Abstract
Background
Proximal humerus fractures are the second-most common fragility fracture in older adults. Although reverse total shoulder arthroplasty (RTSA) is a promising treatment strategy for proximal humerus fractures with favorable clinical and quality of life outcomes, it is associated with much higher, and possibly prohibitive, upfront costs relative to nonoperative treatment and other surgical alternatives.
Questions/purposes
(1) What is the cost-effectiveness of open reduction internal fixation (ORIF), hemiarthroplasty, and RTSA compared with the nonoperative treatment of complex proximal humerus fractures in adults older than 65 years from the perspective of a single-payer Canadian healthcare system? (2) Which factors, if any, affect the cost-effectiveness of ORIF, hemiarthroplasty, and RTSA compared with nonoperative treatment of proximal humerus fractures including quality of life outcomes, cost, and complication rates after each treatment?
Methods
This cost-utility analysis compared RTSA, hemiarthroplasty, and ORIF with the nonoperative management of complex proximal humerus fractures in adults older than 65 years over a lifetime time horizon from the perspective of a single-payer healthcare system. Short-term and intermediate-term complications in the 2-year postoperative period were modeled using a decision tree, with long-term outcomes estimated through a Markov model. The model was initiated with a cohort of 75-year-old patients who had a diagnosis of a comminuted (three- or four-part) proximal humerus fractures; 90% of the patients were women. The mean age and gender composition of the model’s cohort was based on a systematic review conducted as part of this analysis. Patients were managed nonoperatively or surgically with either ORIF, hemiarthroplasty, or RTSA. The three initial surgical treatment options of ORIF, hemiarthroplasty, and RTSA resulted in uncomplicated healing or the development of a complication that would result in a subsequent surgical intervention. The model reflects the complications that result in repeat surgery and that are assumed to have the greatest impact on clinical outcomes and costs. Transition probabilities and health utilities were derived from published sources, with costs (2020 CAD) sourced from regional costing databases. The primary outcome was the incremental cost-utility ratio, which was calculated using expected quality-adjusted life years (QALYs) gained and costs. Sensitivity analyses were conducted to explore the impact of changing key model parameters.
Results
Based on both pairwise and sequential analysis, RTSA was found to be the most cost-effective strategy for managing complex proximal humerus fractures in adults older than 65 years. Compared with nonoperative management, the pairwise incremental cost-utility ratios of hemiarthroplasty and RTSA were CAD 25,759/QALY and CAD 7476/QALY, respectively. ORIF was dominated by nonoperative management, meaning that it was both more costly and less effective. Sequential analysis, wherein interventions are compared from least to most expensive in a pairwise manner, demonstrated ORIF to be dominated by hemiarthroplasty, and hemiarthroplasty to be extendedly dominated by RTSA. Further, at a willingness-to-pay threshold of CAD 50,000/QALY, RTSA had 66% probability of being the most cost-effective treatment option. The results were sensitive to changes in the parameters for the probability of revision RTSA after RTSA, the treatment cost of RTSA, and the health utilities associated with the well state for all treatment options except ORIF, although none of these changes were found to be clinically realistic based on the existing evidence.
Conclusion
Based on this economic analysis, RTSA is the preferred treatment strategy for complex proximal humerus fractures in adults older than 65 years, despite high upfront costs. Based on the evidence to date, it is unlikely that the parameters this model was sensitive to would change to the degree necessary to alter the model’s outcome. A major strength of this model is that it reflects the most recent randomized controlled trials evaluating the management of this condition. Therefore, clinicians should feel confident recommending RTSA for the management of proximal humerus fractures in adults older than 65 years, and they are encouraged to advocate for this intervention as being a cost-effective practice, especially in publicly funded healthcare systems wherein resource stewardship is a core principle. Future high-quality trials should continue to collect both clinical and quality of life outcomes using validated tools such as the EuroQOL-5D to reduce parameter uncertainty and support decision makers in understanding relevant interventions’ value for money.
Level of Evidence
Level III, economic and decision analysis.
Introduction
The incidence of proximal humerus fractures is second only to that of hip fractures among older adults, with related annual healthcare expenditures estimated at CAD 45 million in Canada alone [37]. Current management strategies for displaced proximal humerus fractures include nonoperative management, open reduction and internal fixation (ORIF), or joint arthroplasty with either hemiarthroplasty or reverse total shoulder arthroplasty (RTSA). Although studies comparing operative and nonoperative strategies have not demonstrated meaningful differences in functional outcomes [13, 28, 29], important differences have been observed across surgical strategies. Recent studies have suggested favorable functional outcomes for patients treated with RTSA compared with those treated with hemiarthroplasty, as well as a lower risk of complications and reoperations than with ORIF [8, 12, 34, 38]. Despite these favorable outcomes, the implant costs of an RTSA can be up to four times that of ORIF or hemiarthroplasty. Therefore, orthopaedic surgeons must demonstrate the cost-effectiveness of RTSA in the management of proximal humerus fractures to key stakeholders, including policymakers and hospital administrators, to ensure the widespread adoption of this new technology.
Decision-makers consider multiple evidence sources when recommending the adoption of a new evidence-based treatment strategy. Health economic evidence is an important consideration in these decisions as it weighs clinical outcomes against the corresponding impact to healthcare resource use and costs. Despite recent economic analyses evaluating the cost-effectiveness of RTSA compared with various combinations of nonoperative management, ORIF, or hemiarthroplasty [19, 21, 26], to our knowledge, no evaluation has been made that simultaneously compares nonoperative treatment, ORIF, hemiarthroplasty, and RTSA in the management of proximal humerus fractures in older adults. Further, three randomized controlled trials (RCTs) [11, 15, 18] have been conducted since the publishing of the most recent cost-utility analysis on the subject matter in 2017 [26]. Considering the continued interest in the subject matter as well as the recent publishing of high-quality evidence, an updated cost-utility analysis that simultaneously considers multiple treatment options and reflects the most up-to-date evidence is warranted. The decision to compare the cost-effectiveness of nonoperative treatment, ORIF, hemiarthroplasty, and RTSA simultaneously was based on two primary reasons. First, this analysis sought to reflect the RCTs published to date regarding the subject matter wherein every pairwise combination of the aforementioned treatment options has been studied in at least one trial [2, 5, 9, 11, 15, 18, 22, 23, 34]. Secondly, although certain RCTs exist that assess the clinical and functional efficacy of the various management strategies for complex proximal humerus fractures in older adults, decision makers are also interested in the value associated with an intervention, which is the primary objective of a cost-utility analysis.
Therefore, we asked: (1) What is the cost-effectiveness of open reduction internal fixation (ORIF), hemiarthroplasty, and RTSA compared with the nonoperative treatment of complex proximal humerus fractures in adults older than 65 years from the perspective of a single-payer Canadian healthcare system? (2) Which factors, if any, affect the cost-effectiveness of ORIF, hemiarthroplasty, and RTSA compared with nonoperative treatment of proximal humerus fractures including quality of life outcomes, cost, and complication rates after each treatment?
Patients and Methods
Model Overview
We conducted a cost-utility analysis to compare nonoperative management, ORIF, hemiarthroplasty, and RTSA strategies for adults 75 years and older who presented with three- or four-part proximal humerus fractures from the perspective of the single-payer Ontario, Canada government–administered healthcare system. Incremental cost-utility ratios (ICURs) were calculated using estimates of expected costs and QALYs over a patient’s lifetime, with both discounted at a rate of 3% annually [33]. A lifetime time horizon (25 years, which assumes a maximum life span of 100 years) was used to reflect possible downstream management interventions to treat postoperative complications. This economic evaluation is reported according to the Consolidated Health Economic Evaluation Reporting Standards checklist [14].
Model Structure
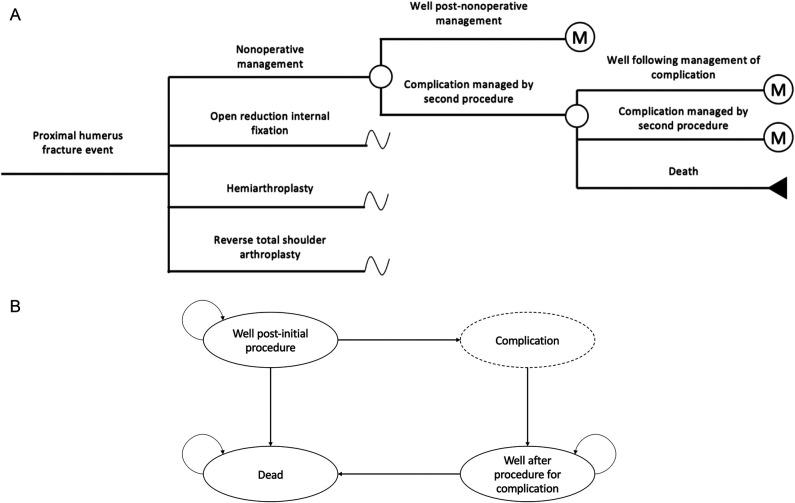
Short-term complications in the 2-year postoperative period were modeled by a decision tree (Fig. 1A), with long-term outcomes to a lifetime time horizon modeled by a Markov model (Fig. 1B). One-year cycle lengths were used in the Markov model. The model structure was informed by previous economic evaluations [6, 19, 21, 26, 30] and an expert clinician panel. The model was developed and programmed in Microsoft Excel according to guidelines published by the Canadian Agency for Drugs and Technologies [12].
Fig. 1.
A-B (A) This decision tree model illustrates the first two postoperative years, where “M” represents entering the long-term Markov model. (B) This Markov model illustrates long-term outcomes.
The model was initiated with a cohort of 75-year-old patients with a diagnosis of a comminuted (three- or four-part) proximal humerus fractures; 90% of patients were women. The mean age was based on a systematic review conducted as part of this analysis. Patients undergoing the nonoperative management strategy were initially immobilized in a sling, with follow-up in an orthopaedic fracture clinic at 2 weeks, 4 weeks, and 6 weeks, and then at 3 months, 6 months, and 12 months after treatment. The three initial surgical treatment options of ORIF, hemiarthroplasty, and RTSA may result in uncomplicated healing or the development of a complication that would result in a subsequent surgical intervention (Fig. 1A). The model reflects the complications that result in repeat surgery and are assumed to have the greatest impact on clinical outcomes and costs. These included nonunion, malunion, avascular necrosis, deep infection (septic loosening), aseptic loosening, and catastrophic implant failure. Complications leading to surgery were assumed to occur at 3 months after the initial treatment.
Model Parameters
Literature Review, Data Abstraction and Data Analysis
We performed a systematic literature review of three databases (MEDLINE, Embase, and Web of Science) to identify studies describing patient demographics, transition probabilities, and health utility model parameters. Two reviewers (HAK, CK) conducted literature screening and data extraction, with conflicts resolved by consulting a senior author (HJ). Searches were run on January 8, 2022 (Supplementary Table 1; http://links.lww.com/CORR/A789). The inclusion criteria was as follows: (1) Level I RCTs; (2) three- or four-part proximal humerus; (3) mean age of included patients being 65 or older; (4) management of three- or four-part proximal humerus fractures either nonoperatively, with ORIF using a locking plate, hemiarthroplasty, or RTSA; (5) reporting of complication rates and/or quality of life measures, specifically, EQ-5D; (6) human studies; and (7) studies published in the English language. Exclusion criteria included (1) biomechanical studies; (2) cadaveric studies; (3) two-part proximal humerus fractures; or (4) no reporting of revision surgery rates or quality of life measures, specifically the EQ-5D score. By limiting the search to Level I evidence, reporting bias was minimized. Relevant data, including patient demographics, fracture type, intervention, secondary surgery rates, and quality of life measures, was abstracted in standardized tables using Google Sheets (Google LLC). Descriptive statistics, including weighted means and SDs were estimated using R (RStudio).
If a parameter’s value could not be identified through the systematic review, a targeted secondary literature search was conducted. Input estimates were based on assumptions only in cases where a parameter was not successfully found in either the primary or secondary literature search.
There were 973 studies initially identified in the electronic search of the literature with nine studies included after applying the review’s eligibility criteria (Supplementary Fig. 1; http://links.lww.com/CORR/A790) [2, 5, 9, 11, 15, 18, 22, 23, 34]. These nine studies were comprised of 574 proximal humerus fractures with a mean age of 76 ± 3 years; 88% ± 5% of patients were female (Supplementary Table 2; http://links.lww.com/CORR/A791) [2, 5, 9, 11, 15, 18, 22, 23, 34].
Transition Probabilities
We obtained transition probabilities used to model patient movement between health states from published sources (Table 1). Where possible, we pooled probabilities across similar studies to obtain an overall weighted average of the probability estimate that was specific to each complication. After failure of nonoperative management, hardware failure after ORIF, or implant failure after either arthroplasty treatment strategies, patients were assumed to have an equal probability of receiving any of the indication-appropriate surgical interventions. The perioperative and 1-year risk of death was derived from large registry study reporting mortality rates after shoulder arthroplasty stratified by cause of surgery [1]. The perioperative and 1-year risk of death after revision surgery was derived from the same registry study and based on the rotator cuff arthropathy patient population, the oldest patient population in the study (mean age 73 ± 8 years) and with a mean age closest to this analysis’ patient cohort (76 ± 3 years) [1]. General mortality estimates, weighted by age and gender, were obtained from Statistics Canada life tables [36]. Hardware and implant failure rates, long-term posttraumatic arthritis rates, and mortality rates after the various treatments were all obtained from a targeted review of the literature [1, 4, 27, 31, 32].
Table 1.
Probabilities, health utility, and cost parameters for the model
| Parameter | Source | Base case (variation for probabilistic sensitivity analysis) | Distribution |
| Transition probabilities | |||
| Death after nonoperative management | Rotman et al. [32] | 2.4% ± 10% | β |
| Death after the index arthroplasty procedure | Amundsen et al. [1] | 3.2% ± 10% | β |
| Death after revision | Amundsen et al. [1] | 3.0% ± 10% | β |
| Nonoperative management | |||
| Posttraumatic arthritis treated with HA or RTSAa | Brophy et al. [4] | 12.0% ± 10% | β |
| Revision ORIF | Boons et al. [2], Fjalestad et al. [9], Lopiz et al. [18], Olerud et al. [23], Olerud et al. [22] | 0.7% ± 10% | β |
| Revision HA | Boons et al. [2], Fjalestad et al. [9], Lopiz et al. [18], Olerud et al. [23], Olerud et al. [22] | 0.7% ± 10% | β |
| Revision RTSA | Boons et al. [2], Fjalestad et al. [9], Lopiz et al. [18], Olerud et al. [23], Olerud et al. [22] | 0.0 | |
| ORIF | |||
| Revision ORIF | Cai et al. [5], Fjalestaed et al. [9], Fraser et al. [11], Olerud et al. [22] | 3.4% ± 10% | β |
| Revision HA | Cai et al. [5], Fjalestad et al. [9], Fraser et al. [11], Olerud et al. [22] | 0.8% ± 10% | β |
| Revision RTSA | Cai et al. [5], Fjalestad et al. [9], Fraser et al. [11], Olerud et al. [22] | 3.1% ± 10% | β |
| Hardware removal | Cai et al. [5], Fjalestad et al. [9], Fraser et al. [11], Olerud et al. [22] | 9.4% ± 10% | β |
| Annual hardware failureb | Robinson et al. [31] | 0.3% ± 10% | β |
| HA | |||
| Revision HA | Cai et al. [5], Jonsson et al. [15], Olerud et al. [23], Sebastiá-Forcada et al. [34] | 2.8% ± 10% | β |
| Revision RTSA | Cai et al. [5], Jonsson et al. [15], Olerud et al. [23], Sebastiá-Forcada et al. [34] | 5.6% ± 10% | β |
| Resection arthroplasty | Cai et al. [5], Jonsson et al. [15], Olerud et al. [23], Sebastiá-Forcada et al. [34] | 0.0 | |
| Annual implant failureb | Assumption | 2.0% ± 10% | β |
| RTSA | |||
| Revision RTSA | Fraser et al. [11], Jonsson et al. [15], Lopiz et al. [18], Sebastiá-Forcada et al. [34] | 1.8% ± 10% | β |
| Resection arthroplasty | Fraser et al. [11], Jonsson et al. [15], Lopiz et al. [18], Sebastiá-Forcada et al. [34] | 0.0 | |
| Annual implant failure rate | Otto et al. [27] | 2.5% ± 10% | β |
| Costs in 2020 CADc | |||
| Nonoperative management | |||
| Treatment cost | OCC data, OHIP Schedule of Benefits | 7422 ± 50% | γ |
| Well after nonoperative treatment | OCC data, OHIP Schedule of Benefits | 142 ± 50% | γ |
| Revision ORIF | OCC data, OHIP Schedule of Benefits | 12,081 ± 50% | γ |
| Revision HA | OCC data, OHIP Schedule of Benefits | 15,486 ± 50% | γ |
| Revision RTSA | OCC data, OHIP Schedule of Benefits | 20,400 ± 50% | γ |
| ORIF | |||
| Treatment cost | OCC data, OHIP Schedule of Benefits | 12,035 ± 50% | γ |
| Well after ORIF | OCC data, OHIP Schedule of Benefits | 142 ± 50% | γ |
| Revision ORIF | OCC data, OHIP Schedule of Benefits | 12,711 ± 50% | γ |
| Revision HA | OCC data, OHIP Schedule of Benefits | 16,308 ± 50% | γ |
| Revision RTSA | OCC data, OHIP Schedule of Benefits | 21,013 ± 50% | γ |
| Hardware removal | OCC data, OHIP Schedule of Benefits | 12,296 ± 50% | γ |
| HA | |||
| Treatment cost | OCC data, OHIP Schedule of Benefits | 15,470 ± 50% | γ |
| Well after HA | OCC data, OHIP Schedule of Benefits | 142 ± 50% | γ |
| Resection arthroplasty | OCC data, OHIP Schedule of Benefits | 12,209 ± 50% | γ |
| Revision HA | OCC data, OHIP Schedule of Benefits | 16,308 ± 50% | γ |
| Revision RTSA | OCC data, OHIP Schedule of Benefits | 20,830 ± 50% | γ |
| RTSA | |||
| Treatment cost | OCC data, OHIP Schedule of Benefits | 20,804 ± 50% | γ |
| Well after RTSA | OCC data, OHIP Schedule of Benefits | 142.00 ± 50% | γ |
| Resection arthroplasty | OCC data, OHIP Schedule of Benefits | 12,208 ± 50% | γ |
| Revision RTSA | OCC data, OHIP Schedule of Benefits | 21,063 ± 50% | γ |
| Health state utilities | |||
| Baseline postfracture | Rangan et al. [29] | 0.41 ± 15% | β |
| Nonoperative management | |||
| Well | Lopiz et al. [18], Olerud et al. [23], Olerud et al. [22] | 0.74 ± 15% | β |
| ORIF | |||
| Well | Cai et al. [5], Olerud et al. [22] | 0.73 ± 15% | β |
| Revision | 80% of uncomplicated healing | 0.58 ± 15% | β |
| HA | |||
| Well | Cai et al. [5], Jonsson et al. [15], Olerud et al. [23] | 0.81 ± 15% | β |
| Revision | 80% of uncomplicated healing | 0.60 ± 15% | β |
| Resection arthroplasty | 70% of uncomplicated healing | 0.70 ± 15% | β |
| RTSA | |||
| Well | Jonsson et al. [15], Lopiz et al. [18] | 0.88 ± 15% | β |
| Revision | 80% of uncomplicated healing | 0.65 ± 15% | β |
| Resection arthroplasty | 70% of uncomplicated healing | 0.73 ± 15% | β |
Denotes equal probability of undergoing outline procedures.
Denotes use in Markov model for 3 or more years.
Reported costs include physician billing (including both surgeon and anesthesiologist billings), in-patient costs, operating room costs, and implant costs), which are all incurred by a single-payer healthcare system; HA = hemiarthroplasty; OCC = Ontario case costing;
OHIP = Ontario Health Insurance Plan.
Health Utilities
Health utility values were obtained from published sources (Table 1). Where possible, EQ-5D values were obtained from RCTs [5, 15, 18, 22, 23]. Assumptions, based on clinical consultation, were used when no Level I evidence was available for a health state and a secondary search was unsuccessful.
Costs
Costs from the publicly funded healthcare system in Ontario, Canada were gathered from local and provincial sources (Table 1). Implant costs were based on the schedule at the Hamilton General Hospital (Hamilton Health Sciences). All other hospital inpatient, outpatient, and acute care costs were obtained from the Ontario Hospital Cost Distribution project (Health Data Branch, Health System Information Management and Investment Division, Ontario Ministry of Health and Long-Term Care; https://hsim.health.gov.on.ca/hdbportal/) [25]. Physician billings were estimated through the Ontario Schedule of Benefits (Physician Services Under the Health Insurance Act, Ontario Ministry of Health and Long-Term Care) [24]. As the model began with patients aged 75 years, and the average age at retirement in Canada is 64.3 years [35], productivity costs were not considered. That is, it was assumed that patients were not working. All costs are presented in 2020 CAD.
Outcomes
The analysis compared nonoperative management with each of the three surgical options. The main outcome was the ICUR, calculated as the difference in average per-patient expected costs divided by the difference in QALYs. A treatment was considered cost-effective if the ICUR was CAD 50,000 or less, as recommended by Nwachukwu and Bozic [20] for cost-effectiveness analyses conducted in orthopaedic surgery from governmental spending perspectives.
Cost-effectiveness Analyses
Because the cost-effective analysis includes more than two interventions, two sets of ICURs are presented. The first set of ICURs is from a pairwise comparison between each surgical option and nonoperative management. Because decision makers often need to compare multiple treatments at once, a second set of ICURs was calculated as part of a sequential analysis where all treatment options are compared simultaneously. In a sequential analysis, the interventions are ordered according to cost, and ICURs are calculated sequentially for the least costly comparator compared with the next most costly comparator, excluding all comparators that are dominated. An intervention is dominated when it is more costly and less effective than another intervention. A dominated intervention would never be considered cost-effective regardless of the threshold used [7].
According to health economic guidelines [4], all results are probabilistic. Probabilistic sensitivity analysis is a technique used to quantify the level of uncertainty associated with individual parameters. In a probabilistic sensitivity analysis, all model parameters are varied simultaneously over many simulations. In this way, the parameter value (such as cost or utility input) is not represented by a static point estimate (such as a mean) but is repeatedly sampled from a range of plausible points (that is, a distribution) over the repeated simulations. The distribution selected to describe each parameter (for example, normal, log-normal, beta, gamma) depends on the type of data and is chosen to represent the characteristics of that input and its plausible bounds. For example, gamma distributions are often used for parameters with skewed data, such as costs, where only a small proportion of individuals typically incur a large cost and therefore skew the mean. In contrast, beta distributions are used for outcomes bounded between 0 and 1, such as probabilities. The results of this analysis are based on 2500 simulations. The ICURs from each simulation are displayed in a scatterplot (such as a cost-effectiveness plane) to show the range of probabilistic sensitivity analysis results, with a greater spread indicating higher levels of parameter uncertainty. The average costs and QALYs across the simulations are then used to calculate the final ICUR.
We also performed one-way sensitivity analyses to explore uncertainty associated with methodologic assumptions (for example, regarding different clinically plausible patient pathways) and uncertainty associated with specific model inputs (for instance, lack of data to inform the utility values for certain health states). In a one-way sensitivity analysis, one parameter is changed at a time and its impact on the ICUR is evaluated to identify what inputs the results are most sensitive to. The values used in the one-way sensitivity analyses were based on previous cost-utility analyses [16, 21] and consultation with an expert panel of clinicians.
Results
RTSA Is the Most Cost-effective of the Treatments Evaluated
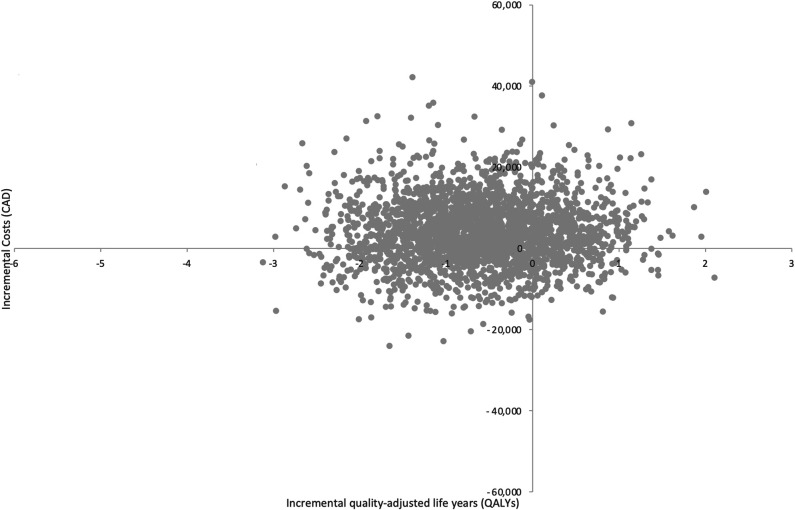
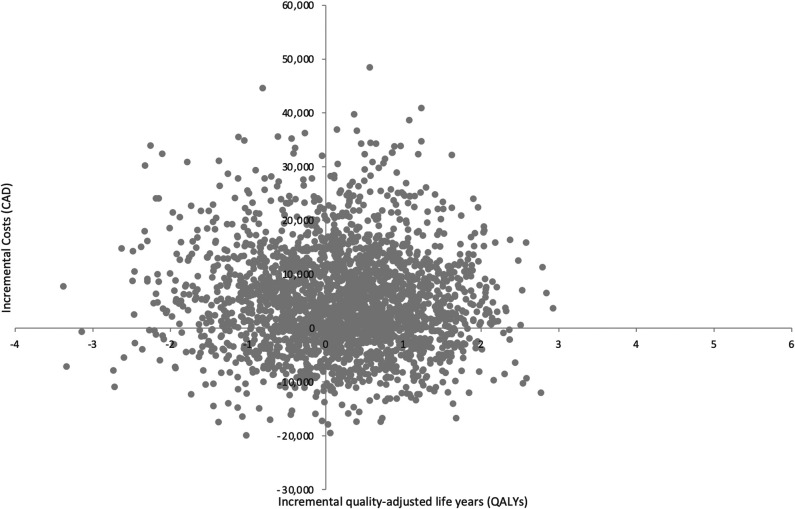
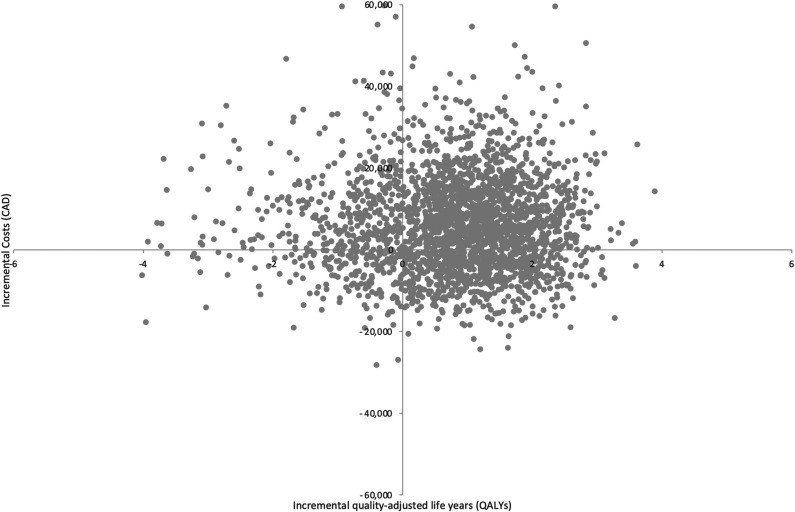
Based on the results of both pairwise and sequential analyses, RTSA was the most cost-effective treatment strategy for the management of proximal humerus fractures in older adults. When nonoperative management is compared individually with each treatment option through a pairwise analysis, ORIF was dominated by nonoperative management (Table 2). The pairwise ICURs of hemiarthroplasty and RTSA were CAD 25,759/QALY, and CAD 7476/QALY, respectively (Table 3). The results from three pairwise 2500 probabilistic sensitivity analysis simulations for nonoperative management compared with ORIF (Fig. 2), hemiarthroplasty (Fig. 3), and RTSA (Fig. 4) have been plotted accordingly. Data points in the northeast quadrant indicate that the comparator intervention is more effective and more costly, those in the southeast quadrant signify that it is more effective and less costly, datapoints in the northwest quadrant show that it is less effective and more costly, and those in the southwest quadrant signal that it is less effective and less costly.
Table 2.
Discounted per-patient results of total costs and outcomes
| Parameter | Nonoperative management | ORIF | HA | RTSA |
| Total life years | 9.44 | 8.86 | 9.29 | 9.21 |
| Total discounted costs in CADa | 18,398 | 22,583 | 23,373 | 24,371 |
| Costs generated during decision tree (years 1-2) | 10,153 | 10,153 | 18,328 | 22,229 |
| Costs generated after decision tree (years 3+) | 8245 | 12,430 | 5044 | 2142 |
| Total discounted QALYs | 7.12 | 6.51 | 7.31 | 7.92 |
| QALYs generated during decision tree (years 1-2) | 1.38 | 1.36 | 1.48 | 1.63 |
| QALYs generated after decision tree (years 3+) | 5.74 | 5.15 | 5.83 | 6.29 |
All life years, costs, and quality adjusted life years are discounted.
Reported costs include physician billing (including both surgeon and anesthesiologist billings), in-patient costs, operating room costs, and implant costs, which are all incurred by a single-payer healthcare system; HA = hemiarthroplasty.
Table 3.
Cost-effectiveness results from pairwise and sequential analyses
| Intervention | Total costs in CADa | Total QALYs | Difference in costs in CAD | Difference in QALYs | Pairwise ICUR vs nonoperative (cost/QALY) | Sequential ICUR vs next best option (cost/QALY)b | Probability of being cost-effective at a threshold of CAD 50,000 |
| Nonoperative | 18,398 | 7.12 | 11% | ||||
| ORIF | 22,583 | 6.51 | 4185 | -0.61 | Dominated | Dominated | 2% |
| HA | 23,373 | 7.31 | 4975 | 0.19 | 25,759 | Extendedly dominated | 21% |
| RTSA | 24,371 | 7.92 | 5972 | 0.80 | 7476 | 7476 | 66% |
Reported costs include physician billing (including both surgeon and anesthesiologist billings), in-patient costs, operating room costs, and implant costs, which are all incurred by a single-payer healthcare system.
In a sequential analysis, ICURs are calculated in order of increasing cost, excluding treatment options that are dominated or extendedly dominated; a dominated treatment is both more expensive and less effective than the reference treatment in the row directly above; HA = hemiarthroplasty.
Fig. 2.
This figure illustrates the cost-effectiveness plane of ORIF versus nonoperative management.
Fig. 3.
This figure illustrates the cost-effectiveness plane of hemiarthroplasty versus nonoperative management.
Fig. 4.
This figure illustrates the cost-effectiveness plane of RTSA versus nonoperative management.
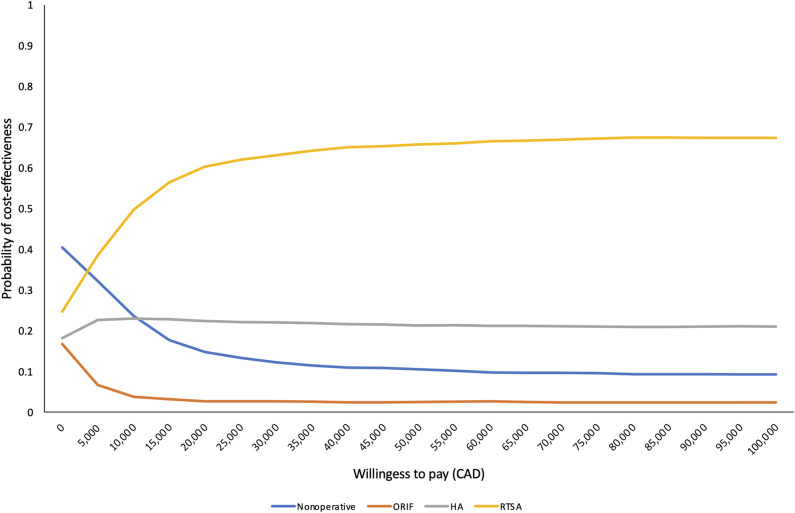
When evaluated sequentially in increasing order of cost, ORIF was dominated by nonoperative management and hemiarthroplasty was extendedly dominated (Table 3). At a willingness-to-pay threshold of CAD 50,000/QALY, RTSA had a 66% probability of being the most cost-effective option (Fig. 5).
Fig. 5.
This figure illustrates the cost-effectiveness acceptability frontier.
One-way Sensitivity Analyses: Situations Where Other Treatment Options Become Preferred
Results from the one-way sensitivity analyses indicated that the model was sensitive to changes in the following parameters: the probability of revision RTSA, the treatment cost of RTSA, and the health utilities scores associated with the well state for nonoperative management, hemiarthroplasty, and RTSA (Table 4). At a threshold of CAD 50,000/QALY, hemiarthroplasty became the preferred treatment option if: (1) the probability of a revision RTSA in the 2-year postoperative period was greater than 11%, (2) the cost of RTSA was greater than CAD 65,000, (3) the utility of being well after RTSA was less than 0.78, or (4) the utility of being well after hemiarthroplasty exceeded 0.90. Nonoperative management became the preferred treatment option if the utility of being well after nonoperative management exceeded 0.93.
Table 4.
Parameters that impacted cost-effectiveness results in the one-way sensitivity analyses
| Parameter | Low value | High value | Base case value | Threshold valuea | Explanation |
| Probability of revision RTSA in the immediate 2-year postoperative period | 0% | 100% | 1.8% | 11% | HA becomes the preferred treatment option when the probability of revision RTSA is greater than 11% |
| Cost of RTSA treatment in CAD | 0 | 100,000 | 20,804 | 65,000 | HA becomes the preferred treatment option when RTSA treatment cost is more than CAD 65,000 |
| Utility score for “Well after nonoperative management” health state | 0 | 1 | 0.74 | 0.93 | Nonoperative management becomes the preferred treatment option if the utility score for being well after nonoperative management is > 0.93 |
| Utility score for “Well after HA” heath state | 0 | 1 | 0.81 | 0.90 | HA becomes the preferred treatment option if the utility score for being well after HA is > 0.90 |
| Utility score for “Well after RTSA” heath state | 0 | 1 | 0.88 | 0.78 | HA becomes the preferred treatment option if the utility score of being well after RTSA is < 0.78 |
The value in which the results change (that is, RTSA no longer considered the most cost-effective option at a willingness-to-pay threshold of CAD 50,000 per QALY); HA = hemiarthroplasty.
Discussion
The incidence of proximal humerus fractures is second only to that of hip fractures in older adults, ultimately posing a large financial burden on healthcare systems [37]. Complex proximal humerus fractures, defined as three- or four-part fractures, can be managed with either nonoperative treatment, ORIF, hemiarthroplasty, and most recently, RTSA. Although RTSA is associated with the highest upfront costs, recent high-quality evidence has demonstrated superior functional and clinical outcomes in favor of RTSA compared with nonoperative management, ORIF, and hemiarthroplasty [8, 12, 34, 38]. To ensure the widespread adoption of an innovative and more expensive technology, its cost effectiveness must be demonstrated. The purpose of this economic analysis was to equip orthopaedic surgeons with the cost-effectiveness data pertaining to the management of complex proximal humerus fractures in older adults.
Key findings of this study were the following: RTSA is a cost-effective treatment strategy for the management of complex proximal humerus fractures with sequential analysis demonstrating that it is the most cost-effective treatment strategy 66% of the time compared with nonoperative management, ORIF, and hemiarthroplasty. Furthermore, the model’s outcome was sensitive to relatively large and clinically improbably changes to (1) the health utilities associated with RTSA, (2) the probability of revision RTSA after RTSA, (3) the cost of RTSA, (4) the health utility associated with nonoperative management, and (5) the health utility associated with hemiarthroplasty. Ultimately, the findings of this study support the widespread adoption of RTSA for the management of complex proximal humerus fractures in adults older than 65 years from both clinical and health economic perspectives by informing both clinicians and policy makers accordingly.
Limitations
As a model-based study, this analysis has its limitations. First, the results of this model depend on parameter estimates obtained from published sources and public databases, as well as assumptions made when certain parameters were not found in the evidence. Fortunately, most inputs used in this model were drawn from high-quality RCTs. When assumptions were used in the model, a panel of expert trauma and upper extremity orthopaedic surgeons was consulted beforehand. Further, assumptions and ranges of values were tested in several sensitivity analyses to evaluate the robustness of our results. Second, our model is from the perspective of a single-payer healthcare system, which may limit the generalizability of the model’s findings to differently funded healthcare systems (such as privately funded healthcare systems). Despite this limitation, the findings of this study can be generalized to numerous other countries that have a universal healthcare system similar to Canada’s (including, Denmark, Finland, France, Germany, and the United Kingdom). This study’s model population was predominantly female, which stems from the epidemiology of this fracture pathology [17]. Therefore, this model’s findings, as well as the proximal humerus fracture RCT data to date may not be directly applicable to male’s presenting with this pathology. As this generalizability challenge was out of the scope of this paper, we recommend that future high-quality RCTs be powered enough to draw substantial conclusions about treating males with this pathology.
RTSA Is the Most Cost-effective of the Treatments Evaluated
We found that RTSA was the most cost-effective of the treatments examined. Not only did RTSA have an ICUR less than the CAD 50,000 willingness-to-pay threshold in the pairwise comparison to nonoperative management, but also it extendedly dominated hemiarthroplasty in the sequential analysis, meaning that RTSA was both cheaper and provided superior quality of life outcomes than the next–most expensive treatment strategy, which was hemiarthroplasty. ORIF was dominated in both pairwise and sequential analyses. Hemiarthroplasty is a cost-effective treatment option relative to nonoperative treatment, although as mentioned earlier, it was dominated by RTSA in the sequential analysis. Our study adds to the body of cost-effectiveness studies favoring RTSA over ORIF and hemiarthroplasty for treating complex proximal humerus fractures in older adults. Similar to our study, a trial-based economic evaluation which compared ORIF and nonoperative management of complex proximal humerus fractures in 50 patients older than 60 years with three- or four-part fractures found that ORIF was not a cost-effective option for these patients, and that nonoperative management was the dominant intervention at 1 year postintervention [10]. The authors estimated an ICUR of CAD 230.556/QALY gained for ORIF relative to nonoperative management, although the uncertainty surrounding this ICUR rendered ORIF not cost-effective relative to nonoperative management [10]. Another economic evaluation which compared RTSA with hemiarthroplasty for treating complex proximal humerus fractures concluded that RTSA is a cost-effective treatment strategy from both payer and hospital perspectives in the United States [21]. From the payer perspective, hemiarthroplasty was dominant over nonoperative management, with the ICUR of RTSA being USD 8100/QALY relative to nonoperative management. From the hospital perspective, the ICUR of RTSA was USD 57,000/QALY relative to hemiarthroplasty, well under the study’s USD 100,000 willingness-to-pay threshold. Our study now extends the generalizability of the aforementioned study’s findings to publicly funded healthcare systems. Further, a previous Canadian economic analysis demonstrated RTSA to be a cost-effective management strategy for complex proximal humerus fractures when compared with hemiarthroplasty with an ICUR of CAD 13,679. In comparison, our model’s sequential analysis produced an ICUR of CAD 7500 when comparing RTSA and hemiarthroplasty after including recent RCT data in our model. This indicates that RTSA seems to be increasingly cost-effective as high-quality RCTs are conducted on the topic. Ultimately, our economic evaluation demonstrates that RTSA is a cost-effective treatment strategy for the management of complex proximal humerus fractures in the setting of universal healthcare systems, and based on updated evidence, RTSA continues to trend in the direction of increasing cost-effectiveness. Our cost-utility analysis’ findings should encourage clinicians to adopt the use of RTSA as a first-line management strategy for complex proximal humerus fractures in older adults. We do acknowledge that although our study was largely based on RCT data, assumptions were made for long-term sequalae of the various treatment strategies analyzed. Future research could better inform the long-term clinical and cost outcomes associated with the various treatment strategies, preferably through RCTs with long-term follow-ups.
One-way Sensitivity Analyses: Situations Where Other Treatment Options Become Preferred
Our model was sensitive to changes to five model parameters: (1) the health utilities associated with RTSA, (2) the probability of revision RTSA after RTSA, (3) the cost of RTSA, (4) the health utility associated with nonoperative management, and (5) the health utility associated with hemiarthroplasty. Notably, RTSA will no longer be the cost-effective treatment option if the probability of revision RTSA after the index procedure increases from 1.8% to 11% in the immediate 2-year postoperative period. Previous systematic reviews have reported pooled reoperation rates of approximately 4% after RTSA, with the rate of revision RTSA ranging from 0.93% to 1% [8, 12]. Therefore, it is unlikely that the real-life probability of revision RTSA will increase by fivefold to render RTSA not cost-effective. For either nonoperative management or hemiarthroplasty to be the cost-effective treatment strategy, the health utilities gained by either treatment must be 0.90 or greater. Preinjury EQ-5D scores from the same patient population range from 0.85 to 0.92, therefore it is improbable that these patients can attain quality-of-life scores greater than their preinjury levels considering the morbidity associated with proximal humerus fractures [5, 15, 22, 23]. For the cost of RTSA to render hemiarthroplasty the cost-effective intervention, it would need to triple from approximately CAD 20,000 to CAD 65,000. Based on our model parameters, this could be possible if a patient undergoes two revision RTSAs. When considering the low probability of a single revision RTSA (1.8%), it can be assumed that undergoing two revision RTSAs would be a rare scenario. Finally, an 11% reduction in health utilities after RTSA from 0.88 to 0.78 would make hemiarthroplasty the cost-effective treatment strategy. Although the SD of the pooled EQ-5D scores after RTSA was 0.01, indicating a narrow distribution of values, we do acknowledge that this outcome was only based on two RCTs [15, 18]. Based on our model’s sensitivity analysis in the context of the evidence to date, RTSA is likely to be the most cost-effective treatment strategy for three- and four-part proximal humerus fractures in most patients older than 65 years who choose to undergo surgery for this injury over a lifetime time horizon.
Conclusion
According to this economic analysis, RTSA is the preferred treatment strategy for complex proximal humerus fractures in adults older than 65 years, despite high upfront costs. Based on the literature to date, it is unlikely that the parameters this model was sensitive to would change to the degree necessary to alter the model’s outcome. A major strength of this model is that it reflects the most recent randomized controlled trials evaluating the management of this condition. Therefore, clinicians should feel confident recommending RTSA for the management of proximal humerus fractures in adults older than 65 years, and they are encouraged to advocate for this intervention as being a cost-effective practice, especially in publicly funded healthcare systems wherein resource stewardship is a core principle. Future high-quality trials should continue to collect both clinical and quality-of-life outcomes using validated tools such as the EQ-5D to reduce parameter uncertainty and support decision makers in understanding relevant interventions’ value for money.
Footnotes
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was not sought.
This work was performed at McMaster University, Hamilton, ON, Canada.
Contributor Information
Brittany Humphries, Email: humphrib@mcmaster.ca.
Michael Zoratti, Email: zorattmj@mcmaster.ca.
Daniel Axelrod, Email: daniel.axelrod@medportal.ca.
Colin Kruse, Email: colin.kruse@medportal.ca.
Bill Ristevski, Email: bill.ristevski@gmail.com.
Krishan Rajaratnam, Email: krishan.rajaratnam@gmail.com.
Michael Gardner, Email: michaelgardner@stanford.edu.
Jean-Éric Tarride, Email: tarride@mcmaster.ca.
Herman Johal, Email: hermanjohal@gmail.com.
References
- 1.Amundsen A, Rasmussen JV, Olsen BS, et al. Mortality after shoulder arthroplasty: 30-day, 90-day, and 1-year mortality after shoulder replacement-5853 primary operations reported to the Danish Shoulder Arthroplasty Registry. J Shoulder Elbow Surg. 2016;25:756-762. [DOI] [PubMed] [Google Scholar]
- 2.Boons HW, Goosen JH, Van Grinsven S, et al. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older a randomized controlled trial. Clin Orthop Relat Res. 2012;470:3483-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy RH, Marx RG. Osteoarthritis following shoulder instability. Clin Sports Med. 2005;24:47-56. [DOI] [PubMed] [Google Scholar]
- 4.CADTH. Guidelines for the Economic Evaluation of Health Technologies: Canada, 4th ed. CADTH; 2017. [Google Scholar]
- 5.Cai M, Tao K, Yang C, et al. Internal fixation versus shoulder hemiarthroplasty for displaced 4-part proximal humeral fractures in elderly patients. Orthopedics. 2012;35:e1340-e1346. [DOI] [PubMed] [Google Scholar]
- 6.Coe MP, Greiwe RM, Joshi R, et al. The cost-effectiveness of reverse total shoulder arthroplasty compared with hemiarthroplasty for rotator cuff tear arthropathy. J Shoulder Elbow Surg. 2012;21:1278-1288. [DOI] [PubMed] [Google Scholar]
- 7.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes, 2nd ed. Oxford University Press; 1998. [Google Scholar]
- 8.Ferrel JR, Trinh TQ, Fischer RA. Reverse total shoulder arthroplasty versus hemiarthroplasty for proximal humeral fractures: a systematic review. J Orthop Trauma. 2015;29:60-68. [DOI] [PubMed] [Google Scholar]
- 9.Fjalestad T, Hole MØ. Displaced proximal humeral fractures: operative versus non-operative treatment—a 2-year extension of a randomized controlled trial. Eur J Orthop Surg Traumatol. 2014;24:1067-1073. [DOI] [PubMed] [Google Scholar]
- 10.Fjalestad T, Hole MØO, Jørgensen JJ, et al. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury. 2010;41:599-605. [DOI] [PubMed] [Google Scholar]
- 11.Fraser AN, Bjørdal J, Wagle TM, et al. Reverse shoulder arthroplasty is superior to plate fixation at 2 years for displaced proximal humeral fractures in the elderly. J Bone Joint Surg Am . 2020;102:477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallinet D, Ohl X, Decroocq L, et al. Is reverse total shoulder arthroplasty more effective than hemiarthroplasty for treating displaced proximal humerus fractures in older adults? A systematic review and meta-analysis. Orthop Traumatol Surg Res. 2018;104:759-766. [DOI] [PubMed] [Google Scholar]
- 13.Gomberawalla MM, Miller BS, Coale RM, et al. Meta-analysis of joint preservation versus arthroplasty for the treatment of displaced 3- and 4-part fractures of the proximal humerus. Injury. 2013;44:1532-1539. [DOI] [PubMed] [Google Scholar]
- 14.Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Heal. 2013;16:231-250. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson EÖ, Ekholm C, Salomonsson B, et al. Reverse total shoulder arthroplasty provides better shoulder function than hemiarthroplasty for displaced 3- and 4-part proximal humeral fractures in patients aged 70 years or older: a multicenter randomized controlled trial. J Shoulder Elbow Surg. 2021;30:994-1006. [DOI] [PubMed] [Google Scholar]
- 16.Klebanoff MJ, Corey KE, Samur S, et al. Cost-effectiveness analysis of bariatric surgery for patients with nonalcoholic steatohepatitis cirrhosis. JAMA Netw Open . 2019;2:e190047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launonen AP, Lepola V, Saranko A, et al. Epidemiology of proximal humerus fractures. Arch Osteoporos. 2015;10:209. [DOI] [PubMed] [Google Scholar]
- 18.Lopiz Y, Alcobía-Díaz B, Galán-Olleros M, et al. Reverse shoulder arthroplasty versus nonoperative treatment for 3- or 4-part proximal humeral fractures in elderly patients: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2019;28:2259-2271. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson JA, Jones R, MacDonald DJ, et al. Cost-effectiveness of the reverse total shoulder arthroplasty. Does indication affect outcome? Shoulder Elbow. 2021;13:90-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nwachukwu BU, Bozic KJ. Updating cost effectiveness analyses in orthopedic surgery: resilience of the $50,000 per QALY threshold. J Arthroplasty. 2015;30:1118-1120. [DOI] [PubMed] [Google Scholar]
- 21.Nwachukwu BU, Schairer WW, McCormick F, et al. Arthroplasty for the surgical management of complex proximal humerus fractures in the elderly: a cost-utility analysis. J Shoulder Elbow Surg. 2016;25:704-713. [DOI] [PubMed] [Google Scholar]
- 22.Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg . 2011;20:747-755. [DOI] [PubMed] [Google Scholar]
- 23.Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. J Shoulder Elbow Surg . 2011;20:1025-1033. [DOI] [PubMed] [Google Scholar]
- 24.Ontario Ministry of Health and Long Term Care. Ontario Health Insurance Plan. OHIP schedule of benefits and fees. Available at: https://www.health.gov.on.ca/en/pro/programs/ohip/sob/. Accessed July 15, 2020.
- 25.Ontario Ministry of Health and Long Term Care. Ontario case costing initiative (OCCI). Available at: https://data.ontario.ca/dataset/ontario-case-costing-initiative-occi#:∼:text=This%20dataset%20tracks%20the%20costs,rehabilitation%20and%20complex%20continuing%20care. Accessed July 15, 2020.
- 26.Osterhoff G, O’Hara NN, D’Cruz J, et al. A cost-effectiveness analysis of reverse total shoulder arthroplasty versus hemiarthroplasty for the management of complex proximal humeral fractures in the elderly. Value Health. 2017;20:404-411. [DOI] [PubMed] [Google Scholar]
- 27.Otto RJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty in patients younger than 55 years: 2- to 12-year follow-up. J Shoulder Elbow Surg. 2017;26:792-797. [DOI] [PubMed] [Google Scholar]
- 28.Rabi S, Evaniew N, Sprague SA, et al. Operative vs non-operative management of displaced proximal humeral fractures in the elderly: a systematic review and meta-analysis of randomized controlled trials. World J Orthop. 2015;6:838-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangan A, Handoll H, Brealey S, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus the PROFHER randomized clinical trial. JAMA . 2015;313:1037-1047. [DOI] [PubMed] [Google Scholar]
- 30.Renfree KJ, Hattrup SJ, Chang YHH. Cost utility analysis of reverse total shoulder arthroplasty. J Shoulder Elbow Surg . 2013;22:1656-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson CM, Stirling PHC, Goudie EB, et al. Complications and long-term outcomes of open reduction and plate fixation of proximal humeral fractures. J Bone Joint Surg Am . 2019;101:2129-2139. [DOI] [PubMed] [Google Scholar]
- 32.Rotman D, Giladi O, Senderey AB, et al. Mortality after complex displaced proximal humerus fractures in elderly patients: conservative versus operative treatment with reverse total shoulder arthroplasty. Geriatr Orthop Surg Rehabil. 2018;9:215145931879524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093-1103. [DOI] [PubMed] [Google Scholar]
- 34.Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, et al. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J Shoulder Elbow Surg . 2014;23:1419-1426. [DOI] [PubMed] [Google Scholar]
- 35.Statistics Canada. Retirement age by class of worker, annual. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410006001. Accessed August 15, 2020.
- 36.Statistics Canada. Life Tables, Canada, provinces and territories. Available at: https://www150.statcan.gc.ca/n1/en/catalogue/84-537-X. Accessed August 15, 2020.
- 37.Tarride JE, Hopkins RB, Leslie WD, et al. The burden of illness of osteoporosis in Canada. Osteoporos Int. 2012;23:2591-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Zhu Y, Zhang F, et al. Meta-analysis suggests that reverse shoulder arthroplasty in proximal humerus fractures is a better option than hemiarthroplasty in the elderly. Int Orthop. 2016;40:531-539. [DOI] [PubMed] [Google Scholar]