TO THE EDITOR:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has high morbidity in individuals receiving cellular therapies.1,2 Immunization with the messenger RNA (mRNA) vaccines, BNT16b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), is immunogenic and reduces severe COVID-19 in the general population,3,4 but there are limited data in individuals receiving chimeric antigen receptor T-cell therapy (CAR-Tx).3,4 A few studies5, 6, 7, 8, 9, 10, 11 demonstrated low humoral immunogenicity after CAR-Tx, and, to our knowledge, there are no data evaluating retention of pre-established SARS-CoV-2 vaccine–induced immunity after CAR-Tx. We evaluated humoral and cellular immunogenicity of up to 3 mRNA SARS-CoV-2 vaccinations after CAR-Tx and retention of pre-established SARS-CoV-2 immunity after CAR-Tx.
We prospectively enrolled adults aged ≥18 years with B-cell malignancies who were planning to or already had received CD19-, CD20-, or B-cell maturation antigen–targeted CAR-Tx and SARS-CoV-2 mRNA vaccinations; participants who had received CAR-Tx were in remission. Healthy control participants are detailed in the supplement. This study was approved by the Fred Hutchinson Cancer Center Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
In the pre–CAR-Tx cohort, blood was obtained ≥2 weeks after a second mRNA vaccine and ∼30 and ∼90 days after CAR-Tx (supplemental Figure 1). In the post–CAR-Tx cohort, blood was obtained before vaccination and ≥2 weeks after second and third vaccinations; patients had not received prior SARS-CoV-2 vaccines. We tested samples for antispike (S) protein immunoglobulin G (IgG) with a semiquantitative total antibody assay (Roche Elecsys Anti-SARS-CoV-2 S), antinucleocapsid (N) IgG (Architect SARS-CoV-2 IgG), and neutralizing antibodies with a D614G SARS-CoV-2 S pseudotyped lentivirus neutralization assay.11 We immunophenotyped B cells with flow cytometry for CD19+ B cells and SARS-CoV-2 S-specific B cells. We assessed cellular immunity with an interferon gamma enzyme-linked immune absorbent spot (ELISPOT) assay (T-SPOT Discovery SARS-CoV-2; Oxford Immunotec, Abingdon, United Kingdom).12 All samples collected after second and third vaccinations were tested; in the pre–CAR-Tx cohort, post–CAR-Tx samples were tested if prior samples had positive results for the relevant assay (supplemental Table 1). Details are provided in the supplement.
We computed Spearman rank correlations between humoral and cellular response measurements using per-individual mean values. We computed receiver operating characteristic curves using anti-S IgG as a continuous marker and an outcome of detection of neutralizing antibodies. To explore predictors of immunogenicity in the post–CAR-Tx cohort, we generated scatterplots of anti-S IgG titers and ELISPOT results stratified by clinical and immunologic characteristics.
We enrolled 45 adults who received SARS-CoV-2 mRNA vaccinations and CAR-Tx (Table 1). Three individuals received intravenous immunoglobulin within 2 months of a sample collection as described in the supplement.
Table 1.
Baseline demographics and clinical characteristics of the pre– and post–CAR-Tx cohorts (N = 45)
| Baseline characteristics | Cohort |
|
|---|---|---|
| Pre–CAR-Tx (n = 21) | Post–CAR-Tx (n = 24) | |
| Age, y (range) | 67 (41-84) | 59 (39-81) |
| Sex, n (%) | ||
| Male | 14 (66.7) | 15 (62.5) |
| Race, n (%) | ||
| White | 19 (90.5) | 24 (100) |
| Disease, n (%) | ||
| Lymphoma | 16 (76.2) | 15 (62.5) |
| ALL | 0 | 1 (4.2) |
| CLL | 0 | 2 (8.3) |
| MM | 3 (14.3) | 6 (25.0) |
| WM | 2 (9.5) | 0 |
| Target, n (%) | ||
| CD19 | 15 (71.4) | 16 (66.7) |
| CD20 | 3 (14.3) | 2 (8.3) |
| BCMA | 3 (14.3) | 6 (25.0) |
| CAR-Tx type, n (%) | ||
| Commercial | 16 (76.2) | 6 (25.0) |
| Investigational | 5 (23.8) | 18 (75.0) |
| Vaccine type, n (%) | ||
| mRNA-1273 | 6 (28.6) | 12 (50.0) |
| BNT162b2 | 15 (71.4) | 12 (50.0) |
| IgG level, median (IQR), mg/dL∗ | — | 458 (363-552) |
| CD-19+ B cells, median (IQR), cells/mL∗ | — | 17 (0-160) |
| CD-4+ T cells, median (IQR), cells/mL∗ | — | 345 (219-559) |
| Time from second vaccine to sample collection, mo, median (IQR) | 4.1 (1.9-5.4) | 2.0 (0.7-2.4) |
| Time from CAR-Tx to second vaccination, mo, median (IQR) | −4.3 (−5.9 to −2.4) | 19.0 (10.5-29.2) |
| Time interval between first and second vaccination, d, median (IQR) | 21 (21-28) | 28 (21-29) |
| Time interval between second and third vaccination, d, median (IQR) | — | 142 (117-206)† |
ALL, acute lymphoblastic leukemia; BCMA, B-cell maturation antigen; CLL, chronic lymphocytic leukemia; IQR, interquartile range; MM, multiple myeloma; WM, Waldenström macroglobulinemia.
Obtained at the time of the first prevaccine blood draw.
Among 15 individuals who received a third dose.
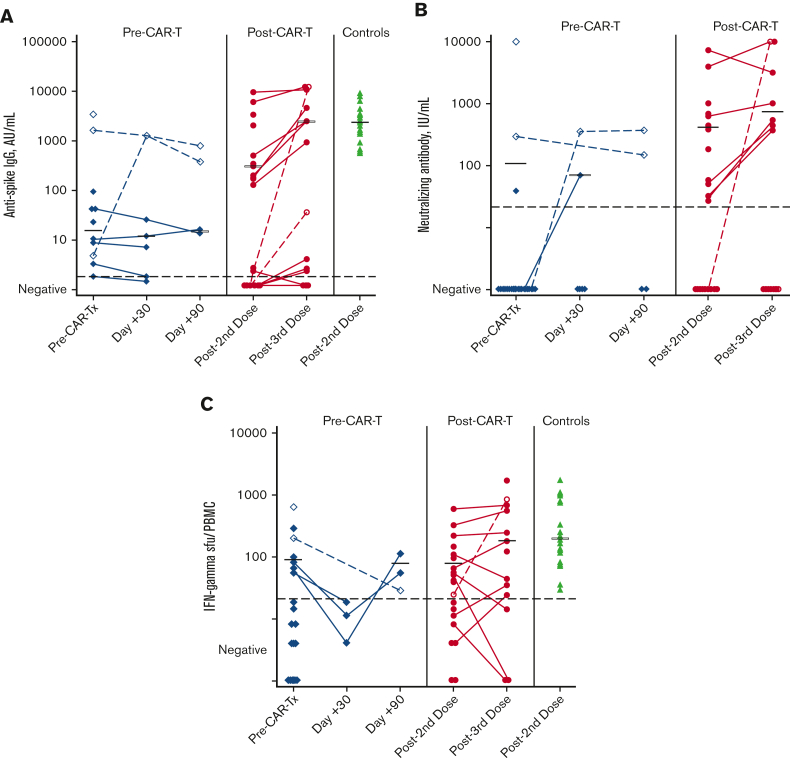
Twenty-one participants received 2 vaccine doses, a median of 4 months (IQR, 2-6 months) before receiving CAR-Tx. One had anti-N antibodies before CAR-Tx, and 2 had anti-N antibodies at either 30 or 90 days after CAR-Tx; data from these time points were excluded from summary statistics. Before CAR-Tx, 10 of 20 (50%) individuals had anti-S IgG, and 2 of 20 (10%) developed neutralizing antibodies (Figure 1A-B). Among 5 individuals with pre–CAR-Tx anti-S IgG detection with available post–CAR-Tx samples, 3 remained positive by day 30 and 2 remained positive at day 90; median titers were stable. Two of 20 (10%) individuals had detectable SARS-CoV-2 S-specific memory B cells before CAR-Tx, both of whom also had anti-S IgG responses; neither maintained these cells after CAR-Tx. (supplemental Figure 2). In addition, 6 of 19 (32%) individuals had SARS-CoV-2–specific T-cell responses (Figure 1C). T-cell responses waned at day 30 after CAR-Tx (0 of 3) but recovered by day 90 in 2 out of 2 individuals who were tested at day 90. Among individuals with positive results, median anti-S IgG titers were 2.2 log10 lower than in healthy control participants but T-cell responses were similar. Overall, SARS-CoV-2 vaccination elicited humoral and/or cellular responses in 13 of 20 (65%) individuals before CAR-Tx.
Figure 1.
Humoral and cellular responses by cohort. (A) Anti-S IgG assay results by cohort. (B) Neutralizing antibody assay results by cohort. (C) ELISPOT T-cell assay results by cohort. The blue diamonds represent the pre–CAR-Tx cohort, the orange circles represent the post–CAR-Tx cohort, and the green triangles represent control samples. Black horizontal bars represent median values among those with a positive result. Open symbols and dashed lines represent individuals who developed antinucleocapsid (N) antibodies and were excluded from median titer calculations on detection of anti-N antibodies. In the pre–CAR-Tx cohort, 1 individual had detectable anti-N antibodies before CAR-Tx, 1 at 30 days after CAR-Tx, and 1 at 90 days after CAR-Tx. In the post–CAR-Tx cohort, 2 individuals became anti-N positive after the third vaccine dose. Dashed lines in black represent the positive cutoff values for each test. Results were log10(x + 1) transformed for figures and before analyses.
Twenty-four individuals received 2 vaccine doses a median of 19 months (IQR, 11-29 months) after CAR-Tx, and 15 individuals received a third dose; for 23 participants, samples collected after second vaccination were available. Thirteen of 23 (57%) had detectable anti-S IgG, of whom all except 1 individual developed neutralizing antibodies (Figure 1A). Except for 2 individuals who developed anti-N antibodies, anti-S IgG titers increased in most of the 7 individuals with detectable anti-S IgG who received third vaccinations, and 3 of 5 (60%) individuals with no initial response seroconverted in the absence of intercurrent SARS-CoV-2 infection. SARS-CoV-2 S-specific memory B cells were detectable in 4 of 23 (17%) individuals, and all 4 also had anti-S IgG responses (supplemental Figure 2). In addition, 12 of 21 (57%) individuals developed SARS-CoV-2–specific T-cell responses, with a slight increase in median responses after a third dose; 2 of 3 (67%) individuals with no initial response developed new T-cell responses in the absence of intercurrent SARS-CoV-2 infection (Figure 1C). After a third vaccine, individuals with prior humoral and cellular responses achieved titers similar to healthy control participants. Overall, SARS-CoV-2 vaccination after CAR-Tx elicited humoral and/or cellular responses in 19 of 23 (83%) individuals after 2 doses and in 11 of 13 (85%) individuals after 3 doses.
Among all 45 individuals, anti-S IgG and neutralizing antibodies were highly correlated (supplemental Figure 3A). An anti-S IgG titer of ≥95 AU/mL after 2 vaccine doses had a sensitivity of 93% and specificity of 100% for the presence of neutralizing antibodies (supplemental Figure 3C). There was limited correlation between anti-S IgG and T-cell responses (supplemental Figure 3B). Finally, detection of SARS-CoV-2 S-specific memory B cells correlated with anti-S IgG responses, but many individuals without detectable S-specific memory B cells also had anti-S IgG (supplemental Figure 4).
There were no strong associations between clinical or laboratory parameters and humoral or cellular responses in the post–CAR-Tx cohort (supplemental Figure 5). However, only 1 of 7 (14%) individuals vaccinated with 2 doses within 12 months of CAR-Tx developed anti-S IgG but 4 of 7 (57%) of these individuals had T-cell responses. Humoral response rates were higher among individuals vaccinated with 2 doses >12 months after receiving CAR-Tx (anti-S IgG detected in 12 of 16, 75%), but T-cell responses were similar (8 of 14, 57%). Notably, positive and negative humoral and cellular responses were observed in individuals with low and high CD19+ total and S-specific naïve B cells, CD4+ T cells, and IgG.
We demonstrate humoral and/or cellular responses in 65%, 83%, and 85% of individuals before and after CAR-Tx, and after a third vaccine, respectively, with responses similar to healthy control participants after a third vaccine dose among responders. Pre-established SARS-CoV-2–specific immunity appeared preserved and/or recovered in the first few months after CAR-Tx. These findings are within the range reported by other studies, which demonstrate humoral responses in 11% to 76% of CAR-Tx recipients after 2 mRNA SARS-CoV-2 vaccines.11,13,14 One study reported an antibody response, T-cell response, or both in 36%, 50%, and 57% of individuals, respectively.7 Nonetheless, limited immunogenicity, or lack thereof, supports the importance of re-vaccination after CAR-Tx in participants who have not yet received CAR-Tx, and vaccination with more than 2 doses in all recipients of CAR-Tx.
We identified strong correlation between anti-S IgG and neutralizing antibodies, and an anti-S IgG titer ≥95 AU/mL with the Roche Elecsys assay was highly predictive of a neutralizing antibody response. A substantial proportion of individuals without antibody responses developed T-cell responses, which may provide protection against severe diseases.15
Overall, clinical and immunologic variables were poor biomarkers for predicting vaccine response. Key observations were that low levels of total IgG, CD19+ B cells, and CD4+ T cells did not preclude antibody responses. Similarly, although most patients with S-specific naïve B cells before vaccination had a humoral response, many patients without detectable levels of these cells also responded, underscoring the limitations of using peripheral blood to assess immunocompetence.
The primary limitation of this study was the relatively small cohort, although this is one of the larger studies to date assessing mRNA SARS-CoV-2 vaccine responses after CAR-Tx, and to our knowledge, the only study assessing retention of previously established SARS-CoV-2 immunity after CAR-Tx. Additional limitations included the small sample size of individuals who were vaccinated within 12 months after CAR-Tx, heterogeneity in the timing of vaccine and sample collection in both cohorts, the relatively low response rates in the pre–CAR-Tx cohort, which limited assessments of retention of SARS-CoV-2 immunity after CAR-Tx, and that most individuals received CD19/20-targeted CAR-Tx for lymphoma. It is expected that immune responses to variants of concern are lower than depicted here, although this study provides direct insights into SARS-CoV-2 vaccine responses and general vaccine immunogenicity concepts in recipients of CAR-Tx. An ongoing CIBMTR (SC21-07) and BMT CTN (2101) study will provide additional insights with a larger sample size. Together, these data support current guidelines for SARS-CoV-2 vaccination in individuals with hematologic malignancies with repeat vaccination as early as 3 months after CAR-Tx.16,17
Conflict-of-interest disclosure: J.A.H. received consulting fees or honoraria from Gilead Sciences, Amplyx, Allovir, Allogene Therapeutics, CRISPR Therapeutics, CSL Behring, OptumHealth, Octapharma, and Takeda, and research funding from Takeda, AlloVir, Karius, Merck, Deverra Therapeutics, and Gilead Sciences, Inc. D.G.M. received consulting fees or honoraria from Bristol Myers Squibb (BMS), Caribou Biosciences, Celgene, Incyte, Juno Therapeutics Inc., Kite, Lilly, Mustang Bio, Novartis, and Umoja, and research funding from Kite Pharma, Juno Therapeutics Inc., and Celgene. J.G. received consulting fees from JMP, Multerra Bio, EUSA Pharma, and Larvol, and research funding from Juno Therapeutics Inc., Celgene, BMS, and Sobi. A.L.G. reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen, and Hologic, and research support from Gilead Sciences, Inc. and Merck, outside of the described work. D.M.K. received research support from Merck, Oxford Immunotec, Sensei Biotherapeutics, and Sanofi Pasteur, and is a scientific advisory board member for Curevo Vaccine and MaxHealth LLC.
Acknowledgments
Acknowledgments: The authors thank the study participants, the Fred Hutchinson Cancer Center, and the participants’ local provider for assistance with sample collection and medical records. The authors also acknowledge Oxford Immunotec (Abingdon, United Kingdom) for providing T-SPOT Discovery SARS-CoV-2 test kits for T-cell evaluation.
This work was supported by grants from the National Institutes of Health National Cancer Institute (NIH/NCI; U01CA247548) (J.A.H.), the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number T32AI118690), the NIH/NCI Cancer Center Support Grants (P30CA0087-48 and P30CA015704-44), and the American Society for Transplantation and Cellular Therapy (J.B.).
Contribution: M.A.G., E.M.K., and J.A.H. designed the study; M.A.G., K.F., J.B., M.-L.H., V.L.C., E.M.K., D.M.K., and J.A.H. interpreted the data; M.A.G., E.M.K., and J.A.H. analyzed the data and created the figures; M.A.G., A.M.B., J.H., S.I., and J.A.H. collected data; M.A.G. drafted the initial manuscript with help from E.M.K. and J.A.H.; and all authors contributed to the writing and revision of the manuscript and approved the final version.
Footnotes
Data are available on request from the author, Joshua A. Hill (jahill3@fredhutch.org).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Yigenoglu TN, Ata N, Altuntas F, et al. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93(2):1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. Spanish Group for the Study of COVID-19 in Transplant Recipients COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2021;21(5):1825–1837. doi: 10.1111/ajt.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gastinne T, Le Bourgeois A, Coste-Burel M, et al. Safety and antibody response after one and/or two doses of BNT162b2 Anti-SARS-CoV-2 mRNA vaccine in patients treated by CAR T cells therapy. Br J Haematol. 2022;196(2):360–362. doi: 10.1111/bjh.17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhakal B, Abedin S, Fenske T, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood. 2021;138(14):1278–1281. doi: 10.1182/blood.2021012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788–794. doi: 10.1016/j.jtct.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abid MB, Rubin M, Ledeboer N, et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell. 2022;40(4):340–342. doi: 10.1016/j.ccell.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamari R, Politikos I, Knorr DA, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2(6):577–585. doi: 10.1158/2643-3230.BCD-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiedmeier JE, Iqbal M, Munoz J, et al. Response to COVID-19 vaccination post-CAR T therapy in patients with non-Hodgkin lymphoma and multiple myeloma. Blood. 2021;138(suppl 1):1750. doi: 10.1016/j.clml.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley BT, Bryan A, Fink SL, et al. Anti-SARS-CoV-2 antibody levels measured by the AdviseDx SARS-CoV-2 assay are concordant with previously available serologic assays but are not fully predictive of sterilizing immunity. J Clin Microbiol. 2021;59(9):e00989–21. doi: 10.1128/JCM.00989-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abid MA, Abid MB. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: a systematic review and preliminary observations. Hematol Oncol. 2022;40(2):287–291. doi: 10.1002/hon.2957. [DOI] [PubMed] [Google Scholar]
- 14.Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Advances. 2022;6(5):1537–1546. doi: 10.1182/bloodadvances.2021006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parvathaneni K, Torres-Rodriguez K, Meng W, et al. SARS-CoV-2 spike-specific T-cell responses in patients with B-cell depletion who received chimeric antigen receptor T-cell treatments. JAMA Oncol. 2022;8(1):164–167. doi: 10.1001/jamaoncol.2021.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darwin R. NCCN shares new guidance principles for vaccinating people with cancer against COVID-19. NCCN. 22 January. 2021. https://bit.ly/3iU496R
- 17.Centers for Disease Control and Prevention Current COVID-19 ACIP vaccine recommendations. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.