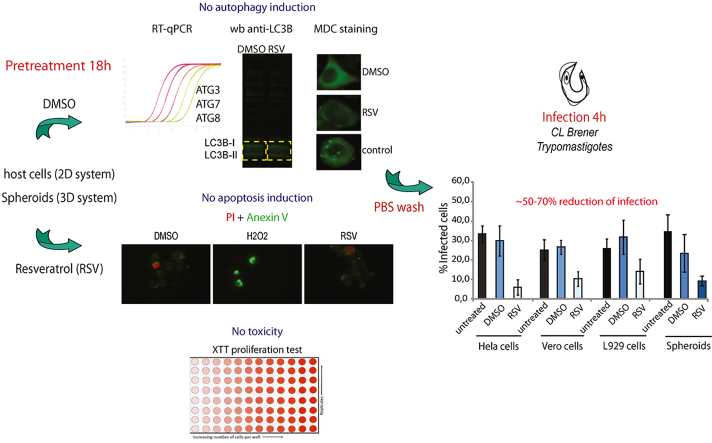
Abstract
Chagas' disease or American trypanosomiasis, caused by Trypanosoma cruzi infection, is an endemic disease in Latin America, which has spread worldwide in the past years. The drugs presently used for treatment have shown limited efficacy due to the appearance of resistant parasites and severe side effects. Some of the most recent studies on anti-parasitic drugs have been focused on protein acetylation, a reversible reaction modulated by Acetyl Transferases (KATs) and Deacetylases (KDACs). We have previously reported the anti-parasite activity of resveratrol (RSV), an activator of KDACs type III (or sirtuins), and showed that this drug can reduce the growth of T. cruzi epimastigotes and the infectivity of trypomastigotes. Since RSV is now widely used in humans due to its beneficial effects as an antioxidant, it has become an attractive candidate as a repurposing drug. In this context, the aim of the present study was to evaluate the ability of this drug to protect three different types of host cells from parasite infection. RSV treatment before parasite infection reduced the percentage of infected cells by 50–70% depending on the cell type. Although the mammalian cell lines tested showed different sensitivity to RSV, apoptosis was not significantly affected, showing that RSV was able to protect cells from infection without the activation of this process. Since autophagy has been described as a key process in parasite invasion, we also monitored this process on host cells pretreated with RSV. The results showed that, at the concentrations and incubation times tested, autophagy was not induced in any of the cell types evaluated. Our results show a partial protective effect of RSV in vitro, which justifies extending studies to an in vivo model to elucidate the mechanism by which this effect occurs.
Keywords: Trypanosoma cruzi, Resveratrol, Drug repurposing, Pre-exposure
Graphical abstract
1. Introduction
American trypanosomiasis, caused by Trypanosoma cruzi infection in humans, is a complex zoonosis involving heterogeneous populations of parasites and a wide variety of Triatomine insect vectors and wild and domestic mammals that could act as parasite reservoirs (Rassi et al., 2010). At present, no vaccines are available to control T. cruzi infection and the drugs used for treatment, Benznidazole and Nifurtimox, have proven efficacy only during early infection and limited benefits in the chronic phase. Moreover, some additional limitations in their application have arisen due to the appearance of resistant parasites and severe side effects (Mejia et al., 2012; Ribeiro et al., 2020). Thus, there is a clear need for development of new therapeutic alternatives. In this regard, a promising starting point to identify new antiparasitic drugs is to focus on regulatory processes essential for parasite growth and development, such as protein acetylation (Andrews et al., 2012a, 2012b; Kelly et al., 2012; Fioravanti et al., 2020). Protein acetylation is a reversible reaction modulated by Acetyl Transferases (KATs) and Deacetylases (KDACs) (Choudhary et al., 2009). These enzymes are involved in regulating important cellular processes related to chromatin structure, gene expression, and metabolic state. Lysine KDACs are generally referred to as histone deacetylases, since the epigenetic modification of histones was described for the first time (Allfrey, 1964). However, many other proteins besides histones have been identified as substrates for these enzymes (Choudhary et al., 2009). KDACs have been classified into two main families according to the description of mammalian enzymes: one that uses zinc as a cofactor and includes four groups differing in size and structural organization, and the sirtuin family (from Sir2-related proteins), which uses nicotinamide adenine dinucleotide (NAD+) as a cofactor (Gray et al., 2001). Coding sequences for several of these enzymes have been found in the T. cruzi genome (El-Sayed et al., 2005), and partially characterized (Moretti et al., 2015; Ritagliati et al., 2015; Marek et al., 2021; Picchi-Constante et al., 2021). Inhibitors of KDACs have been found to affect different parasites, including the pathogen causing malaria (Plasmodium falciparum), kinetoplastids (T. cruzi, Trypanosoma brucei and Leishmania donovani), and nematodes (Brugiamalayi, Dirofilariaimmitis and Haemonchus contortus) (Murray et al., 2001; Andrews et al., 2012a, 2012b; Herrera-Martínez et al., 2020; Ghazy et al., 2022; Ângelo de Souza et al., 2020; Veiga-santos et al., 2014; Verçoza et al., 2017; de Oliveira Santos et al., 2019; Matutino Bastos et al., 2020). Moreover, we have recently reported that resveratrol (RSV), previously described as an activator of sirtuin deacetylases (Andrews et al., 2012b; Dai et al., 2018), reduces the growth of T. cruzi epimastigotes and the infectivity of cell-derived trypomastigotes (Campo, 2017), which agrees with previous reports describing the anti-parasite effects of RSV on Leishmania major (Kedzierski et al., 2007) and other T. cruzi strains (Valera Vera et al., 2016). RSV is a natural occurring phytoalexin found in the skin of red grapes, originally reported as a potential anticancer agent (Jang and Cai, 1997; Boocock et al., 2007; reviewed in Rauf et al., 2018), which has also shown antioxidant, anti-inflammatory and cardioprotective effects (reviewed in Banez et al., 2020). In fact, RSV is able to improve the heart function of chagasic mice by activation of the SIRT1 and AMPK fuel-sensing pathways (Vilar-Pereira et al., 2016). RSV also has the capacity to induce autophagy, both in vitro and in vivo, after cell injury through different mechanisms (Kanamori et al., 2013). In this regard, it has been shown that the autophagic pathway is essential for T. cruzi host cell invasion and that the pre-activation of this process can increase parasite infectivity in vitro (Romano et al., 2009). Since RSV has shown no toxicity for humans (Rauf et al., 2018), it has become an attractive candidate as a repurposing drug. In this context, the aim of the present study was to evaluate, for the first time, the ability of a pre-exposure to RSV to protect three different types of host cells from T. cruzi infection.
2. Materials and methods
2.1. Parasites
Parasites of the T. cruzi CL-Brener strain, the genome project reference clone (El Sayed et al., 2005), were used throughout this study. Cell-derived trypomastigotes were purified from supernatants of Vero cells infections by centrifugation at 5,200g for 10 min and allowing trypomastigotes to swim for 4 h at 37 °C in MEM media supplemented with 4% Fetal Bovine Serum (FBS). Then parasites were collected from the supernatant, concentrated by centrifugation and living parasites were counted for infection assays.
2.2. Resveratrol and sirtinol treatments
The sirtuin activator used was 3, 4′, 5-trihydroxystilbene or RSV; (ab120726, Abcam). For comparison the specific inhibitor for this group of KDACs, 2-[(2-Hydroxynaphthalen-1-ylmethylene)amino]-N-(1-phenethyl)benzamide or Sirtinol (Sir); (S7942, SIGMA) was used. All assays were performed with the corresponding control of untreated cells and cells incubated with equal amounts of the drug's vehicle, dimethylsulfoxide (1% DMSO final concentration). The concentration and incubation time for this compound were set according to previously described (Campo 2017). Briefly, RSV was used at 4 μM and 40 μM (depending on the cell type, according to the data of toxicity shown in Fig. 1) because these concentrations are below the IC50 previously described for CL Brener trypomastigotes (Campo 2017). For comparison, sirtinol concentration was set according to our previous results showing an increment on the percentage of infected cells when parasites were pre-incubated with 25 μM Sir. The incubation time with the drugs or the vehicle was set at 18h as used in our previous report (Campo 2017).
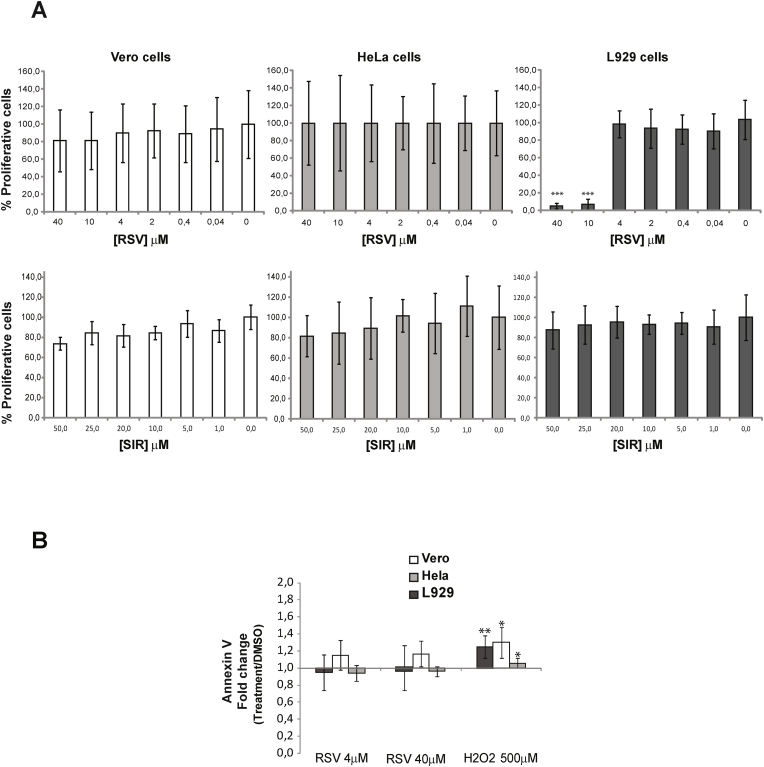
Fig. 1.
A) Resveratrol (RSV) and Sirtinol (SIR) toxicity on mammalian host cells. Host cells (Vero, HeLa, and L929 fibroblasts) were incubated for 18 h with the indicated concentrations of each drug and the vehicle. Cell proliferation was measured using the XTT assay. Values are expressed as the average percentage of three independent experiments corresponding to proliferating treated cells (RSV or SIR) taking as 100% the absorbance values for cells incubated in the drug vehicle (1% DMSO) (drug concentration value = 0). B) Apoptosis assays of host cells. Cells were first treated with the indicated concentration of RSV or the drug vehicle (1% DMSO) or 500 μΜ Η2Ο2 as controls. Then, cells were incubated with annexin V conjugated to Alexa 488 and propidium iodide (1 μg/mL) and fluorescence measured in a 96-well plate reader at an excitation wavelength of 485 nm using emission wavelengths of 535 nm and 620 nm for annexin V and propidium iodide (PI), respectively. Values are expressed as the average of the fold changes from fluorescence values obtained for treated cells compared to control cells incubated with the drug vehicle (1% DMSO). All the assays are expressed as the average of five independent experiments. Asterisks indicate the values showing differences with statistical significance compared to controls (fold change = 1) (∗p < 0.05, ∗∗p < 0.005).
2.3. Cell proliferation assay
Resveratrol and sirtinol toxicity was evaluated using the cell colorimetric proliferation assay based on the production of formazan from the tetrazolium dye (XTT) reduction following the protocol of the cell proliferation kit XTT (Roche). Briefly, 5 × 104 cells were seeded in 100 μL of MEM 10% FBS into wells of a flat-bottom 96-well plate in triplicate, including 3 control wells containing 100 μL of complete growth medium alone as blank for absorbance reading. After 24 h, a different concentration of each drug or the vehicle (DMSO 1%) was added to each well and incubated for an additional 18 h at 37 °C. Then, 50 μL of the Activated-XTT Solution was added to each well and incubated for 4 h. Absorbance was measured in a FilterMax plate reader at a wavelength between 450 nm and at the reference wavelength 620 nm.
2.4. Apoptosis assay
Cells were grown in 24 well plates until confluence and then treated with the indicated concentrations of RSV 1% DMSO for 18 h at 37 °C in MEM 10%. As positive control apoptosis was induced by adding 500 μM H2O2 for 18 h at 37 °C in the same media. After incubation, cells were washed in PBS, treated with trypsin 0,25% for 5 min at 37 °C and washed again in PBS. Then, cells were labeled with annexin V-Alexa 488 and propidium iodide (PI) for 30 min at room temperature. After incubation, 100 μl from each treatment was placed in black 96 wells plates and fluorescence was measured using FilterMax F5 plate reader at excitation wavelength 485 nm using emission wavelength 535 nm and 620 for annexin V and PI, respectively. Measurements were performed in duplicates for each treatment.
2.5. In vitro infections
Vero, HeLa or L929 cells (20,000 cells in 0,5 ml of MEM 10% FBS) were plated onto round coverslips placed in 24 well plates 24 h before treatment. Cells were incubated at 37 °C for 18 h in MEM 10% FBS containing RSV, Sirtinol or vehicle, washed twice with PBS and infected. Infections were performed for 4 h with 2 × 106 trypomastigotes per coverslip in MEM 4% FBS. After infection, cells were washed twice with PBS, and incubated in fresh medium for additional 48 h to allow amastigotes replication. Then, coverslips were washed twice with PBS, fixed with paraformaldehyde (PFA) 4% for 20 min, washed again and mounted in 5 μl of FluorSave Reagent (Calbiochem) and 5 μl of DAPI (100 μg/ml final concentration) for nucleus and kinetoplastid staining and observed and photographed using a Nikon Y-FL fluorescence microscope. Each infection was performed in duplicates and the percentage of infected cells and the number of intracellular amastigotes was calculated using the cell counter plugin from ImageJ software. For this, 20 fields, each containing a mean of 50 cells, were photographed with the 40X magnification objective for each experiment.
Infection of HeLa spheroids was performed as previously described (Rodriguez et al., 2019). Briefly, spheroids of HeLaR2 (HeLa-RFP) cells were generated by the liquid overlay method (Carlsson and Yuhas, 1984). Cells (1,000/well) were added to U-bottom 96-well plates coated with agarose 1% in PBS (w/v) and cultured in MEM 10% FBS. At 72 h, each well contained one spheroid, conformed by ∼9,000 cells. For T. cruzi infection, 12 spheroids were placed on each well of an agarose pre-coated 24 well plate and incubated with 3 × 106/well of CL Brener trypomastigotes (pre-labeled with Carboxy fluorescein succinimidyl ester, CFSE), in MEM supplemented with 4% FBS for 4 h. Then, infected spheroids were disaggregated by addition 0.25% trypsin, cells collected by centrifugation, washed three times and fixed in PBS 0.5% PFA, and acquired on a CyFlow space cytometer (Partec, Germany). HeLaR2 gated by forward and side scatter parameters were selected and 10,000 events were analyzed for each condition. FL1-cells represented uninfected HeLaR2 cells while FL1+ represented cells infected (either with intracellular parasites and/or attached to cell membrane) with CFSE-labeled parasites.
Parasite dissemination inside spheroids was analyzed by fluorescence as described (Rodriguez et al., 2019). Briefly, images of spheroids were acquired with a 40 × objective at 10, 20, 30, 40 and 50 μm in depth from the surface with a confocal Olympus FV1000 microscope. CFSE labeled parasites were imaged at 488 nm while HeLaR2 cells were imaged at 530 nm. Quantification of the parasites in each stack of the infected spheroids was carried out using the particle analysis tool from ImageJ software.
2.6. Quantitative real time PCR
Total RNA from control and treated cells was purified from 1 × 107 host cells (Vero, HeLa or L929 fibroblast), scattered in Trizol reagent (Invitrogen). The cDNA used for real time PCR assays was synthesized from 1 μg of RNA with the SuperScript II system (Invitrogen) and random primers. Specific primers for each gene (ATG3cons_fw1: 5′ AGTTTGTGGCAGCTGGAGATC 3’; ATG3cons_rev1: 5′ CCTGTAGCCCATTGCCATGT3’; ATG7cons_fw1: 5′ GCTTGGCTGCTACTTCTGCAA3’; ATG7cons_rev1: 5′ GGTCCGGTCTCTGGTTGAATC 3’; ATG8cons_fw1: 5′ GGCGCTTACAGCTCAATGC 3’; ATG8cons_rev1: 5′ TGCTGTGCCGTTCACCAA 3′), were designed with Primer Express software using the consensus sequence from alignment of Autophagy-related protein 3, 7 and 8 from mouse (accession numbers: NM_026402.3, XM_006506707.2, NM_026160.4), monkey (accession numbers: NM_001194276.1, NM_001265972.1, NM_001193625.1) and human (accession numbers: NM_001278712.1, XM_011533286.2, NM_022818.4). PCR was carried out in a final volume of 10 μl reaction mixture containing 0,1 μM of each primer, 5 μl of SYBR Green reaction mix (SensiFast SYBR Lo-Rox qPCR kit; Bioline) and 4 μl of cDNA template. Transcript levels were obtained using the 7500 software from Applied Biosystems and results analyzed using LinReg software. qPCR quality was evaluated analyzing the melting curves to ensure that only one product was amplified. Values obtained for each transcript analyzed were normalized by the levels detected for Luciferase 1 mRNA (sequence accession number: MH759210.1; with primers Luciferase_fw: 5′ CGGATTACCAGGGATTTCAG 3′ and Luciferase_rev: 5′ TCACGATCAAAGGACTCTGG 3’) obtained from in vitro transcription using MegaScript transcription kit. Luciferase mRNA was loaded with samples of RNA previously to RT-reaction.
2.7. Western blots
Total protein extracts were obtained from the phenol phase of Trizol preparations following the indications of the manufacturer protocol (Invitrogen). SDS-PAGE was performed by loading 2 × 107 cells per well for each treatment and the corresponding controls in a 12% polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blocked for 1 h in TBS-2% milk and incubated for 16 h at 4 °C with anti-LC3B rabbit polyclonal antiserum (Cell Signaling #2775; 1:1,000 dilution). After incubation, nitrocellulose membranes were washed twice in TBS for 5 min and incubated with IRDye 800CW conjugated Goat anti-Rabbit IgG (H + L) serum in a 1:20,000 dilution for 1 h, washed twice in TBS-Tween 0.2% for 5 min and once in TBS for another 10 min and visualized in an Odyssey scanner. Western blot signals were normalized with the total amount of proteins observed by Coomassie blue staining quantified using the gel analyzer tool of ImageJ software.
2.8. Monodansylcadaverine (MDC) staining
HeLa, Vero and L929 cells were placed in 24-well plates (1 × 106 cells/well) and incubated overnight in MEM 10% FCS at 37 °C. The next day, experimental compounds or vehicle control (1% DMSO) were added and incubated for 18 h at 37 °C. As control for inducing autophagy some wells were starved in Earle's Balanced Salt Solution (EBSS, Gibco) media for 2 h. Following, cells were incubated with 0,05 mM MDC in PBS at 37 °C for 30 min (Biederbick et al., 1995). After incubation, cells were washed four times with PBS, mounted with Fluorsave reagent (Calbiochem) and immediately analyzed by fluorescence microscopy.
2.9. Statistical analysis
All data obtained in each experiment were first analyzed for normal distribution using the Shapiro-Wilk test. If this was true (p > 0.20), then statistical differences between treatments and the corresponding control (untreated cells and cells incubated with the drug vehicle) were analyzed using two way ANOVA and two-tailed Student's-t tests. If data distribution results were not normal, then statistical differences were analyzed by the non parametric tests, Kruskal-Wallis and Mann-Whitney. Differences between the experimental groups were considered significant as follows: p < 0.05 (∗), p < 0,005 (∗∗), p < 0,0005 (∗∗∗), p < 0,00005 (∗∗∗∗). All these tests were applied using the software Graph Pad Prism.
3. Results
3.1. Resveratrol and sirtinol toxicity on different types of host cells
To evaluate the toxicity of RSV and sirtinol on mammalian cells, three types of cells (Vero, HeLa and L929 fibroblasts) were pretreated for 18 h with different concentrations of each drug and their proliferation evaluated using the XTT assay. RSVwas not toxic for Vero and HeLa cells at all the concentrations tested, but inhibited the proliferation of L929 fibroblasts at concentrations higher than 4 μM (Fig. 1A). Thus, since this value is below the toxic concentration for all types of cells tested, it was used in the next assays as the starting point and increased to the highest value tested when Vero and HeLa cells were used. Also, given that sirtinol has been previously described as a specific inhibitor for sirtuins, and for that it should represent the antithesis of RSV, its toxicity was also evaluated on the same cells. The results showed that this drug is not toxic for all three types of cells even at the highest concentration tested (Fig. 1A). Our previous results on in vitro infection assays showed that the effect of parasite pretreatment with 25 μM sirtinol was opposite to that of RSV (Campo, 2017). Thus, we used this condition to test whether it also showed this antagonistic effect on host cells.
Given that, in normal and cancer cells, RSV would exert its effects through apoptotic cell death (Farhadnejad et al., 2019), we next evaluated the modulation of this process on host cells after treatment with RSV at the minimal and maximal concentrations tested, according to the results from the toxicity assays and the IC50 previously described for parasite infective forms (Campo, 2017). As positive control, apoptosis was induced by cell incubation with 500 μM H2O2 for 18 h. To eliminate any effect of the drug vehicle (1% DMSO), the results shown in Fig. 1B are expressed as the average of the fold changes of fluorescence values obtained for treated cells in comparison with the those obtained for cells incubated with the same amount of drug vehicle. These results indicate that RSV was not able to induce apoptosis in any of the cells evaluated under the conditions tested because the signal for annexin V in treated cells showed no statistical differences compared to control cells (fold changes ∼1, Fig. 1B).
3.2. Pre-treatment of host cells with resveratrol reduces parasite infection
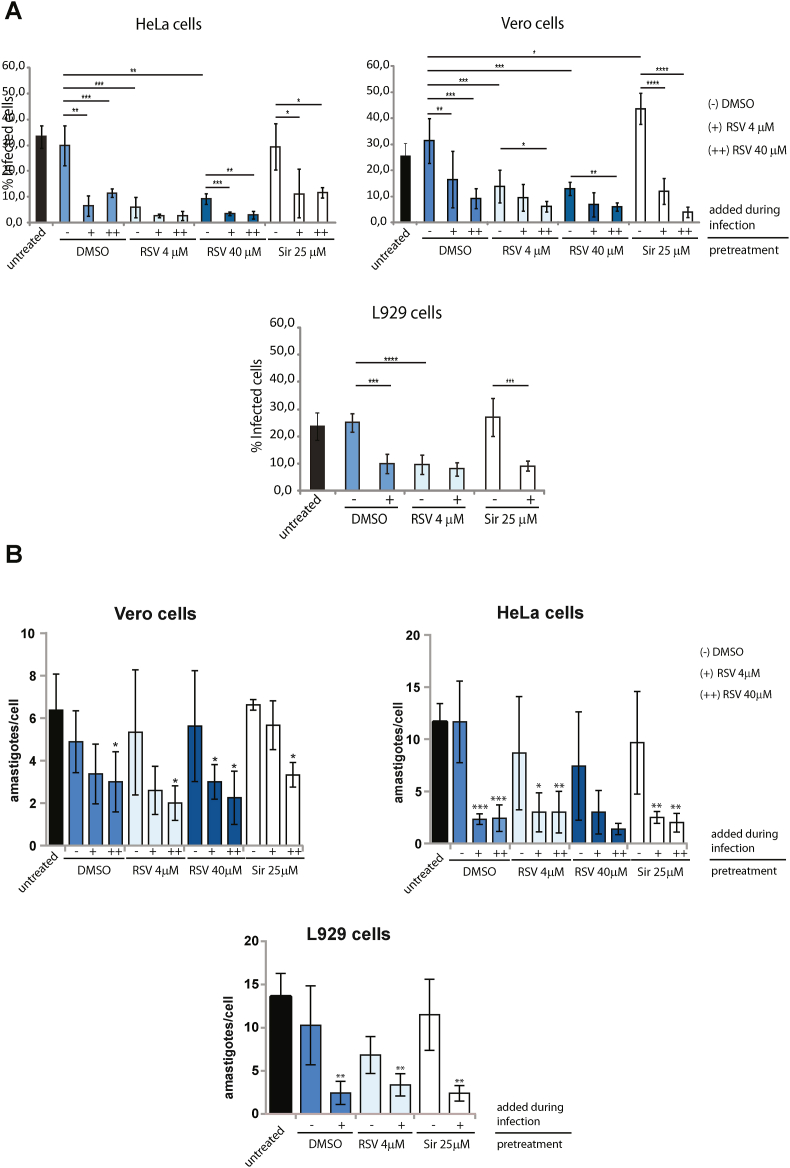
To assess the effect of the pre-treatment of host cells with RSV on in vitro infections, Vero, HeLa and L929 cells were incubated with RSV for 18 h, washed and infected with trypomastigotes for 4 h. To evaluate solely the effects of RSV on the host cells, infections were performed in the absence of the drug and compared with the infections performed with the addition of RSV. Pre-incubation of host cells with RSV reduced the percentage of infected cells by an average of 60% (70% in HeLa, 50% in Vero and 60% in L929 cells) compared to cells incubated with drug vehicle (1% DMSO) or untreated cells (Fig. 2A). Moreover, since Vero and HeLa cells showed to be unaffected by higher concentrations of RSV, the infection assays were also performed increasing the RSV concentration to 40 μM. However, no differences were observed between the two RSV concentrations used for pretreatment of the cells. The comparison of these results with the ones obtained when RSV was added during infection (indicated in Fig. 2A with - for control with DMSO, + for 4 μM and ++ for 40 μM) showed no differences on HeLa and L929 cells pretreated with RSV 4 μM, but an additional 33% reduction of infection was observed for Vero cells when the highest concentration of RSV was added (Fig. 2A). Overall, these results indicate that the addition of RSV during infection reduces parasite invasion more significantly than RSV pretreatment alone for Vero cells but not for HeLa and L929 cells. On the other hand, pretreatment of host cells with sirtinol showed a slight increase in the percentage of infection (about 10%) only for Vero cells. In these assays, the addition of RSV during incubation with trypomastigotes reduced the percentage of infected cells by about 60% and for all types of host cells (Fig. 2A). Also, given that starvation of host cells has been described to increase infection (Vanrell et al., 2013), we performed the in vitro infection assay in cells pre-incubated in amino acid-free medium (EBSS) for 2 h and with or without the addition of RSV during infection. The results showed a 10% increase in the infection for L929 cells, whereas for Vero and HeLa cells, the starvation previous to infection did not result in changes compared to controls. However, the same reduction of infection was obtained when RSV was added during infection but this time in a concentration-dependent manner for Vero and HeLa cells (Supplementary Fig. S1). Also, we observed that the number of intracellular amastigotes was not affected by RSV pretreatment. However, the addition of RSV during infection caused a reduction in the number of intracellular amastigotes in all cell lines tested (Fig. 2B).
Fig. 2.
In vitro infection of host cells pretreated with resveratrol (RSV). A) Percentages of infected HeLa, Vero and L929 cells treated with RSV (for 18 h) or 25 μM Sirtinol (for 18 h) or incubated in EBSS medium (for 2 h) previous infection (indicated in the figure as “pretreatment” under the line). As controls, cells were left untreated or pre-incubated with the drug vehicle (1% DMSO for 18 h). All infections were performed for 4 h with the addition of the drug vehicle (−) or RSV at final concentrations of 4 μM (+) or 40 μM (++) (indicated in the figure as “added during infection” above the line). B) Average numbers of intracellular amastigotes per cell observed in the in vitro infection experiments using Vero, HeLa, and L929 cells pretreated with RSV or Sirtinol and with the addition of DMSO (−) or RSV at 4 μM (+) or 40 μM (++) during infection (indicated in the figure as “added during infection” above the line). As control, cells were left untreated or incubated with the drug vehicle (1% DMSO) previous to infection (indicated in the figure as “pretreatment” under the line).
All assays are expressed as the mean of three independent experiments ± SD, each performed in duplicate (∗p < 0.05, ∗∗p < 0.005). No statistical differences were observed between the controls corresponding to untreated cells and pre-incubation with DMSO. For simplicity, only the statistical analysis between DMSO control and experimental treatments are shown.
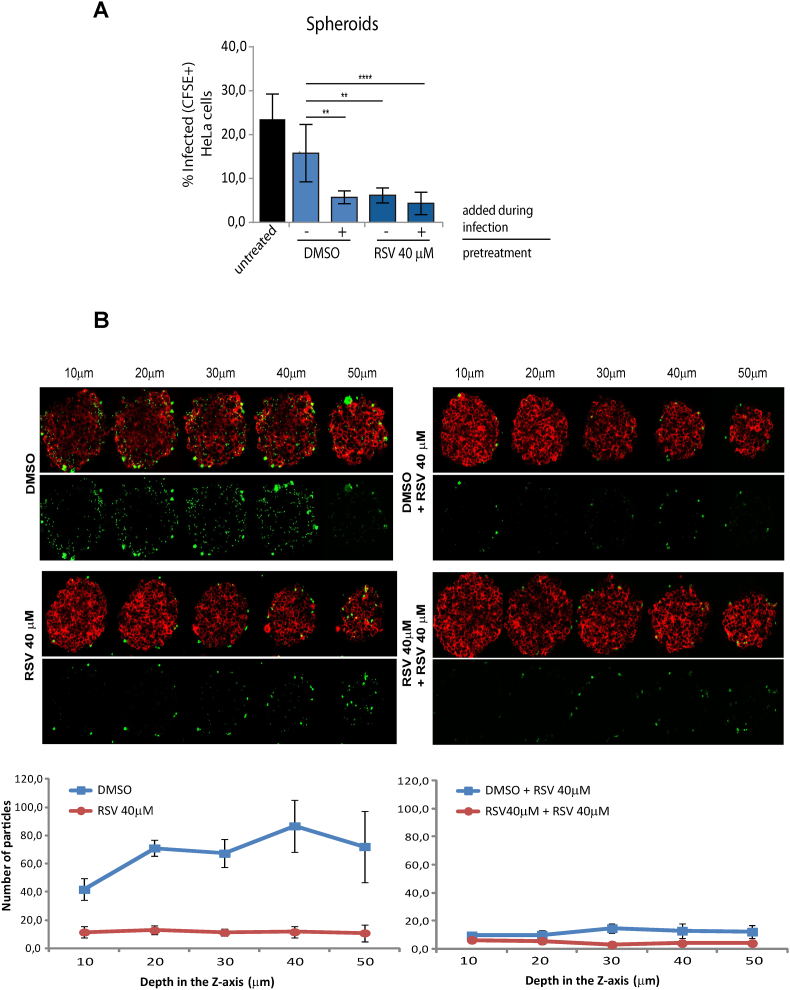
To analyze if pretreatment of cells with RSV can affect trypomastigote invasion and migration, we performed the infection assays using 3D cultures (spheroids). Spheroids have been previously used as a tissue-like model to disclose parasite dissemination and cellular infection. Indeed, CL Brener trypomastigotes were able to transmigrate up to 50 μm in depth and to invade the inner layers of spheroids (Rodriguez et al., 2020). Here, the pretreatment of spheroids with RSV showed a marked reduction (∼50%) in the number of infected cells, independently of the addition of RSV during infection (Fig. 3A). To evaluate the effect of RSV pretreatment in the ability of trypomastigotes to transmigrate inside the spheroid, we next quantified the parasites in each stack of infected spheroids. The total number of parasites inside the spheroids pretreated or incubated during infection with RSV was much lower than in the control due to the reduced invasion. However, transmigration seems not affected because no differences were observed between the numbers of parasites counted in each stack (Fig. 3B). These results suggest that cell pretreatment with RSV seems to be affecting the attachment of trypomastigotes to the cell surface -and therefore cellular invasion- but not their migration.
Fig. 3.
A) Percentages of infected cells using a 3D model with HeLa spheroids pretreated with 40 μM RSV were obtained by quantification of CFSE-positive cells by cytometry analysis. As controls, spheroids were left untreated or pre-incubated with the drug vehicle (1% DMSO for 18 h). All infections were performed for 4 h with the addition of the drug vehicle (−) or RSV at final concentrations of 4 μM (+) or 40 μM (++) (indicated in the figure as “added during infection” above the line). B) Fluorescence images of in vitro infections performed on HeLa spheroids pre-incubated with RSV or DMSO, showing HeLaR2 cells imaged at 530 nm (red) merged with CFSE-labeled parasites imaged with a 488 nm (green). Images were acquired with a 40 × objective, and the spheroid was scanned at 10, 20, 30, 40 and 50 μm in depth from the surface. Graphics below show the quantification of parasites within the spheroids in each stack scanned. Quantification was obtained using the particles analysis tool from ImageJ software of five different spheroids from each treatment per experiment. All assays are expressed as the mean ± SD of three independent experiments (∗p < 0,05; ∗∗p < 0,005). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Overall the results show that RSV can partially protect cells from infection, probably affecting the attachment and/or invasion of trypomastigotes but not the differentiation and/or replication of intracellular amastigotes. Thus, we next intended to analyze what cellular process is being affected by RSV in the host cell that might explain these results.
3.3. Resveratrol pretreatment of host cells does not affect the level of transcripts coding for autophagic proteins or LC3B protein expression
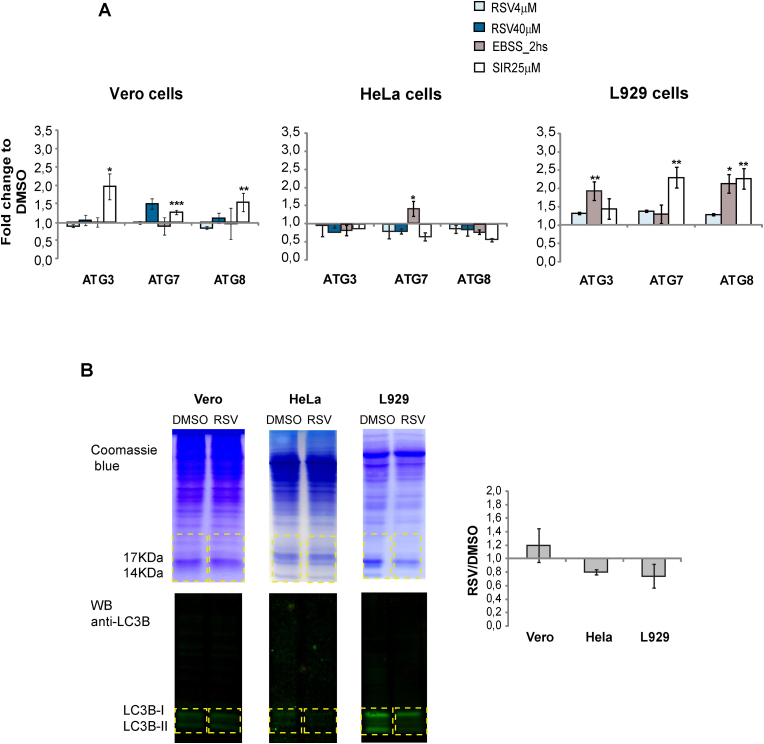
Previous studies describing the importance of autophagy in parasite invasion (reviewed in Romano et al., 2012) together with reports showing the effects of RSV on the modulation of this process (Kanamori et al., 2013) led us to examine the possibility that autophagy might be one of the mechanisms involved in the protective effects of this drug. To this end, the levels of transcripts coding for three of the key proteins involved in induction of autophagy (ATG3, ATG7 and ATG8) were analyzed by RT-qPCR after incubation of host cells with RSV. Results showed no statistically significant changes in any of the cells tested (Fig. 4A). The level of some of these transcripts increased only in L929 cells pretreated with 25 μM sirtinol or incubated in EBSS medium in concordance with the in vitro infection results.
Fig. 4.
A) Quantitative PCR analysis of transcripts coding for the autophagic proteins ATG3, ATG7 and ATG8 in host cells pretreated with the indicated concentrations of RSV, 25 μM Sirtinol, EBSS medium or 1% DMSO. Values are expressed as the mean of fold changes to DMSO obtained from three independent experiments ± SD (∗p < 0,05; ∗∗p < 0,005). B) Analysis by Western blot of the LC3B II/LC3B I + II proteins ratio in cells pretreated with 40 μM RSV (except for L929 cells, where 4 μM RSV was used) or DMSO as control for basal autophagy using specific antibodies. Left panel: Representative Western blot and Coomassie staining. Right panel: The LC3B II/LC3B I + II ratios obtained for each experiment were quantified using the gel analysis tool from ImageJ software. Values are shown as the mean of the fold changes between treated cells with RSV and DMSO obtained from three independent experiments ± SD (∗p < 0,05; ∗∗p < 0,005).
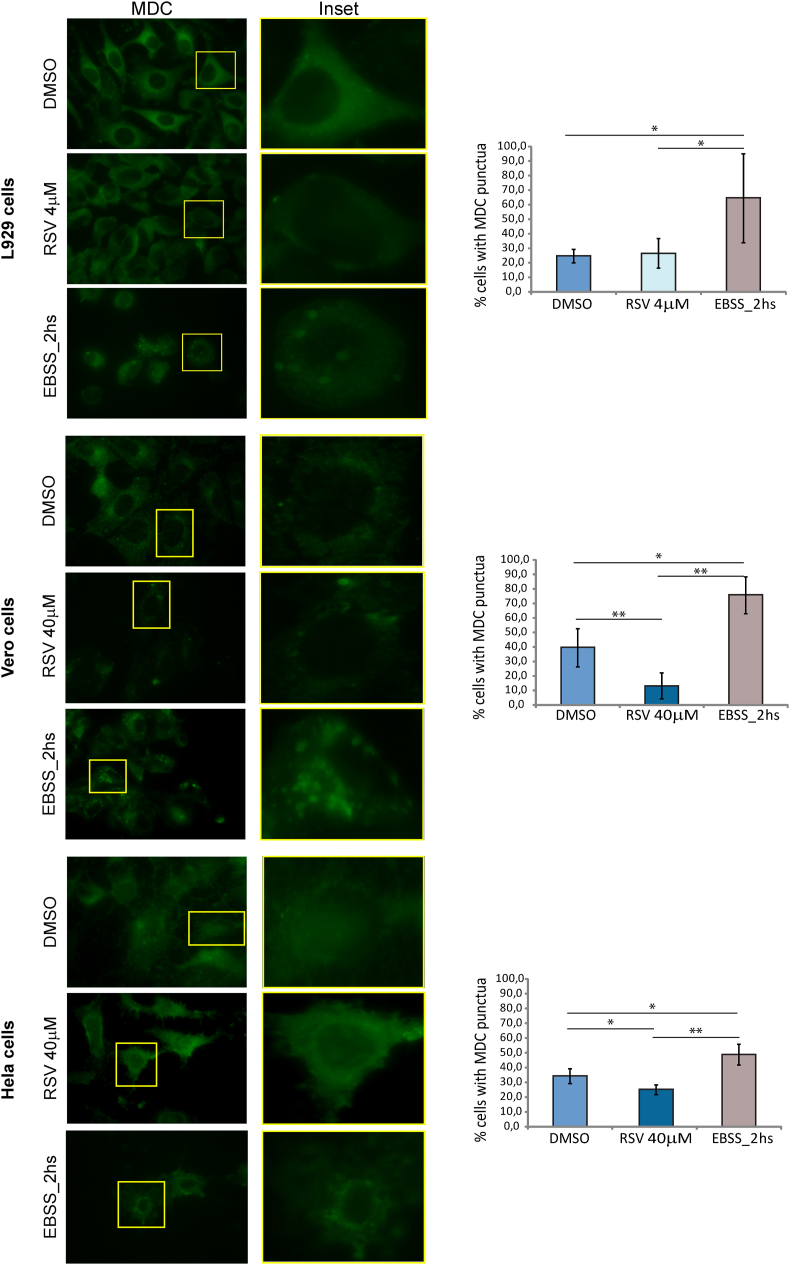
Given that the amount of LC3B-II is closely correlated with the number of autophagosomes, thus being a good indicator of autophagosome formation, we also analyzed changes in the expression of this protein by western blot using specific antibodies directed toward the protein (Mizushima and Yoshimori, 2007). As shown in Fig. 4B, no statistically significant changes in the LC3B-II/LC3B I + II ratio were observed in cells pretreated with RSV in comparison to those incubated with the drug vehicle. To corroborate these results, modulation of autophagy was further analyzed by MDC staining of cells treated with RSV. Taking in consideration the results of toxicity for L929 cells, 4 μM RSV was used for the analysis in these cells. Results showed no changes in the distribution of the signal. However, for HeLa and Vero cells, treatment with 40 μM RSV showed a slight but statistically significant reduction in the signal. On the other hand, starvation by incubation in EBSS medium for 2 h caused accumulation of fluorescence signals with a dot distribution in all cell types (Fig. 5).
Fig. 5.
MDC staining of Vero, HeLa and L929 cells pre-incubated with the indicated concentrations of RSV, EBSS medium or 1% DMSO. Representative photographs (100X) for each treatment are shown indicating the MDC fluorescence signal (green). The insets show details of localization of the signal in one cell. The percentage of cells presenting MDC dots was quantified by analyzing representative photographs from each experiment with the ImageJ software. Values are expressed as means of three independent experiments ± SD (∗p < 0,05; ∗∗p < 0,005); each performed in duplicates. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Taken together, these results show that host cell pretreatment with RSV under the concentrations and incubation times tested is not able to induce autophagy. Although we observed some tendency toward inhibition, given the wide deviation obtained between experiments, we cannot affirm that this is the cause of the protective effect of RSV on host cells.
4. Discussion
KDAC modulators are being pursued as new drugs for the treatment of a wide range of diseases, including infections caused by parasites, like malaria, leishmaniasis (Andrews et al., 2012a), schistosomiasis (Heimburg et al., 2016) and African and American trypanosomiasis (reviewed in Fioravanti et al., 2020). Previously, we analyzed the effect on the biology of the T. cruzi of two different KDAC inhibitors and RSV, an activator of sirtuin deacetylases (Campo, 2017). In that study, we showed that RSV reduced epimastigote growth (thus promoting metacyclogenesis), markedly reduced cell-derived trypomastigote infectivity, and inhibited the differentiation and/or replication of intracellular amastigotes. We also observed a diminished expression of trypomastigote surface proteins important for parasite attachment to the host cell. Moreover, the effect of RSV described in that study can be only partially compared with the effects observed in parasites over-expressing sirtuins (Moretti et al., 2015; Ritagliati et al., 2015), further supporting the notion that targets other than sirtuins might be also responsible for the trypanocidal action of RSV.
In humans, this natural polyphenol is currently being evaluated as a promising anticancer and anti-age-related disease agent (Li et al., 2018; Arabzadeh et al., 2021). The effects of RSV on mammalian cells have been widely reported, showing that it can modulate the cell cycle and multiple pathways involved in cell growth, apoptosis, senescence, autophagy and inflammation (Harikumar and Aggarwal, 2008; Farhadnejad et al., 2019; Malaguarnera, 2019). Moreover, in vitro studies indicate that the biological effects of RSV may vary depending on the cell type (reviewed in Gambini et al., 2015). In this study, we evaluated the potential of RSV to protect host cells from T. cruzi infection and found that the pre-treatment with RSV of three different types of host cells caused a marked reduction of infection in vitro (Fig. 2).
Previous reports have shown that the autophagic pathway is involved in many pathological situations, including the infection by intracellular pathogens. In fact, during host cell invasion, T. cruzi interacts with autophagic compartments (Onizuka et al., 2017). However, the relevance of host autophagy is controversial since opposite effects have been previously observed with in vitro infection assays using cell-derived trypomastigotes and metacyclic trypomastigotes. Induction of autophagy has been found to increase infection by culture-derived trypomastigotes in CHO and HeLa cells and to reduce metacyclic trypomastigote internalization in HeLa cells (Martins et al., 2011; Cortez et al., 2016). Moreover, in other types of host cells like cardiac cells and peritoneal macrophages, the induction of host autophagy has been found to reduce the infection of macrophages (Duque et al., 2019). This led us to investigate whether the modulation of autophagy in host cells by RSV is one of the mechanisms responsible for the protective effect observed. Our results of the monitoring of changes in autophagy protein markers in Vero, HeLa and L929 cells treated with RSV showed that autophagy was not induced under the conditions tested.
To further evaluate the action of RSV on parasite invasion, we performed in vitro infection assays using spheroids, mimicking a tissue infection (Rodriguez et al., 2020). The results showed that, in spheroids, protection was enough to accomplish the same results as in 2D infections under the same conditions of incubation times and concentrations used for pretreatment with RSV. These results highlight the accessibility of the drug even under conditions where the parasite is present. However, we should mention that the protection of host cells was lost when times of infection were extended to 24 h. These results indicate that the protective effect of RSV is reversible, suggesting that adjustment of the conditions or a delivery system might be necessary. However, recent works with RSV in mouse and rat models have shown that this drug is able to reverse established heart damage (Vilar-Pereira et al., 2016; Jiang et al., 2021), to act as a neuroprotector during T. cruzi congenital (Fracasso et al., 2019) and acute infections (Fracasso et al., 2021a), and to ameliorate hepatic injury in the context of acute Chagas disease (Fracasso et al., 2021b), thus showing that RSV can reach target organs in vivo.
Several evidences have indicated that, during progression of Chagas disease, the host antioxidant response is exhausted (Perez-Fuentes et al., 2003; Wen et al., 2008, 2017), supporting the association of the consequent oxidative stress with parasite persistence in host tissues (Paiva et al., 2012) and with the development of cardiac alterations due to mitochondrial dysfunction and chronic inflammation (Lopez et al., 2018; Bonney et al., 2019). As a consequence, some approaches for Chagas disease treatment are focused on reducing the oxidative damage in the host by using an adjuvant therapy with antioxidants (Macao et al., 2007; Budni et al., 2013; Maldonado et al., 2021). Also, Benznidazole, one of the drugs used to treat T. cruzi infection, can induce increased reactive oxygen species (ROS) and DNA damage in host tissues, and antioxidant supplementation has been found to be able to attenuate this effect (Ribeiro et al., 2010; Barbosa et al., 2016). While there is large amount of evidence indicating that antioxidant adjuvant therapy with anti-parasite drugs decreases ROS in the host tissues, eventually resulting in improved heart function in Chagas disease (reviewed in Sánchez-Villamil et al., 2020), our study evaluates for the first time a potential pre-exposure prophylactic drug treatment against T. cruzi infection. Since RSV possesses several cellular targets, the protective effect observed on host cells might be due to a combination of mechanisms. RSV has been used as part of prophylactic formulations against viral infection with HIV (Chan et al., 2017), showing that the protective effect is associated with its previously reported ability to inhibit cellular ribonucleotide reductase, which converts ribonucleotides to deoxyribonucleotides for DNA synthesis during cell proliferation and for DNA repair (Fontecave et al., 1998). If this or other metabolic pathways are being affected during the protective effect of RSV, these pathways should be further studied in more detail. However, overall, our results show that RSV is able to protect cells from infection and, according to previous reports, also acts as an anti-parasitic agent (Valera Vera et al., 2016; Campo, 2017), suggesting that its potential role as a cellular protector from T. cruzi infection should be further investigated in vivo.
Declaration of competing interest
There are none.
Acknowledgments
We would like to thank Dr. Juan Mucci for providing L929 fibroblast cells and Dr. Gabriela Levy for providing luciferase RNA and the RT-qPCR luciferase primers. Special thanks to Agustina Chidichimo and Liliana Sferco for assistance in carrying out the parasite cultures and Francisco Guaimas for assistance with the confocal images. This work was funded by grants awarded to VAC from the “Agencia Nacional de Promoción Científica y Tecnológica” of Argentina (PICT-2017-1516). MR is a postdoc fellow from the National Council for Scientific and Technological Research of Argentina (“Consejo Nacional de Investigaciones Científicas y Técnicas”, CONICET). VT and VAC are researchers from the National Council for Scientific and Technological Research (“Consejo Nacional de Investigaciones Científicas y Técnicas”, CONICET). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2022.08.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
A) Percentages of infected HeLa, Vero and L929 cells incubated in EBSS medium (for 2 h) previous infection as control for autophagy induction (indicated in the figure as “pretreatment” under the line). As control, cells were left untreated previous infection. All infections were performed for 4 h with the addition of the drug vehicle (−) or RSV at final concentrations of 4 μM (+) or 40 μM (++) (indicated in the figure as “added during infection” above the line). Values are expressed as mean ± standard deviation (SD) of three independent experiments each performed in duplicate. B) Average numbers of intracellular amastigotes per cell observed in the in vitro infection experiments using Vero, HeLa, and L929 cells pre-incubated in EBSS media when DMSO (−), RSV at 4 μM (+) or 40 μM (++) was added during infection (indicated in the figure as “added during infection” above the line). Values are expressed as means of three independent experiments ± SD (∗p < 0,05; ∗∗p < 0,005); each performed in duplicates.
References
- Allfrey V.G., Faulner R., MirskyA E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. U.S.A. 1964;51(5):786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews K.T., Tran T.N., Fairlie D.P. Towards histone deacetylase inhibitors as new antimalarial drugs. Curr. Pharmaceut. Des. 2012;18:3467–3479. doi: 10.2174/138161212801327257. [DOI] [PubMed] [Google Scholar]
- Andrews K.T., Haque A., Jones M.K. HDAC inhibitors in parasitic diseases. Immunol. Cell Biol. 2012;90(1):66–77. doi: 10.1038/icb.2011.97. [DOI] [PubMed] [Google Scholar]
- Ângelo de Souza L., Silva E Bastos M., de Melo Agripino J., Souza Onofre T., Apaza Calla L.F., Heimburg T., Ghazy E., Bayer T., Ferraz da Silva V.H., Dutra Ribeiro P., Licursi de Oliveira L., Costa Bressan G., de Almeida Lamêgo M.R., Silva-Júnior A., de Souza Vasconcellos R., Suarez-Fontes A.M., Almeida-Silva J., Vannier-Santos M.A., Pierce R., Sippl W., Lopes Rangel Fietto J. Histone deacetylases inhibitors as new potential drugs against Leishmania braziliensis, the main causative agent of new world tegumentary leishmaniasis. Biochem. Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114191. [DOI] [PubMed] [Google Scholar]
- Arabzadeh A., Mortezazadeh T., Aryafar T., Gharepapagh E., Majdaeen M., Farhood B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: a mechanistic review. Cancer Cell Int. 2021;21(1):391. doi: 10.1186/s12935-021-02099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banez M.J., Geluz M.I., Chandra A., Hamdan T., Biswas O.S., Bryan N.S., Von Schwarz E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020;78:11–26. doi: 10.1016/j.nutres.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Barbosa J.L., Thiers C.A., de Bragança Pereira B., do Nascimento E.M., Ribeiro Frazon C.M., Budni P., Wilhelm Filho D., Pedrosa R.C. Impact of the use of benznidazole followed by antioxidant supplementation in the prevalence of ventricular arrhythmias in patients with chronic Chagas disease: pilot study. Am. J. Therapeut. 2016;23(6):e1474–e1483. doi: 10.1097/MJT.0000000000000137. [DOI] [PubMed] [Google Scholar]
- Biederbick A., Kern H.F., Elsässer H.P. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 1995;66(1):3–14. [PubMed] [Google Scholar]
- Bonney K.M., Luthringer D.J., Kim S.A., Garg N.J., Engman D.M. Pathology and pathogenesis of Chagas heart disease. Annu. Rev. Pathol. 2019;14(1):421–447. doi: 10.1146/annurev-pathol-020117-043711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock D.J., Faust G.E., Patel K.R., Schinas A.M., Brown V.A., Ducharme M.P., Booth T.D., Crowell J.A., Perloff M., Gescher A.J., Steward W.P., Brenner D.E. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007;6:1246. doi: 10.1158/1055-9965.EPI-07-0022. 52. [DOI] [PubMed] [Google Scholar]
- Budni P., Pedrosa R.C., DalmarcoEM, Dalmarco J.B., Frode T.S., Wilhelm Filho D. Carvedilol enhances the antioxidant effect of vitamins E and C in chronic Chagas heart disease. Arq. Bras. Cardiol. 2013;101(4):304–310. doi: 10.5935/abc.20130184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo V.A. Comparative effects of histone deacetylases inhibitors and resveratrol on Trypanosoma cruzi replication, differentiation, infectivity and gene expression. Int. J. Parasitol. Drugs Drug Resist. 2017;7(1):23–33. doi: 10.1016/j.ijpddr.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Yuhas J.M. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1–23. doi: 10.1007/978-3-642-82340-4_1. [DOI] [PubMed] [Google Scholar]
- Chan C.N., Trinité B., Levy D.N. Potent inhibition of HIV-1 replication in resting CD4 T cells by resveratrol and pterostilbene. Antimicrob. Agents Chemother. 2017;61:e00408–e00417. doi: 10.1128/AAC.00408-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Kumar C., Gnad F., Nielsen M.L., Rehman M., Walther T.C., et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Cortez C., Real F., Yoshida N. Lysosome biogenesis/scattering increases host cell susceptibility to invasion by Trypanosoma cruzi metacyclic forms and resistance to tissue culture trypomastigotes. Cell Microbiol. 2016;18(5):748–760. doi: 10.1111/cmi.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H., Sinclair D.A., Ellis J.L., Steegborn C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther. 2018;188:140–154. doi: 10.1016/j.pharmthera.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Santos J., Zuma A.A., de Luna Vitorino F.N., da Cunha J.P.C., de Souza W., Motta M.C.M. Trichostatin A induces Trypanosoma cruzi histone and tubulin acetylation: effects on cell division and microtubule cytoskeleton remodelling. Parasitology. 2019;146(4):543–552. doi: 10.1017/S0031182018001828. [DOI] [PubMed] [Google Scholar]
- Duque T.L.A., Siqueira M.S., Travassos L.H., Moreira O.C., Bozza P.T., Melo R.C.N., Henriques-Pons A., Menna-Barreto R.F.S. The induction of host cell autophagy triggers defense mechanisms against Trypanosoma cruzi infection in vitro. Eur. J. Cell Biol. 2019;99(1) doi: 10.1016/j.ejcb.2019.151060. [DOI] [PubMed] [Google Scholar]
- El-Sayed N.M., Myler P.J., Bartholomeu D.C., Nilsson D., Aggarwal G., Tran A.N., et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309(5733):409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- Farhadnejad H., Emamat H., Zand H. The effect of resveratrol on cellular senescence in normal and cancer cells: focusing on cancer and age-related diseases. Nutr. Cancer. 2019;71(7):1175–1180. doi: 10.1080/01635581.2019.1597907. [DOI] [PubMed] [Google Scholar]
- Fioravanti R., Mautone N., Rovere A., Rotili D., Mai A. Targeting histone acetylation/deacetylation in parasites: an update (2017-2020) Curr. Opin. Chem. Biol. 2020;57:65–74. doi: 10.1016/j.cbpa.2020.05.008. [DOI] [PubMed] [Google Scholar]
- Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998;421:277–279. doi: 10.1016/S0014-5793(97)01572-X. [DOI] [PubMed] [Google Scholar]
- Fracasso M., Bottari N.B., da Silva A.D., Grando T.H., Pillat M.M., Ulrich H., Vidal T., de Andrade C.M., Monteiro S.G., Nascimento L.F.N., Miletti L.C., Schafer da Silva A. Effects of resveratrol on the differentiation fate of neural progenitor cells of mouse embryos infected with Trypanosoma cruzi. Microb. Pathog. 2019;132:156–161. doi: 10.1016/j.micpath.2019.04.040. [DOI] [PubMed] [Google Scholar]
- Fracasso M., Reichert K., Bottari N.B., da Silva A.D., Schetinger M.R.C., Monteiro S.G., da Silva A.S. Involvement of ectonucleotidases and purinergic receptor expression during acute Chagas disease in the cortex of mice treated with resveratrol and benznidazole. Purinergic Signal. 2021;17(3):493–502. doi: 10.1007/s11302-021-09803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracasso M., Dutra da Silva A., Bottari N.B., Gonzalez Monteiro S., Razia Garzon L., Farias de Souza L.A., Schetinger M.R.C., Schafer Da Silva A. Resveratrol impacts in oxidative stress in liver during Trypanosoma cruzi infection. Microb. Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104800. [DOI] [PubMed] [Google Scholar]
- Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., Borras C. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/837042. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazy E., Abdelsalam M., Robaa D., Pierce R.J., Sippl W. Histone deacetylase (HDAC) inhibitors for the treatment of schistosomiasis. Pharmaceuticals. 2022;15(1):80. doi: 10.3390/ph15010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.G., Ekströma T.J. The human histone deacetylase family. Exp. Cell Res. 2001;262(2):75–83. doi: 10.1006/excr.2000.5080. [DOI] [PubMed] [Google Scholar]
- Harikumar K.B., Aggarwal B.B. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- Heimburg T., Chakrabarti A., Lancelot J., Marek M., Melesina J., Hauser A.T., Shaik T.B., Duclaud S., Robaa D., Erdmann F., Schmidt M., Romier C., Pierce R.J., Jung M., Sippl W. Structure-based design and synthesis of novel inhibitors targeting HDAC8 from Schistosoma mansoni for the treatment of schistosomiasis. J. Med. Chem. 2016;59(6):2423–2435. doi: 10.1021/acs.jmedchem.5b01478. [DOI] [PubMed] [Google Scholar]
- Herrera-Martínez M., Orozco-Samperio E., Montaño S., Ariza-Ortega J.A., Flores-García Y., López-Contreras L. Vorinostat as potential antiparasitic drug. Eur. Rev. Med. Pharmacol. Sci. 2020;24(13):7412–7419. doi: 10.26355/eurrev_202007_21909. [DOI] [PubMed] [Google Scholar]
- Jang M., Cai L., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jiang J., Gu X., Wang H., Ding S. Resveratrol improves cardiac function and left ventricular fibrosis after myocardial infarction in rats by inhibiting NLRP3 inflammasome activity and the TGF-β1/SMAD2 signaling pathway. PeerJ. 2021;9 doi: 10.7717/peerj.11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori H., Takemura G., GotoK Tsujimoto A., Ogino A., Takeyama T., Kawaguchi T., Watanabe T., Morishita K., Kawasaki M., Mikami A., Fujiwara T., Fujiwara H., Seishima M., Minatoguchi S. Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am. J. Pathol. 2013;182(3):701–713. doi: 10.1016/j.ajpath.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Kedzierski L., Curtis J.M., Kaminska M., Jodynis-Liebert J., Murias M. In vitro antileishmanial activity of resveratrol and its hydroxylated analogues against Leishmania major promastigotes and amastigotes. Parasitol. Res. 2007;102(1):91. doi: 10.1007/s00436-007-0729-y. 7. [DOI] [PubMed] [Google Scholar]
- Kelly J.M., Taylor M.C., Horn D., Loza E., Kalvinsh I., Björkling F. Inhibitors of human histone deacetylase with potent activity against the African trypanosome Trypanosoma brucei. Biorganic Med. Chem. Lett. 2012;22:1886–1890. doi: 10.1016/j.bmcl.2012.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.R., Li S., Lin C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44(1):69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- Lopez M., Tanowitz H.B., Garg N.J. Pathogenesis of chronic Chagas disease: macrophages, mitochondria, and oxidative stress. Curr. Clin. Micro Rpt. 2018;(5):45–54. doi: 10.1007/s40588-018-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macao L.B., Wilhelm Filho D., Pedrosa R.C., et al. Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int. J. Cardiol. 2007;123(1):43–49. doi: 10.1016/j.ijcard.2006.11.118. [DOI] [PubMed] [Google Scholar]
- Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. 2019;11(946) doi: 10.3390/nu11050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado E., Rojas D.A., Urbina F., Solari A. The use of antioxidants as potential Co-adjuvants to treat chronic Chagas disease. Antioxidants. 2021;10(7):1022. doi: 10.3390/antiox10071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek M., Ramos-Morales E., Picchi-Constante G.F.A., Bayer T., Norström C., Herp D., Sales-Junior P.A., Guerra-Slompo E.P., Hausmann K., Chakrabarti A., Shaik T.B., Merz A., Troesch T., Schmidtkunz K., Goldenberg S., Pierce R.J., Mourão M.M., Jung M., Schultz J., Sippl W., Zanchin N.I.T., Romier C. Species-selective targeting of pathogens revealed by the atypical structure and active site of Trypanosoma cruzi histone deacetylase DAC2. Cell Rep. 2021;37(12) doi: 10.1016/j.celrep.2021.110129. [DOI] [PubMed] [Google Scholar]
- Martins R.M., Melatto Alves R., Macedo S., Yoshida N. Starvation and rapamycin differentially regulate host cell lysosome exocytosis and invasion by Trypanosoma cruzi metacyclic forms. Cell Microbiol. 2011;13(7):943–954. doi: 10.1111/j.1462-5822.2011.01590.x. [DOI] [PubMed] [Google Scholar]
- Matutino Bastos T., Botelho Pereira Soares M., Haddad Franco C., Alcântara L., Antonini L., Sabatino M., Mautone N., Holanda Freitas-Junior L., Moraes C.B., Ragno R., Rotili D., Schenkman S., Mai A., Silvio Moretti N. Identification of inhibitors to Trypanosoma cruzi sirtuins based on compounds developed to human enzymes. Int. J. Mol. Sci. 2020;21:3659. doi: 10.3390/ijms21103659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia A.M., Hall B.S., Taylor M.C., Gómez-Palacio A., Wilkinson S.R., Triana-Chávez O., Kelly J.M. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012;206(2):220. doi: 10.1093/infdis/jis331. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3(6):542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- Moretti N.S., da Silva Augusto L., Clemente T.M., Antunes R.P., Yoshida N., Torrecilhas A.C., Cano M.I., Schenkman S. Characterization of trypanosoma cruziSirtuins as possible drug targets for Chagas Disease.Antimicrob. Agents Chemother. 2015;59(8):4669. doi: 10.1128/AAC.04694-14. 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.J., Kranz M., Ladlow M., Taylor S., Berst F., Holmes A.B., Keavey K.N., Jaxa-Chamiec A., Seale P.W., Stead P., Upton R.J., Croft S.L., Clegg W., Elsegood M.R. The synthesis of cyclic tetrapeptoid analogues of the antiprotozoal natural product apicidin. Bioorg. Med. Chem. Lett. 2001;11(6):773–776. doi: 10.1016/s0960-894x(01)00049-x. [DOI] [PubMed] [Google Scholar]
- Onizuka Y., Takahashi C., Uematsu A., Shinjo S., Seto E., Nakajima-Shimada J. Inhibition of autolysosome formation in host autophagy by Trypanosoma cruzi infection. Acta Trop. 2017;170:57–62. doi: 10.1016/j.actatropica.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Paiva C.N., Feijó D.F., Dutra F.F., Carneiro V.C., Freitas G.B., Alves L.S., Mesquita J., Fortes G.B., Figueiredo R.T., Souza H.S., Fantappié M.R., Lannes-Vieira J., Bozza M.T. Oxidative stress fuels Trypanosoma cruzi infection in mice. J. Clin. Invest. 2012;122(7):2531–2542. doi: 10.1172/JCI58525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Fuentes R., Guegan J.F., Barnabe C., et al. Severity of chronic Chagas disease is associated with cytokine/antioxidant imbalance in chronically infected individuals. Int. J. Parasitol. 2003;33(3):293–299. doi: 10.1016/s0020-7519(02)00283-7. [DOI] [PubMed] [Google Scholar]
- Picchi-Constante G.F.A., Guerra-Slompo E.P., Tahira A.C., et al. Metacyclogenesis defects and gene expression hallmarks of histone deacetylase 4-deficient Trypanosoma cruzi cells. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassi A., Jr., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- Rauf A., Imran M., Butt M.S., Nadeem M., Peters D.G., Mubarak M.S. Resveratrol as an anti-cancer agent: a review. Crit. Rev. Food Sci. Nutr. 2018;58(9):1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- Ribeiro C.M., Budni P., Pedrosa R.C., et al. Antioxidant therapy attenuates oxidative insult caused by benznidazole in chronic Chagas' heart disease. Int. J. Cardiol. 2010;145(1):27–33. doi: 10.1016/j.ijcard.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Ribeiro V., Dias N., Paiva T., Hagström-Bex L., Nitz N., Pratesi R. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020;12:7–17. doi: 10.1016/j.ijpddr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritagliati C., Alonso V.L., Manarin R., Cribb P., Serra E.C. Overexpression of cytoplasmic TcSIR2RP1 and mitochondrial TcSIR2RP3 impacts on Trypanosoma cruzi growth and cell invasion. PLoS Neglected Trop. Dis. 2015;9(5) doi: 10.1371/journal.pntd.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M.E., Rizzi M., Caeiro L., Masip Y., Sanchez D.O., Tekiel V. Transmigration ofTrypanosoma cruzi trypomastigotes through 3D spheroids mimicking host tissues. T. cruzi infection: methods and protocols. Methods Mol. Biol. 2019 doi: 10.1007/978-1-4939-9148-8_12. (chapter 12) [DOI] [PubMed] [Google Scholar]
- Rodriguez M.E., Rizzi M., Caeiro L.D., Masip Y.E., Perrone A., Sánchez D.O., Búa J., Tekiel V. Transmigration of Trypanosoma cruzi trypomastigotes through 3D cultures resembling a physiological environment. Cell Microbiol. 2020;9 doi: 10.1111/cmi.13207. [DOI] [PubMed] [Google Scholar]
- Romano P.S., Arboit M.A., Vázquez C.L., Colombo M.I. The autophagic pathway is a key component in the lysosomal dependent entry of Trypanosoma cruzi into the host cell. Autophagy. 2009;5(1):6–18. doi: 10.4161/auto.5.1.7160. [DOI] [PubMed] [Google Scholar]
- Romano P.S., Cueto J.A., Casassa A.F., Vanrell M.C., Gottlieb R.A., Colombo M.I. Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay. IUBMB Life. 2012;64(5):387–396. doi: 10.1002/iub.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villamil J.P., Bautista-Niño, Serrano N.C., Rincon M.Y., Garg N.J. Potential role of antioxidants as adjunctive therapy in Chagas disease. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/9081813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera Vera E.A., Sayé M., Reigada C., Damasceno F.S., Silber A.M., Miranda M.R., Pereira C.A. Resveratrol inhibits Trypanosoma cruzi arginine kinase and exerts a trypanocidal activity. Int. Jour. of Biol. Macromol. 2016;87:498–503. doi: 10.1016/j.ijbiomac.2016.03.014. [DOI] [PubMed] [Google Scholar]
- Vanrell M.C., Cueto J.A., Barclay J.J., Carrillo C., Colombo M.I., Gottlieb R.A., Romano P.S. Polyamine depletion inhibits the autophagic response modulating Trypanosoma cruzi infectivity. Autophagy. 2013;9(7):1080–1093. doi: 10.4161/auto.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-santos P., Reignault L., Huber K., Bracher F., De Souza W., De CarvalhoT Inhibition of NAD -dependent histone deacetylases (sirtuins) causes growth arrest and activates both apoptosis and autophagy in the pathogenic protozoan Trypanosoma cruzi. Parasitology. 2014;141(6):814–825. doi: 10.1017/S0031182013001704. [DOI] [PubMed] [Google Scholar]
- Verçoza B.R.F., Godinho JLP, de Macedo-Silva S.T., et al. KH-TFMDI, a novel sirtuin inhibitor, alters the cytoskeleton and mitochondrial metabolism promoting cell death in Leishmania amazonensis. Apoptosis. 2017;22:1169–1188. doi: 10.1007/s10495-017-1397-8. [DOI] [PubMed] [Google Scholar]
- Vilar-Pereira G., Carneiro V.C., Mata-Santos H., Vicentino A.R.R., Ramos I.P., Giarola N.L.L., Feijo D.F., Meyer-Fernandes J.A.R., Paula-Neto H.A., Medei E., Bozza M.T., Lannes-Vieira J., Paiva C.N. Resveratrol reverses functional Chagas heart disease in mice. PLoS Pathog. 2016;12(10) doi: 10.1371/journal.ppat.1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.J., Dhiman M., Whorton E.B., Garg N.J. Tissue specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microb. Infect. 2008;10(10–11):1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.J., Porter C., Garg N.J. Inhibition of NFE2L2-antioxidant response element pathway by mitochondrial reactive oxygen species contributes to development of cardiomyopathy and left ventricular dysfunction in Chagas disease. Antioxidants Redox Signal. 2017;27(9):550–566. doi: 10.1089/ars.2016.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Percentages of infected HeLa, Vero and L929 cells incubated in EBSS medium (for 2 h) previous infection as control for autophagy induction (indicated in the figure as “pretreatment” under the line). As control, cells were left untreated previous infection. All infections were performed for 4 h with the addition of the drug vehicle (−) or RSV at final concentrations of 4 μM (+) or 40 μM (++) (indicated in the figure as “added during infection” above the line). Values are expressed as mean ± standard deviation (SD) of three independent experiments each performed in duplicate. B) Average numbers of intracellular amastigotes per cell observed in the in vitro infection experiments using Vero, HeLa, and L929 cells pre-incubated in EBSS media when DMSO (−), RSV at 4 μM (+) or 40 μM (++) was added during infection (indicated in the figure as “added during infection” above the line). Values are expressed as means of three independent experiments ± SD (∗p < 0,05; ∗∗p < 0,005); each performed in duplicates.