Abstract
Purpose
Nitrous oxide (N2O) and methane (CH4) are some of the most important greenhouse gases in the atmosphere of the 21st century. Vegetated riparian buffers are primarily implemented for their water quality functions in agroecosystems. Their location in agricultural landscapes allows them to intercept and process pollutants from adjacent agricultural land. They recycle organic matter, which increases soil carbon (C), intercept nitrogen (N)-rich runoff from adjacent croplands, and are seasonally anoxic. Thus processes producing environmentally harmful gases including N2O and CH4 are promoted. Against this context, the study quantified atmospheric losses between a cropland and vegetated riparian buffers that serve it.
Methods
Environmental variables and simultaneous N2O and CH4 emissions were measured for a 6-month period in a replicated plot-scale facility comprising maize (Zea mays L.). A static chamber was used to measure gas emissions. The cropping was served by three vegetated riparian buffers, namely: (i) grass riparian buffer; (ii) willow riparian buffer and; (iii) woodland riparian buffer, which were compared with a no-buffer control.
Results
The no-buffer control generated the largest cumulative N2O emissions of 18.9 kg ha− 1 (95% confidence interval: 0.5–63.6) whilst the maize crop upslope generated the largest cumulative CH4 emissions (5.1 ± 0.88 kg ha− 1). Soil N2O and CH4-based global warming potential (GWP) were lower in the willow (1223.5 ± 362.0 and 134.7 ± 74.0 kg CO2-eq. ha− 1 year− 1, respectively) and woodland (1771.3 ± 800.5 and 3.4 ± 35.9 kg CO2-eq. ha− 1 year− 1, respectively) riparian buffers.
Conclusions
Our results suggest that in maize production and where no riparian buffer vegetation is introduced for water quality purposes (no buffer control), atmospheric CH4 and N2O concerns may result.
Keywords: Nitrous oxide, Methane, Maize, Vegetated riparian buffer strips
Introduction
Nitrous oxide (N2O) and methane (CH4) are important greenhouse gases that contribute more than 21% of radiative forcing in the greenhouse effect (IPCC 2014). Although N2O and CH4 are less abundant than carbon dioxide (CO2) in the atmosphere, their respective global warming potentials (GWP) over a 100-year period are respectively ~ 310 and ~ 28 times that of CO2 (IPCC 2014; Ramaswamy et al. 2001). Soils play a vital role in N2O and CH4 regulation (Conrad 2007; Firestone 1982; IPCC 2008). Soils of natural and semi-natural agroecosystems, including croplands, grasslands, and forests, are sources or sinks of N2O and CH4 and thus play a significant role in balancing their atmospheric concentrations (Dutaur and Verchot 2007; Smith et al. 2000; Stehfest and Bouwman 2006).
In soils, N2O and CH4 are produced or consumed as a result of microbial processes (Ball et al. 1999; Conrad 2007; Yao et al. 2017). N2O is predominantly produced as a by-product of two microbial processes; nitrification and denitrification (Bowden 1986; Davidson 2009). In the case of CH4, production occurs due to organic material decomposition by methanogens under anaerobic conditions in soils (Smith et al. 2018b; Yamulki and Jarvis 2002). Under such conditions and some aerobic conditions, atmospheric CH4 diffusing into the topsoil can be oxidized by methanotrophs, which subsequently results in CO2 (Jacinthe et al. 2015; Le Mer and Roger 2001).
Agronomic management practices associated with annual row crops may result in soil disturbances that affect soil microbial communities (Friedel et al. 1996), physical properties (Gronle et al. 2015), chemical properties (Neugschwandtner et al. 2014; Wang et al. 2008), temperature (Shen et al. 2018), and moisture content (Ouattara et al. 2006). The previous changes in agricultural land often result in substantial soil and nutrient runoff losses (Bechmann and Bøe 2021; Ulén 1997), including, where they are implemented, into riparian buffer strips. Riparian buffers are primarily implemented between freshwater bodies and upland agricultural land to intercept and process non-point source pollutants, including nitrates (NO3−) sourced from adjacent agricultural land (Groffman et al. 1998; Hill 1990). Their unique location in agrosystems allow riparian buffers to process non-point source pollutants through a range of processes including nitrogen (N) mineralization, N-uptake, leaching, gaseous N emissions (nitrification and denitrification) (Firestone 1982; Müller et al. 2004; Reinsch et al. 2018), CH4 oxidation, and methanogenesis (Le Mer and Roger 2001; Luo et al. 2013; Megonigal and Guenther 2008). These processes are responsible for N2O and CH4 production and/or uptake as well as subsequent exchanges between the soil and atmosphere. Vegetated riparian buffers recycle organic matter through their litter with further increases of soil organic carbon (C) (Tufekcioglu et al. 2001), and seasonal moisture from high soil water tables. When the elevated soil C and high soil moisture in riparian buffers come into contact with NO3−-rich sediments intercepted from agricultural lands, N2O-producing processes including denitrification are promoted (Choi et al. 2006; Garcia and Tiedje 1982). Both soil C and NO3− are energy sources for microbial process (Beauchamp et al. 1989). Anaerobic conditions and elevated soil C prevailing in riparian buffer areas can further increase processes that produce CH4 (Dlamini et al. 2022; Megonigal & Guenther 2008; Wang et al. 2017). Previous studies on N2O (Jacinthe et al. 2012) and CH4 (Mander et al. 2008) emissions from riparian buffers focused on buffer vegetation type and soil and environmental drivers of these gases. For instance, Jacinthe et al. (2012) observed larger N2O emissions in forested riparian buffers compared to grassed sites. Mander et al. (2008), however, found that forested riparian buffers were CH4 sinks.
Despite previous work, understanding N and C trace gas fluxes from adjacent cropped land compared to fluxes from riparian buffer strips remain limited. Therefore, this study evaluated the unintended emissions of N2O and CH4 from maize production from both buffered and un-buffered downslopes. The specific objectives of the study were (i) to understand the soil and environmental controls of soil N2O and CH4 in upslope maize and downslope riparian buffers with varying vegetation, and (ii) to understand whether specific riparian buffer vegetations emitted less N2O and CH4 when introduced for water quality purposes in maize production.
Materials and methods
Experimental site
The replicated plots used in this experiment are located at Rothamsted Research, North Wyke, Devon, United Kingdom (50°46 × 10´´N, 3° 54 × 05´´E). The area is situated at an altitude of 177 m above sea level, has a 37-year (from 1982 to 2018) mean annual precipitation (MAP) of 1033 mm (with the majority of rainfall received between October and November of each year) and mean annual temperature (MAT) of 10.1 °C (Orr et al. 2016). The experimental area has a slope of 8° and is on soils of the Hallsworth series (Clayden and Hollis 1985), or a dystric gleysol (FAO 2006), with a stony clay loam topsoil comprising 15.7% sand, 47.7% clay and 36.6% silt (Armstrong and Garwood 1991) overlying a mottled stony clay, derived from Carboniferous Culm rocks. The subsoil is impermeable to water and is seasonally waterlogged; most excess water moves by surface and sub-surface lateral flow across the clay layer (Orr et al. 2016), thereby making replicated experimental work using hydrologically-isolated plots feasible.
Experimental design and treatments
Experimental set-up
The experiment was laid out as three blocks of four plots corresponding to four treatments each described in detail in Section 2.2.2 and further detail in Dlamini et al. (2022). The cropped upslope area was previously managed as a silage crop, with a permanent pasture dominated by ryegrass (Lolium perenne L.), Yorkshire fog (Holcus lanatus L.) and creeping bentgrass (Agrostis stolonifera L.) planted in 2016, which was ripped and ploughed on the 14th of May 2019 in preparation to plant maize and the riparian buffer areas remained untouched. Maize (Zea mays L.) was planted on the 17th of May 2019 for the experiment. Slurry was applied before ploughing using a slurry spreader fitted with a centrifugal pump and an injector, which supplied N, phosphorus (P), and potassium (K) at respective rates of 20.8, 12, and 46 kg ha− 1. Inorganic fertilizer was applied using a fertilizer spreader to ensure equal coverage at planting as N (Nitram-Ammonium nitrate), P (triple superphosphate; P2O5), and K (muriate of potash; K2O) at respective rates of 100, 85, and 205 kg ha− 1 (Table 1). During fertilizer application and planting, static chambers were removed from the maize fields and positioned at exactly the same place using a hand-held geographical positioning system (GPS; Trimble, California, USA) after the agronomic practices. For example, chambers were removed a day before slurry spreading and re-installed in the afternoon after spreading. During mineral fertilizer application, chambers were removed in the morning before application and re-installed in the afternoon.
Table 1.
Application rates of cattle slurry and inorganic fertilizer during the cropping season
| Date | Application | N-input (kg ha− 1) | P-input (kg ha− 1) | K-input (kg ha− 1) |
|---|---|---|---|---|
| 14 May 2019 | Cattle slurry | 20.8 | 12 | 46 |
| 17 May 2019 | Inorganic fertilizer | 100† | 85¥ | 205‡ |
Nutrient sources: Nitrogen; †Nitram (Ammonium nitrate), Phosphorus; ¥ triple superphosphate (P2O5), Potassium‡ muriate of potash (K2O)
Treatments description
-
i)
No-Buffer control: A downslope area of the maize plots with no-buffer strip at the base of the hydrologically-isolated slope.
-
ii)
Grass Buffer: Novel grass buffer (Festulolium loliaceum cv. Prior). The grass was planted at the end of 2016 at a seeding rate of 5 kg ha− 1, the recommended seeding rate for the species in the Devon area.
-
iii)
Woodland Buffer: Deciduous woodland. Six species, namely Pedunculate oak (Quercus robur L.), hazel (Corylus avellana L.), Hornbeam (Carpinus betulus L.), Small-leaved lime (Tilia cordata Mill.), Sweet chestnut (Castanea sativa Mill.) and Wych elm (Ulmus glabra Huds.) were planted in the woodland buffer strips. Five individual plants (each 40 cm in height and bare rooted) of each species were planted 1.6 m apart in rows 2 m apart in December 2016 in the 10 × 10 m area, with 1.5 m tall protection tubes to remove risk of browsing by wild herbivores (e.g., deer). Planting was done at a density of 3000 plants ha− 1, the recommended planting density for the Devon area.
-
iv)
Willow Buffer: Bio-energy crop included five willow cultivars, namely Cheviot, Mourne, Hambleton, Endurance and Terra Nova (all Salix spp.) of which the first three were newly developed and the rest older cultivars. Whips of willow approximately 30 cm in length were inserted flush into the ground in May 2016 at a population of 200 plants per 10 m x 10 m area, the recommended planting density for willows in the Devon area.
At one month before planting of the different riparian buffer vegetation, each of the three buffer strip areas were sprayed with glyphosate herbicide to remove pre-existing grassland vegetation to enable better establishment of the planted deep rooting grass (Festulolium loliaceum cv. Prior), willow and woodland trees. The deep rooting grass buffer strips were also rotavated prior to seed broadcasting. Each of the buffer strips was composed of two parts – the lower slope area with a 2 m strip of natural grass, and the upslope area with a 10 m strip of treated and planted vegetation. The 2 m strip of natural grass is required for cross-compliance in England; farmers with watercourses must adhere to GAEC (Good Agricultural and Environmental Condition) rule 1, the establishment of buffer strips along watercourses (DEFRA 2019). The 10 m x 10 m area (10 m width) is the GAEC recommended N fertilizer application limit away from surface waters.
Field measurements and laboratory analyses
Greenhouse gas monitoring
Field sampling and analyses
Soil N2O and CH4 fluxes were measured using the static chamber (non-vented) technique (Chadwick et al. 2014; Charteris et al. 2020; De Klein and Harvey 2012). The opaque polyvinyl chloride chambers were square frames with lids (40 cm width x 40 cm length x 25 cm height) with an internal base area of 0.16 m2. Thirty-three chamber collars were inserted to a depth of 5 cm below the soil surface using a steel base, and installation points were marked using a hand-held GPS so that they could be moved into the same positions after periodic removal for agronomic activities (e.g., tillage). In the willow and woodland riparian buffers, maize cropped areas, and no-buffer control, chambers were installed in-between two crop rows. In the grass riparian buffers, chambers were installed in pre-determined positions (Dlamini et al. 2022). At the beginning of the experiment, a gas sampling plan was developed with biweekly samplings after fertilizer application and less frequently (i.e., once or twice a month) afterwards (Dlamini et al. 2022), making a total of 16 measurement events. Gas sampling was conducted periodically from May to October 2019, between 10:00 and 13:00, using 60 mL syringes and pre-evacuated 22 ml vials fitted with butyl rubber septa. All chambers except for linearity chambers were sampled terminally at 40 min after closure (Chadwick et al. 2014). At each occasion, samples were collected at four-time intervals (0, 20, 40, and 60 min) from three chambers (called linearity chambers) to account for the non-linear increase in gas concentration with deployment time (Grandy et al. 2006; Kaiser et al. 1996). The quality of a calculated flux was calculated flux was adequately assessed using the goodness of fit test and/or by visual inspection; plateauing of gas concentration over time, and data that failed to meet the linearity standards were rejected (Collier et al. 2014). Additionally, ten ambient gas samples were collected adjacent to the experimental area with five at the start and five at the end of each sampling event. A Perkin Elmer Clarus 500 gas chromatograph (Perkin Elmer Instruments, Beaconsfield, UK) fitted with a Turbomatrix 110 automated headspace sampler with an electron capture detector (ECD) set at 300oC was used for N2O analysis and a flame ionization detector (FID) was used for CH4 analysis, after applying a 5-standard linear regression calibration. Separation was achieved by Perkin Elmer Elite-PLOT megabore capillary column, 30 m long and 0.53 mm Column Inside Diameter (ID), maintained at 35oC; N2 was used as a carrier gas (Cardenas et al. 2016).
Gas flux determination and GWP calculations
As suggested by Conen and Smith (2000), soil N2O and CH4 fluxes were calculated with the rate of change in concentration (ppm) within the chamber, which was estimated as the slope of a linear regression between concentration and chamber closure time. Cumulative N2O and CH4 fluxes were estimated by calculating the area under the gas flux curve after linear interpolation between sampling points (Mosier et al. 1996). The GWP of CH4 and N2O are respectively 28 and 310 times that of CO2 (IPCC 2014). Therefore, GWP was estimated by multiplying total CH4 and N2O fluxes by 28, and 310, respectively (Del Grosso et al. 2008).
Soil analyses and meteorological variables
Soil pH was measured with a pH meter (Jenway, Stafforshire, UK) using a soil suspension (1:2.5 soil:water ratio), and soil organic matter (OM) was determined using the loss-on-ignition (LOI) technique (Wilke 2005). Composite soil samples (0–10 cm), made up of four random sub-samples, were collected monthly within 1 m of each chamber using a soil corer with a semi-cylindrical gouge auger (2–3 cm diameter) (Poulton et al. 2018). Total oxidized N (TO-N) [nitrite (NO2−) and nitrate (NO3−)] and ammonium N (NH4+) were quantified by extracting field-moist 20 g soil samples using 2 M KCl and a 1:5 soil: extractant ratio; analysis was performed using an Aquakem™ analyser (Thermo Fisher Scientific, Finland). At every gas-sampling occasion, composite soil samples (0–10 cm) made of four random sub-samples were collected within 1 m from each chamber using a soil corer for gravimetric soil moisture determination. Dry bulk density (BD) was determined at the start of the experiment next to each chamber using the core-cutter method (Amirinejad et al. 2011) and used to convert the gravimetric moisture determined during each of the gas sampling events into percent soil water-filled pore spaces (WFPS). Daily precipitation was obtained from a nearby weather station within the Environment Change Network (ECN) at Rowden, North Wyke (Lane 1997; Rennie et al. 2020).
Data processing and statistical analysis
Linear mixed models in Genstat 20 (VSN International, Hemel Hempstead, United Kingdom) were used to determine whether cumulative N2O, and CH4 differed with treatment. The random structure of each model (accounting for the experiment structure) was block/plot/chamber. The fixed structure (accounting for treatment effects) was treatment type/(treatment*distance). This model gave the following four tests in the output: (i) Treatment type – tested main maize cropped area vs. no-buffer control vs. riparian buffers, (ii) Treatment type. treatment – tested for differences between grass, willow, and woodland riparian buffers, (iii) Treatment type. buffer distance – tested for the difference between upper and lower riparian buffer areas, and (iv) Treatment type. treatment. buffer distance – tested for interaction between riparian buffer type and distance. A transformation was required to satisfy the equal variance assumption of the analysis of N2O. Due to the large negative values present for N2O, a modified square root transformation was used, SIGN (N2O)*√ (abs (N2O)). No transformation was required for the analysis of CH4.
Linear mixed models with the same random and fixed structures as those used for N2O, and CH4 were used to determine whether any measured soil variables (BD, pH, NH4+, TO-N, WFPS, and OM) differed with treatment. Pearson’s correlation coefficient (r) was used to evaluate the strength of relationships between soil and environmental factors and N2O/CH4 emissions. If linear mixed models indicated that treatment differences were present, least significant differences (LSD) were calculated to determine which specific treatment pairs resulted in the significant differences in N2O/CH4 emissions. All graphs were generated using Sigma Plot (Systat Software Inc., CA, USA).
Results
Meteorological and soil characteristics
Rainfall patterns
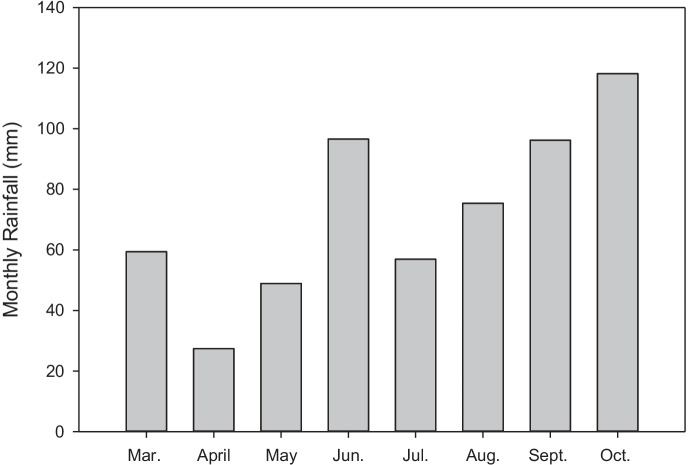
The total rainfall for the experimental period was 492.2 mm, and the highest rainfall event of 118.2 mm fell in October 2019. Before the highest rainfall in October, the second-highest rainfall events of 96.6 and 96.2 mm were recorded in June and September 2019, respectively (Fig. 1).
Fig. 1.
Total monthly rainfall during the experimental period
Soil variables
Soil pH ranged from 5.1 ± 0.17 and 5.5 ± 0.17, with the highest pH of 5.5 ± 0.17 from the willow riparian buffer, which was not significantly (LSD = 0.29) different from the grass or woodland riparian buffers. The largest soil BD of 1.2 ± 0.05 g cm− 3 was recorded in the no-buffer control, which was not significantly different from the upslope maize and the different vegetated riparian buffers (LSD = 0.19). Soil OM ranged from 9.0 (± 3.2) to 17.8 (± 2.3)%, with the largest %OM of 17.8 ± 2.3% recorded in the willow riparian buffer, which was not significantly (LSD = 8.6) different to the woodland riparian buffer (15.98 ± 2.3%). Soil OM in the vegetated riparian buffer strips was different from the upslope maize, but not from the no-buffer control, which was not different from the upslope maize (Tables 2 and 3).
Table 2.
Summary of soil parameters (mean ± standard error) in the upslope maize and downslope riparian buffers with different vegetation (upslope maize: n = 12, no-buffer control: n = 3 and each riparian buffer: n = 6) before the commencement of the experiments in May 2019
| Parameter | Upslope maize | No-buffer control | Grass buffer | Willow buffer | Woodland buffer | LSD |
|---|---|---|---|---|---|---|
| Soil pH | 5.1 ± 0.17 | 5.1 ± 0.19 | 5.4 ± 0.17 | 5.5 ± 0.17 | 5.4 ± 0.17 | 0.29 |
| Bulk density (g cm− 3) | 1.21 ± 0.03 | 1.21 ± 0.05 | 1.1 ± 0.04 | 1.2 ± 0.04 | 1.2 ± 0.04 | 0.19 |
| Organic matter (% w/w) | 9.9 ± 1.3 | 9.0 ± 3.2 | 12.2 ± 2.3 | 17.8 ± 2.3 | 16.0 ± 2.3 | 8.6 |
| NH4+-N (mg kg− 1 dry soil) | 27.4 ± 2.98 | 20.6 ± 4.6 | 6.4 ± 2.7 | 13.6 ± 2.7 | 9.1 ± 2.7 | 7.8 |
| TO-N† (mg kg− 1 dry soil) | 55.7 ± 1.7 | 42.8 ± 3.7 | 13.6 ± 3.0 | 4.99 ± 3.0 | 10.9 ± 3.0 | 10.0 |
| WFPS¥ (%) | 86.9 ± 5.3 | 81.7 ± 9.9 | 86.7 ± 7.2 | 102.9 ± 7.2 | 98.2 ± 7.2 | 18.6 |
†TO-N: total oxidized N; ¥WFPS: water-filled pore spaces
Table 3.
P-values from linear mixed model results for each of the measured soil variables
| Factors and interactions | OM | BD | NH4-N | pH | TO-N | WFPS |
|---|---|---|---|---|---|---|
| Area | 0.04 | 0.29 | < 0.001 | < 0.001 | < 0.001 | 0.23 |
| Area * Treatment crop | 0.31 | 0.13 | 0.16 | 0.238 | 0.173 | 0.24 |
| Area * Buffer area | 0.551 | 1 | 0.97 | 0.959 | 0.349 | 0.9 |
| Area * Treatment crop * Buffer area | 0.079 | 1 | 0.77 | 0.05 | 0.5 | 0.84 |
Soil mineral N-dynamics
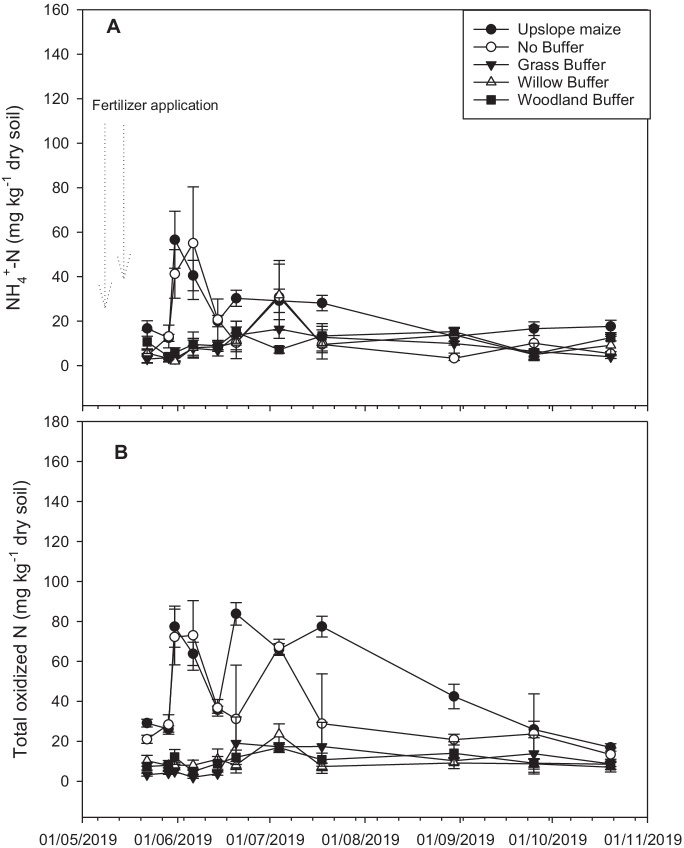
At the commencement of the experiment, NH4+-N was < 17 mg kg− 1 dry soil in all of the treatments, with the largest of 16.7 ± 3.5 mg kg− 1 dry soil observed in the upslope maize. However, after the second sampling event, which had been preceded by two fertilizer application events (Table 1), NH4+-N increased by almost 3-fold in the no-buffer control and upslope maize treatments, but remained relatively low in the vegetated riparian buffers. Despite the high NH4+-N values in the no-buffer control and upslope maize crop areas after fertilization, values dropped to < 30 mg kg− 1 dry soil after the fourth sampling event and remained low until the end of the experimental period (Fig. 2). The average NH4+-N for the experimental period ranged from 6.4 ± 2.78 to 27.4 ± 2.8 mg kg− 1 dry soil, with the largest value of 27.4 ± 2.8 mg kg− 1 dry soil obtained from the upslope maize crop areas, which was not significantly (LSD = 7.8) different to the no-buffer control. It was, however, significantly different (LSD = 7.8) to the vegetated riparian buffers. Soil NH4+-N also differed between areas, but there was no evidence of any other differences between treatments. The NH4+-N in the vegetated riparian buffer strips was different from the upslope maize and no-buffer control, and the upslope maize and no-buffer control were not different from each other (Tables 2 and 3).
Fig. 2.
Soil NH4+ and total oxidized N (TO-N) in the upslope maize and downslope riparian buffers during the experimental period
Total oxidized N was < 30 mg kg− 1 dry soil in all treatments at the commencement of the experiment (Fig. 2). However, after the second sampling event, TO-N increased 4-fold in the upslope maize and no-buffer control, but remained low in the riparian buffers. Despite a drop to ~ 35 mg kg− 1 dry soil in all of the upslope maize and no-buffer control areas during the fifth sampling event, the upslope maize emerged with the highest TO-N of ~ 81 mg kg− 1 dry soil during the sixth sampling event. However, these values dropped gradually up until the end of the experiment. Average TO-N for the experimental period ranged from 4.99 ± 3.0 to 55.7 ± 1.7 mg kg− 1 dry soil, with the highest value of 55.7 ± 1.7 mg kg− 1 dry soil obtained from the upslope maize. This was significantly different (LSD = 10.0) to all other treatments, except for the no-buffer control (Table 2).
%WFPS
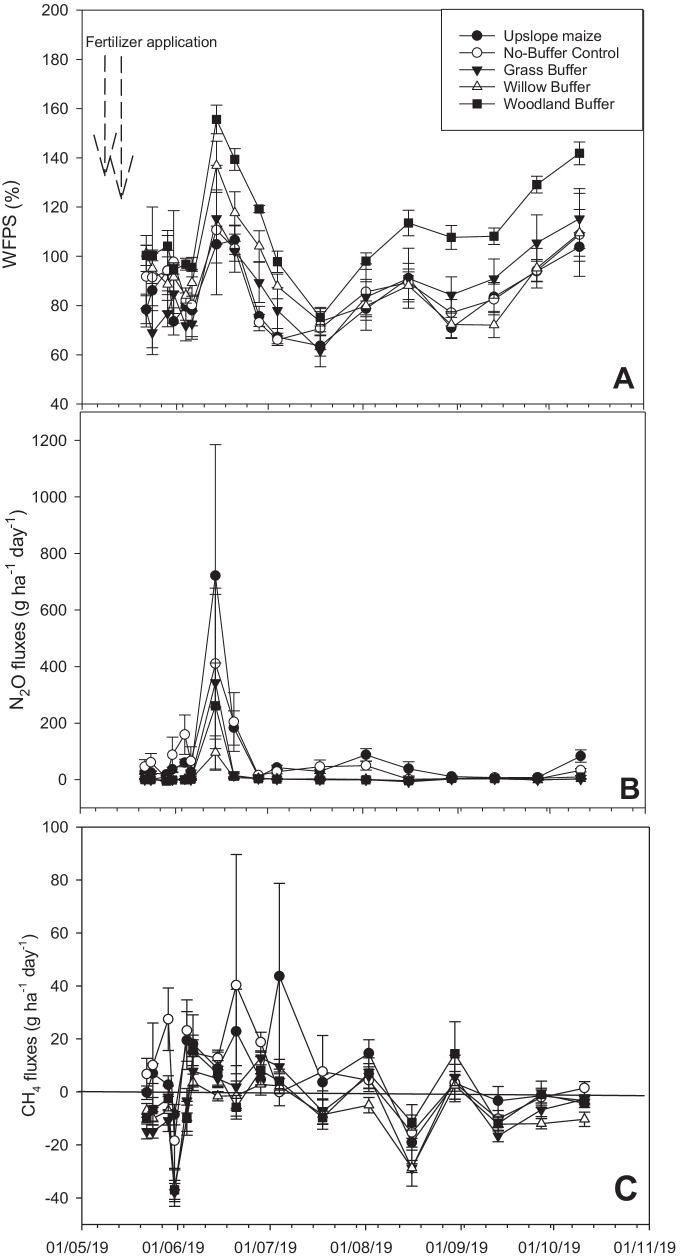
The highest %WFPS was observed during the fifth sampling event, with the overall highest estimate observed in the woodland riparian buffer treatment. The woodland riparian buffer maintained higher %WFPS values than the rest of the treatments during the experiment. The average %WFPS for the experimental period ranged from 81.7 (± 9.9) to 102.9 (± 7.2)%, with the highest value recorded in the willow riparian buffer, which was not significantly (LSD = 18.6) different to the woodland riparian buffer treatment, or any of the other treatments (Fig. 3A and Table 2).
Fig. 3.
Daily (A) soil water filled pore space (WFPS), (B) N2O, and (C) CH4 fluxes in the upslope maize and downslope riparian buffers. Data points and error bars represent the treatment means for cropland (n = 12), no-buffer control (n = 3), grass, woodland and willow buffer (n = 6 for each) and standard errors respectively, during each sampling day. The vertical line in CH4 marks 0 fluxes
Gas emissions
Gas fluxes
Nitrous oxide
Nitrous oxide fluxes measured during each sampling event ranged between − 2.76 ± 1.98 g N2O ha− 1 day− 1 (willow riparian buffer) and 721.1 ± 464.3 g N2O ha− 1 day− 1 (upslope maize) and are shown in Fig. 3(B). The commencement of the experiment was marked by relatively low fluxes in all treatments. The low fluxes were immediately followed by the highest peak in all treatments, observed instantly after fertilizer application, with the maximum mean flux of 721.1 ± 464.3 g N2O ha− 1 day− 1 observed in upslope maize. There was also a smaller peak of 204 ± 5.7 g N2O ha− 1 day− 1 in the upslope maize at around the 1st of August 2019. After that fluxes remained < 10 g N2O ha− 1 day− 1 in all the treatments, with the upslope maize and no-buffer control maintaining predominantly higher fluxes until the end of the experiment.
Methane
Daily CH4 fluxes, which were mostly positive and sometimes negative, ranged between − 37.95 ± 3.43 and 67.45 ± 49.37 g CH4 ha− 1 day− 1 and are illustrated in Fig. 3(C). Similar to N2O fluxes, the commencement of the experiment was marked by low CH4 fluxes, which increased up to ~ 40 g CH4 ha− 1 day− 1 (in the upslope maize and no-buffer control) immediately after fertilizer application. After these peaks, CH4 fluxes remained low and mostly negative in all the treatments until the end of the experiment.
Cumulative gas emissions
Nitrous oxide
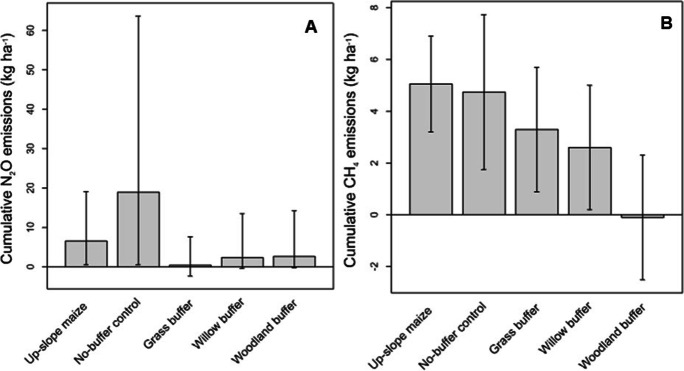
There was no evidence of significant treatment differences in N2O emissions between the upslope maize, no-buffer control and the three vegetated riparian buffers (p = 0.67) (Fig. 4A). Cumulative N2O emissions in descending order were no-buffer control 18.9 kg ha− 1 (95% CI: 0.5–63.6 ) > upslope maize; 6.5 kg ha− 1 (95% CI: 0.55–19.1 ) > woodland riparian buffer; 2.6 kg ha− 1 (95% CI: -0.27–14.2 ), willow riparian buffer; 2.3 kg ha− 1 (95% CI: -0.38–13.5) > grass buffer 0.38 kg ha− 1 (95% CI: -2.3–7.5).
Fig. 4.
Cumulative (A) N2O and (B) CH4 emissions for the experimental period from the upslope maize and different downslope buffer vegetation. Error bars represent 95% confidence intervals for cropland (n = 12), no-buffer control (n = 3), grass, woodland and willow buffer (n = 6 each). Vertical lines are 95% confidence intervals
Methane
The upslope maize and the no-buffer control (not significantly different from each other) emitted significantly higher cumulative soil CH4 fluxes than the three vegetated riparian buffers (p = 0.02) (Fig. 4B). Cumulative soil CH4 fluxes were in the descending order of upslope maize (5.1 ± 0.88 kg ha− 1) > no-buffer control (4.7 ± 1.4 kg ha− 1) > grass riparian buffer (3.3 ± 1.1 kg ha− 1) > willow riparian buffer (2.6 ± 1.1 kg ha− 1) > wood riparian buffer (-0.1 ± 1.1 kg ha− 1).
Global warming potential
Soil N2O-based GWP ranged from 1.2 ± 0.4 (willow riparian buffer) to 10.2 ± 4.7 (no buffer control) Mg CO2-eq. ha− 1 year− 1 (Table 6). A significantly higher GWP was found in the no-buffer control, which was not significantly different from the upslope maize. Soil CH4-based GWP ranged from 0.003 ± 0.36 (woodland riparian buffer) to 0.3 ± 0.03 (no buffer control) Mg CO2-eq. ha− 1 year− 1. Despite the large GWP found in the no buffer control, it was not significantly different to the other treatments, but to the woodland riparian buffer (Table 6).
Table 6.
Land-use (sample mean ± standard error) for upslope maize (n = 12), no-buffer control (n = 3) and each riparian buffer (n = 6) effects on global warming potential (GWP)
| Land-use | GWP (Mg CO2-C equivalent ha− 1 year− 1) | |
|---|---|---|
| N2O | CH4 | |
| Upslope maize | 6.2 ± 3.5 ab¥ | 0.3 ± 0.03 a |
| No-buffer control | 10.2 ± 4.7 a | 0.3 ± 0.04 a |
| Grass buffer | 2.5 ± 1.7 bc | 0.2 ± 0.07 a |
| Willow buffer | 1.2 ± 0.4 c | 0.1 ± 0.07 ab |
| Woodland buffer | 1.8 ± 800.5 bc | 0.003.4 ± 0.04 b |
¥Values within a column for each treatment followed by the same letter are not significantly different at the α = 0.05 probability level
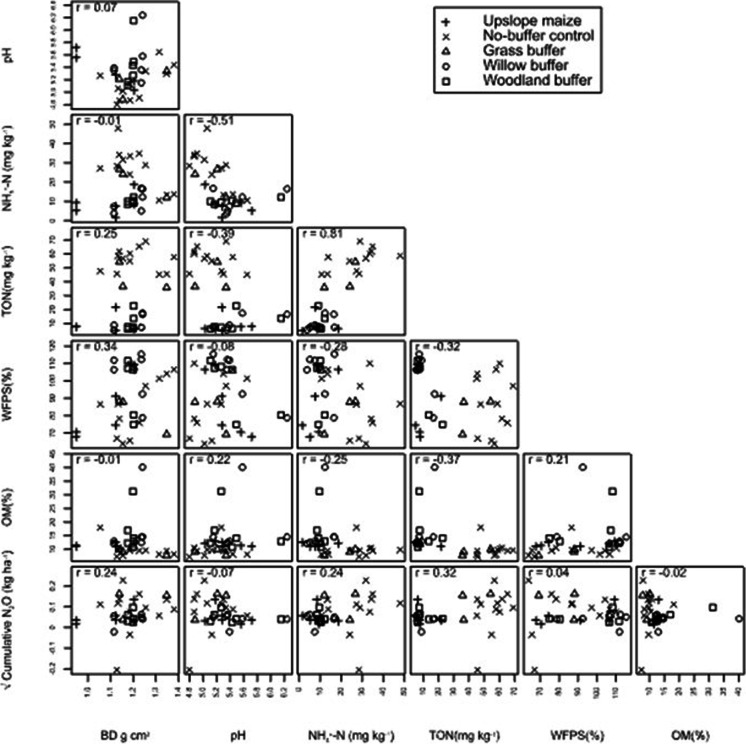
Relationships between gas emissions and soil variables
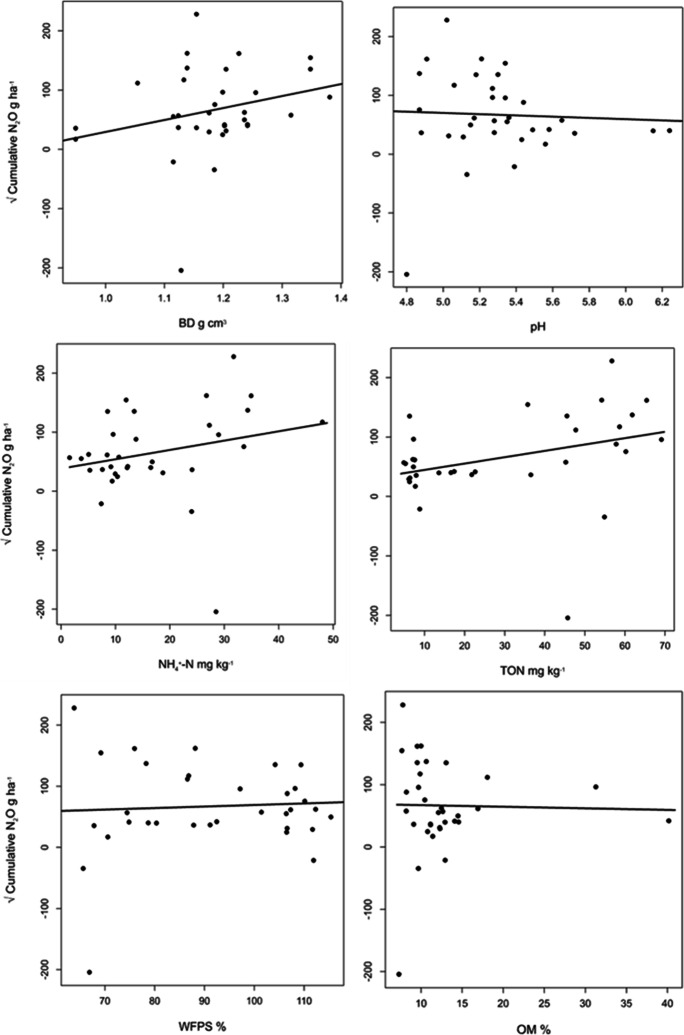
Table 4; Fig. 5 show that none of the soil variables had a significant relationship with cumulative N2O, but a slight relationship with TO-N (r = 0.32; p = 0.065). N2O emissions increased with an increase in soil BD, NH4+-N, TO-N, and %WFPS and decreased with an increase in pH and OM (Fig. 6).
Table 4.
P-values for the slope of the fitted line in the N2O and soil variables model
| Variable | Intercept | Standard error intercept | Slope | Standard error slope | P-value |
|---|---|---|---|---|---|
| BD | -172.6 | 142.1 | 201.9 | 119.98 | 0.126 |
| pH | 122.9 | 191.9 | -10.56 | 36.194 | 0.786 |
| NH4 | 38.29 | 23.48 | 1.58 | 1.1513 | 0.18 |
| TO-N | 33.97 | 18.18 | 1.068 | 0.555 | 0.065 |
| WFPS | 44.16 | 69.45 | 0.2518 | 0.75597 | 0.742 |
| OM | 69.7 | 29.76 | -0.2556 | 2.05029 | 0.902 |
Fig. 5.
Scatterplot showing the relationships between the variables pH, soil NH4+-N, soil total oxized N (TO-N), water filled pore space (WFPS%), organic matter (OM), bulk density (BD) and cumulative N2O emissions for the upslope maize and the downslope riparian buffers with different vegetation treatments. r = Pearson’s correlation coefficient
Fig. 6.
Relationships between cumulative N2O emissions and each of the soil variables
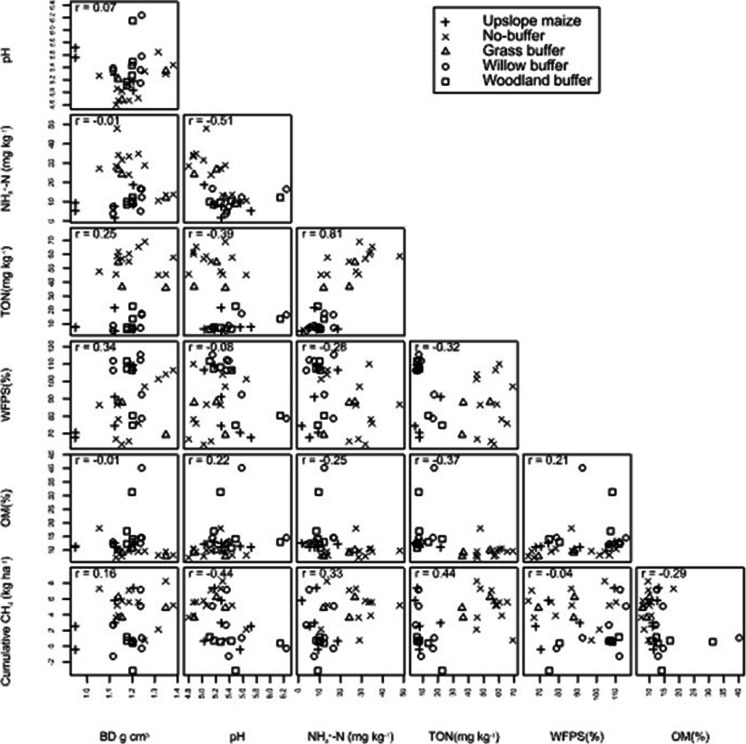
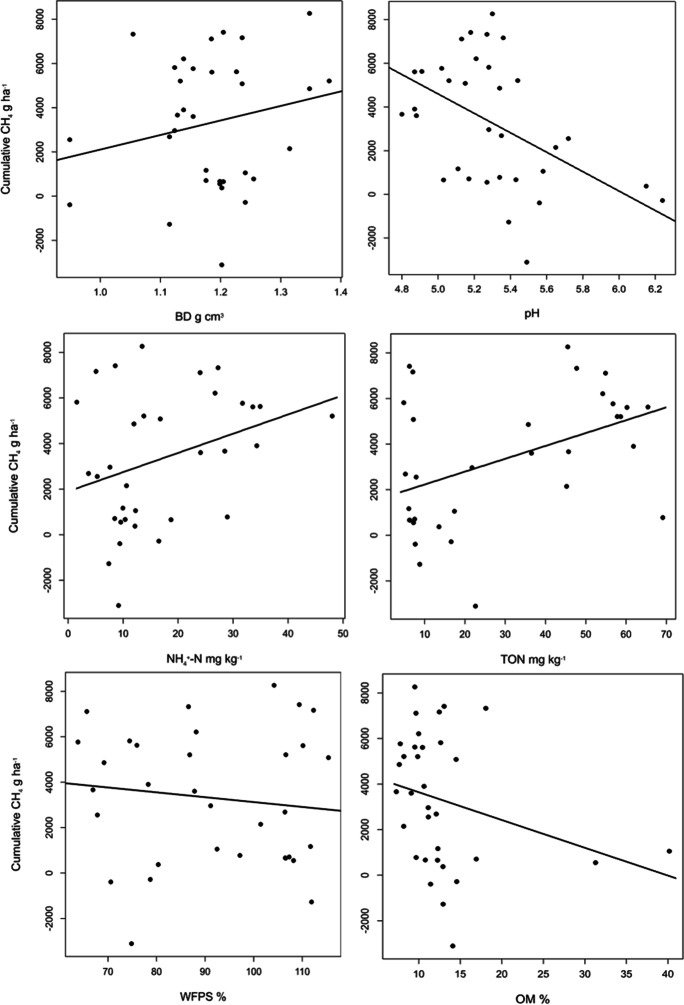
Table 5; Fig. 7 show that pH (r = -0.44; p = 0.042) (perfect linear relationship and negative association), TO-N (r = 0.44; p = 0.005) (perfect linear relationship and positive association), and NH4+-N (r = 0.33; p = 0.056) (perfect linear relationship and positive association) had significant relationships with cumulative CH4 emissions. Soil CH4 emissions increased with increased BD, NH4+-N, and TO-N and decreased with an increase in pH, %WFPS, and OM (Fig. 8, Table 6).
Table 5.
P-values for the slope of the fitted line in the CH4 and soil variables model
| Variable | Intercept | Standard error intercept | Slope | Standard error slope | P-value |
|---|---|---|---|---|---|
| BD | -4469 | 6524 | 6575 | 5467.2 | 0.24 |
| pH | 26,829 | 5813 | -4447 | 1094.7 | 0.042 |
| NH4+ | 1901 | 918.6 | 84.33 | 42.303 | 0.056 |
| TO-N | 1663 | 925.3 | 56.41 | 18.574 | 0.005 |
| WFPS | 5265 | 2916 | -21.41 | 30.548 | 0.489 |
| OM) | 4861 | 1197 | -122 | 74.55 | 0.113 |
Fig. 7.
Scatterplot showing the relationships between the variables pH, soil NH4+-N, soil total oxidized N (TO-N), water filled pore space (WFPS%), organic matter (OM), bulk density (BD) and cumulative CH4 emissions for the upslope maize and the downslope riparian buffers with different vegetation treatments. r = Pearson’s correlation coefficient
Fig. 8.
Relationships between cumulative CH4 emissions and each of the soil variables
Discussion
Gas emissions
Soil and environmental controls of gas fluxes
Nitrous oxide
The largest peak N2O flux observed in the upslope maize coincided largest %WFPS in the treatment. Large peaks also followed N fertilizer application events in the upslope maize and no buffer control (Fig. 3A and B). N2O fluxes following N fertilizer application are known to increase with increasing soil water content; most rapidly above 70% WFPS, wherein denitrification is a dominant process (Abbasi and Adams 2000; Dobbie et al. 1999; Granli and Bockman 1994; Skiba and Ball 2002). Soil moisture is one of the major drivers of N2O production, and directly affects production and consumption by influencing N-substrate availability, soil aeration, and metabolic activity of N2O-producing microorganisms, all of which control the capacity of soil to produce N2O (Di et al. 2014; Khalil and Baggs 2005; Simona et al. 2004). Nitrogen fertilizer has been reported as the main substrate for N2O-producing processes including nitrification and denitrification (Butterbach-Bahl et al. 2013; Dobbie et al. 1999). Thus, the higher fluxes were expected after N fertilizer application in the no-buffer control and the upslope maize (Table 1; Fig. 2) in the current study. Similarly, Halvorson et al. (2008) and Van Groenigen et al. (2004) reported that soil N2O emissions increased linearly with increasing N fertilizer. Additionally, there was an increase in N2O emissions with every increase in soil TO-N and NH4+-N (Fig. 6), which is in agreement with Mosier (1994), Mosier et al. (1996), and Barton and Schipper (2001). Notably, the woodland and willow riparian buffers had the highest %WFPS, but were characterised by lower N2O emissions during the peak flux. Not only the low N substrate due to unfertilized riparian buffers, but the reduced diffusion in the high soil moisture, caused a further reduction of N2O to N2 (Balaine et al. 2013; Hamonts et al. 2013). The no-buffer control and upslope maize had larger fluxes, which highlighted the interactive role of soil moisture and mineral N in enhancing N2O production (Klemedtsson et al. 1988).
The phenomenon of negative N2O fluxes is well documented to be dominant when high soil moisture (%WFPS) coincide with low mineral N (Chapuis-Lardy et al. 2007; La Montagne et al. 2003). Previous studies concur with the current study, for instance, in the first five events for the woodland and two events for grass riparian buffer, we observed negative N2O fluxes. The negative N2O fluxes coincided with high %WFPS and low mineral N (since riparian buffers were not directly fertilized) in the aforementioned treatments, which confirms the findings of other studies.
The larger N2O fluxes, coinciding with higher soil moisture (Fig. 3A and B) in all the treatments after the third sampling event, may have been due to higher N mineralisation potential. Higher mineralisation is known to increase under water saturated conditions and hampered by low soil moisture (Hackl et al. 2004). The larger N2O flux in the upslope maize and no-buffer control meant that the two treatments had higher N mineralisation potential compared to vegetated riparian buffers. It has been previously reported that differences in soil (Reich et al. 1997) and vegetation (Priha and Smolander 1999) characteristics significantly influence N mineralisation. Higher soil pH levels are also known to render conditions favourable for N mineralisation (Hackl et al. 2004). However, in our study, the vegetated riparian buffers with higher soil pH values had lower N2O fluxes compared to the no-buffer control and upslope maize, which had low pH values but maintained high N2O fluxes. This meant that other factors influenced N mineralisation more than high soil pH, but was not confirmed in the current study.
Methane
The overall positive CH4 emissions from all treatments was likely the result of the high %WFPS experienced during most of the experimental period. The upper values (~ 5 kg CH4 ha− 1) are similar to those reported by Groh et al. (2015). Field investigations have identified soil water content as one of the critical controls of CH4 production and consumption in soils from different ecosystems (Khalil and Baggs 2005; Kim et al. 2010; Wu et al. 2010). High soil moisture contents are documented drivers of CH4 production and emissions in soils; as a group of strictly anaerobic bacteria produce the majority of CH4 in reduced environments (Ehhalt et al. 2001; Ehhalt and Schmidt 1978; Yang and Chang 1998). Similar to other studies, our study recorded peak CH4 fluxes immediately after the highest %WFPS occurred (Fig. 3A and C). Soil moisture directly affects the capacity of soil to produce or consume CH4 through its influence on C-substrate availability, soil aeration, and metabolic activity of CH4 producing microorganisms (Khalil and Baggs 2005; Simona et al. 2004). The role of soil moisture in CH4 production and subsequent emissions was verified by the low (sometimes negative) CH4 fluxes, coinciding with low soil %WFPS at the end of August (Fig. 3A). Similarly, Luo et al., (2013) observed that soil moisture affected soil CH4 consumption through its effect on substrate availability and redistribution, soil aeration, and the metabolic activity of microorganisms. In October 2019, low soil CH4 fluxes were observed in all treatments despite the high %WFPS (Fig. 3A and C). We speculate that soil C for CH4-producing processes including mineralisation, may have been exhausted during this time, as was observed by Yu et al. (2013), but we did not verify this in the current study.
Gas emissions in upslope maize and downslope riparian buffer strips
Nitrous oxide
For a riparian buffer to be considered a threat to pollution swopping between air and water, it must emit more N2O than the cropland it serves (Fisher et al. 2014). In the current study, the no-buffer control proved to be an atmospheric concern, since it generated the highest N2O emissions compared to the upslope maize and the three vegetated riparian buffers (Fig. 4A). Despite the large N2O emissions in the no-buffer control, they were not significantly different to the vegetated riparian buffers and the upslope maize. The findings were similar to Baskerville et al. (2021) and De Carlo et al. (2019), who observed no significant differences in N2O emissions amongst the riparian zones. Baskerville et al., (2021) also reported that there were no significant differences when comparing these zones to the agricultural land. The maximum cumulative emissions of 20 kg N2O (~ 12 kg N ha− 1) were similar to Kim et al. (2009) (2-year study) and Groh et al. (2015) (1-year study), who observed 24 and 14.8 kg N2O ha− 1, respectively, in maize in a Humid Continental climate. We acknowledge that the differences in N2O emissions between the current study and the previous studies may have been due to different histories and N fertilization, but we did not have a way to confirm this in the current study. The high N2O emissions in the no-buffer control could have been due to applied fertilizer N (particularly readily available inorganic N), which increased mineral N availability for the N2O-producing nitrification and denitrification processes. Similar findings were reported by Dobbie et al. (1999) and (Butterbach-Bahl et al. 2013). In fact, the high N2O emissions in the no-buffer control showed a downward movement of the fertilizer applied N with rainwater. This was further attested to by the high mineral N (TO-N and NH4+) in the no-buffer control compared to the remainder of the treatments (Table 2) and an increase in N2O emissions with every increase in mineral N (Fig. 6). The vegetated riparian buffers had low N2O emissions; which indicated that they served their purpose of intercepting and processing N to N2 through denitrification induced by their high soil moisture (Groffman et al. 1991; Knowles 1982) before off-site delivery. Interestingly, the riparian buffers had ideal conditions to promote full denitrification (conditions highlighted by Dlamini et al. 2020), reducing NO3− to N2. Especially at the high moisture and in the case of willow and woodland, the high organic matter and potentially available C explained their low N2O compared to the upslope pasture and no buffer control. The low N2O emissions in the vegetated riparian buffers (Fig. 4A) could also have been because the riparian buffer strips were not directly fertilized. This highlighted the role of fertilizer N in increasing mineral N availability for N2O producing processes, as discussed by Davis et al. (2019), Hefting et al. (2003) and Iqbal et al. (2015). The second-highest N2O emissions observed in the upslope maize could have also been due to N fertilizer application.
The N2O emission factor is determined as the percentage of the fertilizer-induced N2O-N emission relative to N fertilization, where fertilizer-induced N2O-N emission is usually measured as the difference in N2O emission between fertilized and unfertilized soil under otherwise identical conditions (Wang et al. 2018). In our study, the N-fertilized upslope maize and the no-buffer control had emission factors of 3.25% and 13.6%, respectively. These were much higher than the default 1% proposed by the IPCC’s Tier-1 model for croplands, assuming a linear response to N fertilization (Eggleston et al. 2006). Despite the emission factors of the upslope maize and no-buffer control being much higher than the IPCC’s suggested 1%, the results of the current study were similar to those of Jungkunst et al. (2006), Kaiser et al. (1996), and Rudaz et al. (1999). These studies recorded N2O-emissions from agricultural soils as a result of applied fertilizer N to vary between 0.005% and 15.5%. Some authors, including Butterbach-Bahl et al. (2013), Rashti et al., (2015), Stehfest & Bouwman (2006), and Venterea et al. (2012) reported that N2O emissions are not only determined by external N fertilizer input, but also by responses controlled by key soil properties.
Methane
The fact that the upslope maize and no-buffer control treatments exhibited high CH4 emissions may have been a result of NH4+-N based fertilizer applied in the two treatments (Table 1; Figs. 2 and 4B). NH4+-N inhibits CH4 oxidation (Hütsch 1998; Kravchenko et al. 2002; Tlustos et al. 1998); which often results in a net increase in CH4 emitted from soil (Bronson and Mosier 1994). This inhibition is either a general salt effect (Gulledge and Schimel 1998) with a competition between ammonia (NH3) and CH4 for methane monooxygenase enzymes (Bédard and Knowles 1989), or non-competitive inhibition by hydroxylamine (NH2OH) or nitrite (NO2−) produced during NH3 oxidation (King and Schnell 1994). To further emphasize the role of mineral N in inhibiting CH4 oxidation, the three vegetated and unfertilized riparian buffers had significantly lower CH4 emissions than the upslope maize and the no-buffer control (Fig. 4B).
Global warming potentials
The high N2O and CH4-based GWP in the no buffer control showed that growing a maize crop without implementing riparian buffer vegetation may have increased the risk of GWP. On a positive note, implementing willow and woodland riparian buffers in tandem with a maize crop may reduce the risk of GWP while simultaneously contributing to their intended use to improve water quality.
Implications of the findings
Our findings have a number of implications especially in research and environmental policy. Although riparian buffer strips are conventionally implemented in intensive farming practices to enhance water quality in the UK and elsewhere, our work demonstrates additional benefits regarding their uptake of gaseous emissions. Many countries have focused on the urgent need to tackle the climate emergency and robust evidence on the efficacy of interventions for reducing harmful gaseous emissions is critical for engaging stakeholders including farmers.
The findings have implications for calibration of process-based models to simulate N2O and CH4 emissions from croplands and/ or riparian buffer areas, which has been challenging due to lack of data availability. Process-based models including the Riparian Ecosystem Management Model (REMM) (Lowrance et al. 2000) have been calibrated to simulate soil processes under riparian buffers. For example, REMM has been used to simulate groundwater movement, water table depths, surface runoff and annual hydrological budgets (Inamdar et al. 1999b). The model has also been used to simulate N, phosphorus (P), and C cycling (Dukes and Evans 2003; Inamdar et al. 1999a) interactions between riparian buffer systems. Other watershed models, such as the Soil and Water Assessment Tool (SWAT), have been calibrated to assess the effectiveness of riparian buffers for reducing total organic N-losses in a watershed (Lee et al. 2020). A landscape model, the Morgan-Morgan-Finney topographic wetness index (MMF-TWI), has been calibrated to simulate erosion reduction using riparian buffers (Smith et al. 2018a). However, to the best of our knowledge, none of these mechanistic models have been calibrated to simulate N2O and CH4 emissions from riparian buffers and further compared with emissions from croplands. Even though process-based models (e.g., Denitrification-Decomposition: DNDC) have been calibrated to simulate biogeochemical cycles including N2O emissions from grass riparian buffers in Illinois, USA (Gopalakrishnan et al. 2012), to the best of our knowledge, this model has not been calibrated to simulate greenhouse gas emissions from riparian buffers in the UK.
Limitations of the study
One of the significant limitations of the study was the use of a replicated plot-scale experimental facility. This meant that our results represented the climate, soil, and environmental conditions prevailing at the experimental site at North Wyke, Devon, UK. Similar conditions in terms of annual rainfall, soil and farming system, are present in 1843 km2 of farmed land across England (Collins et al. 2021). Our results provide robust data on short-term N and C gaseous emissions and clearly, longer-term measurements would help in confirming our findings. Although the static chamber is cheap and easy to use, a possible shortcoming is that it was used to trap gas in the field for the experiment. For instance, Healy et al. (1996) and Rochette (2011) reported that insertion of chambers into the soil may limit lateral gas exchange. However, Rochette (2011) suggested that such limitations may be overcome by inserting chamber collars prior to use. Rochette (2011) also argued that this practice may affect soil temperature by shading the soil, soil moisture by preventing soil run-off, and gas exchange through formation of shrinkage cracks at the collar-soil interface.
Conclusions
Our replicated plot-scale facility experiment showed that the N-fertilized no-buffer control and upslope areas used for maize cropping might be significant N2O and CH4 sources, respectively. Furthermore, the low N2O and CH4-based GWP from the willow and woodland riparian buffers show that willow may mitigate GWP when implemented for water quality protection purposes in maize production. Accordingly, our results attest to the unintended benefits of riparian buffers for reducing gaseous emissions, despite primarily being implemented as water quality protection measures.
Funding
The Department of Higher Education and Training (New Generation Gap of Academics Program) and National Research Foundation-Thuthuka (Grant Number: 117964), both under the South African government, are acknowledged for financial support. The work was also facilitated by the UKRI (UK Research and Innovation) Biotechnology and Biological Sciences Research Council (BBSRC) via grant (awarded to ALC) BB/N004248/1 - “Impacts of different vegetation in riparian buffer strips on hydrology and water quality”. The British Council is acknowledged for a Researcher Links Travel Grant (2017-RLTG9-1069) that initiated the collaboration between J. Dlamini and Rothamsted Research. Rothamsted Research is supported by strategic funding from UKRI-BBSRC via its Institute Strategic Programmes, including BBS/E/C/000I0320 and BBS/E/C/000I0330.
Data availability
Data available from authors on request.
Code availability
Not Applicable.
Declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest/Competing interests
Authors declared no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi M, Adams W. Estimation of simultaneous nitrification and denitrification in grassland soil associated with urea-N using 15 N and nitrification inhibitor. Biol Fertil Soils. 2000;31:38–44. doi: 10.1007/s003740050621. [DOI] [Google Scholar]
- Amirinejad AA, Kamble K, Aggarwal P, Chakraborty D, Pradhan S, Mittal RB. Assessment and mapping of spatial variation of soil physical health in a farm. Geoderma. 2011;160:292–303. doi: 10.1016/j.geoderma.2010.09.021. [DOI] [Google Scholar]
- Armstrong AC, Garwood E. Hydrological consequences of artificial drainage of grassland. Hydrol Process. 1991;5:157–174. doi: 10.1002/hyp.3360050204. [DOI] [Google Scholar]
- Balaine N, Clough TJ, Beare MH, Thomas SM, Meenken ED, Ross JG. Changes in relative gas diffusivity explain soil nitrous oxide flux dynamics. Soil Sci Soc Am J. 2013;77:1496–1505. doi: 10.2136/sssaj2013.04.0141. [DOI] [Google Scholar]
- Ball BC, Scott A, Parker JP. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 1999;53:29–39. doi: 10.1016/S0167-1987(99)00074-4. [DOI] [Google Scholar]
- Barton L, Schipper L. Regulation of nitrous oxide emissions from soils irrigated with dairy farm effluent. J Environ Qual. 2001;30:1881–1887. doi: 10.2134/jeq2001.1881. [DOI] [PubMed] [Google Scholar]
- Baskerville M, Reddy N, Ofosu E, Thevathasan NV, Oelbermann M. Vegetation type does not affect nitrous oxide emissions from riparian zones in agricultural landscapes. Environ Manag. 2021;67:371–383. doi: 10.1007/s00267-020-01419-w. [DOI] [PubMed] [Google Scholar]
- Beauchamp EG, Trevors JT, Paul JW. Carbon sources for bacterial denitrification. In: Stewart BA, editor. Advances in Soil Sciences. New York: Springer; 1989. pp. 113–142. [Google Scholar]
- Bechmann ME, Bøe F. Soil tillage and crop growth effects on surface and subsurface runoff, loss of soil, phosphorus and nitrogen in a cold climate. Land. 2021;10:77. doi: 10.3390/land10010077. [DOI] [Google Scholar]
- Bédard C, Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989;53:68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden WB. Gaseous nitrogen emmissions from undisturbed terrestrial ecosystems: An assessment of their impacts on local and global nitrogen budgets. Biogeochemistry. 1986;2:249–279. doi: 10.1007/BF02180161. [DOI] [Google Scholar]
- Bronson K, Mosier A. Suppression of methane oxidation in aerobic soil by nitrogen fertilizers, nitrification inhibitors, and urease inhibitors. Biol Fertil Soils. 1994;17:263–268. doi: 10.1007/BF00383979. [DOI] [Google Scholar]
- Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci. 2013;368:20130122. doi: 10.1098/rstb.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas LM, Misselbrook TM, Hodgson C, Donovan N, Gilhespy S, Smith KA, Dhanoa MS, Chadwick D. Effect of the application of cattle urine with and without the nitrification inhibitor DCD, and dung on greenhouse gas emissions from a UK grassland soil. Agric Ecosyst Environ. 2016;235:229–241. doi: 10.1016/j.agee.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick D, Cardenas L, Misselbrook T, Smith K, Rees R, Watson C, McGeough K, Williams J, Cloy J, Thorman R. Optimizing chamber methods for measuring nitrous oxide emissions from plot-based agricultural experiments. Eur J Soil Sci. 2014;65:295–307. doi: 10.1111/ejss.12117. [DOI] [Google Scholar]
- Chapuis-Lardy L, Wrage N, Metay A, Chotte JL, Bernoux M. Soils, a sink for N2O? A review. Glob Change Biol. 2007;13:1–17. doi: 10.1111/j.1365-2486.2006.01280.x. [DOI] [Google Scholar]
- Charteris AF, Chadwick DR, Thorman RE, Vallejo A, de Klein CAM, Rochette P, Cardenas LM. Global Research Alliance N2O chamber methodology guidelines; Recommendations for deployment and accounting for sources of variability. J Environ Qual. 2020;49:1092–1109. doi: 10.1002/jeq2.20126. [DOI] [PubMed] [Google Scholar]
- Choi WJ, Lee SM, Han GH, Yoon KS, Jung JW, Lim SS, Kwak JH. Available organic carbon controls nitrification and immobilization of ammonium in an acid loam-textured soil. Appl Biol Chem. 2006;49:28–32. [Google Scholar]
- Clayden B, Hollis JM (1985) Criteria for differentiating soil series. Tech Monograph 17, Harpenden
- Collier SM, Ruark MD, Oates LG, Jokela WE, Dell CJ. Measurement of greenhouse gas flux from agricultural soils using static chambers. J Visual Experiments. 2014;90:52110. doi: 10.3791/52110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Zhang Y, Upadhayay H, Pulley S, Granger S, Harris P, Sint H, Griffith B. Current advisory interventions for grazing ruminant farming cannot close exceedance of modern background sediment loss–Assessment using an instrumented farm platform and modelled scaling out. Environ Sci Policy. 2021;116:114–127. doi: 10.1016/j.envsci.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen F, Smith K. An explanation of linear increases in gas concentration under closed chambers used to measure gas exchange between soil and the atmosphere. Eur J Soil Sci. 2000;51:111–117. doi: 10.1046/j.1365-2389.2000.00292.x. [DOI] [Google Scholar]
- Conrad R. Microbial ecology of methanogens and methanotrophs. Adv Agron. 2007;96:1–63. doi: 10.1016/S0065-2113(07)96005-8. [DOI] [Google Scholar]
- Davidson EA. Microbial processes of production and consumption of nitric oxide, nitrous oxide and methane. In: Matson PA, HArris RC, editors. Biogenic Trace Gases: Measuring Emisions from Soil and Water. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- Davis MP, Groh TA, Jaynes DB, Parkin TB, Isenhart TM. Nitrous oxide emissions from saturated riparian buffers: Are we trading a water quality problem for an air quality problem? J Environ Qual. 2019;48:261–269. doi: 10.2134/jeq2018.03.0127. [DOI] [PubMed] [Google Scholar]
- De Carlo ND, Oelbermann M, Gordon AM. Spatial and temporal variation in soil nitrous oxide emissions from rehabilitated and undisturbed riparian forest. J Environ Qual. 2019;48:624–633. doi: 10.2134/jeq2018.10.0357. [DOI] [PubMed] [Google Scholar]
- DEFRA (2019) The guide to cross compliance in England 2019. In: F. a. R. A. Department for Environment (Ed.), United Kingdom
- De Klein C, Harvey M. Global Research Alliance on Agricultural Greenhouse Gases. Wellington: Ministry for Primary Industries; 2012. Nitrous oxide chamber methodology guidelines. [Google Scholar]
- Del Grosso S, Walsh M, Duffield J. US agriculture and forestry greenhouse gas inventory: 1990–2005. Washington, DC, USA: USDA Technical Bulletin; 2008. [Google Scholar]
- Di HJ, Cameron KC, Podolyan A, Robinson A. Effect of soil moisture status and a nitrification inhibitor, dicyandiamide, on ammonia oxidizer and denitrifier growth and nitrous oxide emissions in a grassland soil. Soil Biol Biochem. 2014;73:59–68. doi: 10.1016/j.soilbio.2014.02.011. [DOI] [Google Scholar]
- Dlamini JC, Cardenas L, Tesfamariam EH, Dunn R, Hawkins J, Blackwell M, Evans J, Collins A (2022) Soil methane (CH4) fluxes in cropland with permanent pasture and riparian buffers with different vegetation. J Plant Nutr Soil Sci 185:132–144. 10.1002/jpln.202000473
- Dlamini JC, Chadwick D, Hawkins JMB, Martinez J, Scholefield D, Ma Y, Cárdenas LM. Evaluating the potential of different carbon sources to promote denitrification. J. Agric. Sci. 2020;158(3):194–205. doi: 10.1017/S0021859620000520. [DOI] [Google Scholar]
- Dobbie K, McTaggart IP, Smith K. Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables, and mean emission factors. J Geophys Res Atmos. 1999;104:26891–26899. doi: 10.1029/1999JD900378. [DOI] [Google Scholar]
- Dukes M, Evans R. Riparian ecosystem management model: Hydrology performance and sensitivity in the North Carolina middle coastal plain. Trans ASABE. 2003;46:1567. doi: 10.13031/2013.15645. [DOI] [Google Scholar]
- Dutaur L, Verchot LV (2007) A global inventory of the soil CH4 sink. Global Biogeochem Cycles 21. 10.1029/2006GB002734
- Ehhalt D, Schmidt U. Sources and sinks of atmospheric methane. Pure Appl Geophys. 1978;116:452–464. doi: 10.1007/BF01636899. [DOI] [Google Scholar]
- Eggleston S, Buendia L, Miwa K, Ngara T, Tanabe K (eds) (2006) 2006 IPCC guidelines for national greenhouse gas inventories. Institute for Global Environmental Strategies, Hayama
- Ehhalt D, Prather M, Dentener F, Derwent R, Dlugokencky EJ, Holland E, Isaksen I, Katima J, Kirchhoff V, Matson P (2001) Atmospheric chemistry and greenhouse gases. Pacific Northwest National Lab. (PNNL), Richland
- FAO . Guidelines for soil description Rome. Italy: Food and Agricultural Organisation of the United Nations; 2006. [Google Scholar]
- Firestone M, American Society of Agronomy, Inc . Biological denitrification. In: Stevenson F, editor. Nitrogen in agricultural soils. Madison: Crop Science Society of America, Inc. Soil Science Society of America Inc.; 1982. pp. 289–326. [Google Scholar]
- Fisher K, Jacinthe P, Vidon P, Liu X, Baker M. Nitrous oxide emission from cropland and adjacent riparian buffers in contrasting hydrogeomorphic settings. J Environ Qual. 2014;43:338–348. doi: 10.2134/jeq2013.06.0223. [DOI] [PubMed] [Google Scholar]
- Friedel J, Munch J, Fischer W. Soil microbial properties and the assessment of available soil organic matter in a haplic luvisol after several years of different cultivation and crop rotation. Soil Biol Biochem. 1996;28:479–488. doi: 10.1016/0038-0717(95)00188-3. [DOI] [Google Scholar]
- Garcia JL, Tiedje J. Denitrification in rice soils. In: Diem H, editor. Microbiology of Tropical Soils and Plant Productivity. London: Springer; 1982. pp. 187–208. [Google Scholar]
- Gopalakrishnan G, Cristina Negri M, Salas W. Modeling biogeochemical impacts of bioenergy buffers with perennial grasses for a row-crop field in Illinois. GCB Bioenergy. 2012;4:739–750. doi: 10.1111/j.1757-1707.2011.01145.x. [DOI] [Google Scholar]
- Grandy AS, Loecke TD, Parr S, Robertson GP. Long-term trends in nitrous oxide emissions, soil nitrogen, and crop yields of till and no-till cropping systems. J Environ Qual. 2006;35:1487–1495. doi: 10.2134/jeq2005.0166. [DOI] [PubMed] [Google Scholar]
- Granli T, Bockman OC. Nitrous oxide from agriculture. Nor J Agric Sci. 1994;12:94–128. [Google Scholar]
- Groffman PM, Axelrod EA, Lemunyon JL, Sullivan WM. Denitrification in grass and forest vegetated filter strips. J Environ Qual. 1991;20:671–674. doi: 10.2134/jeq1991.00472425002000030027x. [DOI] [Google Scholar]
- Groffman PM, Gold AJ, Jacinthe P-A. Nitrous oxide production in riparian zones and groundwater. Nutr Cycl Agroecosyst. 1998;52:179–186. doi: 10.1023/A:1009719923861. [DOI] [Google Scholar]
- Groh TA, Gentry LE, David MB. Nitrogen removal and greenhouse gas emissions from constructed wetlands receiving tile drainage water. J Environ Qual. 2015;44:1001–1010. doi: 10.2134/jeq2014.10.0415. [DOI] [PubMed] [Google Scholar]
- Gronle A, Lux G, Böhm H, Schmidtke K, Wild M, Demmel M, Brandhuber R, Wilbois K-P, Heß J. Effect of ploughing depth and mechanical soil loading on soil physical properties, weed infestation, yield performance and grain quality in sole and intercrops of pea and oat in organic farming. Soil Tillage Res. 2015;148:59–73. doi: 10.1016/j.still.2014.12.004. [DOI] [Google Scholar]
- Gulledge J, Schimel JP. Moisture control over atmospheric CH4 consumption and CO2 production in diverse Alaskan soils. Soil Biol Biochem. 1998;30:1127–1132. doi: 10.1016/S0038-0717(97)00209-5. [DOI] [Google Scholar]
- Hackl E, Bachmann G, Zechmeister-Boltenstern S. Microbial nitrogen turnover in soils under different natural forest. For Ecol Manag. 2004;188:101–112. doi: 10.1016/j.foreco.2003.07.014. [DOI] [Google Scholar]
- Halvorson AD, Del Grosso SJ, Reule CA. Nitrogen, tillage, and crop rotation effects on nitrous oxide emissions from irrigated cropping systems. J Environ Qual. 2008;37:1337–1344. doi: 10.2134/jeq2007.0268. [DOI] [PubMed] [Google Scholar]
- Hamonts K, Balaine N, Moltchanova E, Beare M, Thomas S, Wakelin SA, O’Callaghan M, Condron LM, Clough TJ. Influence of soil bulk density and matric potential on microbial dynamics, inorganic N transformations, N2O and N2 fluxes following urea deposition. Soil Biol Biochem. 2013;65:1–11. doi: 10.1016/j.soilbio.2013.05.006. [DOI] [Google Scholar]
- Healy RW, Striegl RG, Russell TF, Hutchinson GL, Livingston GP. Numerical evaluation of static-chamber measurements of soil—Atmosphere gas exchange: identification of physical processes. Soil Sci Soc Am J. 1996;60:740–747. doi: 10.2136/sssaj1996.03615995006000030009x. [DOI] [Google Scholar]
- Hefting MM, Bobbink R, de Caluwe H. Nitrous oxide emission and denitrification in chronically nitrate-loaded riparian buffer zones. J Environ Qual. 2003;32:1194–1203. doi: 10.2134/jeq2003.1194. [DOI] [PubMed] [Google Scholar]
- Hill AR. Ground-water flow paths in relation to nitrogen chemistry in the near-stream zone. Hydrobiologia. 1990;206:39–52. doi: 10.1007/BF00018968. [DOI] [Google Scholar]
- Hütsch B. Methane oxidation in arable soil as inhibited by ammonium, nitrite, and organic manure with respect to soil pH. Biol Fertil Soils. 1998;28:27–35. doi: 10.1007/s003740050459. [DOI] [Google Scholar]
- Inamdar S, Lowrance R, Altier L, Williams R, Hubbard R. Riparian Ecosystem Management Model (REMM): II. Testing of the water quality and nutrient cycling component for a coastal plain riparian system. Trans ASABE. 1999;42:1691. doi: 10.13031/2013.13360. [DOI] [Google Scholar]
- Inamdar S, Sheridan J, Williams R, Bosch D, Lowrance R, Altier L, Thomas D. Riparian ecosystem management model (REMM): I. Testing of the hydrologic component for a coastal plain riparian system. Trans ASABE. 1999;42:1679. doi: 10.13031/2013.13332. [DOI] [Google Scholar]
- IPCC (2008) IPCC, 2007: climate change 2007: synthesis report. IPCC
- IPCC (2014) Climate change 2014: synthesis report. Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change IPCC
- Iqbal J, Parkin TB, Helmers MJ, Zhou X, Castellano MJ. Denitrification and nitrous oxide emissions in annual croplands, perennial grass buffers, and restored perennial grasslands. Soil Sci Soc Am J. 2015;79:239–250. doi: 10.2136/sssaj2014.05.0221. [DOI] [Google Scholar]
- Jacinthe P, Bills J, Tedesco L, Barr R. Nitrous oxide emission from riparian buffers in relation to vegetation and flood frequency. J Environ Qual. 2012;41:95–105. doi: 10.2134/jeq2011.0308. [DOI] [PubMed] [Google Scholar]
- Jacinthe P-A, Vidon P, Fisher K, Liu X, Baker M. Soil methane and carbon dioxide fluxes from cropland and riparian buffers in different hydrogeomorphic settings. J Environ Qual. 2015;44:1080–1090. doi: 10.2134/jeq2015.01.0014. [DOI] [PubMed] [Google Scholar]
- Jungkunst HF, Freibauer A, Neufeldt H, Bareth G. Nitrous oxide emissions from agricultural land use in German-A synthesis of available annual field data. J Plant Nutr Soil Sci. 2006;169:341–351. doi: 10.1002/jpln.200521954. [DOI] [Google Scholar]
- Kaiser E-A, Munch JC, Heinemeyer O. Importance of soil cover box area for the determination of N2O emissions from arable soils. Plant Soil. 1996;181:185–192. doi: 10.1007/BF00012052. [DOI] [Google Scholar]
- Khalil M, Baggs E. CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol Biochem. 2005;37:1785–1794. doi: 10.1016/j.soilbio.2005.02.012. [DOI] [Google Scholar]
- Kim D-G, Isenhart TM, Parkin TB, Schultz RC, Loynachan TE. Methane flux in cropland and adjacent riparian buffers with different vegetation covers. J Environ Qual. 2010;39:97–105. doi: 10.2134/jeq2008.0408. [DOI] [PubMed] [Google Scholar]
- Kim D, Isenhart TM, Parkin TB, Schultz RC, Loynachan TE, Raich JW. Nitrous oxide emissions from riparian forest buffers, warm-season and cool-season grass filters, and crop fields. Biogeosci Discuss. 2009;6:607. [Google Scholar]
- King GM, Schnell S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microbiol. 1994;60:3508–3513. doi: 10.1128/aem.60.10.3508-3513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemedtsson L, Svensson B, Rosswall T. Relationships between soil moisture content and nitrous oxide production during nitrification and denitrification. Biol Fertil Soils. 1988;6:106–111. doi: 10.1007/BF00257658. [DOI] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982;46:43. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko I, Boeckx P, Galchenko V, Van Cleemput O. Short-and medium-term effects of NH4+ on CH4 and N2O fluxes in arable soils with a different texture. Soil Biol Biochem. 2002;34:669–678. doi: 10.1016/S0038-0717(01)00232-2. [DOI] [Google Scholar]
- Lee S, McCarty GW, Moglen GE, Li X, Wallace CW. Assessing the effectiveness of riparian buffers for reducing organic nitrogen loads in the Coastal Plain of the Chesapeake Bay watershed using a watershed model. J Hydrol. 2020;585:124779. doi: 10.1016/j.jhydrol.2020.124779. [DOI] [Google Scholar]
- Lane A. The UK environmental change network database: An integrated information resource for long-term monitoring and research. J Environ Manage. 1997;51:87–105. doi: 10.1016/S0301-4797(97)80003-5. [DOI] [Google Scholar]
- Le Mer J, Roger P. Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol. 2001;37:25–50. doi: 10.1016/S1164-5563(01)01067-6. [DOI] [Google Scholar]
- La Montagne MG, Duran R, Valiela I. Nitrous oxide sources and sinks in coastal aquifers and coupled estuarine receiving waters. Sci Total Environ. 2003;309:139–149. doi: 10.1016/S0048-9697(02)00614-9. [DOI] [PubMed] [Google Scholar]
- Lowrance R, Altier L, Williams R, Inamdar S, Sheridan J, Bosch D, Hubbard R, Thomas D. REMM: The riparian ecosystem management model. J Soil Water Conserv. 2000;55:27–34. [Google Scholar]
- Luo G, Kiese R, Wolf B, Butterbach-Bahl K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences. 2013;10:3205–3219. doi: 10.5194/bg-10-3205-2013. [DOI] [Google Scholar]
- Mander Ü, Lohmus K, Teiter S, Uri V, Augustin J. Gaseous nitrogen and carbon fluxes in riparian alder stands. Boreal Environ Res. 2008;13:231–241. [Google Scholar]
- Megonigal JP, Guenther AB. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008;28:491–498. doi: 10.1093/treephys/28.4.491. [DOI] [PubMed] [Google Scholar]
- Mosier A. Nitrous oxide emissions from agricultural soils. Fertilizer Res. 1994;37:191–200. doi: 10.1007/BF00748937. [DOI] [Google Scholar]
- Mosier A, Duxbury J, Freney J, Heinemeyer O, Minami K (1996) Nitrous oxide emissions from agricultural fields: Assessment, measurement and mitigation. Progress in Nitrogen Cycling Studies. Springer, Berlin, pp 589–602
- Müller C, Stevens R, Laughlin R, Jäger H-J. Microbial processes and the site of N2O production in a temperate grassland soil. Soil Biol Biochem. 2004;36:453–461. doi: 10.1016/j.soilbio.2003.08.027. [DOI] [Google Scholar]
- Neugschwandtner R, Liebhard P, Kaul H, Wagentristl H. Soil chemical properties as affected by tillage and crop rotation in a long-term field experiment. Plant Soil Environ. 2014;60:57–62. doi: 10.17221/879/2013-PSE. [DOI] [Google Scholar]
- Orr R, Murray P, Eyles C, Blackwell M, Cardenas L, Collins A, Dungait J, Goulding K, Griffith B, Gurr S. The NorthWyke Farm Platform: effect of temperate grassland farming systems on soil moisture contents, runoff and associated water quality dynamics. Eur J Soil Sci. 2016;67:374–385. doi: 10.1111/ejss.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouattara K, Ouattara B, Assa A, Sédogo PM. Long-term effect of ploughing, and organic matter input on soil moisture characteristics of a Ferric Lixisol in Burkina Faso. Soil Tillage Res. 2006;88:217–224. doi: 10.1016/j.still.2005.06.003. [DOI] [Google Scholar]
- Poulton P, Johnston J, Macdonald A, White R, Powlson D. Major limitations to achieving “4 per 1000” increases in soil organic carbon stock in temperate regions: Evidence from long-term experiments at Rothamsted Research, United Kingdom. Glob Change Biol. 2018;24:2563–2584. doi: 10.1111/gcb.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priha O, Smolander A. Nitrogen transformation in soil under Pinus sylestris, Picea abies, and Betula pendula at two forest sites. Soil Biol Biochem. 1999;31:965–977. doi: 10.1016/S0038-0717(99)00006-1. [DOI] [Google Scholar]
- Ramaswamy V, Boucher O, Haigh J et al (2001) Radiative forcing of climate change. In: JT Houghton TJ, Ding Y, Griggs DJ et al (eds) Climate change 2001: the scientific basis. Contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 350– 416
- Rashti MR, Wang W, Moody P, Chen C, Ghadiri H. Fertilizer-induced nitrous oxide emissions fro vegetable production in the world and the regulating factors: A review. Atmos Environ. 2015;112:225–233. doi: 10.1016/j.atmosenv.2015.04.036. [DOI] [Google Scholar]
- Reich PB, Grigal DF, Aber JD, Gower ST. Nitrogen mineralisation and productivity in 50 hardwood and conifer stands on diverse soils. Ecology. 1997;78:335–347. doi: 10.1890/0012-9658(1997)078[0335:NMAPIH]2.0.CO;2. [DOI] [Google Scholar]
- Reinsch T, Loges R, Kluß C, Taube F. Renovation and conversion of permanent grass-clover swards to pasture or crops: Effects on annual N2O emissions in the year after ploughing. Soil Tillage Res. 2018;175:119–129. doi: 10.1016/j.still.2017.08.009. [DOI] [Google Scholar]
- Rennie S, Andrews C, Atkinson S, Beaumont D, Benham S, Bowmaker V, Dick J, Dodd B, McKenna C, Pallett D. The UK Environmental Change Network datasets–integrated and co-located data for long-term environmental research (1993–2015) Earth Syst Sci Data. 2020;12:87–107. doi: 10.5194/essd-12-87-2020. [DOI] [Google Scholar]
- Rochette P. Towards a standard non-steady-state chamber methodology for measuring soil N2O emissions. Anim Feed Sci Technol. 2011;166:141–146. doi: 10.1016/j.anifeedsci.2011.04.063. [DOI] [Google Scholar]
- Rudaz AO, Walti E, Kyburz G, Lehmann P, Fuhrer J. Temporal variation in N2O and N2 fluxes from a permanent pasture in Switzerland in relation to management, soil water content and soil temperature. Agric Ecosyst Environ. 1999;73:83–91. doi: 10.1016/S0167-8809(99)00005-5. [DOI] [Google Scholar]
- Shen Y, McLaughlin N, Zhang X, Xu M, Liang A. Effect of tillage and crop residue on soil temperature following planting for a Black soil in Northeast China. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-22822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simona C, Ariangelo DPR, John G, Nina N, Ruben M, Jose SJ. Nitrous oxide and methane fluxes from soils of the Orinoco savanna under different land uses. Glob Change Biol. 2004;10:1947–1960. doi: 10.1111/j.1365-2486.2004.00871.x. [DOI] [Google Scholar]
- Skiba U, Ball B. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use Manag. 2002;18:56–60. doi: 10.1079/SUM2002101. [DOI] [Google Scholar]
- Smith HG, Peñuela A, Sangster H, Sellami H, Boyle J, Chiverrell R, Schillereff D, Riley M. Simulating a century of soil erosion for agricultural catchment management. Earth Surf Process Landf. 2018;43:2089–2105. doi: 10.1002/esp.4375. [DOI] [Google Scholar]
- Smith K, Ball T, Conen F, Dobbie K, Massheder J, Rey A. Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci. 2018;69:10–20. doi: 10.1111/ejss.12539. [DOI] [Google Scholar]
- Smith K, Dobbie K, Ball B, Bakken L, Sitaula B, Hansen S, Brumme R, Borken W, Christensen S, Priemé A. Oxidation of atmospheric methane in Northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob Change Biol. 2000;6:791–803. doi: 10.1046/j.1365-2486.2000.00356.x. [DOI] [Google Scholar]
- Stehfest E, Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agrosyst. 2006;74:207–228. doi: 10.1007/s10705-006-9000-7. [DOI] [Google Scholar]
- Tlustos P, Willison T, Baker J, Murphy D, Pavlikova D, Goulding K, Powlson D. Short-term effects of nitrogen on methane oxidation in soils. Biol Fertil Soils. 1998;28:64–70. doi: 10.1007/s003740050464. [DOI] [Google Scholar]
- Tufekcioglu A, Raich JW, Isenhart TM, Schultz RC. Soil respiration within riparian buffers and adjacent crop fields. Plant Soil. 2001;229:117–124. doi: 10.1023/A:1004818422908. [DOI] [Google Scholar]
- Ulén B. Nutrient losses by surface run-off from soils with winter cover crops and spring-ploughed soils in the south of Sweden. Soil Tillage Res. 1997;44:165–177. doi: 10.1016/S0167-1987(97)00051-2. [DOI] [Google Scholar]
- Van Groenigen J, Kasper vG, Velthof G, Van den Pol-van Dasselaar A, Kuikman P. Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications. Plant Soil. 2004;263:101–111. doi: 10.1023/B:PLSO.0000047729.43185.46. [DOI] [Google Scholar]
- Venterea RT, Halvorson AD, Kitchen N, Liebig MA, Cavigelli MA, Del Grosso SJ, Motavalli PP, Nelson KA, Spokas KA, Singh BP, Stewart CE, Ranaivoson A, Strock J, Colins H. Challenges and opoortunities for mitigating nitrous oxide emissions from fertilized croppings systems. Front Ecol Environ. 2012;10:562–570. doi: 10.1890/120062. [DOI] [Google Scholar]
- Wang Q, Bai Y, Gao H, He J, Chen H, Chesney R, Kuhn N, Li H. Soil chemical properties and microbial biomass after 16 years of no-tillage farming on the Loess Plateau, China. Geoderma. 2008;144:502–508. doi: 10.1016/j.geoderma.2008.01.003. [DOI] [Google Scholar]
- Wang Y, Gu J, Vogt RD, Mulder J, Wang J, Zhang X. Soil pH as a chief modifier for regional nitrous oxide emissions: New evidence and implications for global estimates and mitigation. Glob Change Biol. 2018;24:e617–e626. doi: 10.1111/gcb.13966. [DOI] [PubMed] [Google Scholar]
- Wang ZP, Han SJ, Li HL, Deng FD, Zheng YH, Liu HF, Han XG. Methane prodouction explained largely by water content in the heartwood of living trees in upland forests. J Geophys Res Biogeosci. 2017;122(10):2479–2489. doi: 10.1002/2017JG003991. [DOI] [Google Scholar]
- Wilke B-M. Determination of chemical and physical soil properties. In: Varma A, editor. Monitoring and assessing soil bioremediation. Berlin Heidelberg: Springer; 2005. pp. 47–95. [Google Scholar]
- Wu X, Yao Z, Brüggemann N, Shen Z, Wolf B, Dannenmann M, Zheng X, Butterbach-Bahl K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol Biochem. 2010;42:773–787. doi: 10.1016/j.soilbio.2010.01.013. [DOI] [Google Scholar]
- Yamulki S, Jarvis S. Short-term effects of tillage and compaction on nitrous oxide, nitric oxide, nitrogen dioxide, methane and carbon dioxide fluxes from grassland. Biol Fertil Soils. 2002;36:224–231. doi: 10.1007/s00374-002-0530-0. [DOI] [Google Scholar]
- Yang S-S, Chang H-L. Effect of environmental conditions on methane production and emission from paddy soil. Agric Ecosyst Environ. 1998;69:69–80. doi: 10.1016/S0167-8809(98)00098-X. [DOI] [Google Scholar]
- Yao P, Li X, Nan W, Li X, Zhang H, Shen Y, Li S, Yue S. Carbon dioxide fluxes in soil profiles as affected by maize phenology and nitrogen fertilization in the semiarid Loess Plateau. Agric Ecosyst Environ. 2017;236:120–133. doi: 10.1016/j.agee.2016.11.020. [DOI] [Google Scholar]
- Yu L, Tang J, Zhang R, Wu Q, Gong M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol Fertil Soils. 2013;49:119–128. doi: 10.1007/s00374-012-0703-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from authors on request.
Not Applicable.