Abstract
Sedentarism and chronic non-communicable diseases have been a worldwide health problem that is drastically exacerbated by the COVID-19 pandemic social impacts. Home-based exercises are widely encouraged during social isolation to counterbalance the physical inactive impacts. Although, in the context of hypertension, are home-based exercises effective in blood pressure controlling? Our objective is to conduct a systematic review of high-quality controlled trials comparing the possible effects of different types of home-based exercises in hypertensive patients. The literature search was carried out in three scientific databases: Medline, Europe PMC, and Lilacs. Articles were included following three criteria: analyzing the effect of home-based exercise programs on blood pressure in treated and untreated hypertensive patients; exercises must perform at home and on the frequency, intensity, time, and type (FITT) principle, and the articles were published in English. From the qualitative analysis of 27 original trials screened through 451 identified studies, the main results are the following: 1) both endurance, isometric strength, and respiratory home-based exercise programs were efficient to decrease blood pressure in hypertensive patients; 2) differences in methodological approaches regarding FITT components, distinct blood pressure values at baseline and specific underlying mechanisms must be considered as a potential bias of each home-based interventions. In conclusion, endurance, isometric strength, and breathing home-based programs seems to be effective to reduce blood pressure in hypertensive patients. However, further randomized controlled trials and mechanistic studies must be performing to guide evidence-based recommendations of home-based exercises as antihypertensive therapy.
Keywords: Hypertension, Breathing Exercises, Endurance Training, Resistance Training, Social isolation
Background
Hypertension remains in the leadership of the causes of deaths globally (> 10.4 million deaths per year). Although billion people worldwide are hypertensive, less than 1 in 5 people have controlled blood pressure [1]. High blood pressure may cause heart damages through the hardening of arteries, decreasing blood flow, and oxygen perfusion to the heart muscle and other tissues. Hypertension is considered as one of the main risk factors for cardiovascular diseases, among others, such as stroke and kidney failure [2].
Sedentarism is the main modifiable risk factor for hypertension development [3]. In opposite, a physically active lifestyle is the best-established non-pharmacological countermeasure to reduce the risk of cardiovascular diseases [4]. According to a reference guideline [5], to be considered physically active adults must perform physical activities for at least 150 min per week of accumulated moderate-intensity or 75 min per week of vigorous-intensity aerobic physical activity (or an equivalent combination of moderate and vigorous activities).
However, sedentarism has a pandemic scale, reaching 28% of adults in the world population [6]. In 2020, the COVID-19 pandemic seems to be increased sedentarism numbers [7], in part due to the adoption of social distancing that suspended many opportunities to exercise, including cardiac rehabilitation services and community health programs [8]. Several position statements have encouraged people to stay active at home, trying to reverse or counterbalance the additional impact of social distance on physical inactivity [9, 10]. Indeed, home-based exercises are considered an alternative for center-based exercise programs so to minimize the discontinuation of regular physical activities.
The effectiveness and safety of exercise training as a frontline non-medication therapy to control blood pressure is well-establish in the literature [11]. Aerobic exercise training has an independent antihypertensive effect that could be added by antihypertensive drugs [12, 13]. Post-exercise hypotension is a common acute effect observed after moderate and dynamic exercise, especially in hypertensive patients. This phenomenon describes the blood pressure falls after a single exercise session due to the persistent reduction in vascular resistance that is not completely offset by the increased cardiac output. Among the possible mechanisms, there are (1) the increment in exercise-induced vasodilator substances; and (2) the arterial baroreflex resetting, which reduces peripheral sympathetic nervous activity [13]. This hypotensive effect of exercise can be extended up to 12 h in hypertensive patients, being plausible to consider that accumulated exercise sessions could provoke a long-lasting effect and a chronic reduction in blood pressure basal values [13, 14].
Therefore, home-based exercises that aimed to control blood pressure in hypertension are the focus of this systematic review. The current study aimed to conduct a systematic review of high-quality controlled trials, following the PRISMA recommendations, to compare the effects of different types of home-based exercises in hypertensive patients.
Main text
Methods
Bibliographic search
The current systematic review was structured according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [15].
A systematic search was conducted in Medline, LILACS, and EUROPE PMC databases on July 7th, 2020. All trials were selected and confirmed by all authors. There were no restrictions on publication dates nor patient’s age in the papers evaluated. The search strategy included the following terms: (“home exer*” OR “home-based” or “home-based exercise” or “home-based rehabilitation” OR “home-based functional training” OR “at-home exercise” OR “home-based physical activity”) AND (“blood pressure” OR “high blood pressure” OR “arterial hypertension” OR “hypert*” OR “hypertensive adults”). Filters selected were: Clinical Study, Clinical Trial, Clinical Trial Protocol, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, and Randomized Controlled Trial.
Inclusion criteria
Only original trials in the English language were included. The population of this study was composed of hypertensive individuals, being classified as hypertensive according to the parameters of the American Heart Association, whose Systolic Pressure value is equal to or higher than 130 mmHg and the Diastolic Pressure value is equal to or higher than 90 mmHg [5]. Treated and untreated hypertensive patients, with or without comorbidities, such as diabetes, hypercholesterolemia, stroke, previous history of smoking, transient ischemic attack, and acute myocardial infarction, were included. Furthermore, the subjects assigned to the present study were submitted to an intervention based on exercises at home and the FITT principle [16]. Thus, the principal measure is the change on blood pressure after a home-based intervention. Research articles not written in English, review articles, and studies in which intervention was not based on exercise at home were excluded.
Results
Literature research
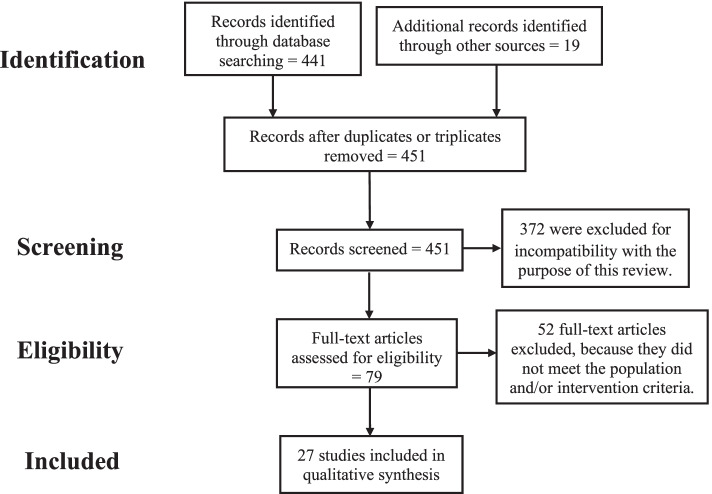
According to pre-established criteria, a total of 441 articles were identified through database searching (Medline = 167; EUROPE PMC = 246; and LILACS = 28), 19 from other sources (study’s references), but 9 were duplicates, remaining 451 articles. After screening the titles, abstracts, and references, 372 were excluded as they did not meet inclusion criteria. Of the remaining 79 eligible full-text articles, and 52 were removed because they did not meet population and/or intervention criteria. Finally, 27 articles were included in the qualitative analysis (Fig. 1). Besides, studies included in qualitative synthesis were evaluated from their risk of bias by each author independently employing the McMaster clinical review form [17]. The consensus was obtained in a later meeting. The results are described in Table 1.
Fig. 1.
Flowchart of the systematic review process according to PRISMA model [18]
Table 1.
Risk of bias from studies included in qualitative synthesis
| Author, year | Purp | Lit | Study Design | Sample | Outcome | Intervention | Results and Statistical analysis | Con | Total (/17) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | |||||
| Coghill and Cooper, 2008 [19] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| Hua, 2009 [20] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Suter et al., 1990 [21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 14 |
| Staffileno et al. 2007 [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Farinatti et al., 2005 [23] | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 12 |
| Farinatti et al., 2016 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Blackwell et al., 2017 [25] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 15 |
| Punia and Kulandaivelan, 2020 [26] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 15 |
| Gordon et al., 2018 [27] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Taylor et al., 2019 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| McCaffrey et al., 2005 [29] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Wolff et al. 2013 [30] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 15 |
| Wolff et al., 2016 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 16 |
| Sujatha and Judie, 2014 [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| Schein et al. 2001 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Viskoper et al. 2003 [34] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 11 |
| Logtenberg et al. 2007 [35] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Anderson et al., 2010 [36] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 13 |
| Meles, 2004 [18] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Rosenthal et al., 2001 [37] | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 11 |
| Elliot et al. 2004 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 15 |
| Schein et al. 2009 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 16 |
| Grossman et al. 2001 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 15 |
| Ublosakka-Jones et al., 2018 [41] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
| Jones et al., 2015 [42] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| Jones et al., 2010 [43] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 17 |
| Sangthong et al., 2016 [44] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 |
I: Controlled; II: Randomized; III: Before and after IV: Described; V: Size justified; VI: Reliable VII: Valid; VIII: Describe in details; IX: Contamination avoided; X: Co-intervention avoided; XI: Reported statistical significance; XII: Analysis appropriate; XIII: Clinical importance reported; XIV: Drop-outs. 1 = Yes; 0 = No; Purp.: Study purpose; Lit.: Literature background. Con.: Clear conclusions. N/A: not applied
Table 2 presents endurance and/or isometric exercise programs. Endurance training was performed from low to moderate [19, 20], moderate to vigorous intensity [22], vigorous [21, 23, 24] and high intensity interval training (HIIT) [25] according to American College of Sports Medicine guideline [16]. Endurance training duration ranged from three to five days a week during four weeks to sixteen months.
Table 2.
Qualitative synthesis of clinical trials from aerobic and strength training included in the systematic review
| Author, year | Sample | Interventions (F.I.T.T) | BP at baseline | Outcomes |
|---|---|---|---|---|
| Coghill and Cooper, 2008 [19] | EXP: 38♂; 54.8 ± 5 yrs CTL: 29♂; 55.6 ± 4.7 yrs |
F: At least 5 days/wk for 12 wks; I: RPE of 12–14; T: at least 30 min T: Walk Briskly |
SBP 138 ± 16; DBP 90 ± 10 mmHg | ↓SBP; ↔ Resting DBP; ↓BMI; ↓BF; ↓Waist-hip-ratio |
|
Hua, 2009 [20] |
EXP: 10♂ 10♀; ♂55.8 + 9.5 yrs; ♀56.3 + 9.6 yrs CTL: 10♂ 10♀; ♂55.9 + 10.2 yrs; ♀58.5 + 11.3 yrs |
F: 4 days/wk for 12 wks; I: 35–40% HR reserve and RPE 11–13; T: 4.8 km/day by the end of 12 weeks T: walking |
Men SBP 140 ± 11; DBP 92 ± 7 mmHg Women SBP 141 ± 16; DBP 87 ± 9 mmHg |
↓SBP and DBP; ↔ HR |
|
Suter et al., 1990 [21] |
EXP: 39 ♂; 38.8 ± 8.9 yrs CTL: 22 ♂; 35.2 ± 7.3 yrs |
F: 2–6 sessions (self-managed) for 4 months I: 75–86% of HRmax T: at least 120 min per wk T: jogging or walking/jogging |
SBP 134 ± 15; DBP 89 ± 11 mmHg | ↔ SBP and DBP; ↓Waist-hip-ratio; ↓BMI; ↑endurance capacity |
| Staffileno et al. 2007 [22] |
EXP: 13 ♀; 38.6 ± 5 yrs CTL: 10 ♀; 40.2 ± 6.1 yrs |
F: 2–3 sessions/day for 8 wks I: 50–60% HRR T: 10 min/session; 150 min/wk T: lifestyle physical activity (e.g., walking, stair climbing) |
SBP 136 ± 7; DBP 91 ± 5 mmHg | ↓SBP ↔ resting DBP |
| Farinatti et al., 2005 [23] |
EXP: 26♂ 52♀; 52 ± 12 yrs CTL: 9♂ 7♀; 48 ± 9 yrs |
F: 3 days/wk for 16 wks I: 60–80% maximum HR for the age T: 30 min T: Aerobic activity and flexibility exercises |
Not reported | ↓SBP and DBP; ↓Weight; ↓WHR; ↓ SM; ↓ %BF; ↑TF |
| Farinatti et al., 2016 [24] |
EXP: 7♂ 22♀; 53 ± 11 yrs CTL: 5♂ 9♀; 48 ± 5 yrs |
F: 3 days/week for 16 months I: 60–85% HRmax (220 – age) T:30 min T: walking and stretching exercises |
SBP 141 ± 20; DBP 85 ± 8 mmHg |
↓SBP, DBP and MBP ↓COL; ↑HDL; ↓TRI; ↓BMI; ↓waist circumference; %BF, ↑TF |
| Blackwell et al., 2017 [25] |
EXP H-HIIT: 6♂♀; 52.2 ± 2 yrs EXP H-IHGT: 6♂♀; 51.5 ± 2.3 yrs |
H-HIIT: F: 3 days/wk for 4 wks; I: max of repetitions with HR over 85% (HRmax [220 – age]) T: 2 min warm-up + 5 × 1 min of equipment-free T: HIIT (star-jumps, squat thrusts, and static sprints) H-IHGT: F: 3 days/wk for 4 wks I: 30% MVC and HR over 85% (HRmax [220 – age]) T: 4 × 2 min T: isometric handgrip exercise |
H-HIIT: SBP 130 ± 5; DBP 81 ± 5 mmHg H-IHGT: SBP 138 ± 4; DBP 93 ± 3 mmHg |
H-HIIT: ↔ SBP; ↔ DBP; ↑AT; ↑VO2max H-IHGT: ↓SBP; ↔ DBP; ↔ AT; ↔ VO2max |
| Punia and Kulandaivelan, 2020 [26] |
EXP: 10♂ 10♀; 30–45 yrs; CTL: 10♂ 10♀; 30–45 yrs |
F: 3 days/wk for 8 wks I: 30% MVC T: 4 × 2 min T: isometric handgrip exercise |
SBP 144 ± 8; DBP 93 ± 5 mmHg | ↓SBP; ↓DBP; ↓MBP; ↓HR; ↔ PP |
|
Gordon et al., 2018 [27] |
EXP: 2♂ 7♀; 47 ± 12 yrs CTL: 2♂ 3♀; 47 ± 9 yrs |
F: 3 days/wk for 12 wks I: 30% MVC T: 4 × 2 min T: isometric handgrip training |
SBP 137.7 ± 4.1; DBP 88.4 ± 0.8 mmHg | ↔ SBP and DBP |
|
Taylor et al., 2018 [28] |
EXP: 24♂; 30–65 yrs; CTL: 24♂; 30–65 yrs |
F: 3 days/wk for 4 wks I: compatible HR from isometric exercise test T: 4 × 2 min T: isometric wall squat exercise |
SBP 137 ± 11; DBP 78 ± 7 mmHg | ↓SBP; ↓DBP; ↓PP; ↔ HR; ↑SV and CO at rest; ↓TPR at rest; ↓LF/HF and ↓LFn at rest; ↑HFn, ↑PSD and ↑BRS at rest |
EXP Experimental group, CTL Control group, H-IHGT Home-Isometric Hand-Grip Training, BMI Body mass index, BF Body fat, COL Total cholesterol, HDL HDL cholesterol, TRI Triglycerides, MVC Maximal Voluntary Contraction, wk Week, wks Weeks, Min Minutes, yrs Years, max Maximum, HRR Heart Rate Reserve, F.I.T.T. Frequency, intensity, time, and type of exercise, TPR Total peripheral resistance, BP Blood pressure, SBP Systolic Blood pressure, DBP Diastolic Blood Pressure, MBP Mean Blood pressure, HR Heart Rate, PP Pulse Pressure, PSD R–R power spectral density, HFn High frequency R-R in normalized units (%), LFn Low frequency R-R in normalized units (%), LF/HF Symphato-vagal balance, BRS Spontaneous baroreflex sensitivity, RPE Rate of perceived exertion, HIIT High-Intensity Interval Training, H-HIIT Home-High-Intensity Interval Training, VO2max Maximum oxygen uptake, AT Anaerobic threshold, WHR Waist-hip measurements, %BF Body fat percentage, SM Sum of skinfols measurements, TF Trunk flexibility, HRmax Maximum heart rate, SV Stroke volume, CO Cardiac output, ↓ decreased, ↑ increased, ↔ unchanged
In three studies, isometric handgrip exercises [26–28] had similar target intensity (30% of maximal voluntary contraction). In one study, isometric wall squat training intensity was controlled by a target heart rate (HR) [45]. Isometric exercise programs were performed three times a week for four to twelve weeks.
Table 3 presents breathing training that includes yoga, device-guided breathing exercises, and slow breathing training with or without inspiratory loading. Yoga programs were composed by breathing and volume-controlled exercises with trunk movements [29–32], device-guided breathing exercises were performed without inspiratory load [18, 33–36], and slow breathing training programs were performed also without load [37–40] or with absolute inspiratory resistive loading (IRL) [18–20 cmH2O] [42–44] or relative IRL defined as 25% of the maximum inspiratory pressure [41]. Regarding training volume, yoga was performed from two days a week to twice-daily sessions (15 min) for 8 to 12 weeks. Device-guided breathing exercises were always performed 7 days/week for 4 to 8 weeks, and slow breathing training (with or without IRL) once or twice daily sessions for 8 weeks.
Table 3.
Qualitative synthesis of clinical trials from breathing training included in the systematic review
| Author, year | Sample | Interventions (F.I.T.T) | BP at baseline | Outcomes |
|---|---|---|---|---|
| McCaffrey et al., 2005 [29] |
EXP: 10♂ 17♀; 56.7 yrs CTL: 9♂ 18♀; 56.2 yrs |
F: 3 days/wk for 8 wks I: unloading breathing I: 63 min T: Yoga |
SBP 161 ± 10; DBP 98 ± 8 mmHg | ↓SBP; ↓DBP; ↓HR; ↓BMI |
| Wolff et al. 2013 [30] |
EXP: 8♂ 20♀; 64 ± 10.3 yrs CTL: 11♂ 16♀; 60.8 ± 11 yrs |
F: 7 days/wk for 12wks I: unloading breathing T: 15 min/day T: Yoga |
SBP 144 ± 14; DBP 88 ± 6 mmHg | ↔ SBP; ↓DBP |
| Wolff et al., 2016 [31] |
EXP: 44♂ 52♀; 64.7 ± 9.2 yrs CTL: 48♂ 47♀; 64.8 ± 7.6 yrs |
F: 7 days/wk; 2 sessions/day for 12wks I: unloading breathing T:15 min T: Yoga |
SBP 149 ± 12; DBP 88 ± 6 mmHg | ↔ SBP; ↔ DBP; Improved self-rated QOL; PSS and HADS |
| Sujatha and Judie 2014 [32] |
EXP: 55♂ 63♀; 30–60 yrs CTL: 55♂ 65♀; 30–60 yrs |
F: 5 days/wk for 12wks I: unloading breathing T: 30–45 min T: Hatha Yoga |
SBP 153 ± 12; DBP 95 ± 7 mmHg | ↓SBP and DBP; ↓HR; ↓BMI ↓Level of stress and anxiety |
| Schein et al. 2001 [33] |
EXP: 18♂ 14♀; 57.8 ± 9.4 yrs CTL: 13♂ 20♀; 56.5 ± 8 yrs |
F: 7 days/wk for 8 wks I: unloading breathing T: 10 min T: Device-guided breathing |
SBP 157 ± 14; DBP 97 ± 9 mmHg | ↓SBP and DBP |
| Viskoper et al. 2003 [34] | EXP: 10♂ 7♀; 66.5 ± 7.6 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 15 min T: Device-guided breathing |
SBP 155 ± 10; DBP 89 ± 8 mmHg | ↓SBP and DBP; ↓HR |
| Logtenberg et al. 2007 [35] |
EXP:3♂ 12♀; 62.7 ± 6 yrs CTL: 10♂ 5♀; 61.0 ± 7.5 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 10 min T: Device-guided breathing |
SBP: 154 ± 8; DBP 83 ± 6.7 mmHg | ↓SBP and DBP |
| Anderson et al., 2010 [36] |
EXP: 12♂ 8♀; 53.4 ± 2.8 yrs CTL: 9♂ 11♀; 52.9 ± 2.8 yrs |
F: 7 days/wk for 4 wks I: < 10 breaths/min, and often ≤ 6 breaths/min T: 15 min T: Device-guided breathing |
SBP 142 ± 3; DBP 88 ± 2 mmHg | ↓MBP; ↓Breathing rate; ↑Tidal volume; ↓PetCO2; ↓24-h BP |
| Meles, 2004 [18] |
EXP:25♂ 19♀; 57 ± 9 yrs CTL: 15♂ 11♀; 49 ± 12 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 15 min T: Device-guided breathing |
SBP 137 ± 12; DBP 83 ± 9 mmHg | ↓SBP and DBP; ↓HR |
| Rosenthal et al., 2001 [37] | EXP: 7♂ 6♀; 50.5 ± 13.9 yrs |
F: 7 days/wk for 8 wks I: Lowest breathing rate for each user T: 15 min T: Slow breathing training |
SBP 146 ± 15; DBP 85 ± 8 mmHg | ↓SBP; ↓DBP |
| Elliot et al. 2004 [38] |
EXP: 89♀♂;59.5 ± 9.6 yrs CTL: 60♀♂; 58.7 ± 10.5 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 15 min T: Slow breathing training |
SBP 150 ± 8; DBP 85 ± 9 mmHg | ↓SBP and ↔ DBP |
| Schein et al. 2009 [39] |
EXP: 20♂ 13♀; 62 ± 9 yrs CTL: 21♂ 12♀; 63 ± 8 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 15 min/day T: Slow breathing training |
SBP 148 ± 11; DBP 81 ± 9 mmHg | ↓SBP and DBP |
| Grossman et al. 2001 [40] |
EXP: 13 ♂ 5♀; 52 ± 12 yrs CTL: 10♂ 5♀; 50 ± 4 yrs |
F: 7 days/wk for 8 wks I: Unloading breathing T: 10 min T: Slow breathing training |
SBP 160 ± 18; DBP 95 ± 7 mmHg | ↓SBP and DBP |
| Ublosakka-Jones et al., 2018 [41] |
EXP: 8♂ 8♀; 66.4 ± 4.2 yrs CTL: 8♂ 8♀; 68.2 ± 4.8 yrs |
F: 7 days/wk; 2 sessions/day for 8wks I: 25% MIP and 50% HRR; T: 6 breaths/min for 5 min/session; 60 breaths/day T: Slow breathing training |
SBP 141 ± 7; DBP 70 ± 3 mmHg | ↓SBP and DBP;↓PP; ↓HR bpm; ↑MIP; ↑SVC; ↑IC; ↑CE; ↑AE |
| Jones et al., 2015 [42] |
EXP Loaded: 10♂♀; 51.4 ± 5.3yrs EXP No Load: 10♂♀; 53.4 ± 4.3yrs CTL: 10♂♀; 50.4 ± 5.4 yrs |
F: 7 days/wk; 2 sessions/day for 8wks I: IRL of 20 cmH2O I: 30 min T: Slow breathing training F: 7 days/wk; 2 sessions/day for 8wks I: Unloading breathing I: 30 min T: Slow breathing training |
IRL group: SBP 137 ± 13; DBP 81 ± 8 mmHg Unloading breathing group: SBP 136 ± 13; DBP 80 ± 6 mmHg |
IRL group: ↓SBP and DBP; ↓HR; ↓MBP Unloading breathing group: ↓SBP and DBP; ↓HR; ↓MBP |
| Jones et al., 2010 [43] |
EXP Loaded: 4♂ 6♀; 51 ± 5 yrs EXP No Load: 4♂ 6♀; 53 ± 4yrs CTL: 3♂ 7♀; 50 ± 5 yrs |
F: 7 days/wk; 2 sessions/day for 8wks I: IRL of 20 cmH2O T: 30 min T: Slow breathing training F: 7 days/wk; 2 sessions/day for 8wks I: Unloading breathing T: 30 min T: Slow breathing training |
IRL group: SBP 142 ± 8.9; DBP 87 ± 5.2 mmHg Unloading breathing group: SBP 141 ± 5.9; DBP 85 ± 4.4 mmHg |
IRL group: ↓SBP and DBP; ↓HR; ↓PP Unloading breathing group: ↓SBP and DBP; ↓HR; ↓PP |
| Sangthong et al., 2016 [44] |
EXP Load: 4♂ 6♀; 60–70 yrs; EXP No Load: 1♂ 9♀; 60–79 yrs CTL: 3♂ 6♀; 60–74 yrs |
F: 7 days/wk; 30 min/day for 8 wks I: IRL of 18 cmH2O T: 6 breaths/min T: Slow breathing training F: 7 days/wk; 30 min/day for 8 wks I: Unloading breathing T: 6 breaths/min T: Slow breathing training |
IRL group: SBP 144 ± 8.7; DBP 81 ± 6.7 mmHg Unloading breathing group: SBP 141 ± 11.1; DBP 81 ± 6.2 mmHg |
IRL group: ↓SBP; ↔ DBP; ↔ HR; ↓PP Unloading breathing group: ↓SBP; ↔ DBP; ↔ HR; ↓PP |
EXP Experimental group, CTL Control group, BMI Body mass index, PetCO2 Partial pressure of carbon dioxide, IRL Inspiratory resistive loading, self-rated QOL World Health Organization Quality of Life Assessment, PSS Perceived stress scale, HADS Hospital anxiety and depression scale, wk week, wks weeks, min minutes, yrs years, HRR Heart Rate Reserve, F.I.T.T. Frequency, intensity, time, and type of exercise, BP Blood pressure, SBP Systolic Blood pressure, DBP Diastolic Blood Pressure, MBP Mean Blood pressure, HR Heart Rate, PP Pulse Pressure, MIP Maximum inspiratory pressure, SVC Slow vital capacity, IC Inspiratory capacity, CE Chest expansion, AE Abdominal expansion
↓: decreased; ↑: increased; ↔ : unchanged
All home-based exercises, except four [19, 21, 27, 31], showed as a primary outcome the blood pressure reduction post-intervention, and secondary outcomes improvements in cardiac autonomic modulation and baroreflex sensitivity [28], inspiratory muscle strength [41], lipids profile and body composition [reduced body fat] [23], quality of life [30], and cardiorespiratory fitness [46].
Risk of bias
The most design used in home-based studies is the randomized-controlled trial with before and after measurements, but some experimental-controlled studies did not perform and/or described the randomization procedures. For all studies, the literature background and purposes were reported. The sample is well described, but the sample size is justified in twelve studies. Home-based interventions were described in detail, and co-interventions had been avoided since groups were not enrolling in any exercise program. However, the follow-up and monitoring of the control group had been not described in most of the studies, increasing the risk of contamination for this group that may influence the studies’ outcomes.
As regards results, in most of the studies, statistical analysis was appropriated and statistical significance was reported. The clinical importance of results was explored, which is expected since blood pressure reduction is often the primary outcome. However, some studies reported drop-outs throughout intervention protocols. Outcomes were reliable and valid, and conclusions were addressed in most of the studies.
Discussion
The current systematic review extracted qualitative data of 27 original trials screened from 451 identified studies. The major findings are 1) Both endurance, isometric strength, and respiratory home-based exercise programs were efficient to decrease blood pressure in hypertensive patients, but FITT components were different among them; 2) Despite the home-based interventions reduced blood pressure as the primary outcome, underlying mechanisms seem to be distinct; 3) differences in blood pressure values at baseline must be considered as a potential bias of each study’s outcomes.
Review studies demonstrated the safety and the effectiveness of home-based exercises in cardiac rehabilitation [47] and elderly’s falls prevention programs [48]. From the current review, in seven home-based endurance studies, five reduced blood pressure in hypertensive. Blood pressure was reduced in studies with moderate [19, 20], moderate to vigorous [22], and vigorous exercise intensities [23, 24]. However, in one intervention of moderate to vigorous intensity [21] and another with HIIT [25], blood pressure was unchanged. Although exercise intensity is one important factor to obtain the optimal dose–response relationship between exercise training and blood pressure reduction, other FITT components must also be addressed [11, 16]. Notably, aerobic exercise training has a major effect on blood pressure reductions in hypertensive than normotensive population since the magnitude of blood pressure reduction after an aerobic exercise program seems to be dependent on baseline values [49, 50].
As regarding training volume, weekly frequency varied from 3 to 5 days/week, the session duration ranged from 7 (e.g., HIIT protocol) to 30 min (e.g., most of the continuous exercise protocols), and the programs’ durations were between 4 weeks to 16 months. Notwithstanding some differences in methodological approaches, it was possible to identify home-based moderate to vigorous endurance exercise programs, with 30 min average duration per day for 8 weeks to 16 months, to reduce blood pressure in hypertensive patients.
Isometric exercise programs were also performed at home. Among four studies included in this review, three used handgrip training [25–27], and one used isometric wall squat training [28]. In handgrip studies, target intensity (30% of maximal voluntary contraction) and session duration (4 sets of 2 min) were similar. Isometric wall squat training was performed similar to handgrip with session duration (4 sets of 2 min), and intensity was controlled by a target HR. The HR should be compatible at the end of each stage from the isometric exercise test in visit 1 [28]. All home-based isometric exercise programs [25, 26, 28], except one handgrip study [27], showed reductions in blood pressure after interventions with a duration from 4 to 8 weeks (3 day/wk).
The resistance or strength training alone (i.e., without the combination of another training modality) reduced blood pressure in hypertensive and pre-hypertensive adults [51]. Among strength training programs, isometric and dynamic resistance exercises are effective to reduce blood pressure [4]. Isometric training is widely recommended because of its safety, low cost, easy application at home, and is effective in reduced blood pressure in hypertensive subjects [52]. Among the underlying mechanisms, the reduction in sympathetic activity and increase in vagal tone [45], acute improvements in left ventricular function [53], and improved endothelial function [54] are the most common findings.
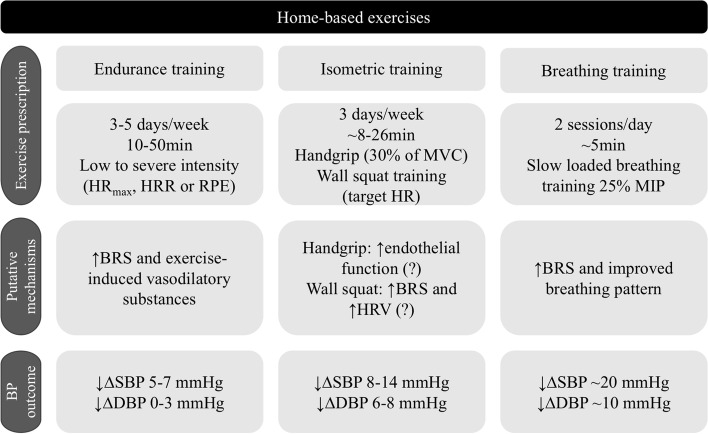
Breathing exercises represent most of the home-based programs included in the current systematic review (n = 18). Breathing exercises include yoga, device-guided breathing exercises, and slow breathing training with or without inspiratory load. Most of these interventions were performed 5 to 7 days a week, and program duration of 4 to 12 weeks. The intensity was controlled by the exercise’s characteristics as yoga (i.e., breathing and volume-controlled exercises with trunk movements) [29–32], the shortness of breathing frequency as device-guided breathing exercises [18, 31, 33, 34, 36], and slow breathing training with [42–44] and without IRL [37–40]. The sessions’ duration ranged from 10 to 15 min daily, except for two yoga studies when the session lasted 30 to 45 min [32] and 63 min [29]. Except for two studies with yoga exercises [30, 31], all home-based breathing training was effective to reduce blood pressure in hypertensive patients. Interestingly, slow breathing with IRL showed more reduction in blood pressure as compared to isometric and endurance exercise interventions, as shown in Fig. 2.
Fig. 2.
Summary of main effects and putative mechanisms from home-based exercise programs on blood pressure in hypertensive. Target HR should be compatible as the end of each stage from the isometric exercise test in visit 1 [46]. HR, heart rate; HRmax, maximal heart rate; HRR, heart rate recovery; HRV, heart rate variability; MVC, maximal voluntary contraction; MIP, maximal inspiratory pressure; RPE, relative perception of effort; BRS, baroreflex sensitivity; SBP, systolic blood pressure; DBP, diastolic blood pressure; (?), represents the unknowing mechanisms
The well-known effects of breathing on blood pressure regulation supported the development of respiratory exercise programs to reduce high blood pressure in hypertensives. The breathing pattern has a strong influence on heart rate and blood pressure dynamics as described by the cardiorespiratory coupling. A slow breathing pattern [55] or a controlled guided-breathing [56] acutely increased the baroreflex sensitivity and the vagal modulation to the heart [57, 58].
Among respiratory exercises, yoga, controlled breathing with and without loading and guided breathing have demonstrated antihypertensive effects [45, 59]. The Yoga trainee executes slow deep breathing as a combination of low frequency and high tidal volume, presenting higher baroreflex sensitivity and lower hypoxic and hypercapnic chemoreflex responses compared to age-matched controls [57]. In hypertensive subjects practicing yoga, breathing exercises and voluntary control of respiration play an important role in acute and chronic blood pressure management [60]. Controlled guided-breathing has been considered for patients who cannot obtain full control of their hypertension with medical therapy alone or cannot tolerate the adverse effects of the treatment, and is recommended for pre-hypertensive or mildly hypertensive individuals to replace drug prescription [61].
Hypertensive patients may have presented reduced blood pressure through different underlying mechanisms that depend on the home-based exercise protocol (i.e., endurance, isometric, and breathing training). Post-exercise hypotension is a common phenomenon observed in hypertensive patients after endurance exercise, which seems to be explained by two putative mechanisms, increased exercise-induced vasodilatory substances and/or the arterial baroreflex resetting [13]. Therefore, it is plausible to consider that accumulated exercise sessions would provoke a long-lasting effect and a chronic reduction in blood pressure basal values [13, 14, 50]. A recent meta-analysis highlighted that aerobic exercise training improved endothelial function contributing to peripheral vascular resistance and blood pressure reductions. Also, a dose–response relationship between exercise intensity and improved flow-mediated dilation was found [62]. It is particularly important, because hypertensive patients show a reduced nitric oxide bioavailability and vasodilatory capacity, exhibiting an increased vasoconstrictor tone [63]. As regards neural mechanisms, endurance exercise modulates the contributions from the autonomic nervous systems in blood pressure regulation normalizing the sympathetic overactivity observed in hypertension and resetting baroreflex sensitivity [13, 64]. Center-based endurance exercise, 60 min three days per week performed at 70% peak VO2 for 4-months, reduced muscle sympathetic nerve activity, improved baroreflex sensitivity and restored blood pressure to normotensive control levels [64]. Besides, home-based endurance training reduced weight, waist-hip-ratio and body fat in hypertensive patients, supporting the reductions in blood pressure [23, 24].
Both endurance and resistance training has been shown to improve baroreflex control as well as vascular function [65]. Otherwise, the putative mechanisms by which isometric exercise training reduces blood pressure in hypertension remain unclear. Cahu Rodrigues et al. [54] demonstrated that 12 weeks of center-based isometric handgrip training improved markers of endothelial function, reducing blood pressure and arterial stiffness in hypertensive patients. Regarding the neural control of the circulation, a study included in the current review [45] showed increased baroreflex sensitivity and reduced sympatho-vagal balance after a home-based isometric wall squat exercise training in hypertensive patients. However, a recent meta-analysis indicates that isometric handgrip training does not improve cardiac autonomic modulation in normotensive as well as in hypertensive subjects [66]. Taken together, some evidence suggests that a low body mass-based isometric training (i.e., handgrip) reduces blood pressure in hypertensive patients due to vascular mechanisms but does not affect neural control of the heart, while a high body mass-based isometric training (i.e., wall squat exercise) improves cardiovascular modulation and reduces blood pressure after a home-based program in hypertensive patients. Little is known about wall squat exercise training, thus the vascular mechanisms, as endothelial function, involved in blood pressure reduction are still to be elucidated.
Among the home-based interventions reviewed in the current study, breathing training have a well-established mechanism that wherefore reduces blood pressure in hypertensive patients. In hypertension, the autonomic imbalance involved in reduced or reset baroreflex sensitivity and chemoreflex induced hyperventilation increases cardiac output, peripheral resistance and blood pressure [67]. The prolonged exhalation in slow or in device-guided breathing exercises, seems to improve baroreflex sensitivity and reduce sympathetic nerve drive and vasoconstriction tone in hypertensive patients. Probably, the activated pulmonary mechanoreceptors that respond to the increased tidal volume (as occurs in slow and deep breathing) act in concert with cardiac mechanoreceptors to inhibit sympathetic outflow to peripheral blood vessels, leading vasodilatation and reducing peripheral resistance and blood pressure [68].
Home-based device-guided breathing training improved the spontaneous breathing pattern at rest in hypertensive patients due to a reduced breathing rate and an increased tidal volume, reducing the blood pressure [36]. Home-based slow breathing training with an IRL also reduced breathing rate and blood pressure in older people with treated and stable isolated systolic hypertension [27]. Finally, a reduction in sympatho-vagal balance and blood pressure was found post-inspiratory muscle training in patients with essential hypertension [67], while an acute IRL increases vagal modulation to the heart in normotensive older women [68]. Taken together, these findings from acute and chronic effects of breathing training, suggest that neural cardiovascular adaptations play a role in blood pressure reductions. Figure 2 summarizes the putative mechanisms in which home-based endurance, isometric and breathing training reduce blood pressure in hypertensive subjects.
In the applicate point of view, all interventions to reduce blood pressure in hypertensive patients could be adapted to a home-based intervention fitting the demand of social isolation. Regarding adherence, home-based exercises could be better than exercises in a sports center or gym. Greater adherence may be explained due to the low-cost characteristic of home-based programs (i.e., without costs with facilities or transportation) and greater flexibility in the participants' routine [9, 10]. Also, obese adults’ enrolled in home-based exercises present greater progress per week when compared to the participants of exercise groups attending a gym. Secondly, home-based exercises are more efficient in a long-term period, but exercise in gym centers remains more effective for short to medium-term benefits [69]. Indeed, both exercise programs (home or centers) improve functional capacity in older adults, but only exercises programs performed three times a week in a fitness center increase strength and cardiorespiratory fitness [70]. Overall, the low adherence in any exercise program seems to involve some concerns as “lack of time” and the accessibility to specific fitness equipment [71, 72]. On the other hand, home-based exercises could increase the accessibility to exercise programs on a large scale and optimize the time expended for physical exercise [47, 73].
Conclusions
All home-based exercise programs (endurance, isometric strength, and breathing training) included in this current systematic review were effective to reduce blood pressure in hypertensive patients. Despite these encouraging findings, additional randomized controlled trials and mechanistic studies are needed to better provide evidence-based recommendations of home-based exercise programs as antihypertensive therapy.
Acknowledgements
Not applicable.
Abbreviations
- FITT
Frequency, intensity, time, and type
- HIIT
High intensity interval training
- HR
Heart rate
- IRL
Inspiratory resistive loading
Authors’ contributions
GDR: Conceptualization, Writing-review & editing; Project administration, Supervision; LSL: Conceptualization, Methodology, Formal analysis, Writing—original draft, Writing-review & editing; NCSS: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review & editing; PGLT: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review & editing; TMMSR: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review & editing; VQAP: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review & editing; VVC: Conceptualization, Methodology, Formal analysis, Writing-original draft, Writing-review & editing; PPSS: Conceptualization, Resources, Writing-original draft, Writing-review & editing, Supervision, Project administration, Funding acquisition. The author(s) read and approved the final manuscript.
Funding
No sources of funding were used in the preparation of this review.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There are no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 3.Pratt M, Ramirez Varela A, Salvo D, Kohl Iii HW, Ding D. Attacking the pandemic of physical inactivity: what is holding us back? Br J Sports Med. 2020;54:760–762. doi: 10.1136/bjsports-2019-101392. [DOI] [PubMed] [Google Scholar]
- 4.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Physical inactivity: a global public health problem. 2020. https://www.who.int/dietphysicalactivity/factsheet_inactivity/en/. Accessed 30 Mar 2020.
- 7.Fitbit, Inc. The Impact of Coronavirus on Global Activity (Online). https://blog.fitbit.com/covid-19-global-activity/. Accessed 25 Mar 2020.
- 8.Chandrasekaran B, Ganesan TB. Sedentarism and chronic disease risk in COVID 19 lockdown - a scoping review. Scott Med J. 2021;66:3–10. doi: 10.1177/0036933020946336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peçanha T, Goessler KF, Roschel H, Gualano B. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318:H1441–H1446. doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentlage E, Ammar A, How D, Ahmed M, Trabelsi K, Chtourou H, et al. Practical recommendations for maintaining active lifestyle during the COVID-19 pandemic: a systematic literature review. Int J Environ Res Public Health. 2020;17:6265. doi: 10.3390/ijerph17176265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, American College of Sports Medicine American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu M, Nadeau A, Lacourcière Y, Cléroux J. Post-exercise reduction in blood pressure in hypertensive subjects: effects of angiotensin converting enzyme inhibition. Br J Clin Pharmacol. 1993;36:331–338. doi: 10.1111/j.1365-2125.1993.tb00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29:65–70. doi: 10.1097/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 14.da Nobrega AC. The subacute effects of exercise: concept, characteristics, and clinical implications. Exerc Sport Sci Rev. 2005;33:84–87. doi: 10.1097/00003677-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson PD, Arena R, Riebe D, Pescatello LS, American College of Sports Medicine ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215–7. doi: 10.1249/JSR.0b013e31829a68cf. [DOI] [PubMed] [Google Scholar]
- 17.Law M, Stewart D, Pollock N, Letts L, Bosch J, Westmorland M. Critical review form – quantitative studies. McMaster University. https://www.unisa.edu.au. Accessed date Mon year.
- 18.Meles E, Giannattasio C, Failla M, Gentile G, Capra A, Mancia G. Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens. 2004;17:370–374. doi: 10.1016/j.amjhyper.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Coghill N, Cooper AR. The effect of a home-based walking program on risk factors for coronary heart disease in hypercholesterolaemic men. A randomized controlled trial. Prev Med. 2008;46:545–551. doi: 10.1016/j.ypmed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Hua LP, Brown CA, Hains SJ, Godwin M, Parlow JL. Effects of low-intensity exercise conditioning on blood pressure, heart rate, and autonomic modulation of heart rate in men and women with hypertension. Biol Res Nurs. 2009;11:129–143. doi: 10.1177/1099800408324853. [DOI] [PubMed] [Google Scholar]
- 21.Suter E, Marti B, Tschopp A, Wanner HU, Wenk C, Gutzwiller F. Effects of self-monitored jogging on physical fitness, blood pressure and serum lipids: a controlled study in sedentary middle-aged men. Int J Sports Med. 1990;11:425–432. doi: 10.1055/s-2007-1024832. [DOI] [PubMed] [Google Scholar]
- 22.Staffileno BA, Minnick A, Coke LA, Hollenberg SM. Blood pressure responses to lifestyle physical activity among young, hypertension-prone African-American women. J Cardiovasc Nurs. 2007;22:107–117. doi: 10.1097/00005082-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Farinatti Pde T, Oliveira RB, Pinto VL, Monteiro WD, Francischetti E. Programa domiciliar de exercícios: efeitos de curto prazo sobre a aptidão física e pressão arterial de indivíduos hipertensos [Home exercise program: short term effects on physical aptitude and blood pressure in hypertensive individuals] Arq Bras Cardiol. 2005;84:473–9. doi: 10.1590/S0066-782X2005000600008. [DOI] [PubMed] [Google Scholar]
- 24.Farinatti P, Monteiro WD, Oliveira RB. Long term home-based exercise is effective to reduce blood pressure in low income brazilian hypertensive patients: a controlled trial. High Blood Press Cardiovasc Prev. 2016;23:395–404. doi: 10.1007/s40292-016-0169-9. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell J, Atherton PJ, Smith K, Doleman B, Williams JP, Lund JN, et al. The efficacy of unsupervised home-based exercise regimens in comparison to supervised laboratory-based exercise training upon cardio-respiratory health facets. Physiol Rep. 2017;5:e13390. doi: 10.14814/phy2.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punia S, Kulandaivelan S. Home-based isometric handgrip training on RBP in hypertensive adults-Partial preliminary findings from RCT. Physiother Res Int. 2020;25:e1806. doi: 10.1002/pri.1806. [DOI] [PubMed] [Google Scholar]
- 27.Gordon BDH, Thomas EV, Warren-Findlow J, Marino JS, Bennett JM, Reitzel AM, et al. A comparison of blood pressure reductions following 12-weeks of isometric exercise training either in the laboratory or at home. J Am Soc Hypertens. 2018;12:798–808. doi: 10.1016/j.jash.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Taylor KA, Wiles JD, Coleman DA, Leeson P, Sharma R, O’Driscoll JM. Neurohumoral and ambulatory haemodynamic adaptations following isometric exercise training in unmedicated hypertensive patients. J Hypertens. 2019;37:827–836. doi: 10.1097/HJH.0000000000001922. [DOI] [PubMed] [Google Scholar]
- 29.McCaffrey R, Ruknui P, Hatthakit U, Kasetsomboon P. The effects of yoga on hypertensive persons in Thailand. Holist Nurs Pract. 2005;19:173–180. doi: 10.1097/00004650-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Wolff M, Sundquist K, Larsson Lönn S, Midlöv P. Impact of yoga on blood pressure and quality of life in patients with hypertension - a controlled trial in primary care, matched for systolic blood pressure. BMC Cardiovasc Disord. 2013;13:111. doi: 10.1186/1471-2261-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff M, Rogers K, Erdal B, Chalmers JP, Sundquist K, Midlöv P. Impact of a short home-based yoga programme on blood pressure in patients with hypertension: a randomized controlled trial in primary care. J Hum Hypertens. 2016;30:599–605. doi: 10.1038/jhh.2015.123. [DOI] [PubMed] [Google Scholar]
- 32.Sujatha T, Judie A. Effectiveness of a 12-week yoga program on physiopsychological parameters in patients with hypertension. Int J Pharm Clin Res. 2014;6:329–335. [Google Scholar]
- 33.Schein MH, Gavish B, Herz M, Rosner-Kahana D, Naveh P, Knishkowy B, et al. Treating hypertension with a device that slows and regularises breathing: a randomised, double-blind controlled study. J Hum Hypertens. 2001;15:271–278. doi: 10.1038/sj.jhh.1001148. [DOI] [PubMed] [Google Scholar]
- 34.Viskoper R, Shapira I, Priluck R, Mindlin R, Chornia L, Laszt A, et al. Nonpharmacologic treatment of resistant hypertensives by device-guided slow breathing exercises. Am J Hypertens. 2003;16:484–487. doi: 10.1016/S0895-7061(03)00571-5. [DOI] [PubMed] [Google Scholar]
- 35.Logtenberg SJ, Kleefstra N, Houweling ST, Groenier KH, Bilo HJ. Effect of device-guided breathing exercises on blood pressure in hypertensive patients with type 2 diabetes mellitus: a randomized controlled trial. J Hypertens. 2007;25:241–246. doi: 10.1097/HJH.0b013e32801040d5. [DOI] [PubMed] [Google Scholar]
- 36.Anderson DE, McNeely JD, Windham BG. Regular slow-breathing exercise effects on blood pressure and breathing patterns at rest. J Hum Hypertens. 2010;24:807–813. doi: 10.1038/jhh.2010.18. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal T, Alter A, Peleg E, Gavish B. Device-guided breathing exercises reduce blood pressure: ambulatory and home measurements. Am J Hypertens. 2001;14:74–76. doi: 10.1016/S0895-7061(00)01235-8. [DOI] [PubMed] [Google Scholar]
- 38.Elliot WJ, Izzo JL, Jr, White WB, Rosing DR, Snyder CS, Alter A, et al. Graded blood pressure reduction in hypertensive outpatients associated with use of a device to assist with slow breathing. J Clin Hypertens (Greenwich) 2004;6:553–559. doi: 10.1111/j.1524-6175.2004.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schein MH, Gavish B, Baevsky T, Kaufman M, Levine S, Nessing A, et al. Treating hypertension in type II diabetic patients with device-guided breathing: a randomized controlled trial. J Hum Hypertens. 2009;23:325–331. doi: 10.1038/jhh.2008.135. [DOI] [PubMed] [Google Scholar]
- 40.Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing-control lowers blood pressure. J Hum Hypertens. 2001;15:263–269. doi: 10.1038/sj.jhh.1001147. [DOI] [PubMed] [Google Scholar]
- 41.Ublosakka-Jones C, Tongdee P, Pachirat O, Jones DA. Slow loaded breathing training improves blood pressure, lung capacity and arm exercise endurance for older people with treated and stable isolated systolic hypertension. Exp Gerontol. 2018;108:48–53. doi: 10.1016/j.exger.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Jones CU, Sangthong B, Pachirat O, Jones DA. Slow breathing training reduces resting blood pressure and the pressure responses to exercise. Physiol Res. 2015;64:673–682. doi: 10.33549/physiolres.932950. [DOI] [PubMed] [Google Scholar]
- 43.Jones CU, Sangthong B, Pachirat O. An inspiratory load enhances the antihypertensive effects of home-based training with slow deep breathing: a randomised trial. J Physiother. 2010;56:179–186. doi: 10.1016/S1836-9553(10)70023-0. [DOI] [PubMed] [Google Scholar]
- 44.Sangthong B, Ubolsakka-Jones C, Pachirat O, Jones DA. Breathing training for older patients with controlled isolated systolic hypertension. Med Sci Sports Exerc. 2016;48:1641–1647. doi: 10.1249/MSS.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;35:251–256. doi: 10.1249/01.MSS.0000048725.15026.B5. [DOI] [PubMed] [Google Scholar]
- 46.Opdenacker J, Delecluse C, Boen F. A 2-year follow-up of a lifestyle physical activity versus a structured exercise intervention in older adults. J Am Geriatr Soc. 2011;59:1602–1611. doi: 10.1111/j.1532-5415.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 47.Poortaghi S, Baghernia A, Golzari SE, Safayian A, Atri SB. The effect of home-based cardiac rehabilitation program on self efficacy of patients referred to cardiac rehabilitation center. BMC Res Notes. 2013;6:287. doi: 10.1186/1756-0500-6-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu-Ambrose T, Davis JC, Best JR, Dian L, Madden K, Cook W, et al. Effect of a home-based exercise program on subsequent falls among community-dwelling high-risk older adults after a fall: a randomized clinical trial. JAMA. 2019;321:2092–2100. doi: 10.1001/jama.2019.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbert JA, Silagy CA, Finucane P, Withers RT, Hamdorf PA, Andrews GR. The effectiveness of exercise training in lowering blood pressure: a meta-analysis of randomised controlled trials of 4 weeks or longer. J Hum Hypertens. 1997;11:641–649. doi: 10.1038/sj.jhh.1000509. [DOI] [PubMed] [Google Scholar]
- 50.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 51.de Sousa EC, Abrahin O, Ferreira ALL, Rodrigues RP, Alves EAC, Vieira RP. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: meta-analysis. Hypertens Res. 2017;40:927–931. doi: 10.1038/hr.2017.69. [DOI] [PubMed] [Google Scholar]
- 52.Punia S, Kulandaivelan S, Punia V. Effect of isometric strength training on blood pressure: systematic review of literature with specific emphasis on Indians. J Exerc Sci Physiother. 2016;12:20–27. [Google Scholar]
- 53.O’Driscoll JM, Taylor KA, Wiles JD, Coleman DA, Sharma R. Acute cardiac functional and mechanical responses to isometric exercise in prehypertensive males. Physiol Rep. 2017;5:e13236. doi: 10.14814/phy2.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahu Rodrigues SL, Farah BQ, Silva G, Correia M, Pedrosa R, Vianna L, et al. Vascular effects of isometric handgrip training in hypertensives. Clin Exp Hypertens. 2020;42:24–30. doi: 10.1080/10641963.2018.1557683. [DOI] [PubMed] [Google Scholar]
- 55.Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, et al. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 56.Krüerke D, Simões-Wüst AP, Kaufmann C, Frank M, Faldey A, Heusser P, et al. Can speech-guided breathing influence cardiovascular regulation and mood perception in hypertensive patients? J Altern Complement Med. 2018;24:254–261. doi: 10.1089/acm.2017.0158. [DOI] [PubMed] [Google Scholar]
- 57.Bernardi L, Gabutti A, Porta C, Spicuzza L. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J Hypertens. 2001;19:2221–2229. doi: 10.1097/00004872-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Elstad M, O’Callaghan EL, Smith AJ, Ben-Tal A, Ramchandra R. Cardiorespiratory interactions in humans and animals: rhythms for life. Am J Physiol Heart Circ Physiol. 2018;315:H6–17. doi: 10.1152/ajpheart.00701.2017. [DOI] [PubMed] [Google Scholar]
- 59.Chaddha A, Modaff D, Hooper-Lane C, Feldstein DA. Device and non-device-guided slow breathing to reduce blood pressure: a systematic review and meta-analysis. Complement Ther Med. 2019;45:179–184. doi: 10.1016/j.ctim.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Johnson BT, Acabchuk RL, Chen S, Lewis HK, Livingston J, et al. Yoga as antihypertensive lifestyle therapy: a systematic review and meta-analysis. Mayo Clin Proc. 2019;94:432–446. doi: 10.1016/j.mayocp.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Cernes R, Zimlichman R. RESPeRATE: the role of paced breathing in hypertension treatment. J Am Soc Hypertens. 2015;9:38–47. doi: 10.1016/j.jash.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, et al. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015;45:279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 63.Durand MJ, Phillips SA, Widlansky ME, Otterson MF, Gutterman DD. The vascular renin-angiotensin system contributes to blunted vasodilation induced by transient high pressure in human adipose microvessels. Am J Physiol Heart Circ Physiol. 2014;307:H25–32. doi: 10.1152/ajpheart.00055.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, et al. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 65.Sabbahi A, Arena R, Elokda A, Phillips SA. Exercise and hypertension: uncovering the mechanisms of vascular control. Prog Cardiovasc Dis. 2016;59:226–234. doi: 10.1016/j.pcad.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Farah BQ, Christofaro DGD, Correia MA, Oliveira CB, Parmenter BJ, Ritti-Dias RM. Effects of isometric handgrip training on cardiac autonomic profile: a systematic review and meta-analysis study. Clin Physiol Funct Imaging. 2020;40:141–147. doi: 10.1111/cpf.12619. [DOI] [PubMed] [Google Scholar]
- 67.Ferreira JB, Plentz RD, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol. 2013;166:61–67. doi: 10.1016/j.ijcard.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 68.Rodrigues GD, Gurgel JL, Gonçalves TR, da Silva Soares PP. Acute effects of inspiratory loading in older women: Where the breath meets the heart. Respir Physiol Neurobiol. 2021;285:103589. doi: 10.1016/j.resp.2020.103589. [DOI] [PubMed] [Google Scholar]
- 69.Perri MG, Martin AD, Leermakers EA, Sears SF, Notelovitz M. Effects of group- versus home-based exercise in the treatment of obesity. J Consult Clin Psychol. 1997;65:278–285. doi: 10.1037/0022-006X.65.2.278. [DOI] [PubMed] [Google Scholar]
- 70.Van Roie E, Delecluse C, Opdenacker J, De Bock K, Kennis E, Boen F. Effectiveness of a lifestyle physical activity versus a structured exercise intervention in older adults. J Aging Phys Act. 2010;18:335–352. doi: 10.1123/japa.18.3.335. [DOI] [PubMed] [Google Scholar]
- 71.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl Physiol Nutr Metab. 2014;39:409–412. doi: 10.1139/apnm-2013-0187. [DOI] [PubMed] [Google Scholar]
- 73.Bergström G, Börjesson M, Schmidt C. Self-efficacy regarding physical activity is superior to self-assessed activity level, in long-term prediction of cardiovascular events in middle-aged men. BMC Public Health. 2015;15:820. doi: 10.1186/s12889-015-2140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.