Abstract
Objective:
Dietary patterns can influence the risk of developing erectile dysfunction (ED). It is unknown whether plant-based diets, which have known health and environmental benefits, are associated with ED. The aim of this study was to evaluate the longitudinal association between plant-based diet index scores and incident ED.
Materials and Methods:
We conducted a prospective analysis of 21,942 men aged 40 to 75 years who were enrolled in the Health Professionals Follow-Up Study. ED was assessed with questionnaires every four years starting in 2000. Dietary data were collected via validated food frequency questionnaires completed every four years and were used to calculate total plant-based diet index (PDI) scores, as well as healthy (hPDI) and unhealthy (uPDI) subscores. Multivariable Cox proportional hazards models were used to compute hazard ratios (HR) for incident ED. All models were stratified by age (<60, 60 to <70, ≥70 years).
Results:
hPDI was inversely associated with incident ED among men aged 60 to <70. Those in the highest quintile of hPDI in that age group had an 18% lower risk of ED (HR = 0.82; 95% CI 0.73–0.91; P-trend<0.001) compared to those in the lowest quintile. Conversely, uPDI was positively associated with ED in men aged <60 (HR = 1.27; 95% CI 1.01–1.60; P-trend=0.02).
Conclusions:
Encouraging a healthy plant-based diet may be an environmentally sustainable intervention for men interested in maintaining erectile function.
Keywords: Plant-based diet, erectile dysfunction, Health Professionals Follow-up Study, men’s health, andrology
Introduction
Estimated to affect at least 18 million men in the United States (US) alone, erectile dysfunction (ED) negatively impacts the quality of life for affected men and their sexual partners.(1–3) Current ED prevention strategies consist of lifestyle modifications such as smoking cessation, exercise, weight loss, and the mitigation of other risk factors shared with cardiovascular disease (CVD), diabetes, and metabolic syndrome.(4)
Diet modification is a promising target for ED prevention, but the link between food intake and sexual health have not been well delineated. Emerging evidence suggests that healthy dietary patterns, such as the Mediterranean diet and the Alternative Healthy Eating Index (AHEI) 2010, are associated with a reduced risk of incident ED.(5) Because these diets encourage the intake of fruits and vegetables over red meat, we hypothesized that plant-based dietary patterns play a key role in these associations. While several cross-sectional studies support this idea,(6–8) a longitudinal analysis of prospectively collected data has not been performed to our knowledge.
Investigating the health effects of plant-based food intake may also have broader social and cultural implications. There is a commonly perceived link between red meat consumption and traditional definitions of masculinity in many cultures.(9–11) Since these perceptions are not supported by evidence, public health messaging may benefit from additional high-quality studies assessing the relationship between plant-based diet and sexual function. Furthermore, as awareness grows of the negative environmental impact of animal product consumption, investigating the health benefits of plant-based foods has great potential to influence change from a conservation and sustainability standpoint.(9, 12)
To continue bridging this gap in knowledge, we explored the associations of the plant-based diet index (PDI), as well as the healthy and unhealthy plant-based diet index (hPDI, uPDI), with incident ED in the Health Professionals Follow-Up Study.
Methods
Participants
Data were collected prospectively from US male health professionals enrolled in the Health Professionals Follow-Up Study since 1986. Food frequency questionnaires (FFQs) were collected every 4 years and surveys regarding lifestyle factors, health outcomes, and medications are collected every 2 years with a 96% response rate. Additional details regarding the methods have been described previously.(13) This study was approved by the Human Subjects Committee at the Harvard T.H. Chan School of Public Health. As approved by the Human Subjects Committee, the return of a questionnaire was considered to imply consent.
There were 51529 men enrolled in 1986, of whom 5510 died before 1998 (Figure S1). We excluded an additional 11,735 men who did not complete the FFQ in 1998, 5 men with multiple records or missing date of birth, 1986 men with a history of prostate, bladder, testicular, or penile cancer to avoid confounding from cancer-related side effects, 2767 men with history of myocardial infarction, 541 men with history of stroke, and 1762 men with diabetes prior to 1998. Finally, we excluded 370 men who did not answer the baseline erectile dysfunction questionnaire upon enrollment and 4911 men with ED at baseline based on self-reported “poor” or “very poor” erectile function prior to 1998. This resulted in 21,942 men who were in the final cohort for analysis.
Assessment of Dietary Patterns
Participants completed a validated FFQ to estimate usual dietary intake (portion size and frequency) of approximately 130 food items. PDI, hPDI, and uPDI were then calculated using a point-based system as previously described.(14) Briefly, all food items from the FFQ were categorized into 12 plant food groups (whole grains, fruits, vegetables, nuts, legumes, vegetable oils, tea/coffee, fruit juices, sugar-sweetened beverages, refined grains, potatoes, and sweets/desserts) and 6 animal food groups (animal fats, dairy, eggs, fish/seafood, meat, and miscellaneous animal-based foods). Plant food groups were further categorized as healthy (whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea/coffee) or unhealthy (fruit juices, sugar-sweetened beverages, refined grains, potatoes, and sweets/desserts) based on established associations with diseases such as diabetes and CVD.(14, 15)
Quintiles were calculated for consumption of each food group measured in servings per day, and each quintile was given a score based on a positive (1–5) or reverse (5–1) designation based on the index. To generate the PDI, all plant food groups were scored positively. For hPDI, only healthy plant food groups were scored positively while unhealthy plant food groups were scored negatively; for uPDI, unhealthy plant food groups were scored positively while healthy plant food groups were scored negatively. Animal food groups were given reverse scores in all three indices.
Outcome Assessment
Starting in 2000, participants reported their ability to maintain an erection sufficient for intercourse without treatment. Response options included very poor, poor, fair, good, or very good. Consistent with previous studies, incident ED was defined as a response of poor or very poor at any point after 2000.(16, 17) Participants were also asked to report their erectile function and historically if/when erectile function changed (before 1986, 1986–1989, 1990–1994, 1995 or later). Subsequent questionnaires after 2004 asked participants to report their erectile function in the past 3 months without treatment. Date of incident ED was defined as date of the questionnaire where ED was first reported for a participant.
Data Analysis
Person-time for each participant was calculated from 1998 until incident ED, genitourinary cancer diagnosis, death, loss to follow-up, or end of the study period (January 1, 2016), whichever occurred first. To account for within-person variation, dietary indices were calculated as the cumulative average of all dietary index scores for each participant until first report of ED, CVD, death, lost to follow-up, or the last FFQ in 2010. Similar to prior studies, dietary index scores were not updated after diagnosis of CVD to minimize confounding since a CVD diagnosis is more likely to lead to both incident ED and dietary modification.(5, 18)
Hazard ratios and 95% CIs for associations between quintiles of dietary index scores and risk of incident ED were calculated using multivariable adjusted Cox proportional hazards models. Based on prior literature and our own previous studies, dividing food groups into quintiles allows for visualization of the shape of an association without assuming a linear relationship.(5, 19, 20) We stratified baseline hazard by calendar year, using age in months as the time scale. Final models were adjusted for smoking status (never, past, or current 1–14, 15–24, or ≥25 cigarettes/day), body mass index (BMI; kg/m2), physical activity (total metabolic equivalent task-hours/week), and time-updated incident CVD during follow-up, self-reported history of diabetes, hypertension, depression, antidepressant or antipsychotic medication use, benzodiazepine medication use, α-blocker or 5-α-reductase inhibitor use,(21) marital status (married, divorced, separated, widowed, or never married), self-reported race, and total caloric intake (kilocalories/day).
To test for linear trends, the median value for each plant-based dietary index score within each quintile was treated as a continuous variable, and the significance of the association was reported as a P-trend. To remain consistent with the prior literature and because age is a strong risk factor for ED(13, 22, 23) and an effect modifier for both dietary and lifestyle factors,(24–26) we stratified all models by age <60, 60 to <70, and ≥70 years. Consistent with previous analyses of this data set, smoking status, history of hypertension or hyperlipidemia, BMI, and physical activity did not modify our observed associations.
All analyses were completed using SAS statistical software version 9.4 (SAS Institute). This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Results
Among the 21,942 men included in the analytic sample, mean age was 61.7 ± 8 years, and 11,292 (51.4%) men developed incident erectile dysfunction over a mean of 12.0 years of follow-up. After stratifying by age, we observed 994 cases per 58102 person-years in men aged <60, 4160 cases per 114457 person-years in men aged 60 to <70, and 6138 per 91661 person-years in men aged ≥70. Compared to the lowest quintile, men in the highest quintile of hPDI were older, more physically active, and they had higher AHEI and Mediterranean dietary index scores (Table 1). Conversely, men in the highest quintile of uPDI were younger, less physically active, and they had lower AHEI and Mediterranean dietary index scores compared to the lowest quintile. PDI and hPDI scores were weakly positively correlated (0.29, p < 0.0001), PDI and uPDI scores were weakly inversely correlated (−0.13, p < 0.0001), and hPDI and uPDI scores were moderately inversely correlated (−0.32, p < 0.0001).
Table 1.
Baseline characteristics of 21,942 men from the Health Professionals Follow-Up Study by extreme quintiles of healthy and unhealthy plant-based dietary index score.
| Healthy plant-based diet score (hPDI) | Unhealthy plant-based diet score (uPDI) | |||
|---|---|---|---|---|
| Characteristic | Lowest quintile (n=4922) | Highest quintile (n=4036) | Lowest quintile (n=4093) | Highest quintile (n=4473) |
| Age, years, mean (SD) | 59.8 (8) | 64.2 (9) | 63.6 (8) | 60.5 (8) |
| BMI, kg/m2, mean (SD) | 26.1 (4) | 25.6 (3) | 26.4 (4) | 25.6 (3) |
| Physical activity, MET-h/wk, mean (SD) | 27.5 (27) | 36.0 (33) | 35.9 (32) | 25.9 (28) |
| Race | ||||
| White | 92.8% | 90.8% | 93.1% | 89.5% |
| Black | 0.5% | 0.6% | 0.5% | 1.1% |
| Asian | 1.0% | 1.8% | 0.7% | 2.8% |
| Other | 5.6% | 6.8% | 5.8% | 6.7% |
| Currently married | 88.6% | 89.6% | 89.8% | 88.7% |
| Smoking Status | ||||
| Never | 54.5% | 51.5% | 47.8% | 59.9% |
| Past | 39.2% | 45.7% | 47.6% | 35.5% |
| Current | 6.3% | 2.9% | 4.6% | 4.6% |
| Self-reported comorbidities | ||||
| Hypertension | 31.0% | 30.6% | 30.0% | 32.1% |
| Hyperlipidemia | 43.0% | 46.2% | 42.3% | 47.8% |
| Depression | 12.6% | 13.2% | 12.9% | 12.2% |
| Medication use | ||||
| Antihypertensive | 22.0% | 22.9% | 20.3% | 24.8% |
| Cholesterol-lowering | 10.0% | 14.7% | 10.2% | 14.6% |
| Antidepressant or antipsychotic | 5.0% | 4.7% | 4.8% | 5.2% |
| Benzodiazepine | 2.7% | 2.3% | 2.6% | 2.6% |
| α-blocker or 5-α-reductase inhibitor | 5.9% | 5.4% | 4.7% | 5.8% |
| Alcohol, g/d, mean (SD) | 10.6 (14) | 11.1 (13) | 13.8 (15) | 8.3 (13) |
| AHEI-2010 score, mean (SD) | 45.1 (10) | 58.8 (11) | 57.0 (10) | 45.3 (10) |
| Mediterranean diet score, mean (SD) | 3.4 (2) | 4.9 (2) | 5.1 (2) | 3.2 (2) |
Abbreviations: AHEI = alternative healthy eating index; BMI = body mass index; MET = metabolic equivalent of task; SD = standard deviation
The associations of PDI, hPDI, and uPDI with incident erectile dysfunction are shown in Figure 1 and Table 2. Higher PDI was associated with a lower risk of incident ED in men aged 60 to <70 years, with those in the highest quintile of PDI having a 9% lower risk of ED compared to those in the lowest quintile (HR = 0.91; 95% CI 0.82–1.01; P-trend = 0.007). We did not observe any associations between PDI and incident ED in men aged <60 or ≥70 (P-trend >0.05 for both). Similarly, hPDI was associated with a lower risk of ED in men aged 60 to <70 (HR = 0.82; 95% CI 0.73–0.91; P-trend<0.001) but not in younger men. For men aged ≥70, the highest quintile of hPDI was associated with a lower risk of ED (HR = 0.92, 95% CI 0.84–0.99), though overall the trend did not reach statistical significance (P-trend = 0.06). Conversely, uPDI was associated with increased risk of ED in men aged <60; men in the highest quintile of uPDI had a 27% higher risk of ED compared to men in the lowest quintile (HR = 1.27; 95% CI 1.01–1.60; P-trend = 0.04), whereas no association was observed between uPDI and ED among men aged 60 and older. When age groups were pooled and analyzed together, multivariable-adjusted models showed an inverse association of higher quintiles of hPDI with incident ED (P-trend <0.001), while a statistically significant association of PDI and uPDI with incident ED was not detected (P-trend 0.09, 0.4 respectively; Figure S2).
Figure 1.
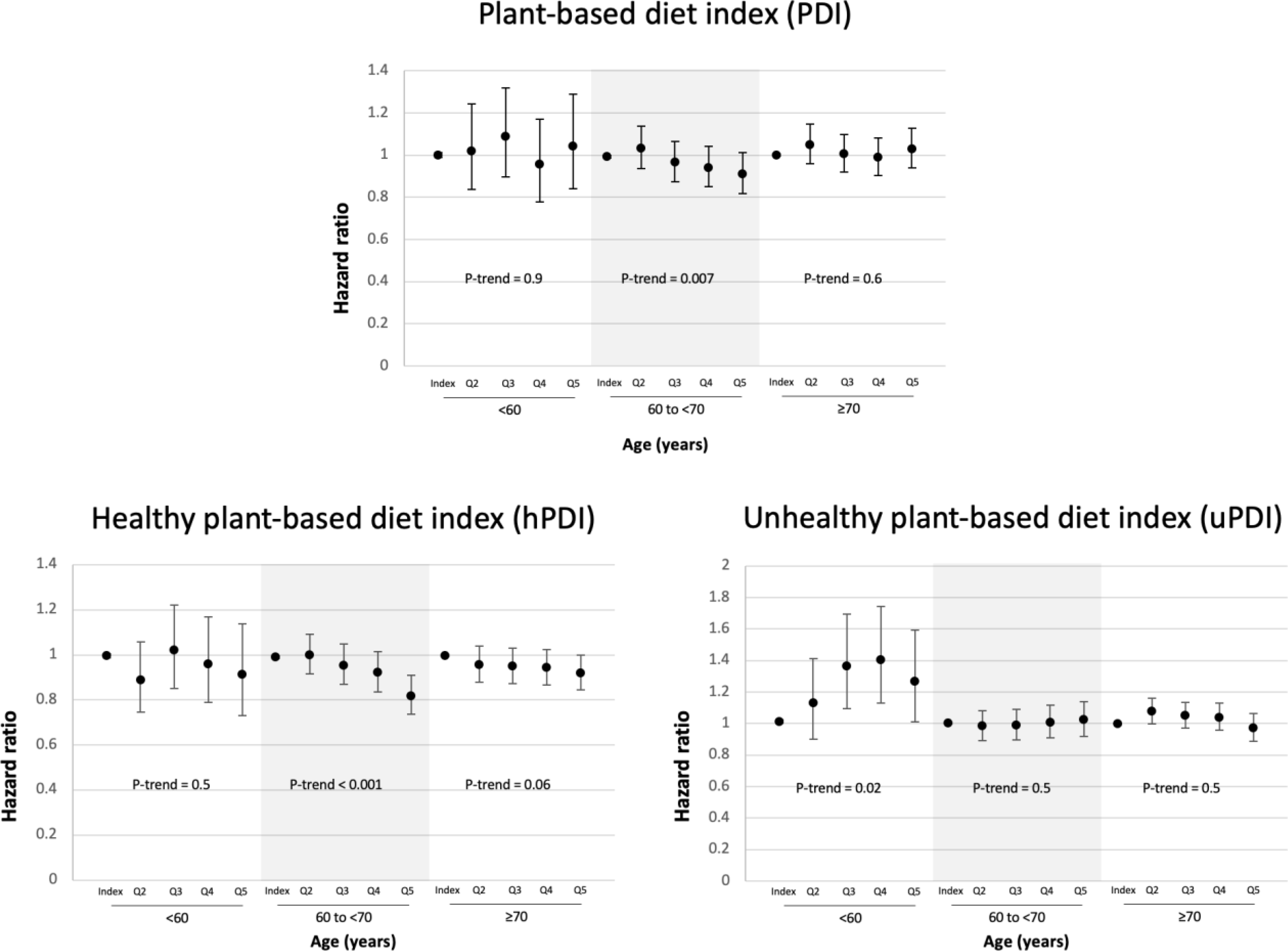
Multivariable-adjusted association of plant-based diet indices with erectile dysfunction stratified by age.
Abbreviations: Q = quintile
Table 2.
Multivariable-adjusted association of plant-based diet index, healthy plant-based diet index, and unhealthy plant-based diet index with incident erectile dysfunction among men from the Health Professionals Follow-Up study stratified by age.
| Quintile of plant-based diet index (PDI) | ||||||
| 1 | 2 | 3 | 4 | 5 | P for trend | |
| Age <60 y | ||||||
| Events/person-years, No. | 199 / 11272 | 197 / 11514 | 213 / 12162 | 194 / 11948 | 191 / 11206 | |
| Event rate, per 1000 person-years | 17.7 | 17.1 | 17.5 | 16.2 | 17.0 | |
| Index score, mean (SD) | 46.0 (2.6) | 51.1 (1.0) | 54.5 (0.9) | 57.9 (1.0) | 63.2 (2.9) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.02 (0.84–1.24) | 1.09 (0.90–1.32) | 0.96 (0.78–1.17) | 1.00 (0.99–1.01) | >0.9 |
| Age 60 to <70 y | ||||||
| Events/person-years, No. | 731 / 19423 | 866 / 22233 | 907 / 24648 | 865 / 24345 | 791 / 23809 | |
| Event rate, per 1000 person-years | 37.6 | 39.0 | 36.8 | 35.5 | 33.2 | |
| Index score, mean (SD) | 45.9 (2.8) | 51.1 (1.1) | 54.5 (1.0) | 57.9 (1.0) | 63.3 (2.8) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.03 (0.94–1.14) | 0.97 (0.87–1.07) | 0.94 (0.85–1.04) | 0.91 (0.82–1.01) | 0.007 |
| Age ≥70 y | ||||||
| Events/person-years, No. | 890 / 13481 | 1172 / 17353 | 1302 / 19303 | 1352 / 20400 | 1422 / 21124 | |
| Event rate, per 1000 person-years | 66.0 | 67.5 | 67.5 | 66.3 | 67.3 | |
| Index score, mean (SD) | 45.7 (2.9) | 51.0 (1.1) | 54.5 (1.0) | 58.0 (1.1) | 63.3 (3.0) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.05 (0.96–1.15) | 1.01 (0.92–1.10) | 0.99 (0.90–1.08) | 1.03 (0.94–1.13) | 0.6 |
| Quintile of healthy plant-based diet index (hPDI) | ||||||
| 1 | 2 | 3 | 4 | 5 | P for trend | |
| Age <60 y | ||||||
| Events/person-years, No. | 304 / 16720 | 208 / 13540 | 204 / 11331 | 160 / 9189 | 118 / 7323 | |
| Event rate, per 1000 person-years | 18.2 | 15.4 | 18.0 | 17.4 | 16.1 | |
| Index score, mean (SD) | 44.5 (3.2) | 50.7 (1.2) | 54.7 (1.1) | 58.6 (1.2) | 64.6 (3.2) | |
| Multivariable model, HR (95% CI) | 1 (index) | 0.89 (0.74–1.06) | 1.02 (0.85–1.22) | 0.96 (0.79–1.17) | 0.91 (0.73–1.14) | >0.9 |
| Age 60 to <70 y | ||||||
| Events/person-years, No. | 1032 / 26949 | 939 / 24695 | 833 / 22864 | 753 / 20578 | 603 / 19371 | |
| Event rate, per 1000 person-years | 38.3 | 38.0 | 36.4 | 36.6 | 31.1 | |
| Index score, mean (SD) | 44.8 (2.9) | 50.8 (1.2) | 54.7 (1.1) | 58.7 (1.2) | 64.8 (3.1) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.00 (0.91–1.09) | 0.95 (0.87–1.05) | 0.92 (0.84–1.02) | 0.82 (0.73–0.91) | <0.001 |
| Age ≥70 y | ||||||
| Events/person-years, No. | 1103 / 16054 | 1188 / 17794 | 1231 / 18389 | 1257 / 19073 | 1359 / 20351 | |
| Event rate, per 1000 person-years | 68.7 | 66.8 | 66.9 | 65.9 | 66.8 | |
| Index score, mean (SD) | 45.2 (2.7) | 50.9 (1.2) | 54.7 (1.1) | 58.7 (1.2) | 64.8 (3.3) | |
| Multivariable model, HR (95% CI) | 1 (index) | 0.96 (0.88–1.04) | 0.95 (0.87–1.03) | 0.94 (0.87–1.02) | 0.92 (0.84–0.99) | 0.06 |
| Quintile of unhealthy plant-based diet index (uPDI) | ||||||
| 1 | 2 | 3 | 4 | 5 | P for trend | |
| Age <60 y | ||||||
| Events/person-years, No. | 132 / 8664 | 177 / 10997 | 215 / 11618 | 241 / 12843 | 229 / 13981 | |
| Event rate, per 1000 person-years | 15.2 | 16.1 | 18.5 | 18.8 | 16.4 | |
| Index score, mean (SD) | 45.4 (2.8) | 50.9 (1.1) | 54.7 (1.0) | 58.4 (1.1) | 64.2 (3.1) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.13 (0.90–1.41) | 1.36 (1.10–1.70) | 1.40 (1.13–1.74) | 1.27 (1.01–1.60) | 0.04 |
| Age 60 to <70 y | ||||||
| Events/person-years, No. | 810 / 21375 | 890 / 24220 | 846 / 23616 | 835 / 23224 | 779 / 22022 | |
| Event rate, per 1000 person-years | 37.9 | 36.8 | 35.8 | 36.0 | 35.4 | |
| Index score, mean (SD) | 45.1 (3.0) | 50.9 (1.1) | 54.6 (1.0) | 58.3 (1.1) | 63.9 (3.0) | |
| Multivariable model, HR (95% CI) | 1 (index) | 0.98 (0.89–1.08) | 0.99 (0.90–1.09) | 1.01 (0.91–1.11) | 1.02 (0.92–1.14) | 0.5 |
| Age ≥70 y | ||||||
| Events/person-years, No. | 1420 / 21198 | 1374 / 19719 | 1309 / 19258 | 1127 / 16823 | 908 / 14664 | |
| Event rate, per 1000 person-years | 67.0 | 69.7 | 68.0 | 67.0 | 61.9 | |
| Index score, mean (SD) | 45.2 (3.1) | 50.9 (1.1) | 54.6 (1.0) | 58.2 (1.1) | 63.9 (2.9) | |
| Multivariable model, HR (95% CI) | 1 (index) | 1.08 (1.00–1.16) | 1.05 (0.97–1.14) | 1.04 (0.96–1.13) | 0.97 (0.89–1.06) | 0.5 |
Abbreviations: CI = confidence interval; HR = hazard ratio; SD = standard deviation
The servings per day of individual dietary score components by lowest and highest quintile of hPDI and uPDI are reported in Table S1 and their individual associations with incident ED are reported in Table S2. In the “healthy” plant-based foods category, fruits and legumes were inversely associated with incident erectile dysfunction while in the animal-based food groups, meat was positively associated with risk of ED. Contrary to the observed relationships with overall dietary patterns, tea and coffee, which is categorized as a “healthy” plant-based food group, was positively associated with incident ED whereas fruit juice, categorized as an “unhealthy” plant-based food group, was inversely associated with ED.
Discussion
This longitudinal cohort study investigates the associations of overall, healthy, and unhealthy plant-based dietary consumption with incident ED. Our data demonstrate that men aged 60 to <70 with the greatest plant-based dietary index—particularly healthy plant-based dietary index—were less likely to develop ED compared to men in this age group with the lowest consumption. Among men aged <60 years, those with the highest consumption of unhealthy plant-based foods had the highest incidence of ED. These findings align with previous studies suggesting a role for healthy plant-based diets in reducing cardiovascular disease. The results also suggest that healthy plant-based dietary patterns may represent an environmentally sustainable intervention for men interested in maintaining erectile function. As a comparison, the hazard ratios reported in our study are similar in magnitude to the associations seen between healthy eating and cardiovascular disease.(27)
To our knowledge, this is the first prospective study to evaluate the association between plant-based dietary indices and incident ED. Our findings are consistent with cross-sectional evidence showing that higher hPDI reduces the risk of ED in men participating in the National Health and Nutrition Examination Survey and that fruit and vegetable intake is associated with lower prevalence of ED in diabetic men(6, 7). A case-control study also showed that men diagnosed with ED have lower PDI and hPDI scores.(8) A single longitudinal study testing the relationship between fruit and vegetable intake and ED was insufficiently powered to detect any significant associations.(28) Our study significantly contributes to the existing literature with a large sample of men and validated dietary questionnaires repeated every 4 years over nearly two decades to better assess long-term dietary patterns.
Compared to previously reported associations between other dietary patterns, such as the Mediterranean diet or AHEI 2010, and ED, associations with plant-based dietary patterns appear to differ across age groups.(5) For example, while both Mediterranean diet score and AHEI 2010 index were most strongly associated with ED risk in men aged <60 years, hPDI was associated with lower ED risk in men aged 60 and older. One possible explanation is that the hPDI was unable to identify small but clinically meaningful associations in men aged <60 due to the smaller number of incident ED cases in this age group. Moreover, this age group is more affected by non-dietary causes of ED,(29, 30) therefore associations between plant-based diets and ED may vary by age groups due to age-related differences in ED pathophysiology. Interestingly, in men aged <60, uPDI was positively associated with an increased risk of developing ED. This is a novel finding that corroborates a large body of evidence supporting a whole-foods plant-based diet for overall cardiovascular health, and it highlights the importance of counseling men about the possible benefit of avoiding not only meat but also unhealthy plant-based foods for optimal sexual health.(31, 32) However, associations observed among younger may also be more susceptible to residual confounding due to psychological stressors which can cause both poor dietary choices and ED. Additional studies are needed to confirm this finding.
The differences in component scoring within each index can provide a degree of mechanistic insight in context of the observed associations of each plant-based index with ED, although these associations may not be causal and must be interpreted cautiously. A healthy plant-based diet emphasizes components that provide higher quantities of dietary fiber, antioxidants such as polyphenols, and unsaturated fatty acids and lower quantities of saturated fat.(14) Dietary fiber, polyphenols, and antioxidants in particular have been shown in randomized clinical trials and prospective studies to improve glucose metabolism, reduce inflammation, and ultimately improve endothelial function.(33–35) Conversely, the unhealthy plant-based diet positively scores components that have a high glycemic index and higher calorie content, qualities that would promote weight gain, insulin resistance, and endothelial dysfunction,(14) although some of these components, such as fruit juice, could also have beneficial effects specific to ED pathophysiology. Ultimately, many components of the hPDI are associated with a reduced risk of CVD and type II diabetes, while many components of uPDI are associated with an increased risk of these conditions. Since these diseases share biological pathways with ED,(36–38) we expected that the corresponding associations of plant-based diet indices with ED would be consistent. Finally, it should be noted that the meat products within each dietary index have been consistently found to be associated with an increased risk of ED.
We acknowledge several limitations to this study. Due to the observational study design and lack of randomization to specific dietary patterns, unmeasured confounding remains possible. For example, psychosocial stressors may promote the development of ED and increased consumption of unhealthy food. However, we expect confounding by psychosocial stressors to be at least partially captured by variables we included in the multivariable model, including history of depression and current antidepressant use. Additionally, we adjusted for a comprehensive set of demographic and health-related behaviors, but we also acknowledge that there are complex relationships between dietary choices, health-related behaviors, and socioeconomic status that may also lead to residual confounding. Future studies would benefit from including a more diverse and international study population.
From a methodology standpoint, both differential and non-differential measurement error are possible when using self-reported diet as predictor. FFQs were used to prospectively evaluate dietary patterns before participants developed ED, however, and non-differential measurement error would bias our results towards the null. The plant-based dietary indices used were not created specifically with respect to ED risk and therefore intake of certain non-plant-based foods that are inversely associated with ED in prior studies, such as fish intake,(5) may not contribute as expected to dietary indices. Conversely, a strength of this study is the use of previously developed dietary indices to estimate the consumption of healthy and unhealthy plant-based foods. Finally, ED was assessed using a single-item question, which may also suffer from misclassification. The use of self-reported ED has been validated, however, and again this would bias our results towards the null.(39)
Conclusions
Encouraging a healthy plant-based diet may be an environmentally sustainable intervention for men interested in maintaining erectile function. Men should be counseled that consuming meat products and unhealthy plant-based foods is associated with an increased risk of developing ED.
Supplementary Material
Acknowledgements
Lydia Liu, SM (Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School) assisted with statistical programming.
Source of Funding
SB is supported by grant 1K12DK111028 from the National Institute of Diabetes, Digestive, and Kidney Disorders. SL is supported by Tricia and Michael Berns. The authors have no other disclosures to report.
References:
- 1.Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. 2007; 120(2): 151–7. [DOI] [PubMed] [Google Scholar]
- 2.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998; 13(3): 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paige NM, Hays RD, Litwin MS, Rajfer J, Shapiro MF. Improvement in emotional well-being and relationships of users of sildenafil. J Urol. 2001; 166(5): 1774–8. [PubMed] [Google Scholar]
- 4.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000; 56(2): 302–6. [DOI] [PubMed] [Google Scholar]
- 5.Bauer SR, Breyer BN, Stampfer MJ, Rimm EB, Giovannucci EL, Kenfield SA. Association of Diet With Erectile Dysfunction Among Men in the Health Professionals Follow-up Study. JAMA Netw Open. 2020; 3(11): e2021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carto C, Pagalavan M, Nackeeran S, Blachman-Braun R, Kresch E, Kuchakulla M, et al. Consumption of a Healthy Plant-based Diet is Associated With a Decreased Risk of Erectile Dysfunction: A Cross-sectional Study of the National Health and Nutrition Examination Survey. Urology. 2022. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Dai S, Wang M, Morrison H. Erectile dysfunction and fruit/vegetable consumption among diabetic Canadian men. Urology. 2013; 82(6): 1330–5. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Kang J, Li Z, Wang X, Liu K, Zhou K, et al. The association between plant-based diet and erectile dysfunction in Chinese men. Basic Clin Androl. 2021; 31(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Backer C, Erreygers S, De Cort C, Vandermoere F, Dhoest A, Vrinten J, et al. Meat and masculinities. Can differences in masculinity predict meat consumption, intentions to reduce meat and attitudes towards vegetarians? Appetite. 2020; 147: 104559. [DOI] [PubMed] [Google Scholar]
- 10.Carroll JA, Capel EM, Gallegos D. Meat, Masculinity, and Health for the “Typical Aussie Bloke”: A Social Constructivist Analysis of Class, Gender, and Consumption. Am J Mens Health. 2019; 13(6): 1557988319885561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prattala R, Paalanen L, Grinberga D, Helasoja V, Kasmel A, Petkeviciene J. Gender differences in the consumption of meat, fruit and vegetables are similar in Finland and the Baltic countries. Eur J Public Health. 2007; 17(5): 520–5. [DOI] [PubMed] [Google Scholar]
- 12.Godfray HCJ, Aveyard P, Garnett T, Hall JW, Key TJ, Lorimer J, et al. Meat consumption, health, and the environment. Science. 2018; 361(6399). [DOI] [PubMed] [Google Scholar]
- 13.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003; 139(3): 161–8. [DOI] [PubMed] [Google Scholar]
- 14.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, et al. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016; 13(6): e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, et al. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J Am Coll Cardiol. 2017; 70(4): 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. A prospective study of risk factors for erectile dysfunction. J Urol. 2006; 176(1): 217–21. [DOI] [PubMed] [Google Scholar]
- 17.Lopez DS, Liu L, Rimm EB, Tsilidis KK, de Oliveira Otto M, Wang R, et al. Coffee Intake and Incidence of Erectile Dysfunction. Am J Epidemiol. 2018; 187(5): 951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladapo JA, Hoffmann U, Lee KL, Coles A, Huang M, Mark DB, et al. Changes in Medical Therapy and Lifestyle After Anatomical or Functional Testing for Coronary Artery Disease. J Am Heart Assoc. 2016; 5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenfield SA, DuPre N, Richman EL, Stampfer MJ, Chan JM, Giovannucci EL. Mediterranean diet and prostate cancer risk and mortality in the Health Professionals Follow-up Study. Eur Urol. 2014; 65(5): 887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010; 92(6): 1429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Nunzio C, Lombardo R, Tema G, Tubaro A. Erectile Dysfunction and Lower Urinary Tract Symptoms. Curr Urol Rep. 2018; 19(8): 61. [DOI] [PubMed] [Google Scholar]
- 22.Fang SC, Rosen RC, Vita JA, Ganz P, Kupelian V. Changes in erectile dysfunction over time in relation to Framingham cardiovascular risk in the Boston Area Community Health (BACH) Survey. J Sex Med. 2015; 12(1): 100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiri R, Koskimaki J, Hakkinen J, Tammela TL, Huhtala H, Hakama M, et al. Effects of age, comorbidity and lifestyle factors on erectile function: Tampere Ageing Male Urological Study (TAMUS). Eur Urol. 2004; 45(5): 628–33. [DOI] [PubMed] [Google Scholar]
- 24.Cassidy A, Franz M, Rimm EB. Dietary flavonoid intake and incidence of erectile dysfunction. Am J Clin Nutr. 2016; 103(2): 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pourmand G, Alidaee MR, Rasuli S, Maleki A, Mehrsai A. Do cigarette smokers with erectile dysfunction benefit from stopping?: a prospective study. BJU Int. 2004; 94(9): 1310–3. [DOI] [PubMed] [Google Scholar]
- 26.Gades NM, Nehra A, Jacobson DJ, McGree ME, Girman CJ, Rhodes T, et al. Association between smoking and erectile dysfunction: a population-based study. Am J Epidemiol. 2005; 161(4): 346–51. [DOI] [PubMed] [Google Scholar]
- 27.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern Med. 2020; 180(8): 1090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu ZM, Wong CKM, Chan D, Tse LA, Yip B, Wong SY. Fruit and Vegetable Intake in Relation to Lower Urinary Tract Symptoms and Erectile Dysfunction Among Southern Chinese Elderly Men: A 4-Year Prospective Study of Mr OS Hong Kong. Medicine (Baltimore). 2016; 95(4): e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HMT, Gabrielson AT, Hellstrom WJG. Erectile Dysfunction in Young Men-A Review of the Prevalence and Risk Factors. Sex Med Rev. 2017; 5(4): 508–20. [DOI] [PubMed] [Google Scholar]
- 30.Rastrelli G, Maggi M. Erectile dysfunction in fit and healthy young men: psychological or pathological? Transl Androl Urol. 2017; 6(1): 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998; 280(23): 2001–7. [DOI] [PubMed] [Google Scholar]
- 32.Loeb S, Fu BC, Bauer SR, Pernar CH, Chan JM, Van Blarigan EL, et al. Association of Plant-Based Diet Index with Prostate Cancer Risk. Am J Clin Nutr. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010; 2(12): 1266–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Keogh JB, Clifton PM. Polyphenols and Glycemic Control. Nutrients. 2016; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butcher JL, Beckstrand RL. Fiber’s impact on high-sensitivity C-reactive protein levels in cardiovascular disease. J Am Acad Nurse Pract. 2010; 22(11): 566–72. [DOI] [PubMed] [Google Scholar]
- 36.McCulloch DK, Campbell IW, Wu FC, Prescott RJ, Clarke BF. The prevalence of diabetic impotence. Diabetologia. 1980; 18(4): 279–83. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki H, Yamasaki H, Ogawa K, Nanjo K, Kawamori R, Iwamoto Y, et al. Prevalence and risk factors for erectile dysfunction in Japanese diabetics. Diabetes Res Clin Pract. 2005; 70(1): 81–9. [DOI] [PubMed] [Google Scholar]
- 38.Miner M, Seftel AD, Nehra A, Ganz P, Kloner RA, Montorsi P, et al. Prognostic utility of erectile dysfunction for cardiovascular disease in younger men and those with diabetes. Am Heart J. 2012; 164(1): 21–8. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med. 2005; 20(6): 515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


