Abstract
Background/Aims
Metabolic dysfunction associated fatty liver disease (MAFLD) has recently been introduced to compensate for the conventional concept of nonalcoholic fatty liver disease (NAFLD). We explored whether fibrotic burden determines the risk of atherosclerotic cardiovascular disease (ASCVD) among subjects with MAFLD.
Methods
We recruited 9,444 participants from the Korea National Health and Nutrition Examination Survey (2008 to 2011). Liver fibrosis was identified using the fibrosis-4 (FIB-4) index and NAFLD fibrosis score. The 10-year ASCVD risk score (>10%) was used to determine a high probability ASCVD risk. For sensitivity analysis, propensity score matching was assessed to subjects with aged 40 to 75 years free from ASCVD.
Results
The prevalence of MAFLD was 38.0% (n=3,592). The ASCVD risk scores stratified in quartile were positively correlated to MAFLD and FIB-4 defined-significant liver fibrosis (p for trend <0.001). Individuals with both MAFLD and FIB-4 defined-significant liver fibrosis had a greater chance of high probability ASCVD risk (odds ratio [OR]=2.40; p<0.001) than those without MAFLD. The impact of MAFLD on high probability ASCVD risk was greater than that of significant liver fibrosis (OR=4.72 for MAFLD vs OR=1.88 for FIB-4 defined-significant liver fibrosis; all p<0.001). Among participants with MAFLD, low muscle mass enhanced the risk of significant liver fibrosis (OR=1.56 to 2.43; p<0.001). When NAFLD fibrosis score was applied to define significant liver fibrosis, similar findings were observed.
Conclusions
Individuals with MAFLD had a substantial ASCVD risk compared to those without MAFLD. Accompanying significant liver fibrosis further enhanced the risk of ASCVD among subjects with MAFLD.
Keywords: Metabolic dysfunction associated fatty liver disease, Nonalcoholic fatty liver disease, Cardiovascular disease, Liver fibrosis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is considered as the most widespread chronic liver diseases in global, and with the fast increase rate of obesity, aged population, and sedentary lifestyle, its incidence is forecasted to sharply rise.1 Although most subjects with simple steatosis without metabolic derangement show favorable long-term outcomes, a certain proportion of individuals with NAFLD may experience liver injury progressing, results in cirrhosis and liver malignancy.2
NAFLD is certainly connected to an advanced risk of atherosclerotic cardiovascular disease (ASCVD), malignancy, and hepatic complication-related mortality.3 Of these, ASCVD accounts for the majority of death cause in subjects with NAFLD.3,4 The dense relationship between NAFLD and ASCVD is presumably appeared from the fundamental role of liver that the metabolism of glucose and lipid shares as common denominator, separated from other risk components involved cardiometabolic disorders including diabetes, obesity, hypertension, and dyslipidemia.5 Radiological atherosclerosis and endothelial dysfunction are also frequent in subjects with NAFLD.6
Besides the presence or absence of NAFLD, the severity of liver fibrosis is significantly linked to ASCVD risk. In a recent U.S. study,7 NAFLD based on ultrasonography-diagnosis was not related with mortality. However, progressed liver fibrosis, as defined by noninvasive fibrosis marker panels, was a convincing predictor of mortality, primarily from ASCVD, separated from other known risk components. In another recent Korean study, fibrotic burden determined by transient elastography, was ultimately correlated with coronary artery calcification.8
Recently, a novel interpretation was proposed for metabolic dysfunction associated fatty liver disease (MAFLD).9 In contrast to the NAFLD criteria, the identification of MAFLD mainly focuses on the existence of metabolic dysregulation among subjects with fatty liver, excluding those with metabolically uncomplicated fatty liver.10 Although further validation is desirable, the transition from NAFLD to MAFLD may facilitate the diagnosis of metabolically dysregulated fatty liver superimposed on other sources of chronic liver diseases including virus or alcoholism.
Thus, we investigated whether MAFLD is significantly related with the risk of ASCVD and whether fibrotic burden determined enhanced the risk of ASCVD among individuals with MAFLD.
MATERIALS AND METHODS
1. Study population
The Korea National Health and Nutrition Examination Survey (KNHANES) is established on civilian population database and covers health checkup and survey to monitor fitness and nutritive conditions of South Koreans. Each KNHANES is consisted of autonomous datasets of the civilian population of South Korea.
Participants were randomly recruited from 600 districts of cities and provinces in South Korea.11 As described in Supplementary Fig. 1, the KNHANES from 2008 to 2011 enrolled 37,753 individuals and, 28,071 adults (≥20 years old, 12,160 men and 15,911 women) were initially selected. Eventually, we excluded 18,627 subjects who satisfied the following conditions: (1) inadequate clinical and laboratory data to estimate the severity of liver steatosis or fibrosis and (2) missing data for ASCVD risk assessments. Thus, 9,444 subjects (4,104 men and 5,340 women) were entered the final analysis.
Written informed consent was obtained from all participants before the health examination and survey started, and the KNHANES was complied with Institutional Review Board of the Korea Center for Disease Control and Prevention (2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, and 2011-02CON-06C).
2. Assessment of clinical and laboratory parameters
KNHANES data include a three-part medical history, nutritional status, and laboratory examination. Medical history included smoking habits, alcohol drinking, physical activity level, and disease diagnosis and/or treatment, conducted on direct interviews and self-reporting. We defined regular exercise as involving in intense athletics, which made one exhausted or gasp for breath, and engaged over 20 minutes per session at least three times a week.5 After overnight (≥8 hours) fasting, participants had blood test and spot urinalysis. The samples were immediately refrigerated, and transferred to a central laboratory (Neodin Medical Institute, Seoul, Korea).5
3. Confirmation of MAFLD and assessment of liver steatosis and fibrosis
We determined the MAFLD in accordance with a recent international expert consensus recommendation.10 Hepatic steatosis was characterized as fatty liver index ≥30,12 besides one of the following three criteria: overweight/obesity (body mass index [BMI] ≥23 kg/m2 in Asian), the presence of diabetes mellitus, or metabolic disorder (at least two metabolic risk components, waist circumference ≥90/80 cm in male and female, blood pressure ≥130/85 mm Hg or if taking anti-hypertensive medications, triglycerides ≥150 mg/dL or taking triglyceride lowering agents, plasma high-density lipoprotein [HDL] cholesterol <40 mg/dL for male and <50 mg/dL for female, prediabetes as fasting blood glucose 100–125 mg/dL, and homeostasis model assessment of insulin resistance [HOMA-IR] score ≥2.5). Diabetes was identified as participants who were taking oral anti-hyperglycemic agents or whose fasting plasma glucose were more than 126 mg/dL.
The severity of liver fibrosis was determined by previously confirmed liver fibrosis prediction models: fibrosis-4 index13 and NAFLD fibrosis score.14 Since the KNHANES did not measure serum albumin concentration; significant liver fibrosis was characterized as either the highest quartile of the NAFLD fibrosis score or fibrosis-4 index ≥2.67.15
4. Assessment of cardiometabolic disease risk and component
The pooled 10-year ASCVD risk estimation equation from the 2013 American College of Cardiology/American Heart Association guidelines was used to calculate each individual’s ASCVD risk.16 ASCVD risk estimation included age, gender, ethnicities, total cholesterol, HDL cholesterol, systolic blood pressure, treatment for hypertension, diabetes, and current cigarette smoking (Supplementary Table 1). American College of Cardiology/American Heart Association ASCVD risk >10% was classified as a “high probability ASCVD risk.”5,16 Hyper-low-density lipoprotein (LDL) cholesterolemia was defined as the subjects’ LDL cholesterol goal recommended by the 2004 update of the Adult Treatment Panel III guidelines or currently taking anti-dyslipidemia drugs.17 We applied the Chronic Kidney Disease Epidemiology Collaboration calculation,18 and chronic kidney disease was categorized if estimated glomerular filtration rate <60 mL/min/1.73 m2. Proteinuria was considered as having more than a trace in urinalysis. Previous ASCVD history included prior history of myocardial infarction, angina, and stroke. The KHNANSE conducts dual-energy X-ray absorptiometry (QDR 4500A; Hologic Inc., Bedford, MA, USA) test and has data on appendicular skeletal muscle mass. The sarcopenia index was considered as total appendicular skeletal muscle mass (kg) divided BMI (kg/m2), and sarcopenia was characterized as the lowest quintile for sex-specific sarcopenia index (<0.877 for male and <0.582 for female) adapted from the Foundation for the National Institutes of Health guideline.19
5. Statistical analysis
We presented data as mean±standard deviation for continuous variables and numbers (n) or percentages (%) for categorical variables. One-way analysis of variance and chi-square tests were used to analyze the subjects’ characteristics. Both were followed by post hoc analyses using the Bonferroni method. In addition, we estimated ASCVD risk score in individuals aged 40 to 75 years free from a prior history of ASCVD in matched subgroup analysis using propensity scores. Sex and age were adjusted for covariates. As MAFLD, fatty liver index, fibrosis-4 index and the ASCVD risk score involved overlapping parameters, variance inflation factors were applied to consider multicollinearity in multivariate analysis. Nevertheless, we could not find any multicollinearity (highest variance inflation factor=3.417). Multivariate logistic regression analysis was used to confirm the independent association between high probability ASCVD risk, MAFLD, and significant liver fibrosis with multi-step adjustment. To demonstrate the independent risk components for significant liver fibrosis among subjects with MAFLD, multivariate logistic regression analysis was conducted in the MAFLD group. For the values which were not normally distributed (triglyceride, HDL cholesterol, LDL cholesterol, insulin, HOMA-IR, aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transpeptidase, and platelet); the results were log-transformed to the original scale to achieve approximately symmetrical distributions. Analysis was conducted using IBM SPSS version 27.0 for Windows (IBM Corp., Armonk, NY, USA). A p<0.05 was considered statistically significant.
RESULTS
1. Population
A total of 9,444 individuals (4,104 male and 5,340 female) were recruited in the final statistical analysis (Supplementary Fig. 1). Table 1 presented the clinical characteristics of the study population. A total of 3,592 subjects (38.0%) had MAFLD, whereas the remaining subjects (n=5,852, 62.0%) did not. Of the subjects who had MAFLD, 1,070 subjects (11.3%) had significant liver fibrosis, whereas the remaining subjects (n=2,522, 26.7%) did not.
Table 1.
Baseline Characteristics
| Variable | Subjects without MAFLD (n=5,852, 62.0%) |
Subjects with MAFLD (n=3,592, 38.0%) | p-value | |
|---|---|---|---|---|
| No significant liver fibrosis by FIB-4 (n=2,522, 26.7%) |
Significant liver fibrosis by FIB-4 (n=1,070, 11.3%) |
|||
| Demographic variables | ||||
| Age, yr | 46.6±15.6 | 47.5±12.9# | 64.9±9.7#,** | <0.001 |
| Male gender | 1,984 (33.9) | 1,488 (59.0)# | 632 (59.1)# | <0.001 |
| Waist circumference, cm | 75.9±7.3 | 89.4±7.2# | 90.0±7.0# | <0.001 |
| Body mass index, kg/m2 | 22.0±2.4 | 26.4±2.8# | 25.7±2.7#,** | <0.001 |
| Appendicular skeletal muscle mass, kg | 17.4±4.3 | 21.6±5.2# | 19.7±4.5#,** | <0.001 |
| Sarcopenia* | 797 (13.6) | 642 (25.5)# | 456 (42.6)#,** | <0.001 |
| ASCVD risk score† | 5.3±9.6 | 6.6±8.4# | 17.9±13.7#,** | <0.001 |
| Systolic blood pressure, mm Hg | 114.9±17.0 | 122.7±16.3# | 128.7±17.3#,** | <0.001 |
| Diastolic blood pressure, mm Hg | 73.5±10.2 | 80.2±11.2# | 79.0±10.2#,** | <0.001 |
| Hypertension | 1,473 (25.2) | 1,220 (48.4)# | 740 (69.2)#,** | <0.001 |
| Metabolic syndrome | 677 (11.6) | 1,446 (57.3)# | 752 (70.3)#,** | <0.001 |
| Diabetes | 327 (5.6) | 354 (14.0)# | 237 (22.1)#,** | <0.001 |
| Current smoker | 962 (16.4) | 787 (31.2)# | 203 (19.0)** | <0.001 |
| Central obesity‡ | 1,016 (17.4) | 1,611 (63.9)# | 715 (66.8)# | <0.001 |
| Overweight§ | 1,922 (32.8) | 2,337 (92.7)# | 911 (85.1)#,** | <0.001 |
| Exercise | 886 (15.1) | 465 (18.4)# | 170 (15.9) | 0.028 |
| Heavy alcohol drinkΙΙ | 705 (12.0) | 590 (23.4)# | 220 (20.6)# | <0.001 |
| Laboratory variables | ||||
| Fasting blood glucose, mg/dL | 93.2±17.2 | 103.4±25.3# | 107.5±27.1#,** | <0.001 |
| Insulin, µIU/mL¶ | 8.9±3.6 | 11.8±5.4# | 11.4±5.4#,** | <0.001 |
| Homeostatic model assessment of insulin resistance¶ | 2.1±1.0 | 3.0±1.8# | 3.1±1.8# | <0.001 |
| Total cholesterol, mg/dL | 181.3±33.2 | 202.2±36.6# | 193.7±39.1# | <0.001 |
| Triglyceride, mg/dL¶ | 92.7±47.0 | 198.1±124.2# | 202.9±175.2# | <0.001 |
| High-density lipoprotein cholesterol, mg/dL¶ | 55.7±12.6 | 47.3±10.6# | 46.7±11.2# | <0.001 |
| Low-density lipoprotein cholesterol, mg/dL¶ | 111.1±30.0 | 122.9±33.7# | 114.5±34.8** | <0.001 |
| Serum creatinine, mg/dL | 0.8±0.2 | 0.9±0.2# | 0.9±0.2#,** | <0.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 96.9±17.5 | 93.5±16.0# | 80.4±16.0#,** | <0.001 |
| Aspartate aminotransferase, IU/L¶ | 20.0±8.5 | 23.3±8.8# | 33.5±30.7#,** | <0.001 |
| Alanine aminotransferase, IU/L¶ | 16.8±10.0 | 28.8±19.1# | 30.1±34.1# | <0.001 |
| Platelet count, 109/L¶ | 254.2±57.6 | 271.0±54.4 | 212.7±45.5#,** | <0.001 |
| Gamma glutamyl-transpeptidase, IU/L¶ | 21.5±30.0 | 51.8±52.5# | 68.6±91.3#,** | <0.001 |
| Liver fibrosis and steatosis | ||||
| NAFLD fibrosis score | 0.3±1.2 | 0.5±1.0# | 2.2±1.0#,** | <0.001 |
| Fibrosis-4 index | 1.0±0.7 | 0.8±0.3 | 2.0±1.3#,** | <0.001 |
| Fatty liver index | 12.4±9.0 | 55.9±17.8# | 55.8±18.2# | <0.001 |
Data are presented as the mean±SD or number (%).
MAFLD, metabolic dysfunction-associated fatty liver disease; FIB-4, fibrosis-4; ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease.
*Sarcopenia was defined as the lowest quintile for sex-specific sarcopenia index; †ASCVD risk score was calculated using the 10-year ASCVD risk score from the 2013 American College of Cardiology/American Heart Association guideline; ‡Central obesity was defined waist circumference ≥90 cm in men and ≥80 cm in women; §Overweight was defined body mass index ≥23 kg/m2; ΙΙHeavy alcohol consumption was defined as those whose alcohol consumption exceeded 140 g/week for men and 70 g/week for women; ¶Log-transformed; #p<0.05 by post hoc analyses when compared without MAFLD; **p<0.05 by post hoc analyses when compared with MAFLD, without significant liver fibrosis.
The mean age, BMI, blood pressure, fasting blood glucose, HOMA-IR, total cholesterol, triglyceride, and LDL cholesterol concentration were significantly greater in subjects with MAFLD than those without MAFLD (all p<0.05). Individuals with both MAFLD and significant liver fibrosis were significantly older and had substantially higher waist circumference, BMI, systolic blood pressure, fasting blood glucose levels, and ASCVD risk score than those with MAFLD but without significant liver fibrosis (all p<0.05), while their kidney function (estimated glomerular filtration rate) was significantly attenuated (p<0.05). The prevalence of hypertension, metabolic syndrome, diabetes, and sarcopenia was significantly greater in subjects with both MAFLD and significant liver fibrosis than in the other groups (all p<0.05).
Similar results were found in NAFLD fibrosis score defined-significant liver fibrosis and propensity score matching analysis (Supplementary Tables 2, 3).
2. Association between ASCVD risk score and MAFLD and fibrotic burden
To demonstrate the association between ASCVD risk score and MAFLD, fibrotic burden, we stratified the ASCVD risk score and fibrotic burden by quartiles, the quartile of ASCVD risk score exhibited a strong positive association with MAFLD and fibrotic burden, irrespective of the fibrosis indices (fibrosis-4 index and NAFLD fibrosis score, both p for trend <0.001). The ASCVD risk score raised from subjects without MAFLD to subjects with both MAFLD and significant liver fibrosis.
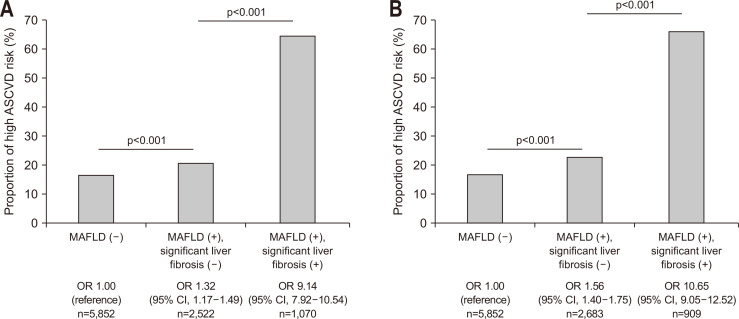
The proportion of high probability ASCVD risk also gradually extended from subjects without MAFLD and subjects with MAFLD but without significant liver fibrosis to subjects with both MAFLD and significant liver fibrosis, in sequence (16.8% vs 21.0% vs 64.8% for dibrosis-4 index; 16.8% vs 23.0% vs 66.4% for NAFLD fibrosis score; all p<0.001) (Fig. 1). Individuals with both MAFLD and significant liver fibrosis had a significantly higher proportion of high probability ASCVD risk than those without NAFLD, independent of significant liver fibrosis (odds ratio [OR]=1.32, 95% confidence interval [CI]=1.17 to 1.49 in subjects without significant liver fibrosis; OR=9.14, 95% CI=7.92 to 10.54 in subjects with significant liver fibrosis) (all p<0.001) (Fig. 1A).
Fig. 1.
High probability of ASCVD risk in agreement with MAFLD and significant liver fibrosis status. The opportunity for high probability of ASCVD risk was predominant in subjects with fibrosis-4 index- (A) or nonalcoholic fatty liver disease fibrosis score-defined (B) significant liver fibrosis and MAFLD (all p<0.001).
ASCVD, atherosclerotic cardiovascular disease; MAFLD, metabolic dysfunction associated fatty liver disease; OR, odds ratio; CI, confidence interval.
When NAFLD fibrosis score was applied to distinguish significant liver fibrosis, similar results were found (Fig. 1B).
3. Cardiometabolic risk factors according to MAFLD and significant liver fibrosis
We analyzed the risk of cardiometabolic risk factors according to MAFLD and significant liver fibrosis after adjusting for confounding factors (Table 2). Individuals with MAFLD had significantly higher risks of chronic kidney disease, hyper-LDL cholesterolemia, hypertriglyceridemia, hypo-HDL cholesterolemia, previous cardiovascular disease history, proteinuria, and sarcopenia (OR=1.48 to 10.36; all p<0.05) than those without. The risk for chronic kidney disease and proteinuria was the highest in participants with both MAFLD and significant liver fibrosis (chronic kidney disease: OR=1.68, 95% CI=1.22 to 2.30 for MAFLD without significant liver fibrosis; OR=1.91, 95% CI=1.46 to 2.48 for both MAFLD and significant liver fibrosis; proteinuria: OR=1.30, 95% CI=1.09 to 1.56 for MAFLD without significant liver fibrosis; OR=1.97, 95% CI=1.54 to 2.52 for both MAFLD and significant liver fibrosis).
Table 2.
Cardiometabolic Risk Factors According to MAFLD and Significant Liver Fibrosis
| Cardiometabolic risk factors |
Fibrosis-4 index, OR (95% CI) | NAFLD fibrosis score, OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| MAFLD (–) | MAFLD (+), significant liver fibrosis (–) |
MAFLD (+), significant liver fibrosis (+) |
MAFLD (–) | MAFLD (+), significant liver fibrosis (–) |
MAFLD (+), significant liver fibrosis (+) |
||
| Chronic kidney disease* | 1.00 (reference) |
1.68 (1.22–2.30) p=0.001 |
1.91 (1.46–2.48) p<0.01 |
1.00 (reference) |
1.50 (1.12–2.00) p=0.022 |
2.22 (1.68–2.93) p<0.001 |
|
| Hyper-LDL cholesterolemia† |
1.00 (reference) |
2.98 (2.64–3.61) p<0.001 |
1.43 (1.22–1.67) p<0.001 |
1.00 (reference) |
2.52 (2.24–2.82) p<0.001 |
1.90 (1.61–2.25) p<0.001 |
|
| Hypertriglyceridemia‡ | 1.00 (reference) |
10.36 (9.23–11.63) p<0.001 |
6.58 (5.63–7.69) p<0.001 |
1.00 (reference) |
10.06 (9.00–11.26) p<0.001 |
6.42 (5.42–7.60) p<0.001 |
|
| Hypo-HDL cholesterolemia§ |
1.00 (reference) |
3.39 (3.04–3.77) p<0.001 |
2.90 (2.49–3.39) p<0.001 |
1.00 (reference) |
3.32 (2.99–3.69) p<0.001 |
3.01 (2.54–3.57) p<0.001 |
|
| Previous ASCVD historyΙΙ | 1.00 (reference) |
1.79 (1.39–2.30) p<0.001 |
1.48 (1.14–1.92) p=0.003 |
1.00 (reference) |
1.52 (1.21–1.95) p<0.001 |
1.80 (1.37–2.37) p<0.001 |
|
| Proteinuria¶ | 1.00 (reference) |
1.30 (1.09–1.56) p=0.004 |
1.97 (1.54–2.52) p<0.001 |
1.00 (reference) |
1.35 (1.13–1.60) p=0.001 |
1.92 (1.47–2.51) p<0.001 |
|
| Sarcopenia# | 1.00 (reference) |
2.61 (2.29–2.96) p<0.001 |
2.20 (1.88–2.58) p<0.001 |
1.00 (reference) |
2.48 (2.20–2.81) p<0.001 |
2.39 (2.01–2.83) p<0.001 |
|
Adjusted for age, sex, smoking, exercise, and alcohol consumption.
MAFLD, metabolic dysfunction-associated fatty liver disease; OR, odds ratio; CI, confidence interval; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ASCVD, atherosclerotic cardiovascular disease.
*Chronic kidney disease was defined as an estimated glomerular filtration rate less than 60 mL/min/1.73 m2; †Hyper-LDL-cholesterolemia was characterized according to the LDL cholesterol goal recommended in the 2004 update of the Adult Treatment Panel III guidelines or current use of anti-dyslipidemia drugs; ‡Hypertriglyceridemia was defined as serum triglycerides ≥150 mg/dL or use of triglyceride-lowering agents; §Hypo-high-density lipoprotein cholesterolemia was defined as HDL <40 mg/dL for men and <50 mg/dL for women; ΙΙPrevious ASCVD history included prior history of myocardial infarction, angina or stroke; ¶Proteinuria was defined as more than trace protein in urinalysis; #Sarcopenia was defined as the lowest quintile of the sex-specific sarcopenia index.
These gradual increases in the risk of cardiometabolic risk factors were similarly observed when the NAFLD fibrosis score was applied to distinguish significant liver fibrosis (data not shown).
4. Association between high probability ASCVD risk and the degree of MALFD and significant liver fibrosis
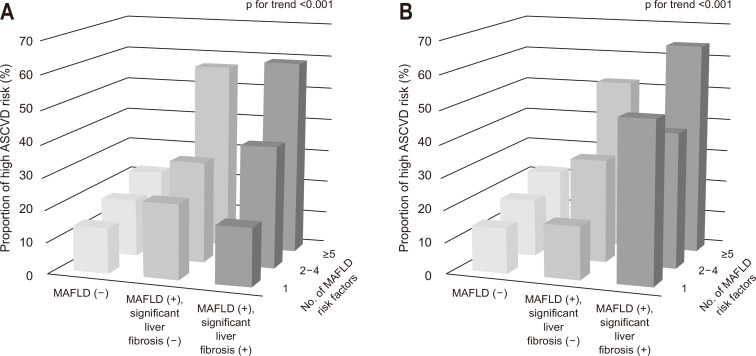
To clarify the relation between high probability ASCVD risk and the presence of MAFLD and significant liver fibrosis, we evaluated the number of MAFLD risk factors (overweight/obesity, central obesity, hypertension, hypertriglyceridemia, hypo-HDL cholesterolemia, prediabetes, and HOMA-IR score ≥2.5) using univariate logistic regression analysis. Subjects with both MAFLD and significant liver fibrosis were more prone to have multiple MAFLD risk factors, regardless of the liver fibrosis prediction models (Fig. 2). If there were comparable MAFLD risk factors, subjects with both MAFLD and significant liver fibrosis had a much higher chance of high probability ASCVD risk.
Fig. 2.
Proportion of subjects with high probability atherosclerotic cardiovascular disease (ASCVD) risk with individual metabolic dysfunction associated fatty liver disease (MAFLD) risk components according to the fibrosis-4 index- (A) or nonalcoholic fatty liver disease fibrosis score-defined (B) significant liver fibrosis. Risk factors included overweight/obesity, central obesity, hypertension, hypertriglyceridemia, hypo-high-density lipoprotein cholesterolemia, prediabetes, homeostatic model assessment insulin resistance score ≥2.5. The number of MAFLD risk factors was significantly greater in subjects with both MAFLD and significant liver fibrosis than in subjects without MAFLD or those with MAFLD but without significant liver fibrosis (all p for trend <0.001). The analysis was conducted after matching groups with propensity scores.
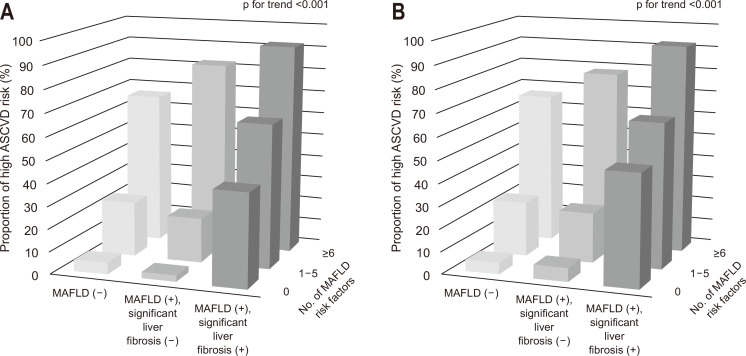
When the number of ASCVD risk factors (hypertension, diabetes, obesity, hypertriglyceridemia, hyper-LDL cholesterolemia, hypo-HDL cholesterolemia, and chronic kidney disease) was evaluated, we found that these risk factors gradually increased from participants without MAFLD to those with both MAFLD and significant liver fibrosis, irrespective of the liver fibrosis prediction models (Fig. 3). If there were comparable ASCVD risk factors, subjects with both MAFLD and significant liver fibrosis had a much higher chance of high probability ASCVD risk.
Fig. 3.
Proportion of subjects with high probability atherosclerotic cardiovascular disease (ASCVD) risk with individual ASCVD risk components according to the fibrosis-4 index- (A) or nonalcoholic fatty liver disease fibrosis score-defined (B) significant liver fibrosis. Risk factors included hypertension, diabetes, obesity, hypertriglyceridemia, hyper-low-density lipoprotein cholesterolemia, hypo-high-density lipoprotein cholesterolemia, and chronic kidney disease. The number of metabolic dysfunction associated fatty liver disease (MAFLD) risk components was significantly greater in subjects with MAFLD and significant liver fibrosis than in subjects without MAFLD or those with MAFLD but without significant liver fibrosis (all p for trend <0.001).
5. High probability ASCVD risk depends on the presence of MAFLD/significant liver fibrosis
The association between high probability ASCVD risk and the existence of MAFLD/significant liver fibrosis after multi-step adjustments is shown in Table 3. When the fibrosis-4 index was used to characterize significant liver fibrosis and the risks for high probability ASCVD risk were evaluated after sufficient adjustment (model 3), subjects with both MAFLD and significant liver fibrosis had a significantly higher risk for high probability ASCVD risk (OR=2.40, 95% CI=1.75 to 3.29; p<0.001), followed by subjects with MAFLD but without significant liver fibrosis (OR=1.44, 95% CI=1.06 to 1.94; p=0.019).
Table 3.
High ASCVD Risk According to the Presence of MAFLD/Significant Liver Fibrosis
| Adjustment | Fibrosis-4 index, OR (95% CI) | NAFLD fibrosis score, OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| MAFLD (–) | MAFLD (+), significant liver fibrosis (–) |
MAFLD (+), significant liver fibrosis (+) |
MAFLD (–) | MAFLD (+), significant liver fibrosis (–) |
MAFLD (+), significant liver fibrosis (+) |
||
| Model 1 | 1.00 (reference) |
1.98 (1.66–2.36) p<0.001 |
2.55 (2.09–3.10) p<0.001 |
1.00 (reference) |
1.78 (1.51–2.10) p<0.001 |
3.56 (2.84–4.45) p<0.001 |
|
| Model 2 | 1.00 (reference) |
2.05 (1.67–2.52) p<0.001 |
2.91 (2.31–3.68) p<0.001 |
1.00 (reference) |
1.90 (1.57–2.31) p<0.001 |
4.05 (3.09–5.30) p<0.001 |
|
| Model 3 | 1.00 (reference) |
1.44 (1.06–1.94) p=0.019 |
2.40 (1.75–3.29) p<0.001 |
1.00 (reference) |
1.60 (1.21–2.12) p=0.001 |
2.77 (1.92–4.01) p<0.001 |
|
| Model 4 | 1.00 (reference) |
3.90 (1.90–8.04) p<0.001 |
4.00 (2.00–7.98) p<0.001 |
1.00 (reference) |
3.95 (2.02–7.73) p<0.001 |
3.98 (1.83–8.63) p<0.001 |
|
Model 1: adjusted for sex and age (per 20 years). Model 2: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, and previous ASCVD history. Model 3: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, previous ASCVD history, systolic blood pressure, fasting blood glucose, body mass index, HOMA-IR, chronic kidney disease, hyper-LDL cholesterolemia, and muscle mass. Model 4: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, previous ASCVD history, systolic blood pressure, fasting blood glucose, body mass index, HOMA-IR, chronic kidney disease, hyper-LDL cholesterolemia, and muscle mass in propensity score matching analysis.
ASCVD, atherosclerotic cardiovascular disease; MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein.
When the NAFLD fibrosis score was reflected to categorize significant liver fibrosis, similar results were found (OR=1.60 in subjects with MAFLD but without significant liver fibrosis [p=0.001] and OR=2.77 in subjects with both MAFLD and significant liver fibrosis [p<0.001]). In addition, subgroup analysis after propensity score matching maintained similar results.
6. Respective impact of MAFLD and significant liver fibrosis on high probability ASCVD risk
The respective impact of MAFLD and significant liver fibrosis on high probability ASCVD risk was further analyzed using another multiple logistic analysis (Table 4). When sex, age, exercise, smoking, alcohol consumption, previous cardiovascular disease history, chronic kidney disease, hyper-LDL cholesterolemia, and muscle mass were adjusted (model 3), the impact of MAFLD was greater than that of significant liver fibrosis using fibrosis-4 index (OR=4.72 for MAFLD vs OR=1.88 for significant liver fibrosis, all p<0.001).
Table 4.
High ASCVD Risk Associated with MAFLD and Significant Liver Fibrosis
| Adjustment | Fibrosis-4 index, OR (95% CI) | NAFLD fibrosis score, OR (95% CI) | |||
|---|---|---|---|---|---|
| MAFLD | Significant liver fibrosis | MAFLD | Significant liver fibrosis | ||
| Model 1 | 4.44 (3.53–5.59) p<0.001 |
1.64 (1.40–1.92) p<0.001 |
3.53 (2.81–4.43) p<0.001 |
2.40 (2.02–2.86) p<0.001 |
|
| Model 2 | 4.52 (3.49–5.86) p<0.001 |
1.76 (1.46–2.13) p<0.001 |
3.61 (2.79–4.67) p<0.001 |
2.53 (2.05–3.13) p<0.001 |
|
| Model 3 | 4.72 (3.57–6.24) p<0.001 |
1.88 (1.54–2.29) p<0.001 |
3.92 (2.97–5.18) p<0.001 |
2.72 (2.18–3.39) p<0.001 |
|
| Model 4 | 1.49 (1.09–2.05) p=0.014 |
1.87 (1.53–2.31) p<0.001 |
1.44 (1.05–1.98) p=0.023 |
1.74 (1.35–2.23) p<0.001 |
|
Model 1: adjusted for sex and age (per 20 years). Model 2: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, and previous ASCVD history. Model 3: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, previous ASCVD history, chronic kidney disease, hyper-LDL cholesterolemia, and muscle mass. Model 4: adjusted for sex, age (per 20 years), exercise, current smoking, alcohol consumption, previous ASCVD history, chronic kidney disease, muscle mass, glycemic status (prediabetes, diabetes), hypertension, and hypertriglyceridemia.
ASCVD, atherosclerotic cardiovascular disease; MAFLD, metabolic dysfunction-associated fatty liver disease; OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; LDL, low-density lipoprotein.
When the NAFLD fibrosis score was used to define significant liver fibrosis, similar findings were observed (OR=3.92 for MAFLD vs OR=2.72 for significant liver fibrosis, all p<0.001). In addition, after adjustment of MAFLD components, the results were still maintained.
7. Risk assessments for significant liver fibrosis in subjects with MAFLD
To demonstrate the risk factors for significant liver fibrosis in subjects with MAFLD, multiple logistic analyses were performed (Table 5). When the fibrosis-4 index was applied to determine significant liver fibrosis, only sarcopenia was associated with a higher risk of significant liver fibrosis (OR=2.43, 95% CI=0.92 to 3.07; p<0.001). When NAFLD fibrosis score was used to characterize significant liver fibrosis, sarcopenia still had the highest risk with statistical significance (OR=1.56, 95% CI=1.22 to 1.98; p<0.001), followed by a higher BMI, fasting blood glucose, systolic blood pressure, HOMA-IR, and chronic kidney disease (all p<0.05).
Table 5.
Risk of Significant Liver Fibrosis in Subjects with MAFLD
| Variable | By fibrosis-4 index | By NAFLD fibrosis score | |||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Sarcopenia* | 2.43 (1.92–3.07) | <0.001 | 1.56 (1.22–1.98) | <0.001 | |
| Body mass index, kg/m2 | 1.02 (0.98–1.06) | 0.425 | 1.19 (1.15–1.24) | <0.001 | |
| Fasting blood glucose, mg/dL | 1.00 (1.00–1.01) | 0.927 | 1.05 (1.01–1.02) | <0.001 | |
| Systolic blood pressure, mm Hg | 1.00 (0.99–1.01) | 0.907 | 1.01 (1.01–1.02) | 0.002 | |
| HOMA-IR† | 0.94 (0.75–1.19) | 0.631 | 1.42 (1.11–1.80) | 0.005 | |
| Chronic kidney disease‡ | 1.37 (0.97–1.94) | 0.071 | 1.52 (1.09–2.12) | 0.015 | |
| Triglyceride† | 1.06 (0.89–1.27) | 0.505 | 0.90 (0.75–1.80) | 0.276 | |
Adjusted for age (per 20 years), age × sarcopenia index, and sex.
MAFLD, metabolic dysfunction-associated fatty liver disease; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance.
*Sarcopenia was defined as the lowest quintile of the sex-specific sarcopenia index; †Log transformed; ‡Chronic kidney disease was defined as an estimated glomerular filtration rate less than 60 mL/min/1.73 m2.
DISCUSSION
Recently, the novel definition of MAFLD was proposed to focus more on the clinical significance of metabolic derangement, regardless of accompanying etiologies for liver diseases. However, it has also been proposed that disease extent can be explained by the level of activity and the stages of liver fibrosis, which is comparable to the definition has been adopted for other chronic liver diseases and recognizes that MAFLD is a continuum.10 However, no study has proven the clinical significance of risk assessment based on fibrotic burden in subjects with MAFLD. In our study, individuals with both MAFLD and significant liver fibrosis had unfavorable demographic and laboratory data similar to subjects with MAFLD but without significant liver fibrosis. When compared to individuals without MAFLD, those MAFLD groups with and without significant liver fibrosis had independently higher risks of having cardiometabolic risk factors. Regarding ASCVD risk, reflected by high probability ASCVD risk, individuals with MAFLD but without significant liver fibrosis showed a 1.44 (by fibrosis index-4) to 1.60 (by NAFLD fibrosis score) fold higher risk of high probability ASCVD risk. However, the risks increased up to 2.40 (by fibrosis index-4) and 2.77 (by NAFLD fibrosis score) increased the risk of high probability ASCVD risk in subjects with MAFLD and significant liver fibrosis. All these might indicate that it is clinically critical to classify subjects with MAFLD who are at a risk of experiencing ASCVD events. Beside to the identification of MAFLD, we showed that further assessment of fibrotic burden in subjects with MAFLD might provide a more detailed prognosis regarding ASCVD outcomes.
Our study has several clinical advantages. First, recently, a new approach of MAFLD was proposed, which is established on histological (biopsy), imaging, or blood biomarker confirmation of hepatic fat accumulation, along with one of the following essential components: overweight/obesity, diabetes mellitus, or presence of metabolic abnormalities. Similar to what has been admitted for other chronic liver diseases, disease severity should be assessed using fibrotic burden in the liver and the grade of activity.10 In parallel with the well-known fact that the stage of liver fibrosis is the only strong predictor of long-term prognosis, including ASCVD development, international experts also recommended risk stratification according to fibrotic burden among subjects with MAFLD,1,7,8 which might be strongly supported by the conclusions of our current study. We found that the risk of having high probability ASCVD risk increased mildly in individuals with MAFLD but without significant liver fibrosis (OR <2), whereas it increased abruptly in individuals with both MAFLD and significant liver fibrosis alone (OR around 10), which might indicate that therapeutic intervention should be considered in the early stage of liver fibrosis progression in subjects with MAFLD. Even after appropriate adjustment and propensity score matching, the significant influence of significant liver fibrosis and MAFLD itself was maintained. However, when the risk of the respective cardiometabolic risk factors was assessed, the risk of chronic kidney disease and proteinuria was found to be higher, whereas the risk of dyslipidemia and sarcopenia was found to be scanty in subjects with both MAFLD and significant liver fibrosis than in those with MAFLD but without significant liver fibrosis.
Second, the risk assessment of ASCVD using the stage of liver fibrosis in our study as a potential link between MAFLD and ASCVD might be powered by several previous studies. A recent study by You et al.8 presented that alanine aminotransferase level and coronary artery calcification score are the sole independent components associated with fibrotic burden, estimated using transient elastography in subjects with NAFLD. In another U.S. study by Kim et al.,7 the National Health and Nutrition Examination Survey from 1988 to 1994 were explored subsequent follow-up data (median, 14.5 years) for mortality was collected. The results clearly showed that advanced fibrosis scores, not NAFLD, was associated with higher mortality. In contrast to participants without liver fibrosis, subjects with a high probability of progressed liver fibrosis had a 69% increase in mortality, which was mostly due to ASCVD events after adjusting for other confounders. Similar to these findings in subjects with NAFLD, this finding does not seem surprising that the severity of liver fibrosis significantly influenced the risk of ASCVD in subjects with MAFLD due to the significant overlap between NAFLD and MAFLD and more focus on metabolic derangements in subjects with MAFLD, which might further increase the risk of ASCVD. This is also supported by our results that the number of MAFLD and ASCVD risk factors significantly increased in association with the status of MAFLD and significant liver fibrosis (from with MALFD and MAFLD without significant liver fibrosis to MAFLD with significant liver fibrosis). The mechanisms by which liver fibrosis may immediately contribute to the pathogenesis of ASCVD are not fully known, and it is likely that several possible pathophysiological mechanisms are engaged. It has been suggested that systemic inflammation, endothelial dysfunction, and oxidative imbalance are related to the degree of fibrosis, thereby inducing atherosclerosis.20,21
Third, the current study observed that sarcopenia was highly prevalent (~43%) among subjects with both MAFLD and significant liver fibrosis, which was higher than that in individuals with MAFLD but without significant liver fibrosis (25.5%) and those without MAFLD (13.6%). Similarly, it has been known that individuals with both NAFLD and liver fibrosis progression also showed a significantly higher prevalence of sarcopenia.22 As we previously demonstrated that the coordinated impact of decreased muscle mass and significant liver fibrosis on ASCVD risks,5 loss of skeletal muscle mass may be responsible for increased ASCVD among subjects with MAFLD. Furthermore, the close relationship between muscle mass loss and significant liver fibrosis was more predominant among subjects with MAFLD (OR, 1.56 to 2.43) than among subjects with NAFLD (OR, 1.30 to 1.92) reported in our previous study,23 although different definitions of sarcopenia were applied. These findings imply that evaluation of sarcopenia, in addition to liver fibrosis assessment, might provide additional clinical implications for the appropriate therapeutic intervention by identifying subjects with MAFLD who are facing ASCVD risk.
Fourth, considering the burden of MAFLD with significant liver fibrosis on ASCVD risks, characterization of individuals with significant liver fibrosis is important to identify high-risk populations among MAFLD. Common risk components including obesity and binge drinking were not linked with the risk of significant liver fibrosis among subjects with MAFLD in our study. However, participants with both MAFLD and significant liver fibrosis showed a much greater prevalence of diabetes, metabolic syndrome, sarcopenia, and chronic kidney disease, regardless of the definition of liver fibrosis indices. Further studies with detailed information on liver fibrosis using liver biopsy or imaging are necessary to establish predictive risk factors of significant liver fibrosis among individuals with MAFLD. In addition, when the respective contribution to the risk of ASCVD of MAFLD and significant liver fibrosis was compared, the presence of MAFLD had a 1.5- to 2.5-fold higher influence on the risk of ASCVD than the presence of significant liver fibrosis (OR=3.92 to 4.72 for MAFLD vs OR=1.88 to 2.72 for significant liver fibrosis in a sufficiently adjusted model [model 3], respectively), indicating that the identification of MAFLD is more important for ASCVD risk stratification and subsequent assessment fibrotic burden in the liver should be followed for more detailed risk stratification.
In spite of the clinical significances of the current study, there were several limitations. First, while we used a well-proven liver fibrosis prediction model,13 data of liver images and histological findings were unavailable by reason of the high cost of image test and the moral arguments respecting the screening of a large national population-based cohort. Nevertheless, we believe that the core present study highlighted could support the cornerstone for prospective and long-term follow-up studies targeting on the prognostic importance of MALFD and accompanying liver fibrosis on ASCVD risk. Second, due to the study design, based on the cross-sectional data, the continuing dynamic interaction between status changes in fibrotic burden and changes in the ASCVD risk could not be determined. We failed to analyze the impacts of nutritional and physical therapies on the disappearance of MAFLD, fibrosis regression, and ASCVD risk. The conclusions in the current study indicated the demand for screening the MAFLD population to identify individuals who are facing ASCVD risk. Third, a pooled cohort risk equation was applied to predict ASCVD risk and we did not investigate the actual risk in subsequent follow-up. The 10-year ASCVD risk estimation model was originally developed for primary prevention based on the blood cholesterol. Moreover, there might be overestimation of ASCVD risk prediction in the Asian ethnicities, our findings should be carefully comprehended.24 Lastly, despite the fact that we adjusted for individuals with prior ASCVD history, there might be feasible that subjects with undiagnosed cerebrovascular disease or obscure coronary artery disease might have affected the results of the present study, since silent stroke or myocardial infarction can be diagnosed in up to 40% of an elderly population with high risk.25
In conclusion, individuals with MAFLD had a substantially increased risk of ASCVD than those without MAFLD. Accompanying significant liver fibrosis further enhanced the risk of ASCVD among subjects with MAFLD. Risk stratification established on the degree of liver fibrosis might be required for subjects with MAFLD. Further studies are necessary to clarify whether current screening strategies for ASCVD risks should be intensified in subjects with MAFLD, especially showing significant liver fibrosis.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210290.
ACKNOWLEDGEMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (number: 2019R1A2C4070136). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
S.U.K. has served as an advisory committee member of Gilead Sciences, GSK, Bayer, and Eisai. He is a speaker for Gilead Sciences, GSK, Bayer, Eisai, Abbvie, EchoSens, MSD, and Bristol-Myers Squibb. He has also received a research grant from Abbvie, Bristol-Myers Squibb. The other authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Study concept and design: Y.H.L., S.U.K. Data acquisition: E.H. Data analysis: E.H., Y.H.L., S.U.K. Data interpretation: E.H., Y.H.L., S.U.K., J.S.L., H.W.L., B.K.K., J.Y.P., D.Y.K., S.H.A., B.W.L., E.S.K., B.S.C. Statistical analysis: E.H. Manuscript drafting: E.H., Y.H.L., S.U.K., J.S.L., H.W.L., B.K.K., J.Y.P., D.Y.K., S.H.A., B.W.L., E.S.K., B.S.C. Study supervision: Y.H.L., S.U.K. Manuscript review & editing: E.H., Y.H.L., S.U.K. All authors read and approved the final manuscript.
REFERENCES
- 1.Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 3.Chitturi S, Wong VW, Chan WK, et al. The Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017-Part 2: management and special groups. J Gastroenterol Hepatol. 2018;33:86–98. doi: 10.1111/jgh.13856. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 5.Han E, Lee YH, Kim YD, et al. Nonalcoholic fatty liver disease and sarcopenia are independently associated with cardiovascular risk. Am J Gastroenterol. 2020;115:584–595. doi: 10.14309/ajg.0000000000000572. [DOI] [PubMed] [Google Scholar]
- 6.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–1365. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You SC, Kim KJ, Kim SU, et al. Hepatic fibrosis assessed using transient elastography independently associated with coronary artery calcification. J Gastroenterol Hepatol. 2015;30:1536–1542. doi: 10.1111/jgh.12992. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Sanyal AJ, George J International Consensus Panel, author. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 10.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 14.Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Bilek S, Rosenbaum S. Recent National Cholesterol Education Program Adult Treatment Panel III update: adjustments and options. Am J Cardiol. 2005;96(4A):53E–59E. doi: 10.1016/j.amjcard.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Musso G, Gambino R, De Michieli F, et al. Association of liver disease with postprandial large intestinal triglyceride-rich lipoprotein accumulation and pro/antioxidant imbalance in normolipidemic non-alcoholic steatohepatitis. Ann Med. 2008;40:383–394. doi: 10.1080/07853890801946515. [DOI] [PubMed] [Google Scholar]
- 22.Wijarnpreecha K, Kim D, Raymond P, Scribani M, Ahmed A. Associations between sarcopenia and nonalcoholic fatty liver disease and advanced fibrosis in the USA. Eur J Gastroenterol Hepatol. 2019;31:1121–1128. doi: 10.1097/MEG.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Kim SU, Song K, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 24.Chia YC, Lim HM, Ching SM. Validation of the pooled cohort risk score in an Asian population: a retrospective cohort study. BMC Cardiovasc Disord. 2014;14:163. doi: 10.1186/1471-2261-14-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.