Abstract
In Bacillus subtilis the citM gene encodes the Mg2+-citrate transporter. A target site for carbon catabolite repression (cre site) is located upstream of citM. Fusions of the citM promoter region, including the cre sequence, to the β-galactosidase reporter gene were constructed and integrated into the amyE site of B. subtilis to study catabolic effects on citM expression. In parallel with β-galactosidase activity, the uptake of Ni2+-citrate in whole cells was measured to correlate citM promoter activity with the enzymatic activity of the CitM protein. In minimal media, CitM was only expressed when citrate was present. The presence of glucose in the medium completely repressed citM expression; repression was also observed in media containing glycerol, inositol, or succinate-glutamate. Studies with B. subtilis mutants defective in the catabolite repression components HPr, Crh, and CcpA showed that the repression exerted by all these medium components was mediated via the carbon catabolite repression system. During growth on inositol and succinate, the presence of glutamate strongly potentiated the repression of citM expression by glucose. A reasonable correlation between citM promoter activity and CitM transport activity was observed in this study, indicating that the Mg2+-citrate uptake activity of B. subtilis is mainly regulated at the transcriptional level.
Carbon catabolite repression (CCR) of many genes in Bacillus subtilis is caused by the availability of glucose or other rapidly metabolized carbon sources during growth. The regulatory process involves several proteins including HPr, Crh, HPr kinase, and CcpA. The HPr protein, encoded by the ptsH gene, functions in CCR as well as in the phosphoenolpyruvate-sugar phosphotransferase system (PTS). Crh (catabolite repression HPr) is a protein homologous to HPr that functions in CCR but not in the PTS (13, 29). After glucose is taken up via the PTS, glycolytic intermediates such as fructose-1,6-bisphosphate activate HPr kinase (14, 19, 25). Then, HPr kinase phosphorylates HPr (14, 36) and Crh (13, 14) at a serine residue (Ser46) in an ATP-dependent manner. The seryl-phosphorylated proteins act as corepressors (11, 20, 24) by forming a complex (6) with the trans-acting CcpA (catabolite control protein A), a member of the LacI/GalR family of regulatory proteins (17). The complex binds to a consensus DNA sequence, the so-called cre site (catabolite-responsive element) (18), located upstream of the target gene, where it may act either as a repressor or as an activator of transcription (18).
The key role of CcpA in carbon regulation in B. subtilis has been demonstrated by inactivating the ccpA gene, which resulted in relief of glucose catabolite repression (11, 17) or the abolition of gene activation (34, 40). Mutating Ser46 of HPr resulted in relief from CCR of gnt (7, 31), in partial relief of lev (13) and xynB (12), and no relief of hut (47) and idh (13). In the last two cases, complete relief was observed in a ptsH1 crh double mutant, in which HPr is mutated and the crh gene is disrupted. This suggests an active role for both P-Ser46-HPr and P-Ser46-Crh in CCR, but the individual roles of the two proteins remain obscure.
Uptake of citrate in B. subtilis is strongly enhanced in the presence of divalent metal ions (2). At least two secondary transport proteins, the paralogues CitM and CitH, encoded by open reading frames yflO and yxiQ, respectively (26), mediate citrate uptake in B. subtilis (3). The transporters were expressed in E. coli and functionally characterized (3). CitM turned out to be the transporter that is likely to be responsible for enhanced uptake in the presence of divalent metal ions: CitM transports citrate in a complex with Mg2+ and several other divalent metal ions (2; B. P. Krom, J. B. Warner, W. N. Konings, and J. S. Lolkema, submitted for publication).
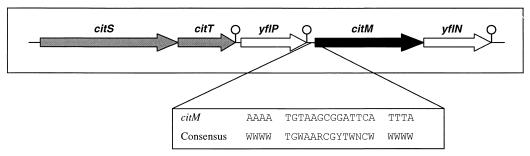
The structural gene coding for CitM is organized in an operon-like structure (Fig. 1), including citM and a second gene, yflN (26), the function of which is not known. Upstream of citM are reading frames citS and citT, which code for a two-component system (9). The CitS-CitT two-component system is essential for the transcription of the citM-yflN operon. The putative CitT target sequence is believed to be located in the region between nucleotides −62 and −113, upstream of the citM transcriptional start point (H. Yamamoto, M. Murata, and J. Sekiguchi, Abstr. 10th Int. Conf. Bacilli, p. 71, 1999). In addition, just in front of the citM gene lies a sequence that matches the consensus sequence of a cre site (41, 46). The functionality of the cre site has been demonstrated in vivo (32). The location of the citM gene on the chromosome of B. subtilis suggests that expression of CitM might be under the control of the metabolic state of the cell.
FIG. 1.
Genetic organization of the citM gene on the B. subtilis chromosome. Arrows, direction of transcription; loops, transcription termination sites. citS and citT code for the two-component signal transduction system, citM codes for the secondary transporter of the Mg2+-citrate complex, and yflP and yflN code for unknown proteins. The genes are drawn to scale. Below is shown the alignment of the cre site located in the CitM promoter region and the consensus sequence as described previously (48). The citM cre site is centered at −24 bp relative to the start codon of the citM gene. Symbols for nucleotides in the consensus sequence: W, A or T; R, A or G; Y, C or T; N, A, G, C, or T.
Induction by citrate and inhibition by glucose of citrate uptake in B. subtilis have been described already 3 decades ago (42). In this study we report on the regulation of synthesis of the Mg2+-citrate transporter of B. subtilis, encoded by the citM gene. Transcription of citM is strictly dependent on the presence of citrate in the growth medium and is under the control of CCR. Repression was observed in media containing several carbohydrates but also nonsugars. Experiments with wild-type and CCR mutant strains revealed the involvement of the different CCR components.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The B. subtilis strains used in this study are listed in Table 1. B. subtilis was grown in C medium (1) in which ferric ammonium citrate was omitted. The C medium was supplemented with 10 mM trisodium citrate (CC medium) or 25 mM myo-inositol (CI medium) or 25 mM myo-inositol and 8 g of potassium glutamate/liter (CIE medium), or 6 g of sodium succinate and 8 g of potassium glutamate/liter (CSE medium). When strain QB5407 was grown in CI medium, 10 mM potassium glutamate was added as nitrogen source (10). Glucose or trisodium citrate was sometimes added at a concentration of 10 mM. Auxotrophic requirements were added at 20-μg/ml final concentration. When appropriate, antibiotics were added at concentrations of 100 μg/ml for spectinomycin (strains QB7097 and QB5407) and 5 μg/ml for kanamycin (QB7102) and chloramphenicol (the PcitM-lacZ fusion strains).
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotypea | Source (reference[s]) |
|---|---|---|
| 168 | trpC2 | Microbiology, University of Groningen |
| SA003 | trpC2 sacB′-′lacZ ptsH1 | J. Deutscher (7) |
| QB7097 | trpC2 crh::spc | I. Martin-Verstraete |
| QB7102 | trpC2 ptsH1 crh::aphA3 | I. Martin-Verstraete (14, 34) |
| QB5407 | trpC2 ccpA::Tn917 spc | I. Martin-Verstraete (10) |
| CM002 | trpC2 amyE::(PcitM-lacZ cat) | This study |
| CM004 | trpC2 sacB′-′lacZ ptsH1 amyE::(PcitM- lacZ cat) | This study |
| CM006 | trpC2 crh::spc amyE::(PcitM-lacZ cat) | This study |
| CM008 | trpC2 ptsH1 crh::aphA3 amyE::(PcitM- lacZ cat) | This study |
| CM010 | trpC2 ccpA::Tn917 spc amyE::(PcitM- lacZ cat) | This study |
Tn917 spc, Tn917 derivative conferring resistance to spectinomycin; aphA3, Enterococcus faecalis kanamycin resistance gene; cat pC194 chloramphenicol acetyltransferase gene.
Overnight cultures of wild-type and mutant B. subtilis strains were inoculated into 20 ml of medium. The cells were grown in 100-ml flasks at 37°C on a rotary shaker operated at 150 rpm. Growth was monitored by measuring the optical density of the cultures at 660 nm (OD660) using a Hitachi U-1100 spectrophotometer. The cells were harvested by centrifugation in the exponential growth phase at an OD660 between 0.3 and 0.6 and washed once with 50 mM PIPES (piperazine-N,N′-bis[2-ethanesulfonic acid]), pH 6.5.
Construction of PcitM-lacZ fusions.
Vector pJM116 (5) contains the promoterless spoVG-lacZ gene between two fragments of the B. subtilis amyE gene and carries the cat gene from pC194 (4). The integration vector pCM160 was constructed by cloning an 819-bp-long PCR fragment of the citM promoter region, PcitM, into the multiple cloning site of pJM116. The citM promoter region including the cre site was amplified by PCR using a forward primer (5′-CTCCAAGGAATTCCAGACGGTTGCATTGCC-3′) that introduced an EcoRI site (boldface) and a backward primer (5′-AAGCCTAAGGATCCTAACACATCCATTCCC-3′) that introduced a BamHI site (boldface). Both pJM116 and the PcitM PCR fragment were digested with BamHI and EcoRI and ligated to yield pCM160. The vector was constructed in Escherichia coli DH5α grown in Luria-Bertani (LB) medium (30) at 37°C. Transformants were selected by including 50 μg of ampicillin/ml in LB agar plates. The construct was checked by restriction and DNA sequence analyses.
Wild-type and mutant B. subtilis strains were transformed with pCM160 to yield the CM series of mutants listed in Table 1. Successful integrants into the amyE locus by homologous recombination were selected for by resistance against chloramphenicol. Integration into the amyE locus was confirmed by an amylase-negative phenotype of cells plated on LB agar containing soluble starch (16). Integrants contained the lacZ gene under the control of the citM promoter region. β-Galactosidase activity was assayed qualitatively on LB agar plates containing the chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) with or without trisodium citrate (10 mM).
Transport assay.
Cells from 20-ml cultures were resuspended in 50 mM PIPES, pH 6.5, to yield an OD660 of 10 and stored on ice until use. Transport activity was determined by the rapid-filtration method (27). Briefly, cells were diluted 10-fold in 50 mM PIPES, pH 6.5–1 mM NiCl2 and incubated for 5 min at 30°C. At time zero, 1 μl of [1,5-14C]citrate (114 mCi/mmol) was added to 99 μl of cell suspension, yielding a final concentration of 4.4 μM citrate. Uptake was stopped by the addition of 2 ml of ice-cold 0.1 M LiCl solution, immediately followed by filtration through a 0.45-μm-pore-size nitrocellulose filter. The filters were washed once with the same LiCl solution. The filters were submerged in scintillation fluid, and the retained radioactivity was counted in a liquid scintillation counter. Samples were taken at time points between 0 and 10 min. Uptake rates were determined from the linear initial part of each uptake curve.
Speciation of Ni2+ in the transport assay buffer was calculated using the MINTEQA2 program (21). The concentration of NiCl2 used during the transport studies was 1 mM, which drives 99.9% of the radiolabeled citrate into the complexed state.
β-Galactosidase assay.
One milliliter of cell culture at an OD660 between 0.3 and 0.6 (exponential growth phase) was harvested by centrifugation for 5 min in an Eppendorf table top centrifuge operated at 14,000 rpm. Cell extracts were obtained by lysozyme treatment, and β-galactosidase activities were determined using o-nitrophenyl-β-d-galactopyranoside as the substrate, as described previously (30). β-Galactosidase activities of PcitM-lacZ integrants were corrected for β-galactosidase activity of B. subtilis 168 transformed with pJM116, which amounted to 0.4 to 2.5 Miller units. Strain CM004 contains the PcitM-lacZ fusion integrated in the chromosome of strain SA003, which already contains another lacZ fusion (Table 1). Under the growth conditions used in our experiments the β-galactosidase activity of strain SA003 was the same as that observed for strain 168 transformed with plasmid pJM116. Consequently, the β-galactosidase activity of strain CM004 reflects citM promoter activity.
RESULTS
Induction and glucose catabolite repression of citM expression.
B. subtilis contains two known transporters for citrate, CitH and CitM. CitM is responsible for citrate-induced citrate uptake activity and transports citrate in complex with Mg2+ and other divalent metal ions (2, 3). To study the expression of CitM, the uptake activity was measured using the Ni2+-citrate complex as the substrate, which is highly specific for CitM (Krom et al., submitted). Ni2+ was chosen rather than Mg2+ because of the higher stability of the Ni2+-citrate complex, which assures that all citrate is in the complexed state (log KA is 5.4 and 3.4 for Ni2+ and Mg2+, respectively [28]).
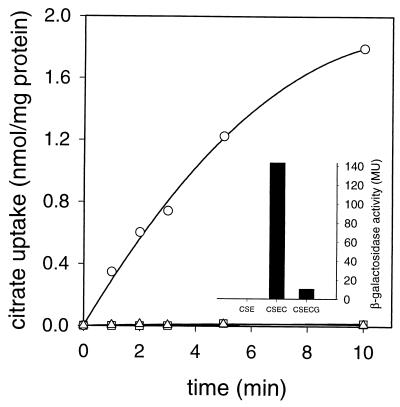
B. subtilis 168 grown in minimal medium containing succinate and glutamate (CSE medium) showed no uptake of Ni2+-citrate, while growth in the same medium with additional citrate resulted in significant uptake (Fig. 2). When, in addition to citrate, glucose was added, the uptake activity dropped dramatically. The experiment suggests that Ni2+-citrate uptake activity is induced by citrate and repressed by glucose.
FIG. 2.
Ni2+-citrate uptake and citM promoter activity. Uptake of [1,5-14C]citrate in whole cells of B. subtilis 168 grown in CSE medium without further additions (□, CSE), with 10 mM citrate (○, CSEC), and with 10 mM citrate plus 10 mM glucose (▵, CSECG). (Inset) β-Galactosidase activity (in Miller units [MU]) of B. subtilis CM002 carrying the lacZ gene under the control of the citM promoter grown in the same media.
To correlate the Ni2+-citrate uptake activity with the expression of the citM gene, the gene encoding β-galactosidase was fused behind the promoter region of citM (PcitM-lacZ fusion) and the construct was integrated in the amyE locus on the genome of B. subtilis 168, yielding strain CM002 (Table 1). The β-galactosidase activity of CM002 correlated with the Ni2+-citrate uptake activity observed in the wild-type strain. No β-galactosidase activity was seen when cells were grown in the absence of citrate, while a high activity was observed in the presence of citrate. Including glucose in the medium in addition to citrate resulted again in very low β-galactosidase activity (Fig. 2, inset). The correlation between Ni2+-citrate uptake activity and citM promoter activity indicates that the lack of uptake activity in cells grown in the absence of citrate or in the presence of glucose was due to the lack of expression of citM.
The same pattern of induction and glucose repression was observed upon growth of B. subtilis in minimal medium containing inositol (CI medium) and inositol plus glutamate (CIE medium) (Table 2). In the absence of citrate neither significant uptake of Ni2+-citrate nor promoter activity was observed. In the presence of citrate both uptake and promoter activities were significantly higher in CSE medium than in CI and CIE media, while repression by glucose was most effective in the two media that contained glutamate in addition to succinate or inositol (CSE and CIE media, respectively).
TABLE 2.
Ni2+-citrate uptake activity and PcitM promoter activity in minimal media
| Medium | Initial uptake rate (nmol/min · mg of protein) | β-Galactosidase activity (Miller units) |
|---|---|---|
| CI | 0.01 | 0 |
| CI + citrate | 0.18 | 35 |
| CI + citrate + glucose | 0.10 | 10 |
| CSE | 0.01 | 0 |
| CSE + citrate | 0.38 | 143 |
| CSE + citrate + glucose | 0.01 | 11 |
| CIE | 0.01 | 0 |
| CIE + citrate | 0.17 | 30 |
| CIE + citrate + glucose | 0.01 | 2 |
Ni2+-citrate uptake and citM promoter activity in different growth media.
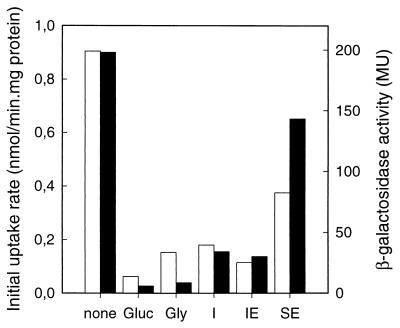
The repressive effects of various growth media on Ni2+-citrate uptake and citM promoter activity were determined by growing B. subtilis in citrate minimal medium supplemented with different carbon sources. Highest uptake and promoter activities were observed when cells were grown in C medium with citrate as the sole carbon and energy source (Fig. 3). Supplementing the growth medium with the carbohydrate glucose, glycerol, or inositol resulted in dramatic loss of both activities. The repressive effect was not restricted to sugars, because, though less prominent, clear decreases in both Ni2+-citrate uptake activity and citM promoter activity were observed with cells grown in medium containing succinate and glutamate. The similar activities of cells grown in inositol and inositol-glutamate media suggest that the latter repression is caused by succinate. In all the growth media tested, there was a fair correlation between CitM transport activity and citM promoter activity, with the relatively low promoter activity in the glycerol medium as the exception. The differences between the two activities most likely represent other regulatory factors acting after the transcription of the gene or differences in the metabolic state of the cells (see Discussion).
FIG. 3.
Effect of different growth substrates on transport and promoter activities. B. subtilis strains 168 and CM002 were grown in C minimal medium with 10 mM citrate in the presence of no further additions (none), glucose (Gluc), glycerol (Gly), inositol (I), inositol and glutamate (IE), and succinate and glutamate (SE). Open bars, initial rates of uptake of [1,5-14C]citrate in the presence of 1 mM NiCl2 by whole cells of B. subtilis 168; solid bars, β-galactosidase activity of B. subtilis CM002 grown in the different media in Miller units (MU).
The highest growth rate was observed in the medium with the lowest Ni2+-citrate uptake activity and citM promoter activity. The doubling time in the media supplemented with glucose, glycerol, or inositol ranged from 120 to 140 min. The lowest growth rate was observed in minimal citrate medium (380-min doubling time), while in CSE medium growth rate and citM expression were intermediate (230-min doubling time).
In conclusion, the expression of the Ni2+-citrate uptake system is under strict control of the components of the growth medium and is repressed by other growth substrates besides glucose. The level of expression of CitM was inversely related to the growth rates in the different media, which is typical for CCR.
Roles of HPr, Crh, and CcpA in repression of citM expression in CI medium.
B. subtilis mutants SA003 (genotype ptsH1), QB7097 (crh), and QB5407 (ccpA) are defective in HPr, Crh, and CcpA, respectively, components involved in CCR in B. subtilis (see the introduction) (Table 1). Mutant QB7102 is a double mutant defective in the two homologous proteins HPr and Crh. The PcitM-lacZ fusion was integrated into the chromosome of each of these mutants to measure the promoter activity in the mutant background under inducing and repressing conditions.
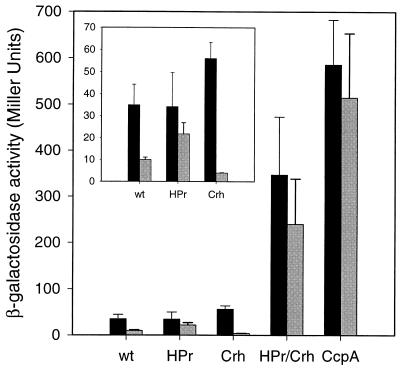
CitM promoter activity in wild-type cells grown in CI medium was approximately sixfold lower than that observed in a medium with only citrate (CC medium) (Fig. 3). The activity was repressed 3.5-fold when glucose was also present in the medium (Fig. 4). Surprisingly, the ccpA mutant strain showed a 17-fold increase in β-galactosidase activity compared to the wild-type level when grown in the absence of glucose in CI medium. As expected, no significant glucose repression was observed in the mutant. The results show that both inositol and glucose have a repressive effect on citM expression in the wild-type strain and that the repression by both is mediated via CcpA. In agreement, a significantly elevated β-galactosidase activity was observed in the ptsH1 crh double mutant, but the activity was somewhat lower than that of the ccpA mutant. HPr, Crh, or both are involved in inositol repression in addition to CcpA. Glucose repression was almost completely alleviated in the double mutant. In contrast to the double mutant, the ptsH1 and crh single-mutant strains showed wild-type promoter activity levels when grown in CI medium. Apparently, both proteins are involved in the repression of citM expression by inositol but they can replace one another. Compared to the wild type, repression by glucose was less strong in the ptsH1 single mutant but was stronger in the crh mutant, where repression was very potent (Fig. 4, inset). This suggests an important role for HPr in glucose repression in CI medium.
FIG. 4.
citM promoter activity of CCR mutants grown in CI medium. β-Galactosidase activities of B. subtilis strains CM002 (wt), CM004 (HPr), CM006 (Crh), CM008 (HPr/Crh), and CM010 (CcpA) grown in CI medium supplemented with citrate (black bars) and citrate plus glucose (gray bars) are shown. All strains carry the PcitM-lacZ fusion integrated in the amyE locus. (Inset) enlarged part of the graph.
Initial uptake rates of Ni2+-citrate in wild-type and mutant cells correlated well with β-galactosidase activity under the same conditions (Table 3). The results showed similar induction and repression features. Transport activity was 1 order of magnitude higher in the ccpA mutant and the ptsH1 crh double mutant than in the wild-type strain. The repression of transport activity by inositol was equally well relieved in the ccpA mutant and the ptsH1 crh double mutant, but in both mutants there was still significant repression by glucose. The transport activities of the crh and ptsH1 single mutants paralleled the promoter activities. Repression by inositol was not relieved in the single mutants, while glucose repression was most potent in the crh mutant and less so in the ptsH1 mutant.
TABLE 3.
Initial uptake rates of Ni2+-citrate in wild-type and mutant B. subtilis strains
| Medium | Initial uptake rate (nmol/min · mg of protein) for straina:
|
||||
|---|---|---|---|---|---|
| 168 (wild type) | SA003 (ptsH1) | QB7097 (crh) | QB7102 (ptsH1 crh) | QB5407 (ccpA) | |
| CI + citrate | 0.18 | 0.20 | 0.21 | 2.42 | 2.50 |
| CI + citrate + glucose | 0.10 | 0.13 | 0.08 | 1.13 | 1.44 |
| CSE + citrate | 0.38 | 0.80 | 0.76 | 2.02 | 1.87 |
| CSE + citrate + glucose | 0.01 | 0.02 | 0 | 0.61 | 1.74 |
| CIE + citrate | 0.17 | 0.06 | 0.08 | 1.03 | 1.89 |
| CIE + citrate + glucose | 0.01 | 0.01 | 0.01 | 1.09 | 1.57 |
The relevant genotype is in parentheses.
Roles of HPr, Crh, and CcpA in repression of citM expression in CSE and CIE media.
Upon growth of cells in the CSE and CIE media, the main features of promoter activity and Ni2+-citrate uptake activity of the mutant and wild-type strains were similar to those observed in CI medium (Tables 3 and 4). Importantly, in the succinate-glutamate medium (CSE medium) both transport and promoter activities were four- to fivefold higher in the ccpA mutant and ptsH1 crh double mutant than in the wild-type strain, indicating that the medium components (succinate and/or glutamate) repressed citM expression via the CCR system. Noteworthy is that in CSE medium the double mutant and the ccpA mutant gave comparable β-galactosidase and transport activities, indicating that repression by succinate and/or glutamate is completely mediated via HPr and/or Crh. In the two single mutants, both transport activity and promoter activity were stimulated by a factor of two relative to those of the wild-type strain, indicating that both HPr and Crh play a role in succinate and/or glutamate repression but that they cannot completely take over each other's roles as was observed in repression by inositol. Additional repression by glucose in the wild-type and mutant strains was much stronger in CSE medium than in CI medium, except for the ccpA mutant, where repression was completely relieved.
TABLE 4.
PcitM promoter activity measured in wild-type and mutant B. subtilis strains
| Medium | β-Galactosidase activity (Miller units) for straina:
|
||||
|---|---|---|---|---|---|
| CM002 (wild type) | CM004 (ptsH1) | CM006 (crh) | CM008 (ptsH1 crh) | CM010 (ccpA) | |
| CI + citrate | 35 | 34 | 56 | 347 | 586 |
| CI + citrate + glucose | 10 | 22 | 4 | 240 | 515 |
| CSE + citrate | 143 | 276 | 460 | 525 | 525 |
| CSE + citrate + glucose | 11 | 22 | 16 | 344 | 526 |
| CIE + citrate | 30 | 29 | 30 | 228 | 430 |
| CIE + citrate + glucose | 2 | 6 | 2 | 304 | 481 |
The relevant genotype is in parentheses.
CitM expression is repressed in CSE medium. Unfortunately, B. subtilis did not grow on C medium containing succinate as the sole carbon and energy source and only poorly on glutamate, which did not allow discrimination between succinate and glutamate as the repressive substrate. As an alternative, all experiments were repeated using CI medium to which glutamate was added (CIE medium). The addition of glutamate had no effect on the repression by inositol (Tables 3 and 4). However, there was a marked difference in the repression by glucose in this medium. The repression was much stronger than in the absence of glutamate and comparable to the repression in CSE medium.
Growth defects of the ptsH1, crh, and ccpA mutant strains.
A (partly) functional CCR system was necessary for normal growth on inositol. Both the ptsH1 crh double mutant and the ccpA mutant grew poorly in CI and CIE minimal media, with growth rates of 0.07 to 0.11 h−1 compared to 0.32 (CI medium) and 0.69 h−1 (CIE medium) for the wild-type strain. Especially long lag phases were observed, and the addition of glucose, citrate, or glutamate (44) had no significant effect on the growth rate.
DISCUSSION
In B. subtilis the uptake of citrate is mediated by at least two known homologous secondary transport proteins (3). One of them, termed CitM, is a proton motive force-driven transporter that mediates the transport of citrate complexed to Mg2+ and several other divalent metal ions in symport with two protons (3). CitM is believed to be the predominant citrate transporter under physiological conditions (2, 3). The conditions under which CitM is expressed were the topic of our research. The expression of citM was monitored at the transcriptional level by measuring the citM promoter activity and at the protein level by measuring Ni2+-citrate uptake activity in whole cells. Our results indicate that citM expression is under strict control of the medium composition. CitM is an inducible protein that is only expressed when citrate is present in the growth medium, which is sensed by the CitS-CitT two-component system, whose coding sequence is located upstream of citM (9; Yamamoto et al., Abstr. 10th Int. Conf. Bacilli). The expression was highest when citrate was the only carbon and energy source in the medium. The carbohydrates glucose, glycerol, and inositol are preferred over citrate, resulting in higher growth rates and a strongly repressed expression of citM. Remarkably, the expression of citM was also repressed during growth on the nonsugars succinate and glutamate, albeit to a lesser extent. In B. subtilis, repression by growth substrates other than glucose has been reported for inositol dehydrogenase, which was repressed by glycerol and mannitol (7), for the hut operon, that was found to be repressed by amino acids (43) and for the first three enzymes of the Krebs cycle were found to be repressed by glutamate and glutamine (8, 33).
Transcription of the structural gene is the first step in the biosynthetic pathway of a protein. The amount of a membrane protein such as CitM that ends up in the cytoplasmic membrane depends on the rate of transcription of the gene (measured by the promoter activity) and on other factors such as messenger stability, efficiency of insertion into the membrane, and protein stability. The activity of the ensemble of protein molecules in the membrane, which is the relevant parameter for the cell, may further depend on regulation of the activity of the individual protein molecules by global cellular factors such as pH and redox potential and by more-specific effectors. Most importantly, for secondary transporters, transport activity depends on the energy status of the cell. Transport activity catalyzed by CitM is driven by the electrochemical proton gradient that is maintained across the cytoplasmic membrane by the cellular energy metabolism (3). In this study, a reasonable correlation between citM promoter activity and Ni2+-citrate uptake activity in the different strains and under different growth conditions was observed; this is somewhat surprising considering the above discussion. Apparently, in the media tested, the uptake of citrate complexed to divalent metal ions in B. subtilis is mainly regulated at the level of transcription of the citM gene.
The promoter region of citM contains a cre sequence centered 24 bp upstream of the citM start codon. The 14-bp DNA sequence with dyad symmetry deviates very little from the consensus sequence (41, 46) (Fig. 1), which suggested that CitM is likely to be subject to carbon catabolite repression. It has been suggested that A- and T-rich regions intensify the interactions of catabolite control protein A (CcpA) with the cre site flanking the citM cre sequence (46). Recently, it was demonstrated that the citM cre site is active in vivo (32). The strong relief of citM repression in all media tested in a defective-CcpA mutant indicates that repression by not only glucose but also inositol and succinate and/or glutamate in the wild-type strain is mediated by the binding of CcpA to the cre site located in the citM promoter region.
In CCR, the binding of CcpA to the cre site is induced by complex formation of CcpA and the phosphorylated forms of HPr and Crh. Repression of citM expression in CI medium was less relieved in the ptsHI crh double mutant than in the ccpA mutant. Since in the double mutant the CcpA molecule is present, this may indicate an affinity of uncomplexed CcpA for the cre site or promotion of binding by factors other than HPr and Crh (15, 23). Complete relief of repression in the double mutant when grown in CSE medium suggests that in CI medium other metabolic intermediates promote CcpA binding to the cre site.
The specific roles of HPr and Crh in CCR are not clear. HPr is one of the general proteins in the phosphoenolpyruvate-dependent PTS that transports sugars into the cell with concomitant phosphorylation. HPr is a phosphocarrier intermediate that is phosphorylated at a histidine residue (His15) by phosphoenolpyruvate in a reaction catalyzed by enzyme I (EI). It donates its phosphoryl group to a sugar-specific enzyme or enzyme domain termed IIA. In CCR, HPr is phosphorylated by ATP at a serine residue (Ser46), a reaction catalyzed by HPr kinase. It has been suggested that the functions of HPr in the PTS and CCR interact when transcription of a gene is repressed by a PTS sugar, for instance, glucose (35). The Crh protein is not operational in the PTS (29), simply because, at the position corresponding to the phosphorylation site (His15) in HPr, a glutamine residue is found in Crh. The CCR phosphorylation site (Ser46) is present, and it has been shown that Crh is phosphorylated by HPr kinase in vitro (13, 14). In a number of studies, it has been observed that HPr and Crh can take over each other's role in the CCR function (34, 46). Similarly, in this study, repression of expression of citM by inositol in the ptsH1 and crh single mutants was similar to that observed in the wild-type strain, while repression was alleviated in the ptsH1 crh double mutant (Fig. 4). It should be noted that inositol is believed to be taken up by the cell by a secondary transporter (encoded by iolF) (45) and, therefore, that there is no turnover of the PTS during growth on inositol. For a PTS sugar, it may be anticipated that the CCR system discriminates between HPr and Crh (35). In agreement, repression of citM by glucose is more effective in the crh mutant than in the HPr mutant, suggesting that repression by HPr is potentiated during turnover of the PTS. Discrimination between HPr and Crh in the repression of citM in medium containing the nonsugars succinate and glutamate (CSE medium) was also observed in this study. Repression was relieved twofold in both single mutants, suggesting that HPr could only partly take over repression exerted by Crh and vice versa. The mechanism by which succinate and/or glutamate metabolism affects the degree of phosphorylation of HPr and Crh is completely unknown.
The addition of glucose to CI medium resulted in an additional threefold repression of citM expression. The addition of glutamate to CI medium had no effect on citM expression. Remarkably, when both glucose and glutamate were added to CI medium, the additional repression was about 15-fold and of the same order of magnitude of the glucose repression in CSE medium, which also contains glutamate. This suggests that the presence of glutamate in the medium makes repression by glucose much stronger. A similar synergistic repression has been observed for the citB gene of B. subtilis, coding for the tricarboxylic acid (TCA) cycle enzyme aconitase. Expression of the citB gene in minimal medium containing glucose or glycerol was fully repressed when a source of 2-ketoglutarate, such as glutamine or glutamate, was present (33, 37, 39). Repression of citB is mediated by a novel regulator termed CcpC (22). A PcitM-lacZ fusion integrated in the amyE locus of B. subtilis CJB8 containing an inactivated ccpC gene resulted in the same β-galactosidase activities when grown on CSE medium in the presence and absence of glucose as those observed for the wild-type strain CM002 (not shown). This strongly suggests that CcpC is not involved in the regulation of CitM expression and that synergistic repression is not restricted to regulation by CcpC.
The ccpA mutant has been reported to be unable to grow on glucose minimal medium with ammonium as the nitrogen source (44). The observation was explained by the inability of the mutant to utilize ammonium as a single source of nitrogen because of the absence of the key enzyme of ammonium assimilation, glutamate synthase (10). Addition of TCA cycle intermediates, such as citrate and glutamate, could restore growth on minimal medium containing glucose and ammonium (44), but not on that containing arabinose (38). In this study, the effect of inositol was as observed with arabinose, i.e., growth in CI and CIE medium was severely slowed down even when citrate or glutamate or both were present. The same was true for the ptsH1 crh double mutant grown in minimal medium in the presence of inositol, whereas the ptsH1 and crh single mutants showed wild-type growth rates under these conditions. Similar growth defects have been described by Zalieckas et al. (47).
ACKNOWLEDGMENTS
We thank J. Deutscher for strain SA003, I. Martin-Verstraete for strains QB7097; QB7102, and QB540, and A. L. Sonenshein for strain CJB8.
This work was supported by grants from the Ministry of Economic Affairs of The Netherlands, in the framework of the “IOP Milieutechnologie/Zware Metalen,” project IZW97404 and by the Fundacion Antorchas (to C.M. and J.L). C.M. is a Career Investigator of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
ADDENDUM IN PROOF
An extensive characterization of the citM promoter region was recently published (H. Yamamoto, M. Murata, and J. Sekiguchi, Mol. Microbiol. 37:898–912, 2000).
REFERENCES
- 1.Aymerich S, Gonzy-Tréboul G, Steinmetz M. 5′-Noncoding region sacR is the target of all identified regulation affecting the levansucrase gene in Bacillus subtilis. J Bacteriol. 1986;166:993–998. doi: 10.1128/jb.166.3.993-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsma J, Konings W N. The properties of citrate transport in membrane vesicles from Bacillus subtilis. Eur J Biochem. 1983;134:151–156. doi: 10.1111/j.1432-1033.1983.tb07545.x. [DOI] [PubMed] [Google Scholar]
- 3.Boorsma A, van der Rest M E, Lolkema J S, Konings W N. Secondary transporters for citrate and the Mg2+-citrate complex in Bacillus subtilis are homologous proteins. J Bacteriol. 1996;178:6216–6222. doi: 10.1128/jb.178.21.6216-6222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byeon W H, Weisblum B. Post-transcriptional regulation of chloramphenicol acetyltransferase. J Bacteriol. 1984;158:543–550. doi: 10.1128/jb.158.2.543-550.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dartois V, Djavakhishvili T, Hoch J A. Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis. J Bacteriol. 1996;178:1178–1186. doi: 10.1128/jb.178.4.1178-1186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher J, Küster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. doi: 10.1111/j.1365-2958.1995.tb02280.x. [DOI] [PubMed] [Google Scholar]
- 7.Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994;176:3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingman D W, Rosenkrantz M S, Sonenshein A L. Relationship between aconitase gene expression and sporulation in Bacillus subtilis. J Bacteriol. 1987;169:3068–3075. doi: 10.1128/jb.169.7.3068-3075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabret C, Feher V A, Hoch J A. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J Bacteriol. 1999;181:1975–1983. doi: 10.1128/jb.181.7.1975-1983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faires N, Tobisch S, Bachem S, Martin-Verstraete I, Hecker M, Stülke J. The catabolite control protein CcpA controls ammonium assimilation in Bacillus subtilis. J Mol Microbiol Biotechnol. 1999;1:141–148. [PubMed] [Google Scholar]
- 11.Fujita Y, Miwa Y, Galinier A, Deutscher J. Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr. Mol Microbiol. 1995;17:953–960. doi: 10.1111/j.1365-2958.1995.mmi_17050953.x. [DOI] [PubMed] [Google Scholar]
- 12.Galinier A, Deutscher J, Martin-Verstraete I. Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J Mol Biol. 1999;286:307–314. doi: 10.1006/jmbi.1998.2492. [DOI] [PubMed] [Google Scholar]
- 13.Galinier A, Haiech J, Kilhoffer M C, Jaquinod M, Stülke J, Deutscher J, Martin-Verstraete I. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc Natl Acad Sci USA. 1997;94:8439–8444. doi: 10.1073/pnas.94.16.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galinier A, Kravanja M, Engelmann R, Hengstenberg W, Kilhoffer M C, Deutscher J, Haiech J. New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression. Proc Natl Acad Sci USA. 1998;95:1823–1828. doi: 10.1073/pnas.95.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gösseringer R, Küster E, Galinier A, Deutscher J, Hillen W. Cooperative and non-cooperative DNA binding modes of catabolite control protein CcpA from Bacillus megaterium result from sensing two different signals. J Mol Biol. 1997;266:665–676. doi: 10.1006/jmbi.1996.0820. [DOI] [PubMed] [Google Scholar]
- 16.Harwood C R, Cutting S M. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons; 1980. [Google Scholar]
- 17.Henkin T M, Grundy F J, Nicholson W L, Chambliss G H. Catabolite repression of α-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacI and galR repressors. Mol Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 18.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 19.Jault J M, Fieulaine S, Nessler S, Gonzalo P, Di Pietro A, Deutscher J, Galinier A. The HPr kinase from Bacillus subtilis is a homo-oligomeric enzyme which exhibits strong positive cooperativity for nucleotide and fructose 1,6-bisphosphate binding. J Biol Chem. 2000;275:1773–1780. doi: 10.1074/jbc.275.3.1773. [DOI] [PubMed] [Google Scholar]
- 20.Jones B E, Dossonnet V, Küster E, Hillen W, Deutscher J, Klevit R E. Binding of the catabolite repressor protein CcpA to its DNA target is regulated by phosphorylation of its corepressor HPr. J Biol Chem. 1997;272:26530–26535. doi: 10.1074/jbc.272.42.26530. [DOI] [PubMed] [Google Scholar]
- 21.Joshi-Tope G, Francis A J. Mechanisms of biodegradation of metalcitrate complexes by Pseudomonas fluorescens. J Bacteriol. 1995;177:1989–1993. doi: 10.1128/jb.177.8.1989-1993.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jourlin-Castelli C, Mani N, Nakano M M, Sonenshein A L. CcpC, a novel regulator of the LysR family required for glucose repression of the citB gene in Bacillus subtilis. J Mol Biol. 2000;295:865–878. doi: 10.1006/jmbi.1999.3420. [DOI] [PubMed] [Google Scholar]
- 23.Kim J H, Voskuil M I, Chambliss G H. NADP, corepressor for the Bacillus catabolite control protein CcpA. Proc Natl Acad Sci USA. 1998;95:9590–9595. doi: 10.1073/pnas.95.16.9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus A, Küster E, Wagner A, Hoffmann K, Hillen W. Identification of a co-repressor binding site in catabolite control protein CcpA. Mol Microbiol. 1998;30:955–963. doi: 10.1046/j.1365-2958.1998.01123.x. [DOI] [PubMed] [Google Scholar]
- 25.Kravanja M, Engelmann R, Dossonnet V, Blüggel M, Meyer H E, Frank R, Galinier A, Deutscher J, Schnell N, Hengstenberg W. The hprK gene of Enterococcus faecalis encodes a novel bifunctional enzyme: the HPr kinase/phosphatase. Mol Microbiol. 1999;31:59–66. doi: 10.1046/j.1365-2958.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 26.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 27.Lolkema J S, Enequist H, van der Rest M E. Transport of citrate catalyzed by the sodium-dependent citrate carrier of Klebsiella pneumoniae is obligatorily coupled to the transport of two sodium ions. Eur J Biochem. 1994;220:469–475. doi: 10.1111/j.1432-1033.1994.tb18645.x. [DOI] [PubMed] [Google Scholar]
- 28.Martell A E, Smith R M. Critical stability constants. Vol. 3. New York, N.Y: Plenum Publishing Corp.; 1977. [Google Scholar]
- 29.Martin-Verstraete I, Galinier A, Darbon E, Quentin Y, Kilhoffer M C, Charrier V, Haiech J, Rapoport G, Deutscher J. The Q15H mutation enables Crh, a Bacillus subtilis HPr-like protein, to carry out some regulatory HPr functions, but does not make it an effective phosphocarrier for sugar transport. Microbiology. 1999;145:3195–3204. doi: 10.1099/00221287-145-11-3195. [DOI] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Miwa Y, Nagura K, Eguchi S, Fukuda H, Deutscher J, Fujita Y. Catabolite repression of the Bacillus subtilis gnt operon exerted by two catabolite-responsive elements. Mol Microbiol. 1997;23:1203–1213. doi: 10.1046/j.1365-2958.1997.2921662.x. [DOI] [PubMed] [Google Scholar]
- 32.Miwa Y, Nakata A, Ogiwara A, Yamamoto M, Fujita Y. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 2000;28:1206–1210. doi: 10.1093/nar/28.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohné M. Regulation of aconitase synthesis in Bacillus subtilis: induction, feedback repression, and catabolite repression. J Bacteriol. 1974;117:1295–1305. doi: 10.1128/jb.117.3.1295-1305.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Presecan-Siedel E, Galinier A, Longin R, Deutscher J, Danchin A, Glaser P, Martin-Verstraete I. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J Bacteriol. 1999;181:6889–6897. doi: 10.1128/jb.181.22.6889-6897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reizer J, Bergstedt U, Galinier A, Küster E, Saier M H, Jr, Hillen W, Steinmetz M, Deutscher J. Catabolite repression resistance of gnt operon expression in Bacillus subtilis conferred by mutation of His-15, the site of phosphoenolpyruvate-dependent phosphorylation of the phosphocarrier protein HPr. J Bacteriol. 1996;178:5480–5486. doi: 10.1128/jb.178.18.5480-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reizer J, Hoischen C, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H, Jr, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 37.Rosenkrantz M S, Dingman D W, Sonenshein A L. Bacillus subtilis citB gene is regulated synergistically by glucose and glutamine. J Bacteriol. 1985;164:155–164. doi: 10.1128/jb.164.1.155-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strauch M A. AbrB modulates expression and catabolite repression of a Bacillus subtilis ribose transport operon. J Bacteriol. 1995;177:6727–6731. doi: 10.1128/jb.177.23.6727-6731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobisch S, Zühlke D, Bernhardt J, Stülke J, Hecker M. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J Bacteriol. 1999;181:6996–7004. doi: 10.1128/jb.181.22.6996-7004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turinsky A J, Grundy F J, Kim J H, Chambliss G H, Henkin T M. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J Bacteriol. 1998;180:5961–5967. doi: 10.1128/jb.180.22.5961-5967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weickert M J, Chambliss G H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willecke K, Pardee A B. Inducible transport of citrate in a gram-positive bacterium, Bacillus subtilis. J Biol Chem. 1971;246:1032–1040. [PubMed] [Google Scholar]
- 43.Wray L V, Jr, Fisher S H. Analysis of Bacillus subtilis hut operon expression indicates that histidine-dependent induction is mediated primarily by transcriptional antitermination and that amino acid repression is mediated by two mechanisms: regulation of transcription initiation and inhibition of histidine transport. J Bacteriol. 1994;176:5466–5473. doi: 10.1128/jb.176.17.5466-5473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wray L V, Jr, Pettengill F K, Fisher S H. Catabolite repression of the Bacillus subtilis hut operon requires a cis-acting site located downstream of the transcription initiation site. J Bacteriol. 1994;176:1894–1902. doi: 10.1128/jb.176.7.1894-1902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida K I, Aoyama D, Ishio I, Shibayama T, Fujita Y. Organization and transcription of the myo-inositol operon, iol, of Bacillus subtilis. J Bacteriol. 1997;179:4591–4598. doi: 10.1128/jb.179.14.4591-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zalieckas J M, Wray L V, Jr, Fisher S H. Expression of the Bacillus subtilis acsA gene: position and sequence context affect cre-mediated carbon catabolite repression. J Bacteriol. 1998;180:6649–6654. doi: 10.1128/jb.180.24.6649-6654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zalieckas J M, Wray L V, Jr, Fisher S H. trans-Acting factors affecting carbon catabolite repression of the hut operon in Bacillus subtilis. J Bacteriol. 1999;181:2883–2888. doi: 10.1128/jb.181.9.2883-2888.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]