Abstract
Inverse association between serum levels of vitamin D and obesity has been pointed out in several studies. Our aim was to identify to the associations between vitamin D levels and a large panel of anthropometric markers and adipokines. Cross-sectional study including 6485 participants. Anthropometric markers included body mass index (BMI), % body fat, waist, waist-to-hip (WHR), waist-to-height (WHtR), conicity index, body roundness index (BRI) and a body shape index (ABSI). 55.7% of women and 60.1% of men presented with vitamin D deficiency. Vitamin D levels were negatively associated with most anthropometric markers, with correlation coefficients ranging between −0.017 (ABSI) and −0.192 (BMI) in women and between −0.026 (weight) and −0.130 (% body fat) in men. Vitamin D levels were inversely associated with leptin levels in both sexes and positively associated with adiponectin levels in women only. The likelihood of vitamin D deficiency increased with increasing adiposity levels, except for ABSI (women) and BMI (men). Total body fat, rather than localized or unevenly distributed body fat, is the adiposity marker most associated with decreased vitamin D levels. Monitoring vitamin D levels in people with overweight/obesity is essential.
Subject terms: Health care, Nutrition
Introduction
Vitamin D deficiency is common among adults1. The causes for vitamin D deficiency include reduced ability to synthesize vitamin D in the skin due to reduced sun exposure or skin pigmentation, decreased vitamin D dietary intake and or intestinal absorption, and increased adiposity2–4. The mechanisms associating increased adiposity (obesity) and vitamin D insufficiency are poorly understood; the main hypothesis is the sequestration of vitamin D by the adipose tissue, as suggested by the well reported negative association between serum vitamin D levels and fat mass in obesity5. In the Swiss Salt study, a one-unit increase in BMI was associated with an 8% decreased likelihood of being in the highest tertile of vitamin D3. Several systematic reviews and meta-analyses found inverse associations between vitamin D levels and waist circumference6 or fat mass7. However, most studies assessing the association between vitamin D and obesity focused on a single anthropometric marker. Several obesity markers such as waist circumference, body composition, the Valdez conicity index, the body roundness index (BRI) and a body shape index (ABSI)8–10 exist, but their joint analysis has seldom been conducted.
Some studies suggest that vitamin D modulates the secretions of many of these adipokines11.The Leptin has been identified as a marker that can be influenced by vitamin D levels, but the mechanisms that explain this association are still controversial11,12.
In this study, we aimed at assessing the associations between vitamin D levels and a large panel of anthropometric markers and adipokines, in a cross-sectional population-based study.
Participants and methods
Study design
The CoLaus (Cohorte Lausannoise) study is a population-based prospective study assessing the clinical, biological, and genetic determinants of cardiovascular disease aged 35 to 75 years at baseline, living in the city of Lausanne, Switzerland)13. In each survey, participants answered questionnaires, underwent a clinical examination and blood samples were drawn for analyses13. Recruitment began in June 2003 and ended in May 2006. For this cross-sectional analysis, all participants at were eligible and no control group was created.
Anthropometry
Anthropometric measurements were conducted using a standard methodology. Body weight and height were measured with participants barefoot and in light indoor clothes. Body weight was measured in kilograms to the nearest 100 g using a Seca® scale (Hamburg, Germany). Height was measured to the nearest 5 mm using a Seca® (Hamburg, Germany) height gauge14. Body mass index (BMI) was computed and categorized into underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obesity (≥ 30 kg/m2)15.
Waist circumference (WC) was measured mid-way between the lowest rib and the iliac crest, and hip was measured at the largest location, using a non-stretchable tape; the average of two measurements was taken14. Abdominal obesity was defined as a waist circumference > 102 cm (men) or > 88 cm (women). A high waist to height ratio (WHtR) was defined as > 0.516; the WHtR is considered as a good indicator of abdominal obesity17.
Fat and fat-free mass (in percent of total body weight) were assessed by electrical bioimpedance in the lying position after a 5-min rest using the Bodystat® 1500 body mass analyzer (Bodystat Ltd, Isle of Man, England)18. This device has been shown to correlate well (r = 0.968) with measurements from dual energy X-ray absorptiometry (DEXA)18.
The conicity index (CI) was calculated according to Valdez9. It is based upon the idea that people accumulate fat around the waist and, the shape of their bodies seems to change from that of a cylinder to that of a “double cone” (two cones with a common base). The CI is determined by the formula
The CI was further categorized as normal if < 1.25 and < 1.18 for men and women, respectively, and as high if ≥ 1.25 and ≥ 1.18 for men and women, respectively19.
Body roundness index (BRI)20 was computed according to and is based on waist circumference and height.
A Body shape index (ABSI)21 was computed according to and is based on waist, BMI and height. Since there are no clinical cutoff values for BRI or ABSI, high BRI and ABSI were defined as those within the highest quartile group (Q4).
Vitamin D levels
Vitamin D was assessed at baseline through an ultra-HPLC tandem-MS system. The calibrators, 3Plus1 Multilevel Serum Calibrator Set 25-OH-Vitamin D3/D2 (ChromoSystems), were standardized against the National Institute of Standards and Technology 972 reference material. Serum 25(OH)D3 and 3-epi-25(OH)D3 were expressed in nanomoles per liter (conversion factor: 1 nmol/L = 0.4006 μg/L). The interday CV% was 4.6% at 40 nmol/L22. Vitamin D levels were further categorized as normal (≥ 30 ng/mL or ≥ 75 nmol/l), insufficiency (21 to 29 ng/mL or 50–75 nmol/l) and deficiency (< 20 ng/mL or < 50 nmol/l)23. Hypovitaminosis D was defined for vitamin D levels < 30 ng/mL or < 75 nmol/l, encompassing insufficiency plus deficiency24.
Other covariates
Educational level was categorized into university, high school, apprenticeship, and mandatory. Nationality as born in Switzerland or not. Smoking status was self-reported and categorized as never, former, and current. Physical activity was considered if the participant reported performing at least twice a week a minimum of 20 min of leisure-time physical activity13.
Adipokines (adiponectin and leptin) were assessed at baseline. Adiponectin was assessed by ELISA (R&D Systems, Inc, Minneapolis, USA), with a maximum inter-assay CV of 8.3% and a maximum intra-assay CV of 8.3%. Leptin was assessed by ELISA (American Laboratory Products Company, Windham, USA) with a maximum inter-assay CV of 12.8% and a maximum intra-assay CV of 5.8%. High sensitive C-reactive protein (CRP) was assessed by immunoassay and latex HS on a Modular P apparatus (Roche Diagnostics, Basel, Switzerland).
Exclusion criteria
Participants were excluded if they lacked any variable needed for the bivariate and the multivariate analyses. Hence, participants devoid of vitamin D data; without anthropometric measurements and any covariate needed for adjustment (education, smoking, BMI, or physical activity) were excluded. The exclusion procedure was conducted sequentially as follows: first, participants devoid of vitamin D data were excluded; of the remaining participants, those without anthropometric measurements were excluded; finally, of the remaining participants, those missing any covariate were excluded.
Statistical analysis
Statistical analyses were performed using Stata version 16.1 for Windows (Stata Corp, College Station, Texas, USA)25. Descriptive results were expressed as number of participants (percentage) for categorical variables and as average ± standard deviation or median [interquartile range] for continuous variables.
As adiposity measures differ between sexes, stratification on the latter was performed. The associations between vitamin D levels and anthropometric markers (BMI, waist, WHR, WHtR, %fat as assessed by bioimpedance, CI, BRI and AABSI) were assessed as follows. First, comparison of vitamin D levels according to categories of anthropometric markers was performed using one-way (bivariate) or multivariate analysis of variance. Multivariate analysis was adjusted for age (continuous), nationality (Swiss, other), month, smoking categories (never, former, current), vitamin D supplementation (yes, no) and physical activity (yes, no) and the results were expressed as adjusted mean ± standard error. Second, bivariate nonparametric Spearman correlations and their 95% CIs were calculated between vitamin D levels and anthropometric markers as continuous variables. A stepwise multivariate linear regression analysis with age (continuous), nationality (Swiss, other), month, smoking categories (never, former, current), vitamin D supplementation (yes, no) and physical activity (yes, no) as locked terms was conducted to identify the anthropometric marker most associated with vitamin D levels. For simplicity, all anthropometric markers were standardized (i.e., zero average and unit standard deviation) before the stepwise regression.
Third, the association between vitamin D deficiency and categories of anthropometric markers was assessed using chi-square (bivariate analysis) and multivariate logistic regression with vitamin D deficiency (yes, no) as the dependent variable and adjusting for age (continuous), nationality (Swiss, other), month, smoking categories (never, former, current), vitamin D supplementation (yes, no) and physical activity (yes, no).
Sensitivity analyses were conducted by excluding participants receiving medically prescribed vitamin D levels. Finally, the analysis of the associations between vitamin D levels and leptin and adiponectin levels as obesity markers was conducted using Spearman correlation and linear regression adjusting for the aforementioned covariates; results of the linear regression were expressed as standardized coefficients. Statistical significance was considered for a two-sided test with p < 0.05.
Ethical statement
The institutional Ethics Committee of the University of Lausanne, which afterwards became the Ethics Commission of Canton Vaud (https://www.cer-vd.ch) approved the baseline CoLaus study (reference 16/03, decisions of 13th January and 10th February 2003). The study was performed in agreement with the Helsinki declaration and its former amendments, and in accordance with the applicable Swiss legislation. All participants gave their signed informed consent before entering the study. Data analysis was conducted in Switzerland and no data was shared with outside groups.
Results
Characteristics of participants
Of the initial 6733 participants, 248 were excluded. The detailed reasons for exclusion are provided in supplementary Fig. 1 and the characteristics of excluded and eligible participants are provided in supplementary Table 1. Excluded participants were younger, less frequently born in Switzerland, had a higher educational level, were less frequently former smokers, less physically active and took vitamin D supplements less frequently than included participants.
The characteristics of the 6485 participants by sex are summarized in Table 1. Women were older, had a lower educational level, smoked less, and took vitamin D supplements more frequently. Women also had higher levels of vitamin D and presented less frequently with vitamin D deficiency. Regarding anthropometric markers, women had higher body fat percentage levels but lower levels for all other anthropometric markers than men (Table 1).
Table 1.
Characteristics of the participants at baseline, by sex, CoLaus|PsyCoLaus study, Lausanne, 2003–2006.
| Women (N = 3401) | Men (N = 3084) | P-value | |
|---|---|---|---|
| Age (years) | 53.1 ± 10.7 | 52.3 ± 10.7 | 0.001 |
| Born in Switzerland (%) | 2108 (62.0) | 1851 (60.0) | 0.106 |
| Education (%) | < 0.001 | ||
| University | 540 (15.9) | 720 (23.4) | |
| High school | 853 (25.1) | 699 (22.7) | |
| Apprenticeship | 1190 (35.0) | 1136 (36.8) | |
| Mandatory | 818 (24.1) | 529 (17.2) | |
| Smoking (%) | < 0.001 | ||
| Never | 1621 (47.7) | 996 (32.3) | |
| Former | 944 (27.8) | 1185 (38.4) | |
| Current | 836 (24.6) | 903 (29.3) | |
| Physically active (%) | 1865 (54.8) | 1578 (51.2) | 0.003 |
| Vitamin D supplement (%) | |||
| Specific | 205 (6.0) | 29 (0.9) | < 0.001 |
| Overall | 425 (12.5) | 182 (5.9) | < 0.001 |
| Vitamin D (nmol/L) median [IQR] | 46.2 [30.8–63.4] | 43.5 [28.5–60.8] | < 0.001§ |
| 48.4 ± 22.6 | 46.2 ± 22.6 | < 0.001 | |
| Vitamin D categories (%) | 0.001 | ||
| Normal | 428 (12.6) | 338 (11.0) | |
| Insufficiency | 1078 (31.7) | 892 (28.9) | |
| Deficiency | 1895 (55.7) | 1854 (60.1) | |
| Anthropometry | |||
| Body mass index (kg/m2) | 25.1 ± 4.8 | 26.5 ± 4.0 | < 0.001 |
| Body mass index categories (%) | < 0.001 | ||
| Underweight | 81 (2.4) | 21 (0.7) | |
| Normal | 1869 (55.0) | 1143 (37.1) | |
| Overweight | 963 (28.3) | 1414 (45.9) | |
| Obesity | 488 (14.4) | 506 (16.4) | |
| Waist (cm) | 83.4 ± 12.3 | 95.5 ± 11.1 | < 0.001 |
| Abdominal obesity (%) | 1115 (32.8) | 798 (25.9) | < 0.001 |
| Hip (cm) | 100.6 ± 10.1 | 102.8 ± 7.9 | < 0.001 |
| Waist to hip ratio | 0.83 ± 0.07 | 0.93 ± 0.06 | < 0.001 |
| Waist to height ratio | 0.51 ± 0.08 | 0.55 ± 0.07 | < 0.001 |
| High waist to height ratio (%) | 1686 (49.6) | 2312 (75.0) | < 0.001 |
| Body fat percentage (%) | 34.3 ± 8.2 | 23.7 ± 6.0 | < 0.001 |
| Conicity index | 1.20 ± 0.10 | 1.29 ± 0.08 | < 0.001 |
| High conicity index (%) | 1863 (54.8) | 2121 (68.8) | < 0.001 |
| Body roundness index | 3.7 ± 1.7 | 4.4 ± 1.4 | < 0.001 |
| Body shape index | 0.077 ± 0.005 | 0.081 ± 0.004 | < 0.001 |
| Adipokines | |||
| Leptin (ng/mL) | 14 [8.2–23] | 6.4 [3.9–10.7] | < 0.001§ |
| Adiponectin (μg/mL) | 10.6 [6.9–15.5] | 6.2 [4.1–9.2] | < 0.001§ |
Results are expressed as average ± standard deviation or median and interquartile range (IQR) and as number of participants and (column percentage). Between-group comparisons performed using t-test or Kruskal–Wallis test (§) for continuous variables and chi-square for categorical variables.
Associations between vitamin D levels and anthropometric markers
The levels of vitamin D according to categories of anthropometric markers are summarized in Table 2. On bivariate analysis, and regardless of the anthropometric markers considered, participants with high adiposity levels had lower vitamin D levels than participants with normal adiposity measures. Those findings were further confirmed by multivariate analysis, where a linear trend for a decrease in vitamin D levels with increasing adiposity was found, except for BMI in men (Table 2). Those findings were further confirmed after excluding participants taking vitamin D supplements (supplementary Table 2).
Table 2.
Bivariate and multivariate comparisons of total vitamin D levels according to adiposity categories, overall and stratified by sex, CoLaus|PsyCoLaus study, Lausanne.
| N | Overall | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | N | Bivariate | Multivariate | N | Bivariate | Multivariate | ||
| Body mass index | |||||||||
| Underweight | 102 | 56.5 ± 29.9 | 52.6 ± 1.9 | 81 | 59.4 ± 30.1 | 55.9 ± 2.2 | 21 | 45.3 ± 26.8 | 42.4 ± 3.9 |
| Normal | 3012 | 50.2 ± 23.3 | 49.5 ± 0.4 | 1869 | 51.4 ± 23.2 | 51.0 ± 0.5 | 1143 | 48.1 ± 23.4 | 47.3 ± 0.5 |
| Overweight | 2377 | 46.3 ± 21.5 | 46.6 ± 0.4 | 963 | 46.1 ± 20.5 | 46.1 ± 0.6 | 1414 | 46.4 ± 22.2 | 46.8 ± 0.5 |
| Obesity | 994 | 40.6 ± 20.3 | 42.1 ± 0.6 | 488 | 39.6 ± 19.7 | 41.8 ± 0.9 | 506 | 41.4 ± 20.9 | 42.2 ± 0.8 |
| p-value | < 0.001 | < 0.001* | < 0.001 | < 0.001* | < 0.001 | 0.945* | |||
| Abdominal obesity | |||||||||
| Normal | 4572 | 49.1 ± 23.0 | 49.0 ± 0.3 | 2286 | 50.7 ± 23.1 | 50.5 ± 0.4 | 2286 | 47.5 ± 22.9 | 47.3 ± 0.4 |
| Obesity | 1913 | 43.3 ± 21.1 | 43.5 ± 0.4 | 1115 | 43.8 ± 20.9 | 44.2 ± 0.6 | 798 | 42.5 ± 21.3 | 43.2 ± 0.7 |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| Waist to height ratio | |||||||||
| Normal | 2487 | 51 ± 23.9 | 50.4 ± 0.4 | 1715 | 51.7 ± 23.5 | 51.3 ± 0.5 | 772 | 49.5 ± 24.7 | 48.5 ± 0.7 |
| Obesity | 3998 | 45.1 ± 21.5 | 45.5 ± 0.3 | 1686 | 45.1 ± 21.1 | 45.5 ± 0.5 | 2312 | 45.1 ± 21.7 | 45.4 ± 0.4 |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| Conicity index | |||||||||
| Normal | 2501 | 49.8 ± 23.2 | 49.6 ± 0.4 | 1538 | 50.2 ± 22.7 | 50.1 ± 0.5 | 963 | 49.3 ± 23.9 | 48.9 ± 0.6 |
| Elevated | 3984 | 45.8 ± 22.1 | 46.0 ± 0.3 | 1863 | 47.0 ± 22.4 | 47.0 ± 0.5 | 2121 | 44.8 ± 21.8 | 45.0 ± 0.4 |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| BRI quartiles | |||||||||
| First | 1625 | 51.6 ± 24.1 | 51.0 ± 0.5 | 854 | 53.1 ± 23.9 | 52.7 ± 0.7 | 772 | 49.5 ± 24.7 | 48.8 ± 0.7 |
| Second | 1622 | 49.3 ± 22.8 | 49.2 ± 0.5 | 861 | 50.3 ± 23.1 | 50.3 ± 0.7 | 772 | 47.6 ± 22.0 | 47.6 ± 0.7 |
| Third | 1622 | 46.6 ± 21.4 | 46.1 ± 0.5 | 836 | 47.7 ± 21.1 | 47.5 ± 0.7 | 773 | 45.4 ± 21.5 | 45.0 ± 0.7 |
| Fourth | 1616 | 42.1 ± 21.0 | 43.1 ± 0.5 | 850 | 42.5 ± 20.9 | 43.1 ± 0.7 | 767 | 42.4 ± 21.4 | 43.4 ± 0.7 |
| p-value | < 0.001 | < 0.001* | < 0.001 | < 0.001* | < 0.001 | < 0.001* | |||
| ABSI quartiles | |||||||||
| First | 1622 | 48.8 ± 22.4 | 48.6 ± 0.5 | 851 | 48.7 ± 22.2 | 49.0 ± 0.7 | 771 | 49.2 ± 23.7 | 49.4 ± 0.7 |
| Second | 1621 | 48.2 ± 22.7 | 48.2 ± 0.5 | 850 | 47.8 ± 21.8 | 47.7 ± 0.7 | 771 | 47.1 ± 21.8 | 46.6 ± 0.7 |
| Third | 1621 | 47.5 ± 22.8 | 47.4 ± 0.5 | 850 | 48.9 ± 23.5 | 49.3 ± 0.7 | 771 | 45.0 ± 22.3 | 45.4 ± 0.7 |
| Fourth | 1621 | 45.0 ± 22.4 | 45.3 ± 0.5 | 850 | 48.3 ± 23.0 | 47.6 ± 0.7 | 771 | 43.6 ± 22.1 | 43.6 ± 0.7 |
| p-value | < 0.001 | < 0.001* | 0.805 | 0.426* | < 0.001 | < 0.001* | |||
*p-value for linear trend. BRI, body roundness index; ABSI, a body shape index. Results are expressed in nmol/L of vitamin D and as mean standard ± deviation for bivariate analyses or as adjusted mean ± standard error for multivariate analyses. Statistical analysis using ANOVA. Multivariate analysis adjusting for age (continuous), nationality (Swiss, other), month, smoking categories (never, former, current), vitamin D supplementation (yes, no) and physical activity (yes, no); for the overall analysis, adjustment on sex (men, women) was also performed.
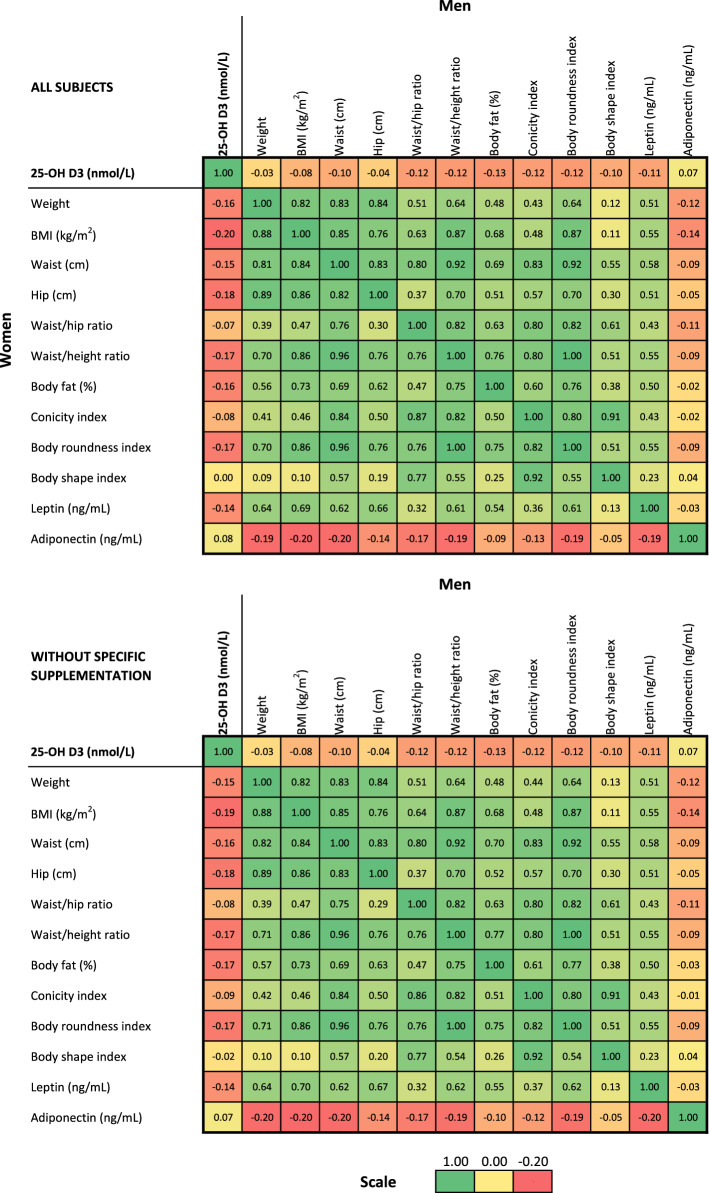
The bivariate Spearman correlations between vitamin D levels and anthropometric markers are summarized in Fig. 1. Negative correlations were found for all anthropometric markers. With the exception of the correlations between vitamin D and ABSI (in women) and weight (in men), all correlations were statistically significant (p < 0.05). Similar findings were obtained after excluding participants taking vitamin D supplements (Fig. 1).
Figure 1.
Bivariate non-parametric Spearman correlations between vitamin D levels and anthropometric markers, stratified by gender, CoLaus|PsyCoLaus study, Lausanne. Top panel: all participants. Bottom panel: participants with prescribed supplemental vitamin D excluded. Within each panel, correlations are provided in the lower left for women and in the top right for men.
The results of the stepwise linear regression analysis are summarized in Table 3. After adjusting for major confounders, % body fat was consistently and negatively associated with vitamin D levels overall and for both sexes. Associations were also found for WHtR (overall), BMI (women) and conicity index (men). Similar findings were obtained after excluding participants taking vitamin D supplements (supplementary Table 3) or adjusting for CRP levels (not shown).
Table 3.
Results of the stepwise linear regression to assess the anthropometric markers most associated with vitamin D levels, overall and stratified by sex, CoLaus|PsyCoLaus study, Lausanne.
| Overall | Women | Men | |
|---|---|---|---|
| Weight (kg) | – | – | – |
| BMI (kg/m2) | – | −3.62 (− 4.62; − 2.62) | – |
| Waist (cm) | – | – | – |
| Hip (cm) | – | – | – |
| Waist/hip ratio | – | – | – |
| Waist/height ratio | −2.78 (−3.51; −2.05) | – | – |
| Body fat (%) | −2.65 (−3.58; −1.72) | −1.84 (−2.92; −0.75) | −1.79 (−2.68; −0.91) |
| Conicity index | – | – | −2.63 (−3.46; −1.80) |
| Body roundness index | – | – | – |
| Body shape index | – | – | – |
Results are expressed as slope and (95% confidence interval) for the markers retained. All anthropometric markers were standardized (i.e., zero average and unit standard deviation) before the stepwise regression. –, not retained.
Association between vitamin D deficiency levels and anthropometric markers
There were 1895 (55.7%) of women and 1854 (60.1%) men presenting with vitamin D deficiency. Table 4 presents the bivariate and multivariate analysis of the associations between vitamin D deficiency and the different measures of adiposity. The likelihood of vitamin D deficiency increased with increasing adiposity levels; the sole exceptions were no associations with ABSI (women) and BMI (men). Similar findings were obtained after excluding participants taking vitamin D supplements (supplementary Table 4).
Table 4.
Bivariate and multivariate associations between vitamin D deficiency (i.e. defined as < 30 ng/mL or < 50 nmol/l) and adiposity measures, overall and stratified by sex, CoLaus|PsyCoLaus study, Lausanne.
| N | Overall | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bivariate | Multivariate | N | Bivariate | Multivariate | N | Bivariate | Multivariate | ||
| Body mass index | |||||||||
| Underweight | 102 | 76 (74.5) | 1.10 (0.70–1.72) | 81 | 35 (43.2) | 1.01 (0.61–1.67) | 21 | 13 (61.9) | 1.74 (0.63–4.83) |
| Normal | 3012 | 2566 (85.2) | 1 (ref.) | 1869 | 932 (49.9) | 1 (ref.) | 1143 | 645 (56.4) | 1 (ref.) |
| Overweight | 2377 | 2141 (90.1) | 1.39 (1.22–1.58) | 963 | 584 (60.6) | 1.65 (1.38–1.98) | 1414 | 854 (60.4) | 1.10 (0.91–1.33) |
| Obesity | 994 | 936 (94.2) | 1.98 (1.66–2.37) | 488 | 344 (70.5) | 2.33 (1.82–2.97) | 506 | 342 (67.6) | 1.55 (1.18–2.03) |
| p-value | < 0.001 | 0.004* | < 0.001 | < 0.001* | < 0.001 | 0.873* | |||
| Abdominal obesity | |||||||||
| Normal | 4572 | 2508 (54.9) | 1 (ref.) | 2286 | 1185 (51.8) | 1 (ref.) | 2286 | 1323 (57.9) | 1 (ref.) |
| Obesity | 1913 | 1241 (64.9) | 1.65 (1.45–1.88) | 1115 | 710 (63.7) | 1.75 (1.48–2.08) | 798 | 531 (66.5) | 1.45 (1.18–1.78) |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| Waist to height ratio | |||||||||
| Normal | 2487 | 1283 (51.6) | 1 (ref.) | 1715 | 866 (50.5) | 1 (ref.) | 772 | 417 (54.0) | 1 (ref.) |
| Obesity | 3998 | 2466 (61.7) | 1.53 (1.34–1.73) | 1686 | 1029 (61.0) | 1.59 (1.35–1.87) | 2312 | 1437 (62.2) | 1.39 (1.13–1.71) |
| p-value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| Conicity index | |||||||||
| Normal | 2501 | 1331 (53.2) | 1 (ref.) | 1538 | 807 (52.5) | 1 (ref.) | 963 | 524 (54.4) | 1 (ref.) |
| Elevated | 3984 | 2418 (60.7) | 1.43 (1.26–1.62) | 1863 | 1088 (58.4) | 1.35 (1.15–1.58) | 2121 | 1330 (62.7) | 1.50 (1.23–1.83) |
| p-value | < 0.001 | < 0.001 | 0.001 | < 0.001 | < 0.001 | < 0.001 | |||
| BRI quartiles | |||||||||
| First | 1625 | 818 (50.3) | 1 (ref.) | 854 | 399 (46.7) | 1 (ref.) | 772 | 417 (54.0) | 1 (ref.) |
| Second | 1622 | 888 (54.8) | 1.18 (1.01–1.39) | 861 | 467 (54.2) | 1.41 (1.14–1.75) | 772 | 442 (57.3) | 1.13 (0.89–1.44) |
| Third | 1622 | 959 (59.1) | 1.53 (1.29–1.82) | 836 | 471 (56.3) | 1.60 (1.28–1.99) | 773 | 481 (62.2) | 1.56 (1.21–2.00) |
| Fourth | 1616 | 1084 (67.1) | 2.06 (1.72–2.47) | 850 | 558 (65.7) | 2.41 (1.90–3.06) | 767 | 514 (67.0) | 1.73 (1.32–2.26) |
| p-value | < 0.001 | < 0.001* | < 0.001 | < 0.001* | < 0.001 | < 0.001* | |||
| ABSI quartiles | |||||||||
| First | 1622 | 904 (55.7) | 1 (ref.) | 851 | 472 (55.5) | 1 (ref.) | 771 | 422 (54.7) | 1 (ref.) |
| Second | 1621 | 907 (56.0) | 0.99 (0.84–1.16) | 850 | 485 (57.1) | 1.14 (0.92–1.41) | 771 | 442 (57.3) | 1.27 (1.00–1.62) |
| Third | 1621 | 937 (57.8) | 1.09 (0.92–1.30) | 850 | 469 (55.2) | 1.00 (0.80–1.23) | 771 | 490 (63.6) | 1.66 (1.29–2.13) |
| Fourth | 1621 | 1001 (61.8) | 1.29 (1.07–1.55) | 850 | 469 (55.2) | 1.09 (0.88–1.37) | 771 | 500 (64.9) | 1.87 (1.43–2.44) |
| p-value | 0.001 | 0.005* | 0.839 | 0.697* | < 0.001 | < 0.001* | |||
*p-value for linear trend for multivariate analysis. BRI, body roundness index; ABSI, a body shape index. Results are expressed as number of participants and row (%) of vitamin D deficiency for bivariate analyses or as adjusted odds ratio (95% confidence interval) for vitamin D insufficiency. Bivariate analysis using chi-square. Multivariate analysis using logistic regression adjusting for age (continuous), nationality (Swiss, other), month, smoking categories (never, former, current), vitamin D supplementation (yes, no) and physical activity (yes, no); for the overall analysis, adjustment on sex (men, women) was also performed.
Association between vitamin D levels and adipokines
The associations between vitamin D, leptin and adiponectin levels are summarized in Fig. 1 (bivariate) and supplementary Table 5 (multivariate). In both univariate and multivariate analyses, leptin levels were negatively associated with vitamin D levels. Adipokine levels were positively associated with vitamin D levels in women, while no association was found in men. Similar findings were obtained after excluding participants taking vitamin D supplements (Fig. 1 and supplementary Table 5).
Discussion
To our knowledge, this is one of the most comprehensive study in a large population-based cohort of apparently healthy subjects associating vitamin D levels with a wide array of obesity markers. Our results show a negative association between various adiposity measurements and vitamin D levels, and that total body fatness is the adiposity marker most associated with vitamin D levels. Our results add further evidence to the hypothesis that low vitamin D levels might be explained by the sequestration of the vitamin by excess body fat stores.
Associations between vitamin D levels and anthropometric markers
Negative associations between vitamin D levels and all markers of adiposity were found, the sole exception being BMI in men, where underweight participants had vitamin D levels close to those of participants with obesity. A possible explanation is that underweight men might have reduced vitamin D dietary intake and/or be affected by pathophysiological conditions affecting vitamin D metabolism, but this hypothesis remains to be assessed. Still, our findings are consistent with the previous literature. Inverse associations have been reported between vitamin D levels and abdominal and visceral fat26; waist circumference and BRI27; the visceral adiposity index28, and with both total and regional adiposity29. Also, De Pergola et al., found that vitamin D circulating levels were progressively lower with the increase of fat mass in a cohort of healthy overweight and subjects with obesity5.
WC, WHR and WHtR are traditional indicators of abdominal obesity. BRI and ABSI have recently been proposed as novel anthropometric measurements and are positively correlated with visceral adiposity10,21,27. Conversely, CI is rarely used as a measure to assess adiposity, despite being a good predictor of abdominal adiposity8. Notwithstanding, total body fat was the sole anthropometric marker consistently associated with low vitamin D levels, suggesting that it is the total amount of body fat, rather than localized or unevenly distributed body fat, that are associated with vitamin D levels. Our results thus add further evidence to the hypothesis that low levels of vitamin D in individuals with obesity can be explained by the sequestration of vitamin D by adipose tissue or by simple volumetric dilution in adipose tissue30.
Association between vitamin D deficiency levels and anthropometric markers
Over half of the sample presented with vitamin D deficiency, hypovitaminosis D (i.e. insufficiency or deficiency) being more frequent in men than in women. Our findings do not replicate recent studies showing that hypovitaminosis D is more prevalent in women than in men10,31,32. Possible explanations include the fact that the women in this study were less frequently were less frequently diagnosed with obesity than men, or an unhealthier dietary intake in men.
Overall, our results show that individuals with higher levels of adiposity are more predisposed to reduced serum concentrations of vitamin D. This reduced bioavailability would trigger a hypothalamic action, stimulating food intake and reducing energy expenditure33. Chronic inflammation linked to obesity could also explain hypovitaminosis D34 due to adipose tissue macrophage infiltration and production of proinflammatory adipokines35. It has also been postulated that the increase in adiposity would be promoted by resistance to stimulation of lipolysis by catecholamines and natriuretic peptide in people with obesity36. Furthermore, this imbalance may lead to reduced release of vitamin D from fat depots, given the fat-soluble nature of vitamin D36.
Importantly, it is unclear whether increasing vitamin D supplementation to correct low vitamin D levels in subjects with increased adiposity is the best option. One study demonstrated that adapting supplementation to both vitamin D deficit and adiposity levels would be more efficient37, but further studies are needed to confirm this finding. Besides, improvements in vitamin D levels have been associated with more weight loss, a result which can be partly attributed to the anti-inflammatory effects of vitamin D33.
Association between vitamin D levels and adipokines
Our study showed that vitamin D levels were inversely associated with leptin and positively associated with adiponectin. Our findings are in agreement with those of Gangloff et al.38, who reported a negative correlation vitamin D levels and leptin. The authors also reported that a decrease in visceral adipose tissue volume and corresponding decrease in leptin levels was associated with an increase in plasma vitamin D concentrations.
Studies have shown that vitamin D affects energy homeostasis by directly regulating leptin expression. However, the exact in vivo effect of vitamin D on leptin expression in humans is controversial11. Some studies have suggested that vitamin D can improve adipose tissue inflammation and suppress expression of leptin39. A recent study demonstrated that vitamin D acts through its receptor (VDR) to inhibit inflammatory pathways and the expression of adipokines in human adipocytes12. Overall, these findings suggest that improving vitamin D status in people with obesity may decrease adipose inflammation, which may contribute to reduce risks of obesity-associated pathophysiological processes. Although more recent studies point to CRP as a marker of inflammation associated with vitamin D levels, our results remained unchanged after adjusting for CRP.
Strengths and limitations
This study was carried out in a representative sample of the population and included a large panel of adiposity markers.
Some limitations should be acknowledged. First, the analysis was conducted in an urban Swiss population and the participants were mostly Caucasian. The results might not be generalizable to other countries. Still, our results are concordant with ones reported in other populations. Second, fat mass was measured by bioimpedance with the known limitations of this technique to accurately quantify adipose tissue particularly using a single time point measurement. Third, our cross-sectional study does not allow to draw any potential causality relationships between the investigated variables (i.e. whether it is obesity that causes vitamin D deficiency or vice versa). However, a prospective study is envisaged. Fourthly, we did not use different cutoff points for vitamin D according to seasonality as indicated in other studies40. Still, we adjusted the analysis considering the month. We chose this methodology as changing the threshold according to season would be difficult to apply in clinical practice.
Conclusions
Our data show a negative association between vitamin D levels and most adiposity markers and that people with excess adiposity are more likely exhibit to present with vitamin D deficiency as measured by total circulating 25-hydroxyvitamin D. Total fat mass is the adiposity marker most consistently associated with decreased vitamin D levels. Monitoring the levels of vitamin D in people with overweight/obesity is essential.
Supplementary Information
Author contributions
The authors had full access to the data and took responsibility for its integrity. All authors have read and agreed to the written manuscript. P.P. and S.R.: conceptualization, investigation, writing—original draft preparation; visualization. I.G.: resources, funding acquisition, writing—review and editing P.M.-V.: methodology, data curation, formal analysis, writing—reviewing and editing.
Funding
The CoLaus study was and is supported by research grants from GlaxoSmithKline, the Faculty of Biology and Medicine of Lausanne, and the Swiss National Science Foundation (grants 33CSCO-122661, 33CS30-139468, 33CS30-148401, and 33CS30_177535/1). The funding source was not involved in the study design, data collection, analysis, and interpretation, writing of the report, or decision to submit the article for publication.
Data availability
The CoLaus|PsyCoLaus cohort data used in this study cannot be fully shared as they contain potentially sensitive patient information. As discussed with the competent authority, the Research Ethic Committee of the Canton of Vaud, transferring or directly sharing this data would be a violation of the Swiss legislation aiming to protect the personal rights of participants. Non-identifiable, individual-level data are available for interested researchers, who meet the criteria for access to confidential data sharing, from the CoLaus Datacenter (CHUV, Lausanne, Switzerland). Instructions for gaining access to the CoLaus data used in this study are available at https://www.colaus-psycolaus.ch/professionals/how-to-collaborate/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19409-9.
References
- 1.Gangloff, A., Bergeron, J., Lemieux, I., & Després, J.-P. Changes in circulating vitamin D levels with loss of adipose tissue. Curr. Opin. Clin. Nutr. Metab. Care [Internet] 19(6), 464–470. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00075197-201611000-00011 (2016). [DOI] [PubMed]
- 2.Cabral MA, Borges CN, Maia JMC, Aires CAM, Bandeira F. Prevalence of vitamin D deficiency during the summer and its relationship with sun exposure and skin phototype in elderly men living in the tropics. Clin. Interv. Aging. 2013;8:1347–1351. doi: 10.2147/CIA.S47058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guessous I, Dudler V, Glatz N, Theler JM, Zoller O, Paccaud F, et al. Vitamin D levels and associated factors: A population-based study in Switzerland. Swiss. Med. Wkly. 2012;2012:142. doi: 10.4414/smw.2012.13719. [DOI] [PubMed] [Google Scholar]
- 4.Zhen D, Liu L, Guan C, Zhao N, Tang X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone [Internet]. 2015;71:1–6. doi: 10.1016/j.bone.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 5.De Pergola G, Martino T, Zupo R, Caccavo D, Pecorella C, Paradiso S, et al. 25 Hydroxyvitamin D levels are negatively and independently associated with fat mass in a cohort of healthy overweight and obese subjects. Endocr. Metab. Immune Disord. Drug Targets. 2019;19(6):838–844. doi: 10.2174/1871530319666190122094039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajhashemy Z, Shahdadian F, Ziaei R, Saneei P. Serum vitamin D levels in relation to abdominal obesity: A systematic review and dose–response meta-analysis of epidemiologic studies. Obes. Rev. 2021;22(2):13134. doi: 10.1111/obr.13134. [DOI] [PubMed] [Google Scholar]
- 7.Golzarand M, Hollis BW, Mirmiran P, Shab-bidar CLWS. Vitamin D supplementation and body fat mass: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018;72:1345. doi: 10.1038/s41430-018-0132-z. [DOI] [PubMed] [Google Scholar]
- 8.Mueller WH, Meininger JC, Liehr P, Chan W, Chandler PS. Conicity: A new index of body fat distribution—What does it tell us? Am. J. Hum. Biol. 1996;8(4):489–496. doi: 10.1002/(SICI)1520-6300(1996)8:4<489::AID-AJHB9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 9.Valdez R. A simple model-based index of abdominal adiposity. J. Clin. Epidemiol. 1991;44(9):955–956. doi: 10.1016/0895-4356(91)90059-I. [DOI] [PubMed] [Google Scholar]
- 10.Zhu XL, Chen ZH, Li Y, Yang PT, Liu L, Wu LX, et al. Associations of vitamin D with novel and traditional anthropometric indices according to age and sex: A cross-sectional study in central southern China. Eat Weight Disord. [Internet]. 2020;25(6):1651–1661. doi: 10.1007/s40519-019-00803-8. [DOI] [PubMed] [Google Scholar]
- 11.Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. [Internet]. 165, 369–381. https://linkinghub.elsevier.com/retrieve/pii/S0960076016302199 (2015). [DOI] [PubMed]
- 12.Nimitphong H, Guo W, Holick MF, Fried SK, Lee M. Vitamin D inhibits adipokine production and inflammatory signaling through the vitamin D receptor in human adipocytes. Obesity [Internet]. 2021;29(3):562–568. doi: 10.1002/oby.23109. [DOI] [PubMed] [Google Scholar]
- 13.Firmann M, Mayor V, Vidal PM, Bochud M, Pécoud A, Hayoz D, et al. The CoLaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008;8:1–11. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmann TG, Roche AF. Human Kinetics Books. Human Kinetics; 1988. [Google Scholar]
- 15.World Health Organization (WHO). WHO Expert Committee on Physical Status: The Use and Interpretation of Anthropometry (1995) [Internet]. Vol. 1. Who Technical Report Series. Vol. 854. https://apps.who.int/iris/handle/10665/37003 (WHO, 1995). [PubMed]
- 16.Ashwell M, Gibson S. A proposal for a primary screening tool: “Keep your waist circumference to less than half your height”. BMC Med. 2014;12(1):1–6. doi: 10.1186/s12916-014-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012;13(3):275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramel A, Geirsdottir OG, Arnarson A, Thorsdottir I. Regional and total body bioelectrical impedance analysis compared with DXA in Icelandic elderly. Eur. J. Clin. Nutr. 2011;65(8):978–983. doi: 10.1038/ejcn.2011.65. [DOI] [PubMed] [Google Scholar]
- 19.Pitanga, F.J.G., & Lessa, I. Anthropometric indexes of obesity as an instrument of screening for high coronary risk in adults in the city of Salvador--Bahia. Arq. Bras. Cardiol. [Internet] 85(1), 26–31. http://www.ncbi.nlm.nih.gov/pubmed/16041451 (2005). [DOI] [PubMed]
- 20.Thomas DM, Bredlau C, Bosy-Westphal A, Mueller M, Shen W, Gallagher D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. 2013;21(11):2264–2271. doi: 10.1002/oby.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce SJ, Rochat B, Béguin A, Pesse B, Guessous I, Boulat O, et al. Analysis and quantification of vitamin D metabolites in serum by ultra-performance liquid chromatography coupled to tandem mass spectrometry and high-resolution mass spectrometry—A method comparison and validation. Rapid Commun. Mass Spectrom. 2013;27(1):200–206. doi: 10.1002/rcm.6439. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017;18(2):153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 25.StataCorp. 2021. Data Management Reference Manual for Stata [Internet]. 17 edn. (College Station, ed.). https://www.stata.com/bookstore/data-management-reference-manual/ (Stata Press, 2017).
- 26.Rafiq R, Walschot F, Lips P, Lamb HJ, de Roos A, Rosendaal FR, et al. Associations of different body fat deposits with serum 25-hydroxyvitamin D concentrations. Clin. Nutr. 2019;38(6):2851–2857. doi: 10.1016/j.clnu.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Sousa-Santos AR, Afonso C, Santos A, Borges N, Moreira P, Padrão P, et al. The association between 25(OH)D levels, frailty status and obesity indices in older adults. PLoS ONE. 2018;13(8):1–16. doi: 10.1371/journal.pone.0198650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivera-Paredez B, Hidalgo-Bravo A, León-Reyes G, León-Maldonado LS, Aquino-Gálvez A, Castillejos-López M, et al. Total, bioavailable, and free 25-hydroxyvitamin D equally associate with adiposity markers and metabolic traits in mexican adults. Nutrients. 2021;13(10):1–15. doi: 10.3390/nu13103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karuppusami R, Antonisami B, Vasan SK, Gowri M, Selliah HY, Arulappan G, et al. Association of serum 25-hydroxy vitamin D with total and regional adiposity and cardiometabolic traits. PLoS One [Internet]. 2021;15:1–13. doi: 10.1371/journal.pone.0243850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savastano S, Barrea L, Savanelli MC, Nappi F, DiSomma C, Orio F, et al. Low vitamin D status and obesity: Role of nutritionist. Rev. Endocr. Metab. Disord. [Internet]. 2017;18(2):215–225. doi: 10.1007/s11154-017-9410-7. [DOI] [PubMed] [Google Scholar]
- 31.Duarte C, Carvalheiro H, Rodrigues AM, Dias SS, Marques A, Santiago T, et al. Correction to: Prevalence of vitamin D deficiency and its predictors in the Portuguese population: A nationwide population-based study. Arch. Osteoporos. 2020;15(1):1–11. doi: 10.1007/s11657-020-0695-x). [DOI] [PubMed] [Google Scholar]
- 32.Muscogiuri G, Barrea L, Di Somma C, Laudisio D, Salzano C, Pugliese G, et al. Sex differences of vitamin D status across BMI classes: An observational prospective cohort study. Nutrients. 2019;11(12):1–12. doi: 10.3390/nu11123034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Da Cunha KA, Magalhães EIDS, Loureiro LMR, Sant’Ana LFDR, Ribeiro AQ, De Novaes JF. Calcium intake, serum vitamin D and obesity in children: Is there an association? Rev. Paul Pediatr. 2015;33(2):222–229. doi: 10.1016/j.rpped.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Z, Xu C, Shu Y, Xie Z, Lu C, Mo X. Serum 25-hydroxy vitamin D is associated with obesity and metabolic parameters in US children. Public Health Nutr. 2020;23(7):1214–1222. doi: 10.1017/S1368980019001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang, E., & Kim, Y. Vitamin D insufficiency exacerbates adipose tissue macrophage infiltration and decreases AMPK/SIRT1 activity in obese rats. Nutrients [Internet]9(4), 338 http://www.mdpi.com/2072-6643/9/4/338 (2017). [DOI] [PMC free article] [PubMed]
- 36.Migliaccio S, Di Nisio A, Mele C, Scappaticcio L, Savastano S, Colao A. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. [Internet]. 2019;9(1):20–31. doi: 10.1038/s41367-019-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drincic A, Fuller E, Heaney RP, Armas LAG. 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. J. Clin. Endocrinol. Metab. 2013;98(12):4845–4851. doi: 10.1210/jc.2012-4103. [DOI] [PubMed] [Google Scholar]
- 38.Gangloff A, Bergeron J, Lemieux I, Tremblay A, Poirier P, Alméras N, et al. Relationships between circulating 25(OH) vitamin D, leptin levels and visceral adipose tissue volume: Results from a 1-year lifestyle intervention program in men with visceral obesity. Int. J. Obes. [Internet]. 2020;44(2):280–288. doi: 10.1038/s41366-019-0347-7. [DOI] [PubMed] [Google Scholar]
- 39.Nimitphong H, Park E, Lee MJ. Vitamin D regulation of adipogenesis and adipose tissue functions. Nutr. Res. Pract. 2020;14(6):553–567. doi: 10.4162/nrp.2020.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schramm S, Lahner H, Jöckel KH, Erbel R, Führer D, Moebus S. Impact of season and different vitamin D thresholds on prevalence of vitamin D deficiency in epidemiological cohorts—A note of caution. Endocrine. 2017;56(3):658–666. doi: 10.1007/s12020-017-1292-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The CoLaus|PsyCoLaus cohort data used in this study cannot be fully shared as they contain potentially sensitive patient information. As discussed with the competent authority, the Research Ethic Committee of the Canton of Vaud, transferring or directly sharing this data would be a violation of the Swiss legislation aiming to protect the personal rights of participants. Non-identifiable, individual-level data are available for interested researchers, who meet the criteria for access to confidential data sharing, from the CoLaus Datacenter (CHUV, Lausanne, Switzerland). Instructions for gaining access to the CoLaus data used in this study are available at https://www.colaus-psycolaus.ch/professionals/how-to-collaborate/.