Abstract
Background
Although essential trace elements (ETEs) play pivotal roles in life-supporting biochemical processes, their function in innate and adaptive immunity has not been fully elucidated, particularly during immunization. Furthermore, the association between anti-SARS-CoV-2 specific IgG antibodies and ETE levels with vaccine responsiveness has not been investigated.
Methods
The present study explored the status of ETEs (Mn, Cu, Zn, and Se) in sera of healthy women before and after vaccination with the anti-SARS-CoV-2 BNT162b2 mRNA vaccine in a follow-up period of six months. The main aim was to explore links between ETE levels and IgG antibodies produced against Spike glycoprotein's Receptor-Binding Domain (RBD).
Results
A recombinant protein of SARS-CoV-2 comprising the receptor binding domain was successfully expressed in HEK-293 T cells. The purified protein was suitable for producing a sensitive antibody detection assay for human serum and monitored seropositivity, indicating a transient response with peak anti-SARS-CoV-2 IgG levels 2 months after vaccination. In parallel to increasing antibody titers, serum concentrations of Cu, Mn, and Se were not affected by vaccination, and concentrations remained relatively constant at the different sampling times during the 6-month observation period. Total serum Zn concentrations were slightly elevated when compared between the first and last sampling dates. Overall, no consistent effects of vaccination on any of the three trace elements analyzed in our study were observed.
Conclusion
Vaccination of adult healthy female volunteers with an mRNA vaccine was not associated with consistent changes in serum trace element concentrations over a six-month observation period.
Keywords: Essential trace elements, Cu, Zn, RBD, mRNA vaccine, (Post)vaccinal immunity
1. Introduction
The novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), the cause of the coronavirus disease (COVID-19) pandemic, is the most dangerous coronavirus identified [1]. On March 11, 2020, the current coronavirus-mediated infection was officially declared a global pandemic by the World Health Organization (WHO).
Еstimates of SARS-CoV-2 infection is over 506 million people worldwide, causing more than 6.2 million deaths [2]. With quite heterogeneous clinical presentations, it is a global priority to encourage collaborative efforts toward developing meaningful and effective therapeutic strategies to treat and reduce disease morbidity [3]. Therapeutic strategies against COVID-19 focus on 1) enhancing the immune system to minimize the intensity of SARS-CoV-2 infection and 2) developing specific virucidal agents against SARS-CoV-2 [1], [4], [5]. The total number of worldwide administered vaccines is over 11 billion doses [2].
The first officially registered case in Serbia occurred on March 06, 2020, and vaccination was initiated on December 24, 2020. As a result, five vaccines are at the disposal of the general population, i.e., Comirnaty (BNT162b2, BioNTech – Pfizer), BBIBP-CorV – Inactivated (Sinopharm), Vaxzevria (ChAdOx1-S [recombinant], AstraZeneca), Sputnik V (Gam-COVID-Vac, Gamaleya), and mRNA-1273 (Moderna). This calls for clinical evaluation and follow-up studies of each available Vaccine to estimate its safety and efficacy profile in the Serbian population. BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine encodes a prefusion stabilized, SARS-CoV-2 full-length spike glycoprotein [6]. A two-dose regimen of BNT162b2 conferred 95% protection against COVID-19. At the same time, reports on safety were similar to that of other viral vaccines, including eliciting high SARS-CoV-2 neutralizing antibody titers and robust antigen-specific CD8 + and Th1-type CD4 + T-cell responses [6], [7].
Following a SARS-CoV-2 infection, most patients develop detectable specific IgG antibodies to the receptor-binding domain (RBD) of the viral spike glycoprotein and associated neutralizing activities [1], [3], [5], [8], [9]. In addition, most infected persons develop detectable IgG levels to structural SARS-CoV-2 antigens that can persist for more than six months post-acute infection [10], [11], [12], [13].
The function of the immune system is supported by essential trace elements (ETEs), which play a pivotal role in maintaining its homeostasis [4], [14]. Deregulation of ETE levels adversely affects the immune system and increases susceptibility to various bacterial and viral microorganisms [12]. Furthermore, ETEs mediate vital biochemical functions by acting as cofactors for many enzymes, such as glutathione peroxidase (GPx), superoxide dismutase (SOD), RNA, and DNA polymerases, but also modulate signaling pathways, including the NF-kB pathway [15], [16]. Zinc (Zn) is an essential dietary ETE that delicately regulates the immune cell functions, including T-dependent antibody response [12], that could be utilized to reduce the intensity of SARS-CoV-2 infection and lessen the respiratory tract infection through antiviral actions [4].
The association between anti-SARS-Cov-2 specific IgG antibodies and ETE levels with vaccine responsiveness has not been fully elucidated. Thus, the present study explored the status of ETEs (Mn, Cu, Zn, and Se) in the sera of healthy women (n = 20) before and after vaccination with the anti-SARS-CoV-2 BNT162b2 mRNA vaccine in a follow-up period of six months (six-time points) and aimed to explore links between ETEs with specific IgG antibodies produced against RBD of Spike glycoprotein.
2. Material and methods
2.1. Study design, sample collection, and processing
Initially, 50 women vaccinated with the BNT162b2 mRNA vaccine were included in this study. However, after strict exclusion criteria (consumers of mineral supplements containing Mn, Cu, Zn, and/or Se, participants with reported liver or kidney diseases, or with signs of respiratory infections), 20 women (SARS-CoV-2 naive, mean age: 47 ± 7) met the criteria for enrollment according to the study design. This created a homogeneous group of participants to gain the best possible insight into their element and immune statuses. Furthermore, all enrolled participants were epidemiologically monitored for six months regarding diet, drug consumption, and other factors that could affect the elemental profile.
Participants were vaccinated with two doses of the BNT162b2 mRNA vaccine (primary and secondary vaccination, three weeks apart). After fasting overnight, a peripheral whole blood specimen (5 mL) was collected by classical venipuncture in the morning. All blood specimens were allowed to coagulate spontaneously, after which they were centrifuged (3000 × g). The resulting serum was separated from the cell fraction into Eppendorf tubes. This protocol was applied six times, starting from the pre-vaccination baseline to six months after the first vaccination point. Blood was collected at six-time points ( Fig. 1.): point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination, and point 6, six months after primary vaccination. Sera were frozen at −80 °C until analysis.
Fig. 1.
Sample collection timeline.
The Ethics Committee approved the study led by the Institute for Oncology and Radiology of Serbia (No. 899–01), and all participants voluntarily agreed to participate in this study.
2.2. Production and purification of the recombinant RBD of SARS-CoV-2
According to the previously published procedure, RBD of the SARS-CoV-2 Spike glycoprotein (UniProt P0DTC2) was expressed in the HEK-293 T cell line [17]. In brief, on day seven post-transfection, the cells were centrifuged (4000 ×g for 10 min), and the supernatant was collected. Then, the supernatant containing the recombinant protein was loaded on an immobilized metal affinity chromatography (IMAC) column (HiTrap FF, 1 mL, G.E. Healthcare, Little Chalfont, U.K.) on Akta Purifier (G.E. Healthcare, Little Chalfont, U.K.). The buffer consisting of 25 mM of Na2HPO4, 25 mM of NaH2PO4, 500 mM of NaCl, and five mM of imidazole was used for the equilibration of the column. Subsequently, elution was carried out using a concentration gradient with a buffer composed of 25 mM of Na2HPO4, 25 mM of NaH2PO4, 500 mM of NaCl, and 500 mM of imidazole. The pH of the buffers was 7.4. Finally, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was employed to analyze the purity of obtained fractions.
2.3. Quantification of circulating SARS-CoV-2 RBD-specific IgG antibodies
Briefly, for antibody quantification, the RBD of the Spike glycoprotein produced in HEK-293 T cells was used to coat flat-bottom 96-well plates (Thermo Scientific NUNC-MaxiSorp, USA) at a final concentration of 10 μg/mL (50 μL/well) in coating buffer (15 mM Na2CO3/35 mM NaHCO3, pH 9.5) at four °C overnight. The plates were then washed with TBS-T (20 mM TRIS, pH 7.4,0.14 M NaCl, 2.7 mM KCI, 0.05% vol/vol Tween 20) and blocked with 1% BSA in TBS-T for two h at 37 °C. Sera were diluted (1:1000 in TBS-T containing 0.1% BSA), added to the wells (50 μL/well), and incubated at 37 °C for one h. The plates were then washed five times with TBS-T. For specific IgG determination, goat anti-Human-IgG-AP conjugated antibody (Novus Biologicals, USA) was diluted 1:1000 in blocking solution and added to the wells (50 μL/well). After incubation for one h at 37 °C, plates were washed five times with TBS-T. The reaction was visualized using p-nitrophenyl phosphate [Sigma–Aldrich, St. Luis, USA] (1.0 mg /mL, 50 μL/well) in 100 mM diethanolamine buffer pH 9.6, and the plate was incubated at 37 °C for 0.5 h. Color development was stopped with 3 M NaOH (50 μL/well). ODs were read by a microplate reader (LKB Vertriebs GmbH, Austria) at 405 nm (minus the OD at 620 nm).
Seropositivity was established using the generated standard curve described in the published procedure [18]. In brief, six standard solutions were prepared by serial dilution based on two COVID-19 patient sera. The undiluted serum and five consecutive two-fold serial dilutions of the serum made in TBS with 0.5% BSA were stored in small aliquots at −80 ⁰C as standard solutions S1 to S6. Upon thawing each solution was diluted 1:10 to obtain the final standard solution (S1 = 1:10; S2 = 1:20; S3 = 1:40; S4 = 1:80; S5 = 1:160 and S6 = 1:320). These solutions were further diluted 1:101 as part of the ELISA assay, just as any other sample to be tested. 4-parameter logistic (4PL) regression analysis (software: GraphPad Prism 8) of the standard curve was used to calculate arbitrary units/milliliter (AU/mL) for the positive samples. Seropositivity was defined as 50 AU/mL or higher.
2.4. Analysis of trace elements
All chemicals used were of high-grade purity and supplied by Merck (Darmstadt, Germany). The purity of concentrated high-grade nitric acid was improved using a Berghof-acid apparatus (BSB-939-IR, Germany). Milli-Q water (resistance of 18.2 MΩ) was prepared using a Milli Q Plus system (Merck, Germany). Glass and plastic materials were immersed in 10% nitric acid for at least 24 h and thoroughly washed with Milli-Q water before use. Medium standard solutions were prepared from stock solutions containing test elements. The standard stock solutions used (10 mg/L) were supplied by VHG Labs (Manchester, USA). Certified reference materials (CRMs) of human blood were provided by Seronorm (Sero AS, Norway) (SERO210105 Level-1 and SERO210205 Level-2). An internal standard solution (100 μg/mL of Sc and 20 μg/mL of In) was supplied by VHG Labs (Manchester, USA).
Sera were diluted 10-fold with an aqueous solution containing 0.01% nitric acid, 0.1% Triton X-100, and 1.5% n-butanol. Four elements (Mn, Cu, Zn, and Se) were quantified using inductively coupled plasma-quadrupole-mass spectrometry, ICP-Q-MS (iCAP Qc, Thermo Scientific, U.K.) in the collision mode. Recorded recoveries for selected isotopes (55Mn, 65Cu, 66Zn, 82Se) ranged from 93.4% to 115%. Also, excellent agreement in detecting the selected isotopes was recorded using the standard addition method (ranging from 96.6% to 103%).
2.5. Data analysis
Parameters of descriptive statistics, Shapiro-Wilk's test, One-way ANOVA, and Pearson's correlation test were performed at a significance level of P = 0.05 using SPSS statistical software (IBM SPSS Statistics v.20). Since the data were normally distributed, the results are presented as the mean ± standard deviation of three independent replicates. Using GraphPad Prism v.5.0 (GraphPad, CA, USA), figures were drawn.
3. Results and discussion
3.1. Expression of the recombinant SARS-CoV-2 RBD in mammalian cells
The SARS-CoV-2 RBD (222 amino acids) is a key part of a virus located on the SARS-CoV-2 Spike glycoprotein (1273 amino acids), which allows the virus to attach to the angiotensin-converting enzyme 2 (ACE2) receptor to entry into host cells. SARS-CoV-2 RBD-specific polyclonal antibodies cross-neutralize SARS-CoV-2 pseudovirus infection [19], [20].
Human embryonic kidney (HEK)− 293 T cells were transiently transfected to produce recombinant RBD. On day seven, after transfection, the cells were centrifuged, and the obtained protein fraction was purified by IMAC. Purified protein was confirmed by SDS-PAGE analysis ( Fig. 2).
Fig. 2.
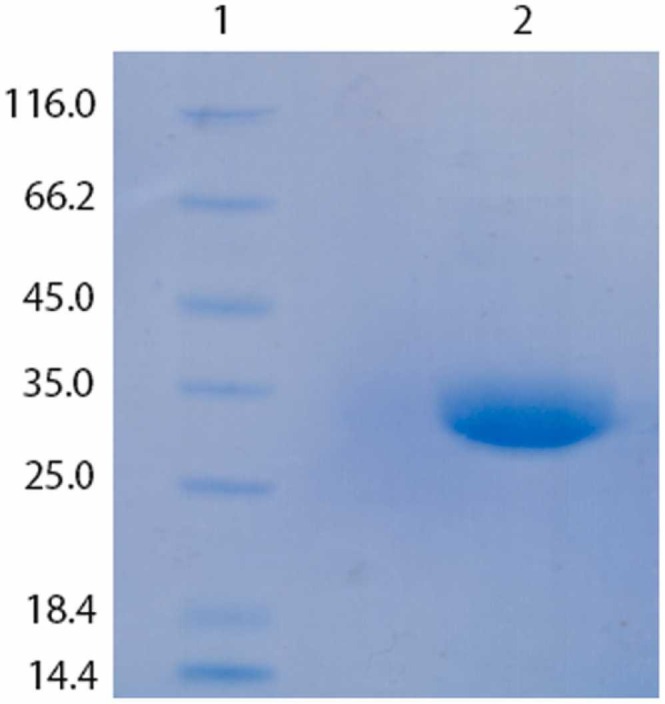
SDS-PAGE analysis of the purified recombinant RBD: 1) molecular markers (kDa), 2) purified recombinant SARS-CoV-2 RBD produced in HEK-293 T cells.
3.2. Quantification of SARS-CoV-2 RBD-specific IgG antibodies
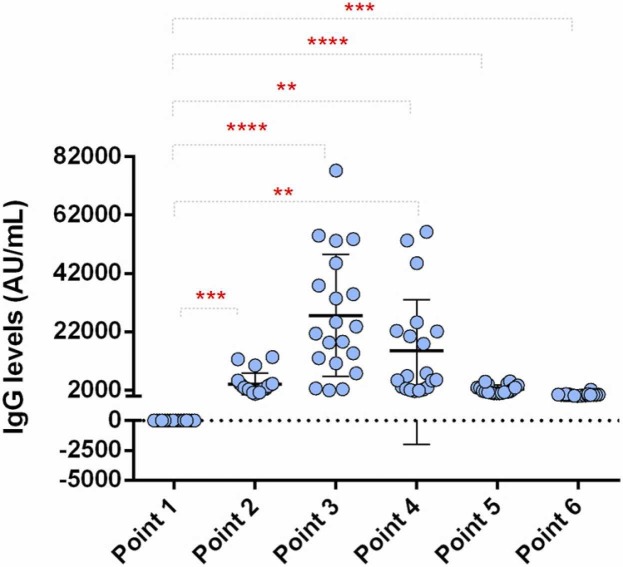
The levels of specific anti-RBD IgG antibodies were analyzed in the sera of the women who entered the study ( Fig. 3). A significant rise in IgG (P ≤ 0.001) was already observed 14 days after the first vaccine dose with a value of 4133 ± 3668 AU/mL. The sera levels of anti-RBD IgG were even higher one-month post-vaccination reaching the maximum of 155,353 ± 17,514 AU/mL. Then a further decrease was detected at six months when the final value measured was 479 ± 429 AU/mL.
Fig. 3.
Levels of SARS-CoV-2 RBD-specific IgG antibodies in the time frame of 6 months: point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination, and point 6, six months after primary vaccination. Statistically significant differences were found between all points (presented with asterisks, ** P ≤ 0.01, *** P ≤ 0.001 **** P ≤ 0.0001).
The duration of response to the SARS-CoV-2 BNT162b2 vaccine (Pfizer-BioNTech) in adults in Serbia has yet to be determined. Among the study participants who received two doses of BNT162b2, a decline in the specific IgG immune response was recorded six months after the first dose. Two doses of the BNT162b2 vaccine in adults were previously associated with remarkably increased IgG titers, especially two weeks after the first dose [1], [3].
Anti-RBD IgG antibody titers were seropositive before and six months after the first dose of BNT162b2 in individuals tested. Although evidence suggests that IgG response correlates with disease protection, cellular immunity also plays an essential role in protecting against SARS-CoV-2 [21].
3.3. Essential trace elements
Maintaining an adequate micronutrient balance should strengthen the host's immune homeostasis and protection against viral infections. ETEs, primarily manganese (Mn), copper (Cu), zinc (Zn), and selenium (Se), have immunomodulatory functions and thus influence the susceptibility to the course and the outcome of a variety of viral infections [12], [22], [23], [24].
3.4. Manganese (Mn)
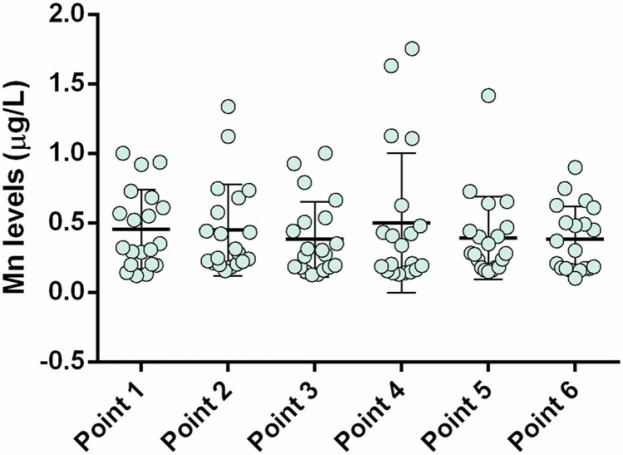
Mean sera Mn levels are given in Fig. 4 and were within the reference levels (0.5 – 1.3 µg/L) [25]. Compared to pre-immunization, no statistically significant changes in serum Mn levels were recorded before and after vaccination, which could be explained by Mn having a primary role in mitochondrial rather than nuclear metabolism [26], [27]. All calculated Mn level correlations with specific anti-SARS-CoV-2 IgG had negative signs immediately after vaccination and up until three months post-vaccination and were of strong intensity (> −0.70). Furthermore, the strength of the negative correlation was lost six months after the first dose and became insignificant (Supplementary Table 1). These findings need to be further elucidated to understand the mechanism of these effects.
Fig. 4.
Mn levels in participants' sera (6-month follow-up): point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination; and; point 6, six months after primary vaccination.
3.5. Copper (Cu)
Copper is an important trace element in many metalloproteins and in the mitochondrial metabolic cascades for energy production to supply different processes, including those of the immune system [28]. The acute-phase reactant ceruloplasmin is a Cu-containing enzyme in plasma whose production strongly increases during inflammation due to the necessity of scavenging oxygen radicals released by immune cells [29]. Copper deficiency results in decreased antibody-producing plasma cell response and increased susceptibility to various microorganisms [24].
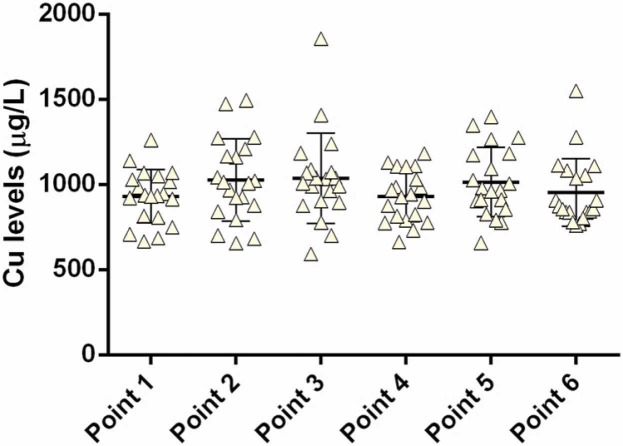
Sera Cu levels are given in Fig. 5, and the mean value before immunization (931 ± 157 µg/L) was in the reference range (700–1300 µg/L) [25]. No significant changes in serum Cu levels were recorded before and after vaccination. As Cu and Zn are antagonists during the absorption process in the gastrointestinal tract (GIT), higher Cu levels in the GIT could be expected to reduce Zn levels [9]. However, the opposite is also true, as zinc intake in high doses (> 150 mg/day) can result in Cu deficiency in healthy individuals [30].
Fig. 5.
Copper levels in the sera (6-month follow-up): point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination; and point 6, six months after primary vaccination.
A negative coefficient between Cu levels and specific anti-RBD IgG antibodies (r = −0.75) was observed before the vaccination. In contrast, post-vaccination analysis at point two (14 days after primary vaccination) showed a positive coefficient of correlation (r = 0.81) between sera Cu levels and specific anti-RBD IgG antibodies. Another interesting finding was detected two months after primary vaccination when the highest correlation coefficient of Cu with serum-specific IgG levels was recorded (r = 0.83) (Supplementary Table 1).
As an ETE, Cu is needed in the body to protect DNA from oxidative stress [31]. Therefore, higher Cu intake (7.8 mg/day) caused greater increases in ceruloplasmin activity, benzylamine oxidase, and SOD compared to lower Cu intake (1.6 mg/day) [32], [33], [34]. Nevertheless, as no Cu deficiency was observed, there is no need for supplemental intake of this trace element, considering the induced immune response to the mRNA vaccine.
3.6. Zinc (Zn)
Zinc is an important trace element that has a critical role in the body. Zn can protect cells from reactive oxygen species (ROS) [34] and is involved in controlling the common cold via its inhibitory mechanism in viral replication [35] and attachment to the nasopharyngeal mucous membrane. There is evidence that Zn decreases the duration of the common cold by about 1.65 days on average [35]. In vitro studies showed that some respiratory pathogens like rhinovirus, respiratory syncytial virus, and SARS-CoV-2 were modified by Zn [36]. Development and maintenance of both the innate and adaptive immune systems require this trace element. The proliferation and function of natural killer (NK) cells, macrophages, neutrophils, and T and B cells depend on optimal Zn amounts [37]. Zn deficiency causes dysfunction in the activation and maturation of lymphocytes, impairment in cellular communication by cytokines, and reduced innate and T- dependent antigen immunity [12], [14], [38], [39].
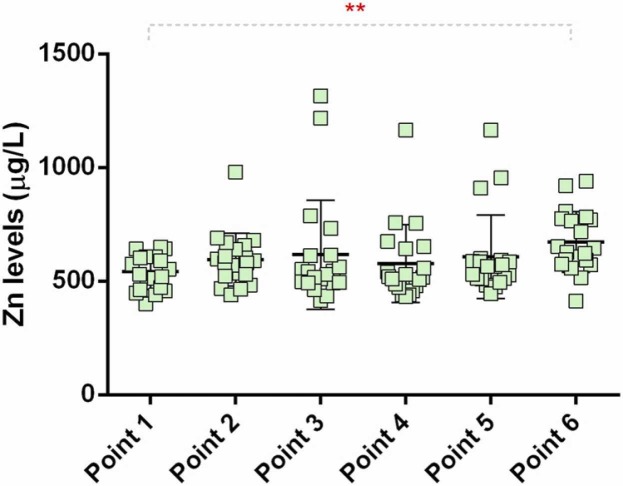
A serum Zn level of 800–1200 µg/L is considered a normal range, < 800 µg/L is considered moderate, while < 400 µg/L is regarded as a clinical Zn deficiency [25]. Serum Zn levels in the analyzed population are shown in Fig. 6 and were found before immunization to be below (544 ± 79 µg/L) the normal reference range (800–1200 µg/L) [25]. This finding indicates moderate Zn deficiency in the study participants, which is in line with the previously reported deficiency of this trace metal in the adult Serbian population [40].
Fig. 6.
Zinc levels in the sera (6-month follow-up); point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination; and; point 6, six months after primary vaccination; * significant difference in sera levels before and six months after the first vaccination; (*P < 0.05).
We noticed that the correlation coefficient of Zn behaved similarly to that of Cu, with a remarkable change (from minus to plus) immediately after immunization. An increase in serum Zn level two weeks after the first dose of the Vaccine indicated the release of this ETE from its storage (Fig. 6). The results suggest a positive contribution of Zn to the synthesis of specific anti-RBD IgG antibodies.
3.7. Selenium (Se)
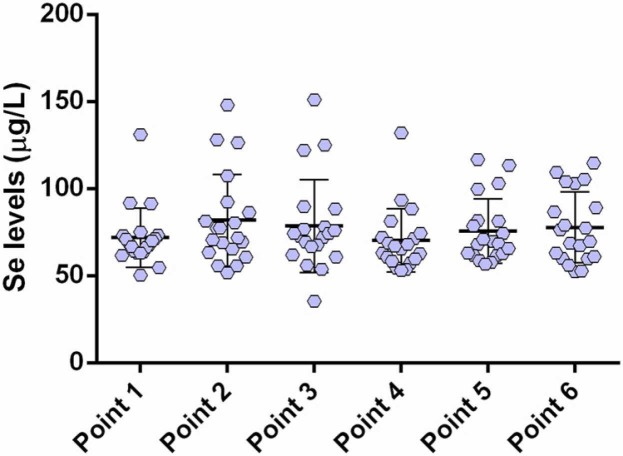
Selenium deficiency is known to negatively impact the host's immune system [41], [42], [43], [44]. Selenium deficiency causes an increase in viral replication and mutation and contributes to pathogenicity and mortality [45]. It has been demonstrated that high Se intake (50–100 μg/day) causes better, especially cellular, immune responses in adults [46]. In this regard, Demicran et al. investigated the possible association of selenium sera levels with the immune response following vaccination with BNT162b2 anti-SARS-CoV-2 vaccine in study participants that were followed for up to 24 weeks, while blood samples were taken at the first and second dose, and at 3 and 21 weeks after the second dose [47]. Serum SARS-CoV-2 IgG titers were significantly correlated at all the time points. In addition, participants subjected to the supplemental intake of Se showed higher sera levels. Our findings suggest low to moderate correlations of selenium levels with specific IgG, in accordance with the results of the previously mentioned study.
We observed no significant differences in serum Se levels between the time points over the six-month observation period ( Fig. 7). This is in accordance with the study of Demicran et al., where there was no significant association of Se status with the humoral immune response to BNT162b2 Vaccine was observed.
Fig. 7.
Selenium levels in the sera (6-month follow-up): point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination; and; point 6, six months after primary vaccination.
The role of Se in immunity has been recognized [15], [42], [46]. Selenium appears important for initiating innate immunity and migration of neutrophils into tissues and triggering inflammation [41]. This ETE is a component of glutathione peroxidase, which inactivates ROS production, prevents its release, and cellular damage. Selenium deficiencies have been associated with reduced neutrophil migration and reduced B-cell response and antibody production. Moreover, after supplementation, higher Se levels contribute to humoral and cell-mediated immune responses [12], [15], [16].
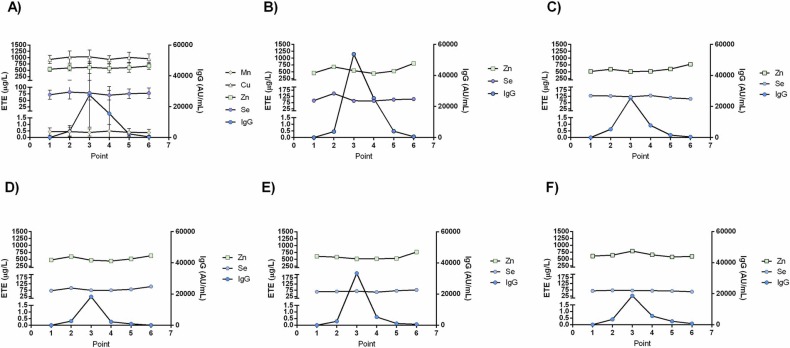
However, our study limitations include the small sample size, short follow-up, and lack of cellular immunity testing. In this paper, we correlated the titer of IgG antibodies produced against RBD, a relatively small part of the Spike glycoprotein (hence a relatively small repertoire of epitopes), the only produced structural antigen upon vaccination with the SARS-CoV-2 BNT162b2 vaccine. In such a study design, only specific IgG production as a component of the humoral immune response was correlated with the concentration of ETEs in the serum. Still, induction of adaptive immunity by the vaccination itself is a much more complex process. The production of specific antibodies necessitates a complex interplay and the activation of antigen-specific B and T cells, a complex network of cytokines that this study has not analyzed. Thus, both humoral and cellular immunity should be evaluated to understand the effects of vaccination and ETEs better. Fig. 8.
Fig. 8.
ETE and specific anti-RBD-IgG antibody levels in the serum of cohorts; point 1, pre-vaccination (baseline); point 2, 14 days after primary vaccination; point 3, one month after primary vaccination; point 4, two months after primary vaccination; point 5, three months after primary vaccination; and; point 6, six months after primary vaccination; A) ETE and anti-RBD IgG serum statuses of the cohort in the examined time, presented as mean ± SD B-F) levels of Zn, Se and anti-RBD IgG antibodies in the serum of individual volunteers (5 of 20).
4. Conclusions
The deficiency of ETEs is an important public health concern in the world. Nevertheless, the molecular mechanisms of many ETEs related to innate and adaptive immunity remain unknown and need to be further explored and elucidated. This is the first longitudinal study highlighting the ETE levels in sera pre- and post-SARS-CoV-2 vaccination with the mRNA vaccine. Only Zn levels in sera differed before and six months after the first immunization. However, the major hallmark of this finding refers to the shift of the correlation sign immediately after vaccination. These alterations are most reflected in both Cu and Zn levels with specific IgG levels (post-vaccinal effects), which could highlight their impact on IgG production. Also, we observed low to moderate correlations of Se status with the humoral immune response to the BNT162b2 vaccine in this study. This study highlighted Cu and Zn status in the adaptive immune response in vaccinated participants with the anti-SARS-CoV-2 BNT162b2 mRNA vaccine. Despite the small sample size representing a limiting factor, longitudinal analysis with several time points after vaccination was conducted. Our study demonstrated that Zn deficiency is observed in adult women in Serbia. Yet, the expected humoral immune response was observed, and no gross alterations in ETE are detectable in healthy vaccinated women with time after vaccination.
Funding
This research was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Contract Numbers (451–03–68/2022–14/200168, 451–03–68/2022–14/200288, 451–03–68/2022–14/200043).
Ethics approval
The procedures in this study were approved by the Ethics Committee of the Institute for Oncology and Radiology of Serbia (No. 899–01) and followed the Helsinki Declaration of 1976 and its further amendments. All participants were interviewed by experienced medical staff and voluntarily signed informed consent.
CRediT authorship contribution statement
A.N., A.S., D.M., and M.G.J. contributed to the design of the study and implementation of the research, the analysis of the results, and the writing of the manuscript. A.N. performed the experiments and wrote the manuscript with support from A.S. and M.G.J. A.S., and J.J. carried out the ICP-MS statistical analysis and processed the experimental data. M.Č. and A.S. contributed to sample preparation, data analysis, standard curve generation, and the interpretation of the results. All authors provided critical feedback and helped shape the research, analysis, and manuscript. M.G.J. supervised the project.
Author statement
This material has not been published previously and is not under consideration for publication elsewhere. Also, we have no conflicts of interest to disclose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jtemb.2022.127079.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Efrati S., et al. Safety and humoral responses to BNT162b2 mRNA vaccination of SARS-CoV-2 previously infected and naive populations. Sci. Rep. 2021;vol. 11(1) doi: 10.1038/s41598-021-96129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center, “COVID-19 Map,” Jul. 03, 2022. https://coronavirus.jhu.edu/map.html (accessed July 03, 2022).
- 3.Gattinger P., et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy: Eur. J. Allergy Clin. Immunol. 2022;vol. 77(1):230–242. doi: 10.1111/all.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razzaque M.S. COVID-19 pandemic: can zinc supplementation provide an additional shield against the infection? Comput. Struct. Biotechnol. J. 2021;19:1371–1378. doi: 10.1016/j.csbj.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang P., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat. Med. . 2021;vol. 27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 6.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;vol. 383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U., et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. 2020;vol. 18(6) doi: 10.1101/2020.12.09.20245175. [DOI] [Google Scholar]
- 8.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;vol. 383(27):2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang N., et al. Effect of different adjuvants on immune responses elicited by protein-based subunit vaccines against SARS-CoV-2 and its delta variant. Viruses. 2022;vol. 14(3) doi: 10.3390/v14030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson J., Murugesan K., Banaei N., Liu A. Interferon-gamma release assay testing to assess COVID-19 vaccination response in a SARS-CoV-2 seronegative patient on rituximab: a case report. Int. J. Infect. Dis. 2021;vol. 110:229–231. doi: 10.1016/j.ijid.2021.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurteva E., et al. Interferon-gamma release assays outcomes in healthy subjects following BNT162b2 mRNA COVID-19 vaccination. Rheuma Int. 2022;vol. 42(3):449–456. doi: 10.1007/s00296-022-05091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das R., et al. Evaluating association of vaccine response to low serum zinc and vitamin D levels in children of a birth cohort study in Dhaka. Vaccine. 2021;vol. 39(1):59–67. doi: 10.1016/j.vaccine.2020.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopandić Z., et al. Igm and igg immunoreactivity of sars-cov-2 recombinant m protein. Int. J. Mol. Sci. 2021;vol. 22(9) doi: 10.3390/ijms22094951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razzaque M.S. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance. Tohoku J. Exp. Med. 2020;251:175–181. doi: 10.1620/tjem.251.175. Tohoku University Medical Press. [DOI] [PubMed] [Google Scholar]
- 15.Razeghi Jahromi S., et al. The correlation between serum selenium, zinc, and COVID-19 severity: an observational study. BMC Infect. Dis. 2021;vol. 21(1) doi: 10.1186/s12879-021-06617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verschelden G., et al. Plasma zinc status and hyperinflammatory syndrome in hospitalized COVID-19 patients: an observational study. Int Immunopharmacol. 2021;vol. 100 doi: 10.1016/j.intimp.2021.108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Cordero J., et al. Recombinant protein expression and purification of n, s1, and rbd of sars-cov-2 from mammalian cells and their potential applications. Diagnostics. 2021;vol. 11(10) doi: 10.3390/diagnostics11101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krähling V., et al. Development and characterization of an indirect ELISA to detect SARS-CoV-2 spike protein-specific antibodies. J. Immunol. Methods. 2021;vol. 490 doi: 10.1016/j.jim.2021.112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes C.O., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;vol. 588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan J., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;vol. 581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 21.Guo L., et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet Microbe. 2022;vol. 3(5):e348–e356. doi: 10.1016/S2666-5247(22)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taheri M., Bahrami A., Habibi P., Nouri F. A review on the serum electrolytes and trace elements role in the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2011 doi: 10.1007/s12011-020-02377-4/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taheri M., Bahrami A., Habibi P., Nouri F. A review on the serum electrolytes and trace elements role in the pathophysiology of COVID-19. Biol. Trace Elem. Res. 2011 doi: 10.1007/s12011-020-02377-4/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharmalingam K., et al. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021;36(4):416–426. doi: 10.1007/s12291-021-00961-6. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nader Rifai. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. sixth ed. Elsevier; 2018. [Google Scholar]
- 26.Li L., Yang X. The essential element manganese, oxidative stress, and metabolic diseases: links and Interactions. Oxid. Med Cell Longev. 2018;vol. 2018:7580707. doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunter T.E. In: Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Collins J.F., editor. Academic Press; Boston: 2017. Chapter 32 - manganese and mitochondrial function; pp. 389–396. [DOI] [Google Scholar]
- 28.Grubman A., White A.R. In: Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. Collins J.F., editor. Academic Press; Boston: 2017. Copper and molecular aspects of cell signaling; pp. 85–99. [DOI] [Google Scholar]
- 29.Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;vol. 12(8):1–12. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis M.S., et al. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am. J. Clin. Pathol. 2005;vol. 123(1):125–131. doi: 10.1309/V6GVYW2QTYD5C5PJ. [DOI] [PubMed] [Google Scholar]
- 31.Uriu-Adams J.Y., Keen C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005;vol. 26(4):268–298. doi: 10.1016/j.mam.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Turnlund J.R., et al. "Long-term high copper intake: effects on indexes of copper status, antioxidant status. Immune Funct. Young Men. 2004:1–3. doi: 10.1093/ajcn/79.6.1037. [Online]. Available: https://academic.oup.com/ajcn/article/79/6/1037/4690211. [DOI] [PubMed] [Google Scholar]
- 33.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;vol. 12(5):1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M.T., Idid S.Z. Can Zn be a critical element in COVID-19 treatment. Biol. Trace Elem. Res. 2021;vol. 199(2):550–558. doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Z., Burwinkel M., Palissa C., Ephraim E., Schmidt M.F.G. Antiviral activity of zinc salts against transmissible gastroenteritis virus in vitro. Vet. Microbiol. 2012;vol. 160(3–4):468–472. doi: 10.1016/j.vetmic.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams K.K., Baker W.L., Sobieraj D.M. Myth busters: dietary supplements and COVID-19. Ann. Pharm. 2020;vol. 54(8):820–826. doi: 10.1177/1060028020928052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iddir M., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;vol. 12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedjimi B. Can trace element supplementations (Cu, Se, and Zn) enhance human immunity against COVID-19 and its new variants. Beni-Suef Univers. J. Basic Appl. Sci. 2021 doi: 10.1186/s43088-021-00123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng H.L., Yang Q., Yuan P., Wang X., Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;vol. 35(3) doi: 10.1096/fj.202002346RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagodić J., et al. Possible zinc deficiency in the Serbian population: examination of body fluids, whole blood and solid tissues. Environ. Sci. Pollut. Res. 2021;vol. 28:47439–47446. doi: 10.1007/s11356-021-14013-2/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;vol. 143(3):1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;vol. 11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma X., Bi S., Wang Y., Chi X., Hu S. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult. Sci. 2019;vol. 98(9):3548–3556. doi: 10.3382/ps/pez207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med Virol. 2020;vol. 92(5):479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;vol. 11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broome C.S., et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004;vol. 80(1):154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 47.Demircan K., et al. Humoral immune response to COVID-19 mRNA vaccination in relation to selenium status. Redox Biol. 2022;vol. 50 doi: 10.1016/j.redox.2022.102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material