Abstract
Background Anticoagulants are high-risk medications and are a common cause of adverse events of hospitalized inpatients. The incidence of adverse events involving anticoagulants has remained relatively unchanged over the past two decades, suggesting that novel approaches are required to address this persistent issue. Electronic medication management systems (eMMSs) offer strategies to help reduce medication incidents and adverse drug events, yet poor system design can introduce new error types.
Objective Our objective was to evaluate the effect of the introduction of an electronic medical record (EMR) on the quality and safety of therapeutic anticoagulation management.
Methods A retrospective, observational pre-/poststudy was conducted, analyzing real-world data across five hospital sites in a single health service. Four metrics were compared 1-year pre- and 1-year post-EMR implementation. They included clinician-reported medication incidents, toxic pathology results, hospital-acquired bleeding complications (HACs), and rate of heparin-induced thrombocytopenia. Further subanalyses of patients experiencing HACs in the post-EMR period identified key opportunities for intervention to maximize safety and quality of anticoagulation within an eMMS.
Results A significant reduction in HACs was observed in the post-EMR implementation period (mean [standard deviation [SD]] =12.1 [4.4]/month vs. mean [SD] = 7.8 [3.5]/month; p = 0.01). The categorization of potential EMR design enhancements found that new automated clinical decision support or improved pathology result integration would be suitable to mitigate future HACs in an eMMS. There was no significant difference in the mean monthly clinician-reported incident rates for anticoagulants or the rate of toxic pathology results in the pre- versus post-EMR implementation period. A 62.5% reduction in the cases of heparin-induced thrombocytopenia was observed in the post-EMR implementation period.
Conclusion The implementation of an EMR improves clinical care outcomes for patients receiving anticoagulation. System design plays a significant role in mitigating the risks associated with anticoagulants and consideration must be given to optimizing eMMSs.
Keywords: anticoagulation, electronic health records, clinical decision support system, medication management, digital platforms, patient care, clinical error types, clinical analytics tools, electronic medication management systems
Background and Significance
Anticoagulant prescribing for hospital inpatients is common. When used appropriately, anticoagulants are effective in the prevention and treatment of a range of thromboembolic disorders. 1 2 3 4 Anticoagulants are deemed high-risk medications as they can cause significant patient harm or death when used inappropriately. 5 6 They are the leading cause of medication-related hospital admissions 7 and are ranked in the top five classes of drugs associated with patient harm, including fatal medication incidents. 8 Anticoagulants are commonly implicated in adverse drug events (ADEs), of which 50 to 70% are potentially preventable. 9 To ensure effectiveness while reducing the risk of ADEs, anticoagulants should be dosed in a narrow therapeutic range which requires regular monitoring. The percentage of clinical incidents related to anticoagulants has remained relatively unchanged in the past two decades (7.2% in 2004, 10 8.3% in 2018, 11 and 6% in 2019). 12 Health care organizations need to develop novel approaches to address this persistent issue.
The digitization of the health care sector continues to advance. 13 Electronic medical records (EMRs) are now implemented within the majority of major hospitals in developed countries. 14 15 EMRs often deploy an electronic medication management system (eMMS) including computerized physician order entry (CPOE) and clinical decision support systems (CDSSs) to enhance patient safety. Additionally, the role of aggregated data and dashboards demonstrating real-time metrics for process and outcome measures of patient care is expanding. 16 17
Limited research exists to date assessing the impact of digitization of inpatient anticoagulation prescribing. 18 Studies focused on CPOE suggest that this may be an effective method to reduce anticoagulant errors or ADEs, with the exception of intravenous unfractionated heparin (UFH), which is notoriously difficult to dose and monitor, and may require additional CDSS methods. 19 20 21 22
Locally, in Queensland, Australia, since the initial implementation of an integrated EMR in 2015, there has been a steady expansion of the single instance EMR to 14 hospitals. We have reported previously on the local strategies employed and lessons learned to manage therapeutic anticoagulation on a digital platform. 23 This study seeks to assess the impact of EMR implementation on the quality and safety of anticoagulation management.
Objectives
The aim of the study was to evaluate the effect of the implementation of an EMR on the quality and safety of anticoagulation management across five hospital sites on a single EMR instance.
The study objectives were to quantify, before and after EMR implementation, the following factors:
Rates, types, and severity of clinician-reported medication errors associated with anticoagulation in hospital inpatients.
Rates and severity of surrogate markers (toxic activated partial thromboplastin time [aPTT], toxic international normalized ratio [INR], and toxic antifactor Xa levels) for poor management of therapeutic anticoagulation (UFH, warfarin, and low molecular weight heparins [LMWHs]).
Rate of hospital-acquired complications (HACs) of bleeding with anticoagulant use.
Rate of hospital-acquired heparin-induced thrombocytopenia (HIT).
Methods
A retrospective, observational pre/poststudy was conducted, analyzing real-world data. 24 25
Study Setting
Study sites consisted of five hospitals across a networked health service which was the first to undergo digital transformation in the region. Site demographics included:
1,033-bed metropolitan quaternary hospital.
479-bed tertiary hospital.
239-bed metropolitan hospital.
199-bed metropolitan hospital.
28-bed rural health care hospital.
Inclusion and Exclusion Criteria
All hospital inpatients on anticoagulation admitted to the study sites 1 year before and 1 year after the implementation of the EMR were eligible. Patients admitted to intensive care units (ICUs) were excluded due to the use of a stand-alone eMMS, unlinked to the EMR. Patients located in the emergency and outpatient departments were excluded as they did not reflect inpatient care. An aPTT < 50 seconds, INR < 1.5, and antifactor Xa <0.4 IU/mL were excluded to minimize inclusion of results for low-dose, prophylactic indications, or normal physiological values.
Outcomes, Data Description, and Collection
The primary outcomes included:
Clinician-reported anticoagulant-related medication incidents.
Toxic pathology results for UFH (aPTT > 110 and >200 seconds), warfarin (INR > 3.5 and >5.5), or LMWH (antifactor Xa > 1.0 IU/mL) as a percentage of total included results.
Hospital-acquired bleeds associated with anticoagulant use.
HIT (a rare but serious immune-mediated adverse drug reaction associated with heparin therapy).
The type, data source, and subanalyses were dependent upon the outcome being measured.
Outcome 1: Clinician-Reported Medication Incidents
RISKMAN is a voluntary self-reporting incident management system used by staff. During the study, a new system was implemented and data could not be accessed from the previously used risk management system. Therefore, the duration of pre- and post-EMR implementation data varied per study site. This resulted in four of the five hospitals being eligible for review over a 4-month pre- and 4-month post-EMR implementation period. Data were reviewed and categorized according to their safety assessment code (SAC) rating (SAC 1 to 4) 26 and type of medication error. 27
Outcome 2: Toxic Pathology Results
Pathology data 12 months before and after implementation of the EMR were reviewed. Toxic aPTT, INR, and antifactor Xa levels were categorized and reported as a percentage of total included results to determine rates of toxicity. An aPTT > 110 seconds, 28 an INR > 3.5 2 , and an antifactor Xa >1.0 IU/mL 29 were defined as a “toxic.” An aPTT > 200 seconds and INR >5.5 were “severely toxic.”
Outcome 3: Hospital Acquired Bleeding Events
HAC 10.2 (hemorrhagic disorder due to circulating anticoagulants during a hospital admission) is coded by the Health Information Management Services (HIMs) using the D-code D68.3. D68.3 data 12-month pre- and post-EMR implementation were collected. The integrity of the HAC data was confirmed via manual chart review for all post-EMR implementation HACs. This enabled subanalyses of the anticoagulant responsible for the bleed, indication for use, concurrent antiplatelet and/or thrombolytic drugs, blood transfusion requirements, bleeding severity scores, and whether the bleed resulted in hospital death. This provided insight into factors which may contribute to the likelihood of a bleeding event and the resultant outcomes. Severity scores were assessed using the Thrombolysis in Myocardial Infarction (TIMI) score 30 which consists of four categories:
Major: experiencing either an intracranial bleed, clinically overt hemorrhage associated with a hemoglobin (Hb) drop ≥5 g/dL, or a fatal bleed resulting in death within 7 days.
Minor: clinically overt bleed resulting in Hb drop 3 to 5 g/dL.
Requiring medical attention: any overt sign of hemorrhage that does not meet the criteria for major/minor bleed above, requiring medical practitioner-guided medical/surgical treatment, prolonged hospitalization, or prompting evaluation (laboratory/imaging).
Minimal: any overt bleeding event that does not meet the criteria above.
All post-EMR HACs were investigated for potential system errors that may have led to the anticoagulant-related bleed. Westbrook et al developed a framework for classifying manifestations and underlying mechanisms of system-related errors in an eMMS. 27 This was adapted to determine the clinical error types responsible for the HACs and whether the EMR was at fault. Adaptation was required given the Westbrook criteria focused solely on prescribing errors. Additional categories included drug combination, inappropriate monitoring, suboptimal workflow, and operational issues (e.g., experiencing an unplanned EMR downtime). While “drug combination” had the potential to fall under the “wrong drug” category, we identified a need to flag this error type given the high volume of drug–drug interaction alerts firing in the eMMS. The classification process was undertaken by one reviewer (J.A.), and then, 20% of charts were independently reviewed and results pooled. Consensus was obtained after deliberation between reviewers (M.B. and C.S.). Potential EMR enhancements to avoid future HACs were also documented.
Outcome 4: Heparin-Induced Thrombocytopenia
Data were obtained from HIMs on patients coded for HITs during hospital admission (D code D69.5—“secondary thrombocytopenia” in conjunction with Y code Y44.2—“ADE associated with anticoagulant use”).
Anticoagulant Usage
To evaluate prescribing trends pre- and post-EMR implementation, a comparison of mean monthly medication use (dispensing and distribution) was analyzed for the five study sites, 1-year pre- and 1-year post-EMR implementation. The quantities of each class of anticoagulant were reviewed ( Table 1 ).
Table 1. Therapeutic anticoagulants included in pharmacy supply comparisons.
| Anticoagulant | Strength/Dosage form |
|---|---|
| Unfractionated heparin | |
| Heparin sodium in sodium chloride | 25,000 units/50-mL prefilled syringe |
| Low molecular weight heparins | |
| Enoxaparin | 60-, 80-, 100-, 120-mg/mL prefilled syringes |
| Dalteparin | 7,500 units/0.75-mL prefilled syringes |
| Direct acting oral anticoagulants | |
| Apixaban | 2.5- and 5-mg tablets |
| Rivaroxaban | 10-, 15-, 20-mg tablets |
| Dabigatran | 110- and 150-mg capsules |
| Vitamin K antagonist | |
| Warfarin | 5, 3, 2, 1-mg tablets |
Data Analysis
Statistical analysis was performed using the software “R.”. All primary outcomes were reported as the number of events/month before and after the implementation of EMR at each site. The mean event rates were compared using a Welch two-sample t -test. It was expected that the large cohorts enabled adequately matched populations.
Results
Outcome 1: Clinician-Reported Medication Incidents
There was no significant difference in the mean monthly incident rates for general anticoagulants pre- and post-EMR implementation: mean (standard deviation [SD]) =15 (6.3)/month versus 17.8 (12.9)/month; t (4.3) 0.38, p = 0.72).
The severity of clinician-reported anticoagulant medication incidents 4-months pre- and 4-months post-EMR implementation is displayed in Fig. 1 . The number of incidents peaked during the 1-month postgo-live period (15 SAC 3 and 22 SAC 4 incidents). No SAC 1 or 2 incidents were reported during the 8-month study period.
Fig. 1.
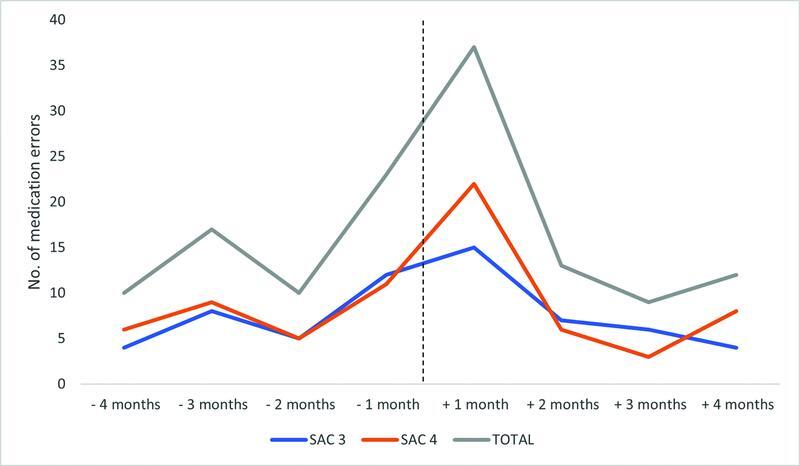
Clinician-reported anticoagulant medication incidents and safety assessment code (SAC) pre- and post-EMR implementation. EMR, electronic medical record.
Fig. 2 summarizes the clinical error type of the reported anticoagulant incidents. Prior to EMR implementation, the most reported error type was an omission ( n = 23/60, 38.3%). Conversely, the most notable error type post-EMR implementation was duplicated orders ( n = 12/71, 16.9%).
Fig. 2.
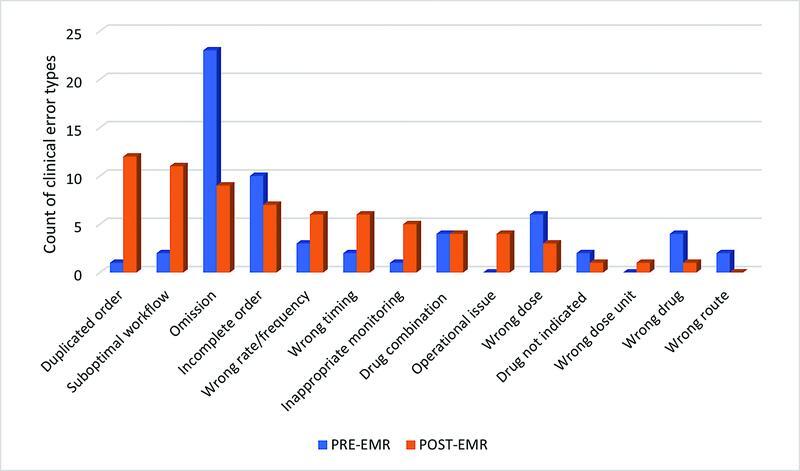
Clinician-reported anticoagulation clinical error types pre- and post-EMR implementation. EMR, electronic medical record.
Table 2 displays anticoagulant clinical error types in relation to their severity during the 12-month pre- versus post-EMR implementation period. In the pre-EMR implementation period, the more severe incidents (SAC 3) were most frequently related to an omission, followed by an incomplete order and wrong dose. The most frequently observed SAC 3 incidents in the post-EMR implementation period related to an omission, and prescribing an incorrect rate or frequency.
Table 2. Severity of clinician-reported anticoagulation clinical error types 12-month pre- and post-EMR implementation.
| Clinical error type | Count of SAC 3 | Count of SAC 4 | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Omission | 10 | 5 | 13 | 4 |
| Wrong rate/frequency | 3 | 5 | 0 | 1 |
| Duplicated order | 0 | 4 | 1 | 8 |
| Inappropriate monitoring | 0 | 4 | 1 | 1 |
| Suboptimal workflow | 0 | 3 | 2 | 8 |
| Incomplete order | 4 | 2 | 6 | 5 |
| Drug combination | 1 | 3 | 3 | 1 |
| Wrong timing | 1 | 2 | 1 | 4 |
| Wrong dose | 4 | 2 | 2 | 1 |
| Operational issue | 0 | 2 | 0 | 2 |
| Drug not indicated | 2 | 0 | 0 | 1 |
| Wrong drug | 2 | 0 | 2 | 2 |
| Wrong route | 2 | 0 | 0 | 0 |
| Wrong dose unit | 0 | 0 | 0 | 1 |
Abbreviation: SAC, safety assessment code.
Outcome 2: Toxic Pathology Results
No statistically significant differences were seen in the percentage of toxic pathology results pre- and postimplementation of the EMR ( Table 3 ).
Table 3. Comparison of percentage of mean monthly toxic pathology results 12-month pre- and post-EMR implementation.
| Toxic pathology result | Pre-EMR (percent of monthly toxic pathology results) | Post-EMR (percent of monthly toxic pathology results) | t -statistic (degrees of freedom), p -value | ||
|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | ||
| aPTT >100 seconds | 21.73 | 1.36 | 21.05 | 2.82 | –0.75(15.86), p = 0.5 |
| aPTT >200 seconds | 5.31 | 0.65 | 5.03 | 1.16 | –0.73(17.36), p = 0.5 |
| INR >3.5 | 8.18 | 1.50 | 7.67 | 1.41 | –0.85(21.92), p = 0.4 |
| INR >5.5 | 0.73 | 0.24 | 0.71 | 0.31 | –0.17(20.75), p = 0.9 |
| Antifactor Xa >1 IU/mL | 30.37 | 15.26 | 24.29 | 12.77 | –1.06(21.34), p = 0.3 |
Abbreviations: aPTT, activated partial thromboplastin time; INR, international normalized ratio; IU, international units.
Outcome 3: Hospital-Acquired Bleeding Events
A statistically significant reduction in HACs was observed from the 12-month pre- to post-EMR implementation: mean (SD) = 12.1 (4.4)/month, versus mean (SD) = 7.8 (3.5)/month; t (21.0) –2.68, p = 0.01).
A total of 93 patients were recorded as experiencing an HAC in the post-EMR-implementation period. During manual chart review, six were excluded due to incorrect coding or the bleed that occurred during an ICU admission and insufficient documentation was available due to the alternative system in use. A total of 87 charts were included in the analysis. Table 4 shows the patient demographics of included participants.
Table 4. Patient demographics for those experiencing a bleed associated with anticoagulant use post-EMR implementation ( n = 87) .
| Characteristic | Count | Percentage | |
|---|---|---|---|
| Age | 20–24 | 1 | 1.1 |
| 25–29 | 1 | 1.1 | |
| 30–34 | 1 | 1.1 | |
| 35–39 | 1 | 1.1 | |
| 40–44 | 1 | 1.1 | |
| 45–49 | 3 | 3.4 | |
| 50–54 | 6 | 6.9 | |
| 55–59 | 7 | 8.0 | |
| 60–64 | 7 | 8.0 | |
| 65–69 | 11 | 12.6 | |
| 70–74 | 7 | 8.0 | |
| 75–79 | 17 | 19.5 | |
| 80–84 | 16 | 18.4 | |
| 85+ | 9 | 10.3 | |
| Sex | Male | 45 | 51.7 |
| Female | 42 | 48.3 | |
| Anticoagulant responsible for bleed | LMWH | 19 | 21.8 |
| IV heparin | 17 | 19.5 | |
| DOAC | 11 | 12.6 | |
| SC heparin | 10 | 11.5 | |
| Warfarin | 7 | 8.0 | |
| Other | 1 | 1.1 | |
| Combination | 22 | 25.3 | |
| Indication for anticoagulant | DVT/PE/embolism | 29 | 33.3 |
| ACS | 16 | 18.4 | |
| VTE prophylaxis | 14 | 16.1 | |
| AF | 13 | 14.9 | |
| Warfarin bridging | 6 | 6.9 | |
| MVR/AVR | 5 | 5.7 | |
| Intraoperative therapy | 4 | 4.6 | |
| Concurrent antiplatelets a | No | 44 | 50.6 |
| Yes | 43 | 49.4 | |
| Concurrent thrombolytic b | No | 84 | 96.6 |
| Yes | 3 | 3.4 | |
| Transfusion required | No | 58 | 66.7 |
| Yes | 29 | 33.3 | |
| TIMI score | Minimal | 7 | 8.0 |
| Requiring medical attention | 44 | 50.6 | |
| Minor | 20 | 23.0 | |
| Major | 16 | 18.4 | |
| Hospital death associated with bleed | No | 84 | 96.6 |
| Yes | 3 | 3.4 | |
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; AVR, aortic valve replacement; DOAC, direct-acting oral anticoagulant; DVT, deep vein thrombosis; IV, intravenous; LMWH, low molecular weight heparin; MVR, mitral valve replacement; PE, pulmonary embolism; SC, subcutaneous; VTE, venous thromboembolism.
Concurrent antiplatelets include aspirin, clopidogrel, dipyridamole, prasugrel, ticagrelor, and combination.
Concurrent thrombolytics include alteplase and tenecteplase.
Drug Factors
Of the patients who experienced a bleed, 22 (25%) were on a combination of anticoagulants. The most common were patients transitioning from a heparin (UFH or LMWH) to warfarin or vice versa (16/22). The most implicated single anticoagulant class were LMWHs ( n = 19, 22%). Fig. 3 demonstrates the number of HACs based upon the type of anticoagulant prescribed. Fig. 4 shows the severity of bleeds according to the TIMI score. 30 A total of 16 (19%) of patients were categorized with a major bleed, most commonly a result of anticoagulant combinations (6/16) followed by UFH infusions (4/16).
Fig. 3.
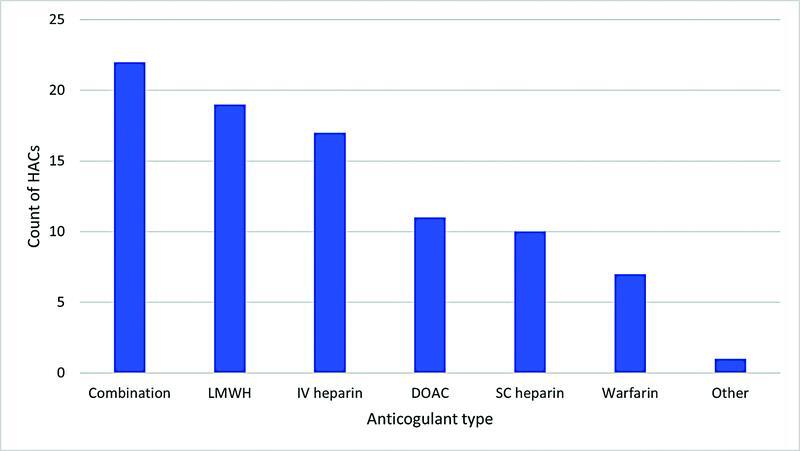
Number of hospital acquired bleeds per anticoagulant type. Abbreviations: DOAC, direct-acting oral anticoagulant; IV, intravenous; LMWH, low molecular weight heparin; SC, subcutaneous.
Fig. 4.
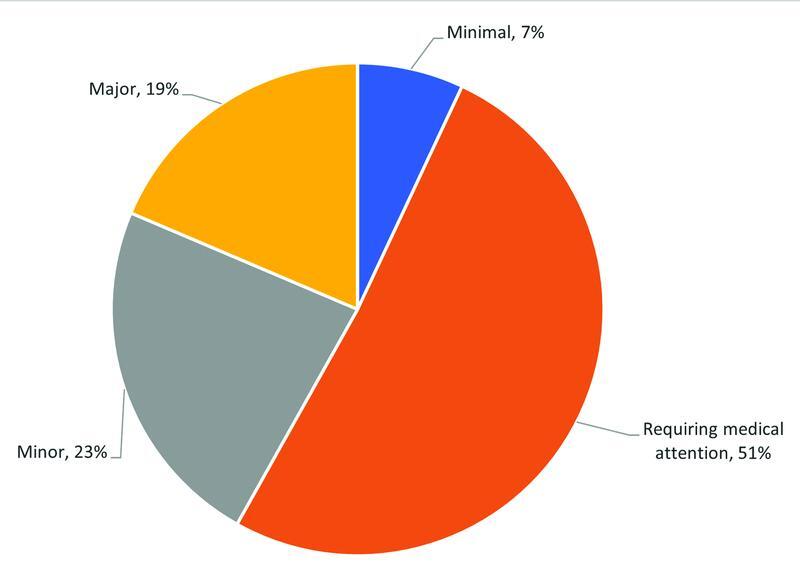
Severity of bleeds (TIMI score)—first year post-EMR implementation ( n = 87). EMR, electronic medical record; TIMI, Thrombolysis in Myocardial Infarction.
System Analysis
Only two of the 87 HACs appeared directly linked to system design. Of these two HACs, both related to selection error during the construction of the order. One related to the incorrect initial UFH infusion rate being ordered. The second scenario related to confusion surrounding duplicate UFH infusion orders being placed (in addition to one order containing the wrong units of 1,500 units/kg/h as opposed to the intended 1,500 units/h. This dose was not administered to the patient).
Using the adapted Westbrook classification system for clinical error types, 27 the chart review was unable to ascertain a reason for error in 33/87 cases. The most cited clinical error type was incorrect drug combination (14/87).
Inappropriate monitoring was the second most cited clinical error type (11/87). Examples include aPTT levels being taken at incorrect times and an inappropriate dose adjustment, lack of antifactor Xa monitoring in obese or renally impaired patients and concurrent direct-acting oral anticoagulant (DOAC) or LMWH prescribed. Fig. 5 shows the count of each clinical error type.
Fig. 5.
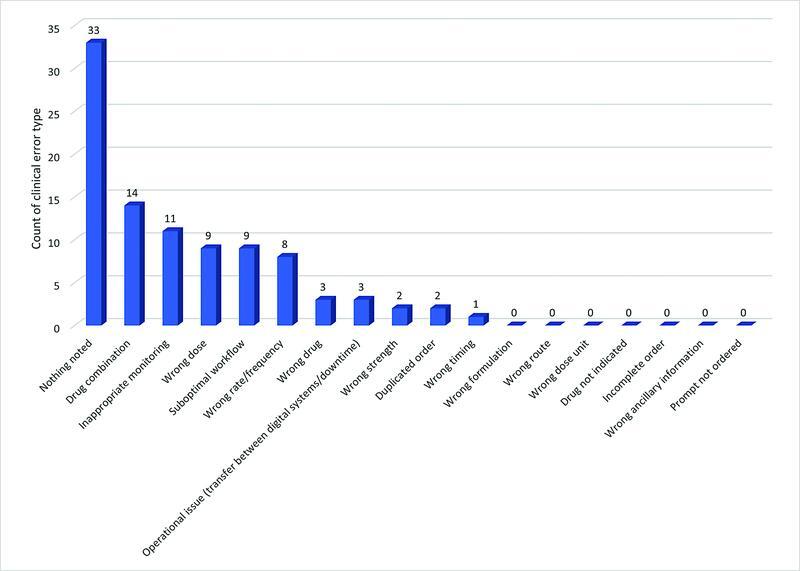
Count of clinical error type for hospital acquired bleeds in the post-EMR implementation period. EMR, electronic medical record.
The chart review included the assessment of potential EMR interventions to prevent the documented HAC from recurring. Fig. 6 provides suggested EMR enhancements that could potentially improve the safety and quality of anticoagulant use. In 39 cases, the system appeared to be functioning appropriately and no obvious interventions were evident.
Fig. 6.
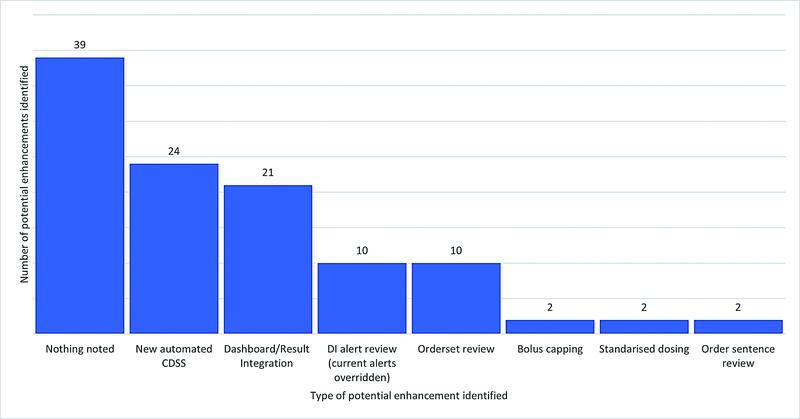
Potential EMR enhancements to improve bleeding complications associated with anticoagulant use. CDSS, clinical decision support system; DI, drug interaction; EMR, electronic medical record.
Outcome 4: Heparin-Induced Thrombocytopenia
There were 16 clinically coded HIT cases in the 12-month pre-EMR implementation versus 6 cases in the 12-month postimplementation period, indicative of a 62.5% reduction in the post-EMR implementation period. Fig. 7 shows the monthly rate of documented cases over the 2-year study period.
Fig. 7.
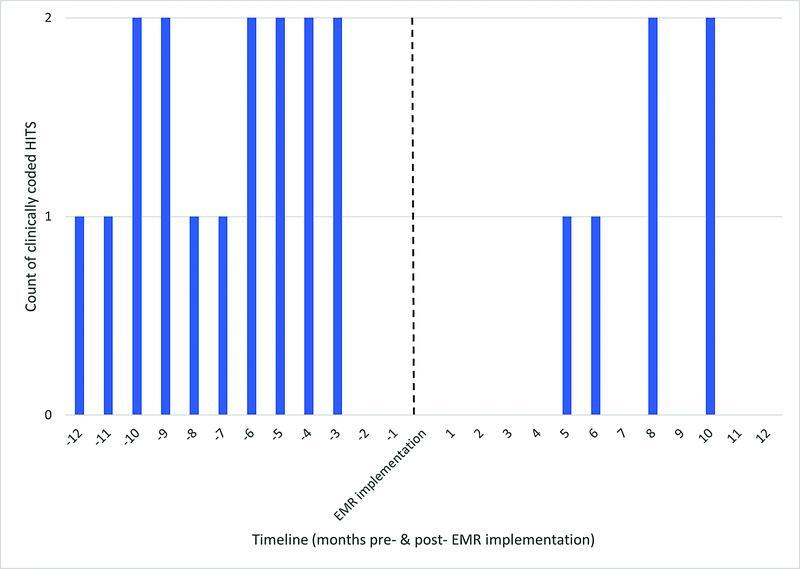
Incidence of heparin induced thrombocytopenia (HITS) (12-month pre- and 12-month post-EMR-implementation). EMR, electronic medical record.
Anticoagulant Usage
Pharmacy supply comparisons for monthly therapeutic anticoagulant usage, 12-month pre- and 12-month post-EMR implementation for all sites are displayed in Table 5 . There were no significant differences for all drugs except for DOACs.
Table 5. Mean monthly quantities of therapeutic anticoagulants supplied by pharmacy across the five study sites, 12-month pre- and post-EMR implementation.
| Anticoagulant class | Pre-monthly mean (SD) | Post- monthly mean (SD) | t -statistic (degrees of freedom) | p -Value |
|---|---|---|---|---|
| IV UFH | 877.5 (186.8) | 813.5 (148.2) | t (20.9) –0.93 | p = 0.36 |
| LMWH | 3192.4 (374.8) | 3400.8 (371.3) | t (22.0) 1.37 | p = 0.19 |
| DOAC | 6512.9 (845.4) | 7860.0 (757.1) | t (21.7) 4.11 | p = 0.0005 |
| Warfarin | 8810.5 (1649.3) | 8105.1 (1178.9) | t (19.9) –1.21 | p = 0.24 |
Abbreviations: DOAC, direct-acting oral anticoagulant; IV, intravenous; LMWH, low molecular weight heparin; SD, standard deviation; UFH, unfractionated heparin.
Discussion
Summary of Main Results
As health care institutions transition to digital platforms medication errors rates may increase initially as staff adjust to new digital workflows. 31 While research suggests eMMSs can reduce prescribing error rates, overall there is still uncertainty on which design features enhance patient safety and which facilitate new error risks. 32 We have previously reported on the key design features locally employed to digitally manage therapeutic anticoagulation. 23 The current study offers evidence that digitization reduces bleeds associated with anticoagulant use and HIT cases and some insight into potential areas for improvement in system design as digital transformation continues to evolve. 33
Given the constraints of an observational pre-/poststudy design, the intent is not to link causality with EMR implementation but to highlight areas of association. Additionally, this study will help to inform the codesign of a near real-time dashboard intervention to improve the quality and safety of digital anticoagulant management. To date, the literature has identified a distinct lack of research surrounding both digital anticoagulant management and clinical analytics products and their impact on patient care outcomes despite these drugs being the leading cause of medication-induced avoidable harm. 18 34 The inclusion of four metrics allowed a broad overview of patient care outcomes that may be impacted and key opportunities to maximize safety and quality.
Medication Incidents
No significant difference in anticoagulation incidents was identified in the pre- versus post-EMR implementation period, suggesting system enhancements are warranted. There was a spike in incidents during the first 4 weeks of implementation. This may be explained by clinicians being particularly vigilant during the go-live period or alternatively indicate the potential for more incidents during the transition as end-users adjust to new digital workflows. A longitudinal study monitoring trends in data errors post-EMR-implementation reported similar observations, with an increase in the error rate over the first three quarters, before decreasing and reaching stability one and a half years after implementation. 31
When reviewing the clinical error type, the most notable during the post-EMR implementation period was duplicated orders (16.9%). While digital platforms offer numerous forms of CDSS to help eradicate order duplication, it would appear system design can play a significant role and introduce such errors if not designed adequately. For example, 8/12 duplicated orders appeared to stem from clinician confusion whereby warfarin or heparin orders were placed both within and outside of order sets (grouping of orders to standardize care). In one instance, triplicate warfarin orders occurred due to the clinician incorrectly reconciling a home medication list into inpatient orders. While EMRs are commonly cited as successful interventions to accurately capture a patient's medical history and reconcile throughout their transitions of care, design flaws have the potential to result in suboptimal outcomes. 35 A recent fatal anticoagulant double-dosing error was reported due to a prescriber's confusion when viewing both inpatient and discharge medications on the medication reconciliation screen. 36
Suboptimal workflow accounted for the second most common self-reported anticoagulation error-type post-EMR implementation (15.5%). An example included nurses signing for medication administration on the wrong day within the Medication Administration Record.
Toxic Pathology Results
Our results demonstrate that the new system has not statistically altered the rate of toxic pathology results. We observed a consistently high rate of toxic results; for example, 21% of aPTT results are toxic both pre- and post-EMR implementation. Our chart review identified inconsistent monitoring as the second most cited clinical error type with the potential to cause the patient's bleed (11/87). Further enhancements within the eMMS are needed to improve the monitoring of anticoagulants. An example may be improving result integration using near real-time clinical analytics products. Recent research evaluates anticoagulants as candidates for machine learning methods to predict heparin and warfarin dose titrations based on pathology results. 37 38
Hospital-Acquired Bleeding Complications
We observed a statistically significant decrease in rates of HACs associated with the digitization of anticoagulant prescription and administration. This is similar to the 41% reduction in clinically coded ADEs associated with oral anticoagulants demonstrated by Daniel et al after the implementation of a real-time clinical surveillance tool. 39
Of patients who experienced an HAC, 19% had a major bleed, most commonly linked to anticoagulant combinations. The consequences of therapeutic duplication of anticoagulants have been studied previously, where 7.4% of cases led to a hemoglobin-relevant bleed. 40 In addition, our study found 3.4% of patients with an HAC linked to an anticoagulant resulted in hospital death. This further emphasizes the high-risk nature of anticoagulants and the need for concerted interventions to ensure their judicious and effective use.
Of the two HACs directly linked to system design, one related to an incorrect initial UFH infusion rate. Currently due to design restraints, while the local EMR will prompt an appropriate weight-based dose, the clinician must remember this rate and then enter manually within subsequent fields to place the order ( Fig. 8 ).
Fig. 8.
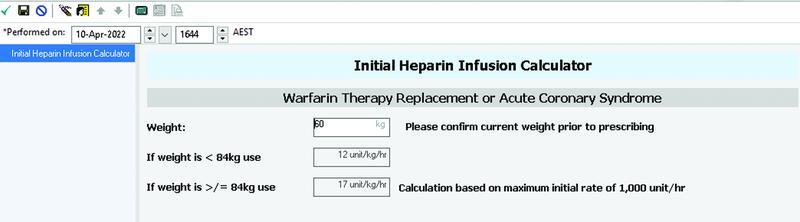
EMR user interface prompting weight-based dosing for UFH infusion orders. EMR, electronic medical record; UFH, unfractionated heparin.
The second scenario related to confusion surrounding duplicate UFH infusion orders being placed. Subsequent system design changes have since been made to alert users to doses that exceed 40 to 45 units/kg/h. 23
Inappropriate drug combinations were the most cited clinical error type. In all cases, drug interaction alerts fired and were overridden, consistent with previously published data suggesting that up to 96% of alerts are overridden. 41 42 Additional functionality has been installed within the local EMR to help minimize nuisance alerts to reduce alert fatigue. There is the potential to incorporate near-hard stops to force prescribing; however this may lead to unintended consequences, for example, delays in essential antibiotic therapy or inadvertent warfarin cessation, with this approach. 43
The categorization of potential EMR design enhancements into seven key areas (noting one HAC may have included more than one potential intervention) found that new automated CDSS or improved result integration/dashboard development would be the most suitable enhancement. For example, ongoing renal function monitoring/alerting for patients prescribed DOACs/LMWHs (not just at the point of prescribing) or improved flagging of patients at higher risk of a bleed on LMWHs (e.g., those with a low body mass index). Improvements to appropriate timing of aPTT testing was another key consideration.
Heparin-Induced Thrombocytopenia
A 62.5% reduction in clinically coded HIT cases was observed in the post-EMR-implementation period. HIT is a rare event (incidence ranging between 0.5 and 6.5%). 44 As predicted, the numbers observed were low and hence not subject to statistical analysis. Incidence varies dependent upon the type of heparin used and patient risk factors. Some risk factors are unavoidable (e.g., female sex), and hence, EMR implementation is irrelevant in terms of risk reduction. Others are modifiable (e.g., duration of UFH therapy >5 days increases the risk, intravenous versus subcutaneous route) and may be improved through various forms of CDSS. Examples include predefined order sentences or enhanced display of recent UFH or LMWH administrations and platelet counts at the time of heparin prescribing. Previous studies evaluating CDSS designed to notify clinicians when patients experience a platelet count decrease consistent with HIT have produced varying results. 45 46 47 One study demonstrated low positive predictive values for alerts resulting in an accepted intervention. 45 The remaining two studies noted a statistically significant increase in HIT test laboratory ordering with no impact on patient harm outcomes, for example, thrombosis, 90-day mortality, and length of stay. 46 47
Anticoagulant Usage
Our comparison of distribution data showed no significant change in prescribing habits for all classes of anticoagulants, except for DOACs. There was significantly higher usage of DOACs in the post EMR-implementation period. This difference is irrelevant in terms of HIT or toxic pathology results given that DOACs do not contribute to these outcomes. The increase in postimplementation DOAC prescribing was still associated with a significant reduction in bleeds associated with anticoagulant use, hence potentially diluting the magnitude of this effect.
Limitations
The observational pre-/poststudy design is inherently at risk of bias. There was an inability to link causality to the implementation of the EMR; however, this research provides valuable insight into the potential benefits of an eMMS. The retrospective nature of the review limits the ability to identify if factors such as new digital workflows or insufficient training contributed to unintended outcomes. Additionally, health information management departments are reliant on clinicians accurately documenting clinical information to retrospectively apply clinical codes. While our study included a manual chart review to capture false positives in the post-EMR-implementation period, the potential for false negatives is unaccounted for.
Using data from a voluntary, self-reporting database such as RISKMAN is associated with some bias as it relies on motivated staff taking the time to submit incidents. This results in the potential for missing or incorrect information. Willingness among nurses to report medication errors varies in the literature between 28.9 and 57.4%. 48 49 However, this is true for both pre- and post-EMR implementation data and still provides valuable clinical process data.
Conclusion
To date, research surrounding patient care outcomes upon transition to a digital anticoagulant prescribing platform is scarce. Our study suggests the implementation of an EMR improves clinical care outcomes for patients receiving anticoagulation, for example, fewer hospital-acquired bleeding complications. System design plays a significant role in mitigating the risks associated with anticoagulants and consideration must be given to optimizing eMMSs.
The rate of toxic pathology results remained unchanged. Further exploration of CDSS and near real-time clinical analytics tools is required, for example, streamlining pathology integration and improving drug interaction and duplicate therapy alert systems. These interventions could help mitigate adverse anticoagulant outcomes.
Clinical Relevance Statement
Anticoagulants are high-risk medications, commonly implicated in adverse effects of hospitalized inpatients. Digitizing the therapeutic management of anticoagulants was associated with improved patient outcomes, for example, a reduction in hospital-acquired bleeding complications and HIT. The number of clinician-reported medication incidents and toxic pathology results remained unchanged and could benefit further from targeted digital design strategies, for example, new automated CDSS or improved result integration.
Multiple Choice Questions
-
Managing anticoagulants within electronic eMMS requires careful design decisions because:
They are expensive medications, causing significant financial burden if prescribed excessively.
They are high-risk medications with the ability to cause significant harm/death if used inappropriately.
They are a new class of medications with limited exposure in clinical practice.
They require standard, consistent doses for all patients.
Correct Answer: The correct answer is option b. Anticoagulants have a narrow therapeutic window and under- or over-anticoagulating a patient can result in significant patient harm or death. They require tight dose-control, centered around regular laboratory monitoring.
-
The digitization of therapeutic anticoagulation management has shown to be associated with:
An increase in the observed cases of HIT.
A reduction in associated supratherapeutic pathology results.
The removal of all common medication clinical error types, for example, duplication of orders.
A reduction in hospital-acquired bleeds associated with anticoagulant use.
Correct Answer: The correct answer is option d. Our study observed a statistically significant reduction in clinically coded bleeds associated with anticoagulant use in the post-EMR implementation period. There were fewer cases of HITs, yet the percentage of toxic pathology results remained unchanged. While eMMS incorporate design strategies to reduce duplicated orders, if not designed adequately they can contribute to the occurrence of such clinical error types.
Conflict of Interest None declared.
Protection of Human and Animal Subjects
Ethics approval to undertake this study was sought and granted by the organization's Human Research Ethics Committee (Ref: HREC/2019/QMS/54368) on 25th June 2019 for low-risk research involving humans.
References
- 1.Australasian Society of Thrombosis and Haemostasis . Tran H, Joseph J, Young L. New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Intern Med J. 2014;44(06):525–536. doi: 10.1111/imj.12448. [DOI] [PubMed] [Google Scholar]
- 2.Australasian Society of Thrombosis and Haemostasis (ASTH) . Tran H A, Chunilal S D, Harper P L, Tran H, Wood E M, Gallus A S. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198(04):198–199. doi: 10.5694/mja12.10614. [DOI] [PubMed] [Google Scholar]
- 3.Whayne T F. A review of the role of anticoagulation in the treatment of peripheral arterial disease. Int J Angiol. 2012;21(04):187–194. doi: 10.1055/s-0032-1330232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmermann A T, Jeffries W S, McElroy H, Horowitz J D.Utility of a weight-based heparin nomogram for patients with acute coronary syndromes Intern Med J 200333(1-2):18–25. [DOI] [PubMed] [Google Scholar]
- 5.Australian Commission on Safety and Quality in Health Care High risk medicines 2018 [cited 2022, 26 Feb]Accessed August 08, 2022 at:https://www.safetyandquality.gov.au/our-work/medication-safety/high-risk-medicines/
- 6.Institute for Safe Medication Practices High-alert medications in acute care settings [website] 2018 [cited 2022, 26 Feb]Accessed August 08, 2022 at:https://www.ismp.org/recommendations/high-alert-medications-acute-list
- 7.Budnitz D S, Lovegrove M C, Shehab N, Richards C L. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 8.The Joint Commission Sentinel Event Alert: Preventing errors relating to commonly used anticoagulants [cited 2022 26 Feb]Accessed August 08, 2022 at:https://www.jointcommission.org/assets/1/18/SEA_41.pdf [PubMed]
- 9.Sennesael A L, Larock A S, Devalet B. Preventability of serious thromboembolic and bleeding events related to the use of oral anticoagulants: a prospective study. Br J Clin Pharmacol. 2018;84(07):1544–1556. doi: 10.1111/bcp.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanikos J, Stapinski C, Koo S, Kucher N, Tsilimingras K, Goldhaber S Z. Medication errors associated with anticoagulant therapy in the hospital. Am J Cardiol. 2004;94(04):532–535. doi: 10.1016/j.amjcard.2004.04.075. [DOI] [PubMed] [Google Scholar]
- 11.Dreijer A R, Diepstraten J, Bukkems V E. Anticoagulant medication errors in hospitals and primary care: a cross-sectional study. Int J Qual Health Care. 2019;31(05):346–352. doi: 10.1093/intqhc/mzy177. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovska T, Fitzsimons K, Ferguson C, Koay A. Types and causes of anticoagulant-related medication incidents across hospitals in Western Australia. J Pharm Pract Res. 2019;49(06):532–537. [Google Scholar]
- 13.World Health Organization Global strategy on digital health 2020–2025 2021:[60 p.]. Accessed August 08, 2022 at:https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf
- 14.McDonald K.Digital Health Institute Summit: 2020 state of the EMR nation 2020 [cited 2022 28 Feb]Accessed August 08, 2022 at:https://www.pulseitmagazine.com.au/australian-ehealth/5820-digital-health-institute-summit-2020-state-of-the-emr-nation
- 15.The Office of the National Coordinator for Health Information Technology (ONC) Non-federal Acute Care Hospital Electronic Health Record Adoption 2021 [cited 2022 28 Feb]Accessed August 08, 2022 at:https://www.healthit.gov/data/quickstats/non-federal-acute-care-hospital-electronic-health-record-adoption
- 16.Barnett A, Winning M, Canaris S, Cleary M, Staib A, Sullivan C. Digital transformation of hospital quality and safety: real-time data for real-time action. Aust Health Rev. 2019;43(06):656–661. doi: 10.1071/AH18125. [DOI] [PubMed] [Google Scholar]
- 17.Ivers N M, Barrett J. Using report cards and dashboards to drive quality improvement: lessons learnt and lessons still to learn. BMJ Qual Saf. 2018;27(06):417–420. doi: 10.1136/bmjqs-2017-007563. [DOI] [PubMed] [Google Scholar]
- 18.Austin J, Barras M, Sullivan C. Interventions designed to improve the safety and quality of therapeutic anticoagulation in an inpatient electronic medical record. Int J Med Inform. 2020;135:104066. doi: 10.1016/j.ijmedinf.2019.104066. [DOI] [PubMed] [Google Scholar]
- 19.Day M, Malone M, Burkeybile A, Deane K. Improving transitions of care for hospitalized patients on warfarin. Jt Comm J Qual Patient Saf. 2016;42(09):425–431. doi: 10.1016/s1553-7250(16)42084-2. [DOI] [PubMed] [Google Scholar]
- 20.Dunn A S, Shetreat-Klein A, Berman J. Improving transitions of care for patients on warfarin: the safe transitions anticoagulation report. J Hosp Med. 2015;10(09):615–618. doi: 10.1002/jhm.2393. [DOI] [PubMed] [Google Scholar]
- 21.Georgiou A, Lang S, Rosenfeld D, Westbrook J I. The use of computerized provider order entry to improve the effectiveness and efficiency of coagulation testing. Arch Pathol Lab Med. 2011;135(04):495–498. doi: 10.5858/2010-0286-SO.1. [DOI] [PubMed] [Google Scholar]
- 22.Roberts D L, Noble B N, Wright M J, Nelson E A, Shaft J D, Rakela J. Impact of computerized provider order entry on hospital medication errors. J Clin Outcomes Manag. 2013;20(03):109–115. [Google Scholar]
- 23.Austin J A, Barras M A, Sullivan C M. Safe and effective digital anticoagulation: a continuous iterative improvement approach. Appl Clin Inform Open. 2021;5(02):e116–e124. [Google Scholar]
- 24.Sherman R E, Anderson S A, Dal Pan G J. Real-world evidence—what is it and what can it tell us? N Engl J Med. 2016;375(23):2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 25.Swift B, Jain L, White C. Innovation at the intersection of clinical trials and real-world data science to advance patient care. Clin Transl Sci. 2018;11(05):450–460. doi: 10.1111/cts.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Queensland Government Best Practice Guide to Clinical Incident Management 2014 [cited 2022, 23 Feb]Accessed August 08, 2022 at:https://clinicalexcellence.qld.gov.au/sites/default/files/2018-01/clinicalincidentguide.pdf
- 27.Westbrook J I, Baysari M T, Li L, Burke R, Richardson K L, Day R O. The safety of electronic prescribing: manifestations, mechanisms, and rates of system-related errors associated with two commercial systems in hospitals. J Am Med Inform Assoc. 2013;20(06):1159–1167. doi: 10.1136/amiajnl-2013-001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Queensland Health; Anticoagulant Guideline for Hospitalised Adult Patients 2022 [cited 2022, 30 Aug]Available at:https://www.health.qld.gov.au/__data/assets/pdf_file/0015/1152213/statewide-anticoagulant-guideline.pdf
- 29.Dose-ranging trial of enoxaparin for unstable angina: results of TIMI 11A. The Thrombolysis in Myocardial Infarction (TIMI) 11A Trial Investigators. J Am Coll Cardiol. 1997;29(07):1474–1482. [PubMed] [Google Scholar]
- 30.Mehran R, Rao S V, Bhatt D L. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 31.Qian S, Munyisia E, Reid D, Hailey D, Pados J, Yu P. Trend in data errors after the implementation of an electronic medical record system: a longitudinal study in an Australian Regional Drug and Alcohol Service. Int J Med Inform. 2020;144:104292. doi: 10.1016/j.ijmedinf.2020.104292. [DOI] [PubMed] [Google Scholar]
- 32.Gates P J, Hardie R-A, Raban M Z, Li L, Westbrook J I. How effective are electronic medication systems in reducing medication error rates and associated harm among hospital inpatients? A systematic review and meta-analysis. J Am Med Inform Assoc. 2021;28(01):167–176. doi: 10.1093/jamia/ocaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan C, Staib A, McNeil K, Rosengren D, Johnson I. Queensland Digital Health Clinical Charter: a clinical consensus statement on priorities for digital health in hospitals. Aust Health Rev. 2020;44(05):661–665. doi: 10.1071/AH19067. [DOI] [PubMed] [Google Scholar]
- 34.Lim H C, Austin J A, van der Vegt A H. Toward a learning health care system: a systematic review and evidence-based conceptual framework for implementation of clinical analytics in a digital hospital. Appl Clin Inform. 2022;13(02):339–354. doi: 10.1055/s-0042-1743243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pevnick J M, Shane R, Schnipper J L. The problem with medication reconciliation. BMJ Qual Saf. 2016;25(09):726–730. doi: 10.1136/bmjqs-2015-004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholefield A.Medical software blamed for fatal anticoagulant double-dosing error. Australian Doctor [Internet]. 2021 [cited 2022, 25 Feb]Accessed August 08, 2022 at:https://www.ausdoc.com.au/news/medical-software-blamed-fatal-anticoagulant-doubledosing-error
- 37.Su L, Liu C, Li D. Toward optimal heparin dosing by comparing multiple machine learning methods: retrospective study. JMIR Med Inform. 2020;8(06):e17648. doi: 10.2196/17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan O, Wu J Z, Yeo K K. Building a predictive model for warfarin dosing via machine learning. Eur Heart J. 2020;41(02):ehaa946. [Google Scholar]
- 39.Daniel J W, Kramer J, Burgess L H.Assessment of oral anticoagulant adverse drug events before and after implementation of a real-time clinical surveillance toolJ Patient Saf 2019 [DOI] [PubMed]
- 40.Rahmanzade R, Cabrera Diaz F, Zaugg C, Schuetz P, Salili A R. Therapeutic duplication of anticoagulants: a retrospective study of frequency and consequences in a tertiary referral hospital. Thromb J. 2020;18(01):14. doi: 10.1186/s12959-020-00227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuperman G J, Gandhi T K, Bates D W.Effective drug-allergy checking: methodological and operational issues J Biomed Inform 200336(1-2):70–79. [DOI] [PubMed] [Google Scholar]
- 42.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(02):138–147. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strom B L, Schinnar R, Aberra F. Unintended effects of a computerized physician order entry nearly hard-stop alert to prevent a drug interaction: a randomized controlled trial. Arch Intern Med. 2010;170(17):1578–1583. doi: 10.1001/archinternmed.2010.324. [DOI] [PubMed] [Google Scholar]
- 44.Arepally G M. Heparin-induced thrombocytopenia. Blood. 2017;129(21):2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibáñez-Garcia S, Rodriguez-Gonzalez C, Escudero-Vilaplana V. Development and evaluation of a clinical decision support system to improve medication safety. Appl Clin Inform. 2019;10(03):513–520. doi: 10.1055/s-0039-1693426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austrian J S, Adelman J S, Reissman S H, Cohen H W, Billett H H. The impact of the heparin-induced thrombocytopenia (HIT) computerized alert on provider behaviors and patient outcomes. J Am Med Inform Assoc. 2011;18(06):783–788. doi: 10.1136/amiajnl-2011-000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riggio J M, Cooper M K, Leiby B E, Walenga J M, Merli G J, Gottlieb J E. Effectiveness of a clinical decision support system to identify heparin induced thrombocytopenia. J Thromb Thrombolysis. 2009;28(02):124–131. doi: 10.1007/s11239-008-0279-x. [DOI] [PubMed] [Google Scholar]
- 48.Jember A, Hailu M, Messele A, Demeke T, Hassen M. Proportion of medication error reporting and associated factors among nurses: a cross sectional study. BMC Nurs. 2018;17(01):9. doi: 10.1186/s12912-018-0280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ulanimo V M, O'Leary-Kelley C, Connolly P M. Nurses' perceptions of causes of medication errors and barriers to reporting. J Nurs Care Qual. 2007;22(01):28–33. doi: 10.1097/00001786-200701000-00007. [DOI] [PubMed] [Google Scholar]


