Abstract
Background and study aims Endoscopic drainage of benign biliary and pancreatic strictures can be challenging, especially when tightness of the stenosis does not allow passage of mechanical and pneumatic dilation catheters. Electroincision of benign biliopancreatic can be considered in selected cases.
Patients and methods Three male patients (mean age 33 years, range 9–60) underwent endoscopic retrograde cholangiopancreatography to drain anastomotic biliary stricture (ABS) following orthotopic liver transplantation (n = 2) and pancreatic duct stenosis due to abdominal trauma (n = 1). The biliopancreatic strictures could be passed only with a thin 0.020-inch hydrophilic guidewire. Conventional mechanical and pneumatic dilators failed to pass the strictures due to weakness of the guidewire. Therefore, electrosurgical incision by over-the-wire 6Fr cystotome or needle-knife was attempted using pure cut current.
Results The two cases of ABS were approached also by cholangioscopy and the 6Fr cystotome easily passed the strictures, allowing subsequent pneumatic dilatation and insertion of multiple plastic stents. The patient with a pancreatic duct stricture underwent electrosurgical incision using a thin needle knife over-the-wire, resulting in insertion of a 7Fr pancreatic stent. No adverse events occurred; all the patients were discharged within 24 to 48 hours.
Conclusions Electrosurgical incision of benign biliopancreatic strictures could be considered in selected patients whom conventional dilation techniques fail.
Introduction
Endoscopy is considered the first-line modality for treatment of benign biliopancreatic stenosis. Benign biliary strictures (BBSs) are more frequently the consequence of postoperative injury (i. e., post-cholecystectomy, liver transplantation, bilioenteric anastomosis), but they can be also due to chronic pancreatitis, chronic cholangiopathies (i. e., primary sclerosing cholangitis, immunoglobulin G4-related cholangiopathy) and trauma. Clinical manifestations vary from incidental elevation on liver function testing in asymptomatic patients to a more severe clinical course such as jaundice and cholangitis. If left untreated, BBS can lead to chronic cholestasis, recurrent sepsis, and secondary biliary cirrhosis.
Benign pancreatic strictures (BPSs) can be caused by chronic pancreatitis, trauma, and surgical injury. Relevant stricture of the main pancreatic duct can increase the intraluminal pressure and cause injury to the gland with abdominal pain and pancreatitis.
Many treatment options (endoscopy, percutaneous, surgery) are currently available for treatment of biliopancreatic strictures 1 2 . Endoscopic retrograde cholangiopancreatography (ERCP) is the preferred option for most patients because it is effective, safe, and minimally invasive 3 . The final goal of endoscopic treatment is permanent resolution of the stricture with mechanical or balloon dilatation and/or stent placement 4 5 . However, deep access of the duct above the stricture can be challenging, depending on stricture severity and anatomic location.
In this study, a novel procedure for recanalization of challenging biliopancreatic strictures is reported.
Patients and methods
Case 1
Video 1 Electroincision of a tight anastomotic biliary stricture following liver transplantation using a 6Fr cystotome.
A 60-year-old man underwent orthotopic liver transplantation (OLT) due to alcohol-related liver cirrhosis in April 2020. Postoperative follow-up visits were uneventful until February 2021, when the patient complained of the onset of itching. Laboratory testing showed elevation in liver function tests. Magnetic resonance cholangiography (MRC) revealed a stricture from the choledocho-choledochostomy. ERCP was attempted to treat the anastomotic biliary stricture (ABS) ( Fig. 1a ); however, deep biliary access with 0.035-inch hydrophilic guidewires (Terumo Radiofocus, Japan) failed, due to the tightness of the stricture. Cholangioscopic-assisted (SpyGlass DS, Boston Scientific, United States) guidewire placement was attempted: only a 0.020-inch angled hydrophilic guidewire (Terumo Radiofocus, Japan) passed the ABS ( Fig. 1b ). However, mechanical (GT 5-4-3, Cook Endoscopy, United States) and pneumatic (Max Force, Boston Scientific, United States) dilation catheters failed to cross the stricture due to the softness of the 0.020-inch guidewire. Therefore, electroincision of the stricture was successfully performed using a small pulse of pure cut current (ERBE VIO3, Tubingen, Germany – High Cut, Effect 1) with an over-the wire 6Fr cystotome ( Fig. 1c ) (Cysto Gastro set, Endoflex, Germany), obtaining effective recanalization as shown on cholangioscopy ( Fig. 1d , Video 1 ). After stricture incision, a larger 0.035-inch wire was placed through the cystotome, 6-mm balloon dilatation was performed, and two 8.5Fr plastic stents were placed through the ABS for biliary drainage; the postoperative course was uneventful. Multiple plastic stents were inserted up to six 10Fr diameter during the subsequent ERCPs. Stents were removed 12 months later due to ABS resolution.
Fig. 1 a.
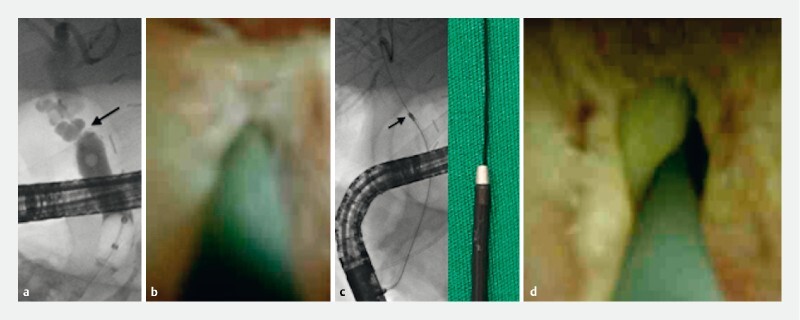
Tight anastomotic biliary stricture (arrow). b Passage of the stricture under cholangioscopic view. c Electroincision of the stricture with a 6Fr cystotome over 0.020-inch guidewire under fluoroscopic control. d Cholangioscopy shows good stricture recanalization.
Case 2
A 29-year-old man with a medical history of OLT due to Wilson’s disease at age 10 years was discovered incidentally to have anicteric cholestasis during follow-up laboratory tests. Clinical and hepatological follow-ups were regular until that time. MRC diagnosed an ABS. Deep cannulation of the ABS using different types of 0.035-inch hydrophilic guidewire (straight, angled – Terumo Radiofocus, Japan) under fluoroscopic control was unsuccessful. Cholangioscopy (SpyGlass DSII, Boston Scientific, United States) showed that ABS was very tight and angled. Under direct vision, a 0.020-inch angled hydrophilic guidewire (Terumo Radiofocus, Japan) passed the stenosis. However, even in this case mechanical (GT 5-4-3, Cook Endoscopy, United States) and a pneumatic (Max Force, Boston Scientific, United States) dilatation catheter failed to cross the stricture due to the softness of the guidewire. Electroincision of the stricture was successfully performed using the 6Fr cystotome (Cysto Gastro set, Endoflex, Germany) and pure cut current setting (ERBE VIO3, Tubingen, Germany – High Cut, Effect 1), as described above. A 0.035-inch hydrophilic guidewire (Terumo Radiofocus, Japan) was placed and 6-mm balloon dilatation was performed, allowing insertion of two 10Fr plastic stents across the stricture. Patient was discharged from the hospital after 24 hours without any adverse events (AEs). After insertion of a maximum of five 10Fr plastic stents, the ABS was resolved; the stents were removed 9 months later.
Case 3
A 9-year-old boy suffered abdominal trauma due to a traffic accident with bike handlebar compression. Computed tomography scan and magnetic resonance pancreatography (MRP) showed pancreatic trauma with rupture of the main pancreatic duct. Due to the patient’s spontaneous clinical improvement, a “watch and wait” strategy was chosen and he was discharged after 2 weeks. Three months later, he was admitted for acute pancreatitis; MRP was repeated and showed main pancreatic duct dilatation above a tight and fibrotic stricture in the head of the pancreas. After multidisciplinary discussion, the decision was made to perform ERCP for pancreatic drainage. During the procedure, the pancreatic stricture ( Fig. 2a ) was passed only with a small 0.020-inch angled hydrophilic guidewire (Terumo Radiofocus, Japan). However, the 3Fr mechanical dilatator (GT 5-4-3, Cook Endoscopy, United States) and the 7Fr Sohendra stent retriever (SSR-7, Cook Endoscopy, United States) ( Fig. 2b ) failed to pass the stricture. An over-the-wire needle-knife (HPC 3, Cook Endoscopy, United States) ( Fig. 2c ) was used to -cut the stricture using pure cut current (ERBE VIO3, Tubingen, Germany – High Cut, Effect 1), allowing passage of a 4-mm balloon dilator, before insertion of a 7Fr pancreatic stent. The patient’s hospital stay was uneventful he was discharged 48 hours after ERCP. Six months later, the patient was asymptomatic; ERCP showed pancreatic stricture improvement and the 7Fr stent was replaced with one with an 8.5Fr diameter.
Fig. 2 a.
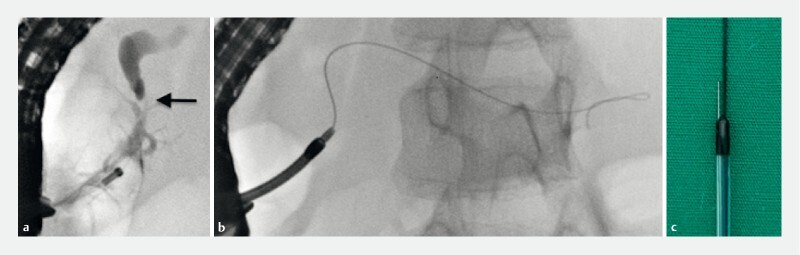
Tight pancreatic duct stricture on pancreatography (arrow). b Failed dilatation with the Soehendra stent retriever. c Over-the-wire needle knife was used for electrosurgical incision of the stricture.
Endoscopic revision of the treatment of ABS and pancreatic strictures were approved by the local Ethics Committee of the Catholic University of Rome (447/12, 1136/1).
Results
Electrosurgical incision of benign biliopancreatic strictures was successful in the three cases reported, without related AEs ( Table 1 ).
Table 1. Results of electrosurgical incision for benign biliopancreatic strictures.
| Patient no. | Stricture site | Stricture etiology | Guidewire diameter | Electrosurgical device | Type of current | No. days after discharge |
| 1 | Biliary | Anastomotic (following liver transplantation) | 0.020-inch | 6F cystotome | Pure cut | 1 |
| 2 | Biliary | Anastomotic (following liver transplantation) | 0.020-inch | 6F cystotome | Pure cut | 1 |
| 3 | Pancreatic | Abdominal trauma | 0.020-inch | Needle-knife | Pure cut | 2 |
Discussion
Endoscopic treatment of benign biliopancreatic strictures can be challenging. Achieving deep cannulation of the duct above the stenotic tract may be difficult, even after papillary cannulation. The difficulty depends on the severity of the stricture (i. e., pinpoint strictures, hard fibrosis, complete obliteration of the lumen) or anatomical issue (i. e., ductal angulation) 6 . Powerful advancement of the guidewire should always be avoided due to the risk of false tract or perforation.
Cholangioscopy-assisted guidewire placement has been described in the literature. Despite the cost, it can be an option in selected cases 7 8 9 10 . However, cholangioscopy-assisted guidewire placement is not a guarantee of treatment success. In fact, in case of hard stenosis, the usual 0.035-inch wire may not pass the stricture even under direct cholangioscopic vision. Smaller-diameter (0.018-, 0.020-, or 0.025-inch) guidewires are available and can be more effective for luminal passage and stricture penetration. However, these wires are softer and may be unsupportive for over-the-wire devices placement, such as dilatators (hydrostatic, mechanical). An effective dilation technique was described using the Soehendra stent retriever 11 , but its use can be limited by small-diameter guidewires.
Hence, there is a need for a new strategy for treatment of severe BBS and BPS. Assuming that benign and especially anastomotic strictures do not usually involve a long segment, but they are more frequently short fibrotic rings, we borrowed the concept of endoscopic incisional therapy (EIT) described in the literature for treatment of benign esophageal 12 or colorectal anastomotic strictures 13 .
Limited literature exists on this topic. Since its introduction by Raskin et al 14 for Schatzki’s ring in 1985, EIT has been used to treat a wide variety of diseases, including benign and refractory esophageal anastomotic strictures 15 16 17 , post-endoscopic submucosal dissection or endoscopic mucosal resection-related esophageal stenosis 18 19 , esophageal corrosive strictures 20 , and colorectal anastomotic stricture 13 21 .
Conclusions
In the present series, EIT was successfully applied to pass difficult biliopancreatic strictures in selected cases without the occurrence of AEs. However, it is a blind procedure because cutting of the strictures is performed only under fluoroscopic guidance and it is based on the endoscopist’s expertise. Probing with a hand while advancing the electrocautery device is important because prompt interruption of the EIT is mandatory to avoid perforation of and/or injury to surrounding structures.
The introduction of dedicated devices that fit the cholangioscope working channel to perform this procedure under direct vision would eliminate this issue in the future.
Footnotes
Competing interests Dr. Tringali is a consultant for Boston Scientific and Olympus.
Dr. Costamagna receives consulting fees from Cook Medical, Olympus, and Boston Scientific Corp.
References
- 1.Savides T J, Varadarajulu S, Palazzo L et al. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3–S7. doi: 10.1016/j.gie.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 2.Bokemeyer A, Müller F, Niesert H et al. Percutaneous-transhepatic-endoscopic rendezvous procedures are effective and safe in patients with refractory bile duct obstruction. United Eur Gastroenterol J. 2019;7:397–404. doi: 10.1177/2050640619825949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumonceau J-M, Tringali A, Papanikolaou I et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 4.Wong M YW, Saxena P, Kaffes A J. Benign biliary strictures: a systematic review on endoscopic treatment options. Diagnostics. 2020;10:221. doi: 10.3390/diagnostics10040221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oracz G, Pertkiewicz J, Kierkus J et al. Efficiency of pancreatic duct stenting therapy in children with chronic pancreatitis. Gastrointest Endosc. 2014;80:1022–1029. doi: 10.1016/j.gie.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y Y, Gwak G-Y, Lee K H et al. Predictors of the feasibility of primary endoscopic management of biliary strictures after adult living donor liver transplantation. Liver Transplant Soc. 2011;17:1467–1473. doi: 10.1002/lt.22432. [DOI] [PubMed] [Google Scholar]
- 7.Parsi M A. Direct peroral cholangioscopy. World J Gastrointest Endosc. 2014;6:1–5. doi: 10.4253/wjge.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo Y S, Lee J K, Noh D H et al. SpyGlass cholangioscopy-assisted guidewire placement for post-LDLT biliary strictures: a case series. Surg Endosc. 2016;30:3897–3903. doi: 10.1007/s00464-015-4695-7. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S. Cholangioscopy-directed endoscopic intervention for post-liver transplantation anastomotic biliary stricture. Gastrointest Endosc. 2015;81:1014–1015. doi: 10.1016/j.gie.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Wright H, Sharma S, Gurakar A et al. Management of biliary stricture guided by the Spyglass Direct Visualization System in a liver transplant recipient: an innovative approach. Gastrointest Endosc. 2008;67:1201–1203. doi: 10.1016/j.gie.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsumi K, Kato H, Sakakihara I et al. Dilation of a severe bilioenteric or pancreatoenteric anastomotic stricture using a Soehendra Stent Retriever. World J Gastrointest Endosc. 2013;5:412–416. doi: 10.4253/wjge.v5.i8.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samanta J, Dhaka N, Sinha S K et al. Endoscopic incisional therapy for benign esophageal strictures: Technique and results. World J Gastrointest Endosc. 2015;7:1318–1326. doi: 10.4253/wjge.v7.i19.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng S, Cao Y, Gu J. Endoscopic diagnosis and treatment of complete anastomosis stenosis after colorectal resection without protective ileostomy: report of two cases and literature review. J Int Med Res. 2020;48 doi: 10.1177/0300060520914833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raskin J B, Manten H, Harary A et al. Transendoscopic electrosurgical incision of lower esophageal (Schatzki) rings: a new treatment modality. Gastrointest Endosc. 1985;31:391–393. doi: 10.1016/s0016-5107(85)72257-2. [DOI] [PubMed] [Google Scholar]
- 15.Simmons D T, Baron T H. Electroincision of refractory esophagogastric anastomotic strictures. Dis Esophagus Off J Int Soc Dis Esophagus. 2006;19:410–414. doi: 10.1111/j.1442-2050.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee T H, Lee S-H, Park J-Y et al. Primary incisional therapy with a modified method for patients with benign anastomotic esophageal stricture. Gastrointest Endosc. 2009;69:1029–1033. doi: 10.1016/j.gie.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Muto M, Ezoe Y, Yano T et al. Usefulness of endoscopic radial incision and cutting method for refractory esophagogastric anastomotic stricture (with video) Gastrointest Endosc. 2012;75:965–972. doi: 10.1016/j.gie.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Yano T, Yoda Y, Satake H et al. Radial incision and cutting method for refractory stricture after nonsurgical treatment of esophageal cancer. Endoscopy. 2013;45:316–319. doi: 10.1055/s-0032-1326016. [DOI] [PubMed] [Google Scholar]
- 19.Minamino H, Machida H, Tominaga K et al. Endoscopic radial incision and cutting method for refractory esophageal stricture after endoscopic submucosal dissection of superficial esophageal carcinoma. Dig Endosc. 2013;25:200–203. doi: 10.1111/j.1443-1661.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka K, Ban S, Aikawa M et al. Electrocautery therapy combined with oral steroid administration for refractory corrosive esophageal stenosis prevents restenosis. Esophagus. 2013;10:230–234. doi: 10.1007/s10388-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan X, Liu W, Ye L et al. Combination of endoscopic incision and balloon dilation for treatment of a completely obstructed anastomotic stenosis following colorectal resection. Medicine (Baltimore) 2019;98:e16292. doi: 10.1097/MD.0000000000016292. [DOI] [PMC free article] [PubMed] [Google Scholar]


