Abstract
The Escherichia coli DNA polymerase III τ and γ subunits are single-strand DNA-dependent ATPases (the latter requires the δ and δ′ subunits for significant ATPase activity) involved in loading processivity clamp β. They are homologous to clamp-loading proteins of many organisms from phages to humans. Alignment of 27 prokaryotic τ/γ homologs and 1 eukaryotic τ/γ homolog has refined the sequences of nine previously defined identity and functional motifs. Mutational analysis has defined highly conserved residues required for activity in vivo and in vitro. Specifically, mutations introduced into highly conserved residues within three of those motifs, the P loop, the DExx region, and the SRC region, inactivated complementing activity in vivo and clamp loading in vitro and reduced ATPase catalytic efficiency in vitro. Mutation of a highly conserved residue within a fourth motif, VIc, inactivated clamp-loading activity and reduced ATPase activity in vitro, but the mutant gene, on a multicopy plasmid, retained complementing activity in vivo and the mutant gene also supported apparently normal replication and growth as a haploid, chromosomal allele.
The dnaX polymerization gene of Escherichia coli encodes two DNA polymerase III components, τ and γ. τ is the full-length translational product of the DnaX reading frame. The shorter γ is identical to the first 430 residues of τ, but its C terminus is generated by a programmed −1 ribosomal frameshift which results in the incorporation of a glutamate as the 431st amino acid followed by a stop codon (3, 19, 63).
τ functions as the replisome organizer, dimerizing the core polymerase (30, 33, 43, 58) and interacting with and stimulating the replicative DnaB helicase and primase (31, 73). τ also contributes to processivity by stabilizing the processivity clamp (32) and the holoenzyme (73) on the leading strand.
γ functions in a five-subunit complex (γ2–4-δ-δ′-ψ-χ) (12, 20, 42, 49, 51, 62) to load and unload the processivity clamp β (2, 5, 25, 26, 45, 47, 59, 67). The binding of two or three ATP molecules by the γ subunit of the complex alters the conformation of the complex, allowing δ to bind directly to and open the clamp and allowing assembly of a primed DNA-open clamp-γ complex structure (25, 26, 45). Hydrolysis of the ATP, required for closing the clamp around primed DNA, occurs in two sequential steps. The first might release β from the γ complex; the second might then release DNA (enclosed within the clamp). Alternatively, the first hydrolysis might release DNA from the γ complex into the open clamp and the second would then release β (encircling the DNA). Another possibility for the second hydrolysis might be resetting of the γ complex for the next cycle (26).
The N-terminal region of τ is identical to γ, except for the 431st residue, and is capable of all the known activities of γ in vitro, including loading β (with δ or δ-δ′) (59) and assembly in vitro or in vivo (from an artificial τ-complex operon) into clamp-loading τ complexes (τ2–4-δ-δ′-ψ-χ) (13, 49, 54). The γ complex is often thought to be the principal clamp-loading machine because it, but not the τ complex, has been isolated from cellular extracts and from holoenzyme (42, 51). Moreover, DNA polymerase III (Pol III) lacking β but assembled with ATP-binding mutant γ could not load β, whereas that assembled with ATP-binding mutant τ could (72). On the other hand, another study found that β clamp loading by the τ complex was more characteristic of the loading reaction of native holoenzyme than the γ-complex-dependent reaction (13) and that the γ subunit is dispensable in vivo whereas τ is essential for growth (4). These data indicate uncertainty as to the individual roles of τ and γ in clamp loading in vivo.
Clamp-loading proteins of various organisms from phage T4 to prokaryotes to humans have significant homology over much of their lengths, including several highly conserved regions (8, 11, 14, 21, 23, 46). We report here highly conserved residues essential for DnaX+-complementing activity in vivo and DNA-dependent ATPase and β clamp loading in vitro.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
The dnaX+ strain C600 was from the laboratory collection. Strain AB27a is a dnaX(Ts)2016 zbb::Tn10 derivative of strain C600. It was constructed by P1 transduction (70) from a zbb::Tn10 derivative of dnaX(Ts) strain AX733 (9). Strain AB28 is a zib::Tn10 dnaX+ rpsL derivative of strain C600, with Tn10 closely linked to dnaA+. Strain BL21(DE3)pLysS was the host for production of τ and γ proteins. Strain SM10 λpir (44a) was used to propagate Pir protein-dependent plasmid pKAS32 (55a) and its derivatives. Phage MO1 consists of a 2,350-bp PstI-SmaI dnaX+ fragment cloned into PstI- and SmaI-cut M13mp19. pET X+ is a derivative of pET 21a+ with the wild-type dnaX gene on a 2.3-kb EcoRI-AvaI fragment from phage MO1 cloned downstream of the T7 promoter. A mutant dnaX allele with the threonine codon 142 changed to alanine (dnaXT142A) was cloned as an amplified 2.35-kb KpnI-HindIII fragment (including some polylinker sequence) from a dnaXT142A derivative of MO1 into similarly cut temperature-sensitive (TS) suicide vector pMAK705 (23a), generating pABMXT142A. The dnaXT142A mutant allele was cloned also as 2.3-kb KpnI-PvuII fragment, including some polylinker, from the dnaXT142A derivative of MO1 into rpsL+ Apr pKAS32 (55a) cut with KpnI and EcoRV, generating pKXT1. Yeast extract (0.5%)-tryptone (1%) medium containing 0.5% NaCl was supplemented with ampicillin (200 μg/ml), chloramphenicol (30 μg/ml), streptomycin sulfate (500 μg/ml), or tetracycline (25 μg/ml), as needed.
DNA preparations.
Plasmids were purified by use of Qiagen Miniprep spin columns and chromosomal DNA was purified by use of Puregene DNA isolation kits (Gentra Systems) according to the manufacturers' directions. For determining the chromosomal dnaX sequence, a 2.2-kb fragment was amplified from chromosomal DNA by the Expand high-fidelity PCR system using Taq and Pwo DNA polymerases (Boehringer Mannheim) and sequenced with an ABI Prism 377 DNA sequencer.
Other sources.
α-ε, δ, δ′, δ-δ′, β, and single-strand binding protein (SSB) were generous gifts from Mike O'Donnell. Other reagents were ultrapure ammonium sulfate (Baker), FastFlow Q and Sp Sepharose (Pharmacia), phenylmethylsulfonyl fluoride (PMSF) (Sigma), pET 21a+ and host strain BL21(DE3)pLysS (Novagen), and molecular mass markers (Bio-Rad). Restriction enzymes were from Promega; T4 DNA ligase was from Ambion, Inc. (Austin, Tex.); oligonucleotides were from Life Sciences or Bio-Synthesis, Inc. (Lewisville, Tex.). All oligonucleotide sequences are available on request.
Mutagenesis.
Phage MO1 was mutagenized by the Kunkel technique (35). The dnaX strand complementary to the messenger was present in the single-strand DNA of the hybrid phage. Confirmed mutant alleles were cloned from replicative-form DNA into pET X+, replacing an AatII-AvaI fragment.
Gene replacement.
Two independent procedures were used to introduce the dnaXT142A allele into the chromosome in the haploid condition. The first procedure used the suicide vector pMAK705 of Hamilton et al. (23a). The T142A mutation in pABMXT142A was verified by sequencing, and the plasmid was electroplated into strain C600 using chloramphenicol resistance at 44°C to select cointegrates. The absence of detectable plasmid DNA was verified in eight transformants, which were then pooled and grown at 30°C for three cycles each of 12 generations in the presence of chloramphenicol to allow resolution. From nine resolution cultures, four plasmids carried the mutant allele and five carried the dnaX+ allele. Two of the cultures carrying the wild-type allele on the plasmid were cured by six generations of growth at 44°C in the absence of chloramphenicol. These no longer contained detectable plasmids, and sequencing the chromosomal dnaX over the region containing codon 142 confirmed that the wild-type allele had been replaced by the dnaXT142A mutant allele.
The second transplacement procedure was based on the Pir-dependent vector pKAS32 (44a, 55a). The dnaXT142A derivative of pKAS32 was transferred by conjugation from strain SM10 λpir to the nonpermissive dnaX+ rpsL host, strain AB28, selecting for ampicillin and tetracycline resistance. Only strains containing pKXT1 integrated into the chromosome and diploid for dnaX+ and dnaXT142A could grow in the presence of both antibiotics. The rpsL+ (on the integrated plasmid) and rpsL (in the normal chromosomal location) cointegrates were streptomycin sensitive, reflecting dominance of the wild-type allele (36a, 55a). Selection of derivatives which had both resolved the cointegrant structure to form again plasmids and which had been cured because the plasmid could not replicate was done in one step by plating on streptomycin-containing medium. The spontaneously arising streptomycin-resistant derivatives occurred with a frequency of about 2 × 10−4 and consisted of strains which carried haploid dnaX+ or dnaXT142A. Sequencing chromosomal DNA over the region of dnaX codon 142 from four such haploid strains confirmed that one was dnaXT142A and three were wild type.
Purification of wild-type and mutant τ and γ.
Strain BL21(DE3)pLysS was transformed with pET X+ or pET X encoding each of the mutations D126N, T142A, and R169A, selecting on yeast extract-tryptone medium containing ampicillin and chloramphenicol (100 and 30 μg/ml, respectively). Six-liter cultures were grown at 30°C to an absorbance of 0.3 and were induced with 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h; the cells were centrifuged, resuspended in lysis solution A (50 mM Tris-HCl [pH 7.5], 10% sucrose, 0.5 mM protease inhibitor PMSF), and lysed by freezing and thawing (56) to produce Fr I. The purification (48) of τ and γ from pET X encoding R169A, which was typical, will be described. Fr I consisted of 48 ml containing 8 mg of protein/ml. Buffer A consisted of 50 mM Tris-HCl, 10% glycerol, and 0.1 mM EDTA. All solutions used in purification contained 0.5 mM PMSF. Ammonium sulfate precipitation, backwashing (41), and dialysis against buffer A plus 20 mM NaCl yielded Fr II: 40.7 mg of protein in 3.7 ml. A 2-ml Fast Flow Q Sepharose column was equilibrated with buffer A plus 1 mM dithiothreitol (DTT) and loaded with 40 mg of Fr II, washed with 20 ml of the same solution, and eluted with a 200-ml linear NaCl gradient in the same buffer. Both τ and γ eluted in (2-ml) fractions 48 to 69. After precipitation with 0.488 g of AmSO4/ml, dissolving in buffer A, and dialysis against 3 liters of buffer A containing DTT, Fr III contained 8.8 mg of protein in 4.6 ml. The mixture of τ and γ was loaded onto a 4-ml Fast Flow Sp Sepharose column, equilibrated with buffer A plus DTT, and washed with 40 ml of the same solution. Elution with a NaCl gradient in buffer A plus DTT (50 ml of 0 to 100 mM NaCl linear gradient, 50 ml of 100 mM NaCl, and 50 ml of 100 to 400 mM NaCl linear gradient) and collection of 2-ml fractions yielded Fr IV γ in fractions 16 to 20 and Fr IV τ in fractions 57 to 59. These fractions were dialyzed separately against buffer A plus DTT. The total yield of γ was 810 μg in 8.75 ml; the τ yield was 370 μg in 5.25 ml.
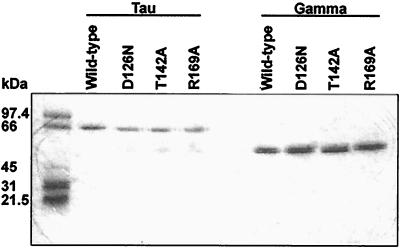
The mutant proteins eluted from both columns at conductivities similar to those at which the wild-type proteins eluted, suggesting that the mutations did not cause major disruption of structures. Electrophoresis and staining by Coomassie blue indicated that the γ preparations were essentially homogenous; all the τ preparations contained minor amounts of γ or γ-like material, which tended to accumulate during storage (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of wild-type and mutant τ and γ. Two micrograms of each protein was electrophoresed on a 10% polyacrylamide–0.1% SDS gel at 200 V and stained with Coomassie blue. Molecular mass markers are shown on the left.
ATPase assay.
The assay of Onrust and O'Donnell (50) was used. Each 20-μl assay mixture contained 840 ng of single-strand M13Gori1 DNA, 0.4 μCi of [α-32P]ATP in 2,000 pmol, and 0.5 to 2.0 pmol of τ or γ all in 20 mM Tris-HCl, pH 7.5–8 mM MgCl2–1 mg of bovine serum albumin/ml. If δ or δ′ was included, it was added at the level of 8 pmol. The assay mixtures were mixed on ice, and the assays were started by transferring the mixtures to 37°C; duplicate 1-μl samples were collected at 0, 15, and 30 min and spotted onto polyethyleneimine thin-layer chromatography plates which had been washed with deionized water and dried. The samples were spotted onto areas which had been prespotted with 5 μl of stop buffer (50 mM EDTA [pH 8.0], 1 mM [each] ATP and ADP) and dried. The labeled ATP and ADP were separated by chromatography with 750 mM K2HPO4 and quantitated by a Molecular Dynamics PhosphorImager and ImageQuaNT software. For kinetic studies, the ATP concentration was varied as shown in Fig. 3 and five samples were taken at 3-min intervals.
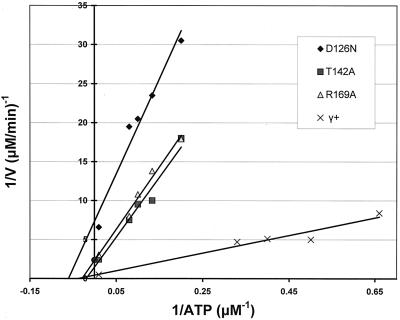
FIG. 3.
Lineweaver-Burk plot of wild-type and mutant γ ATPase activities with single-strand DNA and δ-δ′. The solid circle on the y axis represents +1 standard deviation from the mean (1.43) of the intercepts of the three lines representing γ+, γ T142A, and γ R169A.
Replication assay.
The assay for wild-type and mutant τ and γ in β clamp loading was based on the Onrust et al. (51) procedure. The 25-μl assay mixtures contained 0.5 or 2.0 pmol of γ or τ, 250 ng of uniquely primed (57) M13Gori1 single-strand DNA, 22.5 ng of α-ɛ complex (0.14 pmol), 10.5 ng of the β subunit (0.14 pmol as a dimer), 2 ng of δ-δ′ (0.025 pmol each), 980 ng of SSB (13.6 pmol as a tetramer), 1 μCi/500 pmol of [α-32P]dATP in a solution of 20 mM Tris-HCl (pH 7.5), 8 mM MgCl2, 500 μM ATP, 30 μM TTP, 60 μM (each) dGTP and dCTP, 18 mM NaCl, 5 mM DTT, 100 μM EDTA, 4% glycerol, and 40 μg of bovine serum albumin/ml. After 30 min at 37°C, 20 μl of the assay mixture was added to 1 ml of cold 5% trichloroacetic acid containing 10 mM sodium pyrophosphate, filtered onto Whatman 2.4-cm-diameter filters, and quantitated with a PhosphorImager as described above.
RESULTS
Motifs of τ and γ homologs.
A BLAST search (1) of GenBank release 118 using E. coli τ and γ residues 1 to 223, with a C substituted for the P-loop (GxxGxGKT) lysine to reduce the number of unrelated ATPases detected, identified 27 prokaryotic τ and γ, 10 prokaryotic δ′, and 33 eukaryotic clamp loader (replication factor C [RFC] or activator 1) (7, 17, 37, 38, 64, 65, 66) subunits. A hypothetical protein of eukaryotic origin, that of Arabidopsis thaliana (40) (GenBank accession no. AAC18938), is more closely related to prokaryotic τ and γ than to RFC subunits and has been included with τ and γ for motif identification. It is 26.5% identical to E. coli γ over the first 430 residues and is designated a τ and γ homolog. The 27 prokaryotic τ and γ homologs plus the Arabidopsis τ and γ homolog were aligned by CLUSTAL W (60), which employs the BLOSUM series of weight matrices (24). Nine highly conserved regions were identified and compared to previously defined clamp loader protein motifs. With the rare exceptions noted below, all 28 τ and γ homologs contained all nine motifs.
The N-terminal motif II was previously defined as (PL)WV(ED)KYrPxxU in RFC subunits (11, 21). The τ and γ consensus extends to 15 residues: r(KR)yRPx2Fx(ed)UUGQ(ed) (Table 1). (For the τ and γ motifs defined in this paper, boldface uppercase letters indicate invariant residues, uppercase letters indicate conservation in at least 90% of the proteins, lowercase letters indicate conservation in the majority of proteins, and parentheses indicate that one of the two reported residues is found at that position. U indicates aliphatic residues I, L, M, or V; & indicates bulky hydrophobic I, L, M, V, F, W, or Y. For previously reported consensus sequences, the conventions used by the authors to indicate degree of conservation are repeated here. The underlining provides a reference residue for comparing the RFC and τ and γ motifs.) Although both RFC subunits and τ and γ contain the KYRP region, the R is not strongly conserved in RFC proteins but both R and P are invariant in τ and γ. τ and γ also contain an invariant Q. Motif II is not present in the shorter Zymomonas mobilis τ and γ (GenBank accession no. AAF12838); its N terminal residue corresponds to E. coli residue 24. However, the Z. mobilis homolog contains all eight remaining motifs.
TABLE 1.
Clamp loader τ- and γ-subunit motifsc
| Motif | N residuea | Consensusb | C residuea |
|---|---|---|---|
| II | 8 | r(KR)yRPx2Fx(ed)UUGQ(ed) | 22 |
| III | 36 | (RK)x2ha(YF)UfsGxRGxGKT(ST)xA(RK)U | 57 |
| Zn | 63 | nCx6-13Cx2Cx2C | 79 |
| IV | 89 | D&&EUD(AG)ASx2gU(de)(de)xRxU | 107 |
| V | 121 | (KR)U&UUDE(VA)H(ML)U(ST) | 132 |
| VIc | 136 | fnaLLKtUEEPP | 147 |
| Sensor 1 | 152 | F&(FL)aTT(ED) | 158 |
| VII | 161 | (KR)UpxT(IV)xSR(CT) | 170 |
| VIII | 212 | GsxRDx2(ST)Ux(DE)q | 223 |
The numbers refer to N and C residues of E. coli τ and γ.
Single-letter abbreviations indicate amino acids. For details concerning the terminology, see Results.
The 28 organisms represented (GenBank accession numbers) are C. crescentus (AAB61695), Z. mobilis (AAF12838), E. coli (P06710), Salmonella enterica serovar Typhimurium (P74876), Haemophilus influenzae (P43746), Xylella fastidiosa (AAF84614), Neisseria meningitidis (CAB84884), Deinococcus radiodurans (AAF11953), Thermus thermophilus (AAB82595), Mycobacterium leprae (CAA19155), Mycobacterium tuberculosis (O69688), Streptomyces coelicolor (CAB56347), Synechocystis sp. (BAA17547), Bacillus subtilis (S13786), Thermotoga maritima (AAD35768), Borrelia burgdorferi (AAC66831), Treponema pallidum (AAC65953), Chlamydia trachomatis (AAC67929), Chlamydia muridarum (AAF39442), Chlamydophila pneumoniae (AAD18193), Helicobacter pylori (AAD07767), Campylobacter jejuni (CAB73411), Mycoplasma genitalium (P47659), Mycoplasma pneumoniae (P75177), Ureaplasma urealyticum (AAF30492), Rickettsia prowazekii (CAA15289), Aquifex aeolicus (AAC07663), and the eukaryote A. thaliana (ACC18938).
Motif III includes Walker phosphate-binding motif A with the typical GxxGxGKT P loop (68). The τ and γ box III consensus is (RK)x2ha(YF)UfsGxRGxGKT(ST)xA(RK)U; the RFC version is hUUuyGPPGtGKT(ST)t (11, 21). A major difference is the presence of the charged (RK) residues bracketing the τ and γ P loop.
A zinc-binding module (23) is present in all τ and γ subunits, with one exception. In the E. coli τ and γ, the pattern is nCx8Cx2Cx2C (Table 1). Among 27 proteins, the number of residues between the first two cysteines ranged from 6 to 13, with 8 being the most common. The one exceptional protein, that from Caulobacter crescentus (71) (GenBank accession no. AAB61695), has no Zn module. The N-terminal N and the C-terminal C are conserved, but the internal 15 residues are not. The Caulobacter protein contains the remaining eight motifs.
τ and γ motif IV consists of D&&EUD(AG)ASx2gU(de)(de)xRx(U), the invariant D (underlined) replacing the highly conserved N (underlined) of RFC proteins: LEUNaSDxR (11, 21). τ and γ motif IV is strongly conserved, with three invariant residues and five residues consisting of one of a group of related amino acids.
Motif V contains the DExx motif common to ATPases and helicases (6, 22, 27) and consists of 12 highly conserved residues, (KR)U&UUDE(VA)H(ML)U(ST), 3 of which are invariant and 5 of which are always hydrophobic. The RFC DExx motif is (FHY)kUUUUDExD (11, 21). The DExx motif has been divided into DEAD, DEAH, and DExH subfamilies (10, 34, 55). The τ and γ homologs belong to the DExH group, whereas the RFC subunits belong to a DExD group. The Synechocystis τ and γ (29, 40a) GenBank accession no. BAA17547) DECH is interrupted by a 430-residue intein between the DE and the CH. Otherwise, this protein contains the remaining eight conserved τ and γ motifs.
τ and γ motif Vlc is fnaLLKtUEEPP compared to s(ML)TxxAQxALRRxxE of eukaryotic RFC subunits 2 to 5 (11, 21). A distinctive feature of RFC subunits is QxAL, whereas that of τ and γ is fnaLL. The C-terminal PP is also distinctive for τ and γ; those residues are not present in RFC subunits. The τ and γ region is designated VIc to distinguish it from VIa and VIb of RFC 1 and 2 to 5 subunits, respectively (11).
The sensor 1 motif, proposed by Guenther et al. (23) as common to RFC, RuvB, and τ and γ, consists of residues FLLATT. Among 28 τ and γ proteins, this motif is strongly conserved as F&(FL)aTT(ED).
The τ and γ SRC region, motif VII, is (KR)UpxT(IV)xSR(CT). The S and R are invariant, whereas the C, invariant in RFC subunits (11, 21), is present in 22 of 28 τ and γ proteins but is replaced by the T in the remaining 6.
Motif VIII of τ and γ consists of GsxRDx2(ST)Ux(DE)q, with the GsxRD being invariant. This region overlaps the sensor 2 motif proposed by Guenther et al. (23) as GSLRDA (in E. coli) and RFC motif VIII, gdURxx(LI)xxlq, of Cullman et al. (11).
The BLAST search also detected fragments of a 28th prokaryotic τ and γ homolog. Two short Clostridium perfringens (GenBank accession no. X86478) fragments of 23 and 50 residues, encoded by different reading frames, were 65 and 48% identical to E. coli τ and γ residues 11 to 33 and 36 to 85. These fragments, which contained a portion of motif II, the P loop, and Zn module, were not included in the CLUSTAL W compilation.
Motif residues essential for DnaX+ activity in vivo.
Single-base substitutions were introduced into the P loop and the DExx, VIc, and SRC motifs of dnaX, cloned in the pET 21a vector, and the resulting plasmids were transferred into TS dnaX mutant strain AB27a for complementation analysis. The TS mutation in this strain changes codon 118 from a glycine to an aspartate codon (4) in both τ and γ. DNA polymerization stops abruptly when this mutant is shifted to a nonpermissive temperature (18). Specific mutations included the P loop G45A and K51A, DExx motif D126E and D126N, motif VIc T142A, and SRC motif R169A. Transformants selected at 30°C were tested for growth at the nonpermissive 42°C by streaking plates and quantitatively by efficiency of plating (Table 2). Although the wild-type dnaX allele on the plasmid restored growth at high temperature, all the point mutations, except T142A, inactivated complementing activity. (The pET 21a vector is designed to limit uninduced expression by virtue of a lacI gene and the presence of the lac operator upstream of the cloning site, but the basal [i.e., uninduced] level of dnaX expression from this vector was adequate to provide wild-type complementing activity.) The dnaXT142A allele restored recipient growth at 42°C.
TABLE 2.
τ and γ residues essential for DnaX+ complementation activity in strain AB27a, a dnaX(Ts) host
| Plasmid/mutation | Motif | Growth
at 42°C by:
|
|
|---|---|---|---|
| Streaka | EOPb | ||
| None | − | 2 × 10−7 | |
| pET X+ | + | 1.0 | |
| pET X/G45A | P loop | − | 2 × 10−4 |
| pET X/K51A | P loop | − | 2 × 10−4 |
| pET X/D126E | DExx | − | 5 × 10−4 |
| pET X/D126N | DExx | − | 3 × 10−4 |
| pET X/T142A | Vlc | + | 0.87 |
| pET X/R169A | SRC | − | 6 × 10−4 |
+, colony formation on yeast extract-tryptone plates; −, no growth or residual growth.
Relative to growth at 30°C. EOP, efficiency of plating.
Moreover, the dnaXT142A allele supported normal growth when transferred into the chromosome as a haploid gene. Two different strategies were used to generate mutants carrying only the dnaXT142A allele. The first was based on the TS suicide vector pMAK705 (23a) and produced five haploid dnaXT142A derivatives of strain C600, one of which is designated AB1000, after resolution of a heterozygous cointegrate strain. The corresponding wild-type strain, also produced by resolution, is designated AB1001. The second transplacement strategy made use of Pir-dependent pKAS32 (55a) and produced one haploid dnaXT142A derivative of strain AB28 (designated strain RCC2C). The corresponding wild-type is strain RCC1A. These dnaXT142A mutants grew in liquid medium under laboratory conditions at approximately the same rates as their respective wild-type strains and plated with efficiencies of approximately 1 at 20, 34, 39, and 42°C.
ATPase activity of wild-type and mutant γ.
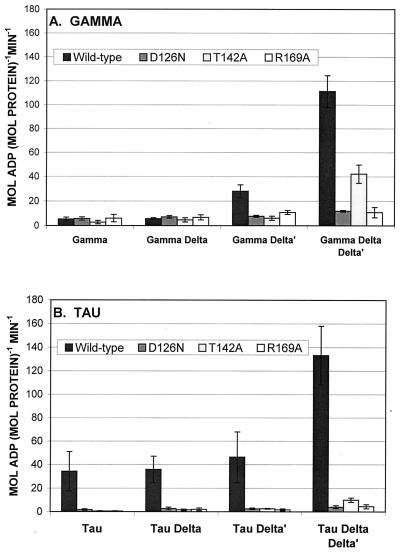
Although γ has inefficient DNA-dependent ATPase activity, with a kcat (maximum catalytic rate at saturating substrate) on the order of 3 × 10−3 s−1 (25, 50, 51), that low activity is thought to be intrinsic to γ rather than the result of a contaminating ATPase. δ and δ′, which are not ATPases themselves, stimulate the intrinsic γ ATPase (50, 51). Each of the wild-type and mutant γ proteins was assayed for DNA-dependent ATPase activity alone, with δ, with δ′, and with δ-δ′ (Fig. 2A). As individual proteins, wild-type and mutant γ had ATPase activities that were essentially background and δ had no detectable effect on any of them. δ′, on the other hand, stimulated the wild-type γ ATPase about sixfold and each of the mutants about twofold. The combination of δ-δ′ was more active than either δ or δ′ alone and stimulated the wild-type γ about 22-fold. Addition of both δ and δ′ stimulated the residual activity of mutant γ also, the D126N mutant about threefold, the T142A mutant about eightfold, and the R169A mutant about twofold. Interestingly, R169A mutant residual activity was stimulated about twofold by δ′ alone and by the combination δ-δ′. This mutation apparently interferes with γ ability to respond to the synergistic effect of δ and δ′.
FIG. 2.
ATPase activities of wild-type and mutant γ (A) and τ (B). Each protein was assayed alone or with δ, δ′, or δ-δ′, as indicated. The assay mixtures all contained single-strand DNA (as described in Materials and Methods), 0.5 to 2.0 pmol of τ or γ, and 8 pmol each of δ and δ′. Error bars, ±1 standard deviation.
In assay mixtures containing DNA and both δ and δ′, all three mutations reduced the kcat (Fig. 3, Table 3). The T142A and R169A changes reduced the kcat values to 30 and 18%, respectively, of the wild-type value of 92 min−1, whereas the D126N change reduced the kcat by 94%. Catalytic efficiencies of all three mutant proteins were 1/7 to 1/10 that of wild-type γ. The D126N mutation had the most dramatic effect on both kcat and on catalytic efficiency.
TABLE 3.
Kinetic analysis of wild-type and mutant DNA-dependent, δ-δ′-stimulated γ ATPase
| Enzyme | kcat (min−1) | kcat/Km (μmol−1 min−1) |
|---|---|---|
| γ+ | 92 | 3.6 |
| γD126N | 5.4 | 0.32 |
| γT142A | 27 | 0.52 |
| γR169A | 17 | 0.49 |
ATPase activity of wild-type and mutant τ.
The wild-type τ has weak, but significant, DNA-dependent ATPase activity, even in the absence of δ-δ′ (39, 62). With the M13Gori1 single-strand DNA as the effector, wild-type τ hydrolyzed about 35 mol of ATP/min/mol of protein (Fig. 2B). All three mutations, D126N, T142A, and R169A, reduced that activity to neligible levels.
The ATPase activity of τ also responds to the δ-δ′ combination. Although neither δ nor δ′ stimulated τ significantly, the combination of both stimulated τ activity about eightfold (50). With the M13Gori1 effector used in this study, δ-δ′ stimulated τ about fourfold (Fig. 2B). All three mutant τ proteins also responded slightly to δ-δ′ stimulation and, quantitatively, in the same pattern as the mutant γ response to δ-δ′. That is, τ with the T142A mutation (τ T142A) was stimulated more than τ D126N and τ R169A.
Replication activity of wild-type and mutant γ and τ.
Replication assays were done as described by Onrust et al. (51) to measure β clamp loading by wild-type and mutant γ and τ. Each was mixed with uniquely primed SSB-coated M13Gori1 single-strand DNA, α-ɛ, and β and incubated with substrates, including radioactive dATP, with and without δ-δ′, at 37°C for 30 min. Total incorporation of nucleotides into replicative-form DNA was measured. The wild-type γ and τ, with δ-δ′, supported the incorporation of 0.4 and 2.2 mol of nucleotide into DNA product/min/mol of protein, respectively. All three mutant forms of both γ and τ were essentially inactive (Fig. 4). Increasing the mutant γ and τ concentrations from 0.5 to 2.0 pmol/assay did not significantly change their clamp-loading specific activities (Fig. 4), suggesting that the mutant proteins have defects in intrinsic catalytic activity rather than affinity differences.
FIG. 4.
Replication assay of β clamp loading of wild-type and mutant γ and τ. The assay mixtures contained uniquely primed single-strand DNA, α-ɛ, β, single-strand binding protein, and γ or τ, each with and without δ-δ′. For each condition, γ and τ were added at both 0.5 and 2.0 pmol/assay. Because the increasing concentrations did not change specific activities significantly, the averages of all assays are presented. Error bars, ±1 standard deviation. Each assay was done in quadruplicate.
DISCUSSION
Identity and functional motifs have previously been described in clamp-loading proteins from phage T4, bacteria, and eukaryotes (8, 11, 21, 23, 46), and the structure of the N-terminal 80% of γ has been proposed, based on γ homology with δ′, for which the structure has been solved (23). The N-terminal 330 residues of γ are organized into three domains with a “C” shape, and ATP is thought to bind within the inner surface of the “C” (23). In this study, we have used the BLAST program (1) to identify 28 homologs of the E. coli τ and γ clamp-loading subunits (27 prokaryotic proteins and 1 eukaryotic protein) plus 10 prokaryotic homologs of the δ′ subunit and 33 eukaryotic RFC subunits. The 28 τ and γ homologs contain nine highly conserved motifs within the N-terminal halves of the molecules (Table 1). Also identified by the BLAST search were seven closely related prokaryotic homologs of a hypothetical E. coli protein (GenBank accession no. P45526) with significant homology to τ and γ and RuvB (H. R. Neely and J. R. Walker, unpublished).
τ and γ functional motifs include motif III, the P loop or phosphate-binding region (68), DExx motif V, common to helicases and nucleases (6, 22, 27), and a Zn binding motif. Although the Zn binding sequence is atypical, γ contains Zn2+ (cited by Guenther et al. [23]). Two sensor motifs (Table 1) which would respond to ATP have been proposed by Guenther et al. (23) by analogy to crystal structures for known nucleotide binding proteins complexed with substrates (or their analogs). Sensor 1, proposed to be residues 152 to 157 (FLLATT) of E. coli τ and γ, is highly conserved. It is extended to F&(FL)aTT(ED). This motif is strongly conserved, with three residues being invariant; the second position is always a bulky hydrophobic residue (&), the third position is always F or L, and the (ED) is present in at least 90% of 28 homologs. The second proposed sensor motif, motif 2, is GSLRDA (residues 212 to 217) of τ and γ (23) and is part of motif VIII described by Cullman et al. (11). The R, invariant in all known τ and γ proteins, might bind the phosphate groups of ATP (23). The sensor 2 (motif VIII) highly conserved region is extended to include GsxRDx2(ST)Ux(DE)q (Table 1). Four additional motifs (II, IV, VIc, and VII) are identified by high degrees of homology among all the known τ and γ homologs (Table 1).
P loop residues bind nucleotide α- and β-phosphates (15, 28, 36, 61). The E. coli τ and γ P-loop residues are essential in vivo and in vitro. Both G45A and K51A mutations eliminated DnaX activity in vivo (Table 2). Xiao et al. (72) mutated the P-loop lysine to alanine (K51A) and showed that mutant τ and γ did not bind ATP with high affinity and that mutant τ and γ complexes assembled with τ K51A or γ K51A neither hydrolyzed ATP nor loaded β clamps. They used the mutant proteins to provide evidence that the γ complex, but not the τ complex, loads β in the holoenzyme. Pol III* (holoenzyme without β [44, 69]) assembled with wild-type τ and mutant γ was inactive in loading β and polymerizing DNA in vitro, but Pol III* assembled with mutant τ and wild-type γ was active. The γ complex assembled with γ K51R had reduced ATPase activity and was inactive in clamp loading (25).
DExx aspartates are situated at the carboxy ends of β strands and participate in catalysis by binding magnesium ions, directly or indirectly through water, which bridge the nucleotide β-γ phosphates (see, e.g., references 16, 28, and 52). Pause and Sonenberg (53) mutagenized the DEAD box of the mammalian translation initiation factor eIF-4A. Changing the invariant first aspartate to asparagine eliminated ATPase and helicase activity, but ATP binding was not severely affected. The charge on that aspartate is critical for hydrolysis but not for nucleotide binding. All the prokaryotic τ and γ homologs contain invariant DExH residues within the DExx motif, and the invariant aspartate in E. coli τ and γ, D126, is similarly situated at the carboxy end of a β strand (23). A D126N mutation reduced both the ATPase kcat and catalytic efficiency, eliminated clamp loading in vitro, and eliminated DnaX activity in vivo (Fig. 2 to 4; Table 2). γ complexes assembled with γ D126N should support one round of clamp opening, because ATP binding is adequate to open the clamp (25, 26), but would not be expected to complete the catalytic cycle.
The T142A mutation altered a conserved residue within motif VIc. This motif has three forms; Cullman et al. (11) described VIa and VIb for RFC subunits 1 and 2 to 5, respectively. Motif VIb [s(ML)TxxAQxLRRtmE] is more closely related to VIc (fnaLLKtUEEPP). In all subunits with motif VIb or VIc, the underlined E is invariant. In τ and γ, the naLLKtL comprises alpha helix 5; the invariant glutamate is the first residue in a solvent-exposed loop at the lower exterior of the “C”-shaped molecule (23). Mutation of the conserved T to A reduced the γ-δ-δ′ ATPase catalytic efficiency to about 14%, and kcat to about 30%, of the wild-type levels. The γ T142A protein residual ATPase responded to δ-δ′ stimulation. τ T142A had greatly reduced ATPase activity, Neither γ T142A nor τ T142A had significant clamp-loading activity.
Unexpectedly, the dnaXT142A mutant allele retained sufficient activity in vivo to support growth. The mutant allele on a multicopy plasmid complemented a dnaX(Ts) host (although the expression level was basal and not specifically induced) and a haploid dnaXT142A mutant grew normally under laboratory conditions. Perhaps the mutant DnaXT142A activity was restored in vivo by the mass action effect of other DnaXT142A molecules in replication complexes (which might not have assembled in the in vitro reactions) or was stabilized by interaction with other replication factors. Studies with mutant dna alleles in vivo and mutant proteins in vitro have defined another paradox. The principal clamp loader in vitro was concluded to be the γ complex, and not the τ complex (73), but γ is dispensable in vivo, whereas τ is indispensable (4).
Motif VII is characterized by the invariant SR followed by C or T. The arginine is located in a solvent-exposed loop leading from α helix 6 to β-strand 5, the last structured region of domain 1 (23). Changing the arginine to alanine reduced ATPase activity of τ (with δ-δ′); catalytic efficiency was reduced to 14% of the wild-type level, and kcat was reduced to 18% of the wild-type level (Table 3). In τ, this mutation greatly reduced the ATPase activity of τ and eliminated clamp loading by τ or γ. The R169A mutation did not abolish ATPase stimulation of τ or γ by δ-δ′, although the overall activity was reduced. This mutation also abolished DnaX activity in vivo.
The degree of wild-type γ DNA-dependent ATPase stimulation by δ and δ′ is dependent on the primer template. In all reported cases, the δ-δ′ combination stimulated more than the sum of δ and δ′ alone, but responses to δ or δ′ individually varied with the identity of the DNA effector. The γ ATPase with poly(dA):oligo(dT) was stimulated more by δ than by δ′ (51). With M13mp18 single-strand DNA, δ′ was more stimulatory than δ (50). With M13Gori1 single-strand DNA, γ did not respond significantly to δ but was stimulated by δ′ (Fig. 2A). The nature of the δ, δ′, and δ-δ′ stimulation of γ ATPase remains unknown.
ACKNOWLEDGMENTS
We thank Mike O'Donnell, Manju Hingorani, and Frank Leu for generous gifts of purified proteins and advice, Sidney Kushner for pMAK705, Karen Skorupski and Ronald Taylor for pKAS32, Lawrence Poulsen for numerous discussions, Wen-Ling Lin for help with some experiments, Kimberly Severson for help with the figures, and Indu Ghosh, Ningna Xiao, and Cecil Harkey of the University of Texas Institute for Cellular and Molecular Biology Core Facility for DNA sequencing.
This work was supported by Welch Foundation grant F-1349.
REFERENCES
- 1.Altschul S F, Madden T J, Schäfer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertram J G, Bloom L B, Turner J, O'Donnell M, Beechem J M, Goodman M F. Pre-steady state analysis of the assembly of wild type and mutant circular clamps of Escherichia coliDNA polymerase III onto DNA. J Biol Chem. 1998;273:24564–24574. doi: 10.1074/jbc.273.38.24564. [DOI] [PubMed] [Google Scholar]
- 3.Blinkova A, Walker J R. Programmed ribosome frameshift generates the Escherichia coliDNA polymerase III γ subunit from within the τ subunit reading frame. Nucleic Acids Res. 1990;18:1725–1729. doi: 10.1093/nar/18.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blinkova A, Hervas C, Stukenberg P T, Onrust R, O'Donnell M E, Walker J R. The Escherichia coli DNA polymerase III holoenzyme contains both products of the dnaXgene, τ and γ, but only τ is essential. J Bacteriol. 1993;175:6018–6027. doi: 10.1128/jb.175.18.6018-6027.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom L B, Turner J, Kelman Z, Beechem J M, O'Donnell M, Goodman M F. Dynamics of loading the β sliding clamp of DNA polymerase III onto DNA. J Biol Chem. 1996;271:30699–30708. doi: 10.1074/jbc.271.48.30699. [DOI] [PubMed] [Google Scholar]
- 6.Bork P, Koonin E V. An expanding family of helicases within the 'DEAD/H' superfamily. Nucleic Acids Res. 1993;21:751–752. doi: 10.1093/nar/21.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgers P M J. Saccharomyces cerevisiaereplication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases δ and ɛ. J Biol Chem. 1991;266:22698–22706. [PubMed] [Google Scholar]
- 8.Carter J R, Franden M A, Aerobersold R, McHenry C S. Identification, isolation, and characterization of the strucutural gene encoding the δ′ subunit of Escherichia coliDNA polymerase III holoenzyme. J Bacteriol. 1993;175:3812–3822. doi: 10.1128/jb.175.12.3812-3822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu H, Malone M M, Haldenwang W H, Walker J R. Physiological effects of growth of an Escherichia coli temperature-sensitive dnaZmutant at non-permissive temperatures. J Bacteriol. 1977;132:151–158. doi: 10.1128/jb.132.1.151-158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Company M, Arenas J, Abelson J. Requirement of the RNA helicase-like protein PRP22 for release of messenger RNA from spliceosomes. Nature. 1991;349:487–493. doi: 10.1038/349487a0. [DOI] [PubMed] [Google Scholar]
- 11.Cullman G, Fien K, Kobayashi R, Stillman B. Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:4661–4671. doi: 10.1128/mcb.15.9.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallmann H G, McHenry C S. DnaX complex of DNA polymerase III holoenzyme. Physical characterization of the DnaX subunits and complexes. J Biol Chem. 1995;270:29563–29569. [PubMed] [Google Scholar]
- 13.Dallmann HG, Thimmig R L, McHenry C S. DnaX complex of Escherichia coliDNA polymerase III holoenzyme. Centrol role of τ in initiation complex assembly and in determining the functional asymmetry of holoenzyme. J Biol Chem. 1995;270:29555–29562. [PubMed] [Google Scholar]
- 14.Dong Z, Onrust R, Skangalis M, O'Donnell M. DNA polymerase III accessory proteins. I. holA and holBencoding δ and δ′. J Biol Chem. 1993;268:11758–11765. [PubMed] [Google Scholar]
- 15.Dreusicke D, Karplus F A, Schulz G C. Refined structure of porcine cytosolic adenylate kinase at 2.1 Å resolution. J Mol Biol. 1988;199:359–371. doi: 10.1016/0022-2836(88)90319-1. [DOI] [PubMed] [Google Scholar]
- 16.Egner U, Tomasselli A G, Schultz G E. Structure of the complex of yeast adenylate kinase with the inhibitor P1,P5-di(adenosine-5′-)pentaphosphate at 2.6 Å resolution. J Mol Biol. 1987;195:649–658. doi: 10.1016/0022-2836(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 17.Fien K, Stillman B. Identification of replication factor C from Saccharomyces cerevisiae:a component of the leading-strand DNA replication complex. Mol Cell Biol. 1992;12:155–163. doi: 10.1128/mcb.12.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filip C C, Allen J S, Gustafson R A, Allen R G, Walker J R. Bacterial cell division regulation: Characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974;119:443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flower AM, McHenry C S. The γ subunit of DNA polymerase III holoenzyme of Escherichia coliis produced by ribosomal frameshift. Proc Natl Acad Sci USA. 1990;87:3713–3717. doi: 10.1073/pnas.87.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fradkin L G, Kornberg A. Prereplicative complexes of components of DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1992;267:10318–10322. [PubMed] [Google Scholar]
- 21.Gary S L, Burgers P M J. Identification of the fifth subunit of Saccharomyces cerevisiaereplication factor C. Nucleic Acids Res. 1995;23:4986–4991. doi: 10.1093/nar/23.24.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Two related superfamilies of putative helicases involved in replication, recombination, repair, and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guenther B, Onrust R, Sali A, O'Donnell M, Kuriyan J. Crystal structure of the δ′ subunit of the clamp-loader complex of E. coli DNA polymerase III. Cell. 1997;91:335–345. doi: 10.1016/s0092-8674(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 23a.Hamilton C M, Aldea M, Washburn B K, Babitzke P, Kushner S R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989;171:4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henikoff S, Henikoff J G. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani M M, O'Donnell M. ATP binding to the Escherichia coliclamp loader powers opening of the ring-shaped clamp of DNA polymerase III holoenzyme. J Biol Chem. 1998;273:24550–24563. doi: 10.1074/jbc.273.38.24550. [DOI] [PubMed] [Google Scholar]
- 26.Hingorani M M, Bloom L B, Goodman M F, O'Donnell M. Division of labor—sequential ATP hydrolysis drives assembly of a DNA polymerase sliding clamp around DNA. EMBO J. 1999;18:5131–5144. doi: 10.1093/emboj/18.18.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodgman T C. A new family of replicative proteins. Nature. 1988;333:22–23. doi: 10.1038/333022b0. . (Erratum, 333:578.) [DOI] [PubMed] [Google Scholar]
- 28.Jurnak R. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to rasoncogene proteins. Science. 1985;230:32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystissp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 30.Kim D R, McHenry C S. Biotin tagging deletion analysis of domain limits involved in protein-macromolecular interactions. Mapping the τ binding domain of the DNA polymerase III α subunit. J Biol Chem. 1996;271:20690–20698. doi: 10.1074/jbc.271.34.20690. [DOI] [PubMed] [Google Scholar]
- 31.Kim S, Dallmann H G, McHenry C S, Marians K J. Coupling of a replicative polymerase and a helicase: a τ-DnaB interaction mediates rapid replication fork movement. Cell. 1996;84:643–650. doi: 10.1016/s0092-8674(00)81039-9. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Dallmann H G, McHenry C S, Marians K J. τ protects β in the leading-strand polymerase complex at the replication fork. J Biol Chem. 1996;271:4315–4318. doi: 10.1074/jbc.271.8.4315. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Dallmann H G, McHenry C S, Marians K J. τ couples the leading- and lagging-strand polymerases at the Escherichia coliDNA replication fork. J Biol Chem. 1996;271:21406–21412. doi: 10.1074/jbc.271.35.21406. [DOI] [PubMed] [Google Scholar]
- 34.Koonin E V. Similarities in RNA helicases. Nature. 1991;352:290. doi: 10.1038/352290c0. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:499–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaCour T F M, Nyborg J, Thirup S, Clark B F C. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. colias studied by X-ray crystallography. EMBO J. 1985;4:2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Lederberg J. Streptomycin resistance: a genetically recessive mutation. J Bacteriol. 1951;61:549–550. doi: 10.1128/jb.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S-H, Hurwitz J. Mechanism of elongation of primed DNA by DNA polymerase δ, proliferating cell nuclear antigen, and activator 1. Proc Natl Acad Sci USA. 1990;87:5672–5676. doi: 10.1073/pnas.87.15.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S-H, Kwong A D, Pan Z Q, Hurwitz J. Studies on the activator 1 protein complex, an accessory factor for proliferating cell nuclear antigen-dependent DNA polymerase δ. J Biol Chem. 1991;266:594–602. [PubMed] [Google Scholar]
- 39.Lee S-H, Walker J R. Escherichia coliDnaX product, the τ subunit of DNA polymerase III, is a multifunctional protein with single-stranded DNA-dependent ATPase activity. Proc Natl Acad Sci USA. 1987;84:2713–2717. doi: 10.1073/pnas.84.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X, Kaul S, Rounsley S D, Shea T P, Benito M-I, Town C D, Fujii C Y, Mason T M, Bowman C L, Barnstead M E, Feldblyum T V, Buell C R, Ketchem K A, Lee J J, Ronning C M, Koo H, Moffat K S, Cronin L A, Shen M, VanAken S E, Umayam L, Tallon L J, Gill J E, Adams M D, Carrera A J, Creasy T H, Goodman H M, Somerville C R, Copenhaver G P, Preuss D, Nierman W C, White O, Eisen J A, Salzberg S L, Fraser C M, Venter J C. Sequence and analysis of chromosome II of Arabidopsis thaliana. Nature. 1999;402:761–768. doi: 10.1038/45471. [DOI] [PubMed] [Google Scholar]
- 40a.Liu X-Q, Hu Z. Identification and characterization of a cyanobacterial DnaX intein. FEBS Lett. 1997;408:311–314. doi: 10.1016/s0014-5793(97)00393-1. [DOI] [PubMed] [Google Scholar]
- 41.Maki S, Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. I. Purification and distinctive functions of subunits τ and γ, the dnaZXgene products. J Biol Chem. 1988;263:6547–6554. [PubMed] [Google Scholar]
- 42.Maki S, Kornberg A. DNA polymerase III holoenzyme of Escherichia coli.II. A novel complex including the γ subunit essential for processive synthesis. J Biol Chem. 1988;263:6555–6560. [PubMed] [Google Scholar]
- 43.McHenry CS. Purification and characterization of DNA polymerase III′: Identification of τ as a subunit of the DNA polymerase III holoenzyme. J Biol Chem. 1982;257:2657–2663. [PubMed] [Google Scholar]
- 44.McHenry C S, Kornberg A. DNA polymerase III of Escherichia coli.Purification and resolution into subunits. J Biol Chem. 1977;252:6478–6484. [PubMed] [Google Scholar]
- 44a.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naktinis V, Turner J, O'Donnell M. A molecular switch in a replication machine defined by an internal competition for protein rings. Cell. 1996;84:137–145. doi: 10.1016/s0092-8674(00)81000-4. [DOI] [PubMed] [Google Scholar]
- 46.O'Donnell M, Onrust R, Dean F B, Chen M, Hurwitz J. Homology in accessory proteins of replicative polymerases—E. colito humans. Nucleic Acids Res. 1993;21:1–3. doi: 10.1093/nar/21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Donnell M E. Accessory proteins bind a primed template and mediate rapid cycling of DNA polymerase III holoenzyme from Escherichia coli. J Biol Chem. 1987;262:16558–16565. [PubMed] [Google Scholar]
- 48.Onrust R. The structure and function of accessory proteins of the E. coli DNA polymerase III holoenzyme. Ph.D. thesis. New York, N.Y: Cornell University Medical College; 1993. [Google Scholar]
- 49.Onrust R, Finkelstein J, Naktinis V, Turner J, Fang L, O'Donnell M. Assembly of a chromosomal replication machine: two DNA polymerases, a clamp loader and sliding clamps in one holoenzyme particle. I. Organization of the clamp loader. J Biol Chem. 1995;270:13348–13357. doi: 10.1074/jbc.270.22.13348. [DOI] [PubMed] [Google Scholar]
- 50.Onrust R, O'Donnell M. DNA polymerase III accessory proteins. II. Characterization of δ and δ′. J Biol Chem. 1993;268:11766–11772. [PubMed] [Google Scholar]
- 51.Onrust R, Stukenberg P T, O'Donnell M. Analysis of the ATPase subassembly which initiates processive DNA synthesis by DNA polymerase III holoenzyme. J Biol Chem. 1991;266:21681–21686. [PubMed] [Google Scholar]
- 52.Pai E G, Krengel U, Petsko G A, Goody R S, Kabasch W, Wittinghofer A. Refined structure of the triphosphate conformation of H-ras-p21 at 1.35 Å resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pause A, Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor elF-4A. EMBO J. 1992;11:2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pritchard A E, Dallmann H G, McHenry C S. In vivo assembly of the τ-complex of the DNA polymerase III holoenzyme expressed from a five-gene artificial operon. Cleavage of the τ-complex to form a mixed γ-τ-complex by the OmpT protease. J Biol Chem. 1996;271:10291–10298. doi: 10.1074/jbc.271.17.10291. [DOI] [PubMed] [Google Scholar]
- 55.Schmid S R, Linder P. DEAD protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–292. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 55a.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 56.Studier W F, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 57.Studwell P S, O'Donnell M. Processive replication is contingent on the exonuclease subunit of DNA polymerase III holoenzyme. J Biol Chem. 1990;265:1171–1178. [PubMed] [Google Scholar]
- 58.Studwell-Vaughn P S, O'Donnell M. Constitution of twin polymerase of DNA polymerase III holoenzyme. J Biol Chem. 1991;266:19833–19841. [PubMed] [Google Scholar]
- 59.Stukenberg P T, Studwell-Vaughn P S, O'Donnell M. Mechanism of the sliding β clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991;266:11328–11334. [PubMed] [Google Scholar]
- 60.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tong L, deVos A M, Milburn M V, Kim S-H. Structure of rasproteins. Science. 1989;245:244. doi: 10.1126/science.2665078. [DOI] [PubMed] [Google Scholar]
- 62.Tsuchihashi Z, Kornberg A. ATP interactions of the τ and γ subunits of DNA polymerase III holoenzyme of Escherichia coli. J Biol Chem. 1989;264:17790–17795. [PubMed] [Google Scholar]
- 63.Tsuchihashi Z, Kornberg A. Translational frameshifting generates the γ subunit of DNA polymerase III holoenzyme. Proc Natl Acad Sci USA. 1990;87:2516–2520. doi: 10.1073/pnas.87.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsurimoto T, Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases α and δ. EMBO J. 1989;8:3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsurimoto T, Stillman B. Purification of a cellular replication factor, RF-C, that is required for coordinated synthesis of leading and lagging strands during simian virus 40 DNA replication in vitro. Mol Cell Biol. 1989;9:609–619. doi: 10.1128/mcb.9.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specfic recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991;266:1950–1960. [PubMed] [Google Scholar]
- 67.Turner J, Hingorani M M, Kelman Z, O'Donnell M. The internal workings of a DNA polymerase clamp-loading machine. EMBO J. 1999;18:771–783. doi: 10.1093/emboj/18.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker J E, Sarasti M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wickner W, Schekman R, Geider K, Kornberg A. A new form of DNA polymerase III and a copolymerase replicate a long single-stranded primer template. Proc Natl Acad Sci USA. 1973;70:1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willetts N S, Clark A J, Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969;97:244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winzeler E, Shapiro L. Translation of the leaderless Caulobacter dnaXmRNA. J Bacteriol. 1997;179:3981–3988. doi: 10.1128/jb.179.12.3981-3988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao H, Naktinis V, O'Donnell M. Assembly of chromosomal replication machine: two DNA polymerases, a clamp loader, and sliding clamps in one holoenzyme particle. IV. ATP-binding site mutants identify the clamp loader. J Biol Chem. 1995;270:13378–13383. doi: 10.1074/jbc.270.22.13378. [DOI] [PubMed] [Google Scholar]
- 73.Yuzahakov A, Turner J, O'Donnell M. Replisome assembly reveals the basis for symmetric function in leading and lagging strand replication. Cell. 1996;86:877–886. doi: 10.1016/s0092-8674(00)80163-4. [DOI] [PubMed] [Google Scholar]