Abstract
Gastric outlet obstruction (GOO) is a relatively common condition in which mechanical obstruction of the pylorus, distal stomach, or duodenum causes severe symptoms such as nausea, vomiting, abdominal pain, and early satiety. Its etiology includes both benign and malignant disorders. Currently, GOO has many treatment options, including initial conservative therapeutic protocols and more invasive procedures, such as surgical gastroenterostomy, stent placement and, the most recently implemented procedure, endoscopic ultrasound-guided gastroenterostomy (EUS-GE). Each procedure has its merits, with surgery often prevailing in patients with longer life expectancy and stents being used most often in patients with malignant gastric outlet stenosis. The newly developed EUS-GE combines the immediate effect of stents and the long-term efficacy of gastroenterostomy. However, this novel method is a technically demanding process that requires expert experience and special facilities. Thus, the true clinical effectiveness, as well as the duration of the effects of EUS-GE, still need to be determined.
Keywords: Gastric outlet obstruction, Gastric outlet stenosis, Gastroenterostomy, Self expandable metal stent, Endoscopic ultrasound-guided gastroenterostomy
INTRODUCTION
Gastric outlet obstruction (GOO), otherwise called pyloric obstruction or stenosis, is a debilitating condition that results from the mechanical compression and blockage of the distal stomach, pyloric antrum, or duodenum. It can result from both benign and malignant conditions with the most common causes including peptic ulcer and periampullary and gastric cancer respectively.1
Though it usually presents at an advanced stage in various progressive diseases, it adversely affects the patients’ quality of life, due to its related symptoms. Patients present with clinical manifestations, depending on the extent of stenosis and the duration of gastrointestinal discontinuity, starting with pain and vomiting that will lead to weight loss and deterioration of the general nutritional and hydration status.1,2
Treatment strategies range from open or laparoscopic surgery to various endoscopic approaches that need to be selected only after thorough inclusion of several criteria in a personalized treatment plan, as numerous alternatives may prove to be useful for the management of this complex entity. The traditional surgical bypass option by creating a gastrojejunostomy (GJ) is gradually being abandoned due to the risk of short-term complications, although it is still applied in cases of a relatively prolonged life expectancy, where a durable therapy is required. The endoscopic approach, which is currently under rapid development includes the placement of self-expandable metallic stents and endoscopic ultrasound-guided gastroenterostomy (EUS-GE), using lumen-apposing metal stents (LAMS). Each procedure presents different benefits and limitations; thus, they should be selected only after careful consideration of the patient’s needs and characteristics. In addition, both necessitate the fulfillment of a demanding learning curve on the part of the physician in order to achieve maximum technical performance.3-5
ETIOLOGY–EPIDEMIOLOGY
Upper gastrointestinal tract diseases that can be complicated with GOO comprise both benign and malignant disorders. In the recent past, gastric or duodenal ulcers used to be the prevailing etiology for GOO.6 Although they still account for most cases with a benign cause, their incidence has significantly decreased due to extensive eradication of Helicobacter pylori infection and the equally extensive use of proton pump inhibitors. Other non-neoplastic causes of GOO include strictures from chronic pancreatitis, nonsteroidal anti-inflammatory drugs or Crohn’s disease, caustic ingestion, surgical anastomosis scarring, or adhesions.7,8 Among the malignant causes of GOO, gastric cancer and periampullary tumors (pancreatic adenocarcinoma, cholangiocarcinoma, cancer of the ampulla of Vater, and duodenal adenocarcinoma) predominate. Other neoplasms have also been reported to cause GOO, including hepatocellular carcinoma, lymphoma, metastases to the duodenum or jejunum, and lymph node adenopathy.2,9,10
CLINICAL MANIFESTATIONS
Patients with GOO generally present with progressive symptoms like nausea, intractable vomiting, regurgitation, and pain. Vomiting may initially manifest following intake of solid meals and then gradually progress to solid intolerance due to the inability of emptying of the stomach. Epigastric pain, abdominal distention, and early satiety should be of primary concern since they can lead to weight loss, dehydration, electrolyte disturbances, and hypoalbuminemia due to malnutrition.2 Overall, this combination of symptoms experienced by patients’ needs to be investigated in-depth so that they are not mistakenly attributed to the primary condition’s manifestations or complications of treatment (e.g., radiation or chemotherapy).1,2
DIAGNOSIS
Auscultation of the upper abdomen in patients suffering from GOO can reveal a characteristic succussion splash due to undigested food retained in the stomach for more than 3 hours after its consumption. This sign may also be heard, even without the use of a stethoscope. Other findings on clinical examination may include cachexia, volume depletion, and clinical signs suggestive of the underlying disease, for example, a palpable mass or lymph nodes.1
Laboratory tests reveal abnormalities deriving from insufficient oral intake and emesis like hyperchloremic metabolic alkalosis and hypokalemia, and hypoalbuminemia. Imaging studies can also be of value. An initial abdominal X-ray can show an enlarged gastric bubble, a dilated proximal duodenum, and limited presence of air in the small bowel. Further contrast studies using barium or water-soluble contrast and computed tomography or magnetic resonance imaging are helpful to unveil the anatomic obstruction site and severity. However, the test of choice for the diagnosis of GOO is upper gastrointestinal endoscopy which offers a direct visual assessment of the stricture and can allow tissue sampling to help for a histological differentiation between benign and malignant blockages. Finally, in patients with a malignant cause of GOO, endoscopic ultrasonography can also be a valuable tool in tissue sampling using fine-needle-aspiration of otherwise inaccessible tumors, thus facilitating diagnosis, as well as locoregional staging.1,2,7
MANAGEMENT
Initial management of patients with a confirmed diagnosis of GOO requires correction of their general status, initially avoiding ingestion of solid foods in case the patient cannot ingest them, administration of intravenous fluids with the aim of correcting an electrolyte imbalance, gastric decompression by inserting a nasogastric tube and proton pump inhibitors to decrease the secretions of the stomach. Afterwards, targeted therapy depending on the etiology of the obstruction is indicated and may include management of peptic ulcer disease (by eradicating respective risk factors, such as H. pylori infection and/or cessation of nonsteroidal anti-inflammatory drugs) and radiotherapy or chemotherapy of malignant conditions.2 Conservative management might ultimately lead to more invasive options, as patients who initially respond well to nonoperative management, might relapse and may ultimately require surgery.11
Traditional therapeutic options for GOO due to peptic ulcer disease that persist despite conservative treatment as well as endoscopic dilation include mainly surgical therapeutic options (open or laparoscopic), such as vagotomy and pyloroplasty.2 Other treatment modalities are mainly palliative aiming to achieve the patients’ symptomatic relief and to ameliorate the quality of life of these patients, whose life expectancy is relatively short, ranging from months to a few years. Such options include the following options.
1. Surgical gastrojejunostomy
Surgical gastrojejunostomy (SGJ) is a technique that was developed to create a bypass in patients with mostly malignant GOO, in order to maintain adequate oral nutrition. It is performed under general anesthesia, either as an open procedure or laparoscopically. It is most commonly performed in patients with tumors deemed as unresectable, who may well benefit from a long-lasting palliative gastric anastomosis, in case they are expected to have a life expectancy of several months, which should be long enough to overcome the short-term morbidity and mortality burden of the surgical procedure.9 The laparoscopic version of the technique has been associated with improved morbidity and mortality rates, as well as improved outcomes (including shorter hospitalization, shorter intervals to resume eating, reduced intraoperative hemorrhage and reduced needs for postoperative opiates).12 There are two variants of the laparoscopic technique, that is, the antecolic and retrocolic method, with the former being the most commonly used.12 It should be noted that for patients in whom the aforementioned surgical therapies are not an option (especially those at risk of not tolerating such an approach) or have failed, an alternative could be to place a percutaneous gastrostomy for gastric decompression, combined with subsequent placement of a jejunal feeding-tube.10,13-16
2. Self-expandable metal stents
A less invasive intervention for the palliation of GOO of malignant origin is endoscopic enteral stenting. The technique is performed using a flexible gastroscope that should be inserted to the obstruction site, which is usually evaluated by a combination of endoscopy and/or injection of contrast proximal to and immediately distal to the stricture, in order to assess its length and endoscopic characteristics, including the degree of luminal obstruction it causes; occasionally, a computed tomography can also be helpful not only in the pre-interventional assessment of the stricture, but also to evaluate the patients status overall (e.g., presence of metastases, further obstructions downstream).12 Since the first report of stent placement for the management of GOO, a variety of stent types has become available.12 Among them, self-expandable metal stents (SEMS) are those that seem to be the most effective choice to recanalize a significantly narrowed lumen. Designs of SEMS that are nowadays available include uncovered (USEMS), covered (CSEMS), or partially covered (PCSEMS) designs, the latter two with a special coating. The selection of SEMS that suits best in a specific case should be personalized, based on the stent’s technical features, taking into account potential complications, in combination with the specific characteristics of the patient’s underlying cause of GOO.1,7,14,16-18 SEMS are mostly available in a compressed manner on a delivery device, which is then passed through the working channel of an endoscope over a wire that has already been passed through the stricture under fluoroscopic guidance; stent deployment over the stricture is the following step of the procedure (also performed under endoscopic and/or fluoroscopic control); finally, the stent self-expands in the following 24 to 48 hours in order to reach its maximum diameter (Figs 1, 2). Several studies have so far compared the different types of SEMS currently available. Studies suggest that all types of SEMS are comparable in terms of technical success (ranging between 89% and 98%)7 and clinical success (usually defined as the relief of obstructive symptoms and improvement of oral intake, ranging between 63% and 93%),7,12,18-20 as well as overall adverse events (AE).7,21 The relatively wide clinical success rates published seem to be influenced by definitions of terms used in the relative publications, which are not always homogenous, thus causing confusion;21 In a recently published systematic review from the Netherlands that included exclusively prospective data reporting on clinical success of SEMS placement for malignant GOO (19 prospective studies in the period 2009 to 2016, including 1,281 patients), overall pooled technical success rate was 97.3% whereas clinical success rates reached as high as 85.7%.17
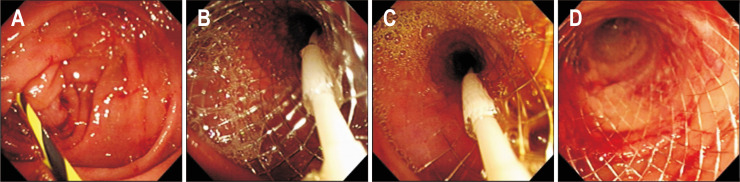
Fig. 1.
Placement of a self-expandable metal stent (SEMS) under endoscopic view. Note the placement of a guidewire over the obstruction (A), gradual deployment of the stent over the delivery system (B, C), as well as the SEMS in situ, gradually expanding to its full size (D).
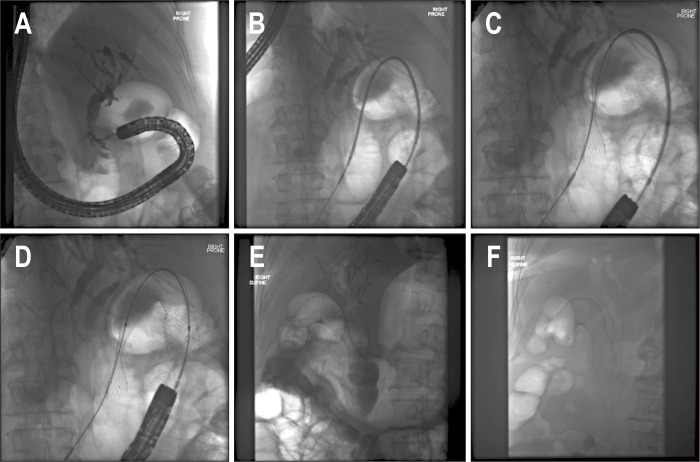
Fig. 2.
Placement of a self-expandable metal stent under fluoroscopic assistance. Note a biliary stent already placed, with the biliary tracts opacified (A), placement of the delivery system over the obstruction (B) and gradual opening of the stent (C, D), which was finally placed (E). Four days later the stent was finally expanded to its full size (F).
The most notable differences between the several SEMS types that are currently used can be explained by the dynamics of the obstruction and the migration rate. Migration is more commonly seen after placement of CSEMS or PCSEMS, as the cover prevents effective anchorage that is more likely to possible when using USEMS; on the other hand, the benefit of reduced migration of USEMS should be balanced by the increased risk of stent occlusion (and thus recurrence of symptoms of the obstruction) in USEMS due to tumor ingrowth or overgrowth, or even mucosal hyperplasia as a result of increased pressure by the stent on the duodenal mucosa causing non-neoplastic tissue ingrowth.15,22,23 Despite this drawback, USEMS are currently most frequently used for managing malignant GOO in the majority of cases, as they combine reduced migration rates, more flexibility and also allow for better bile outflow (through the stent’s mesh interstices) in case the stent is placed across the duodenal papilla.7 Design changes and precautions to reduce migration rates of PCSEMS and CSEMS have been attempted (e.g., anti-migration modifications of the stent or applying clips or sutures to fix the stent to the wall), but most of these efforts were found to be not successful.18 In general, AE rates linked to the use of SEMS have been reported to range between 0% and 30%.18 This great variation of AE rates that has been reported is often based on the variation of definitions of AE that are used in the published studies; AE are usually divided into minor and major (i.e., life threatening), with these two categories appearing not only in different rates, but also resulting in different consequences for the patient; thus, the kind of AE that is reported should be strictly defined; minor AE of SEMS placement include among others epigastric pain or nausea and vomiting, whereas major counterparts include bleeding, perforation and fracture of the stents. When strict definitions are applied, AE rates are lower. This was reaffirmed in the aforementioned Dutch systematic review of prospective reports with duodenal SEMS, where obstruction and migration were reported in 12.6% and 4.3% of cases respectively, whereas the perforation rate was 1.2% and major bleeding was reported in 0.8% of patients (although overall bleeding was somewhat higher, i.e., 4.1% of cases).17 Moreover, except for stent occlusion and migration, and cholangitis (due to obstruction of bile flow), the other AEs seem to be balanced between USEMS on the one hand and PCSEMS/CSEMS on the other hand.22 This was also highlighted in a more recent systematic review and meta-analysis including nine studies and 849 patients, in which CSEMS were found to have a lower obstruction rate (relative risk [RR], 0.42; 95% confidence interval [CI], 0.24 to 0.73; p=0.002), at the cost of a higher migration rate (RR, 3.48; 95% CI, 2.16 to 5.62; P<0.00001).19 As previously stated, technical and clinical success, post-stenting dysphagia, stent patency, AEs and post-stenting reinterventions were similar between USEMS and CSEMS.19 In general, it seems that both USEMS and CSEMS are comparably effective, and in cases where the literature shows some–in most cases only a small–benefit for one of the two types, this is usually at the cost of more AEs, or an issue specific for that particular stent design. This was further illustrated, in an even more recent systematic review and metanalysis, in which five randomized controlled trials (RCTs), one prospective, and seven retrospective studies were analyzed (1,642 patients included; 800 with a USEMS and 688 with a CSEMS), with equal technical and clinical success for both stent types. Moreover, in a sub-analysis including only the RCTs, a trend towards less dysfunction for CSEMS was found (RR, 0.63; 95% CI, 0.45 to 0.88); however, this was counterbalanced by a higher migration risk, as well as a higher cumulative AE rate (RR, 1.75; 95% CI, 1.09 to 2.83).20
3. Endoscopic ultrasound-guided gastroenterostomy
EUS has evolved in recent years from a merely diagnostic procedure to an indispensable interventional endoscopic technique that offers solutions when other endoscopic techniques reach their limits or are no longer possible. As the technique combines endoscopy with ultrasonography it allows, with the help of recently developed accessories, for expansion of therapeutic endoscopic options even outside the luminal gastrointestinal tract. Based on this, interventional EUS has shown promising results with regard to decompression of the pancreatobiliary tract, but more recently also in establishing patency between the stomach and distal duodenum/proximal jejunum in case of GOO.12,18,23,24 The techniques that involve EUS for the management of GOO are referred to as EUS-GE. Although EUS-GE has been around since 2002, it has only recently been more widely implemented and this could partly be attributed to a change in mentality towards EUS as therapeutic instrument, which is therefore now evolving to an interventional technique, and partly by the more recent development of new EUS-dedicated accessories, with the most characteristic example being the introduction of LAMS. LAMS are an evolution of CSEMS, with the basic difference of LAMS from a “typical” CSEMS being the design of its stent ends: LAMS are made with wide flanges on both ends, providing improved anchoring and an even distribution of pressure on the luminal walls. Three types of LAMS are nowadays widely used, namely: AXIOS (Boston Scientific Corp., Marlborough, MA, USA), NAGI (Taewoong Medical Co., Goyang, Korea), and the Niti-S Spaxus (Taewoong Medical Co.). The AXIOS stent consists of double-walled flanges perpendicular to the lumen that hold the tissue walls in apposition and is the type that was initially introduced in 2012, marking the beginning of the new era of EUS-GE.7,12,18,23,24 LAMS are preloaded on a 10.8 F through-the-scope delivery system, that is compatible with a EUS-scope, endowed with a working channel of at least 3.7 mm. The rationale of the procedure consists of puncturing the small bowel from the stomach to a point distal to the obstruction, usually the distal duodenum or proximal jejunum under EUS-guidance, also assisted by fluoroscopy, which is then followed by LAMS-placement and hence, the creation of a tight lumen-to-lumen anastomosis.12,18 Three basic techniques have been used to create this bypass, the direct technique, the balloon-assisted technique and the EPASS (EUS-guided double-balloon-occluded gastrojejunostomy bypass) technique.12,18 During direct EUS-GE (Supplementary Video 1), a puncture of the small bowel loop that is adjacent to the gastric wall is performed using a needle (usually 19-gauge). Ultrasonographic visualization of this adjacent loop is facilitated by injecting saline or diluted contrast through a duodenal tube that was previously inserted distally to the obstruction using endoscopic guidance. The water- (or contrast-) filled segment is identified with the linear echoendoscope and after it is punctured, either a guidewire is passed through the needle, which is then used to guide placement of a LAMS, or alternatively a cautery-enhanced LAMS (HOT AXIOS; Boston Scientific Corp.) can be used directly, thus avoiding use of a guidewire and minimizing the risk of “pushing away” the small bowel loop from the stomach. In the balloon-assisted procedures, a guidewire is usually placed across the stenosis under endoscopic or radiologic guidance. Then, a balloon device (e.g., a retrieval or dilation balloon, single-balloon overtube) is advanced over the guidewire and inflated in order to help localizing the indicated puncture site, usually by puncturing (and bursting) the balloon under EUS-guidance, followed by advancement of a guidewire through the puncture needle, which is then used to guide LAMS placement. There are at least three variants of this technique that have been described.13,18 The final variant of EUS-GE is the EPASS technique, which uses a dedicated device, i.e., a double-balloon-occlusion catheter endowed with two balloons (with 20 cm distance between them) which is inserted through the narrowed segment in the more distal bowel lumen under endoscopic control. Both balloons are filled with saline or contrast material to hold the small intestine in between open. Then saline with contrast material is introduced into the space between the two balloons to distend the small bowel lumen and thus facilitating its stabilization; the next step includes direct puncture and deployment of the LAMS.12,18,24-27
Irrespective of the exact technique or the diameter of the LAMS that is used for EUS-GE, both technical and clinical success rates are excellent. In a recent clinical review including eight studies (seven retrospective and one prospective, with at least 10 patients), technical success was reported to range between 87% and 96% (reaching even 100% after some technical modifications), whereas clinical success was reported to range between 81% and 92%.27 Similarly, in a recent systematic review and meta-analysis including four retrospective and one prospective study, technical success was reported to be 92.9% and clinical success 90.11%. It is important to note that these excellent results were achieved at the cost of 5.6% serious AE (95% CI, 2.8% to 10.6%; I2=1.6%), among these peritonitis, perforation, bleeding and abdominal pain and an 11.4% (95% CI, 7.2% to 17.4%; I2= 17.3%) reintervention rate.24 In other reports AE ranges from an impressive 0% to an alarming 21%.18 This wide spectrum illustrates the need to use clearly defined definitions as outcomes when attempting to pool data, but it should also be noted that expertise of the endoscopist is a major factor that influences outcomes in interventional EUS, as it remains a method that requires extensive training.
The various advantages and disadvantages of the aforementioned procedures that are used to manage GOO are summarized in Table 1.
Table 1.
Advantages and Disadvantages of Procedures Used to Manage Gastric Outlet Obstruction
| EUS-guided gastroenterostomy | Enteral stenting using SEMS | Surgical gastrojejunostomy | |
|---|---|---|---|
| Advantages | Minimally invasive, also suitable for patients unable to undergo surgery | Minimally invasive, also suitable for patients unable to undergo surgery | Durable results (long-lasting palliation) |
| Fewer complications (compared with surgery) | Simple, fast, and easy procedure | ||
| Short time to oral intake | |||
| Disadvantages | Adequately trained endoscopist with expertise in EUS required | Symptom recurrence | Requires general anesthesia |
| Short-term complications | |||
| Delayed gastric emptying | |||
| Increased costs |
EUS, endoscopic ultrasound; SEMS, self-expandable metal stents.
COMPARISON OF OUTCOMES
1. SGJ versus SEMS
Various studies (including RCTs, non-RCTs, systematic reviews, and meta-analyses) have been published that compared outcomes, i.e., mainly technical, and clinical success, AE, time to resumption of oral intake and hospital stay, of SGJ with those of SEMS placement.3,12,22,28-30 In most reports, high technical and clinical success rates of both methods hardly allow statistical differences to be found.22,30 On the other hand, in the few studies in which the surgical option was shown to achieve higher technical success rates, this difference was in fact questionable, as was shown in the study by Zheng et al.,3 in which their meta-analysis showed a significantly higher technical success rate for SGJ (odds ratio, 0.10; 95% CI, 0.02 to 0.47; I2=0%, p=0.003), but with the difference being significant due to the non-RCTs that were also included and two of the included studies reporting a 100% technical success for both groups. Clinical success was found to be comparable in most of the aforementioned reports. Not surprisingly, enteral stenting requires less time to be performed compared to SGJ and procedure-related mortality was not different between both groups.30 In contrast, time to start oral intake was in favor of SEMS placement (around 3 to 5 days in most studies).3,16,30,31 As already thoroughly discussed above, AEs after SEMS placement are mainly associated with stent dysfunction, usually re-obstruction and migration,17 both leading to an increased need for reinterventions. This was highlighted in a recent systematic review and meta-analysis of 27 studies that compared GJ to SEMS in patients with malignant GOO (2,354 patients were included; 1,306 in the SEMS and 1,048 in the GJ group, respectively), in which SEMS placement was associated with a 3-fold increased reintervention rate.31 On the other hand, hospital stay (and overall costs) were significantly reduced when placing SEMS, due to the minimally invasive nature of stenting compared to SGJ.1,3,15,31 Mortality has not been analyzed with uniform criteria in the reviews available and thus is hard to assess, although it seems that there are no striking differences between the two methods of palliation.3,12 On the other hand, two studies showed a link between performing SGJ and prolonged survival,30,31 but this association should be interpreted with caution, as various factors might influence survival (among others chemotherapy administration32) and as patients selected for a surgical intervention are often those with longer life expectancies.33 Comparison of AEs between the two groups is also largely associated to definitions.12 It seems that overall complication rates are not very different comparing both SGJ and SEMS,22 although SGJ is more often complicated by minor complications, while SEMS placement is more often associated with major complications.12,30 Overall, the decision for the most appropriate form of therapy for a specific patient should be individualized depending on whether the patient is fit enough to undergo a surgical procedure, the patient’s predicted survival and the cause of the obstruction. SGJ seems to provide better long-term results with fewer reinterventions but should be reserved for those with a more prolonged predicted survival. A 2-month cutoff has been proposed.33
2. SGJ versus EUS-GE
There is only a limited number of comparative studies between SGJ and EUS-GE available. The studies by Khashab et al.13 and Perez-Miranda et al.34 were pooled in a systematic review and meta-analysis and demonstrated a significantly higher technical success rate for SGJ, but similar clinical success rates and significantly fewer AEs for EUS-GE (RR, 0.28; 95% CI, 0.11 to 0.68; I2=0%, p=0.005).35 Length of hospital stay, GOO recurrence and mean time to reintervention appeared to be similar in the study by Khashab et al.13 Of note, the study by Perez-Miranda et al.34 also showed equal technical success rates between SGJ and EUS-GE, but the pooled data shifted the scale in favor of the surgical option.35 In any case, EUS-GE should be considered a minimally invasive treatment, which however needs special training with a possibly significant learning curve and which, for the moment, should be performed in specialized centers with significant expertise. Thus, more data, especially RCTs are required, incorporating real-life results to draw more reliable conclusions.
3. SEMS versus EUS-GE
Four studies tried to compare EUS-GE to SEMS.4,5,18,36 EUS-GE was demonstrated to have lower technical and higher clinical success rates, but without a significant statistical difference. Therefore, for now both techniques should be considered with regard to these outcomes.5,36 AEs, both major and overall, were also comparable, but the difference in these techniques seems to lie in obstruction recurrence and need for reintervention, which according to Chen et al.36 was significantly lower for EUS-GE (4.0% vs 28.6%, p=0.015). This was a multicenter retrospective study including 30 consecutive patients from four centers who had EUS-GE in the period 2013 to 2015, that were compared to 52 patients from one center who underwent SEMS placement for malignant GOO in the period 2008 to 2010. As previously mentioned, other outcomes were similar between the two groups in this study: technical success was 86.7% for EUS-GE versus 94.2% for SEMS (p=0.2), clinical success 83.3% for EUS-GE versus 67.3% for SEMS (p=0.12) whereas AE rates were 16.7% versus 11.5% respectively (p=0.5).36 Of note, a more recent retrospective study including 100 patients (22 underwent EUS-GE and 78 SEMS placement) reported higher initial clinical success for the EUS-GE group (95.8% vs 76.3%, p=0.042).37 Other outcomes were similar, besides need for reintervention which was lower for EUS-GE (32.0% vs 8.3%, p=0.021): whether this difference in clinical success reflects a further improvement in technical skills to perform EUS-GE needs to be evaluated in future randomized studies, but the evidence so far seems to suggest that EUS-GE is a safe and effective minimally invasive technique, overall doing better than SEMS and with results comparable to those of SGJ. The results of the studies that directly compare outcomes of the procedures that are used to treat GOO38-41 are summarized in Table 2.
Table 2.
Comparative Studies of Procedures Used to Treat Gastric Outlet Obstruction
| Study (year) | Technical success | Clinical success | Adverse events | Recurrence of GOO±reintervention |
|---|---|---|---|---|
| SGJ vs SEMS† | ||||
| Fiori et al., (2004)38 | 9/9 (100) vs 9/9 (100) | 9/9 (100) vs 9/9 (100) | 1/9 (11.1) vs 1/9 (11.1) | NR |
| Mehta et al., (2006)39 | 13/14 (92.9) vs 10/13 (76.9) | NR | 8/14 (57.1) vs 0/13 (0) | NR |
| Jeurnink et al., (2010)33 | 17/18 (94.4) vs 20/21 (95.2) | NR | 5/18 (27.8) vs 8/21 (38.1) | 2/18 (11.1) vs 7/21 (33.3)* |
| Johnsson et al., (2004)40 | 15/15 (100) vs 21/21 (100) | 12/15 (80.0) vs 21/21 (100) | 1/15 (6.7) vs 2/21 (9.5) | |
| Schmidt et al., (2009)41 | 10/10 (100) vs 24/34 (70.6) | NR | NR | NR |
| EUS-GE vs SGJ | ||||
| Khashab et al., (2016)13 | 26/30 (86.7) vs 63/63 (100)* | 26/30 (86.7) vs 57/63 (90.5) | 5/30 (16.7) vs 16/63 (25.4) | 1/30 (3.3) vs 9/63 (14.3) |
| Perez-Miranda et al., (2017)34 | 22/25 (88.0) vs 29/29 (100) | 21/25 (84.0) vs 28/29 (96.6) | 3/25 (12.0) vs 12/29 (41.4)* | NR |
| EUS-GE vs SEMS | ||||
| Chen et al., (2017)36 | 26/30 (86.7) vs 49/52 (94.2) | 25/30 (83.3) vs 35/52 (67.3) | 5/30 (16.7) vs 6/52 (11.5) | 1/25 (4.0) vs 10/35 (28.5)* |
| Ge et al., (2019)37 | 24/24 (100) vs 97/97 (100) | 23/24 (95.8) vs 74/97 (76.3)* | 5/22 (22.7) vs 39/78 (50.0) | 2/24 (8.3) vs 31/97 (32.0)* |
GOO, gastric outlet obstruction; SGJ, surgical gastrojejunostomy; SEMS, self-expandable metal stent; EUS-GE, endoscopic ultrasound-guided gastroenterostomy; NR, not reported.
*p-value <0.05; †For comparison of SGJ versus SEMS only randomized controlled studies and prospective cohort studies were included.
CONCLUSION AND FUTURE PROSPECTS
This review of the current literature seems to support that all available therapeutic tools, including SGJ, SEMS or EUS-GE are safe and effective options for the treatment of GOO, with each of them having its own advantages but also special properties. The decision which one to perform should to a large extent depend on clinical criteria of the patient, the location and type of obstruction in the distal stomach or proximal duodenum, as well as availability and expertise of the endoscopic team. When taking this into consideration, SGJ could be the best choice for patients with malignant GOO and a better life expectancy, whereas SEMS placement as a minimally invasive alternative would be preferable for patients with a worse prognosis. Nonetheless, SEMS placement remains the endoscopic technique most commonly performed due to the advanced stage many patients present. Finally, EUS-GE is definitely the “new kid on the block” for the minimally invasive endoscopic management of GOO and could even be efficiently utilized as a technique that combines the best aspects of the other two methods. In our opinion, EUS-GE likely will eventually replace SGJ in the future, of course when it is clearly shown that EUS-GE is at least as effective and safe as SGJ. For the time being, EUS-GE still requires further specialization of endoscopists, whereas its outcomes need more validation through comparative prospective randomized data. In any case, though, it needs to be stressed that patients with GOO can be treated most effectively in a multidisciplinary environment, with more than one specialist available, with the aim to achieve better outcomes for patients with GOO.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210327.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Brimhall B, Adler DG. Enteral stents for malignant gastric outlet obstruction. Gastrointest Endosc Clin N Am. 2011;21:389–403. doi: 10.1016/j.giec.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Koop AH, Palmer WC, Stancampiano FF. Gastric outlet obstruction: a red flag, potentially manageable. Cleve Clin J Med. 2019;86:345–353. doi: 10.3949/ccjm.86a.18035. [DOI] [PubMed] [Google Scholar]
- 3.Zheng B, Wang X, Ma B, Tian J, Jiang L, Yang K. Endoscopic stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Dig Endosc. 2012;24:71–78. doi: 10.1111/j.1443-1661.2011.01186.x. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal U, Khara HS, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: a systematic review and meta-analysis. Endosc Ultrasound. 2020;9:16–23. doi: 10.4103/eus.eus_70_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandan S, Khan SR, Mohan BP, et al. EUS-guided gastroenterostomy versus enteral stenting for gastric outlet obstruction: systematic review and meta-analysis. Endosc Int Open. 2021;9:E496–E504. doi: 10.1055/a-1341-0788.08a49fdab78342d7822cda2f28f9ad40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Annamaraju P. StatPearls [Internet] StatPearls Publishing; Treasure Island: 2022. Jan, [cited 2021 Sep 21]. Gastric outlet obstruction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557826/ [DOI] [Google Scholar]
- 7.Jeong SJ, Lee J. Management of gastric outlet obstruction: focusing on endoscopic approach. World J Gastrointest Pharmacol Ther. 2020;11:8–16. doi: 10.4292/wjgpt.v11.i2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tantillo K, Dym RJ, Chernyak V, Scheinfeld MH, Taragin BH. No way out: causes of duodenal and gastric outlet obstruction. Clin Imaging. 2020;65:37–46. doi: 10.1016/j.clinimag.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Upchurch E, Ragusa M, Cirocchi R. Stent placement versus surgical palliation for adults with malignant gastric outlet obstruction. Cochrane Database Syst Rev. 2018;5:CD012506. doi: 10.1002/14651858.CD012506.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potz BA, Miner TJ. Surgical palliation of gastric outlet obstruction in advanced malignancy. World J Gastrointest Surg. 2016;8:545–555. doi: 10.4240/wjgs.v8.i8.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dada SA, Fuhrman GM. Miscellaneous disorders and their management in gastric surgery: volvulus, carcinoid, lymphoma, gastric varices, and gastric outlet obstruction. Surg Clin North Am. 2011;91:1123–1130. doi: 10.1016/j.suc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Cheung SLH, Teoh AYB. Optimal management of gastric outlet obstruction in unresectable malignancies. Gut Liver. Epub. 2021 May 31; doi: 10.5009/gnl210010. https://doi.org/10.5009/gnl210010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khashab MA, Bukhari M, Baron TH, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–E281. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manuel-Vázquez A, Latorre-Fragua R, Ramiro-Pérez C, López-Marcano A, la Plaza-Llamas R, Ramia JM. Laparoscopic gastrojejunostomy for gastric outlet obstruction in patients with unresectable hepatopancreatobiliary cancers: a personal series and systematic review of the literature. World J Gastroenterol. 2018;24:1978–1988. doi: 10.3748/wjg.v24.i18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tringali A, Giannetti A, Adler DG. Endoscopic management of gastric outlet obstruction disease. Ann Gastroenterol. 2019;32:330–337. doi: 10.20524/aog.2019.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki Y, Takiguchi S, Takahashi T, et al. Treatment of gastric outlet obstruction that results from unresectable gastric cancer: current evidence. World J Gastrointest Endosc. 2016;8:165–172. doi: 10.4253/wjge.v8.i3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Halsema EE, Rauws EA, Fockens P, van Hooft JE. Self-expandable metal stents for malignant gastric outlet obstruction: a pooled analysis of prospective literature. World J Gastroenterol. 2015;21:12468–12481. doi: 10.3748/wjg.v21.i43.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troncone E, Fugazza A, Cappello A, et al. Malignant gastric outlet obstruction: which is the best therapeutic option? World J Gastroenterol. 2020;26:1847–1860. doi: 10.3748/wjg.v26.i16.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan YM, Pan J, Guo LK, Qiu M, Zhang JJ. Covered versus uncovered self-expandable metallic stents for palliation of malignant gastric outlet obstruction: a systematic review and meta-analysis. BMC Gastroenterol. 2014;14:170. doi: 10.1186/1471-230X-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamada T, Hakuta R, Takahara N, et al. Covered versus uncovered metal stents for malignant gastric outlet obstruction: systematic review and meta-analysis. Dig Endosc. 2017;29:259–271. doi: 10.1111/den.12786. [DOI] [PubMed] [Google Scholar]
- 21.Tringali A, Costa D, Anderloni A, Carrara S, Repici A, Adler DG. Covered versus uncovered metal stents for malignant gastric outlet obstruction: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92:1153–1163. doi: 10.1016/j.gie.2020.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Minata MK, Bernardo WM, Rocha RS, et al. Stents and surgical interventions in the palliation of gastric outlet obstruction: a systematic review. Endosc Int Open. 2016;4:E1158–E1170. doi: 10.1055/s-0042-115935.68f759a66a8f457384e9dd723956a5a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakai Y, Hamada T, Isayama H, Itoi T, Koike K. Endoscopic management of combined malignant biliary and gastric outlet obstruction. Dig Endosc. 2017;29:16–25. doi: 10.1111/den.12729. [DOI] [PubMed] [Google Scholar]
- 24.McCarty TR, Garg R, Thompson CC, Rustagi T. Efficacy and safety of EUS-guided gastroenterostomy for benign and malignant gastric outlet obstruction: a systematic review and meta-analysis. Endosc Int Open. 2019;7:E1474–E1482. doi: 10.1055/a-0996-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YI, Kunda R, Storm AC, et al. EUS-guided gastroenterostomy: a multicenter study comparing the direct and balloon-assisted techniques. Gastrointest Endosc. 2018;87:1215–1221. doi: 10.1016/j.gie.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Rimbas M, Larghi A, Costamagna G. Endoscopic ultrasound-guided gastroenterostomy: are we ready for prime time? Endosc Ultrasound. 2017;6:235–240. doi: 10.4103/eus.eus_47_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbajo AY, Kahaleh M, Tyberg A. Clinical review of EUS-guided gastroenterostomy (EUS-GE) J Clin Gastroenterol. 2020;54:1–7. doi: 10.1097/MCG.0000000000001262. [DOI] [PubMed] [Google Scholar]
- 28.Fiori E, Lamazza A, Demasi E, Decesare A, Schillaci A, Sterpetti AV. Endoscopic stenting for gastric outlet obstruction in patients with unresectable antro pyloric cancer. Systematic review of the literature and final results of a prospective study: the point of view of a surgical group. Am J Surg. 2013;206:210–217. doi: 10.1016/j.amjsurg.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraja V, Eslick GD, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction: a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol. 2014;5:92–98. doi: 10.3978/j.issn.2078-6891.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian SB, Shen WS, Xi HQ, Wei B, Chen L. Palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer: a meta-analysis comparison of gastrojejunostomy with endoscopic stenting. Chin Med J (Engl) 2016;129:1113–1121. doi: 10.4103/0366-6999.180530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mintziras I, Miligkos M, Wächter S, Manoharan J, Bartsch DK. Palliative surgical bypass is superior to palliative endoscopic stenting in patients with malignant gastric outlet obstruction: systematic review and meta-analysis. Surg Endosc. 2019;33:3153–3164. doi: 10.1007/s00464-019-06955-z. [DOI] [PubMed] [Google Scholar]
- 32.Chang KB, Ye BW, Chou CK, et al. Outcomes of enteral metallic stent in patients with pancreatic carcinoma and gastric outlet obstruction: a single center experience. J Formos Med Assoc. 2020;119(1 Pt 2):238–246. doi: 10.1016/j.jfma.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Miranda M, Tyberg A, Poletto D, et al. EUS-guided gastrojejunostomy versus laparoscopic gastrojejunostomy: an international collaborative study. J Clin Gastroenterol. 2017;51:896–899. doi: 10.1097/MCG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 35.Fan W, Tan S, Wang J, et al. Clinical outcomes of endoscopic ultrasound-guided gastroenterostomy for gastric outlet obstruction: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:159–167. doi: 10.1080/13645706.2020.1792500. [DOI] [PubMed] [Google Scholar]
- 36.Chen YI, Itoi T, Baron TH, et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–2952. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 37.Ge PS, Young JY, Dong W, Thompson CC. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33:3404–3411. doi: 10.1007/s00464-018-06636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures: gastroenterostomy vs. endoscopic stenting: a randomized prospective trial. Anticancer Res. 2004;24:269–271. [PubMed] [Google Scholar]
- 39.Mehta S, Hindmarsh A, Cheong E, et al. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–242. doi: 10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 40.Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg. 2004;28:812–817. doi: 10.1007/s00268-004-7329-0. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt C, Gerdes H, Hawkins W, et al. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. 2009;198:92–99. doi: 10.1016/j.amjsurg.2008.09.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.