Abstract
Two Letters to Blood address the risks of COVID-19 in populations with precursors of hematological disease. In the first article, Miller and colleagues report on whether clonal hematopoiesis of intermediate potential (CHIP) is associated with adverse outcomes with COVID-19, finding no association between CHIP and 28-day mortality while providing data indirectly linking IL-6 signaling and patient outcomes. In the second article, Ho and colleagues investigate the outcomes of patients with monoclonal gammopathy of undetermined significance (MGUS) with COVID-19, reporting that one-fourth had a severe infection and that on multivariable analysis, adverse outcomes are more likely if immunoparesis is present.
TO THE EDITOR:
Clonal hematopoiesis is an age-related phenomenon in which a clonal population of blood cells emerges and is often detected by the presence of a mutation present in a peripheral blood sample. Clonal hematopoiesis of indeterminate potential (CHIP) is a subtype of clonal hematopoiesis found in individuals without a hematologic malignancy in which a somatic pathogenic mutation in a gene mutated in myeloid neoplasia is present in at least 2% of the sequenced blood DNA (termed variant allele fraction [VAF]).1 , 2 CHIP is associated with increased mortality and risk of adverse outcomes including cardiovascular and pulmonary disease (eg, chronic obstructive pulmonary disease).3, 4, 5 These associations, which are largely driven by CHIP clones with a VAF of greater than 0.1, are thought to arise from augmentation of inflammasome-mediated interleukin-1β (IL-1β) and IL-6 production by mutant macrophages.4, 5, 6, 7
Complications from COVID-19 are thought to result from an enhanced inflammatory state, potentially mediated by macrophage activation and elevated levels of IL-6, though clinical trials assessing the clinical benefit of IL-6 blockade in patients with COVID-19 have yielded conflicting results.8, 9, 10, 11, 12, 13, 14, 15, 16 Clinical risk factors for poor outcomes from COVID-19 include older age, cardiovascular disease, and pulmonary disease. Given the shared risk factors between CHIP and COVID-19 severity, we examined whether there was an association between CHIP and risk of death in patients hospitalized with COVID-19.
The Massachusetts General Brigham cohort included patients hospitalized between March 10, 2020, and April 4, 2021, at Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) (Figure 1 A). All studies were performed with institutional review board approval. Clinical and laboratory data were obtained using the Massachusetts General Brigham Electronic Data Warehouse.17 The tocilizumab cohort included patients from a randomized trial assessing the efficacy of tocilizumab in patients with COVID-19 (supplemental Figure 1, available on the Blood website).10 Genomic sequencing to identify the presence of CHIP was performed on all samples using either whole genome or targeted as previously described (supplemental Tables 1 and 2).18, 19, 20 The primary end point was the association between CHIP with a VAF of 0.1 or greater and in-hospital mortality within 28 days of admission. We used multivariable logistic regression to adjust for age at blood draw, sex, cardiovascular disease, pulmonary disease, diabetes, cancer, and admitting hospital.4 , 5 Exploratory end points were evaluated using a Wilcoxon rank-sum test when continuous and a Fisher’s exact test when categorical. A P value of less than .05 was considered statistically significant. Additional information for all methods can be found in the supplemental Methods.
Figure 1.
CHIP with VAF ≥ 0.1 is not associated with increased 28-day in-hospital mortality in primary cohort. (A) Generation of the primary MGH cohort. (B) Distribution of genes mutated in patients found to have CHIP with a VAF of 0.1 or greater in the MGB cohort. (C) Forest plot of multivariable analysis within MGB cohort (adj P value, adjusted P value). (D) Cumulative 28-day mortality among patients with and without CHIP (VAF ≥ 0.1) in the MGB cohort.
Our final cohort included 1338 patients (BWH, n = 530; MGH, n = 758) who did not have a hematologic malignancy, were admitted for at least 24 hours, and had a peripheral blood sample available. The majority of patients had a blood draw on the day of admission (median time between blood draw and admission, <1 hour; interquartile range, <24 hours). The cohort characteristics are shown in supplemental Table 3.
CHIP was identified in the BWH patients using a targeted sequencing platform and in the MGH patients using whole genome sequencing (see supplemental Methods) Given the stronger associations reported for larger CHIP clones with adverse outcomes, our primary analyses focused on CHIP clones with a VAF of 0.1 or greater.4 , 5 We identified 73 individuals (5.5%) with CHIP at a VAF of 0.1 or greater, with most mutations occurring in DNMT3A, TET2, and ASXL1 (Figure 1B, supplemental Table 4).
Individuals with CHIP at a VAF of 0.1 or greater were significantly older (median age, 74.0 years vs 61.3 years; P < .001) and more likely to have a history of cardiovascular disease (46.6% vs 33.7%; P = .03) (supplemental Table 5; supplemental Figure 2A).6 , 21 In univariable analysis, older age (odds ratio [OR], 4.2 for age ≥65 years old; 95% confidence interval [CI], 2.9-6.1; P < .001), a history of cardiovascular disease (OR, 1.7; 95% CI, 1.2-2.3; P = .002), renal disease (OR, 1.8; 95% CI, 1.1-3.0; P = .014), and cancer (OR, 1.7; 95% CI, 1.0-2.7; P = .029) were significantly associated with an increased risk of 28-day mortality, whereas CHIP with a VAF of 0.1 or greater was not (OR, 0.76; 95% CI, 0.3-1.6; P = .50) (supplemental Table 6). In multivariable analysis only age (OR, 4.1 for age ≥65 years old; 95% CI, 2.8-6.1; P < .001) was significantly associated with an increased risk of 28-day mortality (Figures 1C-D), whereas CHIP with a VAF of 0.1 or greater was not (OR, 0.53; 95% CI, 0.21-1.1; P = .121). Exploratory analysis of patients aged more than 65 years, those with a DNMT3A-mutant CHIP (VAF ≥ 0.1), or those with CHIP at a VAF of 0.05 or greater also showed no association between CHIP and 28-day mortality (supplemental Figures 2B-C, 3A-C, and 4A-B; supplemental Table 7).
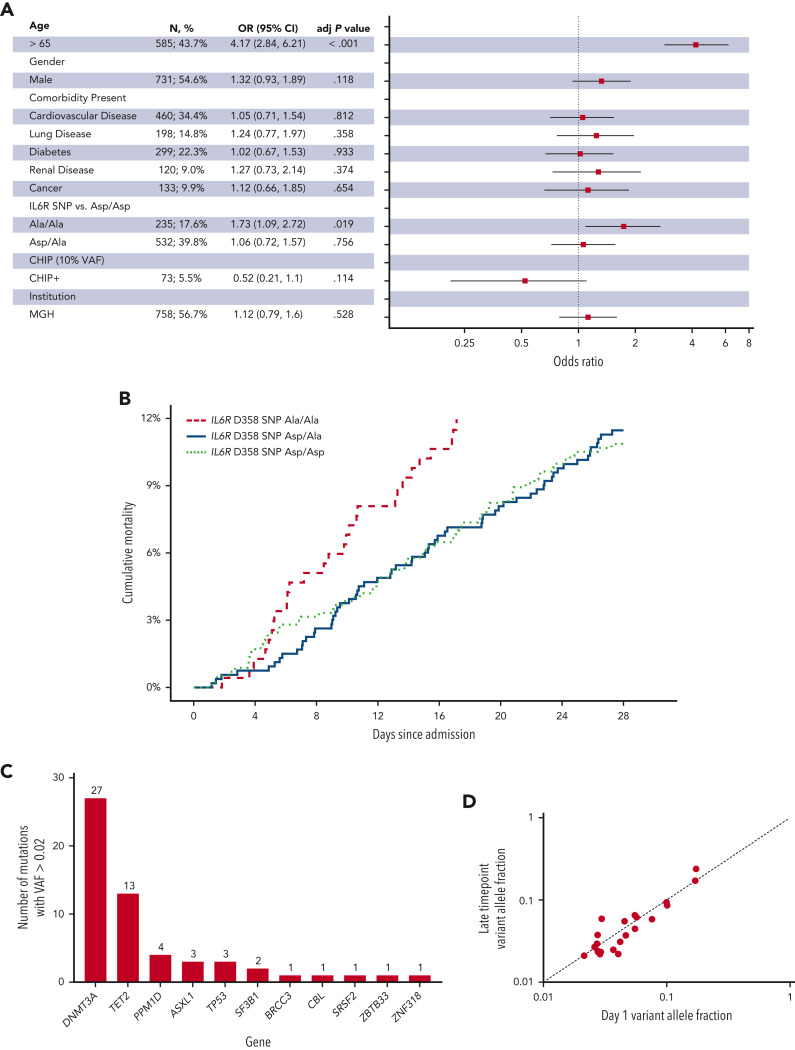
We genotyped all patients for the IL-6 receptor (IL6R) D358A single nucleotide polymorphism (SNP) that is associated with decreased IL-6 signaling and a decreased risk of adverse cardiovascular outcomes.4 , 22 In multivariable analysis, the risk of 28-day mortality was again associated with older age (OR, 4.2; 95% CI, 2.8-6.2; P < .001 for age greater than 65 years), but also with the presence of the IL6R A/A SNP (OR, 1.7; 95% CI, 1.1-2.7; P = .019) (Figure 2 A-B).
Figure 2.
IL6R D358 SNP A/A is associated with increased 28-day in-hospital mortality in the primary cohort. (A) Forest plot of multivariable analysis within Massachusetts General Brigham (MGB) cohort (adj P value, adjusted P value). (B) Cumulative 28-day mortality among patients with IL6R D/D, D/A, and A/A in the MGB cohort. (C) Distribution of genes mutated in patients found to have CHIP with a VAF of 0.02 or greater in the tocilizumab cohort. (D) Comparison of mutant VAF between day 1 and late timepoint in serial samples from the tocilizumab cohort (see supplemental Figure 5).
Finally, we performed targeted sequencing on peripheral blood samples from 231 patients enrolled in a phase III trial that did not find a benefit of tocilizumab for decreasing the risk of intubation or death in patients hospitalized with COVID-19 (supplemental Figure 1).10 We identified 40 patients (17%) with a VAF of 0.02 or greater, but given the small number of deaths in this cohort (11 patients [4.7%]), we were not powered to assess for associations between CHIP and mortality (Figure 2C and supplemental Table 8). Among 92 patients with serial blood samples (sample interval ranged from 1 to 28 days) we found very little change in the clone size regardless of mutations type or treatment arm, despite the intense inflammatory stimulus of severe acute respiratory syndrome coronavirus 2 infection (Figure 2D and supplemental Figure 5).
We did not observe an association between CHIP and the risk of dying within 28 days of admission with COVID-19.5 , 7 , 9 Our findings are consistent with and extend those of a recent study of 568 patients with COVID-19, and differ from other, smaller studies, in several important ways.23 Our cohort was more than double the size of prior studies, we used mortality as our primary end point given challenges with other severity metrics (inaccurate data abstraction and evolving treatment patterns), and focused on large CHIP clones carrying pathogenic mutations, which are more well-established risk modifiers. We also found that the homozygous IL6R D358A SNP A/A, which has been shown to dampen IL-6 signaling, was independently associated with a significantly increased risk of death. Further analysis of primary patient samples and mechanistic work to understand this association is needed.24 A recent study using the UK Biobank found no association between impaired IL-6 signaling and death or need for intubation in patients with COVID-19, but did find an association with increased risk of hospitalization and infection, a question our study was not designed to address.25
Our study has several limitations. The study was performed retrospectively, but did include patients admitted over a year to 2 separate hospitals. Additionally, a 28-day in-hospital mortality primary end point may have missed patients who died after discharge from the hospital. We also did not assess smaller CHIP clones, given their weak associations with adverse outcomes in prior studies.4 , 5 Finally, our analyses were not structured to interrogate causality or define specific mechanisms that may link CHIP or the IL6R D358A SNP to mortality, but was rather performed to identify the presence of an association that could have implications for risk stratification and defining future clinical and basic studies.
Taken together, our data do not support an association between the presence of clonal hematopoiesis and the risk of 28-day mortality in patients with COVID-19, but do support the potential role of IL-6 signaling in mediating patient outcomes.
Conflict-of-interest disclosure: P.G.M. reports consulting fees from Foundation Medicine; A.S.S. reports consulting fees from Adaptive Technologies; B.L.E. has received research funding from Celgene and Deerfield Ventures, consulting fees from GRAIL, and is on the scientific advisory boards for Exo Therapeutics and Skyhawk Therapeutics. P.N. reports investigator-initiated grants from Amgen, Apple, AstraZeneca, Boston Scientific, and Novartis; personal fees from Apple, Allelica, AstraZeneca, Blackstone Life Sciences, Foresite Labs, Novartis, and Roche/Genentech; is a co-founder of TenSixteen Bio; is a scientific advisory board member of Esperion Therapeutics, geneXwell, and TenSixteen Bio; and spousal employment at Vertex, all unrelated to the present work. M.J.F. reports consulting fees from Novartis, BMS, Iovance, Kite/Gilead, Cytoagents, Legend/Johnson and Johnson and Arcellx; and research support from Novartis and Kite/Gilead. A.S.K. received research funding from the Multiple Myeloma Research Foundation and consulting fees from LabCorp, Inc, and Quanterix, Inc. M.K.M. received an unrestricted research grant from Genentech. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH), National Cancer Insitute (NCI), and National Heart, Lung, and Blood Institute (NHLBI) (K08-CA263181 [P.G.M.], K08-CA252174 [A.S.S.], R01-HL082945 [B.L.E.], P01-CA066996 [B.L.E. and D.N.], R01HL148050 [P.N.], R01HL151283 [P.N.], R01HL148565 [P.N.], R01DK125782 [P.N.], RO1-AI132638 [M.K.M.], P30DK34854 [L.B.]), the Office of the Director (DP5-OD02958 [A.G.B.]), the EvansMDS Foundation (P.G.M. and B.L.E.), the Burroughs Wellcome Foundation (A.G.B.), Fondation Leducq (TNE-18CVD04 [P.N.]), and the Howard Hughes Medical Institute (B.L.E.).
Authorship
Contribution: P.G.M., G.G.F., P.N., A.S.K., and B.L.E. designed and conceived the study; P.G.M., G.G.F., A.S.S., B.H.F., J.M.H., and D.N. performed the statistical analyses and analyzed and interpreted the data; J.O.K., C.J.G., T.N., M.M.U., B.B.B., and A.G.B. generated the somatic mutation calls; A.K.S., M.K.M., A.S.K., H.W., L.B., O.P., and M.J.F. provided samples and clinical data; and P.G.M., G.G.F., D.N., and B.L.E. drafted the manuscript.
Footnotes
The original sequencing data are available upon request.
The online version of this article contains a data supplement.
Supplementary Material
References
- 1.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coombs CC, Zehir A, Devlin SM, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017;21(3):374–382.e374. doi: 10.1016/j.stem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller PG, Qiao D, Rojas-Quintero J, et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139(3):357–368. doi: 10.1182/blood.2021013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bick AG, Pirruccello JP, Griffin GK, et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141(2):124–131. doi: 10.1161/CIRCULATIONAHA.119.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal S, Natarajan P, Ebert BL. Clonal hematopoiesis and atherosclerosis. N Engl J Med. 2017;377(14):1401–1402. doi: 10.1056/NEJMc1710381. [DOI] [PubMed] [Google Scholar]
- 6.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 9.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas IO, Diaz G, Gottlieb RL, et al. Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: a randomized clinical trial. Intensive Care Med. 2021;47(11):1258–1270. doi: 10.1007/s00134-021-06507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Group RC Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 17.Foy BH, Carlson JCT, Reinertsen E, et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763–768. doi: 10.1038/s41586-020-2819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35(14):1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uddin MM, Zhou Y, Bick AG, et al. Cost effective sequencing enables longitudinal profiling of clonal hematopoiesis. medRxiv. Published February 2, 2022 doi: 10.1101/2022.01.31.22270028. [DOI] [Google Scholar]
- 21.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai T, Zhang Y, Ho YL, et al. Association of interleukin 6 receptor variant with cardiovascular disease effects of interleukin 6 receptor blocking therapy: a phenome-wide association study. JAMA Cardiol. 2018;3(9):849–857. doi: 10.1001/jamacardio.2018.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Shalhoub R, Rogers SN, et al. Clonal hematopoiesis is not significantly associated with COVID-19 disease severity. Blood. 2022;140(14):1650–1655. doi: 10.1182/blood.2022015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Bovijn J, Lindgren CM, Holmes MV. Genetic variants mimicking therapeutic inhibition of IL-6 receptor signaling and risk of COVID-19. Lancet Rheumatol. 2020;2(11):e658–e659. doi: 10.1016/S2665-9913(20)30345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.