Abstract
Norepinephrine stimulates the growth of a range of bacterial species in nutritionally poor SAPI minimal salts medium containing 30% serum. Addition of size-fractionated serum components to SAPI medium indicated that transferrin was required for norepinephrine stimulation of growth of Escherichia coli. Since bacteriostasis by serum is primarily due to the iron-withholding capacity of transferrin, we considered the possibility that norepinephrine can overcome this effect by supplying transferrin-bound iron for growth. Incubation with concentrations of norepinephrine that stimulated bacterial growth in serum-SAPI medium resulted in loss of bound iron from iron-saturated transferrin, as indicated by the appearance of monoferric and apo- isoforms upon electrophoresis in denaturing gels. Norepinephrine also caused the loss of iron from lactoferrin. The pharmacologically inactive metabolite norepinephrine 3-O-sulfate, by contrast, did not result in iron loss from transferrin or lactoferrin and did not stimulate bacterial growth in serum-SAPI medium. Norepinephrine formed stable complexes with transferrin, lactoferrin, and serum albumin. Norepinephrine-transferrin and norepinephrine-lactoferrin complexes, but not norepinephrine-apotransferrin or norepinephrine-albumin complexes, stimulated bacterial growth in serum-SAPI medium in the absence of additional norepinephrine. Norepinephrine-stimulated growth in medium containing 55Fe complexed with transferrin or lactoferrin resulted in uptake of radioactivity by bacterial cells. Moreover, norepinephrine-stimulated growth in medium containing [3H]norepinephrine indicated concomitant uptake of norepinephrine. In each case, addition of excess iron did not affect growth but significantly reduced levels of radioactivity (55Fe or 3H) associated with bacterial cells. A role for catecholamine-mediated iron supply in the pathophysiology of infectious diseases is proposed.
Bacterial growth and virulence have long been known to be influenced by environmental parameters such as temperature, pH, and osmolarity. The effect of host signaling molecules on bacteria, however, has only recently become apparent (30, 33–35), leading to the concept of microbial endocrinology (16, 17), which proposes that infectious organisms utilize hormones present within the host as environmental cues to initiate growth and pathogenic processes. Supporting this concept are the observations that elevated catecholamine levels preceded the development of acute infectious disease episodes (12) and that levels of epinephrine and norepinephrine (NE) were significantly higher in postoperative patients who developed severe sepsis than in those with uncomplicated recovery (11). Systemic infections are often caused by translocation of commensal organisms from the gastrointestinal tract following traumatic injury, such as burns or major surgery, even when there is no direct injury to the gastrointestinal tract itself (5, 25, 28). One consequence of severe tissue injury is the release of NE into the peripheral circulation due to the destruction of noradrenergic neurons innervating the traumatized tissue (28, 37). Lyte and Bailey (19) used a mouse model of neurotoxin-induced trauma to show a direct correlation between experimentally elevated systemic NE levels and overgrowth and translocation of indigenous gut bacteria, particularly Escherichia coli.
A number of in vitro studies also indicate that NE has marked stimulatory effects on the growth of important microbial pathogens (3, 4, 9, 18–23). The fact that such studies typically involve iron-restricted growth media raises the intriguing possibility that NE acts by facilitating the supply of iron to stressed bacterial cells. In this paper, we demonstrate that NE does indeed stimulate bacterial iron uptake and growth in iron-restricted conditions imposed by the high-affinity iron-binding glycoproteins transferrin and lactoferrin.
MATERIALS AND METHODS
Organisms, media, and reagents.
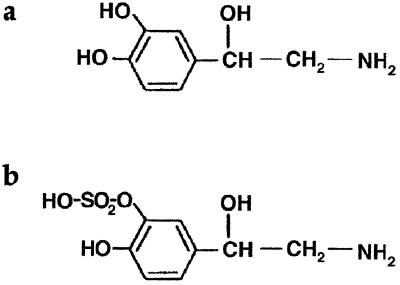
Enteropathogenic E. coli strain E2348/69, serotype O127:H6 (9, 15, 26), was used as the test organism throughout this study. SAPI medium and growth conditions have been described previously (9, 21); growth assays and plate counts were carried out in triplicate, and all experiments were performed on at least two separate occasions. Bovine serum albumin (BSA); iron-saturated, partially iron-saturated, and apo- forms of human transferrin (Tf), human lactoferrin (Lf), 2,3-dihydroxybenzoic acid (DHBA), NE, and bovine serum were all purchased from Sigma, Poole, United Kingdom. Superdex 75, Sephadex G25, 1-[7,8-3H]norepinephrine (TRK584; specific activity, 35 Ci/mmol) and 55FeCl3 (IES; specific activity, 5 mCi/mg of Fe) were obtained from Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom. Norepinephrine-3-O-sulfate (NE-S; Fig. 1) was synthesized by Research Biochemicals, Inc., Natrick, Mass., as part of the National Institute of Mental Health's Chemical Synthesis and Drug Supply Program.
FIG. 1.
Structure of (a) NE and (b) its pharmacologically inactive metabolite NE-S.
Size fractionation of serum components.
Bovine serum (1 ml) was fractionated using a Pharmacia Superdex 75 column (HR 10/30) equilibrated in phosphate-buffered saline (PBS) and eluted at a flow rate of 0.33 ml/min. Samples of 1-ml column fractions were diluted 1:1 with 100 mM Tris-HCl (pH 6.8) containing 10% (vol/vol) glycerol and 1% (wt/vol) sodium dodecyl sulfate (SDS), heated to 100°C for 3 min, and analyzed by electrophoresis on SDS–7% polyacrylamide gels. For analysis of protein profiles, gels were fixed and stained after electrophoresis in 0.05% (wt/vol) Coomassie blue in 40% (vol/vol) methanol and 10% (vol/vol) acetic acid and destained in 10% (vol/vol) methanol and 10% (vol/vol) acetic acid. For identification of proteins by N-terminal sequencing, gels were electroblotted onto polyvinylidene difluoride (PVDF) membranes at 0 to 4°C in 25 mM Tris, 192 mM glycine, and 0.037% (wt/vol) SDS, and 10% (vol/vol) methanol. Protein bands of interest were excised and sequenced using an Applied Biosystems 470A gas-phase sequencer.
Iron removal from Tf and Lf.
Iron-saturated Tf (50 μg) was incubated with NE (at concentrations indicated in the text) at 37°C for 15 h in 50-μl assay volumes containing 100 mM Tris-HCl (pH 7.5) and 10% (vol/vol) glycerol (8). NE was omitted from negative controls; 10 mM DHBA was included in positive controls. Samples were analyzed by electrophoresis in 6% polyacrylamide gels containing 6 M urea (24) in a BioRad Protean II vertical minigel system. Electrophoresis was at 70 V for 5 h. Gels were fixed and stained as described above. A partially iron-saturated human Tf preparation (Sigma), comprising iron-depleted, iron-saturated, and both N-terminal and C-terminal domain monoferric isoforms, was used as a marker standard. The equivalent isoforms of Lf resolve poorly on urea-acrylamide gels; however, removal of iron from Fe-Lf is characterized by an increase in apparent molecular mass from 70 to 78 kDa following electrophoresis in nonreducing SDS-polyacrylamide gels (10, 38). Assays were therefore performed as described for Tf except that Lf protein samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) as described above.
55Fe labeling of Tf, Lf, and serum.
[55Fe]Tf was prepared by an adaptation of the method of Cavill (1). Apo-Tf was incubated at 37°C for 5 h with 25 μCi of 55FeCl3 in a reaction mixture containing a total of 1.5 μg of Fe per mg of protein, using sodium citrate (2 mM) as the iron donor (1); this labeling method gives a Tf preparation of approximately 30% iron saturation. To prepare [55Fe]Lf, iron-saturated Lf was first depleted of iron by sequential dialysis against 0.2 M citric acid (pH 2.3), distilled water, and 100 mM Tris-HCl (pH 7.5). Labeling conditions were as for Tf except that incubation with 55Fe was for 15 h. For both [55Fe]Tf and [55Fe]Lf, unincorporated 55Fe was removed by two rounds of spin column chromatography (Micro Bio-spin 6 columns; Bio-Rad Laboratories, Hemel Hempstead, U.K.). Note that because of the very high affinity of Lf for ferric ions, it is difficult to obtain completely iron-depleted preparations of the protein. Consequently, the specific activity of the 55Fe-labeled Lf used in this study was less than a third of that of the 55Fe-labeled Tf. Serum-SAPI medium was 55Fe-labeled by incubating 5 μCi of 55FeCl3 per ml of medium at 37°C for a minimum of 3 h to ensure sequestration of all free iron.
[3H]NE binding assays.
Tf, Lf, and BSA (50 μg of protein) were incubated for 15 h at 37°C in triplicate 50-μl assays containing 1 μCi of [3H]NE in 50 μM unlabeled NE–100 mM Tris-HCl (pH 7.5)–10% (vol/vol) glycerol. Unbound label was removed by at least two rounds of spin column chromatography. [3H]NE binding was measured by mixing samples with 2 ml of Emulsifier-safe scintillant (Canberra-Packard, Pangbourne, U.K.) for counting in the tritium channel of a Minaxi Tri-Carb 400 series scintillation counter (Canberra-Packard). All assays were performed on at least three occasions and were quantitatively similar between experiments, typically varying by less than 10%.
NE-Tf complex formation.
The interaction of Tf with NE was analyzed by Sephadex G-25 gel chromatography (column size, 0.5 by 10 cm) in PBS at a flow rate of 0.3 ml/min; 0.5-ml fractions were collected, and 30-μl aliquots were measured for radioactivity as described above.
Bacterial uptake of 55Fe and [3H]NE.
Bacteria were grown for 18 h at 37°C in SAPI medium supplemented with serum, [55Fe]Tf, [55Fe]Lf, or 55FeCl3, as indicated in the text. Bacteria were in contact with the radioligands throughout the assay. Cells from triplicate 1-ml cultures were harvested by centrifugation, washed once with 1 ml of ice-cold PBS, and suspended in 50 μl of PBS. Radioactivity was measured as described above. All incorporation assays were performed on at least three occasions and were quantitatively similar between experiments, with values typically varying by less than 10%. Note that multiple washes with PBS or with PBS containing excess iron, cold NE, or cold apo-Tf gave essentially identical results. Moreover, this method gave more reproducible results than the more conventional filter capture method of harvesting radiolabeled bacteria; serum-SAPI is an osmotically stressful medium, and bacteria were susceptible to lysis in hypotonic washing solutions in filter assays.
Cellular localization of incorporated [3H]NE or 55Fe.
To determine the cellular location of [3H]NE or 55Fe, harvested bacteria were disrupted by sonication (four times for 15 s each; 6-μm amplitude), and after removal of nonlysed bacteria by centrifugation, the cell-free supernatant was separated into soluble (periplasmic and cytoplasmic) and membrane fractions by centrifugation at 50,000 × g for 10 min. The pellet was washed twice with 10 mM Tris-HCl (pH 7.5) containing 1 mM EDTA (TE buffer), recovered by centrifugation at 50,000 × g, and suspended in TE buffer. Supernatants and pellets were analyzed for radioactivity (as described above) and for the presence of Tf by Western blotting. Samples were separated by electrophoresis on SDS–12% PAGE gels, electroblotted onto PVDF membranes as described above, and probed with a 1:2,000 dilution of anti-Tf polyclonal antiserum (Sigma; T-6265); visualization of cross-reactivity was done by horseradish peroxidase-conjugated secondary antibodies (Sigma; A-5420; 1:3,000 dilution) and enhanced chemiluminescence (Amersham Pharmacia Biotech).
RESULTS
Involvement of Tf in stimulation of growth by NE.
We previously reported the use of a serum-containing growth medium (serum-SAPI) to examine the responsiveness of gram-negative and gram-positive bacteria to NE (9, 21). The SAPI minimal salts component of this medium is nutritionally rather poor, supporting bacterial growth to a low level (approximately 107 CFU/ml, Fig. 2a) compared with media such as M9 salts (9). Addition of NE (50 μM) to SAPI minimal medium did not stimulate growth of any bacterial species we have tested and indeed was slightly inhibitory to some, including E. coli. Addition of serum (30%, vol/vol) severely restricted growth in SAPI medium. However, as we have shown previously (9, 18–23), addition of NE to serum-supplemented SAPI cultures not only overcame the inhibitory effect of serum, but also significantly stimulated growth to a level (>108 CFU/ml; Fig. 2a) comparable to that in conventional media. It is interesting to note that the ability of the clinical E. coli isolate E2348/69 to synthesize and utilize the siderophore enterobactin (15) is not sufficient to allow growth in serum-SAPI medium to a level equivalent to that attainable in the presence of NE.
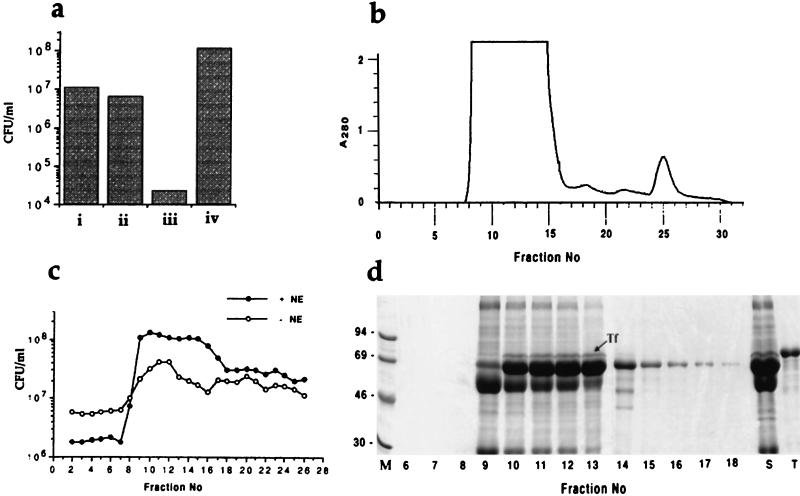
FIG. 2.
Effects of fractionated serum components on NE-induced stimulation of bacterial growth. (a) Viable counts of E. coli strain E2348/69 after 18 h of incubation from an inoculum of 102 CFU/ml in (i) SAPI minimal salts medium, (ii) SAPI medium supplemented with 50 μM NE, (iii) SAPI medium supplemented with 30% bovine serum, and (iv) SAPI medium supplemented with 30% bovine serum and 50 μM NE. (b) Superdex 75 elution profile of 1 ml of bovine serum. (c) viable counts of strain E2348/69 after 18 h of incubation from an inoculum of 102 CFU/ml in SAPI minimal medium supplemented (10%, vol/vol) with filter-sterilized fractions from the column shown in panel b. Assays were performed in the absence (○) or presence (●) of 50 μM NE. (d) SDS-PAGE of proteins in 5-μl aliquots of fractions 6 to 18 of the column shown in panel b; the position of Tf is indicated. Lane M contains marker proteins. Sizes are shown in kilodaltons. Lane S contains 0.5 μl of unfractionated bovine serum, and lane T contains 2 μg of purified human Tf.
To identify the components of serum required for growth stimulation by NE, we separated 1 ml of bovine serum into high- and low-molecular-weight fractions by size exclusion chromatography (Fig. 2b), added each fraction to SAPI medium, and assayed growth of our E. coli test strain in the presence and absence of NE (Fig. 2c). Fractionated serum did not show the growth-inhibitory potential of whole serum (size fractionation results in significant dilution), and so growth was observed with all fractions, even in the absence of NE. However, significant stimulation of growth by NE was still apparent, particularly with the high-molecular-weight protein fractions. PAGE in the presence of SDS showed that the active fractions contained several particularly abundant proteins of approximately 50, 60, and 75 kDa (Fig. 2d). N-terminal sequencing of the 60- and 75-kDa proteins gave sequences (DTHKSEIA and DPERTVR) that identified them as serum albumin and mature Tf, respectively; the 50-kDa protein was N-terminally modified and failed to sequence, but based on its size and relative abundance, it may be a component of complement. Immunoblotting with antiserum against bovine Tf confirmed the presence of Tf in all fractions that stimulated bacterial growth in the presence of NE (data not shown).
NE-induced loss of iron from Tf and Lf.
The bacteriostatic effect of serum is due primarily to the iron-binding capacity of Tf (although it is likely that other components, including low-molecular-weight molecules, may also be involved [unpublished data]). The intriguing possibility arises, therefore, that NE overcomes this effect in serum-SAPI medium by sequestering and supplying Tf-bound iron for bacterial growth. The catecholate structure of NE (Fig. 1) is certainly consistent with the ability to form bidentate complexes with ferric ions. Moreover, incubation of purified diferric Tf with NE resulted in marked loss of bound iron, as analyzed by denaturing urea-PAGE (Fig. 3a). The effect was concentration dependent, although we were never able to demonstrate complete removal, as achieved with DHBA (8). Nevertheless, the appearance of some apo-Tf at higher NE concentrations (see also Fig. 4c) indicates loss of iron from diferric and both monoferric isoforms of Tf, an effect that was prevented by addition of excess iron to the incubation mixtures (Fig. 3a). Significantly, the concentrations of NE that resulted in loss of iron from Tf were also able to stimulate the growth of E. coli in serum-SAPI medium (Fig. 3b). On the other hand, the pharmacologically inactive metabolite NE-S, whose structure is inconsistent with bidentate complex formation with ferric iron (Fig. 1), was unable to remove iron from Tf (Fig. 3a) or to support bacterial growth in serum-SAPI medium (Fig. 3b). DHBA also stimulated growth of E. coli in serum-SAPI (Fig. 3b), although a concentration of 10 mM was required to attain growth levels comparable to those achievable with 50 μM NE.
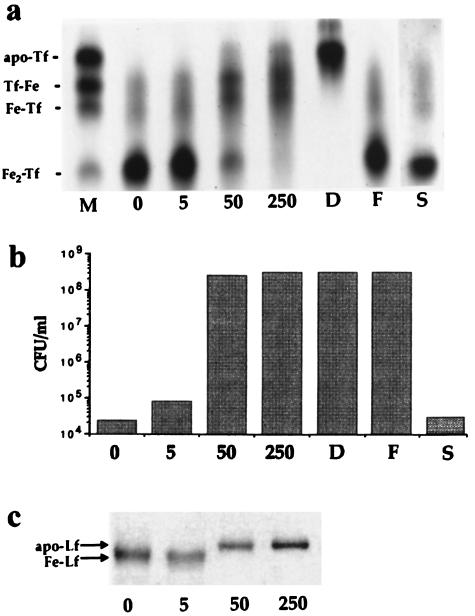
FIG. 3.
NE-mediated loss of iron from Tf and Lf. (a) Urea-PAGE of iron-saturated Tf after incubation for 18 h at 37°C in the absence of NE (lane 0) or in the presence of 5, 50, or 250 μM NE or 10 mM DHBA (lanes 5, 50, 250, and D, respectively). Lanes D, F, and S show the effect of incubation with DHBA (10 mM), 250 μM NE plus excess iron [1 mM Fe(NO3)3], and 250 μM NE-S, respectively. Lane M contains marker isoforms of Tf, fully saturated (diferric, Fe2Tf), monoferric isoforms with iron occupying the N-terminal or C-terminal domain (Fe-Tf and Tf-Fe, respectively), and iron-free Tf (apo-Tf). (b) Viable counts of E. coli strain E2348/69 after 18 h of incubation from an inoculum of 102 CFU/ml in serum-SAPI medium supplemented with NE or NE-S at the concentrations indicated for panel a above. (c) SDS-PAGE of iron-saturated Lf after incubation for 18 h at 37°C in the absence of NE (lane 0) or in the presence of 5, 50, or 250 μM NE (lanes 5, 50, and 250, respectively). Fe-Lf and apo-Lf indicate the mobility of iron-bound and iron-free isoforms of Lf, respectively.
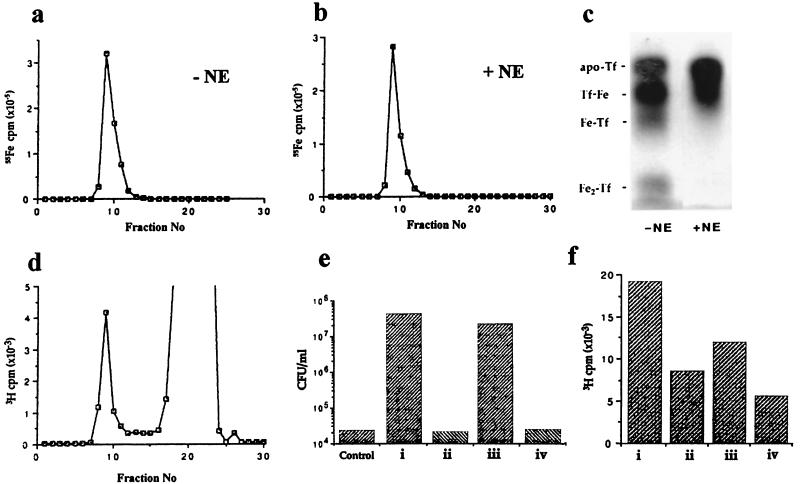
FIG. 4.
Formation of NE-Tf complexes. (a and b) Sephadex G-25 elution profiles of 100 μg of [55Fe]Tf in the absence of NE and following incubation with 50 μM NE, respectively. The radioactivity in 30-μl aliquots of 0.5-ml column fractions was determined by scintillation counting. (c) Coomassie-stained urea-polyacrylamide gel showing protein profiles of peak material from the Sephadex G-25 columns shown in panels a and b. Mobilities of marker isoforms of Tf are as described in the legend to Fig. 3. (d) Sephadex G-25 elution profile of 100 μg of iron-saturated Tf following incubation with [3H]NE. The radioactivity in 30-μl aliquots of 0.5-ml column fractions was determined by scintillation counting; the label associated with the Tf peak represents approximately 8% of the total radioactivity. (e) Viable counts of E. coli strain E2348/69 after 18 h of incubation from an inoculum of 102 CFU/ml in unsupplemented serum-SAPI medium (control) or in serum-SAPI medium supplemented with NE complexes of (i) the Tf isoform mixture used as markers in panel c above, (ii) Apo-Tf, (iii) iron-bound Lf, or (iv) BSA. (f) Binding of [3H]NE, expressed as 3H cpm per 30 μg of protein, to (i) partially iron-saturated Tf, (ii) Apo-Tf, (iii) iron-bound Lf, or (iv) BSA; the values shown are the means of triplicate assays.
Although we routinely use a test medium containing serum Tf to study the effects of NE, we are aware that it is in the Lf-rich mucosal secretions of the gastrointestinal tract that E. coli is most likely to encounter neuroendocrine hormones in vivo. Because of the physical characteristics of Lf, it was not possible to analyze iron removal by NE in urea gels. However, mobility changes in SDS-polyacrylamide gels were observed that indicated that NE also caused loss of iron from Lf (Fig. 3c). NE-S, on the other hand, was unable to remove iron from Lf (data not shown).
Formation of an NE-Tf complex.
To determine whether NE was able to supply Tf-derived iron for bacterial growth, we attempted to prepare 55Fe-complexed NE by incubating NE with [55Fe]Tf and separation on a Sephadex G25 column. To our surprise, however, identical elution profiles for [55Fe]Tf were obtained in the absence (Fig. 4a) and presence (Fig. 4b) of NE; no low-molecular-weight peak of radioactivity corresponding to [55Fe]NE was observed in the nondenaturing conditions of the Sephadex column, despite the fact that urea-PAGE clearly indicated NE-dependent loss of iron from [55Fe]Tf in denaturing conditions (Fig. 4c). Sephadex G25 fractionation of unlabeled Tf that had been incubated with [3H]NE gave a major peak of label corresponding to unbound [3H]NE and a minor peak with the expected elution volume of Tf (Fig. 4d). These results indicate the formation of a relatively stable complex between NE and native Fe-Tf from which iron is lost only in denaturing separation conditions. Any iron whose removal is facilitated by NE does not remain associated with Tf (as demonstrated by scintillation counting the electrophoresis buffer following analysis of NE treatment of [55Fe]Tf). Similarly, NE complexed with Tf is also dissociated during electrophoresis (again demonstrated by scintillation counting the electrophoresis buffer following analysis of [3H]NE treatment of Tf).
Significant [3H]NE binding to all isoforms of Tf, including apo-Tf, and to Lf was observed (Fig. 4f). However, while spin column-purified NE-Tf and NE-Lf complexes supported bacterial growth in serum-SAPI medium in the absence of additional NE, iron-free NE-apo-Tf did not, even at 100 μg/ml (Fig. 4e), confirming the importance of iron supply in growth stimulation. Note that [3H]NE also bound to BSA (Fig. 4f), but this complex did not stimulate bacterial growth in serum-SAPI medium (Fig. 4e).
NE-mediated bacterial iron uptake.
We previously reported that NE is nonfunctional in standard siderophore growth assays (9). The data presented above strongly suggest that NE stimulation of bacterial growth in the presence of serum is dependent on the formation of a ternary complex comprising NE, iron, and Tf or Lf. The question remains, therefore, whether NE mediates the supply of Tf- or Lf-sequestered iron to growing bacteria. To address this, we grew E. coli in SAPI medium containing serum labeled with 55Fe (Fig. 5a) or in serum-SAPI medium supplemented with [55Fe]Tf (Fig. 5b) or [55Fe]Lf (Fig. 5c). In all three media, NE-stimulated growth was accompanied by substantial association of 55Fe label with bacterial cells. Moreover, essentially identical results were obtained in growth assays performed in serum-SAPI medium supplemented with [3H]NE (Fig. 5d); stimulation of growth was accompanied by association of 3H label with bacterial cells. Even in the absence of the inhibitory factor(s) in serum, NE significantly enhanced the level of 55Fe associated with growing bacteria when SAPI was supplemented with [55Fe]Tf (Fig. 6a), even though it did not stimulate growth above unsupplemented control levels (indeed, NE was slightly inhibitory, perhaps due to its iron-chelating ability). When 55Fe (as 55FeCl3) was added to SAPI in the absence of Tf, levels of growth and of radioactivity associated with bacterial cells were essentially identical in the absence and presence of NE (Fig. 6b). Iron uptake in the absence of NE is presumably siderophore mediated. More efficient iron uptake in the presence of NE, regardless of the presence of serum, strongly suggests that formation of a complex with Tf-bound iron is essential for the effects that we observe with NE.
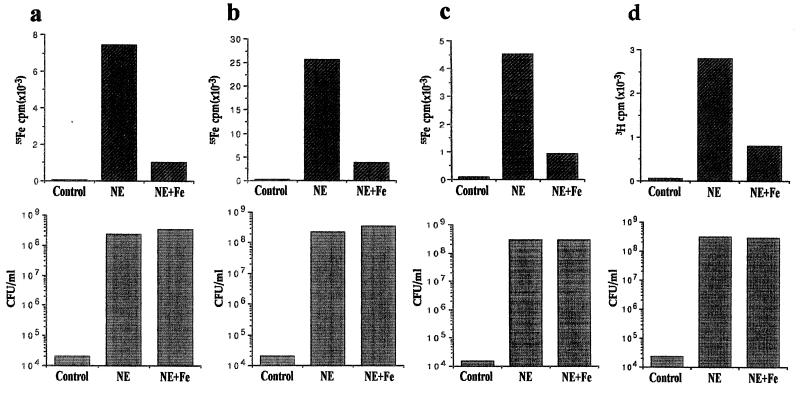
FIG. 5.
Bacterial acquisition of iron from NE-Tf and NE-Lf complexes. Lower panels show viable counts of E. coli strain E2348/69 grown in serum-supplemented SAPI medium after 18 h of incubation from an inoculum of 102 CFU/ml; upper panels show uptake of radioactivity (55Fe or 3H) associated with the 1-ml cultures shown in the lower set of histograms, i.e., radiolabel per 108 CFU (approximately); the values shown are the means of triplicate assays. Assay conditions were (a) SAPI medium containing 55Fe-labeled serum, (b) serum-SAPI medium supplemented with [55Fe]Tf (105 cpm), (c) serum-SAPI medium supplemented with [55Fe]Lf (105 cpm), and (d) unlabeled serum-SAPI medium. Unlabeled NE was used in assays a to c; 1 μCi of [3H]NE plus 50 μM unlabeled NE was used in assay d. In each case, bacteria were incubated in the absence of NE (control), in the presence of 50 μM NE (NE), or in the presence of 50 μM NE plus 200 μM Fe(NO3)3(NE+Fe).
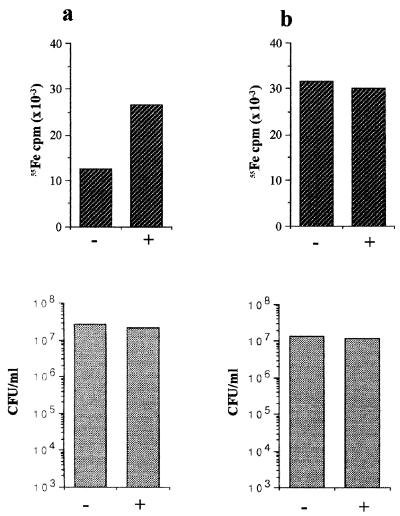
FIG. 6.
Bacterial acquisition of iron from NE-Tf complexes in the absence of serum. Lower panels show viable counts of E. coli strain E2348/69 grown in non-serum-supplemented SAPI medium after 18 h of incubation from an inoculum of 102 CFU/ml; upper panels show uptake of radioactivity (55Fe) associated with the 1-ml cultures shown in the lower set of histogram; the values shown are the means of triplicate assays. Assay conditions were (a) SAPI medium supplemented with [55Fe]Tf in the absence (−) or presence (+) of 50 μM NE and (b) SAPI medium supplemented with 105 cpm of 55FeCl3 in the absence (−) or presence (+) of 50 μM NE.
Cellular localization of 55Fe and [3H]NE.
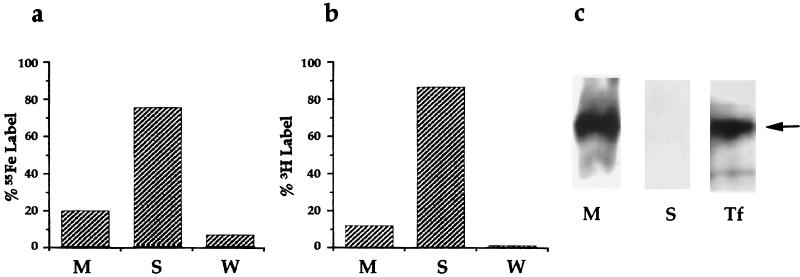
Sonication of the bacteria and separation of membrane from soluble fractions by high-speed centrifugation indicated that 75% of the 55Fe label and 87% of the [3H]NE were present in the soluble (cytoplasmic/periplasmic) fractions (Fig. 7a and b), indicating that both NE and Tf- or Lf-derived iron are internalized by growing bacteria. Western blotting of separated proteins from membrane and soluble fractions with anti-Tf antibodies indicated that associated Tf remained bound to the bacterial membrane (Fig. 7c), and indeed may account for the significant minority of 55Fe counts (approximately 20% of the total) that remained in the membrane fraction. The incorporation of NE and Tf-bound 55Fe is markedly influenced by environmental iron levels (Fig. 5a to d); addition of 200 μM iron to NE-supplemented cultures had no appreciable effect on bacterial growth, but significantly reduced both 55Fe and 3H uptake.
FIG. 7.
Localization of cell-associated iron, NE, and Tf in growing bacteria. (a and b) Distribution of Tf-derived 55Fe and [3H]NE, respectively, in membrane (M), soluble (S), and wash (W) fractions following ultracentrifugation of sonicated bacteria. (c) Western blot analysis of membrane (M) and soluble (cytoplasmic/periplasmic, S) proteins (approximately 25 μg of protein separated by SDS-PAGE) probed with anti-Tf antibodies. Lane Tf contains 1 μg of purified human Tf; the arrow indicates the position of the mature protein.
DISCUSSION
Iron is an essential nutrient for the growth of most bacteria. Moreover, in the case of pathogenic bacteria, effective iron acquisition in the face of the defensive hypoferremic response of the infected host may be crucial to the outcome of an infection (36). Bacteria use a variety of mechanisms to acquire iron, including ferric reductase activity, interactions with host iron-binding proteins, and the use of siderophores, low-molecular-weight secreted molecules with high affinity for ferric iron (13, 27, 36). Many species possess the genetic capacity both to synthesize and to take up siderophores, and some species express uptake systems for exogenous siderophores secreted by other microorganisms (14, 27). Furthermore, certain compounds present in the growth milieu, such as the primary metabolites α-keto- and α-hydroxyacids (14, 31), can act in a siderophore-like fashion, supplying iron for bacterial growth in the absence of other high-affinity iron uptake systems. The data presented in this report suggest that opportunistic utilization of the neuroendocrine hormone NE represents yet another method by which bacteria acquire iron. Previous work by Coulanges and coworkers proposed that Listeria monocytogenes uses ferric reductase activity to obtain iron bound to catecholamine hormones in vitro (3, 4). However, since NE stimulates the growth of a wide range of both gram-negative and gram-positive bacteria in iron-restricted conditions (9), it is unlikely that a single mechanism applies to all species. Here we demonstrate that NE at physiological concentrations complexes with the host iron-binding glycoproteins Tf and Lf and that a clinical E. coli strain has the ability to both bind and use these complexes as a source of iron for growth in vitro. Uptake of iron and NE by growing bacteria is significantly reduced in iron-supplemented cultures, indicating that NE-mediated acquisition of iron is modulated by environmental iron concentrations. NE-Tf and NE-Lf complexes appear to be relatively stable, although analysis by PAGE in the presence of urea or SDS indicated NE-dependent loss of iron from Tf and Lf, respectively, in native conditions, there was no measurable transfer of protein-bound iron to free NE. We suggest that the affinity of Tf and Lf for iron is reduced by complex formation with NE, presumably due to structural or conformational changes in the proteins, and that this facilitates iron removal and assimilation by bacteria. Preliminary examination of the UV and visible spectra of diferric Tf before and after incubation with NE suggests shifts in absorption maxima consistent with changes in iron-binding affinity (unpublished data). Although it is clear that NE can facilitate removal of iron from Tf and Lf, we do not yet know the mechanism by which E. coli takes up iron from NE-Tf and Lf-Tf complexes. Our data are compatible with two possible models, one in which NE simply releases iron from Tf, which is then assimilated via microbial iron acquisition systems, the other in which NE acts in a siderophore-like fashion, removing iron from Tf and delivering it directly to the bacteria. Although we have yet to demonstrate chelation of iron by NE, we favor the latter model, since both NE and Tf- and Lf-derived iron are internalized. However, the question of how bacteria acquire iron from NE still remains. Previous in vitro work has shown that agonists or antagonists of vertebrate adrenergic receptors had no measurable effect on NE-stimulated growth of gram-negative bacteria in iron-restricted medium (22). Moreover, database searches of genome sequences have so far revealed no evidence for vertebrate-type adrenoreceptors among NE-responsive bacteria. The E. coli strain used in this work is wild type for enterobactin synthesis and utilization (15), but this alone is not sufficient to promote growth in serum-SAPI medium unless NE is also present. Mutants (entA and entF) deficient in enterobactin synthesis do not grow at all in serum-SAPI medium even in the presence of NE (unpublished data), suggesting that enterobactin is involved in some way in the NE responsiveness of E. coli, but whether the effect is direct or indirect is not yet known. This is currently under investigation in our laboratories.
The physiological importance of the interaction of NE with Tf or Lf is unclear. However, from the infectious disease standpoint, it is apparent that the formation of these complexes in vivo is potentially dangerous if, as demonstrated in vitro, they supply iron for bacterial growth. It may also be significant that NE binds to the very abundant serum protein albumin; since the NE-albumin complex does not support bacterial growth in iron-restricted medium, such binding may be important in reducing the effective systemic concentration of microbiologically active NE. It would be interesting to know if NE retains pharmacological activity in complexes with Tf, Lf, or albumin. Serum Tf is normally about 30% iron saturated in healthy individuals and maintains free iron in the blood at levels too low to support microbial growth; Lf limits iron availability at mucosal surfaces and in secretions (36). We propose that formation of NE-Tf and NE-Lf complexes in vivo creates environments in which iron is more readily available for bacterial growth. We previously demonstrated that release of NE into the gastrointestinal tract of mice following chemical sympathectomy resulted in a greater than 100,000-fold increase in viable E. coli in the cecum within 24 h, with concomitant tissue invasion (19). In trauma and burn patients, in whom the development of intra-abdominal sepsis due to commensal bacteria is frequently observed, severe tissue damage is associated with massive systemic release of NE, which eventually spills over into the gastrointestinal tract (5, 25, 28, 29, 37). Moreover, many patients in intensive care receive intravenous catecholamine hormone infusions to maintain heart function (32); such patients are particularly susceptible to infection from a variety of opportunistic pathogens despite intensive antibiotic prophylaxis. Thus, while effects of trauma on mucosal integrity and decreased resistance to infection due to impaired immune status may partly account for the occurrence of sepsis in critically ill patients, a direct effect of catecholamine hormones, either released naturally as a consequence of stress or administered therapeutically, on bacterial growth should not be discounted.
Catecholamine levels in vivo are tightly controlled, in terms of both production (in response to stress) and metabolism. Indeed, a significant proportion of NE in the body at any time is likely to be in the pharmacologically inactive sulfated form NE-S, due to the activity of catecholamine sulfotransferases located primarily within the gastrointestinal tract (2, 6, 7). NE-S is unable either to remove iron from Tf and Lf in vitro or to stimulate bacterial growth in iron-restricted medium. In the mammalian body, the gastrointestinal tract is the site at which bacteria are most likely to encounter NE and Lf. We may speculate, therefore, that inactivation of NE by sulfation, in addition to its generally recognized purpose of damping down nervous system activity, may play an important physiological role in maintaining a stable commensal flora in healthy individuals by at least partially abrogating the bacterial growth-stimulatory effects of NE-mediated iron supply. Experiments to test these hypotheses are currently in progress in our laboratories.
ACKNOWLEDGMENTS
This work was supported by grant F/212/W from the Leverhulme Trust (to P.W.) and National Institutes of Health grants MH-01371, MH-50431, and A144918 (to M.L.). C.N. was in receipt of a Wolfson Foundation Intercalated Award.
We are grateful to Kathryn Lilley of the Protein and Nucleic Acid Chemistry Laboratory, University of Leicester, for assistance with protein sequencing.
REFERENCES
- 1.Cavill I. Preparation of 59Fe-labelled transferrin for ferrokinetic studies. J Clin Pathol. 1971;24:472–474. doi: 10.1136/jcp.24.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughtrie M W H. Sulphation catalyzed by the human cytosolic sulphotransferases—chemical defence or molecular terrorism? Hum Exp Toxicol. 1996;15:547–555. doi: 10.1177/096032719601500701. [DOI] [PubMed] [Google Scholar]
- 3.Coulanges V, Andre P, Vidon D J-M. Effect of siderophores, catecholamines, and catechol compounds on Listeria spp. growth in iron-complexed medium. Biochem Biophys Res Commun. 1998;249:526–530. doi: 10.1006/bbrc.1998.9184. [DOI] [PubMed] [Google Scholar]
- 4.Coulanges V, Andre P, Ziegler O, Bucheit L, Vidon D J-M. Utilization of iron-catecholamine complexes involving ferric reductase activity in Listeria monocytogenes. Infect Immun. 1997;65:2278–2785. doi: 10.1128/iai.65.7.2778-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deitch E A, Maejima K, Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J Trauma. 1985;25:385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhofer G, Aneman A, Hooper D, Rundqvist B, Friberg P. Mesenteric organ production, hepatic metabolism, and renal elimination of norepinephrine and its metabolites in humans. J Neurochem. 1996;66:1565–1573. doi: 10.1046/j.1471-4159.1996.66041565.x. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhofer G, Coughtrie M W H, Goldstein D S. Dopamine sulfate: an enigma resolved. Clin Exp Pharmacol Physiol. 1999;26:S41–S53. [PubMed] [Google Scholar]
- 8.Ford S, Cooper R A, Evans R W, Hider R C, Williams P H. Domain preference in iron removal from human transferrin by the bacterial siderophores aerobactin and enterochelin. Eur J Biochem. 1988;178:477–481. doi: 10.1111/j.1432-1033.1988.tb14473.x. [DOI] [PubMed] [Google Scholar]
- 9.Freestone P P E, Lyte M, Haigh R D, Williams P H. Stimulation of bacterial growth by heat stable norepinephrine-induced autoinducers. FEMS Microbiol Lett. 1999;172:53–60. doi: 10.1111/j.1574-6968.1999.tb13449.x. [DOI] [PubMed] [Google Scholar]
- 10.Groenink J, Walgreen-Weterings E, Nazmi K, Bolscher J G M, Veerman E C I, van Winkelhoff A J, Nieuw Amerongen A V. Salivary lactoferrin and low-Mr mucin MG2 in Actinobacillus actinomycetemcomitans-associated periodontitis. J Clin Peridontol. 1999;26:269–275. doi: 10.1034/j.1600-051x.1999.260501.x. [DOI] [PubMed] [Google Scholar]
- 11.Groves A C, Griffiths J, Leung F, Meek R N. Plasma catecholamines in patients with serious postoperative infection. Ann Surg. 1973;178:102–107. doi: 10.1097/00000658-197307000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruchow H W. Catecholamine activity and infectious disease episodes. J Hum Stress. 1979;5:11–17. doi: 10.1080/0097840X.1979.9934523. [DOI] [PubMed] [Google Scholar]
- 13.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 14.Kingsley R, Rabsch W, Roberts M, Reissbrodt R, Williams P H. TonB-dependent iron supply in Salmonella by α-ketoacids and α-hydroxyacids. FEMS Microbiol Lett. 1996;140:65–70. doi: 10.1111/j.1574-6968.1996.tb08316.x. [DOI] [PubMed] [Google Scholar]
- 15.Law D, Wilkie K M, Freeman R, Gould F K. The iron uptake mechanisms of enteropathogenic Escherichia coli: the use of haem and haemoglobin during growth in iron-limited environment. J Med Microbiol. 1992;37:15–21. doi: 10.1099/00222615-37-1-15. [DOI] [PubMed] [Google Scholar]
- 16.Lenard J. Mammalian hormones in microbial cells. Trends Biochem Sci. 1992;17:147–150. doi: 10.1016/0968-0004(92)90323-2. [DOI] [PubMed] [Google Scholar]
- 17.Lyte M. The role of microbial endocrinology in infectious disease. J Endocrinol. 1993;137:343–345. doi: 10.1677/joe.0.1370343. [DOI] [PubMed] [Google Scholar]
- 18.Lyte M, Arulanandam B P, Frank C D. Production of Shiga-like toxins by Escherichia coli O157:H7 can be influenced by the neuroendocrine hormone norepinephrine. J Clin Lab Med. 1996;128:392–398. doi: 10.1016/s0022-2143(96)80011-4. [DOI] [PubMed] [Google Scholar]
- 19.Lyte M, Bailey M T. Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J Surg Res. 1997;70:195–201. doi: 10.1006/jsre.1997.5130. [DOI] [PubMed] [Google Scholar]
- 20.Lyte M, Erickson A K, Arulanandam B P, Frank C D, Crawford M A, Francis D H. Norepinephrine-induced expression of the K99 pilus adhesin of enterotoxigenic Escherichia coli. Biochem Biophys Res Commun. 1997;232:682–686. doi: 10.1006/bbrc.1997.6356. [DOI] [PubMed] [Google Scholar]
- 21.Lyte M, Ernst S. Catecholamine induced growth of Gram negative bacteria. Life Sci. 1992;50:302–212. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- 22.Lyte M, Ernst S. Alpha and beta adrenergic receptor involvement in catecholamine-induced growth of Gram-negative bacteria. Biochem Biophys Res Commun. 1993;190:447–452. doi: 10.1006/bbrc.1993.1068. [DOI] [PubMed] [Google Scholar]
- 23.Lyte M, Frank C D, Green B T. Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol Lett. 1996;39:155–159. doi: 10.1111/j.1574-6968.1996.tb08196.x. [DOI] [PubMed] [Google Scholar]
- 24.Makey D G, Seal U S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976;453:250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- 25.Marshall J C, Christou N V, Horn R, Meakins J L. The microbiology of multiple organ failure: the proximal gastrointestinal tract as an occult reservoir of pathogens. Arch Surg. 1988;123:309–315. doi: 10.1001/archsurg.1988.01400270043006. [DOI] [PubMed] [Google Scholar]
- 26.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;55:2370–2377. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwen Huijzen G A, Deitch E A, Goris R J. Infection, the gut and the development of the multiple organ dysfunction syndrome. Eur J Surg. 1996;162:259–273. [PubMed] [Google Scholar]
- 29.Plunkett J J, Reeves J D, Ngo L, Bellows W, Shafer S L, Roach G, Howse J, Herskowitz A, Mangano D T. Urine and plasma catecholamine and cortisol concentrations after myocardial revascularization—modulation by continuous sedation. Anesthesiology. 1997;86:785–796. doi: 10.1097/00000542-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Powell B L, Drutz D J, Hulpert M, Hun S H. Relationship of progesterone- and estradiol-binding proteins in Coccidioides immitis to coccidioidal dissemination in pregnancy. Infect Immun. 1983;40:478–485. doi: 10.1128/iai.40.2.478-485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reissbrodt R, Kingsley R, Rabsch W, Beer W, Roberts M, Williams P H. Iron-regulated excretion of α-keto acids by Salmonella typhimurium. J Bacteriol. 1997;179:4538–4544. doi: 10.1128/jb.179.14.4538-4544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smythe M A, Melendy S, Jahns B, Dmuchowski C. An exploratory analysis of medication utilization in a medical intensive care unit. Crit Care Med. 1993;37:1319–1326. doi: 10.1097/00003246-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Sonnex C. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74:11–19. doi: 10.1136/sti.74.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss M, Ingbar S H, Winblad S, Kasper D L. Demonstration of a saturable binding site for thyrotropin in Yersinia enterocolitica. Science. 1983;219:1331–1333. doi: 10.1126/science.6298936. [DOI] [PubMed] [Google Scholar]
- 35.Woods D E, Jones A L, Hill P J. Interaction of insulin with Pseudomonas pseudomallei. Infect Immun. 1993;61:4045–4050. doi: 10.1128/iai.61.10.4045-4050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 37.Woolf P D, McDonald J V, Feliciano D V, Kelly M M, Nichols D, Cox C. The catecholamine response to multi-system trauma. Arch Surg. 1992;127:899–903. doi: 10.1001/archsurg.1992.01420080033005. [DOI] [PubMed] [Google Scholar]
- 38.Ying L, He J L, Furmanski P. Iron-induced conformational change in human lactoferrin—demonstration by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analysis of effects of iron-binding to the N-lobe and C-lobe of the molecule. Electrophoresis. 1994;15:244–250. doi: 10.1002/elps.1150150142. [DOI] [PubMed] [Google Scholar]