Abstract
Purpose of review:
Basic transplant immunology has primarily focused on the definition of mechanisms, but an often-stated aspirational goal is to translate basic mechanistic research into future therapy. Pre-transplant donor specific antibodies (DSA) mediate hyperacute as well as early antibody-mediated rejection (AMR), while DSA developing late post-transplantation may additionally mediated chronic rejection. While contemporary immunosuppression effectively prevents early cellular rejection after transplant in non-sensitized patients, it is less effective at controlling pre-existing HLA antibody responses or reversing DSA once established, thus underscoring a need for better therapies.
Recent Findings:
We here review the development of a bench-to-bedside approach involving transient proteasome inhibition to deplete plasma cells, combined with maintenance co-stimulation blockade, with CTLA-4Ig or belatacept, to prevent the generation of new antibody-secreting cells (ASCs).
Summary:
This review discussed how this a treatment regimen that was rationally designed and validated to reverse established DSA responses in mouse models, translated into reversing active AMR in the clinic, as well as desensitizing highly-sensitized patients on the transplant waitlist.
Keywords: Donor-specific antibodies, antibody-mediated rejection, desensitization, proteasome inhibition, co-stimulation blockade
Introduction
Seminal studies by Billingham and colleagues established a critical role for T cells in mediating skin allograft rejection(1, 2), while studies by Terasaki and colleagues established a role for humoral immunity in mediating kidney rejection in the clinic (3, 4). Additional direct evidence for antibodies mediating kidney rejection came from Feucht and colleagues who reported on complement C4d deposits in the peritubular capillaries of rejected kidney allografts(5, 6). These and subsequent studies firmly establish the pathogenicity of high-titer DSA pre-existing at the time of transplantation, or when produced early post-transplantation, in mediating hyperacute and early AMR of kidney and heart allografts.
While there is a strong clinical correlation between DSA and poor graft outcomes (7-9), some controversy exists as to whether DSA is the initiator and/or driver of ongoing chronic rejection or is serving simply as a biomarker for an uncontrolled cellular response that is actually mediating graft rejection (10). One way to resolve this uncertainty would be to test if reversing DSA responses improves graft outcome. However, currently there is no treatment that reliably reverses established DSA responses especially in a chronic setting. In this review, we will discuss a mechanistic-based bench-to-bedside approach we took to developing an effective therapeutic strategy, involving co-stimulation blockade and proteasome inhibition, to reverse established donor-specific responses that is applicable to treating clinical AMR and in desensitization (Figure 1).
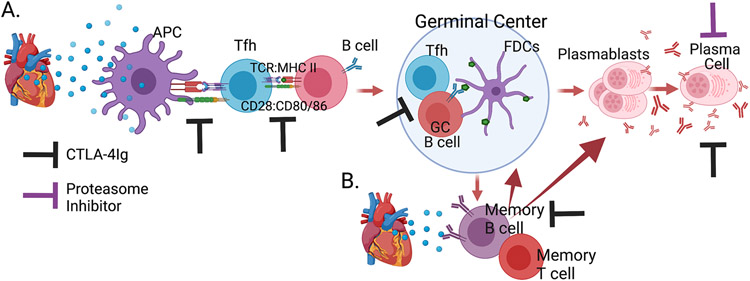
FIGURE 1.
(a) The cellular interactions that result in the differentiation of naive alloreactive B cells into antibody-secreting plasmablasts and plasma cells. CTLA-4Ig interferes with CD28 binding to CD80/CD86, which facilitates cognate T-cell : B-cell interactions at the T: B interface and in the germinal center. As a result, the differentiation of extrafollicular and postgerminal center memory B cells and antibody-secreting cells is prevented. (b) Upon alloantigen reencounter, memory B cells preferentially differentiate into antibody-secreting cells in a T-cell-dependent manner. CTLA-4Ig inhibits memory B-cell differentiation into antibody-secreting cells. Proteasome inhibitors rapidly deplete plasma cells, whereas CTLA-4Ig induces a more gradual depletion of plasma cells. APC, antigen presenting cells; FDC, follicular dendritic cells presenting intact antigen complexes to B cells in the germinal center; GC, germinal center; MHC, major histocompatibility complex; Tfh, T-follicular helper; TCR, T-cell receptor.
Preventing and reversing primary DSA responses
The T-cell dependent nature of the anti-donor HLA IgG response is clinically underscored by the fact that calcineurin inhibitor (CNI) withdrawal leads to an unacceptably high incidence of DSA(11, 12) and by the observation that non-adherence and CNI minimization, as is necessary to reduce CNI toxicities, increases the risk of developing DSA especially in patients where the antigenic eplet mismatch is high (13-15). T-dependent B cell responses develop in two phases: an earlier extrafollicular B cell phase that generates short-lived plasmablasts and memory B cells, and a later germinal center (GC) phase that generates short-lived plasmablasts and long-lived plasma cells (PC), as well as memory B cells. While extrafollicular and post-GC memory B cells and PCs exhibit somatic hypermutation (SHM) and class-switching, post-GC B cells tend to have higher levels of somatic mutation and undergo affinity maturation that is driven by limited access to GC Tfh help(16).
Our early studies into the splenic architecture after allogeneic heart transplantation revealed a significant increase in the T cell zones and GCs compared to isografted rats, whereas the B-cell follicles and MZ were not statistically different(17). Those observations are consistent with allografts preferentially eliciting T-dependent GC B cell responses with minimal extrafollicular and MZ responses, whereas significant increases in the B-cell follicles and MZ were observed after xenografting with hamster hearts. These observations prompted us to investigate if established GC responses could be dissolved and developing antibody responses halted with co-stimulation blockade that interferes with T:B cell activation. Indeed, anti-CD154 or CTLA-4Ig starting on day 7 after allogeneic donor splenocyte immunization rapidly dissolved established GCs and halted further development of the alloantibody response(18). Furthermore, delaying the initiation of CTLA-4Ig treatment until day 6 after a fully mismatched heart transplantation inhibited alloantibody production and prevented acute rejection, while the adoptive transfer of immune sera reversed the effects of delayed CTLA-4Ig. These observations underscored the efficacy of co-stimulation blockade at inhibiting ongoing B cell responses even when graft-specific T cell and GC B cell responses had been established(19, 20). Furthermore, delaying CTLA-4Ig treatment to day 7 post-sensitization was able to prevent the generation of memory B cells(20) (Figure 1).
In contrast, when CTLA-4Ig was administered starting on day 14 post-immunization, it no longer was able to reduce donor-specific IgG responses. The lack of efficacy was not due to the inability of CTLA-4Ig to dissolve late GC responses, but because plasmablasts had already been generated and CTLA-4Ig was not able to inhibit their production of donor-specific IgG(19). These observations suggested that inhibiting the production of donor-specific IgG by newly generated plasmablasts is necessary. One way to accomplish this is by adding a proteasome inhibiter such as bortezomib, to CTLA-4Ig therapy. Indeed, transient bortezomib in combination with sustained CTLA-4Ig, starting at day 14 after donor splenocyte immunization or allogeneic skin-transplantation was able to prevent further increase in DSA(21). Most impressively, this treatment combination starting as late as day 35 post-skin transplant was still able to reduce circulating DSA levels. This raises the intriguing question of whether the combination of a proteasome inhibitor and co-stimulation blockade could be translated to the clinical setting to halt and/or reverse established DSA responses.
Preventing and reversing recall DSA responses
Recent studies by Mesin et al.(22) using prime-boost models in mice showed that, contrary to expectation, secondary B cell responses were characterized by a bottleneck that restricted the engagement of the large diversity of memory B cell clones generated in the primary response. In fact, only a few higher-affinity memory B cell clones accounted for the majority of secondary antibody responses, whereas GC responses observed with secondary antigen encounter were predominantly populated by B cells without prior GC experience and are most likely naïve. One possible explanation for this observation was suggested by the early studies of Pape et al.(23), where the presence of high-affinity neutralizing serum immunoglobulin prevented low affinity memory B cells from participating in the recall response.
We investigated the fate of memory B cells upon re-encounter with the same alloantigen as in the primary sensitizing event, and showed that recall class-switched DSA responses were associated with memory B cells responding to donor-MHC Class I or Class II antigens by rapidly differentiating into plasmablasts, with minimal involvement of GC responses(20, 24). The appearance of donor-specific plasmablasts occurred more quickly in sensitized compared to naïve mice, correlating with more rapid increases in DSA, and was inhibited by CTLA-4Ig administration started at the time of transplant. Thus the recall B cell response upon re-encounter of the same alloantigens was extrafollicular and T cell-dependent. When CTLA-4Ig treatment was delayed for 14 days until the recall DSA was at its peak, and combined with transient administration of bortezomib, a significant and rapid reversal of the recall DSA response was also observed(21), suggesting a therapeutic treatment for sensitized patients undergoing AMR early post-transplantation. Similarly, Burghuber et al.(25) reported that the combination of co-stimulation blockade and transient bortezomib was able to significantly reduce AMR in sensitized non-human primates, through the targeting of both PC and upstream GC responses.
Investigations into the B cells participating in recall antibody responses in humans have only recently become possible through the use of ultrasound-guided fine needle aspiration to serially sample the draining lymph nodes and investigate the dynamics and specificity of GC B cell and PC responses after influenza vaccination(26). In response to seasonal influenza vaccination, GC responses were detected in 3 of 8 individuals, and between 12% and 88% of the responding GC B cell clones overlapped with B cells detected among early circulating plasmablasts. The shared B cell clones had high frequencies of somatic hypermutation consistent with memory B cells differentiation into PC and undergoing affinity maturation in the GC for new influenza epitopes. For the remaining vaccine-induced B cell clones detected only in the GC but not as plasmablasts, the GC B cells exhibited significantly lower frequencies of somatic hypermutation and predominantly encoded strain-specific antibodies consistent with naive B cells originating the GC. A reasonable explanation for the high frequency of memory B cells entering the GC is that the response is driven by new epitopes in the seasonal influenza vaccine, and that for identical epitopes, the entry of memory B cells into the GC response might be substantially reduced. An inference from these observations is that reencounter of identical HLA alleles might preferentially induce memory alloreactive B cells to differentiate into plasmablasts, but challenge with non-identical HLA epitopes/eplets might result in memory B cells entering into a GC response. In either case, susceptibility to co-stimulation blockade is predicted (Figure 1).
Based on the potency of CTLA-4Ig in combination with transient bortezomib in reversing established B cells primary or recall responses in our model, we conducted in a series of proof-or-principle investigation to test whether co-stimulation blockade with belatacept, in combination with transient bortezomib, was able to reverse established DSA responses and AMR in 6 kidney transplant recipients (21). Compassionate use of this regimen was initiated for the first patient who developed early, severe acute AMR after his third kidney transplant that was unresponsive to steroids, plasmapheresis, and intravenous immunoglobulin. Remarkably, belatacept in combination with two doses of bortezomib resulted in a rapid reversal of AMR and the reduction of 3 of 4 (including presumed anamnestic) DSA within 30 days. A 30-month follow-up showed sustained control of DSA responses and a well-functioning graft. These early observations prompted the treatment of five additional patients who also resolved their acute AMR episodes and had sustained disappearance of circulating DSA. Notably, case 4 was treated successfully with bortezomib and belatacept on post-transplant day 11, and DSA become undetectable by day 17. However, DSA was again detected on post-transplant day 31, and positive C4d deposition detected 50 days later prompted a second round of bortezomib on post-transplant day 115. This resulted in loss of all DSA within 119 days, and remained controlled at 18 months post-transplant follow-up. Likewise, case 6 presented with DSA and biopsy confirmed AMR at routine follow-up on day 291 post-transplantation. Treatment with bortezomib and belatacept also rapidly reversed 3 of 4 DSAs within 9 days. This small case series confirm that a strategy combining plasmablast and PC depletion with co-stimulation blockade is effective at reversing antibody responses in both an acute and subacute settings, thus raising the possibility that it might be efficacious in desensitization.
Targeting serological memory to HLA
Long-lived serological memory has long been thought to be mediated by long-lived PCs that reside in the bone marrow(27). An important feature of the long-lived PC cells is that they are not intrinsically long-lived, but depend on continually receiving survival signals from the microenvironment. In the bone marrow, this comprises soluble components such as cell-releasing (cytokines, growth factors, and chemokines) and extracellular matrix (ECM) components (fibronectin, collagen, laminin, and heparan sulfate (HS), and cellular components such as mesenchymal stromal/stem cells, dendritic cells (DC), monocyte/macrophages, megakaryocytes, eosinophils, basophils, and regulatory T cells. These cells support PC longevity through their production of survival cytokines, such as CXCL12, APRIL, and/or IL-6, and contact-dependent signals by CD138, CD28, CD44, VLA-4, and CD93. Notably, CD28 facilitates PC survival by stimulating IL-6 production by dendritic cells upon CD80/86 engagement(28, 29), and by promoting PC metabolic fitness through upregulating IRF4 and inducing ROS production(30).
Antibody producing cells (ASCs) are comprised of the short-lived plasmablasts produced by extrafollicular and GC responses, and long-lived PCs. In mice, Wilmore et al.(31) used B220, CD138 and Sca-1 (detecting Ly6A/E) to identify ASCs, while Pracht et al.(32) distinguished plasmablasts from PC based on expression of CD19 and B220, CD138 and TACI, with plasmablasts expressing TACI+CD138+B220intCD19int, and PC further down-regulating the B cell markers, B220 and CD19. PCs are a heterogenous population of cells that can reside in secondary lymphoid organs and sites of inflammation, in addition to the bone marrow(33). Studies by Halliley et al.(27) used the markers CD19, CD38, and CD138 to identify four PC subsets in human bone marrow (BM), with the CD19(−)CD38(hi)CD138(+) subset being the longest-lived population that expressed CD28 and genes enriched in the autophagy pathway, that has been reported to be critical for the survival and function of mouse PCs.
The unique properties of PC provide opportunities for depleting these cells in the transplantation setting. To this point, PC are vulnerable to proteasome inhibition, due to their vigorous production of secreted antibodies and propensity to accumulate misfolded immunoglobulins, resulting in endoplasmic reticulum stress, a misfolded protein response, and cell apoptosis (Figure 1)(34). There are a number of reports confirming the ability of proteasome inhibitors, such as bortezomib and carfilzomib, to reduce circulating DSA(35-38). However, the major caveat of these studies was the incomplete reduction of anti-HLA antibodies and their rebound when treatment was stopped. One hypothesis for the resistance to proteasome inhibition is differential susceptibility of PC subsets, with the long-lived PC being the most resistant. We tested this hypothesis in mice treated with bortezomib, and demonstrated, unexpectedly, that it was the more mature PC in the BM that were most sensitive to bortezomib compared to the proliferating plasmablasts(21). We reasoned that this susceptibility could be explained by the PC being more efficient producers of antibody compared to plasmablasts. Furthermore, investigations into resistance to carfilzomib by human CD138+ PC using a transcriptomic profiling revealed an acquired genomic signature, including increased expression of the immunoproteasome that mediated resistance to the proteasome inhibitors carfilzomib, bortezomib, and ixazomib(39).
Experimental and clinical data indicate that proteasome inhibition alone cannot sustainably reduce circulating anti-HLA antibodies; indeed Kwun et al.(40) reported that repeated bortezomib monotherapy of allosensitized non-human primates resulted in humoral compensation that manifested as a rapid but transient induction of circulating IgG+ B cells and increased GC responses in the lymph nodes. We reasoned that CTLA-4Ig or belatacept would work synergistically with proteasome inhibition to not only inhibit memory B cells differentiation into plasmablasts and PC, but also to deplete long-lived CD28+ PC. Indeed, non-human primate and clinical data are consistent with this possibility, where long-term treatment with belatacept results in the gradual reduction of pre-transplant donor-specific as well as third-party HLA alloantibody in highly sensitized kidney transplant recipients(35, 41-43). Furthermore, Jain et al.(21) reported that sustained belatacept treatment after acute AMR resulted in the gradual reduction of anti-donor HLA Class I and Class II IgG. These observations raised the possibility that this approach of transient proteasome inhibition in combination with belatacept might be successful in desensitizing highly-sensitized patients on the transplant waitlist. This possibility was affirmed by the observations in non-human primates by Kwun, Knechtle and colleagues(25, 44-46).
Successful desensitization using this approach has recently been suggested in a small cohort of highly sensitized (cPRA>99%) heart transplant candidates(47). All four patients were female, multiparous, had a history of prior blood transfusions, and three were supported on a left ventricular assist device (LVAD) all with complications related to the device. Desensitization with multiple cycles of a proteasome inhibitor (with or without plasmapheresis) under continuous co-stimulation blockade with belatacept sequentially reduced the mean fluorescence intensity of both class I and II HLA antibodies, and allowed for transplant with a negative CDC crossmatch across multiple historically C1q binding DSA.
In this case series, participants received consecutive cycles of bortezomib and/or carfilzomib along with belatacept that was continued after each cycle was completed. Bortezomib, a boronic acid dipeptide, is a reversible first-generation proteasome inhibitor associated with off-target effects including peripheral neuropathy(48, 49). Carfilzomib has an epoxyketone as its active moiety, binds irreversibly offering a potential efficacy advantage, and has less neurotoxic effects but has been associated with cardiotoxicity in the myeloma literature raising concern for its use in desensitization(50). The rapid rebound observed with carfilzomib-based desensitization without costimulation blockade(38) reinforces the need for synergistic therapies that prevent ASC differentiation. A desensitization study in kidney transplantation combining carfilzomib with belatacept (NCT05017545, Table 1) will help further address both safety and efficacy of the approach. Recently, the results of a phase 2 study using the second-generation oral proteasome inhibitor, ixazomib, in highly sensitized kidney transplant recipients suggested a reduction in class I and II HLA antibodies in some participants although the effect was not consistent across all specificities (51). Its use in combination with belatacept has yet to be determined but this strategy, if successful, would simplify the regimen and potentially reduce some of the cost associated with infusions or injections required for carfilzomib and bortezomib, respectively. Finally, proteasomal adaptations may limit efficacy over time and thus, alternative options are warranted(39).
Table 1.
Clinical trials of HLA antibody reduction with belatacept and plasma cell targeting therapies
| Carfilzomib and Belatacept for Desensitization (ADAPT) | Desensitization | NCT05017545 | Recruiting | Phase 1/2 open-label non-randomized clinical trial to address the safety and efficacy of belatacept and carfilzomib in highly HLA sensitized kidney transplant candidates. |
| Lung Transplant Plasmapheresis/Belatacept/Carfilzomib for Antibody Mediated Rejection and Desensitization | Desensitization and AMR | NCT03805178 | Withdrawn | Phase 2 open-label non-randomized clinical trial to address safety and tolerability in highly sensitized lung transplant candidates and lung transplant recipients with antibody mediated rejection (AMR). |
| Novel Desensitization Kidney Transplantation | Desensitization | NCT05345717 | Recruiting | Open-label pilot study to test the safety and effectiveness of desensitization therapy with belatacept and proteasome inhibitor to increase the likelihood of finding an acceptable donor for highly HLA sensitized kidney transplant candidates. |
| Daratumumab and Belatacept for Desensitization (ATTAIN) | Desensitization | NCT04827979 | Recruiting | Phase 1/2 open-label non-randomized, clinical trial to assess the safety and efficacy of daratumumab and belatacept in highly HLA sensitized kidney transplant candidates. |
| The Safety/Efficacy Of Daratumumab With Belatacept In Highly HLA-Sensitized Patients Awaiting Kidney Transplantation (COMBAT) | Desensitization | NCT05145296 | Recruiting | Non-randomized, single-center pilot trial assessing the safety and efficacy of targeting peripheral and central humoral alloimmune memory with daratumumab and belatacept in highly HLA sensitized patients awaiting kidney transplantation. |
Daratumumab, a human IgG1 kappa monoclonal anti-CD38 antibody has successfully been used to target malignant plasma cells in treatment-refractory multiple myeloma and AL amyloidosis through a variety of Fc-dependent effector mechanisms, as well as directly inducing apoptosis(52-54). Its distinct mechanism of action from proteasome inhibitors may afford less off-target systemic effects (neurological, cardiac, and gastrointestinal). Daratumumab has also shown efficacy in cases of refractory lupus where both clinical improvement and a reduction in autoantibodies was observed(55), and in a case of pure red-cell aplasia after allogeneic stem-cell transplant(56). In the non-human primate sensitization model, daratumumab with plerixafor (anti-CXCR4) reduced circulating DSA and lymph node plasmablasts but not T follicular helper cells or proliferating germinal center B cells. DSA rebound was observed after transplant (57) emphasizing the need for synergistic strategies as are being studied in two clinical desensitization trials using daratumumab with belatacept in highly sensitized kidney transplant candidates (NCT04827979, NCT05145296; Table 1).
Daratumumab also reduces the frequency of CD38 expressing NK cells, suggesting a potential adjunctive therapeutic effect. However, the remaining NK cells may continue to be functional and promote immune cell priming, and the implications for allogeneic transplantation are unclear(58, 59). CD38 is additionally expressed on suppressor lineages including regulatory B cells, myeloid cells, and a subset of Tregs with enhanced suppressive function, further suggesting that off-target effects could be of concern (60). In heavily pre-treated multiple myeloma patients enrolled in two clinical trials, the CD8+ T cell: Treg ratio, CD8+ T cell clonality, and response to viral antigens increased during treatment with daratumumab suggesting immune activation(60). Indeed, in the aforementioned non-human primate model acute T-cell mediated rejection was observed(57). Consistent with this observation, cellular rejection or tubulointerstitial inflammation have also been described in select cases where daratumumab was used to treat AMR (61, 62). The implications for desensitization will be defined in ongoing clinical trials (Table 1). Other plasma cell therapies with the potential to be used in synergistic desensitization strategies have been discussed elsewhere(63).
Although this review focuses on the rational and evidence for combining plasma cell depletion with costimulation blockade to block the development of new antibody secreting cells, the implications for infection and protective immunity are also noteworthy. In the non-human primate models, CMV infection was a limiting factor while pre-desensitization vaccine-induced protective immunity remained intact(25, 46). In our case series, there were two mild infections (C. difficile and E.coli urinary tract infection), both of which responded to treatment after which desensitization was resumed without recurrence(47). Consistent with our proposed mechanism and supported by the poor response to primary vaccine series under costimulation blockade(64), in our program, we recommend comprehensive vaccination prior to desensitization while acknowledging that some reduction in protective immunity may occur with multiple cycles of desensitization.
Implications of the approach for thoracic organ transplantation
The importance of sustaining the response to treatment in the pre-transplant setting is critical for thoracic organ transplant candidates as desensitization is optimally undertaken when the patient is clinically stable and the timing of transplant cannot be ascertained a priori. Thus, the brisk rebound following proteasome inhibitor-based desensitization(38) presents a major challenge to this population. The sustained response to CTLA4-Ig in the mouse model, inhibition of proteasome inhibitor mediated homeostatic proliferation in the non-human primate studies(40), combined with the clinical observation that belatacept can constrain pre-existing DSA (65) and reduce non-DSA in the clinical setting(43), along with our preliminary experience(47) suggests that continuing belatacept in the pre-transplant setting may help overcome this limitation.
End-stage heart and lung disease is unique in its urgent need for effective desensitization strategies to permit life-saving organ transplantation where, unlike patients with chronic kidney disease, living donor or paired exchange is not an option. While left ventricular support devices (LVADs) may be an alternative for some heart transplant candidates, not all patients qualify and complications limit duration of support, thus transplantation remains the gold-standard. Moreover, LVADs further drive sensitization serving as a unique risk factor to this population(66). Immunologically, LVAD-related sensitization is often characterized by a robust HLA antibody response early after implant that decays over time(67). Nonetheless, memory persists as suggested by the increased risk of rejection (both cellular and antibody mediated) after transplant. This raises the possibility that targeting the plasmablast/early PC subsets could be particularly effective and that long-term inhibition of memory T cell – B cell interactions is required. Consistent with the mechanistic principles discussed above, belatacept may be particularly efficacious in this setting reducing plasmablasts(68) as well as memory and isotype switched B cell responses(44, 46, 68). Multiple cycles of plasma cell targeted therapies are likely to be needed to effectively control long-lived plasma cells, particularly when there are additional risk factors, such as remote pregnancies, as was the situation in all our cases.
HLA sensitization related to congenital heart disease (CHD) repair with allogeneic homograft material is often considered a formidable barrier to transplant yet CHD is now the 2nd most common indication for heart transplant between 18-39 years of age(69). Chronic exposure to foreign HLA in the absence of immunosuppression results in immunological sensitization implying an important role for targeting long-lived plasma cells and preventing rebound in the setting of established memory and ongoing presence of the homograft. While the dose and dosing strategy of belatacept in our protocol was modeled after the FDA licensed approach in de novo kidney transplant recipients for rejection prophylaxis, whether higher or more frequent dosing would enhance the effect or improve tissue penetration in the setting of established HLA sensitization, remains to be defined.
Conclusion
There is an urgent need for effective, mechanistically-driven strategies to treat established HLA antibody responses both in the pre- and post-transplant setting. Herein we review a bench-to-bedside approach to addressing this unmet need. Building on the pre-clinical efficacy of CTLA4-Ig at inhibiting memory B cell responses and reversing established DSA, we address the clinical limitation of rebound seen with proteasome-inhibitor based strategies by combining costimulation blockade with proteasome inhibition first in mouse models and in the clinical setting to treat AMR and for desensitization in highly sensitization heart transplant candidates. This approach is further supported by work in non-human primate models. Collectively, this review highlights the utility of a rational, collaborative, mechanistically-driven approach to humoral desensitization to improve access to and long-term outcomes in solid organ transplantation.
Key points.
Pre-transplant donor specific antibodies (DSA) mediate hyperacute as well as early antibody-mediated rejection (AMR), while DSA developing late post-transplantation may additionally mediated chronic rejection.
The combination of transient proteasome inhibition to deplete plasma cells, together with maintenance co-stimulation blockade, CTLA-4Ig or belatacept, to prevent the generation of new antibody-secreting cells (ASCs), effectively reverses established DSA responses in mouse models.
Treatment regimen of proteasome inhibition and belatacept reverses active antibody-mediated rejection in the clinic and is an effective desensitizing strategy for highly-sensitized patients on the transplant waitlist.
Disclosure:
This work was supported in part by by grants from the National Institutes of Health to ASC (R01 AI142747 and R01 AI148705), and MVH (R34 AI152962, U01 AI136816). MVH also received grant support from the Mendez National Institute of Transplantation Foundation, Nelson Faculty Development Award and an Investigator Initiated Research Grant from Bristol Myers Squibb.
Discussion of the off-label use of belatacept and proteasome inhibitor.
Footnotes
Conflict of interest
The authors have no additional conflicts of interest.
References
- 1.Billingham RE, Silvers WK, Wilson DB. Further Studies on Adoptive Transfer of Sensitivity to Skin Homografts. The Journal of experimental medicine. 1963;118:397–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–6. [DOI] [PubMed] [Google Scholar]
- 3.Terasaki PI. Humoral theory of transplantation. Am J Transplant. 2003;3(6):665–73. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735–9. [DOI] [PubMed] [Google Scholar]
- 5.Lederer SR, Kluth-Pepper B, Schneeberger H, Albert E, Land W, Feucht HE. Impact of humoral alloreactivity early after transplantation on the long-term survival of renal allografts. Kidney Int. 2001;59(1):334–41. [DOI] [PubMed] [Google Scholar]
- 6.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43(6):1333–8. [DOI] [PubMed] [Google Scholar]
- 7.Lai X, Zheng X, Mathew JM, Gallon L, Leventhal JR, Zhang ZJ. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Frontiers in immunology. 2021;12:661643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith JD, Banner NR, Hamour IM, Ozawa M, Goh A, Robinson D, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(2):312–9. [DOI] [PubMed] [Google Scholar]
- 9.Kobashigawa J, Colvin M, Potena L, Dragun D, Crespo-Leiro MG, Delgado JF, et al. The management of antibodies in heart transplantation: An ISHLT consensus document. J Heart Lung Transplant. 2018;37(5):537–47. [DOI] [PubMed] [Google Scholar]
- 10.Lakkis FG, Chalasani G, Hariharan S. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med. 2018;379(26):2580. [DOI] [PubMed] [Google Scholar]
- 11.Hricik DE, Formica RN, Nickerson P, Rush D, Fairchild RL, Poggio ED, et al. Adverse Outcomes of Tacrolimus Withdrawal in Immune-Quiescent Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26(12):3114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugast E, Soulillou JP, Foucher Y, Papuchon E, Guerif P, Paul C, et al. Failure of Calcineurin Inhibitor (Tacrolimus) Weaning Randomized Trial in Long-Term Stable Kidney Transplant Recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(11):3255–61. [DOI] [PubMed] [Google Scholar]
- 13.Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, et al. Class II Eplet Mismatch Modulates Tacrolimus Trough Levels Required to Prevent Donor-Specific Antibody Development. J Am Soc Nephrol. 2017;28(11):3353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon S, Colovai A, Del Rio M, Hayde N. Tacrolimus variability is associated with de novo donor-specific antibody development in pediatric renal transplant recipients. Pediatr Nephrol. 2020;35(2):261–70. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigo E, Segundo DS, Fernandez-Fresnedo G, Lopez-Hoyos M, Benito A, Ruiz JC, et al. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation. 2016;100(11):2479–85. [DOI] [PubMed] [Google Scholar]
- 16. *. Elsner RA, Shlomchik MJ. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity. 2020;53(6):1136–50. A review on the current understanding of the two major fates of activated B cells in extrafollicular or germinal center responses, that result in the generation of memory B cells, short-lived plasmablasts, and long-lived plasma cells.
- 17.Shen J, Short J, Blinder L, Karademir S, Foster P, Sankary H, et al. Quantitation of the changes in splenic architecture during the rejection of cardiac allografts or xenografts. Transplantation. 1997;64(3):448–53. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(9):2280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young JS, Chen J, Miller ML, Vu V, Tian C, Moon JJ, et al. Delayed Cytotoxic T Lymphocyte-Associated Protein 4-Immunoglobulin Treatment Reverses Ongoing Alloantibody Responses and Rescues Allografts From Acute Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(8):2312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Chen J, Young JS, Wang Q, Yin D, Sciammas R, et al. Tracing Donor-MHC Class II Reactive B cells in Mouse Cardiac Transplantation: Delayed CTLA4-Ig Treatment Prevents Memory Alloreactive B-Cell Generation. Transplantation. 2016;100(8):1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. **. Jain D, Rajab A, Young JS, Yin D, Nadasdy T, Chong AS, et al. Reversing donor-specific antibody responses and antibody-mediated rejection with bortezomib and belatacept in mice and kidney transplant recipients. Am J Transplant. 2020;20(10):2675–85. A proof-of-principle demonstration that mouse models can identify mechanistically rational therapies that translate to the clinic. An approach that prevents the generation of new plasma cells with co-stimulation blockade and depletes pre-existing plasmablasts and plasma cells with a proteasome inhibitor in mice was shown to be safe and efficiacious in reversing antibody-mediated rejection in the clinic.
- 22. *. Mesin L, Schiepers A, Ersching J, Barbulescu A, Cavazzoni CB, Angelini A, et al. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell. 2020;180(1):92–106 e11. Mechanistic studies on the basis of the anamnestic antibody responses in mice revealed that memory B cells of higher-affinity account for the majority of the secondary antibody response, but that the germinal center response consisted predominantly of B cells without prior GC experience.
- 23.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol. 2015;195(9):4069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M, et al. Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19(3):724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. **. Turner JS, Zhou JQ, Han J, Schmitz AJ, Rizk AA, Alsoussi WB, et al. Human germinal centres engage memory and naive B cells after influenza vaccination. Nature. 2020;586(7827):127–32. Investigation into the response to seasonal influenza vaccination in humans, following fine-needle aspiration of lymph nodes, revealed that the antibody-secreting plasmablasts were predominantly of memory B cell origin while germinal center B cell responses detected in only 3 of 8 participants were predominantly of naïve B cell origin with a substantial contribution by memory B cells.
- 27.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity. 2015;43(1):132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, et al. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. The Journal of experimental medicine. 2011;208(7):1435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozanski CH, Utley A, Carlson LM, Farren MR, Murray M, Russell LM, et al. CD28 Promotes Plasma Cell Survival, Sustained Antibody Responses, and BLIMP-1 Upregulation through Its Distal PYAP Proline Motif. Journal of immunology. 2015;194(10):4717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. *.Utley A, Chavel C, Lightman S, Holling GA, Cooper J, Peng P, et al. CD28 Regulates Metabolic Fitness for Long-Lived Plasma Cell Survival. Cell Rep. 2020;31(12):107815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilmore JR, Jones DD, Allman D. Protocol for improved resolution of plasma cell subpopulations by flow cytometry. European journal of immunology. 2017;47(8):1386–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pracht K, Meinzinger J, Daum P, Schulz SR, Reimer D, Hauke M, et al. A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. European journal of immunology. 2017;47(8):1389–92. [DOI] [PubMed] [Google Scholar]
- 33. *. Nguyen DC, Duan M, Ali M, Ley A, Sanz I, Lee FE. Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators. Immunological reviews. 2021;303(1):138–53. A in-depth review on the molecular drivers of long-lived plasma cells from B cell differentiation, migration to survival sites and survival in specialized bone marrow niches.
- 34.Parodis I, Stockfelt M, Sjowall C. B Cell Therapy in Systemic Lupus Erythematosus: From Rationale to Clinical Practice. Front Med (Lausanne). 2020;7:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Everly MJ, Roberts M, Townsend R, Bray RA, Gebel HM. Comparison of de novo IgM and IgG anti-HLA DSAs between belatacept- and calcineurin-treated patients: An analysis of the BENEFIT and BENEFIT-EXT trial cohorts. Am J Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 36.Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(1):101–18. [DOI] [PubMed] [Google Scholar]
- 37.Ejaz NS, Alloway RR, Halleck F, Durr M, Budde K, Woodle ES. Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid Redox Signal. 2014;21(17):2401–18. [DOI] [PubMed] [Google Scholar]
- 38.Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL, et al. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20(2):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodle ES, Tremblay S, Brailey P, Girnita A, Alloway RR, Aronow B, et al. Proteasomal adaptations underlying carfilzomib-resistance in human bone marrow plasma cells. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20(2):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwun J, Burghuber C, Manook M, Iwakoshi N, Gibby A, Hong JJ, et al. Humoral Compensation after Bortezomib Treatment of Allosensitized Recipients. J Am Soc Nephrol. 2017;28(7):1991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons RF, Zahid A, Bumb S, Decker H, Sullivan HC, Eun-Hyung Lee F, et al. The impact of belatacept on third-party HLA alloantibodies in highly sensitized kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20(2):573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwun J, Burghuber C, Manook M, Ezekian B, Park J, Yoon J, et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv. 2017;1(24):2115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. **. Schroder PM, Schmitz R, Fitch ZW, Ezekian B, Yoon J, Choi AY, et al. Preoperative carfilzomib and lulizumab based desensitization prolongs graft survival in a sensitized non-human primate model. Kidney Int. 2021;99(1):161–72. In non-human primate kidney transplantation, a clinically translatable desensitization protocol that targets CD28 with an anti-CD28 domain antagonist antibody, lulizumab, in combination with a proteosome inhibitor, carfizomib, was shown to significantly reduce antibody-mediated rejection.
- 46.Ezekian B, Schroder PM, Mulvihill MS, Barbas A, Collins B, Freischlag K, et al. Pretransplant Desensitization with Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients. J Am Soc Nephrol. 2019;30(12):2399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. **. Alishetti S, Farr M, Jennings D, Serban G, Uriel N, Sayer G, et al. Desensitizing highly sensitized heart transplant candidates with the combination of belatacept and proteasome inhibition. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20(12):3620–30. Report on four highly sensitized heart transplant candidates successfully desensitized with costimulation blockade and proteasome inhibition, thus faciliating heart transplantation in 3 individuals with a negative CDC crossmatch against historically strong, C1q binding antibodies.
- 48.Yamamoto S, Egashira N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int J Mol Sci. 2021;22(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pero ME, Meregalli C, Qu X, Shin GJ, Kumar A, Shorey M, et al. Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy. Proc Natl Acad Sci U S A. 2021;118(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(3):e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. **. Wilson N RS, Ptak L, Aziz F, Parajuli S, Jucaud V, Denham S, Mishra A, Hematti P, Djamali A. Ixazomib for Desensitization (IXADES): Results of a Phase II Clinical Trial. Am J Transplant 2022;22. Pilot exploratory, proof-of-concept, open-label, single-center phase II trial of Ixazomib, a second-generation oral proteasome inhibitor, in highly sensitized kidney transplant candidates.
- 52.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840–8. [DOI] [PubMed] [Google Scholar]
- 53.Usmani SZ, Nahi H, Plesner T, Weiss BM, Bahlis NJ, Belch A, et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020;7(6):e447–e55. [DOI] [PubMed] [Google Scholar]
- 54. *. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N Engl J Med. 2021;385(1):46–58. In a randomized phase 3 clinical study of 388 patients, targeting CD38 with Daratumumab demonstrated efficacy, when combined with bortezomib, chclophosphamide and dexamethasone, to successfully treat newly diagnosed systemic immunoglobulin light-chain amyloidosis.
- 55.Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, et al. Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus. N Engl J Med. 2020;383(12):1149–55. [DOI] [PubMed] [Google Scholar]
- 56.Chapuy CI, Kaufman RM, Alyea EP, Connors JM. Daratumumab for Delayed Red-Cell Engraftment after Allogeneic Transplantation. N Engl J Med. 2018;379(19):1846–50. [DOI] [PubMed] [Google Scholar]
- 57.Kwun J, Matignon M, Manook M, Guendouz S, Audard V, Kheav D, et al. Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use. J Am Soc Nephrol. 2019;30(7):1206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viola D, Dona A, Caserta E, Troadec E, Besi F, McDonald T, et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia. 2021;35(1):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casneuf T, Xu XS, Adams HC 3rd, Axel AE, Chiu C, Khan I, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017;1(23):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38(+) immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128(3):384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jordan SC VR, Toyoda M, Ammerman N, Huang E, Peng A, Sethi S, Najjar R, Lim K, Vo A. . Daratumumab for Treatment of Antibody-Mediated Rejection in a Kidney Transplant Recipient. Am J Transplant. 2019;19. [Google Scholar]
- 62. **.Doberer K, Klager J, Gualdoni GA, Mayer KA, Eskandary F, Farkash EA, et al. CD38 Antibody Daratumumab for the Treatment of Chronic Active Antibody-mediated Kidney Allograft Rejection. Transplantation. 2021;105(2):451–7. [DOI] [PubMed] [Google Scholar]
- 63.Woodle ES, Tremblay S, Rossi A, Rojas CC, Alloway R, Roskin K, et al. Plasma cell targeting to prevent antibody-mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2020;20 Suppl 4:33–41. [DOI] [PubMed] [Google Scholar]
- 64.Chavarot N, Morel A, Leruez-Ville M, Vilain E, Divard G, Burger C, et al. Weak antibody response to three doses of mRNA vaccine in kidney transplant recipients treated with belatacept. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2021;21(12):4043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(7):1774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. *. Habal MV. Current Desensitization Strategies in Heart Transplantation. Front Immunol. 2021;12:702186. Review on the current state of desensitization in heart transplantation, focusing on newer approaches that modulate circulating HLA antibodies and alloimmune memory.
- 67.Ko BS, Drakos S, Kfoury AG, Hurst D, Stoddard GJ, Willis CA, et al. Immunologic effects of continuous-flow left ventricular assist devices before and after heart transplant. J Heart Lung Transplant. 2016;35(8):1024–30. [DOI] [PubMed] [Google Scholar]
- 68.Leibler C, Thiolat A, Henique C, Samson C, Pilon C, Tamagne M, et al. Control of Humoral Response in Renal Transplantation by Belatacept Depends on a Direct Effect on B Cells and Impaired T Follicular Helper-B Cell Crosstalk. J Am Soc Nephrol. 2018;29(3):1049–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D Jr., Kucheryavaya AY, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018;37(10):1155–68. [DOI] [PubMed] [Google Scholar]