Abstract
The impact of the COVID-19 pandemic on bloodstream infections (BSIs) due to Streptococcus pneumoniae and Streptococcus pyogenes was assessed in 25 university hospitals of Paris. Monthly BSIs incidence rates that appeared stable in 2018 and 2019, decreased for the 2 pathogens during the 2 COVID-19 lockdown periods of 2020. Containment policies, including social distancing, masking and hand hygiene strengthening in both community and hospital settings are likely to reduce BSIs due to these pathogens.
Key Words: COVID-19, Bloodstream infection incidence, Streptococcus pneumoniae, Streptococcus pyogenes
During the coronavirus disease 2019 (COVID-19) pandemic, several containment measures have been implemented to limit the spread of the virus. The diversity of these measures has been previously described and their impact on the COVID-19 pandemic has been largely commented.1 Nevertheless, data on the collateral effects2 on such large containment measures on the transmission of pathogens other than the one causing the pandemic should be further analyzed.
Measures adopted to mitigate the COVID-19 pandemic, that is, masking, social distancing, and strengthening of hand hygiene, are also those implemented in hospitals and in the community to control pathogens with droplet transmission. Streptococcus pneumoniae and Streptococcus pyogenes are pathogens with nasopharyngeal carriage and known to be mainly transmitted via droplets. Both have the ability to cause invasive diseases, including bloodstream infections (BSIs).3 , 4 Hence, COVID-19 prevention could favorably impact the epidemiology of these infections.
On the opposite, coughing during COVID-19 infection worsened the spread of those bacteria and super-infections are reported in COVID-19 patients, particularly with S. pneumoniae.5 In addition, inpatients accumulate risk factors for hospital-acquired infections (invasive support and monitoring, corticosteroid therapy).6 Therefore, we sought to analyze the impact of the COVID-19 pandemic on the epidemiology of BSIs due to S. pneumoniae and S. pyogenes in a large public multihospital institution in the Paris region.
Assistance Publique - Hôpitaux de Paris is a consortium of 37 university-hospitals (20,000 beds) covering the Paris region (12 million inhabitants). This work was conducted in a subgroup of 25 hospitals (70% of the beds) using the same laboratory information system, allowing standardized data extraction of BSIs. The study covers 2020, the first year of COVID-19 pandemic and, as control, the years 2018 and 2019. A blood culture set (BC set) was defined as the combination of one aerobic and one anaerobic blood culture bottles drawn through the same puncture. Only the first BSI episode with the same microorganism for a given patient was included in the study when several BC sets were positive. Blood cultures drawn in the first two days of admission were considered community-acquired and those >48 hours after admission, hospital-acquired. All laboratories participate in the national accreditation process “COFRAC” with internal and external quality controls and use the EUCAST European quality standards for drug susceptibility testing.
Time-series modelling was used to quantify changes in incidence rates of BSIs during 2018 and 2019. The model was calculated with a linear trend and seasonal components to account for possible changes in the incidence over time, independent of the pandemic. The predicted values for 2020 were calculated with 95% prediction intervals. The validity of the model was tested with Durbin-Watson and Shapiro-Wilk normality tests. All BSIs data were extracted anonymously following current French rules.
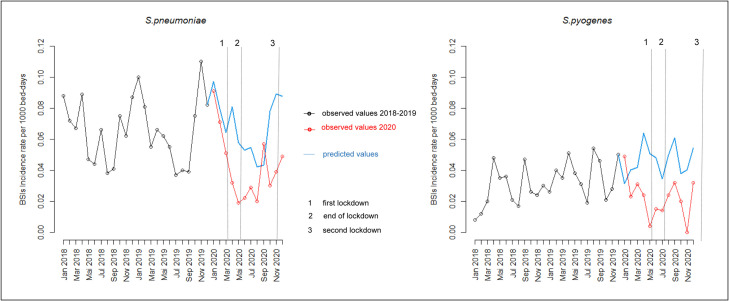
We observed a global decrease of BSIs incidence rates in 2020 by 34% for S. pneumoniae and 28% for S. pyogenes as compared to 2018 and 2019. The temporal course showed a substantial reduction in the monthly incidence rates of BSIs between March and May 2020, coinciding with the implementation of COVID-19 first drastic containment measures (Fig 1 ). At the end of the lockdown, there was a slight increase in BSI incidence rates, but rates remained lower than expected according to the two previous years. During the second lockdown, at the end of the year 2020, containment measures were more relaxed than in the first quarter (back to office work, opened schools). Concomitantly, there was a small increase in BSI rates, remaining lower than predicted values during the winter season, and no classical seasonal peak for S. pneumoniae (Fig 1). All rates observed during 2020 remained below predicted values of the model and during lockdowns, several rates were even lower than the predicted 95% lower limits. The decrease in the BSIs incidence in 2020 remained significant after stratification on the origin of the BSIs, that is, community- or hospital-acquired.
Fig 1.
Impact of containment measures on monthly incidence rates per 1000 bed-days of BSIs due to S. pneumoniae and S. pyogenes. Red lines represent observed data; blue lines represent model estimates based on observed data. The start and the end of the containment measures are indicated by a vertical dashed line.
Discussion
We witnessed a decrease in both community- and hospital-acquired bloodstream infections due to S. pneumoniae and S. pyogenes concomitantly to the large implementation of preventive measures to curb the COVID-19 pandemic. These findings are in line with several reports based on either the whole population with data issued from nationwide surveillance systems,7 , 8 or on inpatients such as the pediatric population.9 , 10 On the opposite, one study reported an increase of such infections during intervals when COVID-19 incidence slowed down, that is, to date not clearly explained.11
The issue of poor case ascertainment and notification could be raised to explain the decreases while countries were responding to the surge of COVID-19. However, we studied S. pneumoniae and S. pyogenes invasive infections diagnosed in the hospital setting, where an increase in the number of blood cultures collected, in particular during the first wave has been reported.12 Hence, the reductions by 34% for S. pneumoniae and 28% for S. pyogenes observed in 2020 in our facilities are likely to be consistent with actual decreases in incidence rates. These reductions are probably linked to basic COVID-19 bundle prevention procedures. Regarding hospital-acquired cases, the consumption of alcohol-based hand products was multiplied by 2.5 in 2020 in the 25 participating hospitals, which likely has an impact on cross transmission. In addition, universal masking adopted during the pandemic was not recommended in hospitals before that period, and besides performing high-risk invasive procedures, facemasks were only recommended in specific situations such as childbirth to prevent S. pyogenes transmission, or for all droplet-generating procedures. In the community, similar precautions were widely adopted and the adherence to those were enforced by lockdown periods, which decreases opportunities for transmission.
The major strength of the present study is that it covers a large regional population and allowed to distinguish community- from hospital-acquired cases. Nevertheless, we had no access to comprehensive medical records preventing analysis of data at the individual level (COVID-19 status, prior hospitalization, antibiotic use). Similarly, analysis of rates of lower respiratory tract infections was not feasible because it requires clinical and radiological data for interpretation.
The containment measures implemented during the COVID-19 pandemic appear to have positively impacted other infections transmitted by the same routes, including influenza. Decreasing such infections should conduct to prevent antibiotic use, which is a worldwide priority. However, the highly restrictive measures may not be accepted outside a health crisis. Therefore, health authorities should promote lighter hygienic measures that are acceptable on the long term to influence public health by decreasing transmission of respiratory pathogens.
Members of the Collégiale de Bactériologue – Virologie – Hygiène (CBVH)
Guillaume Arlet, Laurence Armand Lefevre, Alexandra Aubry, Laurent Belec, Béatrice Bercot, Stéphane Bonacorsi, Vincent Calvez, Emmanuelle Cambau, Etienne Carbonnelle, Stéphane Chevaliez, Jean-Winoc Decousser, Constance Delaugerre, Diane Descamps, Florence Doucet-Populaire, Jean-Louis Gaillard, Antoine Garbarg-Chenon, Elyanne Gault, Jean-Louis Herrmann, Vincent Jarlier, Jérôme Le Goff, Jean-Christophe Lucet, Jean-Luc Mainardi, Anne-Geneviève Marcellin, Laurence Morand-Joubert, Xavier Nassif, Jean-Michel Pawlotsky, Jérôme Robert, Anne-Marie Roque Afonso, Martin Rottman, Christine Rouzioux, Flore Rozenberg, François Simon, Nicolas Veziris, David Skurnik, Jean-Ralph Zahar, Guilene Barnaud, Typhaine Billard Pomares, Gaëlle Cuzon, Dominique Decré, Alexandra Doloy, Jean-Luc Donay, Laurence Drieux-Rouzet, Isabelle Durand, Agnès Ferroni, Vincent Fihman, Nicolas Fortineau, Camille Gomart, Nathalie Grall, Christelle Guillet Caruba, Françoise Jaureguy, Valérie Lalande, Luce Landraud, Véronique Leflon, Patricia Mariani, Liliana Mihaila, Didier Moissenet, Latifa Noussair, Isabelle Podglajen, Isabelle Poilane, Hélène Poupet, Emilie Rondinaud, Valérie Sivadon Tardy, David Trystram, Charlotte Verdet, Emmanuelle Vigier, Sophie Vimont Billarant
Acknowledgments
The authors thank the members of the Collégiale de Bactériologue – Virologie – Hygiène (CBVH) whose daily work made possible to obtain quality data in each of the 25 participating hospitals.
Footnotes
Conflicts of interest: None to report.
Consent for publication: All patients admitted to APHP hospitals are informed about the potential use of data for research purpose.
Members of ‘la Collegiale de Bactériologie-Virologie-Hygiène de l'Assistance Publique, Hôpitaux de Paris’ are given are the end of the article.
Contributor Information
la Collégiale de Bactériologie-Virologie-Hygiène de l'Assistance Publique-Hôpitaux de Paris:
Guillaume Arlet, LaurenceArmand Lefevre, Alexandra Aubry, Laurent Belec, Béatrice Bercot, Stéphane Bonacorsi, Vincent Calvez, Emmanuelle Cambau, Etienne Carbonnelle, Stéphane Chevaliez, Jean-Winoc Decousser, Constance Delaugerre, Diane Descamps, Florence Doucet-Populaire, Jean-Louis Gaillard, Antoine Garbarg-Chenon, Elyanne Gault, Jean-Louis Herrmann, Vincent Jarlier, Jérôme Le Goff, Jean-Christophe Lucet, Jean-Luc Mainardi, Anne-Geneviève Marcellin, Laurence Morand-Joubert, Xavier Nassif, Jean-Michel Pawlotsky, Jérôme Robert, Anne-Marie Roque Afonso, Martin Rottman, Christine Rouzioux, Flore Rozenberg, François Simon, Nicolas Veziris, David Skurnik, Jean-Ralph Zahar, Guilene Barnaud, Typhaine Billard Pomares, Gaëlle Cuzon, Dominique Decré, Alexandra Doloy, Jean-Luc Donay, Laurence Drieux-Rouzet, Isabelle Durand, Agnès Ferroni, Vincent Fihman, Nicolas Fortineau, Camille Gomart, Nathalie Grall, Christelle Guillet Caruba, Françoise Jaureguy, Valérie Lalande, Luce Landraud, Véronique Leflon, Patricia Mariani, Liliana Mihaila, Didier Moissenet, Latifa Noussair, Isabelle Podglajen, Isabelle Poilane, Hélène Poupet, Emilie Rondinaud, Valérie Sivadon Tardy, David Trystram, Charlotte Verdet, Emmanuelle Vigier, and Sophie Vimont Billarant
References
- 1.The Institute of Medicine’s Forum on Microbial Threats . National Academies Press; Washington, D.C.: 2007. Ethical and Legal Considerations in Mitigating Pandemic Disease: Workshop Summary. [PubMed] [Google Scholar]
- 2.Cheng VC-C, Wong S-C, So SY-C, et al. Decreased antibiotic consumption coincided with reduction in bacteremia caused by bacterial species with respiratory transmission potential during the COVID-19 pandemic. Antibiotics. 2022;11:746. doi: 10.3390/antibiotics11060746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20:45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 4.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 5.Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 2021;29:930–941. doi: 10.1016/j.tim.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ripa M, Galli L, Poli A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. 2021;3:e360–e370. doi: 10.1016/S2589-7500(21)00077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steens A, Knol MJ, Freudenburg-de Graaf W, de Melker HE, van der Ende A, van Sorge NM. Pathogen- and type-specific changes in invasive bacterial disease epidemiology during the first year of the COVID-19 pandemic in the Netherlands. Microorganisms. 2022;10:972. doi: 10.3390/microorganisms10050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil JC, Flores AR, Kaplan SL, Hulten KG. The Indirect Impact of the SARS-CoV-2 Pandemic on Invasive Group a Streptococcus, Streptococcus Pneumoniae and Staphylococcus Aureus Infections in Houston Area Children. Pediatr Infect Dis J. 2021;40:e313–e316. doi: 10.1097/INF.0000000000003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skurnik D, Rybak A, Yang DD, et al. Unexpected lessons from the coronavirus disease 2019 lockdowns in France: low impact of school opening on common communicable pediatric airborne diseases. Clin Infect Dis. 2021;73:e2830–e2832. doi: 10.1093/cid/ciaa1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khongyot T, Moriyasu T. Invasive pneumococcal disease diminish during the coronavirus disease 2019 in Japan between 2019 and 2022. Int J Infect Dis. 2022;122:307–309. doi: 10.1016/j.ijid.2022.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amarsy R, Trystram D, Cambau E, et al. Surging bloodstream infections and antimicrobial resistance during the first wave of COVID–19: a study in a large multihospital institution in the Paris region. Int J Infect Dis. 2022;114:90–96. doi: 10.1016/j.ijid.2021.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]