Abstract
In the cariogenic organism, Streptococcus mutans, low pH induces an acid tolerance response (ATR). To identify acid-regulated proteins comprising the ATR, transposon mutagenesis with the thermosensitive plasmid pGh9:ISS1 was used to produce clones that were able to grow at neutral pH, but not in medium at pH 5.0. Sequence analysis of one mutant (IS1A) indicated that transposition had created a 6.3-kb deletion, one end of which was in dltB of the dlt operon encoding four proteins (DltA-DltD) involved in the synthesis of d-alanyl-lipoteichoic acid. Inactivation of the dltC gene, encoding the d-alanyl carrier protein (Dcp), resulted in the generation of the acid-sensitive mutant, BH97LC. Compared to the wild-type strain, LT11, the mutant exhibited a threefold-longer doubling time and a 33% lower growth yield. In addition, it was unable to initiate growth below pH 6.5 and unadapted cells were unable to survive a 3-h exposure in medium buffered at pH 3.5, while a pH of 3.0 was required to kill the wild type in the same time period. Also, induction of the ATR in BH97LC, as measured by the number of survivors at a pH killing unadapted cells, was 3 to 4 orders of magnitude lower than that exhibited by the wild type. While the LTA of both strains contained a similar average number of glycerolphosphate residues, permeabilized cells of BH97LC did not incorporate d-[14C]alanine into this amphiphile. This defect was correlated with the deficiency of Dcp. Chemical analysis of the LTA purified from the mutant confirmed the absence of d-alanine-esters. Electron micrographs showed that BH97LC is characterized by unequal polar caps and is devoid of a fibrous extracellular matrix present on the surface of the wild-type cells. Proton permeability assays revealed that the mutant was more permeable to protons than the wild type. This observation suggests a mechanism for the loss of the characteristic acid tolerance response in S. mutans.
Lipoteichoic acids (LTAs) are surface components of most gram-positive bacteria comprised of phosphodiester-linked poly(alditolphosphate) chains covalently anchored to membrane glycolipid. The LTA of many oral streptococci is comprised of polyglycerolphosphate [poly(GroP)] (30), with each GroP unit potentially glycosylated and selectively acylated with d-alanine ester residues (16). d-Alanyl esters of LTA have important functions in growth and physiology, including a role in the synthesis of wall teichoic acid (34, 45), regulation of autolytic activity (16), and the binding of Mg2+ for enzyme activity (2). The polyanionic properties of LTA are associated with adherence and cell-cell coaggregation through the binding of cations (36), proteins and polysaccharides (14), as well as hydroxyapatite (9). These are important factors in the formation of dental plaque biofilms. Streptococcus mutans, an organism associated with dental caries, synthesizes considerable LTA (25), particularly under the low growth rates typical of the plaque biofilm (7).
The biosynthesis of d-alanyl-LTA, studied in Lactobacillus rhamnosus (12, 27, 28, 42) and Bacillus subtilis (46), requires the d-alanyl-carrier protein (Dcp) for incorporation of the activated d-alanine into membrane-associated LTA (mLTA). The activation and ligation of d-alanine to Dcp is catalyzed by d-alanine:Dcp ligase (d-alanine-Dcl) having a function similar to the acid thiol ligases (28). Unlike the first reaction, the transfer of activated d-alanine to mLTA is highly specific for d-alanine-Dcp. Recent genetic analysis (11, 42) has indicated that the proteins for d-alanine incorporation reside in the dlt operon comprised of four genes:dltA, encoding Dcl; dltC, encoding Dcp; dltB, encoding a putative transmembrane protein involved in the secretion of activated d-alanine; and dltD, a membrane-associated thioesterase for mischarged carrier protein.
Two recent reports have indicated that oral streptococci possess the dlt operon comprised of dltA, dltB, dltC, and dltD having homology to the operons of L. rhamnosus and B. subtilis. In one study, Spatafora et al. (49) observed that the inactivation of the S. mutans UA130 dlt by transposon insertion into a site upstream of dltA resulted in the diminished synthesis of intracellular polysaccharide. Interestingly, the expression of the dlt operon was induced in the exponential phase when the cells were grown with sugars transported by the phosphoenolpyruvate-sugar phosphotransferase system (PTS) but was expressed constitutively when grown with the non-PTS sugars raffinose and melibiose. A mutant of S. mutans defective in PTS activity showed constitutive expression, suggesting a relationship between the dlt operon and sugar transport via the PTS. In a second study (10), insertional inactivation of dltA in S. gordonii DL1 (Challis) not only resulted in the loss of d-alanylation of LTA, but also the loss of intrageneric coaggregation with other oral streptococci. Such inactivation also resulted in the loss of a 100-kDa surface protein adhesin known to be associated with this aggregation. In spite of this defect, the mutant was, nevertheless, able to carry out intergeneric coaggregation with human oral actinomyces. It was postulated that the d-alanyl-LTA, but not d-alanine-free LTA, provided binding sites for the adhesin to facilitate intrageneric coaggregation.
In human dental plaque, S. mutans is subjected to daily cycles of acid shock created by the accumulation of acid end products generated during the metabolism of dietary carbohydrate by the acidogenic microflora. The rate of acid formation in human subjects, as measured by pH telemetry, has indicated that the intake of carbohydrate can lower the plaque pH from 7.0 to 4.0 in as little as 3 min (31, 32). Our earlier studies (24, 50, 51) demonstrated that the acid shock (pH 7.5 to 5.5) of log-phase cells of S. mutans resulted in the induction of an acid tolerance response (ATR) that enhanced survival at pH 3.0. This log-phase ATR requires protein synthesis and involved the transient formation of acid-responsive proteins over a 2-h period (24). This acid shock results in the upregulation of 64 proteins within 30 min of the pH change (51). These are undoubtedly related to the variety of physiological changes induced in cells during pH downshifts in continuous culture (21).
Our goal is to identify the key global regulators involved in the ATR in S. mutans. To this end, transposon mutagenesis has been used to isolate clones that are acid sensitive. During this process, an acid-sensitive clone was isolated carrying a 6.3-kb deletion, which included dltA and a portion of dltB of the dlt operon as well as four complete genes upstream from that operon. To test whether the defect in the dlt operon was associated with the acid-sensitive phenotype, the d-alanylation of LTA in S. mutans LT11, was blocked by the inactivation of dltC. The resulting mutant, BH97LC, exhibited enhanced acid sensitivity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids are listed in Table 1. S. mutans strains were maintained anaerobically at 37°C (or 30 or 42°C when appropriate) on Todd-Hewitt plates (Difco or BBL) and grown for DNA isolation in Todd-Hewitt broth supplemented with 0.3% yeast extract (THYE). For selection of acid-sensitive colonies, THYE plates containing 50 mM sodium acetate at pH 5.3 and pH 5.0 were used. Growth studies and morphological comparisons by scanning electron microscopy involved growing cells in tryptone-yeast extract-glucose (TYEG) broth and a minimal defined medium (MM4) (24), modified to contain Na/K phosphate buffer rather than phosphate-citrate buffer, and with the addition of 19 mM sodium carbonate. Escherichia coli was maintained at 37°C (or 30°C when appropriate) on Luria-Bertani plates and grown for plasmid isolations in Luria-Bertani broth. When appropriate, the following antibiotics were used at the indicated concentrations: erythromycin at 500 μg/ml for E. coli and 10 μg/ml for S. mutans; tetracycline at 10 μg/ml for E. coli; and ampicillin at 100 μg/ml for E. coli.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genetic marker(s) or description | Source or reference |
|---|---|---|
| Strains | ||
| S. mutans | ||
| LT11 | Highly transformable mutant of UA159 | 53 |
| G9IS1A | LT11 containing multiple tandem integrated copies of pGh9:ISS1 at dlt-abcX locus | This work |
| IS1A | Single copy of ISS1 at dlt-abcX locus | This work |
| IS1A:r | IS1A with pISS1:r integrated via single crossover at ISS1 | This work |
| BH97LC | LT11 with Emr gene inserted into dltC gene | This work |
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| Plasmids | ||
| pBluescript | Ap ColE1 ori f1 ori multipurpose cloning vector | Stratagene |
| pΩ | Emr, pBluescript-based streptococcal integration vector | 37 |
| pISS1:r | pGh9:ISS1 0.89 kb BamHI/SalI fragment containing ISS1 cloned into pΩ | This work |
| pIS1A/E:r | Contains opposite ISS1-genomic DNA junctions rescued from S. mutans IS1A:r | This work |
| pIS1A/H:r | Contains opposite ISS1-genomic DNA junctions rescued from S. mutans IS1A:r | This work |
| p7B/H-BgNs | The insert in p7H/B-Bg/Ns is a 702 bp BglI-NspV fragment spanning the last 164 bp of dltC and the first 542 bp of dltD | This work |
| pBS-Em | pBluescript containing Emr gene from pAMβ1 cloned as 1.8-kb BamHI fragment | R. Burne |
| pIS1A-MN | 1.35-kb MunI/NspV fragment from dlt operon cloned into pBluescript | This work |
| pDLTC-Em | Emr gene from pBS-Em cloned into BglII site of pIS1A-MN | This work |
DNA methodology.
The isolation of chromosomal and plasmid DNA, agarose gel electrophoresis, Southern hybridizations, DNA ligations, and transformation of E. coli were carried out as previously described (5), while the basic transformation procedures for S. mutans were as described by Perry et al. (46). Sequencing was carried out manually using Sequenase (version 2.0; Amersham) with the modifications described by Mytelka and Chamberlin (40) and automatically using fluorescent dye terminators provided by the University of Florida (Gainesville) DNA Sequencing Core Laboratory. Custom-made primers for manual sequencing or for PCR were synthesized by the University of Calgary, University Core DNA Services. PCRs were carried out using native Pfu polymerase (Stratagene) or the Expand Long Template PCR system (Boehringer Mannheim) according to manufacturers' instructions.
Isolation of S. mutans IS1A, G9IS1A, and BH97LC.
S. mutans LT11 was transformed with pGh9:ISS1 essentially as described for Lactococcus lactis (38) with selection for plasmid-containing colonies at 30°C on THYE-erythromycin plates. Plasmid-containing cultures were diluted 1:100 into fresh prewarmed medium without antibiotic and incubated for 3 h at 30°C to allow exponential growth to resume. For transposition of pGh9:ISS1 into LT11, the culture was shifted to 37°C for 30 min and then to 42°C for 3 h. Samples were diluted and spread on plates containing erythromycin (10 μg/ml) and incubated at 42°C for 1 to 2 days. To select for plasmid integrants, pH-sensitive mutants were isolated by picking ∼3,000 erythromycin-resistant colonies in duplicate onto THYE plates at pH 5.3 and pH 7.0. Approximately 90 colonies whose growth appeared to be impaired at pH 5.3 were picked in duplicate and plated onto THYE at pH 5.0 and pH 7.0. One colony (G9IS1A) that showed no growth at pH 5.0 was selected.
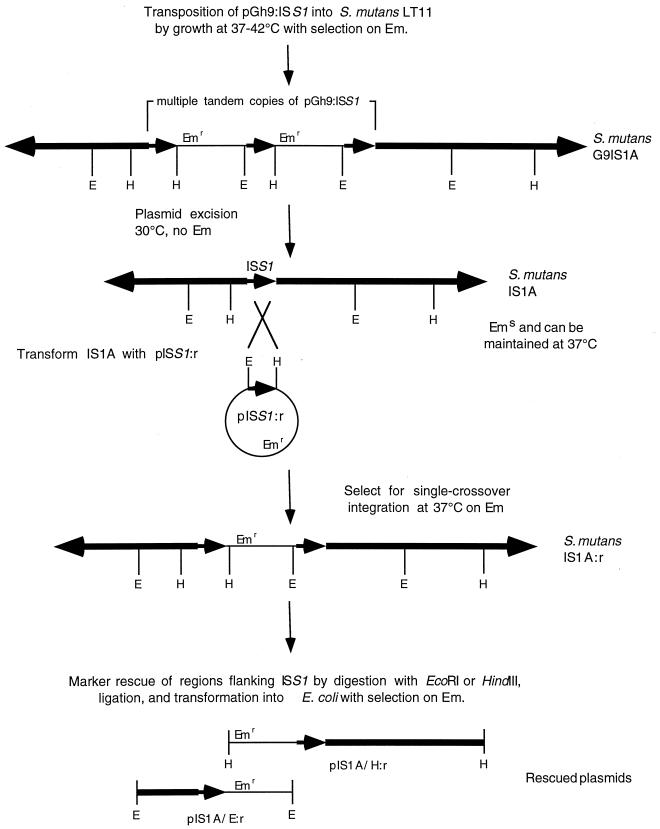
Excision of the vector backbone was accomplished by growth without antibiotic at 30°C, plating dilutions onto solid medium. Individual colonies were then picked in duplicate and plated onto selective (erythromycin [10 μg/ml] and non-selective medium and grown overnight at 37°C. One colony out of 95 picked was erythromycin sensitive at 37°C, and the presence of ISS1 in the genome of this strain (IS1A) was confirmed by Southern hybridization (Fig. 1). Isolation of the regions flanking ISS1 were carried out by transforming IS1A with pISS1:r and selecting for erythromycin-resistant transformants at 37°C. The presence of a pISS1:r intergrated via single-crossover at the genomic copy of ISS1 was confirmed for five transformants by Southern analysis. One was selected to attempt marker rescue and was named IS1A:r. Upstream and downstream regions were isolated by rescue of the integrated copy of pISS1:r plus flanking DNA by digestion of IS1A:r DNA with either EcoRI or HindIII, ligation of the cut DNA, and transformation into E. coli XL1-Blue with selection for erythromycin resistance.
FIG. 1.
Construction and isolation of S. mutans G9IS1A and IS1A using pGh9:ISS1 and isolation of plasmids carrying DNA flanking the ISS1 element in IS1A. Thick lines and arrowheads represent genomic DNA, thin lines represent plasmid DNA, and small arrowheads represent ISS1 DNA. Abbreviations for restriction enzymes: E, EcoRI; H, HindIII.
To inactivate dltC in S. mutans LT11, plasmid pIS1A-MN was constructed by cloning the 1.35-kb MunI/NspV fragment from pIS1A/H:r, containing the 3′ end of dltB, dltC, and the 5′ end of dltD, into EcoRI/ClaI-digested pBluescript KS. The dltC was disrupted by cloning an erythromycin resistance gene, isolated as a 1.8-kb BamHI fragment from pBS-Em, into the BglII site of pIS1A-MN to give pDLTC-Em (Fig. 2). Plasmid pDLTC-Em was linearized by digestion with KpnI (single site in the vector) and used to transform LT11 to erythromycin resistance. The presence of the interrupted dltC gene was confirmed for six transformants by Southern analysis, and one acid-sensitive clone was picked and named S. mutans BH97LC.
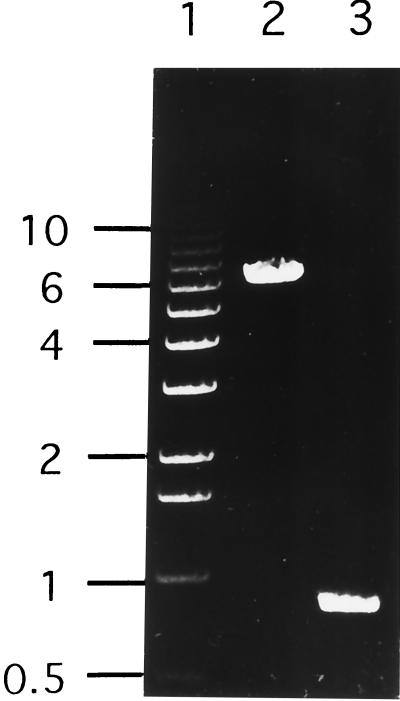
FIG. 2.
PCR products generated using S. mutans LT11 genomic DNA (lane 2), and S. mutans IS1A (lane 3) genomic DNAs as templates in reactions with primers ABC-UP and DLT-UP. Lane 1 shows the 1-kb ladder (Life Technologies) with sizes (in kilobases) shown on the left.
RNA isolation and RNA dot blotting.
To isolate S. mutans RNA, log-phase cells were resuspended in 1 ml of TRIZOL reagent (Gibco-BRL) and disrupted by rapid shaking for 24 s with glass beads using a Fast prep FP120 cell disruptor (Savant, Holbrook, N.Y.) according to the manufacturer's instructions. RNA preparations were treated with 20 U of RQ1 DNase (Promega) for 30 min at 37°C, extracted with TRIZOL and chloroform, and precipitated with ethanol. The message for dltC and dltD was detected by RNA dot blotting using the Genius nonradioactive nucleic acid labeling and detection system (Roche Diagnostics, Laval, Quebec, Canada). A DNA probe was generated by amplifying a segment of dltCD by PCR, using primer T7 and T3 with p7B/H-BgNs (Table 1) as the template. The probe was labeled directly during PCR using digoxigenin-dUTP following the manufacturer's (Roche) protocol. Equal amounts of total RNA extracted from parent strain LT11 and mutant BH97LC were applied to the membrane.
Characterization of BH97LC.
The mutant was grown anaerobically in TYEG medium at pH 7.5, and log-phase cells were used to determined survival over the pH range pH 7.5 to 2.5 as previously described (50). Tests to examine the ability of the mutant to initiate growth over the pH range 7.5 to 4.5 were carried out by inoculating (2%) the same medium with an overnight culture of either the wild-type or mutant strain. The ability of BH97LC to induce an ATR at sublethal pH values (pH 7.5 to 3.5) that would enhance survival over a 3-h period at pH 3.0 was determined by methods previously described (50).
d-Alanine incorporation assay.
Incorporation of d-[14C]alanine into LTA by membrane and cytosolic (supernatant) fractions of the wild-type (LT11) and the mutant, BH97LC, was performed in reaction mixtures (50 μl) which contained 30 mM bis-Tris (pH 6.5) 10 mM ATP, 10 mM MgCl2, 1 mM dithiothreitol, and 0.11 mM d-[14C]alanine (43 mCi/mmol). The amount of incorporation was measured by the method of Heaton and Neuhaus (28). For the preparation of membranes and supernatant fractions, cultures (500 ml) were grown in TYEG medium and harvested at an optical density at 600 nm of 1.6. The cells were washed in ice-cold water, suspended in 10 ml of buffer containing 20% sucrose, 60 mM Tris-HCl (pH 7.8), 2 mM MgCl2, 0.25 g of lysozyme, and 1,000 U of mutanolysin (Sigma Chemical Co.); and incubated at 37°C for 2 h with gentle shaking. The resulting suspension was diluted 2.5 times with the same buffer without sucrose and one tablet of the protease inhibitor cocktail (Boehringer Mannheim) was added. The disrupted cells were further treated with a French press (three times at 15,000 metric tons) and, subsequently, incubated with DNase and RNase. The supernatant fraction was centrifuged at 200,000 × g for 90 min, and the membrane pellet was homogenized in a minimum amount of 30 mM Tris-HCl buffer (pH 7.8), 2 mM MgCl2, and 10% glycerol. Membrane fragments were washed by two cycles of differential centrifugation at 9,000 and 200,000 × g and homogenized in the same buffer with aliquots frozen in liquid nitrogen and stored at −80°C. The 200,000 × g supernatant was dialyzed against 30 mM Tris-HCl buffer (pH 7.0) containing 2 mM MgCl2 and 1 mM dithiothreitol and concentrated with a Filtron centrifugal concentrator (10K; FiltronTechnology Corp.).
Chemical analysis of LTA.
LTA was isolated from early-stationary-phase cells following disruption in a Mickle disintegrator with Ballotini beads (60/40) and extraction by hot phenol-water. LTA was purified from the aqueous layer followed by hydrophobic chromatography on octyl-Sepharose using the centrifugation method of Koch et al. (35). The presence of LTA in the final 50% 1-propanol fraction, following freeze-drying and reconstitution in water, was confirmed in a precipitin reaction with an anti-LTA antibody generated from L. rhamnosus ATCC 7469. The purified LTA was subjected to chloroform-methanol extraction (6) and analyzed essentially by the procedure of Perego et al. (45). One fraction was hydrolyzed in 2 M HCl at 100°C for 2.5 h and analyzed for phosphorus (58), alanine (18), glucose (33), and glycerol (41) (analysis for glycerol following treatment with alkaline phosphatase). Alanine as the alanine ester and alanyl glycerol were determined in the second fraction following mild alkaline treatment in 0.1 M NaOH at 37°C for 1 h. The chain length of LTA was calculated from the amounts (micromoles gram of dry cells−1) of phosphorus and glucose using the following equation (45): phosphorus/0.5 glucose × 1.1.
Thin-section and scanning electron microscopy.
Thin-section electron microscopy was performed essentially as previously described (26). Briefly, exponential-phase cells were fixed overnight with 25% freshly purified glutaraldehyde, washed, and fixed for 1 h in 1% osmium tetroxide in SC-Mg buffer. Following additional washing and resuspension in 2% aqueous uranyl acetate for postfixing, cell pellets were resuspended in an equal volume of 3% low-melting-point agarose, and the resulting agarose-cell blocks were diced and dehydrated prior to thin sectioning. Silver sections were mounted on uncoated hexagonal 400-mesh copper grids, stained first with saturated ethanolic uranyl acetate followed by 0.25% aqueous lead citrate in 0.1 M NaOH, and viewed and photographed at machine magnifications ranging from ×15,000 to ×100,000 in a Philips model 201 electron microscope at an acceleration voltage of 60 KeV.
For scanning microscopy, cells were grown to exponential phase in TYE and in MM4 (24), the latter supplemented with 24 mM sodium bicarbonate, and then fixed overnight in cacodylate buffer (pH 7.4) containing 2.5% (wt/vol) glutaraldehyde at 4°C. Samples were then washed four times in 0.1 M cacodylate buffer, fixed to glass coverslips by a graded series of ethanol dehydrations, dried as previously described (10), and viewed with a Hitachi S-2500 scanning electron microscope.
Proton permeability.
The rate of proton uptake by intact cells of the wild-type and mutant strain was tested in a proton conductance assay using energy-depleted cells (20 mg [dry weight] ml−1) equilibrated at pH 6.0, 5.0, or 4.5 and receiving a pulse of 10 mM HCl–140 mM KCl sufficient to drop the pH ∼0.2 pH units (3, 22). Washed cells were considered depleted of endogenous energy reserves when acid was no longer generated during anaerobic incubation at pH 7.0 in a pH-stat (22). The minimum pH of the suspension immediately after acid addition (pHα) was reversed as protons entered the cell and the extracellular pH increased. The pH recording was continued for approximately 10 min before butanol (6% final concentration) was added to permit complete equilibrium between the cells and the external medium for the estimation of the final equilibrated pH (pHω). The results are expressed as the time (in minutes) required for the pH to reach a value (t1/2) halfway between pHα and pHω and are reported as the means of at least three separate determinations.
Computer-aided analysis.
Protein sequence analysis was carried out using the current version of the BLAST v2.0 homology search software (1) via the World Wide Web interface of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.goc/BLAST). The sequence of the genes shown in Fig. 2 has been submitted to the GenBank database under accession number AF051356.
RESULTS
Isolation of acid-sensitive mutants.
To isolate mutants of S. mutans LT11 unable to grow at pH 5.0, transposon mutagenesis with pGh9:ISS1 was used. Southern analysis of one erythromycin-resistant acid-sensitive colony (G9IS1A) indicated that a tandem transposition had occurred. From this strain, the plasmid backbone was excised leaving behind a single copy of the ISS1 element (IS1A) (Fig. 1). Like G9IS1A, IS1A failed to grow on THYE (pH 5.0) plates. The DNA flanking ISS1 was rescued by transforming IS1A with pISS1:r to integrate a copy of the plasmid at this locus. This permitted recovery of genomic DNA fragments carrying the plasmid backbone plus additional flanking DNA (pIS1A/H:r and pIS1A/E:r [Fig. 1]). Sequence analysis of these flanking regions followed by searches of the GenBank database showed that one end of ISS1 was inserted into a gene that had homology to dltB of the dlt operon (42), while the other end was inserted just upstream of a gene whose product shows homology to proteins belonging to the ATP-binding cassette (ABC) protein superfamily. Since the flanking regions were not genetically colinear, it was apparent that a deletion had occurred during construction of G9IS1A and IS1A. PCR analysis, using primers to regions within the ABC and dltB sequences and using the LT11 genomic DNA as a template, revealed a 6.3-kb product representing the region deleted from IS1A (Fig. 2, lane 2) and this was confirmed using IS1A genomic DNA as a template (Fig. 2, lane 3). This 6.3-kb PCR product and the ISS1 flanking regions from pIS1A/E:r and pIS1/H:r were sequenced to give 11,202 bp of contiguous sequence comprised of nine complete open reading frames (ORFs), eight of which were arranged in the same orientation (Fig. 3).
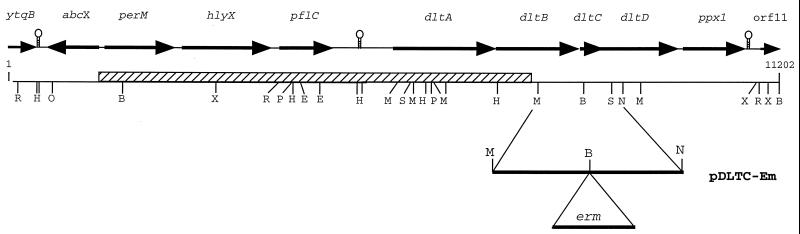
FIG. 3.
Physical map and ORF characterization of 11,202 bp of the S. mutans LT11 genome based on DNA sequence analysis of pIS1A/E:r, pIS1A/H:r (plasmids rescued from strain IS1A carrying the flanking ends of the transposon and their junction sites), and the PCR product (hatched bar) produced using primers of the adjacent S. mutans DNA. The MunI/NspV fragment that was disrupted by insertion of an Emr gene and used to construct pDLTC-Em is also shown. Hairpin structures represent putative transcriptional terminators. Genetic designations: ytqB, unknown; abcX, ABC transport protein; perM, permease; hlyX, hemolysin; pflC, pyruvate-formate lyase activase; dltABCD, genes of the dlt operon; ppx1, exopolyphosphatase. Abbreviations for restriction sites: B, BglII; E, EcoRI; H, HindIII; M, MunI; N, NspV; P, PstI, R, RcaI; S, SphI; X, XbaI.
As the size of the deletion precluded the possibility of assigning the acid phenotype of IS1A to any one gene, further work was directed at the specific genes affected by the deletion. An obvious starting point was the dlt operon, comprised of four genes encoding the proteins involved in the incorporation of d-alanine esters into LTA. Since these esters determine the polyanionic charge of the cell surface, we addressed the role of this operon in determining the ATR of S. mutans LT11. The operon shared a high degree of similarity to previously reported sequences encoding genes proven to be involved in d-alanyl-LTA synthesis in other bacteria. For example, the deduced protein sequence of dltA (d-alanine-Dcp ligase) from S. mutans LT11 shares 50% sequence identity with the product of dltA of L. rhamnosus and 41% with the dltA product of B. subtilis (12, 45). As with the dlt operon of Lactobacillus casei (27) and Streptococcus gordonii (10), dltA and dltB in S. mutans LT11 overlap as do dltC and dltD. In B. subtilis, the dlt operon also includes dltE, which encodes a protein belonging to a large family of oxidoreductases (45). We found no homolog of dltE in the S. mutans LT11 sequence, but 61 bp downstream of dltD is an ORF whose product has 76% sequence identity with a protein (Cog) from S. gordonii, which is involved in intrageneric coaggregation (57). Further study of the BLAST search results revealed that Cog was homologous to exopolyphosphatase encoded by the ppx1 gene.
For inactivation of the dlt operon in S. mutans LT11, the gene (dltC) for the carrier protein, d-alanyl-Dcp, was disrupted since the high specificity of the d-alanylation process appears to be associated with this protein (42). We inactivated dltC by transforming S. mutans LT11 with linearized pDLTC-Em containing the disrupted dltC gene (Fig. 3). Six erythromycin-resistant transformants were shown by Southern analysis to have integrated the inactive dltC gene and all were acid-sensitive. One transformant, BH97LC, was subjected to further study.
Acid tolerance of BH97LC.
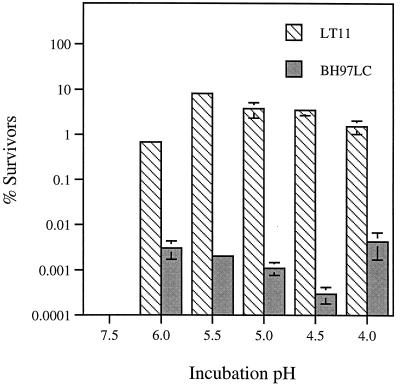
From a variety of experiments, it was shown that BH97LC and IS1A were less acid tolerant than the wild-type strain LT11 (Table 2). The data for these mutants showed that they were unable to initiate growth below pH 6.5 compared to pH 5.0 for LT11 and had a higher killing pH (3.5) than LT11 (3.0). Further testing of BH97LC demonstrated that the mutant exhibited a reduced capacity to induce an ATR. In the latter experiment, log-phase cells, grown at pH 7.5, were incubated for 2 h in the fresh medium at sublethal pH values (pH 6 to 4) to induce the ATR (24, 50) followed by a 3-h challenge at pH 3.0. This pH kills 100% of unadapted control cells maintained at pH 7.5 (50). As shown in Fig. 4, the response generated by BH97LC was significantly compromised, since the number of survivors at pH 3.0 was 3 to 4 orders of magnitude lower than that exhibited by LT11. Of particular interest for the interpretation of this experiment was the fact that the terminal pH achieved during growth was only slightly higher (4.64) than that of the wild-type strain (4.50), suggesting that intracellular pH homeostasis could be maintained in the mutant once growth was initiated. In addition to the difference in ATR, the doubling time of BH97LC was 2.8 times that observed for LT11, and the cell biomass per mole of glucose was 0.66 times that of LT11 (Table 2).
TABLE 2.
Growth and acid-tolerant characteristics of the wild-type strain S. mutans LT11 and acid-sensitive mutants
| Strain | Doubling time (min) | Yielda | Terminal pH during growth | Killing pHb | Lowest pH for growth initiation |
|---|---|---|---|---|---|
| Wild type | 56 ± 3 | 44.5 ± 1.2 | 4.50 ± 0.05 | 3.0 | 5.0 |
| BH97LC | 155 ± 15 | 29.2 ± 1.4 | 4.64 ± 0.08 | 3.5 | 6.5 |
| IS1A | 230 ± 15 | 23.2 ± 1.7 | 4.66 ± 0.08 | 3.5 | 6.5 |
Grams of cells (dry weight)/mole of glucose.
As measured by the absence of survivors following a 3-h incubation in TYEG.
FIG. 4.
Effect of prior pH conditioning on the survival of the wild-type S. mutans LT11 and the mutant BH97LC in TYEG following a 3-h exposure to pH 3.0. Log-phase cells growing at pH 7.5 were transferred to fresh TYEG medium buffered at pH 6.0 to 3.5 and incubated at 37°C for 2 h prior to acidification to pH 3.0. The control cells (pH 7.5) were similarly treated. Error bars, standard deviations.
Incorporation of d-[14C]alanine into LTA.
To confirm that the defect in the dltC gene had blocked d-alanine incorporation into LTA of BH97LC, permeabilized cells were incubated with d-[14C]alanine and ATP according to the method of Ntamere et al. (43), and compared with permeabilized cells from parent LT11. Permeabilized cells provide a test system for assaying the ligation of d-alanine to Dcp and its subsequent incorporation into mLTA. The incorporation of d-alanine in either toluene- or toluene-acetone-permeabilized cells was 3% and <1% of that observed for the parent (data not shown). Thus, the insertion of the erm cassette in the dltC gene resulted in the defective d-alanylation of LTA.
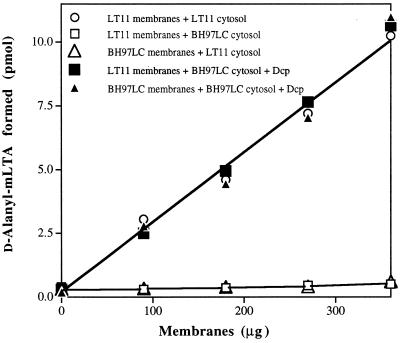
By recombining the cytosolic (supernatant) fractions and membranes containing LTA from the parent and mutant in various combinations, it was determined that the defect was expressed in the cytosolic fraction. For example, the cytosolic fraction of BH97LC could not reconstitute the system with wild-type membranes (Table 3). To establish that BH97LC is deficient for Dcp, the cytosolic fraction from the mutant was reconstituted with recombinant Dcp from L. rhamnosus. Addition of 12.5 nM Dcp to this fraction reconstituted the maximum d-alanine incorporation observed with parent membranes and cytosolic fraction from the parent (Fig. 5). Thus, the dltC mutant, which is deficient for d-alanine incorporation, is deficient in Dcp.
TABLE 3.
Incorporation of d-[14C]alanine into LTA by in vitro combinations of membranes and cytosolic fractions from the wild-type strain S. mutans LT11 and the acid-sensitive mutant BH97LC
| Membrane | Activity (pmol)a
|
|
|---|---|---|
| LT11 cytosol | BH97LC cytosol | |
| LT11 | 7.2 ± 0.3 | 0.50 ± 0.05 |
| LT11 (heat treated) | 0.46 ± 0.07 | 0.35 ± 0.07 |
| BH97LC | 7.5 ± 0.2 | 0.38 ± 0.08 |
| BH97LC (heat treated) | 0.41 ± 0.06 | 0.33 ± 0.04 |
The d-alanine incorporation assay as described in Materials and Methods was used to measure the incorporation of d-[14C]alanine. The amounts of membrane and cytosol were 360 and 50 μg of protein, respectively.
FIG. 5.
Reconstitution of the DltC-deficient cytosol fraction from BH97LC with Dcp. The d-alanine incorporation assay used was described in Materials and Methods, with the indicated amounts of membrane from either LT11 or BH97LC, cytosol (supernatant) fraction (3 μg of protein), and 12.5 nM Dcp.
Chemical analysis of wild-type and mutant LTA.
In order to confirm the absence of d-alanylation of LTA in the mutant, the chemical compositions of the LTA from the wild type and BH97LC were determined by extracting and purifying LTA from disrupted early stationary-phase cells under conditions maintaining d-alanine ester substitution of the LTA. The LTA from both the wild type and mutant was composed of poly(GroP) chains as indicated by the equimolar ratio of glycerol and phosphorus (1.01 and 0.96, respectively), confirming values obtained earlier with purified LTA of S. mutans AHT (4). Furthermore, the LTA chain lengths of the mutant and wild-type strains were essentially the same, e.g., 32 versus 36 glycerophosphate residues/chain, respectively. The molar ratio of d-alanine to LTA phosphorus for the wild type, LT11, was 0.67, slightly higher than that obtained with strain AHT (0.52) (4). The mutant, BH97LC, on the other hand, was devoid of d-alanine ester. Thus, chemical analysis confirms that the inactivation of dltC blocked d-alanine esterification of LTA and the formation of the poly(GroP) moiety of LTA was unaltered.
Electron microscopy.
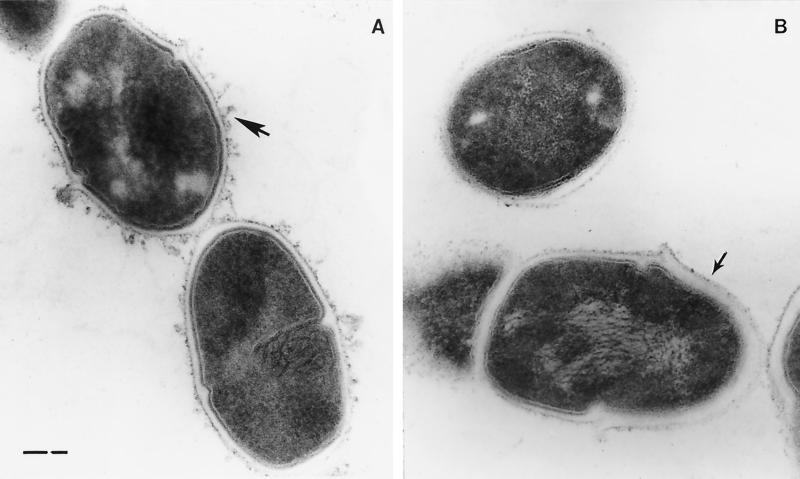
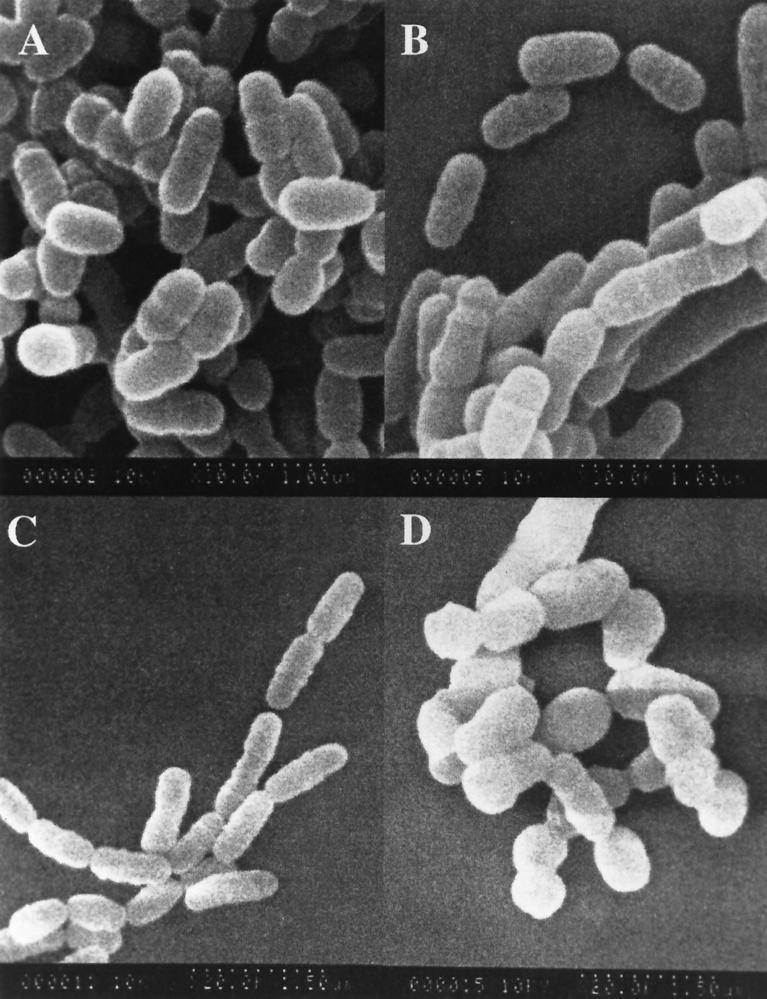
Electron micrographs showed S. mutans LT11 to possess a fibrous extracellular matrix constituent that has also been reported for S. aureus (54). (Fig. 6A). There was no evidence of this matrix material with the mutant BH97LC (Fig. 6B). Furthermore, unlike the wild type, cell wall thickness of the mutant varied within the same cell or diplococcal unit. One cell, or the end of a single cell, exhibited a thickness similar to LT11 cells, while polar caps of unequal thickness (Fig. 6B) were observed at the opposite ends of the diplococcal units (29). As seen in Fig. 7, comparisons of scanning electron micrographs of the wild-type and mutant cells grown on rich medium (TYEG) showed virtually no difference in morphology, while a transfer to minimal medium was correlated with a transition of the mutant cells from diplococcus to spheres which were not seen with the wild-type strain, LT11.
FIG. 6.
Transmission electron micrographs of thin sections of the wild-type strain, S. mutans LT11, showing cell surface structures (A) (arrow) that are absent in the dltC-defective mutant, BH97LC (B) (arrow).
FIG. 7.
Scanning electron micrographs of log-phase cells of the wild-type strain, S. mutans LT11 (A and C), and in the dltC-defective mutant, BH97LC (B and D), grown in complex medium (TYEG) (A and B) and minimal defined medium (MM4) (C and D).
Proton permeability of the dltC mutant BH97LC.
As the acid tolerance of oral streptococci has been shown to be related to the membrane permeability of protons (3, 22), we reasoned that the acid-sensitive mutant, BH97LC, may have a different permeability to protons than the wild-type strain, LT11. To test for differences in proton permeability, energy-depleted, log-phase cells of LT11 and BH97LC were equilibrated at pH 6.0, 5.0, or 4.5 and then subjected to an acid pulse sufficient to drop the pH ∼0.2 pH units (3). Proton uptake was determined by extrapolation of the pH-versus-time curve from pHα to pHω achieved by the addition of butanol. Permeability was recorded as the t1/2 value. As seen in Table 4, the lower t1/2 values for BH97LC at all pH values indicated the mutant was more permeable to protons than the wild-type strain. For example, at pH 4.5 the mutant was almost twofold more permeable to protons that LT11. These results indicate that the defect in the dltC gene resulted in cells more permeable to the passive inflow of protons than wild-type cells.
TABLE 4.
Proton uptake by deenergized cells of S. mutans LT11 (wild type) and the acid-sensitive mutant BH97LC as a function of the external pHa
| Initial pH |
t1/2 (min)b
|
|
|---|---|---|
| LT11 | BH97LC | |
| 7.0 | 8.8 ± 3.3 | 7.2 ± 2.3 |
| 6.0 | 7.7 ± 1.2 | 5.3 ± 1.4 |
| 5.0 | 10.9 ± 2.6 | 6.1 ± 1.3c |
| 4.5 | 10.0 ± 3.3 | 5.4 ± 1.5d |
Cell suspensions were equilibrated at each pH value for 2 min prior to the addition of an acid pulse to reduce the pH to pHα.
t1/2 is defined as one-half the time for the pH to increase (proton uptake) from pHα to pHω.
P = 0.0004 (significant).
P < 0.0001 (significant).
DltD expression in BH97LC.
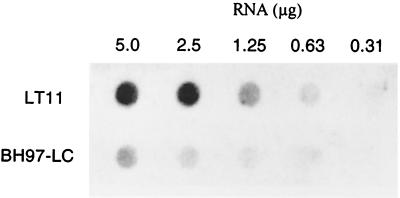
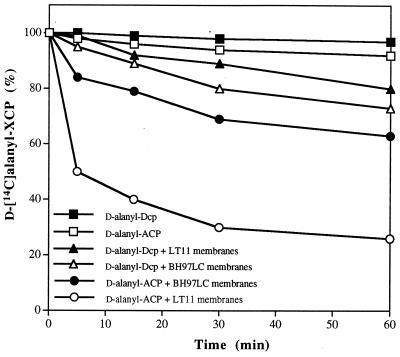
The insertion of the erm cassette into dltC may affect the synthesis of the complete polycistronic message. RNA dot blot analysis of the message for dltD indicated that the expression of this message was defective in BH97LC (Fig. 8). One of the functions of DltD is to hydrolyze mischarged d-alanyl-ACP (13). Thus, hydrolysis of d-alanyl-ACP may be absent or greatly decreased in the mutant membranes. In Fig. 9, the hydrolytic cleavage of the mischarged d-alanyl-ACP by the parent and mutant membranes is shown. At 5 min, 50% of the d-alanyl-ACP is cleaved by the parent membranes, whereas only 15% of this ligated-carrier protein is hydrolyzed by the mutant membranes. In contrast, d-alanyl-Dcp, the normal carrier protein, is not hydrolyzed more than 5% by either the mutant or wild-type membranes. Thus, the mutant described in this paper would appear not only to be defective in the expression of dltC, i.e., Dcp, but also for the expression of DltD. This deficiency of DltD results from the upstream insertion of the resistance cassette.
FIG. 8.
Levels of message for dltC and dltD in the wild-type strain, S. mutans LT11, and the dltC-defective mutant, BH97LC, as detected by RNA dot blotting using a DNA probe of the dltC and dltD genes on p7B/K-BgNs as the template (Table 1).
FIG. 9.
Hydrolysis of d-alanyl-acyl carrier protein (ACP) and d-alanyl-Dcp by membranes from BH97LC and from LT11. Membranes from either the parent or mutant were incubated in a reaction mixture (250 μl) containing either 6.5 nmol of d-[14C]alanyl-ACP or d-[14C]alanyl-Dcp in 10 mM bis-Tris (pH 6.5) and 30 mM MgCl2. Aliquots (50 μl) were removed from the mixtures at the indicated times, and the amount of d-[14C]alanyl-ACP or d-[14C]alanyl-Dcp remaining was measured by precipitation with 10% trichloroacetic acid according to the method of Heaton and Neuhaus (22).
DISCUSSION
The selection of the dlt operon for further study in relation to the acid-sensitive phenotype of mutant S. mutans BH97LC was based on the importance of d-alanyl-LTA in the growth and physiology of gram-positive bacteria (2, 17, 34, 45, 56). To test for a link between d-alanylation of LTA and acid sensitivity, we inactivated the dltC gene of the dlt operon in LT11, which encodes the d-alanyl carrier protein, to create the mutant BH97LC. In addition to being unable to d-alanylate LTA, BH97LC was shown to be acid-sensitive, displaying a defective ATR and an increased permeability to protons compared to wild-type LT11.
Inactivation of genes in the dlt operon in various bacteria shows an array of phenotypic changes. Insertional activation of the dltA-dltD genes in B. subtilis was without effect on LTA chain length, cellular morphology, cell growth, and basic metabolism but resulted in a greater susceptibility to methicillin and an increased rate of autolysis (45, 55, 56). The latter is postulated to occur by the increased binding of cationic autolysins to the more negative, d-alanine deficient LTA. Similarly, recent studies with Staphylococcus aureus and Staphylococcus xylosus demonstrated that inactivation of the dlt operon resulted in enhanced susceptibility of cells to positively charged antimicrobial peptides, such as defensin, protegrins, and similar compounds (47). Mutation of dltD in L. lactis resulted in slower growth than the wild-type strain in addition to increased sensitivity to UV light (15). Inactivation of this gene in L. casei 102S resulted in an increase in cellular length and enhanced antimicrobial activity of the cationic detergents cetyltrimethylammonium bromide and chlorhexidine (13). In addition to the present study, two studies have examined mutants defective in genes of the dlt operon in the oral streptococci. In one study, insertion of Tn916 upstream of the dlt operon in S. mutans UA130 resulted in cells deficient in glycogen-like storage material (49), while defects in dltA of S. gordonii DL1 resulted in loss of d-alanine esterification with the concomitant loss of intrageneric coaggregation and a 100-kDa surface protein associated with this aggregation (10).
In this study, the defect in the dltC gene resulted in a variety of alterations in growth characteristics in addition to the increased acid sensitivity when compared to the wild-type strain LT11. For example, the doubling time was almost threefold longer than that the wild-type, while the yield was 66% of that of LT11. Moreover, electron micrographs (Fig. 6) showed that the mutant was devoid of the fibrous extracellular matrix observed with LT11, was typical of strains of S. mutans (39), and exhibited polar caps of unequal thickness within the diplococcal unit. Comparisons of the wild-type and mutant cells grown on TYEG showed virtually no difference in morphology, while a transfer to minimal medium was correlated with a transition of the mutant cells from rods to spheres that was not seen with the wild-type strain LT11. Such rod-to-sphere transition has been observed with S. mutans when the HCO3−/K+ ratio of the medium was increased (52). In the case of the rod-to-sphere transition of the B. subtilis rodB1 mutant, the degree of d-alanylation of wall teichoic acid decreased from 0.22 to 0.10 at the restrictive temperature (48). Insertional inactivation of the dltA gene of S. gordonii DL1 resulted in a mutant (PK3241) with multiple septation sites, which also exhibited a smooth and unstructured surface with a thickened, cap-like cell wall similar to S. mutans BH97LC (10).
The relationship between the acid sensitivity of the mutant, BH97LC, and inactivation of LTA alanine esterification is currently unknown but may be related to alterations in normal pH homeostatic mechanisms. S. mutans responds to external acid over the short term by extruding protons from the cell via the membrane-associated, proton-translocating ATPase (H+ ATPase) (3, 22) and by acid end product efflux (11). Sustained growth at low pH (5.5 to 5.0) results in increased H+ ATPase (22) and glycolytic activity (23). This is also supported by a lowering of the pH optimum for sugar transport and glycolysis (22), as well as a shift in cellular regulation to increased lactic acid formation (21) to support the efflux mechanism. Unlike the enteric bacteria (44), S. mutans does not maintain a constant intracellular pH (pHi) as the external pH falls but supports a relatively consistent transmembrane pH gradient (∼1.0 U) that must be sustained by a carbon source (20). Thus, adaptation to growth at lower pH values permits the organism to maintain transmembrane pH gradients at lower pH values (22).
These physiological characteristics can be used to explain the apparent paradoxical differences seen in Table 2 with respect to the acid sensitivity of BH97LC. The mutant was unable to initiate growth below pH 6.5 and yet was able to lower the pH of an established growing culture to pH 4.64, just slightly higher than that of the wild-type strain. The carbon source, glucose, was essentially depleted during growth, and yet the yield was a third less than that of the wild-type. This observation suggests that the intracellular pH was maintained adequately by the H+ ATPase and lactate-efflux mechanisms (3), however, at a greater cost in ATP than that of the wild-type, resulting in a loss of biomass. This would suggest some alteration in the permeability of the mutant cells to protons that required cells to expend more energy to maintain the pH gradient. The inability to initiate growth below pH 6.5 and the higher killing pH suggest that the cells are “leaky” to protons and can sustain a suitable intracellular pH only during active growth and glycolysis. The increased proton permeability of deenergized cells of BH97LC compared to the wild-type strain, particularly at pH 5.5 and 4.5, supports this proposition.
Data presented here are consistent with our earlier findings (19), indicating a role for de novo membrane biogenesis in maintaining an ATR. Specifically, we have shown that the signal recognition particle-associated FFh ribonucleoprotein, which acts as a chaperone for the expedited insertion of newly synthesized proteins into procaryotic membranes, is essential for a normal ATR. In that work, we demonstrated reduced amounts of H+ ATPase in membranes of ffh mutants created by Tn917 insertions. It will be of great interest to determine if proteins associated with the dlt operon are also translocated by signal recognition particle-associated mechanisms.
To our knowledge, the inactivation of dltC provides the first evidence linking increased proton permeability and the failure to induce a significant ATR, ensuring survival at a killing pH. This log-phase ATR requires protein synthesis and has been shown to involve the transient formation of proteins over a 2-h period (24). Thus, one might postulate that an alteration in the dlt operon resulting from the inactivation of dltC placed the cells under a condition of physiological stress, in which energy normally required for protein synthesis during the ATR is diverted to pH homeostasis. While one cannot exclude the possibility that the lower intracellular pH resulting from proton leakage may influence the synthesis of specific proteins involved in the ATR, it is more likely that the weak acid-induced adaptation is due to a general lack of biochemical or physiological fitness.
ACKNOWLEDGMENTS
This research was supported by operating grants to I.R.H. (MT-3546) and D.G.C. (MT-15431) from the Medical Research Council of Canada, by a grant to A.S.B. (DE-08007) from NIDCR, and by a Public Health Service grant (R01 GM51623) to F.C.N. from the National Institute for General Medical Sciences.
We acknowledge the excellent technical assistance of Elke Greif and thank Paul Hazelton (University of Manitoba) for the electron micrographs and Robert Chernecky (University of Toronto) for the scanning electron micrographs.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald A R, Baddiley J, Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim Biophys Acta. 1973;291:629–634. doi: 10.1016/0005-2736(73)90468-9. [DOI] [PubMed] [Google Scholar]
- 3.Bender G R, Sutton S V W, Marquis R E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53:331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleiweis A S, Craig R C. A model system for the purification of amphophiles: the preparation of lipoteichoic acid using liposomes. In: Shockman G D, Wicken A J, editors. Chemistry and biological activities of bacterial surface amphophiles. New York, N.Y: Academic Press; 1981. pp. 43–49. [Google Scholar]
- 5.Boyd D, Cvitkovitch D G, Hamilton I R. Sequence and expression of the HPr (ptsH) and enzyme I (ptsI) genes of the phosphoenolpyruvate-dependent phosphotransferase transport system from Streptococcus mutans. Infect Immun. 1994;62:1156–1165. doi: 10.1128/iai.62.4.1156-1165.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brautigan V M, Childs III W C, Neuhaus F. Biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei: d-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981;146:239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brecx M, Theilade J, Attström R. An ultra structural quantitative study of the significance of microbial multiplication during early plaque growth. J Periodontal Res. 1983;18:177–186. doi: 10.1111/j.1600-0765.1983.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson J, Hamilton I R. Differential toxic effects of lactate and acetate on the metabolism of Streptococcus mutans and Streptococcus sanguis. Oral Microbiol Immunol. 1996;11:412–419. doi: 10.1111/j.1399-302x.1996.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciardi J E, Rölla G, Bowen W H, Reilly J A. Adsorption of Streptococcus mutans lipoteichoic acid to hydroxyapatite. Scand J Dent Res. 1977;85:387–391. doi: 10.1111/j.1600-0722.1977.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 10.Clemans D L, Kolenbrander P E, Debabov D V, Zhang Q, Lunsford R D, Sakone H, Whittaker C J, Heaton M P, Neuhaus F C. Insertional inactivation of genes responsible for the d-alanylation of lipoteichoic acid in Streptococcus gordonii DL1 (Challis) affects intrageneric coaggregation. Infect Immun. 1999;67:2464–2474. doi: 10.1128/iai.67.5.2464-2474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dashper S G, Reynolds E C. Lactic acid excretion by Streptococcus mutans. Microbiology. 1996;142:33–39. doi: 10.1099/13500872-142-1-33. [DOI] [PubMed] [Google Scholar]
- 12.Debabov D V, Heaton M P, Zhang Q, Stewart K D, Lambalot R H, Neuhaus F C. The d-alanyl carrier protein in Lactobacillus casei: cloning, sequencing, and expression of dltC. J Bacteriol. 1996;178:3869–3876. doi: 10.1128/jb.178.13.3869-3876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debabov D V, Kiriukhin M Y, Neuhaus F C. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J Bacteriol. 2000;182:2855–2864. doi: 10.1128/jb.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle R J, Chatterjee A N, Streips U N, Young F E. Soluble macromolecular complexes involving bacterial teichoic acid. J Bacteriol. 1975;124:341–347. doi: 10.1128/jb.124.1.341-347.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duwat P, Cochu A, Ehrlich S D, Gruss A. Characterization of Lactococcus lactis UV-sensitive mutants obtained by ISS1 transposition. J Bacteriol. 1997;179:4473–4479. doi: 10.1128/jb.179.14.4473-4479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer W. Physiology of lipoteichoic acids. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 17.Fischer W, Rösel P, Koch H U. Effect of alanine ester substitution and other structural features of lipoteichoic acid on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981;146:467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grassl M, Supp M. d-Alanine. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods in enzymatic analysis. Vol. 8. Weinheim, Germany: Verlag Chemie; 1986. pp. 336–240. [Google Scholar]
- 19.Gutierrez J A, Crowley P J, Cvitkovitch D G, Brady L J, Hamilton I R, Hillman J D, Bleiweis A S. Streptococcus mutans ffh, a gene encoding a homologue of the fifty-four kilodalton subunit of the signal recognition particle, is involved in resistance to acid stress. Microbiology. 1999;145:357–366. doi: 10.1099/13500872-145-2-357. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton I R. Growth, metabolism and acid production by Streptococcus mutans. In: Hamada S, Michalek S U, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunobiology of Streptococcus mutans. Amsterdam, The Netherlands: Elsevier; 1986. pp. 255–262. [Google Scholar]
- 21.Hamilton I R. Effect of changing environment on sugar transport and metabolism by oral bacteria. In: Reizer J, Peterkofsky A, editors. Sugar transport and metabolism by Gram-positive bacteria. Chichester, United Kingdom: Ellis Horwood; 1987. pp. 94–133. [Google Scholar]
- 22.Hamilton I R, Buckley N D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol Immunol. 1991;6:65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton I R, Ellwood D C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978;19:434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton I R, Svensäter G. Acid-regulated proteins induced by Streptococcus mutans and other oral bacteria during acid shock. Oral Microbiol Immunol. 1998;13:292–300. doi: 10.1111/j.1399-302x.1998.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 25.Hardy L, Jacques N A, Forester F, Campbell L K, Knox K W, Wicken A J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981;31:78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazelton P R, Coombs K M. The reovirus mutant tsA279 has temperature-sensitive lesions in the M2 and L2 genes: the M2 gene is associated with decreased viral protein production and blockade in transmembrane transport. Virology. 1995;207:46–58. doi: 10.1006/viro.1995.1050. [DOI] [PubMed] [Google Scholar]
- 27.Heaton M P, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid: cloning, nucleotide sequence, and expression of the Lactobacillus casei gene for the d-alanine-activating enzyme. J Bacteriol. 1992;174:4707–4717. doi: 10.1128/jb.174.14.4707-4717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaton M P, Neuhaus F C. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J Bacteriol. 1994;176:681–690. doi: 10.1128/jb.176.3.681-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins M L, Daneo-Moore L. Surface growth of streptococci in the presence and absence of beta-lactam antibiotics. In: Actor P, Daneo-Moore L, Higgins M L, Salton M R J, Shockman G D, editors. Antibiotic inhibition of bacteria cells surface assembly and function. Washington, D.C.: American Society for Microbiology; 1988. pp. 91–97. [Google Scholar]
- 30.Hogg S D, Whiley R A, De Soet J J. Occurrence of lipoteichoic acid in oral streptococci. Int J Syst Bacteriol. 1997;47:62–66. doi: 10.1099/00207713-47-1-62. [DOI] [PubMed] [Google Scholar]
- 31.Imfeld T, Lutz F. Intraplaque acid formation assessed in vivo in children and young adults. Pediatr Dent. 1980;2:87–93. [Google Scholar]
- 32.Jensen M E, Polansky P J, Schachtele C F. Plaque sampling and telemetry for monitoring acid production on human buccal tooth surfaces. Arch Oral Biol. 1982;27:21–31. doi: 10.1016/0003-9969(82)90172-8. [DOI] [PubMed] [Google Scholar]
- 33.Kingsley G R, Getchell G. Direct ultra micro glucose oxidase method for the determination of glucose in biological fluids. Clin Chem. 1960;6:466–475. [PubMed] [Google Scholar]
- 34.Koch H U, Fischer W, Fielder F. Influence of alanine ester and glycosyl substitution on the lipoteichoic acid carrier activity of lipoteichoic acids. J Biol Chem. 1982;257:9473–9479. [PubMed] [Google Scholar]
- 35.Koch H U, Haas R, Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur J Biochem. 1984;138:357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- 36.Kolenbrander P E, Andersen R N, Moore L V H. Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl Environ Microbiol. 1990;56:3890–3894. doi: 10.1128/aem.56.12.3890-3894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lunsford R D. A Tn4001 delivery system for Streptococcus gordonii (Challis) Plasmid. 1995;33:153–157. doi: 10.1006/plas.1995.1016. [DOI] [PubMed] [Google Scholar]
- 38.Maguin E, Prévost H, Ehrlich S D, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moro I, Toda Y, Koga T, Hamada S. Morphological aspects of Streptococcus mutans. In: Hamada S, Michalek S M, Kiyono H, Menaker L, McGhee J R, editors. Molecular microbiology and immunology of Streptococcus mutans. New York, N.Y: Elsevier; 1986. pp. 81–90. [Google Scholar]
- 40.Mytelka D S, Chamberlin M J. Analysis and suppression of DNA polymerase pauses associated with a trinucleotide consensus. Nucleic Acids Res. 1996;24:2774–2781. doi: 10.1093/nar/24.14.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nägele U, Wahlefeld A-W, Ziegenhorn J. Fatty acids and derivatives. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods in enzymatic analysis vol. 8. Weinheim, Germany: Verlag Chemie; 1986. pp. 2–12. [Google Scholar]
- 42.Neuhaus F C, Heaton M P, Debabov D V, Zhang Q. The dlt operon in the biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei. Microb Drug Res. 1996;2:77–84. doi: 10.1089/mdr.1996.2.77. [DOI] [PubMed] [Google Scholar]
- 43.Ntamere A S, Taron D J, Neuhaus F C. Assembly of d-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the d-alanyl ester content of this amphiphile. J Bacteriol. 1987;169:1702–1711. doi: 10.1128/jb.169.4.1702-1711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padan E, Zilberstein D, Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981;650:151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- 45.Perego M, Glaser P, Minutello A, Strauch M A, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J Biol Chem. 1995;270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 46.Perry D, Wondrack L M, Kuramitsu H K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983;41:722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 48.Pollack J H, Neuhaus F. Changes in wall teichoic acid during rod-sphere transition of Bacillus subtilis 168. J Bacteriol. 1994;176:7252–7259. doi: 10.1128/jb.176.23.7252-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spatafora G, Sheets M, June R, Luyimbazi D, Howard K, Hulbert R, Barnard D, El Janne M, Hudson M C. Regulated expression of the Streptococcus mutans dlt genes correlates with intracellular polysaccharide accumulation. J Bacteriol. 1999;181:2363–2372. doi: 10.1128/jb.181.8.2363-2372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensäter G, Larsson U-B, Greif E C G, Cvitkovitch D G, Hamilton I R. Acid tolerance response and survival by oral bacteria. Oral Microbiol Immunol. 1997;12:266–273. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 51.Svensäter G, Sjögreen B, Hamilton I R. Multiple stress responses in Streptococcus mutans and the induction of general and stress-specific proteins. Microbiology. 2000;146:107–117. doi: 10.1099/00221287-146-1-107. [DOI] [PubMed] [Google Scholar]
- 52.Tao L, MacAlister T J, Tanzer J M. Factors influencing cell shape in the mutans group of streptococci. J Bacteriol. 1988;170:3752–3755. doi: 10.1128/jb.170.8.3752-3755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao L, MacAlister T J, Tanzer J M. Transformation efficiency of EMS-induced mutants of Streptococcus mutans of altered cell shape. J Dent Res. 1993;72:1032–1039. doi: 10.1177/00220345930720060701. [DOI] [PubMed] [Google Scholar]
- 54.Umeda A, Yokoyama S, Arizono T, Amako K. Location of peptidoglycan and teichoic acid on the cell wall surface of Staphylococcus aureus as determined by immunoelectron microscopy. J Electron Microsc Tokyo. 1992;41:46–52. [PubMed] [Google Scholar]
- 55.Wecke J, Madela K, Fischer W. The absence of D-alanine from lipoteichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology. 1997;143:2953–2960. doi: 10.1099/00221287-143-9-2953. [DOI] [PubMed] [Google Scholar]
- 56.Wecke J, Perego M, Fischer W. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects autolytic activity. Microb Drug Resist. 1997;2:2953–2960. doi: 10.1089/mdr.1996.2.123. [DOI] [PubMed] [Google Scholar]
- 57.Whittaker C J, Clemens D L, Kolenbrander P E. Insertional inactivation of an intrageneric coaggregation-relevant adhesion locus from Streptococcus gordonii DL1 (Challis) J Bacteriol. 1996;64:4147–4142. doi: 10.1128/iai.64.10.4137-4142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou X, Arthur G. Improved procedures for the determination of lipid phosphorus by malachite green. J Lipid Res. 1992;33:1233–1236. [PubMed] [Google Scholar]