Abstract
Temperate bacteriophage D3, a member of the virus family Siphoviridae, is responsible for serotype conversion in its host, Pseudomonas aeruginosa. The complete sequence of the double-stranded DNA genome has been determined. The 56,426 bp contains 90 putative open reading frames (ORFs) and four genes specifying tRNAs. The latter are specific for methionine (AUG), glycine (GGA), asparagine (AAC), and threonine (ACA). The tRNAs may function in the translation of certain highly expressed proteins from this relatively AT-rich genome. D3 proteins which exhibited a high degree of sequence similarity to previously characterized phage proteins included the portal, major head, tail, and tail tape measure proteins, endolysin, integrase, helicase, and NinG. The layout of genes was reminiscent of lambdoid phages, with the exception of the placement of the endolysin gene, which parenthetically also lacked a cognate holin. The greatest sequence similarity was found in the morphogenesis genes to coliphages HK022 and HK97. Among the ORFs was discovered the gene encoding the fucosamine O-acetylase, which is in part responsible for the serotype conversion events.
Upon infection of sensitive cells, the genomes of temperate bacteriophages have two pathways open to them: development associated with cell lysis and the release of progeny (lytic response), or repression of lytic development usually associated with integration into the host chromosome and maintenance in a quiescent state (lysogenic response). The best studied of the temperate phages is bacteriophage lambda, which infects Escherichia coli strains. This phage is the archetype of a group of phylogenetically related viruses called the lambdoid phages. In many cases temperate phages also alter the phenotype of the lysogenized cells, resulting in the production of toxins or expression of surface components resulting in alterations to the cells' antigenicity. This phenomenon is called lysogenic conversion.
Bacteriophage D3 was obtained from a clinical isolate of Pseudomonas aeruginosa by Holloway et al. (35), who noted subsequently that lysogenization of host cells by phage D3 resulted in a change in the cells' serological properties (34). Kuzio and Kropinski showed that the lipopolysaccharide isolated from the lysogens [PAO(D3)] lacked receptor activity for this phage and that the O-antigenic polysaccharide side chains were chemically altered (41). Specifically, the hydroxyl group at position 4 of the d-fucosamine residues became acetylated, and the bonding between the trisaccharide repeats changed from α1→4 to β1→4. This results in the change of serotype from International Antigenic Typing Scheme O5 to O16/20 (42, 43). Simultaneously, the cells become both immune and resistant to superinfection by D3. Extrapolating from the work with Salmonella phage ɛ15 (6, 45, 46), I hypothesized that three phage gene products might be involved in the conversion: an inhibitor of the α-polymerase, a new β-polymerase, and a fucosamine O-acetylase. Extensive early attempts to clone the conversion genes failed.
Work by Cavenagh and Miller (12) demonstrated that phage D3 integrates at two distinct loci on the P. aeruginosa genome, and subsequent studies showed that it probably utilized the Campbell model of insertion (25). Induction of D3 prophage results in low-frequency-transducing lysates in which genes adjacent to the attB sites are transduced (12). Sharp and colleagues cloned the D3 cos sequences into a broad-host-range plasmid and demonstrated efficient transductional transfer between P. aeruginosa strains with wild-type D3 (60). The latter observation served as the basis for the development of a cosmid cloning system for P. aeruginosa (62).
Electron microscopic studies have shown that D3 is a member of the B1 (isometric head) subgroup of the family Siphoviridae, possessing a head with a diameter of 55 nm and a long flexible tail (7 by 113 nm) possessing six tail fibers with terminal knobs (26). The genome is double-stranded DNA of 56.4 kb (25) with 9-bp 3′-extended cohesive ends (61). The reported base composition is 50 mol% GC (49), which is unusually low relative to that (67 mol% GC) of its host, P. aeruginosa.
We have previously described the cloning and analysis of those regions involved in immunity (21, 22) and capsid morphogenesis (26). The immunity region (repressor gene [c1] and antirepressor [cro], together with the left operator-promoter [OLPL] and right operator-promoter [ORPR] complexes) from D3, is clearly homologous in structure and function to the immunity region of lambdoid phages (21, 22). Furthermore, in the case of both D3 and λ, the repressor mRNAs originating from the promoter for repressor maintenance (PRM) lack the typical prokaryotic ribosome-binding sites (Shine-Dalgarno box), and the first three nucleotides of the message code for formylmethionine. Another point, which supports our contention that D3 is a lambdoid phage, was the observation that the capsid morphogenesis genes (portal-protease-major head protein) are laid out in the same orientation as in coliphage HK97. In addition, the portal and head proteins of these two phages show considerable sequence identity. Last, the D3 capsid protein undergoes proteolytic processing and cross-linking during head morphogenesis in a manner identical to that of the coliphage (26).
While exhibiting considerable similarities, these two phages differ in some significant ways. Their host range is restricted to either E. coli (λ) or P. aeruginosa (D3), making the generation of recombinant phages problematic. Coliphage λ DNA exhibits extensive segmented base composition, while melting temperature (Tm) analysis with D3 DNA indicates that this occurs to only a limited extent in D3 (A. Kropinski, unpublished results). The two phages are heteroimmune; that is, their repressors do not bind to the other's operator sequences. In the case of D3, the mRNA transcript from PRM originates from within OR3, rather than upstream of OR3 as it does in λ. Analysis of 2 kb of D3 sequence data to the left of the OLPL complex failed to demonstrate open reading frames (ORFs) with homology to proteins in lambda (e.g., N) or its relatives. While the DNA of coliphage λ possesses 5′-extended termini, that of phage D3, along with coliphages HK97 and HK022, has 3′-extended cohesive ends. These are 9 bases in the case of D3 (61) and 10 bases in the case of the latter two coliphages (38). In addition, D3, unlike other lambdoid phages which have been sequenced, encodes four tRNAs (63). Last, the sole cellular receptor for coliphage λ is the LamB protein, while the receptor for D3 appears to be lipopolysaccharide (41).
To fully elucidate the relationship between D3 and other phages, particularly of the lambdoid group, I have completed sequencing the genome of D3. Analysis of the phage gene data indicates once gain that viral evolution is a far more complex issue than simple family relationships would explain.
MATERIALS AND METHODS
Cloning and sequencing.
Specific D3 HindIII, SalI, EcoRI, SphI, PstI, XbaI, BclI, and partial Sau3A fragments were cloned into pGEM3Zf(+) and grown in E. coli DH5α (F− φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF) U169] (Gibco/BRL). The cultures were routinely grown in Terrific broth (Difco Laboratories) at 37°C. Plasmid DNA was isolated by the alkaline lysis procedure with treatments with boiled RNase A and extractions with phenol-chloroform-isoamyl alcohol (27:24:1, vol/vol/vol) in the presence of silicone high-vacuum grease (Dow Corning) (52) prior to ethanol precipitation. DNA sequencing was carried out at the Institute for Molecular Biology and Biotechnology (McMaster University, Hamilton, Ontario, Canada) and the Guelph Molecular Supercenter (Guelph, Ontario, Canada), using the dideoxy-chain termination method (58) and Applied Biosystems automated fluorescence sequencers. The complete sequence was obtained through sequencing the termini of cloned fragments and primer walking on the clones. On two occasions, PCR was required to obtain intervening sequence for adjacent contigs.
Sequence assembly and analysis.
The results of automated sequencing were collected, stripped of poor-quality data and vector sequences, and assembled into contigs using Seqman II (DNASTAR Inc.). ORFs were analyzed using ORF Finder at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and WebGeneMark.HMM (http://genemark.biology.gatech.edu/GeneMark/whmm.cgi). In addition, the Find ORF feature of SeqEdit (DNASTAR) was used to manually scan the sequence for potential genes. A compendium of online tools (http://www.queensu.ca/micr/faculty/kropinski/online.html) was used for analysis of the putative genes. Proteins translated at ORF Finder or “translate tool” (http://www.expasy.ch/tools/dna.html) were scanned for homologs using BLASTP (2, 3) at http://www.ncbi.nlm.nih.gov; their masses and isoelectric points were determined online at ProtParam tools (http://www.expasy.ch/tools/protparam.html). Where homologs were identified, the sequences were compared using CLUSTAL W (70) at the EMBL-European Bioinformatics Institute (http://www2.ebi.ac.uk/clustalw/). In addition, Genestream's (Institute de Génétique Humaine) program ALIGN at its website (http://www2.igh.cnrs.fr /bin/align-guess.cgi) was used to compare two sequences. Proteins were also examined using PROSITE (4, 32) for conserved motifs at ExPASy-Swiss Institute of Bioinformatics homepage (http://www.expasy.ch/tools/scnpsit1.html). To predict transmembrane proteins, two online programs were used: TMPred (33) at European Molecular Biology network-Swiss node (http://www.ch.embnet.org/software/TMPRED form.html) and TMHMM (65) at the Center for Biological Sequence Analysis at the Technical University of Denmark (http://www.cbs.dtu.dk/services/TMHMM-1.0/).
For basic analysis of the DNA sequence, including restriction sites and motifs, DNAMAN (Lynnon BioSoft, Vaudreuil, Quebec, Canada) and Omiga (Oxford Molecular Group, Campbell, Calif.) were used. The DNA sequence was scanned for putative tRNA species using tRNAScan (18, 47) at its website (http://www.genetics.wustl.edu/eddy/tRNAscan-SE/) and FAStRNA (19) at http://bioweb. pasteur.fr/seqanal/interfaces/fastrna.html. Potential integration host factor (IHF)-binding sites were assessed using MacTargsearch (27) at SEQSCAN (http://www.bmb.psu.edu/seqscan/seqform1.htm), while potential transcriptional terminators (7, 8) were assessed using the Genetics Computer Group program terminator at Bionavigator (http://www.bionavigator.com).
Nucleotide sequence accession number.
The phage D3 sequence has been deposited with GenBank (accession no. AF165214).
RESULTS AND DISCUSSION
Sequence of D3.
We have previously published reports describing specific aspects of D3 and its sequence: analysis of the cos (61) and immunity regions (21), the existence of tRNA genes (63), and the identification of those proteins involved in DNA packaging and capsid morphogenesis (26). The complete nucleotide sequence of D3 is 56,426 bp, making it the largest Pseudomonas phage genome sequenced to date, the next largest being the 35.5-kb, cytotoxin-converting phage φCTX (53). This value agrees completely with the mass determined by restriction mapping (56.4 kb [25]) but is significantly less than values calculated from the sedimentation coefficient measured by Davison and colleagues (14) or the mass calculated from electron micrographs by Miller and colleagues (49). The work of Davison et al. (14) indicated that D3 DNA was significantly larger than the genome of phage F116, which Caruso and Shapiro have measured at 61.7 kb (10). Furthermore, a size of 65 kb can be calculated from the data based on electron micrographs and zone sedimentation by Miller and coworkers (49). While estimations of mass based on sedimentation would be influenced by the cohesive ends of the phage DNA which display a strong tendency to form hydrogen-bonded circles (62), the reason for the discrepancy based on electron microscopic data is unknown.
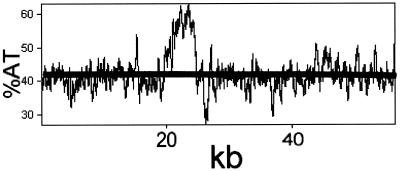
The overall base composition (57.8 mol% GC) is considerably higher than the published value (50 mol% GC [49]) but very similar to the value (58 mol% GC) derived on the basis of Tm analysis for the phage DNA (Kropinski, unpublished). This value is still significantly less than that of the host bacterium (67 mol% GC), which is unusual since the GC contents of temperate phage genomes usually closely match those of their hosts (Kropinski, unpublished). This may result in codon usage problems in P. aeruginosa (see below and reference 63). Unlike coliphage λ DNA, which displays segmented base composition (64), the Tm profile revealed only one region of differing base composition. This was verified by measuring variations in base composition across the genome length (Fig. 1). The only region of higher than expected AT content corresponds to the position of the serotype conversion gene (orf28), suggesting that this may have arisen by lateral gene transfer from another bacterium or phage with a lower GC content.
FIG. 1.
Variation in AT content of D3 DNA presented as a function of genome length. The horizontal line corresponds to the average AT content (42.2 mol%), with the peak corresponding to the region associated with conversion.
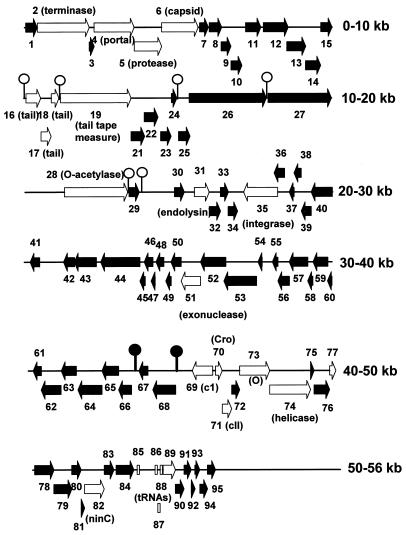
The restriction map published by Miller and Kokjohn (48) suggested multiple BamHI sites, which I have been unable to verify. The map of Gertman et al. (25) also contains several errors of fragment order. The genetic map (Fig. 2) is presented in the same orientation as that of coliphage λ, with the repressor gene located to the right rather than the left, as published previously (25). The previous HindIII map (25) indicated that the fragments were arrayed C-A-G-I-H-D-E-B-F; this has been corrected to C-G-I-A-H-D-E-B-F.
FIG. 2.
Arrangement of ORFs in D3 DNA, indicating those which have significant sequence similarity or could otherwise be identified (open arrows) and those of unknown function (filled arrows). Potential Rho-independent terminators in the top and complementary strands are presented as open and filled lollipops, respectively.
DNA motifs and their implication.
A number of DNA motifs have been found in D3 DNA, including long direct repeats, Rho-independent terminators, and putative CII- and IHF-binding sites. Between nucleotides 29768 and 41830, seven direct repeats of 18, 23, 27, 28, 35, 210 and 247 bp were discovered. The significance of these repeats is unknown.
Lambda protein CII is an activator, stimulating transcription by binding to the opposite face of the DNA from that which the RNA polymerase binds in three promoters: PRE (promoter for repressor establishment), PI (integrase promoter), and PaQ (anti-Q promoter). The P22 homolog, C1 protein, recognizes TTGCN6TTGY (30), while the lambda protein recognizes TTGCN6TTGC (31). A search for TTGCN6TTGY identified five sites in D3 DNA, two of which may play a role in development. A λ consensus CII-binding site (TTGCN6TTGC), probably representing PRE, was found within the cII gene (orf71). A site upstream of integrase (int) gene may represent a region homologous to the phage P22 promoter Pal, which is a C1-activated anti-integrase promoter (30). Farinha and colleagues (21) identified a number of promoters, including PL, PR, and PRM in D3 DNA through cloning into a chloramphenicol acetyltransferase reporter gene vector. They also reported that two strong promoters existed downstream of the immunity region which were not affected by repression in lysogens. One of these, contained within the SphI R fragment, lies between bases 53879 and 54693, that is, between the tRNA genes and orf90, and may represent the late promoter for this phage. The fact that it is constitutive suggests that we have stripped it away from its normal regulatory circuits. While a number of potential ς54 promoters lie in this region, the exact location of the promoter and its function remain unresolved. This also applies to the promoter found in the SphI B fragment (21).
In bacteria and their phages, termination of transcription occurs in a factor (Rho)-dependent (54) or -independent (13, 57) manner. The intrinsic mode of transcription termination is often characterized by a GC-rich sequence that could form a stem-loop structure immediately followed by a run of thymidine residues. Using the search algorithm of Brendel and colleagues (7, 8) I was able to identify many potential Rho-independent terminators. Those that fell between genes are listed in Table 1 and illustrated in Fig. 2. Interestingly, all of the putative terminators lie to the left of the repressor (c1) gene. Since one might expect that the morphogenesis genes would be expressed from the late promoter, the presence of sites following orf15 and orf18 suggests that these may function to modulate expression of downstream genes. The existence of factor-independent termination motifs flanking orf29, followed by a region apparently devoid of ORFs, suggests that the endolysin gene (orf30) may have its own promoter.
TABLE 1.
Putative Rho-independent terminators found in the D3 DNA sequence
| Sequencea | Location |
|---|---|
| Direct | |
| CCCGCCTTGAGCGGGTTTTTTTGT | 10019–10039 |
| GCCCGGATCATTCCGGGCTTTTTCATT | 11255–11274 |
| CCCGCTTCGGCGGGTTTTTTATT | 15432–15451 |
| CCCGCCATAGAGCGGGCTTTCTTAT | 18597–18618 |
| GCCCATCTAAATGTGGGCTTTATTTTAT | 23373–23397 |
| CGCCGGCCCTCGAGCCGGCGTTTTTGTT | 24707–24728 |
| Complement | |
| GCCCGCCTAGCGCGGGCTTTTTCAT | 45047–45065 |
| GCCCGGCAAGTCCGGGCATTTTTT | 43446–43466 |
The region which might assume a stem-loop structure is bold-faced.
In many lambdoid phages, the switch from immediate-early to delayed-early gene expression is regulated through antitermination by a small (98- to 127-amino-acid) basic (pI 8.1 to 11.7) protein which in lambda is called N (24). In addition, antitermination is dependent on the presence of several host proteins, including RNA polymerase, NusA, -B, -E, and -G, Rho, and ribosomal protein S10, and cis-acting sites on the RNA. The Nut (N-utilization) region is comprised of two nucleotide motifs, boxA, which is conserved in sequence (CGCTCTTTAACA), and a downstream sequence (boxB) which is not (23). The latter is capable of forming a small stem-loop structure and is associated with RNA polymerase-NusA-NusG-S10 binding, while NusB and N bind to the former. We have detected a boxA site between genes 68 and 69, suggesting that transcriptional antitermination catalyzed by an N-type protein may exist in phage D3 early transcription (51) (Fig. 3). All attempts to identify N-like proteins using BLASTP or sequence examination for conserved motifs such as those found in P22, ES18, L (ICNIIDSIF), H-19B, HK97, or 933W (MTRRTQFKNSR) failed to identify an N homolog in D3. The possible exception is the product of orf67, which has a short motif (KARR) in common with φ21 gpN. A hyphenated stem-loop structure (GAGCCAACAGGCGC) is found 9 bp downstream of boxA and may function as the boxB analog in this phage.
FIG. 3.
DNA sequence of the putative immediate-early leftward transcription region showing locations of the terminator downstream of c1, operators (OL1 and OL2), and NutL (boxA sequence), each in boldface and underlined. Asterisks mark PL, which overlaps the operators; the position of orf68 is also indicated.
Description of selected ORFs.
ORFs were designated very carefully, and as a result five regions apparently lack ORFs. These are between orf27 and -28, orf41 and 42, orf68 and 69, and the first and second tRNA genes and from orf95 to the end of the sequence. Otherwise, the ORFs were fairly densely packed, with many incidences of overlapping gene sequences. A total of 94 potential genes were discovered, of which 60 (63%) encoded polypeptides that showed no homology to proteins in the GenBank databases using the BLASTP search algorithm (Fig. 2; Table 2). The properties of some of those which showed sequence similarity will be discussed below, with special emphasis on the 17 ORFs that encode proteins that are significantly similar to proteins of characterized lambdoid phages.
TABLE 2.
Data on genes found in D3 DNA
| Gene | Position
|
Initiation codon | Size of protein (kDa) | pI | Sequence similarity | |
|---|---|---|---|---|---|---|
| Left end | Right end | |||||
| 1 | 37 | 417 | ATG | 14.1 | 8.7 | |
| 2 | 419 | 2110 | ATG | 63.3 | 5.2 | D3 terminase large subunitE. coli hypothetical 50.9-kDa protein; 3e-77; P75978 (YMFN_ECOLI) Phage φ105 ORF22; 8e-43; BAA36628 Phage A2 ORF5; 4e-35; CAA66178 Phage HK97 terminase large subunit; e-33; AAF31098 Phage HK022 terminase large subunit; e-33; AAF30354 Phage φPVL ORF2; 2e-28; BAA31875 |
| 3 | 2107 | 2271 | ATG | 5.5 | 8.3 | |
| 4 | 2264 | 3568 | GTG | 47.3 | 8.8 | D3 portal protein Phage HK022 portal protein; 6e-57; AAF30355 Phage HK97 gp3 portal protein; 3e-56; P49859 (VP3_BPHK7) Phage φC21 gp34; 7e-35; CAA07104 |
| 5 | 3572 | 4462 | ATG | 31.9 | 4.7 | D3 capsid protease Phage φadh hypothetical protein; 3e-23; CAB52520 Phage φ7201 ORF25; 5e-18; AAF43518 Phage DT1 scaffolding protein; 6e-18; AAD21883 Phage Sfi21 orf-221 Clp protease; e-15; AF115103 |
| 6 | 4459 | 5646 | ATG | 42.9 | 4.8 | D3 major capsid protein Phage HK97 major capsid protein precursor GP5; 2e-69; P49861 (COAT_BPHK7) |
| 7 | 5691 | 5978 | ATG | 10.2 | 9.7 | |
| 8 | 6005 | 6409 | ATG | 15.0 | 8.5 | |
| 9 | 6390 | 6710 | ATG | 11.6 | 4.2 | |
| 10 | 6711 | 7067 | ATG | 13.7 | 5.6 | Phage HK022 gp9; 6e-11; AAF30361 |
| 11 | 7184 | 7684 | ATG | 18.9 | 8.8 | Phage HK97 gp73; 6e-24; AAF31121 |
| 12 | 7763 | 8575 | ATG | 30.2 | 9.0 | |
| 13 | 8586 | 9143 | ATG | 21.2 | 5.6 | |
| 14 | 9136 | 9630 | ATG | 17.8 | 9.9 | Phage HK022 gp10; 9e-21; AAF30362 |
| 15 | 9633 | 9998 | ATG | 13.3 | 4.9 | Phage HK022 gp11; 7e-31; AAF30363 |
| 16 | 10074 | 10595 | ATG | 18.7 | 4.3 | D3 major tail protein Pseudomonas aeruginosa prophage 28 (similar to V gene of lambda and gene13 of N15) major tail protein; 1e-55; BAA83172 Phage HK022 gp12 (major tail protein); 8e-53; AAF30364 |
| 17 | 10605 | 10964 | ATG | 12.3 | 4.6 | D3 tail protein Phage HK022 gp13 (lambda gpG analog); 2e-47; AAF30365 |
| 18 | 10970 | 11242 | GTG | 10.3 | 8.0 | D3 tail protein Phage HK022 gp14 (lambda gpT analog); 1e-10; AAF30366 |
| 19 | 11292 | 13796 | ATG | 87.8 | 4.9 | D3 tail tape measure protein Phage HK022 gp15 (tail length tape measure protein); e-82; AAF30367 Phage HK97 (tail length tape measure protein) gp16; 2e-25; AAF31092 |
| 21 | 13793 | 14272 | ATG | 18.4 | 5.4 | |
| 22 | 14256 | 14747 | ATG | 18.1 | 4.8 | Phage MB78 hypothetical protein; 4e-04; CAB36891 |
| 23 | 14837 | 15208 | ATG | 13.2 | 4.5 | |
| 24 | 15219 | 15413 | ATG | 7.4 | 8.2 | |
| 25 | 15468 | 15875 | ATG | 14.9 | 8.4 | |
| 26 | 15847 | 18576 | GTG | 99.5 | 5.0 | |
| 27 | 18636 | 20879 | ATG | 80.3 | 5.3 | |
| 28 | 21282 | 23345 | ATG | 75.5 | 9.1 | D3 acetylase Salmonella serotype Typhimurium O-antigen acetylase; 2e-53; U65941 |
| 29 | 23383 | 23715 | ATG | 12.5 | 8.8 | |
| 30 | 24857 | 25180 | GTG | 11.3 | 4.5 | |
| 31 | 25505 | 25987 | ATG | 17.3 | 9.0 | D3 endolysin Phage HK97 lysin; 2e-41; AAF31145 Phage HK022 lysin; 2e-41; AAF31145 Phage lambda endolysin (gpR); 5e-41; P03706 (LYCV_LAMBD) Phage 186 P protein; 3e-32; AAC34155 Phage P2 lysozyme; 3e-32; P51771 (LYCV_BPP2) |
| 32 | 25984 | 26352 | GTG | 12.6 | 6.9 | P. aeruginosa prophage hypothetical protein; 1e-16; BAA83169 |
| 33 | 26349 | 26609 | ATG | 9.4 | 9.9 | P. aeruginosa prophage hypothetical protein; 2e-14; BAA83170 |
| 34 | 26600 | 26908 | ATG | 11.4 | 6.3 | |
| 35 | 27104 | 28213C | ATG | 41.4 | 9.8 | D3 integrase Phage Sfx integrase; 8e-33; AAD10295E. coli prophage DLP12 integrase; 4e-31; P24218 (INTD_ECOLI) Phage V integrase; 1e-30; AAB72135 Phage P22 integrase; 2e-30; CAA27685 Phage APSE-1 integrase; e-29; AAF03981 Phage SfII integrase; 2e-19; AAC39270 |
| 36 | 28099 | 28440C | ATG | 12.5 | 8.9 | |
| 37 | 28588 | 28749C | ATG | 6.1 | 3.8 | |
| 38 | 28746 | 28979C | ATG | 8.5 | 6.1 | |
| 39 | 28979 | 29305C | ATG | 11.8 | 6.6 | |
| 40 | 29302 | 29991C | ATG | 25.8 | 4.9 | |
| 41 | 30192 | 30503C | ATG | 11.7 | 4.6 | |
| 42 | 31282 | 31641C | ATG | 13.4 | 5.1 | |
| 43 | 31634 | 32356C | ATG | 26.5 | 4.5 | |
| 44 | 32492 | 33772C | GTG | 47.7 | 5.4 | |
| 45 | 33769 | 33942C | GTG | 6.3 | 9.7 | |
| 46 | 33911 | 34195C | GTG | 10.4 | 5.4 | |
| 47 | 34135 | 34266C | ATG | 5.0 | 9.8 | |
| 48 | 34294 | 34551C | ATG | 10.2 | 11.8 | |
| 49 | 34611 | 34787C | GTG | 6.7 | 8.7 | |
| 50 | 34780 | 35115C | ATG | 12.7 | 9.5 | P. aeruginosa regulatory protein AlgR; 4e-10; P26275 (ALGR_PSEAE) P. putida PprA; 9e-10; CAA56559 |
| 51 | 35112 | 35738C | ATG | 23.3 | 5.0 | D3 exonuclease Phage VT-Sa exonuclease; 5e-06; BAA84296 Phage lambda exonuclease; 8e-06; P03697 (EXO_LAMBD) Phage 933W exonuclease; 8e-06; AAD25418 |
| 52 | 35742 | 36569C | ATG | 29.9 | 6.7 | |
| 53 | 36503 | 37567C | ATG | 40.2 | 5.4 | |
| 54 | 37615 | 37752C | ATG | 4.8 | 10.3 | |
| 55 | 38086 | 38268C | ATG | 6.6 | 10.2 | |
| 56 | 38265 | 38639C | ATG | 14.1 | 5.1 | |
| 57 | 38636 | 39244C | ATG | 22.8 | 5.1 | |
| 58 | 39237 | 39407C | ATG | 6.5 | 9.1 | |
| 59 | 39410 | 39895C | ATG | 18.1 | 5.6 | |
| 60 | 39880 | 40026C | GTG | 5.5 | 4.5 | |
| 61 | 40161 | 40430C | ATG | 10.0 | 5.7 | |
| 62 | 40423 | 41079C | ATG | 25.0 | 5.5 | |
| 63 | 41083 | 41580C | GTG | 19.1 | 8.6 | |
| 64 | 41630 | 42418C | ATG | 29.5 | 4.5 | |
| 65 | 42415 | 42963C | ATG | 20.6 | 4.6 | |
| 66 | 42960 | 43385C | ATG | 15.6 | 4.5 | |
| 67 | 43628 | 43921C | ATG | 11.1 | 8.8 | |
| 68 | 44080 | 44829C | ATG | 27.4 | 7.7 | |
| 69 | 45369 | 46040C | ATG | 24.6 | 5.6 | D3 C1 repressor Phage D3112 repressor; e-14; S13498 |
| 70 | 46128 | 46349 | ATG | 7.9 | 7.9 | D3 Cro |
| 71 | 46351 | 46659 | ATG | 11.4 | 5.7 | D3 CII Phage lambda CII protein; e-04; P03042 (RPC2_LAMBD) Phage 434 CII protein; 2e-04; P03043 (RPC2_BP434) |
| 72 | 46659 | 46922 | ATG | 9.6 | 10.2 | |
| 73 | 46919 | 47908 | ATG | 37.0 | 8.9 | D3 DNA replication protein O Phage HK97 gp54; e-06; AAF31132 Phage lambda replication protein O; 3e-05; AAA96584 |
| 74 | 47905 | 49245 | ATG | 48.9 | 5.4 | D3 DNA helicaseBacillus subtilis helicase; e-75; P37469 Phage P1 Ban protein; e-69; CAA09719 |
| 75 | 49245 | 49364 | ATG | 4.6 | 7.9 | |
| 76 | 49361 | 49870 | GTG | 19.0 | 7.9 | |
| 77 | 49872 | 50075 | ATG | 7.8 | 4.5 | Phage φCTX ORF39; 6e-09; AB008550 |
| 78 | 50065 | 50697 | ATG | 24.1 | 9.7 | Phage APSE-1 P46; 4e-23; AAF03989 Phage φadh ORF188; 3e-16; CAB52497 |
| 79 | 50690 | 51277 | ATG | 22.1 | 6.6 | |
| 80 | 51274 | 51582 | ATG | 11.5 | 5.3 | |
| 81 | 51579 | 51692 | ATG | 4.1 | 5.7 | |
| 82 | 51689 | 52330 | ATG | 23.9 | 9.9 | D3 NinG Phage P22 NinG; 2e-21; CAA55163 Phage lambda ORF204; e-20; P03770 (Y204_LAMBD) Phage 21 NinG; 2e-20; CAB39991 Phage 933W ORF15; 2e-15; CAB39297 Phage H-19B ORF204; 4e-15; AAD04653 |
| 83 | 52330 | 52647 | ATG | 11.8 | 9.5 | |
| 84 | 52715 | 53293 | ATG | 21.5 | 9.5 | |
| 85 | 53398 | 53473 | Met-tRNA (CAU) | |||
| 86 | 53980 | 54055 | Gly-tRNA (UCC) | |||
| 87 | 54068 | 54142 | Asn-tRNA (GUU) | |||
| 88 | 54147 | 54222 | Thr-tRNA (UGU) | |||
| 89 | 54241 | 54645 | ATG | 14.0 | 6.5 | |
| 90 | 54638 | 54922 | ATG | 10.5 | 10.0 | Phage φCTX ORF10; 4e-6; AB008550 |
| 91 | 54922 | 55155 | ATG | 8.3 | 5.1 | |
| 92 | 55155 | 55280 | ATG | 4.6 | 10.0 | |
| 93 | 55273 | 55434 | ATG | 5.9 | 3.8 | |
| 94 | 55434 | 55685 | ATG | 9.4 | 9.7 | |
| 95 | 55682 | 55942 | ATG | 9.2 | 6.7 | |
Emphasis is given to phage homologs. The similar protein is followed by the BLAST E value and the GenBank accession number (and in some cases sequence designation) of that protein. Proteins clearly identifiable by experimental or BLAST analysis are in boldface.
Morphogenesis genes.
We have previous shown that the pathway for capsid morphogenesis closely resembles that of coliphage HK97, with the prohead undergoing a number of transformations including proteolysis and cross-linking (26). Recent data from Juhala et al. on the completed sequence of lambdoid coliphages HK022 (GenBank accession no. AF069308) and HK97 (AF069529) proved most useful in the determination of which of the D3 gene products corresponded to other structural proteins (38). The sequence of ORF16 was 60% identical to that of HK022 gp12, which is the major tail protein. Interestingly, it is also shares 62% identity with a P. aeruginosa prophage protein. The product of D3 orf17 is a protein of 119 amino acids that shares 56% identity with the 161-amino-acid-containing tail protein (gp13) of phage HK022. In addition, the products of D3 orf10, -14, and -15 show 41, 42, and 58% identity with HK022 gp9, 10, and 11, respectively. Gene 19 specifies a 88-kDa protein with sequence similarity (32% identity) to tail tape measure protein of coliphage HK022.
Conversion.
The 687-amino-acid product of orf28 shows homology to a variety of proteins which have been identified as acetylases. These include the product of the Salmonella enterica serovar Typhimurium oaf gene and the WbpC protein of P. aeruginosa (GenBank accession no. 1545849; E [expect] value of 4 × 10−41). In both cases, the overall sequence identity is 26%. Other hypothetical or inferred acetylases which show sequence relatedness include those from Rhizobium (1531614), Mycobacterium (CAA17305), Bacillus, and Caenorhabitis (2291126), with E values of 4 × 10−21 to 5 × 10−36. This protein gave no hits with PROSITE but did so with pfam, indicating motifs found in a number of transport proteins including the xanthine/uracil permease family. Using TMPred and THMMH to evaluate this protein, 11 strong transmembrane regions are predicted with the amino terminus in the cytoplasm and the carboxy terminus in the periplasm. The original hypothesis had suggested that three proteins would be involved in the conversion event: an O-acetylase, a β-polymerase, and an inhibitor of the host α-polymerase (41). While the sequence evidence is strong for the first protein, neither of the others was identified in this study. In view of the fact that adjacent to orf28 are regions apparently deficient in ORFs, it is possible that the other conversion genes map in these regions but are sufficiently dissimilar from the normal D3 ORFs to have been ignored. It is interesting that this protein has such a high molecular weight, while those acetyltransferases which modify antibiotics are significantly smaller (39). The multiple transmembrane domain of this protein may facilitate acetyl coenzyme A transport to periplasms or organization of a macromolecular membrane complex involved in O-antigen biosynthesis.
Lysis.
The product of orf31 is a polypeptide of 160 amino acids with strong homology to certain bacteriophage lyzozymes. The degrees of sequence identity to the λ, P2, 186, HK022, and HK97 endolysins are 54.5, 48.8, 48.2, 55.1, and 55.1%, respectively. Lambda endolysin, which has a high affinity for GlcNAc polymers (16), functions as a transglucosylase to cleave glycosidic bonds between the C-1 of MurNAc and C-4 of GlcNAc residues in cell wall peptidoglycan. Its active site has been defined by site-directed mutagenesis (37) as Glu19, which lies, with its side chain exposed, in a cleft between the two domains of the protein (20). In the case of the homologous protein in D3, which has a slightly longer amino-terminal region, this corresponds to Glu25. D3, HK97, HK022, and λ endolysins have two oligopeptides in common at the amino (AFLDMLAWSEGT) and carboxyl (CSNIWASLPGAGYGQ) termini of the proteins.
With the possible exception of coliphage T4 and Staphylococcus aureus phage 187, in all phages studied the endolysin gene is preceded or overlapped by a gene encoding a holin. This protein creates pores in the inner or cytoplasmic membrane permitting the endolysin to access the peptidoglycan layer in the periplasm, resulting in cell lysis and release of progeny viruses. In the case of T4, the holin gene is unlinked to the endolysin (E. Cutter, personal communication), while in phage 187 the holin gene is completely embedded within the endolysin gene (44). These proteins are characterized by their relatively small size (71 to 161 amino acid residues), the existence of two to three membrane-spanning helices, and poor sequence identity to other members of this group of functionally similar proteins (28, 72, 73). Furthermore, they sometimes possess a dual-translation start regulatory motif; for example, the holins of coliphages λ, HK97, and HK022 begin MetLysMet, whereas that of the phage 187 holin starts with MetLeuMet. With the possible exception of the O-acetylase, no D3 protein displaying these features was identified in the D3 sequence.
Integration.
The sequence of integrase (ORF35) was 27.1% identical to the integrase sequence from filamentous Shigella flexneri phage SfX, which is a member of the virus family Inoviridae. It contained within it two conserved motifs, HDLRHT and RYAH. This gene and my analysis of the integration of the phage will be the subject of a future communication.
Immunity region.
Our first published data on D3 (21) suggested that the immunity region was arranged very similarly to that of coliphage λ. Furthermore, while sequence similarity between λ CI and D3 C1 proteins was poor, residues which were shown be to essential for λ repressor function were strongly conserved in the D3 repressor. The ORPR complex contained three operators and two promoters (PR and PRM) as it did in phage λ. One fundamental difference exists between the spatial arrangement of genes in the classical lambdoid phages and in Pseudomonas phage D3, that is, the lack of intervening nucleotide sequence between cro and cII. In λ this region contains a Rho-independent transcriptional terminator (tR1) in addition to sites involved in antitermination.
The region to the left of the c1 gene is illustrated in Fig. 3. This region contains two putative Rho-independent terminators (tL1 and tc1), the Nut site, and two genes (orf67 and orf68). Overlapped orf67 is another potential genes (orf67A) which is not listed in Table 1 but contains a sequence (THW-P-PEPPQ) which is also found in two hypothetical proteins, the products of genes L0065 and orf4 from coliphages 933W (GenBank accession no. AAD25410) (57) and VT2-Sa (BA84287) (50), respectively. This protein would have a mass of 6.9 kDa and a pI of 5.8. Furthermore, the alleged PL overlaps the OL1 and OL2 and has the sequence TTGACAACGAATATGAGCAATCTCATACT. The bases in bold match the consensus ς70 promoter sequence in the −35 box completely and at the −10 site by four out of six bases.
DNA replication.
We have shown that replication of D3 DNA involves a switch, as it does in λ, from a theta to a sigma mode during lytic development (60, 62). DNA replication in coliphage λ involves assembly of an activated replication complex involving gpO, gpP, and a variety of host proteins, including primase (DnaG) and helicase (DnaB), at the origin of replication oriλ (69). This region also contains DnaA-binding sites (68). While D3 DNA encodes a 37-kDa basic protein with homology to λ gpO, there is no indication of a gpP homolog or DnaA-binding sites (consensus TTWTNCACA [59]). In its stead D3 possesses, as do certain other phages including Salmonella serovar Typhimurium phage P22 (36), a helicase homolog with a mass of 49 kDa. In this case, the greatest sequence similarity is shown to the Bacillus subtilis DnaB helicase.
tRNAs.
Using tRNAscan-SE (47) and FAStRNA (19), Sibbald and Kropinski identified four tRNA genes in the delayed-early region of the bacteriophage D3 genome (63). These are specific for methionine (AUG), glycine (GGA), asparagine (AAC), and threonine (ACA). In D3, Thr- and Gly-tRNAs recognize codons which are rarely used in P. aeruginosa and presumably influence the rate of translation of phage proteins. Two codons, AGA (Arg) and AUA (Ile), are rarely used in E. coli but employed more frequently in coliphage λ. It has been noted that the λ integrase has a higher proportion of the rare arginine codons AGA and AGG and that this influences expression of this gene (74). Taking two pairs of proteins which one would expect to be expressed at different levels, capsid (orf6) and major tail protein (orf16), compared with repressor (orf69) and integrase (orf35), it was noted that certain codons are favored in the highly expressed genes. These include UUC (Phe) and AAC (Asn), while UCA (Ser), AUA (Ile), ACA (Ala), AGG (Arg), and both GGA and GGG (Gly) are selected against in the highly expressed proteins. This presents a conundrum as to why this phage should have tRNA genes for GlyGGA, AsnAAC, and MetATG.
Phage evolution.
The phylogeny of phages has been discussed in two excellent reviews by Campbell (9) and Casjens et al. (11). Relationships have been hypothesized through similarities in morphology, conservation of gene arrangement, ability to recombine, cross-hybridization patterns, and sequence. Hendrix and colleagues have stated that while conserved patterns exist, which indicates familial relationships, the overall picture suggests that considerable intervirus or virus-host recombination has occurred, often between distant bacterial groups (29). Their proposition is that all double-stranded DNA phage genomes are “mosaics with access, by horizontal exchange, to a large common genetic pool but in which access to the gene pool is not uniform for all phages.” The data presented for D3 show clear evidence that this type of evolutionary process may have operated in the evolution of this Pseudomonas phage, making it the first bacteriophage outside the family Enterobacteriaceae that clearly shows phylogenetic relatedness to members of the lambdoid family of coliphages. With minor exceptions, including the placement of the lyzozyme gene, the genomic layout, particularly the morphogenesis and immunity-replication regions, mimics that of lambdoid phages. This is borne out by the sequence data which suggest that D3 is most closely related to HK022 and HK97, both well-recognized members of the lambdoid group. In part, this may be expected since the databases are overrepresented by data from enterobacterial phages and have only a limited selection of genome data from the large Pseudomonas phages. While E. coli and its relatives and the fluorescent pseudomads (55) are both members of the γ subdivision of the phylum Proteobacteria (66), they are only distantly related. Other intriguing data suggest a relationship between D3 and members of the Siphoviridae infecting gram-positive bacteria, particularly those of the lactic acid bacteria of the genera Streptococcus and Lactobacillus. D3 proteins involved in packaging (terminase large subunit) show sequence similarity to analogous proteins of B. subtilis phage φ105 (K. Kobayashi, K. Okamura, T. Inouse, T. Sato, and Y. Kobayashi, unpublished data [GenBank accession no. BAA36628]) and Lactobacillus casei phage A2 (J. E. Suarez, unpublished data [GenBank accession no. LCA251790]), while the capsid maturation protease appears related to ClpP proteases from Lactobacillus gasseri phage φadh (1) and Streptococcus thermophilus phages φ7201 (67), DT1 (71), and Sfi21 (15). Furthermore, in the case of many of the phage genomes for gram-positive bacteria, the endolysin genes are found downstream of those involved in morphogenesis whereas in the classical lambdoid phages these genes are located before those genes involve in morphogenesis. These results suggest a bipartite ancestry to D3 involving recombination between phages of gram-positive bacteria and a protolambdoid phage with the packaging originating among the phage of gram-positive bacteria, while the remainder of the phage genome evolved from lambdoid phages of gram-negative bacteria. This could occur following the superinfection of a common host cell by two different species of phages (or DNA) or through recombination between superinfecting and resident prophage genomes. Those phages that had the ability to infect different species could then pass on the new genomic segments, ultimately resulting in unrelated bacteriophages possessing homologous genes. From an ecological perspective, lactic acid bacteria and pseudomonads have been isolated from the rumen (5), while both Bacillus species and pseudomonads are soil microorganisms, resulting in the potential for genetic exchange through transformation (17, 40).
In all phages examined to date, a considerable percentage of the ORFs do not encode proteins with homologs in the current database. It is imperative that GenBank contain more annotated phage sequence data, representing complete phage genomes, so that useful conclusions can drawn about the nature of these unknown genes and the evolution of phages.
ACKNOWLEDGMENTS
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada.
Thanks are extended to Brad Cooney (Guelph Molecular Supercentre, Guelph, Ontario, Canada) and Brian Allore (MOBIX, The Institute for Molecular Biology and Biotechnology, McMaster University, Hamilton, Ontario, Canada) for the DNA sequencing. To all my students and research associates, particularly Mark Farinha, Robert Sharp, Mali Galakjan, and Mary-Jo Sibbald, many thanks for their contributions to this project, and thanks also to Harrald Bruessow for useful discussions on phage evolution.
REFERENCES
- 1.Altermann E, Klein J R, Henrich B. Primary structure and features of the genome of the Lactobacillus gasseritemperate bacteriophage φadh. Gene. 1999;236:333–346. doi: 10.1016/s0378-1119(99)00236-x. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–4022. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairoch A. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 1992;11:2013–2088. doi: 10.1093/nar/20.suppl.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesheuvel M H, Bijker P G, Urlings H A. Some aspects of the gastrointestinal microflora of veal calves fed different rations: a pilot study. Vet Q. 1991;13:97–104. doi: 10.1080/01652176.1991.9694291. [DOI] [PubMed] [Google Scholar]
- 6.Bray D, Robbins P W. Mechanism of ɛ15 conversion studied with bacteriophage mutants. J Mol Biol. 1967;30:457–475. doi: 10.1016/0022-2836(67)90362-2. [DOI] [PubMed] [Google Scholar]
- 7.Brendel V, Hamm G H, Trifonov E N. Terminators of transcription with RNA polymerase from Escherichia coli: what they look like and how to find them. J Biomol Struct Dyn. 1986;3:705–723. doi: 10.1080/07391102.1986.10508457. [DOI] [PubMed] [Google Scholar]
- 8.Brendel V, Trifonov E N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984;12:4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell A. Comparative molecular biology of lambdoid phages. Annu Rev Microbiol. 1994;48:193–222. doi: 10.1146/annurev.mi.48.100194.001205. [DOI] [PubMed] [Google Scholar]
- 10.Caruso M, Shapiro J A. Interactions of Tn7 and temperate phage F116L of Pseudomonas aeruginosa. Mol Gen Genet. 1999;188:292–298. doi: 10.1007/BF00332690. [DOI] [PubMed] [Google Scholar]
- 11.Casjens S, Hatfull G F, Hendrix R. Evolution of dsDNA tailed bacteriophage genomes. In: Koonin E, editor. Seminars in virology. London, England: Academic Press; 1992. pp. 383–397. [Google Scholar]
- 12.Cavenagh M M, Miller R V. Specialized transduction of Pseudomonas aeruginosaPAO by bacteriophage D3. J Bacteriol. 1986;165:448–452. doi: 10.1128/jb.165.2.448-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das A. Control of transcription termination by RNA-binding proteins. Annu Rev Biochem. 1993;62:893–930. doi: 10.1146/annurev.bi.62.070193.004333. [DOI] [PubMed] [Google Scholar]
- 14.Davison P F, Freifelder D, Holloway B W. Interruptions in the polynucleotide strands in bacteriophage DNA. J Mol Biol. 1964;8:1–10. doi: 10.1016/s0022-2836(64)80142-x. [DOI] [PubMed] [Google Scholar]
- 15.Desiere F, Lucchini S, Brüssow H. Evolution of Streptococcus thermophilusbacteriophage genomes by modular exchanges followed by point mutations and small deletions and insertions. Virology. 1998;241:345–356. doi: 10.1006/viro.1997.8959. [DOI] [PubMed] [Google Scholar]
- 16.Duewel H S, Daub E, Honek J F. Investigations of the interactions of saccharides with the lysozyme from bacteriophage lambda. Biochim Biophys Acta. 1995;1247:149–158. doi: 10.1016/0167-4838(94)00207-w. [DOI] [PubMed] [Google Scholar]
- 17.Duncan S H, Doherty C J, Govan J R, Neogrady S, Galfi P, Stewart C S. Characteristics of sheep-rumen isolates of Pseudomonas aeruginosa inhibitory to the growth of Escherichia coliO157. FEMS Microbiol Lett. 1999;180:305–310. doi: 10.1111/j.1574-6968.1999.tb08810.x. [DOI] [PubMed] [Google Scholar]
- 18.Eddy S R, Durbin R. RNA sequence analysis using covariance models. Nucleic Acids Res. 1994;22:2079–2088. doi: 10.1093/nar/22.11.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Mabrouk N, Lisacek F. Very fast identification of RNA motifs in genomic DNA. Application to tRNA search in the yeast genome. J Mol Biol. 1996;264:46–55. doi: 10.1006/jmbi.1996.0622. [DOI] [PubMed] [Google Scholar]
- 20.Evrard C, Fastrez J, Declercq J P. Crystal structure of the lysozyme from bacteriophage lambda and its relationship with V and C-type lysozymes. J Mol Biol. 1998;276:151–164. doi: 10.1006/jmbi.1997.1499. [DOI] [PubMed] [Google Scholar]
- 21.Farinha M A, Allan B J, Gertman E M, Ronald S L, Kropinski A M. Cloning of the early promoters of Pseudomonas aeruginosabacteriophage D3: sequence of the immunity region of D3. J Bacteriol. 1994;176:4809–4815. doi: 10.1128/jb.176.16.4809-4815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farinha M A, Kropinski A M. Overexpression, purification, and analysis of the c1 repressor protein of Pseudomonas aeruginosabacteriophage D3. Can J Microbiol. 1997;43:220–226. doi: 10.1139/m97-030. [DOI] [PubMed] [Google Scholar]
- 23.Franklin N C. Conservation of genome form but not sequence in the transcription antitermination determinants of bacteriophages lambda, phi 21 and P22. J Mol Biol. 1984;181:75–84. doi: 10.1016/0022-2836(85)90325-0. [DOI] [PubMed] [Google Scholar]
- 24.Franklin N C. “N” transcription antitermination proteins of bacteriophages λ, φ21 and P22. J Mol Biol. 1985;181:85–91. doi: 10.1016/0022-2836(85)90326-2. [DOI] [PubMed] [Google Scholar]
- 25.Gertman E, Berry D, Kropinski A M. Serotype-converting bacteriophage D3 of Pseudomonas aeruginosa: vegetative and prophage restriction maps. Gene. 1987;52:51–57. doi: 10.1016/0378-1119(87)90394-5. [DOI] [PubMed] [Google Scholar]
- 26.Gilakjan Z A, Kropinski A M. Cloning and analysis of the capsid morphogenesis genes of Pseudomonas aeruginosa bacteriophage D3: another example of protein chainmail? J Bacteriol. 1999;181:7221–7227. doi: 10.1128/jb.181.23.7221-7227.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich J A, Schwartz M L, McClure W R. Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF) Nucleic Acids Res. 1990;18:4993–5000. doi: 10.1093/nar/18.17.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundling A, Blasi U, Young R. Biochemical and genetic evidence for three transmembrane domains in the class I holin, lambda S. J Biol Chem. 2000;275:769–776. doi: 10.1074/jbc.275.2.769. [DOI] [PubMed] [Google Scholar]
- 29.Hendrix R, Smith M C, Burns R N, Ford M E, Hatfull G F. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc Natl Acad Sci USA. 1999;96:2192–2197. doi: 10.1073/pnas.96.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho Y S, Pfarr D, Strickler J, Rosenberg M. Characterization of the transcription activator protein C1 of bacteriophage P22. J Biol Chem. 1992;267:14388–14397. [PubMed] [Google Scholar]
- 31.Ho Y S, Rosenberg M. Characterization of a third, cII-dependent, coordinately activated promoter on phage lambda involved in lysogenic development. J Biol Chem. 1985;260:11838–11844. [PubMed] [Google Scholar]
- 32.Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann K, Stoffel W. TMbase—a database of membrane spanning proteins segments. Biol Chem Hoppe-Seyler. 1993;347:166–175. [Google Scholar]
- 34.Holloway B W, Cooper G N. Lysogenic conversion in Pseudomonas aeruginosa. J Bacteriol. 1962;84:1324. doi: 10.1128/jb.84.6.1321-1324.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holloway B W, Egan J B, Monk M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol. 1960;38:321–330. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- 36.IIyina T V, Gorbalenya A E, Koonin E V. Organization and evolution of bacterial and bacteriophage primase-helicase systems. J Mol Evol. 1992;34:351–357. doi: 10.1007/BF00160243. [DOI] [PubMed] [Google Scholar]
- 37.Jespers L, Sonveaux E, Fastrez J. Is the bacteriophage lambda lysozyme an evolutionary link or a hybrid between the C and V-type lysozymes? Homology analysis and detection of the catalytic amino acid residues. J Mol Biol. 1992;228:529–538. doi: 10.1016/0022-2836(92)90840-g. [DOI] [PubMed] [Google Scholar]
- 38.Juhala R J, Ford M E, Duda R L, Youlton A, Hatfull G F, Hendrix R W. Genetic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J Mol Biol. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- 39.Kato T, Hirai K, Okuda K. Identification of a chromosomally encoded kanamycin acetylase in Porphyromonas gingivalis. FEMS Microbiol Lett. 1995;131:301–306. doi: 10.1111/j.1574-6968.1995.tb07791.x. [DOI] [PubMed] [Google Scholar]
- 40.Kozak S, Forsberg C W. Transformation of mercuric chloride and methylmercury by the rumen microflora. Appl Environ Microbiol. 1979;38:626–636. doi: 10.1128/aem.38.4.626-636.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuzio J, Kropinski A M. O-antigen conversion in Pseudomonas aeruginosaPAO1 by bacteriophage D3. J Bacteriol. 1983;155:203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu P V, Matsumoto H, Kusama H, Bergan T. Survey of heat-stable, major somatic antigens of Pseudomonas aeruginosa. Int J Syst Bacteriol. 1983;33:256–264. [Google Scholar]
- 43.Liu P V, Wang S. Three new major somatic antigens of Pseudomonas aeruginosa. J Clin Microbiol. 1990;28:922–925. doi: 10.1128/jcm.28.5.922-925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loessner M J, Gaeng S, Scherer S. Evidence for a holin-like protein gene fully embedded out of frame in the endolysin gene of Staphylococcus aureusbacteriophage 187. J Bacteriol. 1999;181:4452–4460. doi: 10.1128/jb.181.15.4452-4460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Losick R. Isolation of a trypsin-sensitive inhibitor of O-antigen synthesis involved in lysogenic conversion by bacteriophage ɛ15. J Mol Biol. 1969;42:237–246. doi: 10.1016/0022-2836(69)90040-0. [DOI] [PubMed] [Google Scholar]
- 46.Losick R, Robbins P W. Mechanism of ɛ15 conversion studied with a bacterial mutant. J Mol Biol. 1967;30:445–455. doi: 10.1016/0022-2836(67)90361-0. [DOI] [PubMed] [Google Scholar]
- 47.Lowe T M, Eddy S R. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller R V, Kokjohn T A. Cloning and characterization of the c1 repressor of Pseudomonas aeruginosabacteriophage D3: a functional analog of phage lambda cI protein. J Bacteriol. 1987;169:1847–1852. doi: 10.1128/jb.169.5.1847-1852.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller R V, Pemberton J M, Richards K E. F116, D3, and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974;59:566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto H, Nakai W, Yajima N, Fujibayashi A, Higuchi T, Sato K, Matsushiro A. Sequence analysis of Stx2-converting phage VT2-Sa shows a great divergence in early regulation and replication regions. DNA Res. 1999;6:235–240. doi: 10.1093/dnares/6.4.235. [DOI] [PubMed] [Google Scholar]
- 51.Mogridge J, Mah T F, Greenblatt J. Involvement of boxA nucleotides in the formation of a stable ribonucleoprotein complex containing the bacteriophage lambda N protein. J Biol Chem. 1998;273:4143–4148. doi: 10.1074/jbc.273.7.4143. [DOI] [PubMed] [Google Scholar]
- 52.Mukhopadhyay T, Roth J A. Silicone lubricant enhances recovery of nucleic acids after phenol-chloroform extraction. Nucleic Acids Res. 1993;21:781–782. doi: 10.1093/nar/21.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakayama K, Kanaya S, Ohnishi M, Terawaki Y, Hayashi T. The complete nucleotide sequence of φCTX, a cytotoxin-converting phage of Pseudomonas aeruginosa: implications for phage evolution and horizontal gene transfer via bacteriophages. Mol Microbiol. 1999;31:399–419. doi: 10.1046/j.1365-2958.1999.01158.x. [DOI] [PubMed] [Google Scholar]
- 54.Opperman T, Richardson J P. Phylogenetic analysis of sequences from diverse bacteria with homology to the Escherichia coli rhogene. J Bacteriol. 1994;176:5033–5043. doi: 10.1128/jb.176.16.5033-5043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palleroni N J, Kunisawa R, Contopoulou R, Doudoroff M. Nucleic acid homologies in the genus Pseudomonas. Int J Syst Bacteriol. 1973;23:333–339. [Google Scholar]
- 56.Plunkett G 3, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coliO157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynolds R, M. R, Chamberlin M J. Parameters affecting transcription termination by Escherichia coliRNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol. 1992;224:31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- 58.Sanger F, Coulson A R, Hong G F, Hill D F, Peterson G B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982;162:729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- 59.Schaper S, Messer W. Interaction of the initiator protein DnaA of Escherichia coliwith its DNA target. J Biol Chem. 1995;270:17622–17626. doi: 10.1074/jbc.270.29.17622. [DOI] [PubMed] [Google Scholar]
- 60.Sharp R, Gertman E, Farinha M A, Kropinski A M. Transduction of a plasmid containing the bacteriophage D3 cos site in Pseudomonas aeruginosa. J Bacteriol. 1990;172:3509–3511. doi: 10.1128/jb.172.6.3509-3511.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharp R, Jansons I S, Gertman E, Kropinski A M. Genetic and sequence analysis of the cos region of the temperate Pseudomonas aeruginosabacteriophage, D3. Gene. 1996;177:47–53. doi: 10.1016/0378-1119(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 62.Sharp R W. Development of a cosmid cloning system for Pseudomonas aeruginosa. Doctoral dissertation. Kingston, Ontario, Canada: Queen's University; 1991. [Google Scholar]
- 63.Sibbald M J, Kropinski A M. Transfer RNA genes and their significance to codon usage in the Pseudomonas aeruginosalamboid bacteriophage D3. Can J Microbiol. 1999;45:791–796. [PubMed] [Google Scholar]
- 64.Skalka A, Burgi E, Hershey A D. Segmental distribution of nucleotides in the DNA of bacteriophage lambda. J Mol Biol. 1968;34:1–16. doi: 10.1016/0022-2836(68)90230-1. [DOI] [PubMed] [Google Scholar]
- 65.Sonnhammer E L L, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. In: Glasgow J, Littlejohn T, Major F, Lathrop R, Sankoff D, Sensen C, editors. Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology. Menlo Park, Calif: AAAI Press; 1998. pp. 175–182. [PubMed] [Google Scholar]
- 66.Stackbrandt E, Murray R G E, Truper H. Proteobacteria classisnov., a name for the phylogenetic taxon that includes the purple bacteria and their relatives. Int J Syst Bacteriol. 1988;38:321–325. [Google Scholar]
- 67.Stanley E, Walsh L, van der Z A, Fitzgerald G F, van Sinderen D. Identification of four loci isolated from two Streptococcus thermophilusphage genomes responsible for mediating bacteriophage resistance. FEMS Microbiol Lett. 2000;182:271–277. doi: 10.1111/j.1574-6968.2000.tb08907.x. [DOI] [PubMed] [Google Scholar]
- 68.Szalewska-Palasz A, Weigel C, Speck C, Srutkowska S, Konopa G, Lurz R, Marszalek J, Taylor K, Messer W, Wegrzyn G. Interaction of the Escherichia coliDnaA protein with bacteriophage lambda DNA. Mol Gen Genet. 1998;259:679–688. doi: 10.1007/s004380050863. [DOI] [PubMed] [Google Scholar]
- 69.Taylor K, Wegrzyn G. Replication of coliphage lambda DNA. FEMS Microbiol Rev. 1995;17:109–119. doi: 10.1111/j.1574-6976.1995.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 70.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tremblay D M, Moineau S. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology. 1999;255:63–76. doi: 10.1006/viro.1998.9525. [DOI] [PubMed] [Google Scholar]
- 72.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young R, Blasi U. Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev. 1995;17:191–205. doi: 10.1111/j.1574-6976.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 74.Zahn K, Landy A. Modulation of lambda integrase synthesis by rare arginine tRNA. Mol Microbiol. 1996;21:69–76. doi: 10.1046/j.1365-2958.1996.6201335.x. [DOI] [PubMed] [Google Scholar]