Abstract
Background
Patterns of overall and disease-free survival after esophagectomy for esophageal cancer in older adults have not been carefully studied.
Methods
Retrospective analysis of all patients with esophageal cancer undergoing esophagectomy from 2005 to 2020 at our institution was performed. Differences in outcomes were stratified by age groups, < 75 and ≥ 75 years old, and two time periods, 2005–2012 and 2013–2020.
Results
A total of 1135 patients were included: 979 (86.3%) patients were < 75 (86.3%), and 156 (13.7%) were ≥ 75 years old. Younger patients had fewer comorbidities, better nutritional status, and were more likely to receive neoadjuvant and adjuvant therapy (all p < 0.05). However, tumor stage and operative approach were similar, except for increased performance of the McKeown technique in younger patients (p = 0.02). Perioperatively, younger patients experienced fewer overall and grade II complications (both p < 0.05). They had better overall survival (log-rank p-value < 0.001) and median survival, 62.2 vs. 21.5 months (p < 0.05). When stratified by pathologic stage, survival was similar for yp0 and pathologic stage II disease (both log-rank p-value > 0.05). Multivariable Cox models showed older age (≥ 75 years old) had increased hazard for reduced overall survival (HR 2.04 95% CI 1.5–2.8; p < 0.001) but not disease-free survival (HR 1.1 95% CI 0.78–1.6; p = 0.54). Over time, baseline characteristics remained largely similar, while stage became more advanced with a rise in neoadjuvant use and increased performance of minimally invasive esophagectomy (all p < 0.05). While overall complication rates improved (p < 0.05), overall and recurrence-free survival did not. Overall survival was better in younger patients during both time periods (both log-rank p < 0.05).
Conclusions
Despite similar disease-free survival rates, long-term survival was decreased in older adults as compared to younger patients. This may be related to unmeasured factors including frailty, long-term complications after surgery, and competing causes of death. However, our results suggest that survival is similar in those with complete pathologic responses.
Keywords: Esophagectomy, Older adults, Survival, Recurrence, Outcomes
Introduction
Esophageal cancer is primarily a disease of older adults with a median age at diagnosis of 68 within the USA. More than half of patients with newly diagnosed esophageal cancer are now over the age of 65.1
Older adults historically are less likely to undergo esophagectomy.2,3 The reasons for this are multifactorial including perceived physician bias, patient and family concern about permanent functional decline, and uncertainty of long-term survival benefit to justify the morbidity associated with esophagectomy.4,5 However, despite these concerns, esophagectomy, compared to definitive chemoradiation, demonstrates a clear survival benefit in older patients.3
With the advancement in neoadjuvant regimens,6 perioperative care, and minimally invasive techniques, it is important to re-examine long-term outcomes after esophagectomy. Particularly with the emergence of geriatric surgery centers, precise data on surgical outcomes in older adults is important for clinicians, including geriatricians and anesthesiologists who perform preoperative assessments and for patients and families for shared decision-making that is evidence-based.7 Therefore, the first aim of our study was to compare long-term outcomes between older and younger adults after esophagectomy for esophageal cancer. The secondary aim was to examine the impact of improvements in treatment over time.
Methods
Study Population
This study was a retrospective analysis of all patients ≥ 18 years old who underwent an esophagectomy for esophageal cancer by the Division of Thoracic Surgery from 2005 to 2020 at a single tertiary care academic center. The study was approved by the Institutional Review Board (#2015P000752).
All patients underwent either Ivor Lewis, modified McKeown,8,9 transhiatal, or thoracoabdominal esophagectomy by open, totally minimally invasive (MIE), video-assisted thoracoscopic surgery (VATS), robotic, or hybrid open and minimally invasive approach according to surgeon discretion. Preoperative and postoperative care was standardized according to our institution’s practice and has previously been described.10
Variables
Variables were retrieved from the medical record and included: (1) demographics (age, gender, Body Mass Index (BMI), date of surgery); (2) comorbidities (Barrett’s esophagus, congestive heart failure (CHF), coronary artery disease (CAD), atrial fibrillation (afib), history of other malignancy, chronic obstructive pulmonary disease (COPD), hypertension (HTN), diabetes, smoking history (never, current, former), smoking pack-years, preoperative albumin, Eastern Cooperative Oncology Group (ECOG) performance score, neoadjuvant and adjuvant therapy details; (3) tumor characteristics (tumor size, histology, margin status, lymph node count and positivity, presence of lymphovascular invasion, venous invasion, and/or perineural invasion, tumor location; 8th edition clinical and pathologic TNM stage;11 HER-2 status, signet ring cell status); (4) operative details (technique (Ivor Lewis, McKeown, other), thoracic approach (VATS, robotic, thoracotomy), abdominal approach (laparoscopy, robotic, laparotomy), conversion rates); (5) complications: overall complications, and complications classified according to Clavien-Dindo grade (II–V);12 (6) the long-term outcomes under consideration were: overall survival and overall, local–regional, and distant disease-free survival.
Statistical Analysis
Patients were stratified into two age groups: age < 75 years old, and ≥ 75 years old. Categorical variables were analyzed using either the chi-square or Fisher exact test where appropriate. Continuous variables were described using medians and interquartile range (IQR), or means and standard deviation (SD), with comparisons performed using the Wilcoxon rank-sum test or Kruskal–Wallis test where needed.
The Kaplan–Meier estimators were used to calculate overall survival (OS) and disease-free survival, local–regional disease-free survival, and distant disease-free survival (DFS, LRDFS, DDFS) which were measured from the time of surgery to death or last known follow-up or radiologic or pathologic confirmation of recurrence respectively. If both local and distant occurrences occurred, recurrence was timed based on the first appearance of either. Testing was done using a log-rank test for differences in survival.
Univariable and multivariable Cox regression models were used to assess the effects of clinicopathologic, operative, and postoperative covariates on the risk of OS and DFS, LRDFS, and DDFS. Inclusion into the multivariable model was based on a priori selection due to clinical significance followed by the removal of variables that led to unstable estimates.
A sub-analysis was performed to examine temporal trends on survival between 2005–2012 and 2013–2020 with the breakpoint at 2012 chosen due to wide implementation of neoadjuvant therapy after the publication of the Dutch Chemoradiotherapy for Oesophageal cancer followed by surgery study (CROSS).6 In practice, our institution has used a platinum-based regimen since before 2013 but neoadjuvant protocols were updated after this time. All outcomes were analyzed within each time period between the two age groups, and then separately between each time period for the overall cohort and for each age group.
Significance was determined as p ≤ 0.05. All statistical analysis was performed using R statistical software 4.0.3 (R Core Team, 2020).
Results
Baseline Patient Characteristics
Patient Characteristics of the Overall Cohort
A total of 1135 esophagectomies were included in the final analysis with a median age of 64.9 years (interquartile range (IQR) 13.6). Overall, 979 (86%) were < 75 years old (median 63.3 (IQR 11.4)) and 156 (14%) were ≥ 75 years old (median 78.7, (IQR 3.9)). The overall cohort was primarily male (82%), with a good performance score (median ECOG 0). Most patients received neoadjuvant chemoradiation (81%), while only 12.7% received adjuvant therapy and 9.3% received neoadjuvant chemoradiation and adjuvant therapy (Table 1).
Table 1.
Baseline demographics and comorbidities
| Variable | Age < 75 | Age 75 + | Overall | p-value |
|---|---|---|---|---|
| Number of patients | 979 | 156 | 1135 | |
|
| ||||
| Age | < 0.001 | |||
| Mean (SD) | 62.16 (8.40) | 79.10 (2.90) | 64.49 (9.80) | |
| Median (IQR) | 63.32 (11.38) | 78.66 (3.86) | 64.88 (13.59) | |
| Gender (male) | 799 (81.61%) | 126 (80.77%) | 925 (81.50%) | 0.888 |
| Body Mass Index | 0.001 | |||
| Mean (SD) | 27.94 (5.72) | 26.49 (4.58) | 27.74 (5.59) | |
| Median (IQR) | 27.41 (6.81) | 26.00 (5.17) | 27.20 (6.61) | |
| Comorbidities | ||||
| Barrett’s esophagus | 260 (26.56%) | 43 (27.56%) | 303 (26.70%) | 0.868 |
| Congestive heart failure | 14 (1.43%) | 2 (1.28%) | 16 (1.41%) | > 0.999 |
| Coronary artery disease | 123 (12.56%) | 38 (24.36%) | 161 (14.19%) | < 0.001 |
| Chronic obstructive pulmonary disease | 82 (8.38%) | 13 (8.33%) | 95 (8.37%) | > 0.999 |
| Hypertension | 506 (51.69%) | 108 (69.23%) | 614 (54.10%) | < 0.001 |
| Diabetes mellitus | 178 (18.18%) | 29 (18.59%) | 207 (18.24%) | 0.991 |
| Smoking history | < 0.001 | |||
| Never | 279 (28.50%) | 38 (24.36%) | 317 (27.93%) | |
| Current | 220 (22.47%) | 16 (10.26%) | 236 (20.79%) | |
| Former | 480 (49.03%) | 102 (65.38%) | 582 (51.28%) | |
| Smoking pack-years | 0.866 | |||
| Mean (SD) | 33.01 (24.09) | 33.64 (24.12) | 33.10 (24.08) | |
| Median (IQR) | 30.00 (30.00) | 30.00 (36.50) | 30.00 (30.00) | |
| Preoperative albumin | < 0.001 | |||
| Mean (SD) | 4.01 (0.40) | 3.87 (0.45) | 3.99 (0.41) | |
| Median (IQR) | 4.00 (0.50) | 3.90 (0.40) | 4.00 (0.50) | |
| ECOG | ||||
| Median (IQR) | 0.00 (1.00) | 1.00 (1.00) | 0.00 (1.00) | 0.001 |
| Neoadjuvant CT | 820 (83.76%) | 110 (70.51%) | 930 (81.94%) | < 0.001 |
| Neoadjuvant RT | 809 (82.64%) | 110 (70.51%) | 919 (80.97%) | < 0.001 |
| Combined neoadjuvant CXRT | 807 (82.43%) | 110 (70.51%) | 917 (80.79%) | < 0.001 |
| Adjuvant therapy | 136 (13.95%) | 7 (4.58%) | 143 (12.68%) | 0.002 |
SD standard deviation, IQR interquartile range, CT chemotherapy, CXRT chemoradiation, ECOG Eastern Cooperative Oncology Group, RT radiotherapy
Tumors were primarily adenocarcinoma (85%), locally advanced, and located at the distal esophageal/gastroesophageal junction (GEJ) (88%). R0 resection occurred in 97% of surgeries (Table 2).
Table 2.
Tumor characteristics
| Variable | Age < 75 | Age 75 + | Overall | p-value |
|---|---|---|---|---|
| Number of patients | 979 | 156 | 1135 | |
|
| ||||
| Pathologic tumor sizea | 0.159 | |||
| Mean (SD) | 3.43 (2.23) | 3.72 (2.27) | 3.47 (2.24) | |
| Median (IQR) | 3.00 (2.50) | 3.10 (2.80) | 3.00 (2.50) | |
| Histologyb | 0.671 | |||
| Adenocarcinoma | 835 (85.55%) | 130 (83.33%) | 965 (85.25%) | |
| Squamous cell carcinoma | 129 (13.22%) | 23 (14.74%) | 152 (13.43%) | |
| Other | 12 (1.23%) | 3 (1.92%) | 15 (1.33%) | |
| Histologic gradec | 0.174 | |||
| 1 | 63 (6.58%) | 5 (3.27%) | 68 (6.13%) | |
| 2 | 324 (33.86%) | 63 (41.18%) | 387 (34.86%) | |
| 3 | 429 (44.83%) | 66 (43.14%) | 495 (44.59%) | |
| Not defined | 141 (14.73%) | 19 (12.42%) | 160 (14.41%) | |
| Positive margin | ||||
| Overall | 32 (3.27%) | 7 (4.49%) | 39 (3.44%) | 0.59 |
| Radiald | 24 (2.48%) | 4 (2.65%) | 28 (2.50%) | > 0.999 |
| Longitudinal | 9 (0.92%) | 4 (2.56%) | 13 (1.15%) | 0.165 |
| Lymph node count | ||||
| Median (IQR) | 18.00 (9.00) | 19.00 (10.00) | 18.00 (9.00) | 0.293 |
| Positive lymph node count | ||||
| Median (IQR) | 0.00 (1.00) | 0.00 (1.00) | 0.00 (1.00) | 0.542 |
| Lymphovascular invasion present | 179 (18.28%) | 40 (25.64%) | 219 (19.30%) | 0.04 |
| Venous invasion presente | 33 (3.54%) | 8 (5.44%) | 41 (3.80%) | 0.374 |
| Perineural invasion present | 162 (16.55%) | 28 (17.95%) | 190 (16.74%) | 0.749 |
| Tumor location inf esophagus | 0.007 | |||
| Upper/proximal | 9 (0.92%) | 6 (3.85%) | 15 (1.32%) | |
| Middle | 100 (10.24%) | 20 (12.82%) | 120 (10.59%) | |
| Distal/gastroesophageal junction | 868 (88.84%) | 130 (83.33%) | 998 (88.08%) | |
| Clinical T stage | 0.499 | |||
| T0/is | 3 (0.31%) | 0 (0.00%) | 3 (0.26%) | |
| 1 | 121 (12.36%) | 18 (11.54%) | 139 (12.25%) | |
| 2 | 191 (19.51%) | 38 (24.36%) | 229 (20.18%) | |
| 3 | 484 (49.44%) | 68 (43.59%) | 552 (48.63%) | |
| 4 | 14 1.43%) | 1 (0.64%) | 15 (1.32%) | |
| Not defined/missing | 166 (16.96%) | 31 (19.87%) | 197 (17.36%) | |
| Clinical N stage | 0.153 | |||
| 0 | 374 (38.20%) | 68 (43.59%) | 442 (38.94%) | |
| 1 | 385 (39.33%) | 47 (30.13%) | 432 (38.06%) | |
| 2 | 82 (8.38%) | 11 (7.05%) | 93 (8.19%) | |
| 3 | 5 (0.51%) | 1 (0.64%) | 6 (0.53%) | |
| Not defined/missing | 133 (13.59%) | 29 (18.59%) | 162 (14.27%) | |
| Clinical M stage | 0.187 | |||
| 0 | 14 (0.43%) | 5 (3.21%) | 19 (1.67%) | |
| 1 | 5 (0.51%) | 0 (0.00%) | 5 (0.44%) | |
| Not defined/missing | 960 (98.06%) | 151 (96.79%) | 1111 (97.89%) | |
| Clinical TNM stage | 0.843 | |||
| 1 | 100 (11.79%) | 16 (12.60%) | 116 (11.90%) | |
| 2 | 196 (23.11%) | 33 (25.98%) | 229 (23.49%) | |
| 3 | 472 (55.66%) | 68 (53.54%) | 540 (55.38%) | |
| 4 | 80 (9.43%) | 10 (7.87%) | 90 (9.23%) | |
| Missing | 131 | 29 | 160 | |
| Pathologic T stage | 0.77 | |||
| 0 | 246 (25.13%) | 34 (21.79%) | 280 (24.67%) | |
| Is | 1 (0.10%) | 0 (0.00%) | 1 (0.09%) | |
| 1 | 242 (24.72%) | 36 (23.08%) | 278 (24.49%) | |
| 2 | 154 (15.73%) | 30 (19.23%) | 184 (16.21%) | |
| 3 | 319 (32.58%) | 53 (33.97%) | 372 (32.78%) | |
| 4 | 12 (1.23%) | 3 (1.92%) | 15 (1.32%) | |
| Not defined/missing | 5 (0.51%) | 0 (0.00%) | 5 (0.44%) | |
| Pathologic N stage | 0.893 | |||
| 0 | 666 (68.03%) | 104 (66.67%) | 770 (67.84%) | |
| 1 | 173 (17.67%) | 29 (18.59%) | 202 (17.80%) | |
| 2 | 95 (9.70%) | 17 (10.90%) | 112 (9.87%) | |
| 3 | 40 (4.09%) | 6 (3.85%) | 46 (4.05%) | |
| Not defined/missing | 5 (0.51%) | 0 (0.00%) | 5 (0.44%) | |
| Pathologic M stage | 0.957 | |||
| 0 | 849 (86.72%) | 134 (85.90%) | 983 (86.61%) | |
| 1 | 11 (1.12%) | 2 (1.28%) | 13 (1.15%) | |
| Not defined/missing | 119 (12.16%) | 20 (12.82%) | 139 (12.25%) | |
| Pathologic TNM stage | 0.928 | |||
| 1 | 534 (54.71%) | 82 (52.56%) | 616 (54.42%) | |
| 2 | 123 (12.60%) | 22 (14.10%) | 145 (12.81%) | |
| 3 | 269 (27.56%) | 43 (27.56%) | 312 (27.56%) | |
| 4 | 50 (5.12%) | 9 (5.77%) | 59 (5.21%) | |
| Missing | 3 | 0 | 3 | |
| HER 2 status | 0.7 | |||
| Negative | 152 (15.53%) | 21 (13.46%) | 173 (15.24%) | |
| Positive | 32 (3.27%) | 4 (2.56%) | 36 (3.17%) | |
| Not reported | 795 (81.21%) | 131 (83.97%) | 926 (81.59%) | |
| Signet cell histology present on biopsy or final pathology | 142 (14.50%) | 13 (8.33%) | 155 (13.66%) | 0.05 |
IQR interquartile range, SD standard deviation, TNM tumor, node, metastases
Reported out of n = 978
Reported out of n = 1132
Reported out of n = 1110
Reported out of n = 1118
Reported out of n = 1079
Reported out of n = 1133
Esophagectomies were primarily performed by either Ivor Lewis (50%) or modified McKeown technique (48%) with total MIE approach most commonly (66%). The conversion rate to an open procedure was 5% (Table 3).
Table 3.
Operative details
| Variable | Age < 75 | Age 75 + | Overall | p-value |
|---|---|---|---|---|
| Number of patients | 979 | 156 | 1135 | |
|
| ||||
| Surgical technique | 0.02 | |||
| McKeown | 479 (48.93%) | 62 (39.74%) | 541 (47.67%) | |
| Ivor Lewis | 482 (49.23%) | 87 (55.77%) | 569 (50.13%) | |
| Transhiatal | 12 (1.23%) | 3 (1.92%) | 15 (1.32%) | |
| Thoracoabdominal | 6 (0.61%) | 4 (2.56%) | 10 (0.88%) | |
| Overall approach | 0.718 | |||
| Total MIE | 645 (65.88%) | 107 (68.59%) | 752 (66.26%) | |
| Open | 176 (17.98%) | 24 (15.38%) | 200 (17.62%) | |
| Hybrid | 158 (16.14%) | 25 (16.03%) | 183 (16.12%) | |
| Thoracic approacha | 0.696 | |||
| Minimally Invasive | 642 (65.58%) | 107 (68.59%) | 749 (65.99%) | |
| Thoracotomy | 289 (29.52%) | 43 (27.56%) | 332 (29.25%) | |
| Robotic | 48 (4.90%) | 6 (3.85%) | 54 (4.76%) | |
| Abdominal approach | 0.713 | |||
| Minimally invasive | 642 (65.58%) | 107 (68.59%) | 749 (65.99%) | |
| Laparotomy | 289 (29.52%) | 43 (27.56%) | 332 (29.25%) | |
| Robotic | 48 (4.90%) | 6 (3.85%) | 54 (4.76%) | |
| Convert to open surgeryb | 0.154 | |||
| None | 905 (94.86%) | 142 (94.04%) | 1047 (94.75%) | |
| Thoracic portion only | 26 (2.73%) | 2 (1.32%) | 28 (2.53%) | |
| Abdominal portion only | 18 (1.89%) | 4 (2.65%) | 22 (1.99%) | |
| Both thoracic and abdominal portions | 5 (0.52%) | 3 (1.99%) | 8 (0.72%) | |
SD standard deviation, IQR interquartile range, MIE minimally invasive esophagectomy (includes video-assisted or robotic-assisted thoracoscopic surgery)
Out of N = 1119
Out of N = 1105
Patient Characteristics by Age Group
Table 1 describes baseline characteristics by age. Patients < 75 years old as compared to those ≥ 75 had higher BMI (median 27.4 vs. 26.0), better performance status (ECOG 0 vs. 1), and lower rates of CAD (12.6% vs. 24.4%) and HTN (51.7% vs. 69.2%) (all p < 0.05). Meanwhile, they were more likely to be active smokers (22.5% vs. 10.3%) and receive neoadjuvant chemoradiation therapy)82.4% vs. 70.5%), and adjuvant therapy (14.0% vs. 4.6%) (all p < 0.05). Tumor characteristics varied by age with respect to tumor location, with increased rates of distal/GEJ junction tumors in younger adults (88.8% vs. 83.3%) (p = 0.007), as well as lower rates of lymphovascular invasion, but a higher rate of signet cell presence (both p ≤ 0.05). Tumor type, as well as clinical and pathologic stage, and rate of positive margins (longitudinal and radial) were statistically similar (Table 2).
In terms of operative details, apart from younger patients undergoing more modified McKeown technique (48.9% vs. 39.74%; p = 0.02) as compared to the Ivor Lewis technique, operative approach (overall, thoracic, and abdominal) and rates of conversion were statistically similar (p > 0.05) (Table 3).
Perioperative Outcomes by Age
The overall complication rate was 58.3%. Complication rates by grade (II–V) were 47.8%, 29.3%, 6.7%, and 1.2% respectively. Patients < 75, with respect to those 75 or older, experienced lower rates of overall (56.7% vs. 68.6%; p = 0.007) and grade II complication (44.9% vs. 66.0%; p < 0.001) (Table 4). Grade III–V complication rates were similar between the groups.
Table 4.
Outcomes
| Variable | Age < 75 | Age 75 + | Overall | p-value |
|---|---|---|---|---|
| Number of patients | 979 | 156 | 1135 | |
|
| ||||
| Any complicationa (II-IV) | 555 (56.69%) | 107 (68.59%) | 662 (58.33%) | 0.007 |
| Grade of complicationa | ||||
| Grade 2 | 440 (44.94%) | 103 (66.03%) | 543 (47.84%) | < 0.001 |
| Grade 3 | 284 (29.01%) | 48 (30.77%) | 334 (29.25%) | 0.723 |
| Grade 4 | 63 (6.44%) | 13 (8.33%) | 76 (6.70%) | 0.479 |
| Grade 5 | 10 (1.02%) | 3 (1.92%) | 13 (1.15%) | 0.563 |
| Hospital length of stay | 0.003 | |||
| Mean, days (SD) | 12.99 (8.96) | 15.31 (12.14) | 13.31 (9.49) | |
| Median, days (IQR) | 10.00 (6.00) | 11.00 (6.50) | 10.00 (6.00) | |
| Discharge disposition | < 0.001 | |||
| Death | 7 (70.72%) | 2 (1.29%) | 9 (0.80%) | |
| Home | 30 (3.08%) | 4 (2.58%) | 34 (3.01%) | |
| Home with services | 796 (81.72%) | 80 (51.61%) | 876 (77.59%) | |
| Inpatient rehabilitation | 141 (14.48%) | 67 (43.23%) | 208 (18.42%) | |
| Hospice | 0 (0.00%) | 2 (1.29%) | 2 (0.18%) | |
| Missing | 5 | 1 | 6 | |
| Return to the operating room | 205 (20.94%) | 34 (21.79%) | 239 (21.06%) | 0.891 |
| 1-year mortality | 139 (14.57%) | 41 (26.97%) | 180 (16.27%) | < 0.001 |
| 5-year mortality | 353 (36.92%) | 85 (55.56%) | 438 (39.50%) | < 0.001 |
IQR interquartile ratio, SD standard deviation
grade evaluated by Clavien-Dindo system
Patients < 75, with respect to those 75 or older, were found to have shorter hospital LOS (median 10 vs. 11 days respectively; p = 0.003) and lower re-admission within 30 days (11.6% vs. 18.6%; p = 0.022) and were more likely to go home with services (81.7% vs. 51.6%) as compared to inpatient rehabilitation (14.5% vs. 43.2%) (p < 0.001). Re-operation rate within 30 days (rates included return for: bleeding, anastomotic leak, fistula, empyema, chyle leak or “other” (inclusive of tracheostomy, wound vac placement, dilation, wound debridement, vocal cord paralysis)) overall was 21.1% and was not statistically different between age groups (Table 4).
Long-term Outcomes by Age
Overall Survival
The Kaplan–Meier (K-M) estimator of overall survival by age (OS) (Fig. 1) showed improved OS in younger patients (p-value < 0.001). Patients < 75, compared to patients 75 or older, had better median OS (62.2 months, 95% CI 52.5–86.4 vs. 21.5 months, 95% CI 17.0–31.8), 1-year OS (84.3%, 95% CI 81.9–86.7%, vs. 71%, 95% CI 63.9–79.0%) and 5-year OS (50.7%, 95% CI 46.9–54.8%, vs. 32.3%; 95% CI 24.7–42.4%). When K-M were stratified by clinical and pathologic stage (Supplemental Figs. 1 and 2 respectively) a statistically significant overall survival benefit was observed for younger patients compared to older patients’ groups for clinical stages I, II, and III disease (all p < 0.001) and pathologic stages I, III, and IV (all p-value < 0.05). Meanwhile, those with complete pathologic response (yp0) and pathologic stage II disease did not have statistically significant survival differences between age groups. Five-year OS by pathologic stage was only significantly different for pathologic stage I disease (64.9%, 95% CI 58.7–71.7%, vs. 33.3%, 95% CI 21.5–51.5%).
Fig. 1.
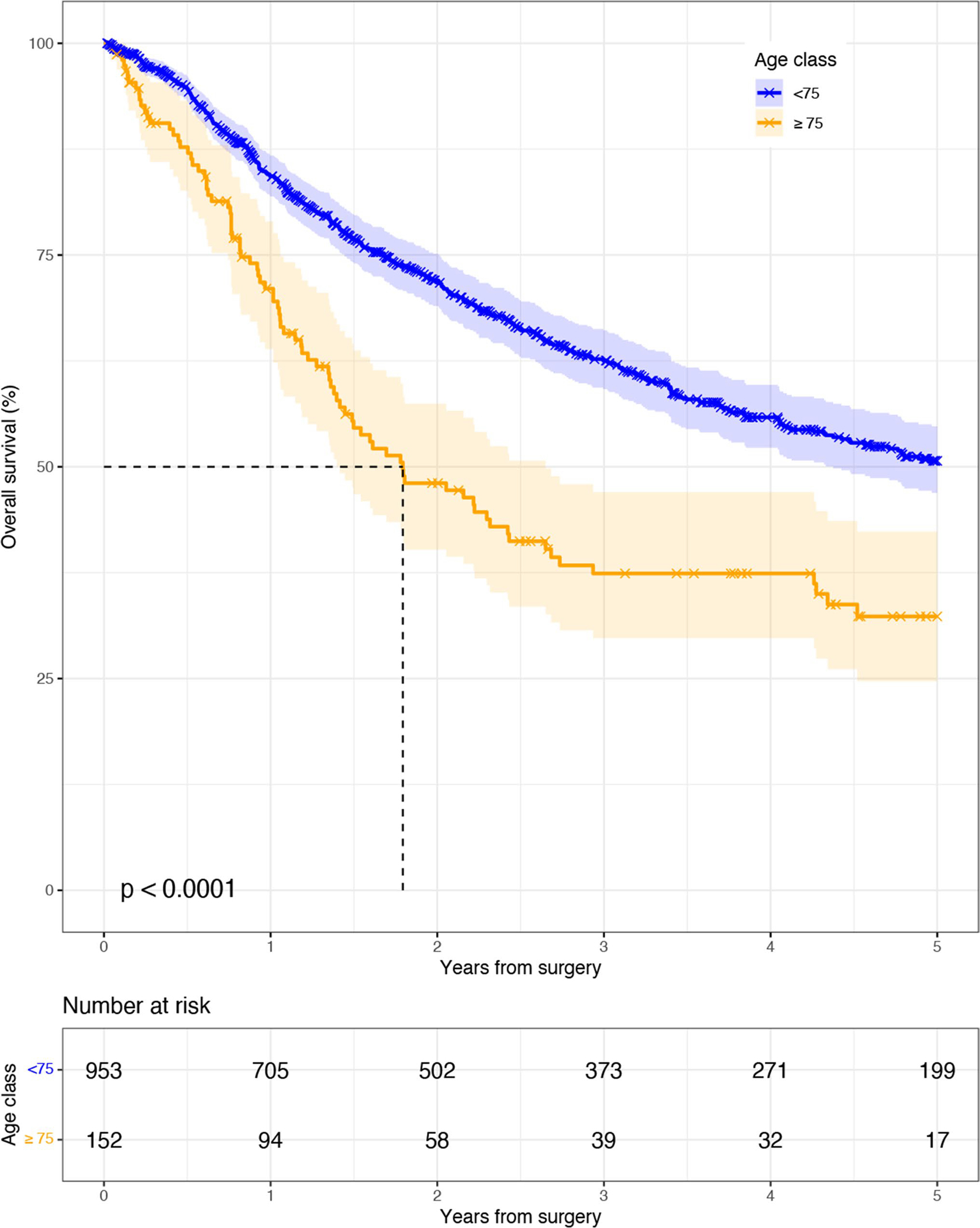
Kaplan–Meier overall survival after esophagectomy by age
Univariable and multivariable Cox proportional hazard models for overall survival are depicted in Supplemental Table 1. In our multivariable Cox model after adjustment for baseline characteristics, comorbidities, tumor characteristics, operative and postoperative outcomes, age ≥ 75 (ref. < 75) demonstrated a significantly increased hazard for death (HR 2.0, 95% CI 1.5–2.8). Furthermore, positive radial margin (ref. negative), any recurrence (ref. none), worse tumor differentiation (ref. well differentiated), and pathologic stage III disease (reference yp0) had increased hazard for death, meanwhile BMI 25–30 (ref. < 18) had a decreased hazard for death (all p < 0.05).
Disease-ree Survival
K-M estimators of DFS, LRDFS, DDFS (Fig. 2a–c) did not show worse disease-free survival by age (all p-value > 0.05). Median DFS was not reached for either group at 5-years; the 5-year DFS of patients < 75 was 53.3% (95% CI 49.7–57.1%) vs 56.8% (95% CI 47.8–67.5%) for ≥ 75. Age ≥ 75 (ref < 75) was not associated with worse ORFS, LRFS, or DRFS in our univariable and multivariable Cox models (Supplemental Tables 2–4 respectfully).
Fig. 2.
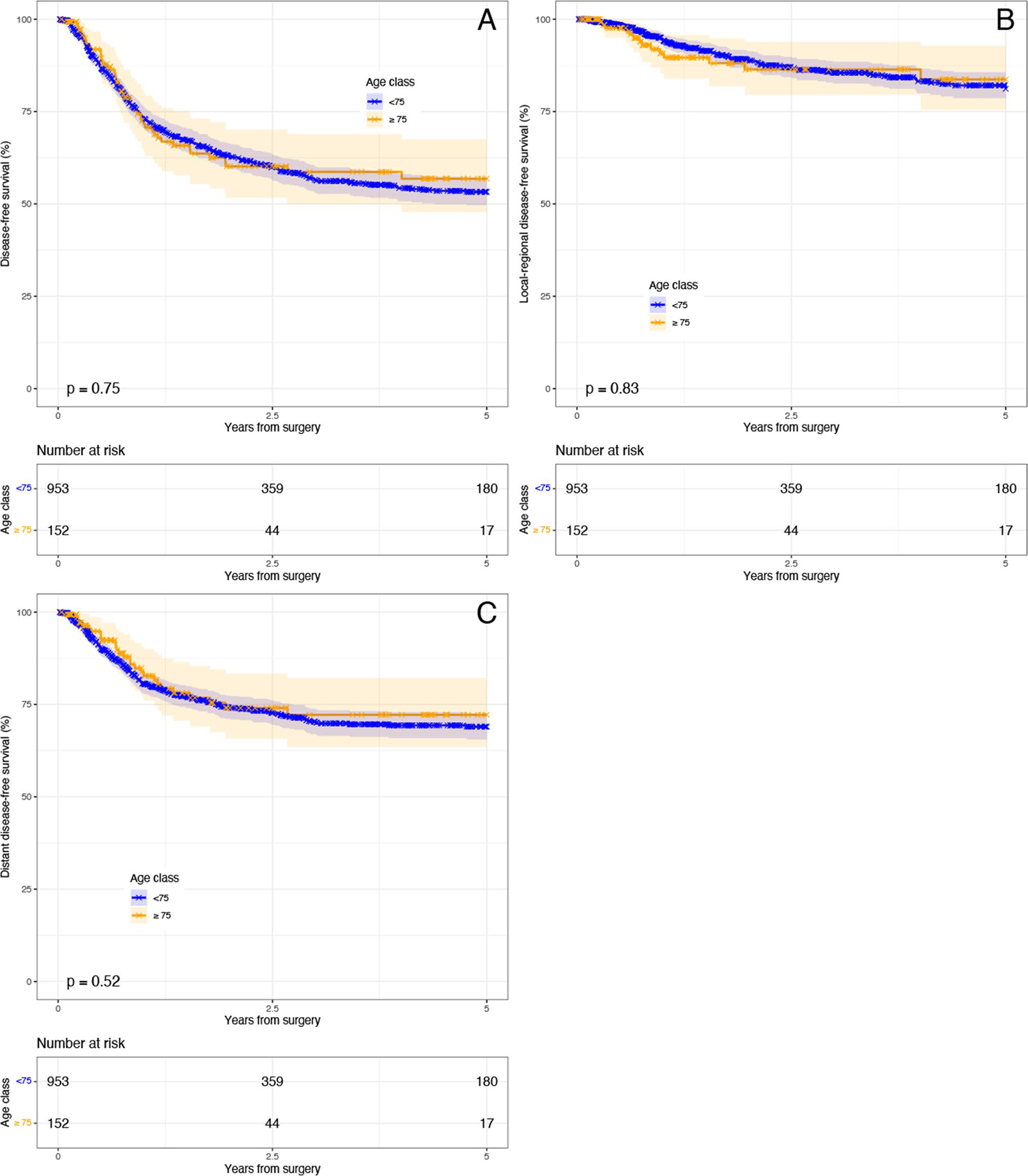
Kaplan–Meier disease-free survival by age. a Overall disease-free survival; b local–regional disease-free survival; c distant disease-free survival
Temporal Analysis Between 2005–2012 and 2013–2020
Characteristics of Overall Cohort
In total, 389 patients underwent esophagectomy between 2005 and 2012 (n = 338 < 75; n = 51 ≥ 75) and 746 patients underwent esophagectomy from 2013 to 2020 (n = 641 < 75; n = 105 ≥ 75).
Baseline characteristics were largely similar for the overall cohort between time periods apart from the number of smoking pack-years, median preoperative albumin, and rates of neoadjuvant therapy (rose from 70.4 to 86.2%) and adjuvant therapy (rose from 8.7 to 14.6%) respectively (all p < 0.05). Tumor characteristics were more advanced in the latter time period. Notably, tumor size (median size 2.8 cm vs. 3.2 cm), differentiation (less grade 2/3 more undefined grade), clinical and pathologic tumor, and nodal descriptors as well as overall stage became more advanced (p < 0.05).
As compared to 2005–2012, from 2013–2020, the performance of the Ivor Lewis technique became more common (63.4% from 24.7%) with a corresponding decrease in the modified McKeown technique. Notably, the overall approach became increasingly totally minimally invasive (77.2% from 43.7%) with open technique dropping from 36 to 9% in the latter time period (all p < 0.001).
In terms of complications, overall, grade II, and grade III complications decreased over time (p < 0.05) (Supplemental Appendix 1). Consequently, median hospital LOS, re-operation rates, and discharge requirements improved with time (all p < 0.05).
Characteristics by Age
Baseline characteristics between age groups differed during each time period which is detailed in Supplemental Appendix 1. But overall, in the latter time period, younger patients tended to have fewer comorbidities and slightly better performance and nutritional status vs older patients. Initially, younger patients had statistically higher rates of neoadjuvant chemoradiation and adjuvant therapy, but in the latter time period, this was only significant for adjuvant therapy (p < 0.05). Despite some change in stage distribution in younger patients, clinical and pathologic stages remained similar between age groups during both time periods.
In terms of operative factors, younger patients in 2005–2012 were likelier to undergo modified McKeown (p < 0.05), but rates became similar by age in the latter time period with Ivor Lewis most commonly performed in either age group. Both age groups had an equivalent rise in all forms of minimally invasive approach for the thoracic and abdominal portions of esophagectomies between 2005–2012 and 2013–2020. These increases also coincided with a rise in total minimally invasive esophagectomies (from 42 to 78.5% and 54.9 to 75.2% for those < 75 and ≥ 75 respectively) and a corresponding drop in open or hybrid methods (all p < 0.05). Consequently, the approach remained similar during both time periods.
Outcomes as a Function of Time by Age
Those < 75 had improved rates of overall and grade II complications over time, while those ≥ 75 had improvement in only overall complication (all p < 0.05). As a result, from 2013 to 2020, overall complication rates became similar with only differences in minor grade II complications (42.0% for < 75-year-old patients vs. 61.0% for ≥ 75-year-old patients, p < 0.001). Over time, patients < 75 experienced improvement in hospital LOS, discharge requirements, and rates of re-operation. Patients ≥ 75 had improved hospital LOS and rates of re-operation. Despite these changes in 2013–2020, older adults still had longer hospital LOS, more discharge needs, and higher re-admission rates as compared to younger patients (p < 0.05).
Overall Survival Changes as a Function of Time
K-M estimators of OS between the two time periods for the overall cohort were not statistically different (p-value = 0.15) (Fig. 3a). When stratified by age (Fig. 3b), neither age group experienced an improvement in overall survival (both p-value > 0.05). However, OS remained significantly better in younger patients compared to older patients in both time periods (both p-value < 0.05). However, in the latter time period, 1- and 5-year survival was not different among age groups (p > 0.05), with similarities in 5-year survival attributable to low event rate and wide confidence intervals.
Fig. 3.
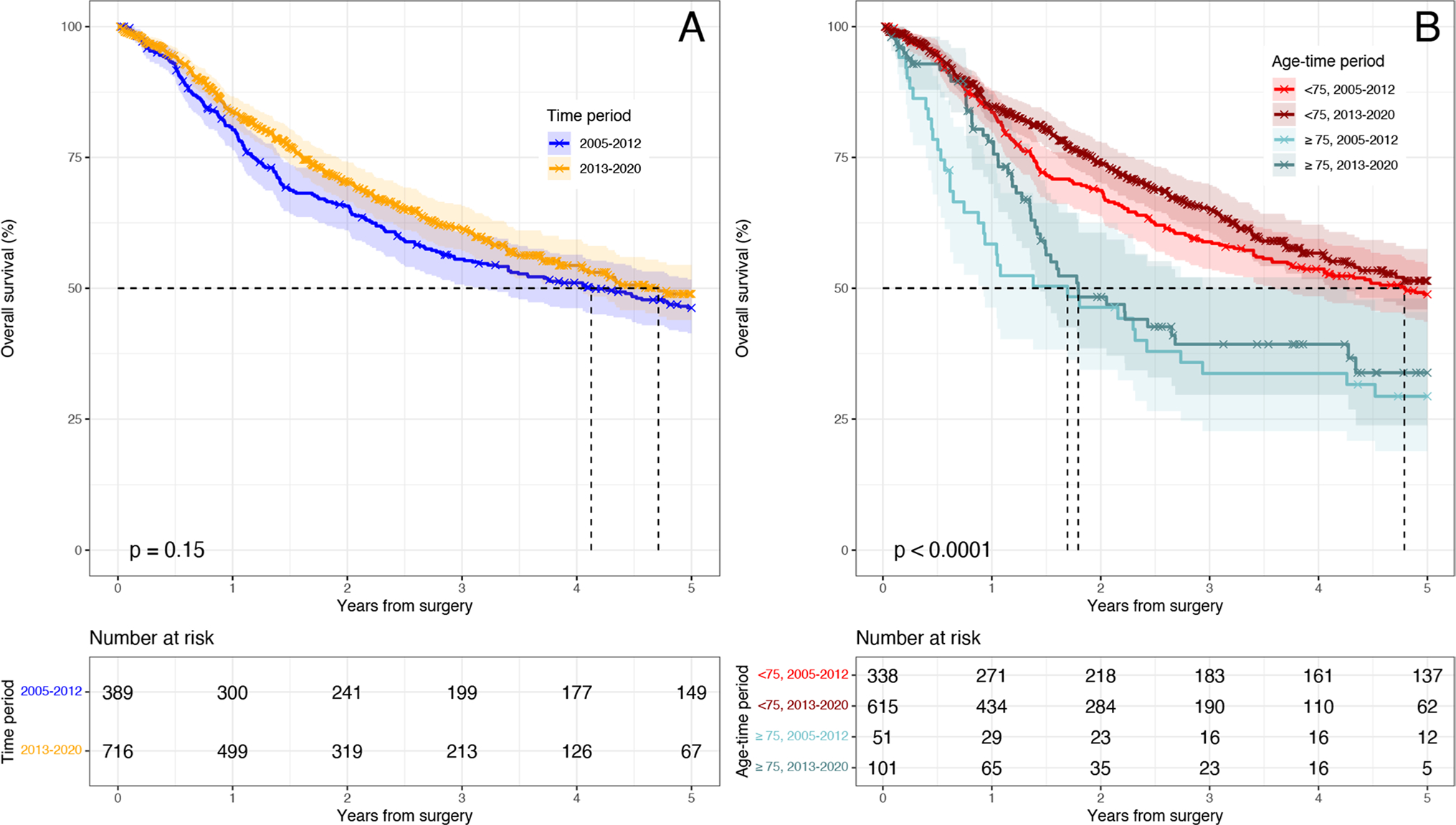
Kaplan–Meier Overall survival between time periods. a Overall survival between time periods; b overall survival between age groups and time periods
After adjusting for time period in our multivariable Cox model, while patients ≥ 75 in 2005–2012 (ref < 75) had a hazard for worse OS (HR 2.1 95% CI 1.2–3.5; p = 0.0059), this became nonsignificant from 2013 to 2020. Furthermore, the following variables were associated with increased risk of death: positive radial margin, recurrence, worse tumor differentiation, and pathologic stage 3 disease (ref. yp0) (all p < 0.05).
Disease-Free Survival as a Function of Time
KM curves examining DFS, LRDFS, and DDFS for the overall cohort between time periods were not statistically different, nor was DFS after stratification for time and age (all p-value > 0.05) (Fig. 4). In our time adjusted Cox model, age was not independently associated with DFS, LRDFS, and DDFS (all p < 0.05).
Fig. 4.
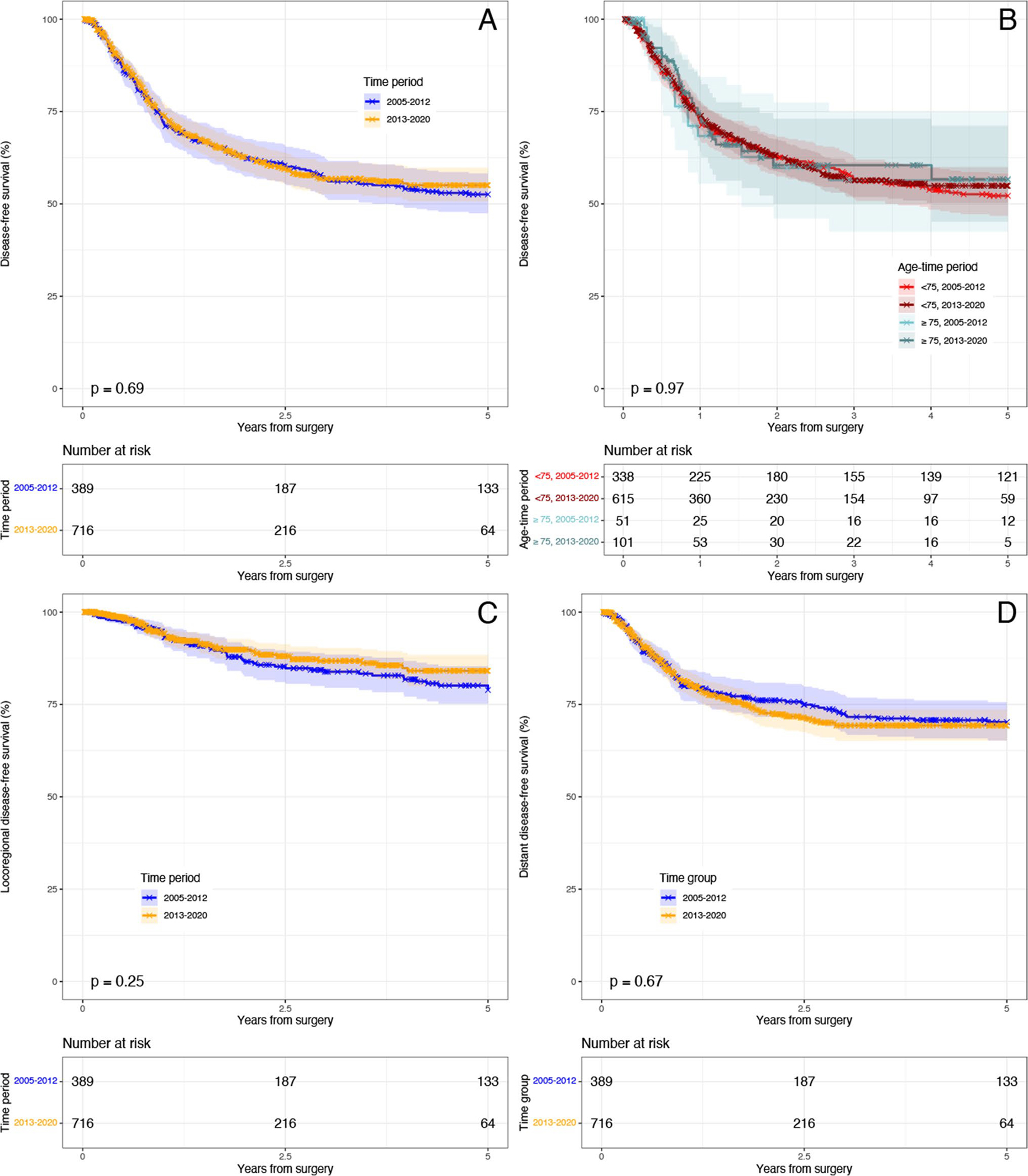
Kaplan–Meier disease-free survival between time periods. a Overall disease-free survival by time; b overall disease-free survival by age and time; c locoregional disease-free survival by time; d distant disease-free survival by time
Discussion
This is a retrospective study of 1135 esophagectomies which analyzed outcomes and survival in younger (< 75) and older (≥ 75) adults, over a 15-year time period at a large academic tertiary referral center. This surgical era saw the development of numerous improvements in minimally invasive surgical techniques, postoperative care, and increased use of preoperative preparation of the patient. We found that older adults experienced an increase in only minor perioperative complications and clinically insignificant prolonged LOS (net difference of 1 day) compared to their younger counterparts. Furthermore, these specific outcomes improved over time, reflective of increased performance of minimally invasive methods and improved perioperative care.
In terms of overall survival, younger patients were found to have a clinically and statistically significant survival advantage compared to older patients which diverged after 1 year after surgery, while disease-free survival was similar between the age groups and median DFS was not reached for either group at 5 years. However, when adjusted for clinical and pathologic stage, the survival benefit was not uniform, and notably, we found no survival difference by age in those with complete pathologic treatment response which was likely due to reduced risk of recurrence in those who experienced a complete pathologic response. This finding further supports the benefit of appropriate utilization of neoadjuvant therapy in older adults. As our study demonstrated, neoadjuvant and adjuvant therapy were used less frequently in older adults, which is consistent with previous studies.13,14 This further exemplifies the concept of undertreatment in older adults,15 the need to provide better equity of care, and the increased willingness to offer neoadjuvant therapy and surgery as experience was gained.
Another possible explanation for larger differences in survival for the clinical stage (that was not as present in pathologic stage) may be attributed to potential clinical mis-staging. However, despite the above-mentioned improvements in perioperative care, surgical technique, and utilization of neoadjuvant therapy, we did not see improvement in overall survival over time within our own cohort. These findings likely reflect unmeasured frailty factors, more statistically advanced stage disease in the latter time period, and other competing mortality causes in older adults. Improvements in perioperative care have improved short-term outcomes as demonstrated by a confluence of 1-year survival in the latter time period, but without considerable effect on long-term outcomes. These findings further support the need for a comprehensive geriatric assessment prior to treatment in older adults7 and guidelines supporting the use of validated tools to estimate noncancer-based life expectancy.16
The survival we found in older adults is in line with other studies3 which suggest a survival benefit as compared to definitive chemoradiation for these patients. Our survival does appear better than historic studies, such as that by Cijs et al.4 which examined cases from 1985 to 2005, and more in line with findings by Morita et al.17 While our survival was worse than that observed by Tapias et al.18 in their analysis of long-term survival by age, we believe this was a reflection of the more advanced stage distribution in our cohort.
From an oncologic perspective, our results are reassuring as we found that recurrence rates remained similar and confer the benefit of esophagectomy; recurrence was more related to stage and pathologic factors than surgery. Our findings are in-line with similar studies from large academic centers in older adults.18,19 However, unlike the study by Tapias et al.,18 RFS was notably worse in younger patients, which we attribute in part due to subtle differences in age categorization and more advanced tumor stage within our cohort. In order to achieve the long-term benefit from recurrence-free survival, better risk-stratification and optimization are required as noted above. As such, despite the perceived survival benefit with esophagectomy, older adults and their families should have clear goals of care discussion to weigh the trade-offs and risks of surgery to achieve oncologic resection with the potential for reduced long-term survival.
This study has several limitations. First, this is a retrospective analysis of a single-center prospectively collected database. As such, certain variables such as baseline kidney disease, frailty measures, and neoadjuvant and adjuvant regimen and toxicity as well as causes of death outside the hospital were often not reported. Second, we acknowledge the potential for selection bias as we only analyzed data for those patients who were selected for surgery, and particularly at a large tertiary referral center.
Our future work will focus on perioperative geriatric assessment and interventions to both quantify and optimize frailty within our patient population.
Conclusion
Select older adults at tertiary hospitals can undergo esophagectomy with similar short-term morbidity and mortality to younger patients. While recurrence-free survival is similar, long-term survival is significantly better in younger patients, and despite improvements in perioperative care, these outcomes did not improve with time. However, our results suggest that survival may be similar in those with complete pathologic response which suggest a benefit to neoadjuvant therapy in older adults.
Supplementary Material
Funding
This study received support in part through the generous donations of Bruce Bartlett and Family and the Jack Mitchell Thoracic Oncology Fellowship (ARD). This work is supported by the Harvard Translational Research in Aging Training Program (National Institute on Aging of the National Institutes of Health: T32AG023480) (CD). The authors report no conflicts of interest.
Footnotes
Declarations
Conflicts of interest Doctors Aaron R. Dezube, Luis E. De-Leon, Suden Kucukak, Lisa Cooper, Daniel Dolan, Michael T. Jaklitsch, Abby White, Bayonle Adenoma, Laura Frain, and Jon O. Wee have no conflicts of interest or financial ties to disclose as well as Mr. Daniel N. Lee and Ms. Emily Polhemus. Dr. Swanson is a consultant for Ethicon. Dr. Bueno has grants from NCI, NIBIB, NHLBI, DoD, Roche, Genetech, Merck, Siemens, Verastem, Gristone, Northpond, Epizyme, and Intuitive Surgical as well as receives consulting fees from Regeneron and receives payment for expert testimony to Thornton Law Firm LLP, Blakinship & Keith, PC, Dolan Dobrinsky Rosenblum and Bluestein, Kelley and Uustal, Foster & Eldridge LLP, and Adler Cohen Harvey Wakeman Guekguezian LLP; he further has patents licensed to BWH and participates on Data Safety Monitoring Board or Advisory Board for Novocure; finally, he has equity in Navigation Sciences. Emanuele Mazzola receives consulting feeds from the VeraMedica Institute LLC.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11605-022-05295-z.
References
- 1.Cancer of the Esophagus - Cancer Stat Facts. SEER, https://seer.cancer.gov/statfacts/html/esoph.html (accessed 13 May 2020).
- 2.Chang AC, Lee JS. Resection for Esophageal Cancer in the Elderly. Thorac Surg Clin 2009; 19: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrow NE, Raman V, Jawitz OK, et al. Impact of Age on Surgical Outcomes for Locally Advanced Esophageal Cancer. The Annals of Thoracic Surgery 2021; 111: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg 2010; 90: 900–907. [DOI] [PubMed] [Google Scholar]
- 5.Schlottmann F, Strassle PD, Nayyar A, et al. Postoperative outcomes of esophagectomy for cancer in elderly patients. Journal of Surgical Research 2018; 229: 9–14. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. New England Journal of Medicine 2012; 366: 2074–2084. [DOI] [PubMed] [Google Scholar]
- 7.Launching a Geriatric Surgery Center: Recommendations from the Society for Perioperative Assessment and Quality Improvement - Cooper - 2020 - Journal of the American Geriatrics Society - Wiley Online Library, 10.1111/jgs.16681 (accessed 17 May 2021). [DOI] [PubMed] [Google Scholar]
- 8.Wee JO, Bueno R, Swanson SJ. Minimally invasive esophagectomy: the Brigham and Women’s Hospital experience. Ann Cardiothorac Surg 2017; 6: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson SJ, Sugarbaker DJ. The three-hole esophagectomy. The Brigham and Women’s Hospital approach (modified McKeown technique). Chest Surg Clin N Am 2000; 10: 531–552. [PubMed] [Google Scholar]
- 10.Risk of chyle leak after robotic versus video-assisted thoracoscopic esophagectomy | SpringerLink, 10.1007/s00464-021-08410-4 (accessed 5 March 2021). [DOI] [PubMed] [Google Scholar]
- 11.Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017; 6: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper L, Dezube AR, De León LE, et al. Outcomes of trimodality CROSS regimen in older adults with locally advanced esophageal cancer. Eur J Surg Oncol. Epub ahead of print 17 April 2021. 10.1016/j.ejso.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin P, O’Leary E, Deady S, et al. The Uptake and Efficacy of Neoadjuvant Therapy in Older Adults with Locally Advanced Esophogastric Cancer. J Gastrointest Canc 2020; 51: 893–900. [DOI] [PubMed] [Google Scholar]
- 15.DuMontier C, Loh KP, Bain PA, et al. Defining Undertreatment and Overtreatment in Older Adults With Cancer: A Scoping Literature Review. J Clin Oncol 2020; 38: 2558–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohile SG, Dale W, Somerfield MR, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 2018; 36: 2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita M, Otsu H, Kawano H, et al. Advances in esophageal surgery in elderly patients with thoracic esophageal cancer. Anticancer Res 2013; 33: 1641–1647. [PubMed] [Google Scholar]
- 18.Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg 2013; 95: 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda M, Koike M, Tanaka C, et al. Feasibility of subtotal esophagectomy with systematic lymphadenectomy in selected elderly patients with esophageal cancer; a propensity score matching analysis. BMC Surg; 19. Epub ahead of print 15 October 2019. 10.1186/s12893-019-0617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


