Abstract
Knowledge about ticks and Rickettsiae in Colombia is still limited and the areas of the country where studies have been conducted are scarce. In this study, ticks (Acari, Ixodidae) associated with reptiles and amphibians in San Juan de Carare, Santander department, were morphologically and molecularly identified and tested for the presence of Rickettsia. For the molecular characterization of ticks, CO1, 12S and 16S sequences were generated and compared with other sequences available in genbank. Our analyses confirmed that the collected ticks were Amblyomma dissimile, and we provide the first report of this species parasitizing the snake Leptodeira septentrionalis. Of the samples analyzed, 69% were positive for Rickettsia sp. using the gltA, ompA and sca1 genes. Rickettsia sequences generated in this study were also compared to sequences downloaded from genbank by Bayesian inference (BI) and maximum likelihood (ML) phylogenetic analyzes. The presence of a single Rickettsia species, Candidatus Rickettsia colombianensi, was identified. This study expands the knowledge regarding the distribution of A. dissimile ticks and Rickettsiae in Colombia.
Keywords: Reptiles, Amphibians, Ectoparasites, Hosts, Amblyomma dissimile, Candidatus Rickettsia colombianensi
Graphical abstract
Highlights
-
•
First report of A. dissimile parasitizing Leptodeira septentrionalis.
-
•
69% of tested ticks were positive for Rickettsia sp. amplifying gltA, sca1 and ompA genes.
-
•
Candidatus Rickettsia colombianensi was the only Rickettsia identified.
-
•
Candidatus R. colombianensi is phylogenetically related to pathogenic species.
1. Introduction
The genus Rickettsia has been the causal agent of epidemics in different areas of the world, and biological groups such as mites, ticks, lice, and fleas are the main vectors (Vélez et al., 2012). One of the diseases that this group of bacteria transmits is rickettsiosis, which has taken a leading role as an emerging and re-emerging disease affecting both animals and humans (Hidalgo et al., 2013). Rickettsia species have been found in various taxa, such as amphibians, reptiles, birds, mammals, and arthropods, the latter acting as vectors (Bernabeu and Segura, 2005).
Herpetofauna has been studied more in recent years from an epidemiological perspective, evaluating its possible role in the epidemiology of various zoonotic diseases (Aguillón et al., 2007). In some scenarios, the rapid settlement of human communities in rural areas makes it possible for them to become part of the epidemiological cycle of zoonotic pathogens and alters the existing relationships between vectors and reservoirs, causing the emergence and resurgence of diseases (Monsalve et al., 2009).
Different tick species have been associated with reptiles and amphibians in the region. Amblyomma dissimile was reported as a parasite of herpetofauna, especially reptiles in the Caribbean, Andean and Orinoquia regions of Colombia (Carrascal et al., 2009; Cotes-Perdomo et al., 2018; Mora-Rivera et al., 2020; Osorno-Mesa, 2006; Santodomingo et al., 2018), while A. rotundatum was found in the Pacific region (Benavides-Montaño et al., 2018; Osorno-Mesa, 2006).
There are reports of Rickettsia associated with ticks that have been collected in reptiles and amphibians in northern regions of Colombia such as La Guajira, Magdalena, and Cordoba. These studies report the same Rickettsia strain, Candidatus Rickettsia colombianensi, a Ricketssia of unknown pathogenicity, but closely related to old world pathogenic species such as R. monacencis and R. tamurae (Miranda et al., 2012; Cotes-Perdomo et al., 2018; Santodomingo et al., 2018, 2019). Rickettsia monacencis and R. tamurae, together with R. slovaca, R. aeschlimannii, and R. massiliae were initially considered non-pathogenic, but today they are known to cause disease in humans (Parola et al., 2005; Jado et al., 2007). This makes it imperative to have a record of the presence of Rickettsiae in the region, its distribution and possible hosts, in order to be able to design adequate prevention and control strategies.
The objective of this work was to evaluate the presence of Rickettsia spp. in ticks associated with reptiles and amphibians in San Juan de Carare region in the department of Santander, Colombia, a region in where such studies have not been previously performed.
2. Material and methods
2.1. Study area and herpetofauna sampling
The samples were collected in the locality of San Juan de Carare, Cimitarra municipality, Santander Department, Colombia; located at 6° 42 ′58 0.20″N and 74° 08 ′02 0.71″W during five visits between March 2017 and May 2018. This locality exhibits temperature ranges from 23.4 °C to 32.9 °C, an approximate altitude of 92 m above sea level (Alfonso, 2009), and comprises a series of perpetual and seasonal wetlands along the San Juan River. We carried out vertebrate collections in two types of environments: around villages, hereafter referred to as the “urban environment”, and inside forest patches, hereafter referred to as the “sylvatic environment”. Hosts were captured by visual encounter search method, physically restrained, placed in cloth bags individually and identified morphologically at species level. We identified the specimens based on Rengifo and Lundberg (1999), Páez et al. (2002), and Castro-Herrera. and Vargas-Salinas (2008). We followed the taxonomy proposed by Uetz (2016: www.reptile-database.org) for reptiles. Ticks were removed with forceps from each individual host and stored in 1.5 ml vials with 96% ethanol until processing in the laboratory.
2.2. Morphological identification of ticks
The morphological identification of the adult ticks was done using the taxonomic keys of Voltzit (2007), Osorno-Mesa (1940) and Jones et al. (1972). For nymphs, the key developed by Martins et al. (2010) and for the larvae the key of Osorno-Mesa (1940) was used. Ticks were separated by stage and host.
2.3. Molecular characterization of ticks
Fourteen tick samples, including larvae analyzed individually and in pools of five, 4 nymphs (analyzed individually), and 6 adults, collected in three different host were molecularly confirmed (Supplementary Table 1). The DNA was extracted following the “ISOLATE II Genomic DNA” Kit (BIOLINE, USA) protocol. DNA extractions and its quality were confirmed by performing 2% agarose gel electrophoresis stained with “GelRed” (Biotium). CO1, 12S and 16S genes were amplified using the primers shown in Supplementary Table 2. Amplifications were carried out in a thermocycler (Eppendorf Mastercycler. Pro), in a final volume of 25 μl, including in each reaction 2 μl of DNA, 0.25 μl of Taq polymerase (5 u/μL BIOLASE ™, Bioline USA), 1 μl of MgCl (50 mM), 2.5 μl of buffer PCR (10X), 0.5 μl of dNTP (10 mM) and 0.5 μl of each primer (10 μM). The products were visualized by electrophoresis on 2% agarose gels with GelRed staining (Biotium). Each of the samples obtained in the amplification was purified using the SureClean purification kit (Bioline USA). The PCR products were sequenced in both directions.
2.4. Molecular detection of Rickettsiae
Amplifications of the genes gltA, ompA and sca1 were performed using a conventional PCR in an “Eppendorf Mastercycler Pro” thermal cycler, using the primers and specifications shown in Supplementary Table 2. The final reaction volume corresponded to 25 μL, which contained 1 μL of MgCl (50 mM), 2.5 μL of buffer (10X, PCR buffer), 1 μL of dNTPs (10 mM), 1 μL of each primer (10 pmol, forward and reverse), 15 μL of water, 0.5 μL of Taq polymerase (5 U/μL, BIOLASE TM, Bioline) and 3 μL of the extracted DNA. Positive amplification products were purified using the kit “E.Z.N.A Cycle Pure Kit” (Omega Bio-tek), and sequenced in both directions.
2.5. Analyses of tick and Rickettsia sequences
Tick sequences were verified using the BLAST tool (www.ncbi.nlm.nih.gov/Blast.cgi). Rickettsia sequences were also verified using BLAST (www.ncbi.nlm.nih.gov) and subsequently edited with the ProSeq V3 software (Filatov, 2009) and Geneious Prime 2022.0.2 (https://www.geneious.com). The ClustalW algorithm (Thompson et al., 1994), implemented in MEGA11 program (Tamura et al., 2021), was used for the alignment of the Rickettsia sequences obtained in the present study and others available in GenBank (Supplementary Table 3). A phylogenetic reconstruction by Bayesian inference and maximum likelihood was performed using the programs MrBayes 3.2.2 (Ronquist et al., 2012) and RAxML 8.0.24 (Stamatakis, 2006) respectively, using a concatenated data set of the three genes. The best partition schemes and the most suitable substitution models were chosen using the Partition Finder program (Lanfear et al., 2012), according to the Bayesian Information Criterion (Schwarz, 1978). The GTR + G model was used for all the positions in the three genes.
3. Results
3.1. Diversity of herpetofauna species and taxonomic identification of ticks
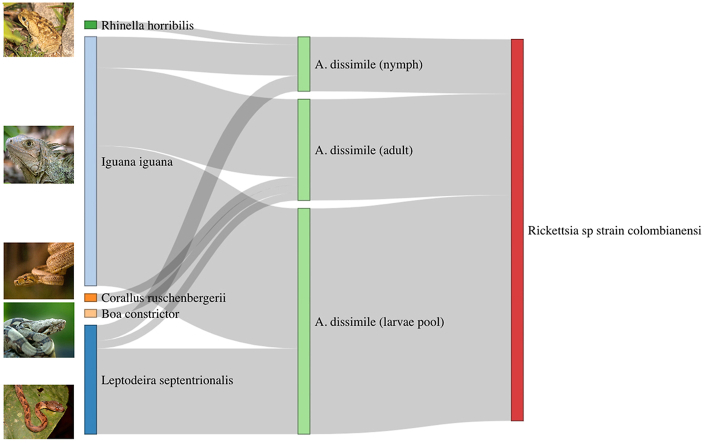
A total of 47 individuals from 11 species were recorded, of which 1 was amphibian and 10 reptiles. In total, reptiles belonged to 10 genera of the order Squamata (n = 45) and the amphibian was Rhinella horribilis (n = 2). Fourteen of the 47 individuals were captured in the urban environment, while 33 were captured in the sylvatic environment. Ten individuals were found infested with ticks, six from the urban and four from the sylvatic environment (Table 1). The prevalence of tick infestation in the herpetofauna population examined was 21.3%. A total of 247 ticks were collected in five hosts species. All the samples were taxonomically identified as Amblyomma dissimile and were categorized between larvae, nymph, and adult stages (Table 2).
Table 1.
Number of amphibians and reptiles captured and infested with ticks in San Juan de Carare, Colombia (6° 42 ′58 0.20″N/74° 08 ′02 0.71″W), between March 2017 and May 2018.
| Urban environment |
Sylvatic environment |
|||||
|---|---|---|---|---|---|---|
| Order | Species | Abundance | Number of hosts infested with ticks | Abundance | Number of hosts infested with ticks | Prevalence (%) |
| Squamata | Basiliscus basiliscus | 3 | – | 5 | – | 0 |
| Squamata | Boa constrictor | 1 | 1 | – | – | 100 |
| Squamata | Chironius spixii | – | – | 1 | – | 0 |
| Squamata | Corallus ruschenbergerii | 4 | – | 16 | 2 | 10 |
| Squamata | Iguana iguana | 5 | 4 | 4 | – | 44.4 |
| Squamata | Leptoderia septemptrionalis | – | – | 1 | 1 | 100 |
| Squamata | Leptophis ahaetulla | – | – | 2 | – | 0 |
| Squamata | Mastigodryas pleei | – | – | 1 | – | 0 |
| Squamata | Pseudoboa neuwiedii | – | – | 1 | 1 | 100 |
| Anura | Rhinella horribilis | 2 | 1 | – | – | 50 |
| Squamata | Tupinambis teguixin | – | – | 1 | – | 0 |
| TOTAL | 14 | 6 | 33 | 4 | 21.3 | |
Table 2.
Number of ticks collected per host species and number of ticks positive for Rickettsia.
| Host species | Total of ticks collected (larvae/nymphs/adults) | No. of larva or larvae pools Rickettsia positive/total tested for Rickettsia | No. of nymphs Rickettsia positive/total tested for Rickettsia | No. of adults Rickettsia positive/total tested for Rickettsia |
|---|---|---|---|---|
| Leptodeira septentrionalis | 21 (18/2/1) | 11/12 (92%) | 2/2 (100%) | 1/1 (100%) |
| Iguana iguana | 220 (179/8/33) | 18/31 (58%) | 4/6 (57%) | 10/14 (72%) |
| Corallus ruschenbergerii | 3 (0/1/2) | – | 0/1 (0%) | 1/2 (50%) |
| Boa constrictor | 1 (0/0/1) | – | – | 1/1 (100%) |
| Rhinella horribilis | 2 (0/2/0) | – | 1/2 (50%) | – |
| Total | 247 | 29/43 (67%) | 7/11 (64%) | 13/18 (72%) |
3.2. Molecular characterization of ticks and molecular detection of Rickettsiae
Verifications with the NCBI BLAST tool showed that all the analyzed tick sequences belong to the species A. dissimile with 99–100% identity. Our second match was with A. rotundatum with a much lower percentage of identity (92–94%). On the other hand, a total of 71 samples were tested for Rickettsia, of which 49 samples (29/43 larvae, 7/11 nymphs, and 13/18 adults) were positive, which is equivalent to 69% (Table 2). The largest number of individuals collected corresponded to larvae, which were processed individually or in pools of five specimens (just the ones collected on Iguana iguana), while all nymphs and adults were processed individually. From the positive samples, 15 gltA, 10 sca1 and 11 ompA sequences were generated (Supplementary Table 1 includes detailed information of the samples from which those sequences were obtained). The success of the PCRs with the gltA primers was high, however, the sequences were not long or had bad quality. All positive samples were corroborated with the ompA and sca1 primers. In the Blast analyses, most of the gltA sequences showed an identity percentage from 99,68–100% with Rickettsia tamurae (MN947696) and one of them (the longest one) of 100% with Candidatus Rickettsia colombianensi (MW384861); the ompA sequences showed 98,74–100% identity with Candidatus Rickettsia colombianensi (MN058024); and for sca1, most of the sequences showed a 100% of identity with uncultured Rickettsia sp. (MG020421 and MG020420), and one of them a 99,39% with Rickettsia monacensis (LN794217), mainly because there are not sca1 sequences named as Rickettsia colombianensi. Further analyzes using Bayesian inference and maximum likelihood grouped our samples into the same clade with Candidatus Rickettsia colombianensi, Rickettsia sp strain colombianensi, and Rickettsia endosymbiont Amblyomma dissimile (Supplementary Fig. 1).
Sequences generated in this study were uploaded to GenBank under accession numbers: ON138709-ON138719 (16S Amblyomma), ON138623-ON138635 (CO1 Amblyomma), ON149661-ON149672 (12S Amblyomma), ON159963 – ON159972 (Sca 1 Rickettsia), ON159973 – ON159987 (gltA Rickettsia), ON159988 – ON159998 (ompA Rickettsia).
4. Discussion
Reptiles may serve as a suitable host for various tick species and contribute to transmission of pathogenic organisms (Mendoza-Roldan et al., 2021a). However, despite the great diversity of amphibian and reptile species distributed in Colombia, tick-reptile associations are still poorly documented for most of the country. This is the first study related to the molecular detection of Rickettsia in ticks associated with herpetofauna in Santander, Colombia.
The ectoparasite found in the samples collected in this study corresponded exclusively to Amblyomma dissimile. This tick is commonly found as an ectoparasite of reptiles and some amphibians like toads. Its distribution goes from Florida to South America and the Caribbean, except Chile and Uruguay (Voltzit, 2007; Jones et al., 1972). In Colombia, other records correspond to the departments of Cesar, La Guajira, Magdalena and Córdoba (Miranda et al., 2012; Cotes-Perdomo et al., 2018; Santodomingo et al., 2018, 2019). Amblyomma dissimile was identified parasitizing Boa constrictor, Corallus ruschenbergerii, Iguana iguana, Leptodeira septentrionalis and Rhinella horribilis. These hosts, with the exception of L. septentrionalis, were also identified as hosts of A. dissimile in other studies (Álvarez-Calderón et al., 2005; Guglielmone and Nava, 2010; Santodomingo et al., 2018).
The species L. septentrionalis, locally known as Mapaná or false Mapaná, has a distribution that overlaps that of A. dissimile (Dávila and Buelvas, 2009; McKelvy et al., 2013). Also sharing a variety of habitats such as the dry forest, rainforest, riverside forest, savannah, and others (Medina-Rangel, 2011). However, its wide distribution led to this species having divergency processes, for which currently three clades of this biological group are recognized, two of these in Central America and one in South America (Daza et al., 2009), which makes it necessary to evaluate whether Central American clades would be parasitized by the same ticks. This snake, like the other species collected in the sylvatic environment during our surveys, was collected in the rainforest understory, within a forest patch of primary vegetation. It had high levels of tick infestation harboring all parasitic stages of A. dissimile (18 larvae, two nymphs, and one adult), with ticks scattered in its ventral area. These infestation levels could reflect the great host-tick adaptation, explaining why they don't appear to have any deleterious effects (Bower et al., 2019).
Of the samples infesting L. septentrionalis, 93% were positive for Rickettsia. In general, we found high percentages of Rickettsia positivity in ticks from hosts collected in both the sylvatic and the urban areas, but parasitic loads were lower in the sylvatic environments. Urban expansion can represent a wider range of host availability for ticks, which can be related to higher parasitic loads in urban areas (Calegaro-Marques and Amato, 2014). In our study, one of the urban reptiles that presented the highest tick infestations was Iguana iguana. Four out of the five iguanas collected in the urban environment had 213 ticks, while none of the iguanas collected in the sylvatic environment were found with ectoparasites. Additionally, 63% of the iguana ticks tested were positive to Rickettsia. Iguanas are one of the most trafficked reptiles in Colombia, mainly for their meat and eggs (Martinez and Gomez, 2013; Janssen, 2021). This is of concern as illegal animal trade and traffic has been associated with the dispersion of ticks and their associated pathogens (Erster et al., 2015; Mihalca, 2015). Moreover, as this species was captured in both environments, the connectivity between its populations remains a risk for the movement of pathogens between wild and urbanized environments (Corn et al., 2011).
Despite the sampling effort in each field trip, the number of individuals collected and infested with ticks was limited, largely because searching for, manually capturing, and handling these vertebrate species is physically challenging. However, this data remains relevant from the One Health perspective, as pathogenic organisms for humans such as Rickettsia monacensis and Rickettsia helvetica had been found in reptile tissue suggesting that these vertebrates could have a role as amplifiers of these bacteria (Mendoza-Roldan et al., 2021b). Ca. Rickettsia colombianensi is phylogenetically close to R. monacensis and Rickettsia tamurae, and it has been previously evidenced in several studies in the departments of Córdoba and Magdalena in the Colombian Caribbean region (Miranda et al., 2012; Cotes-Perdomo et al., 2018; Santodomingo et al., 2018), and now in the Santander department, which suggest this rickettsia is widely distributed and probably abundant in the country. The high prevalence of Ca. R. colombianensi in A. dissimile ticks indicates a well-established arthropod-bacteria relationship, which makes it necessary to evaluate if this bacterium could be harmful and/or confers and advantage for A. dissimile over other tick species.
Although in the Blast searchers only the ompA sequences match with Ca. R. colombianensi, mainly due to the lack of samples named as R. strain colombianensi for sca1 and the short sequences obtained for the gltA, the concatenated phylogenetic analyses grouped the Rickettsia sequences reported in this study in a clade with Ca. Rickettsia colombianensi sequences. As Quintero et al. (2017) points out, the detection of Ca. R. colombianensi in ticks that are potential ectoparasites to humans, as is the case for A. dissimile, is of concern, as the pathogenic potential of Ca. R. Colombianensi has not been discarded. Oteo et al. (2014), also proposes that any species of Rickettsia described in ticks, despite the lack of knowledge regarding its ecology, should be considered a potential pathogen for humans.
The wide host range of A. dissimile has been acknowledged in previous works, as well as ours, all the parasitic stages of this tick are mostly found on reptiles and amphibians, but as seen in our results, adult stages are most likely to appear on bigger hosts such as turtles, boas or iguanas, as well as mammals including humans (Guglielmone and Nava, 2010). The ecology of these generalist hematophagous arthropods is of special interest as they may serve as a bridge for the spread of pathogens from sylvatic to urban environments and vice versa (Rosenberg and Beard, 2011). In addition to Rickettsia, A. dissimile has also been reported infected with a wide variety of microorganisms belonging to well-known pathogenic groups such as Anaplasma, Borrelia, and Hepatozoon among others (Ogrzewalska et al., 2018). Although many of these strains remain of unknown pathogenicity, preventing their spread, through strict control measures on hosts traffic (such as iguanas), agricultural frontier expansion, and changes in land use, to wild, and native populations should be a priority.
5. Conclusion
The ticks collected in San Juan de Carare in the department of Santander correspond entirely to the Amblyomma dissimile species. In this study, A. dissimile is recorded for the first time as an ectoparasite of Leptodeira septentrionalis. Likewise, the amplification and sequencing of the gltA, ompA and sca1 genes allowed the identification of Candidatus Rickettsia colombianensi, a Rickettsia of unknown pathogenicity, but closely related to old world pathogenic species that should remain of concern.
Declaration of competing interest
We have no conflicts of interest to disclose.
Acknowledgments
This study was funded by the patrimonial fund for research (Fonciencias) of Universidad del Magdalena. Fieldwork was made possible through support provided by the Office of Infectious Disease, Bureau for Global Health, U.S. Agency for International Development, under the terms of an Interagency Agreement with CDC, and the Faculty of Sciences at Uniandes. We wish to thank all the team that provided support during fieldwork: C. Cárdenas, N. Gomez, M. De Meo, J. Panche, A. Montoya. Also, A.M. Eguis, A. Oviedo and M. Rodríguez for their help in the laboratory.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.08.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aguillón D., Lazcano D., Ramírez R., Aguirre A., Zárate J., Wong A. Bacterias cloacales y evaluación física de la herpetofauna del Parque Ecológico Chipinque. Ciencia UANL. 2007;10(2):168–174. [Google Scholar]
- Alfonso F. Pontificia Universidad Javeriana; Bogotá, Colombia: 2009. Densidad poblacional y ecología de Ateles hybridus (I. Geoffroyi-St. Hilaire, 1829) en un fragmento de bosque húmedo tropical en la Hacienda San Juan de Carare, Municipio de Cimitarra, Departamento de Santander, Colombia (tesis de pregrado)http://hdl.handle.net/10554/14590 [Google Scholar]
- Álvarez-Calderón, V., Hernández-Fonseca, V., Hérnandez-Gamboa., J., 2005. Cátalogo de garrapatas suaves (Acari: Argasidae) y duras (Acari: Ixodidae) de Costa Rica. Brenesia 63-64, 81-88.
- Benavides-Montaño J., Jaramillo-Cruz C., Mesa-Cobo N. Ticks (Acari Ixodidae) in Valle del Cauca, Colombia. Bol. Cient. Mus. His Nat. 2018;22(1):131–150. doi: 10.17151/bccm.2018.22.1.12. [DOI] [Google Scholar]
- Bernabeu M., Segura F. Enfermedades producidas por Rickettsia. Enferm Infecc Microbiol Clin. 2005;23(3):163–172. doi: 10.1157/13072167. [DOI] [PubMed] [Google Scholar]
- Bower D.S., Brannelly L.A., McDonald C.A., Webb R.J., Greenspan S.E., Vickers M., Gardner M.G., Greenlees M.J. A review of the role of parasites in the ecology of reptiles and amphibians. Austral Ecol. 2019;44(3):433–448. doi: 10.1111/aec.12695. [DOI] [Google Scholar]
- Calegaro-Marques C., Amato S.B. Urbanization breaks up host-parasite interactions: a case study on parasite community ecology of rufous-bellied thrushes (Turdus rufiventris) along a rural-urban gradient. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0103144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascal J., Oviedo T., Monsalve S., Torres A. Amblyomma dissimile (Acari: Ixodidae) parásito de Boa constrictor en Colombia. Rev MVZ Córdoba. 2009;14(2):1745–1749. [Google Scholar]
- Castro-Herrera F., Vargas-Salinas F. Anfibios y reptiles en el departamento del Valle del Cauca. Biota Colombiana. 2008;9:251–277. [Google Scholar]
- Corn J.L., Mertins J.W., Hanson B., Snow S. First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J. Med. Entomol. 2011;48(1):94–100. doi: 10.1603/me10065. [DOI] [PubMed] [Google Scholar]
- Cotes-Perdomo A., Santodomingo A., Castro L.R. Hemogregarine and Rickettsial infection in ticks of toads from northeastern Colombia. Int J Parasitol: Parasites Wildl. 2018;7(2):237–242. doi: 10.1016/j.ijppaw.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila J., Buelvas J. Reporte de algunas especies de ofidios presentes en el departamento de Sucre, Colombia. Revista Colombiana de Ciencia Animal-RECIA. 2009;1(2):273–278. [Google Scholar]
- Daza J., Smith E., Páez V., Parkinson C. Complex evolution in the Neotropics: the origin and diversification of the widespread genus Leptodeira (Serpentes: colubridae) Mol. Phylogenet. Evol. 2009;53(3):653–667. doi: 10.1016/j.ympev.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Erster O., Rotha A., Avnib Z., Kingc R., Shkap V. Molecular detection of Rickettsia bellii in Amblyomma rotundatum from imported red-footed tortoise (Chelonoides carbonaria) Ticks Tick-borne. Dis. 2015;6:473–477. doi: 10.1016/j.ttbdis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Filatov D.A. Processing and population genetic analysis of multigenic datasets with ProSeq3 software. Bio. informatics. 2009;25(23):3189–3190. doi: 10.1093/bioinformatics/btp572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmone A., Nava S. Hosts of Amblyomma dissimile koch, 1844 and Amblyomma rotundatum koch, 1844 (Acari: Ixodidae) Zootaxa. 2010;2541(1):27. doi: 10.11646/zootaxa.2541.1.2. [DOI] [Google Scholar]
- Guglielmone A.A., Nava S. Hosts of Amblyomma dissimile Koch, 1844 and Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae) Zootaxa. 2010;2541(1):27–49. doi: 10.11646/ZOOTAXA.2541.1.2. [DOI] [Google Scholar]
- Hidalgo M., Faccini Á., Valbuena G. Rickettsiosis transmitidas por garrapatas en las Américas: avances clínicos y epidemiológicos, y retos en el diagnóstico. Biomédica. 2013;33(1):161–178. doi: 10.7705/biomedica.v33i0.1466. [DOI] [PubMed] [Google Scholar]
- Jado I., Oteo J., Aldámiz M., Gil H., Escudero R., Ibarra V., Portu J., Portillo A., Lezaun M., García C., Rodríguez I., Anda P. Rickettsia monacensis and human disease, Spain. Emerg. Infect. Dis. 2007;13(9):1405–1407. doi: 10.3201/eid1309.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J. Wildlife Biodiversity Conservation. Springer; Cham: 2021. A primer to the global trade of reptiles: magnitude, key challenges, and implications for conservation; pp. 439–461. [DOI] [Google Scholar]
- Jones E., Clifford C., Keirans J., Kohls G. Ticks of Venezuela (Acarina: ixodoidea) with a key to the species of Amblyomma in the western hemisphere. Brigham. Young university. Science. Bulletin, Biological. Series. 1972;17(4):1–40. [Google Scholar]
- Lanfear R., Calcott B., Ho S., Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012;29(6):1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- McKelvy A., Figureoa A., Lewis T. First record of ophiophagy in the widely distributed snake Leptodeira septentrionalis (Kennicott, 1859) (Ophidia, Colubridae) Herpetol. Notes. 2013;6:177–178. [Google Scholar]
- Martinez D., Gomez J.R. The use of green iguanas in Fonseca, Colombia. TRAFFIC Bulletin. 2013;25(2):73–78. https://www.traffic.org/site/assets/files/3002/traffic_pub_bulletin_25_2_green_iguanas_fonseca_colombia.pdf [Google Scholar]
- Martins T., Onofrio V., Barros D., Labruna M. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks. Tick-Borne Dis. 2010;1(2):75–99. doi: 10.1016/j.ttbdis.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Medina-Rangel G.F. Diversidad alfa y beta de la comunidad de reptiles en el complejo cenagoso de Zapatosa, Colombia. Rev. Biol. Trop. 2011;59(2):935–968. [PubMed] [Google Scholar]
- Mendoza-Roldan J.A., Mendoza-Roldan M.A., Otranto D. Reptile vector-borne diseases of zoonotic concern. Int J Parasitol: Parasites Wildl. 2021;15:132–142. doi: 10.1016/j.ijppaw.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Roldan J.A., Ravindran Santhakumari Manoj R., Latrofa M.S., et al. Role of reptiles and associated arthropods in the epidemiology of rickettsioses: a one health paradigm. PLoS Negl. Trop. Dis. 2021;15(2) doi: 10.1371/journal.pntd.0009090. https://doi:10.1371/journal.pntd.0009090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalca A. Ticks imported to Europe with exotic reptiles. Vet. Parasitol. 2015;213:67–71. doi: 10.1016/j.vetpar.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Miranda J., Portillo A., Oteo J., Mattar S. Rickettsia sp. Strain Colombianensi (Rickettsiales: Rickettsiaceae): a New Proposed Rickettsia Detected in Amblyomma dissimile (Acari: Ixodidae) from Iguanas and Free-Living Larvae Ticks from Vegetation. J. Med. Entomol. 2012;49(4):960–965. doi: 10.1603/ME11195. [DOI] [PubMed] [Google Scholar]
- Monsalve S., Mattar S., Gonzalez M. Zoonosis transmitidas por animales silvestres y su impacto en las enfermedades emergentes y reemergentes. Rev. MVZ. Córdoba. 2009;14(2):1762–1773. [Google Scholar]
- Mora-Rivera C., Suarez-Páez F., Pacheco-Sierra G., Vargas-Cuevas L., Padilla-Barreto M. Tick infection of Caimancrocodilus fuscus at the Hidroprado hydroelectric dam in Colombia: new records, parasite prevalence, and blood loss rate. South. Am. J. Herpetol. 2020;16:42–49. doi: 10.2994/SAJH-D-18-00080.1. [DOI] [Google Scholar]
- Ogrzewalska M., Machado C., Rozental T., Forneas D., Cunha L.E., de Lemos E.R. Microorganisms in the ticks Amblyomma dissimile koch 1844 and Amblyomma rotundatum koch 1844 collected from snakes in Brazil. Med. Vet. Entomol. 2018;33(1):154–161. doi: 10.1111/mve.12341. [DOI] [PubMed] [Google Scholar]
- Osorno-Mesa E. Las garrapatas de la República de Colombia. Rev. Acad. Colomb. Cies. Exact. Fis. Nat. 1940;4(13):6–24. [Google Scholar]
- Osorno-Mesa E. Clásicos del INS. Biomédica. 2006;(4):178–193. http://www.scielo.org.co/scielo.php?pid=S0120-41572006000200002&script=sci_pdf [Google Scholar]
- Oteo J.A., Nava S., Sousa R., Mattar S., Venzal J., Abarca K., Labruna M., Zavala J. Guías Latinoamericanas de la RIICER para el diagnóstico de las rickettsiosis transmitidas por garrapatas. Rev. Chilena. Infectol. 2014;31(1):54–65. doi: 10.4067/S0716-10182014000100009. [DOI] [PubMed] [Google Scholar]
- Páez V.P., Bock B.C., Estrada J.J., Ortega A.M., Daza J.M., Gutierrez-C P.D. Guía de campo de algunas especies de anfibios y reptiles de Antioquia. Primera Edición. Colciencias, Universidad de Antioquia y Universidad Nacional. Medellin-Colombia. 2002;136 [Google Scholar]
- Parola P., Davoust B., Raoult D. Tick and flea-borne rickettsial emerging zoonoses. Vet. Res. 2005;36(3):469–492. doi: 10.1051/vetres:2005004. [DOI] [PubMed] [Google Scholar]
- Quintero J., Paternina L., Uribe A., Muskus C., Hidalgo M., Gil J., Cienfuegos A., Osorio L., Rojas C. Eco-epidemiological analysis of rickettsial seropositivity in rural areas of Colombia: a multilevel approach. PLOS Negl. Trop. Dis. 2017;11(9):1–19. doi: 10.1371/journal.pntd.0005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengifo J., Lundberg M. Skanska Conciviles; Colombia: 1999. Anfibios y reptiles de Urra; p. 96. [Google Scholar]
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M., Huelsenbeck J. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R., Beard C. Vector-borne infections. Emerg Infect Dis. 2011;17(5):769–770. doi: 10.3201/eid1705.110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santodomingo A., Cotes A., Foley J., Castro L.R. Rickettsial infection in ticks (Acari: Ixodidae) from reptiles in the Colombian Caribbean. Ticks. Tick-Borne. Dis. 2018;9(3):623–628. doi: 10.1016/j.ttbdis.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Santodomingo A., Sierra K., Cotes A., Castro L.R. Molecular detection of Rickettsia spp., Anaplasma platys and Theileria equi in ticks collected from horses in Tayrona National Park, Colombia. Exp. Appl. Acarol. 2019;77(3):411–423. doi: 10.1007/s10493-019-00354-8. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6(2):461–464. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA 11: molecular evolutionary genetics analysis. 11. Mol. Biol. Evol. 2021 doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Higgins D., Gibson T. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids. Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez J., Hidalgo M., González J. Rickettsiosis, una enfermedad letal emergente y re-emergente en Colombia. Univ. Sci. 2012;17(1):82–99. [Google Scholar]
- Voltzit O.V. A review of neotropical Amblyomma species (Acari: Ixodidae) Acarina. 2007;15(1):3–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.