator cuff status and glenoid morphology have recently been added to the AOANJRR shoulder form, but insufficient longitudinal data were available for analysis. Over time, implementation techniques have changed slightly, and the effect of these on revision risk was not examined as part of this study. We did not investigate the potential effect of stem length and prostheses using shorter stems on the risk of revision because sufficient data on these prosthesis attributes in this study are limited; however, the AOANJRR has recently reported that shorter stem shoulder prostheses are not an outlier in terms of revision risk [4]. When performing the prosthesis-specific analysis, most implant combinations did not have enough of both XLPE and non-XLPE glenoid components for analysis, and not all prostheses have both XLPE and non-XLPE options available. Although the inclusion criteria for the prosthesis-specific analysis only allowed shoulders from a single manufacturer to be included, this allowed a comparative analysis of the two types of polyethylene, with reductions in many of the variables that occur when all XLPE gleniods from all manufacturers are compared as a single group. The prosthesis-specific analysis also only accounted for a small portion of the difference in the revision difference when all all-polyethylene glenoid components were considered. There are insufficient numbers of TSAs performed for reasons other than OA to determine whether the findings of this paper extend to TSAs performed for other diagnoses. The AOANJRR has collected data on American Society of Anesthesiologists class and BMI only since 2012 and 2015, respectively. We did not control for these factors in the analysis, but they are unlikely to have much impact on the revision question, particularly as the proportions were similar in the two polyethylene groups.
Although the revision rate is an important outcome, primary procedures associated with poor patient outcomes such as pain, stiffness, and instability that were not revised were not captured in this analysis. Furthermore, ROM, additional complications, and patient-reported and radiologic outcomes were not collected by the registry for this group of patients.
All-cause Revision Surgery
Our results showed that XLPE had a lower risk of revision rate than non-XLPE after 1.5 years. We excluded metal-backed glenoid components in this study as they were disproportionately used with non-XLPE (XLPE [811 of 3889] and non-XLPE [3078 of 3889]) and they have previously been reported to have higher revision rates [2] unrelated to particle-induced osteolysis. With these removed, aseptic glenoid loosening became the predominant reason for revision in the non-XLPE group after 5 years, sharply diverging from all other causes of revision, a pattern not seen in the XLPE group. Given the difference in revision risk favoring XLPE over non-XLPE, where possible, XLPE should be selected as the bearing surface. Further registry studies are needed when sufficient longitudinal data are available to control for additional variables. The AOANJRR has started collecting data relating to cuff status, glenoid morphology, ASA, and BMI, which will be able to be analyzed in the future.
Head Size Analysis
When a subgroup analysis was performed to examine head size, non-XLPE had a higher CPR when heads 44 mm or larger were used. Although an anatomic TSA aims to replicate the patient’s native anatomy, surgeons should be aware of this to ensure that a shoulder system with XLPE available is used with larger head sizes. A simulation study comparing 32 mm and 40 mm glenospheres in reverse shoulder arthroplasty found that similar to hips, in the shoulder, larger glenospheres demonstrate greater volumetric wear and smaller glenospheres demonstrate greater linear wear [10]. Given the findings of this study, further retrieval studies, especially in anatomic shoulder replacements, would help understand wear patterns and assist surgeons in implant positioning to help reduce wear.
Prosthesis-specific Analysis
Although a higher CPR was seen for non-XLPE than for XLPE, this was not observed for all prostheses. The Global AP/Global showed a higher revision rate when non-XLPE was used, but no difference was seen with the Global Advantage/Global, even though the same glenoid component was used with both combinations, both humeral heads had the same radius of curvature and height, and both were made of the same material. These prosthesis combinations also had similar proportions of pegged and keeled glenoid designs, and the registry has reports of no statistical difference in revision rates between these designs over the past decade [4]. The prosthesis-specific analysis was not controlled for surgeon volume because of small numbers. The authors postulate that the lack of difference in revision rates between XLPE and non-XLPE when the Global Advantage/Global combination was assessed was because only a small number of surgeons use non-XLPE with this older-design implant (23% [163 of 723]), and these are experienced surgeons who can accurately position the glenoid component, thereby reducing wear in resulting from implant malposition in the non-XLPE group.
Conclusion
The AOANJRR data demonstrate that XLPE all-polyethylene glenoid components have a lower revision rate than non-XLPE all-polyethylene glenoid components when used in stemmed anatomic TSA for OA. This indicates that polyethylene material is likely an important determinant in revision risk in stemmed primary TSA. The authors recommend that, when available, XLPE be used in TSA, particularly with medium and larger humeral head sizes, to reduce the risk of loosening and the requirement for revision surgery. Further studies should be conducted to eliminate other confounding factors when the data are available, particularly to look at additional prosthesis combinations and contemporary prostheses.
Acknowledgments
We thank the AOANJRR staff, orthopaedic surgeons, hospitals, and patients whose data made this work possible.
Footnotes
One of the authors (ACAP) certifies receipt of personal payments or benefits, during the study period, in an amount of USD 10,000 to USD 100,000 from the study institution (St. John of God Hospital) for fellowship support, which was provided to the hospital from DePuy Synthes.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Ethical approval for this study was obtained from the Commonwealth of Australia.
This work was performed at the the Australian Orthopaedic Association National Joint Replacement Registry, based at the South Australian Health and Medical Research Institute, Adelaide, South Australia.
Contributor Information
Richard S. Page, Email: richardpage@geelongortho.com.au.
Sophia Rainbird, Email: srainbird@aoanjrr.org.au.
Stephen E. Graves, Email: segraves@aoanjrr.org.au.
Richard N. de Steiger, Email: Richard.Desteiger@epworth.org.au.
Yi Peng, Email: andrea.pengyi@gmail.com.
Carl Holder, Email: carl.holder@sahmri.com.
Michelle F. Lorimer, Email: michelle.lorimer@sahmri.com.
Stephen D. Gill, Email: stephen.gill2@deakin.edu.au.
References
- 1.Alexander JJ, Bell SN, Coghlan J, Lerf R, Dallmann F. The effect of vitamin E-enhanced cross-linked polyethylene on wear in shoulder arthroplasty-a wear simulator study. J Shoulder Elbow Surg. 2019;28:1771-1778. [DOI] [PubMed] [Google Scholar]
- 2.AOANJRR. Hip, knee & shoulder arthroplasty: 2019 annual report. Available at: https://aoanjrr.sahmri.com/annual-reports-2019. Accessed December 21, 2021.
- 3.AOANJRR. Hip, knee & shoulder arthroplasty: 2020 annual report. Available at: https://aoanjrr.sahmri.com/annual-reports-2020. Accessed December 21, 2021.
- 4.AOANJRR. Hip, knee & shoulder arthroplasty: 2021 annual report. Available at: https://aoanjrr.sahmri.com/annual-reports-2021. Accessed December 21, 2021.
- 5.Braun S, Schroeder S, Mueller U, Sonntag R, Buelhoff M, Kretzer JP. Influence of joint kinematics on polyethylene wear in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg. 2018;27:1679-1685. [DOI] [PubMed] [Google Scholar]
- 6.de Steiger R, Lorimer M, Graves SE. Cross-linked polyethylene for total hip arthroplasty markedly reduces revision surgery at 16 years. J Bone Joint Surg Am. 2018;100:1281-1288. [DOI] [PubMed] [Google Scholar]
- 7.de Steiger RN, Muratoglu O, Lorimer M, Cuthbert AR, Graves SE. Lower prosthesis-specific 10-year revision rate with crosslinked than with non-crosslinked polyethylene in primary total knee arthroplasty. Acta Orthop. 2015;86:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernstbrunner L, Andronic O, Grubhofer F, Camenzind RS, Wieser K, Gerber C. Long-term results of reverse total shoulder arthroplasty for rotator cuff dysfunction: a systematic review of longitudinal outcomes. J Shoulder Elbow Surg. 2019;28:774-781. [DOI] [PubMed] [Google Scholar]
- 9.Gill DRJ, Page RS, Graves SE, Rainbird S, Hatton A. The rate of 2nd revision for shoulder arthroplasty as analyzed by the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Acta Orthop. 2021;92:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haggart J, Newton MD, Hartner S, et al. Neer Award 2017: wear rates of 32-mm and 40-mm glenospheres in a reverse total shoulder arthroplasty wear simulation model. J Shoulder Elbow Surg. 2017;26:2029-2037. [DOI] [PubMed] [Google Scholar]
- 11.Harris WH. Osteolysis and particle disease in hip replacement. A review. Acta Orthop Scand. 1994;65:113-123. [DOI] [PubMed] [Google Scholar]
- 12.Kiet TK, Feeley BT, Naimark M, et al. Outcomes after shoulder replacement: comparison between reverse and anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:179-185. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin Orthop Relat Res. 2011;469:2262-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzyk PR, Saccone M, Sprague S, Simunovic N, Bhandari M, Schemitsch EH. Cross-linked versus conventional polyethylene for total hip replacement: a meta-analysis of randomised controlled trials. J Bone Joint Surg Br. 2011;93:593-600. [DOI] [PubMed] [Google Scholar]
- 15.McBride AP, Ross M, Duke P, et al. Shoulder joint arthroplasty in young patients: analysis of 8742 patients from the Australian Orthopaedic Association National Joint Replacement Registry. J Shoulder Elbow Surg. 2021;30:E419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam D, Kepler CK, Nho SJ, Craig EV, Warren RF, Wright TM. Observations on retrieved humeral polyethylene components from reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19:1003-1012. [DOI] [PubMed] [Google Scholar]
- 17.Page RS, Navarro RA, Salomonsson B. Establishing an international shoulder arthroplasty consortium. J Shoulder Elbow Surg. 2014;23:1081-1082. [DOI] [PubMed] [Google Scholar]
- 18.Page RS, Pai V, Eng K, Bain G, Graves S, Lorimer M. Cementless versus cemented glenoid components in conventional total shoulder joint arthroplasty: analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Shoulder Elbow Surg. 2018;27:1859-1865. [DOI] [PubMed] [Google Scholar]
- 19.Paxton EW, Inacio MC, Namba RS, Love R, Kurtz SM. Metal-on-conventional polyethylene total hip arthroplasty bearing surfaces have a higher risk of revision than metal-on-highly crosslinked polyethylene: results from a US registry. Clin Orthop Relat Res. 2015;473:1011-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peers S, Moravek JE, Jr, Budge MD, et al. Wear rates of highly cross-linked polyethylene humeral liners subjected to alternating cycles of glenohumeral flexion and abduction. J Shoulder Elbow Surg. 2015;24:143-149. [DOI] [PubMed] [Google Scholar]
- 21.Radnay CS, Setter KJ, Chambers L, Levine WN, LU Bigliani, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg. 2007;16:396-402. [DOI] [PubMed] [Google Scholar]
- 22.van de Sande MA, Brand R, Rozing PM. Indications, complications, and results of shoulder arthroplasty. Scand J Rheumatol. 2006;35:426-434. [DOI] [PubMed] [Google Scholar]
- 23.Wirth MA, Klotz C, Deffenbaugh DL, McNulty D, Richards L, Tipper JL. Cross-linked glenoid prosthesis: a wear comparison to conventional glenoid prosthesis with wear particulate analysis. J Shoulder Elbow Surg. 2009;18:130-137. [DOI] [PubMed] [Google Scholar]