Introduction
Chronic pancreatitis (CP) represents a fibro-inflammatory disease of the exocrine pancreas characterized by irreversible damage to the pancreas, and ultimately loss of pancreatic function.1 With an estimated global incidence of 9.62 cases/100,000 person-years, the burden of CP is increasing as reflected by an increasing prevalence and hospital visits secondary to CP in the last decade.1–4 In examining the etiology of CP, the role of cigarette smoking has garnered much interest due to emerging preclinical and epidemiological research.
The prevalence of smoking worldwide has decreased over several decades with estimates of 25% in men and 5.4% in women as of 2015, reflecting decreases of 28.4% in men and 34.4% in women since 1995.5 Despite these global improvements, there remains a high rate of current smoking in the CP population with a prevalence of 51.1% reported in the North American Pancreatitis Study-2 (NAPS-2) population and 53% in a Scandinavian cohort.6, 7
This review will focus on the interplay between CP and cigarette smoking to: (i) provide an overview of how smoking contributes to the pathogenesis of CP, (ii) describe the association of smoking with disease progression, (iii) describe the association of smoking with patient-reported outcomes in CP, and (iv) highlight unique challenges for smoking cessation in CP.
Search Strategy
We identified potential references for this narrative review using a search of PubMed for articles published until January 10th, 2022 with the following Medical Subject Headings (MeSH): (chronic pancreatitis or acute pancreatitis) AND (smoking OR nicotine OR tobacco). We identified additional articles by examining the references of selected articles. The final articles were selected with preference for original articles and those with high relevance to the goals of this review.
Smoking and the Pathogenesis of Chronic Pancreatitis
Of the approximately 4000 chemicals found in cigarettes, precisely which components contribute to the pathogenesis of CP is challenging to tease apart. In fact, there are multiple potential pathways, which may function additively or synergistically (Figure). One key mechanism is ductal dysfunction related to the dysregulation of the cystic fibrosis transmembrane conductance regulator (CFTR), which may occur through the elevation of cytoplasmic calcium which prevents normal sorting and degradation of CFTR.8 CFTR in turn controls transport of chloride and bicarbonate ions, the dysfunction of which leads to decreased fluid secretion from the pancreas into the pancreatic duct, resulting into a low-flow, high protein concentration in the pancreas juice that can lead to premature activation of pancreatic enzymes and lead to acinar cell damage.9 In both mice and humans, smoking has been found to lead to CFTR dysfunction, with one study finding smoking to be independently associated with low bicarbonate secretion into the duodenum (indicating impaired secretory function).10–12
Figure.
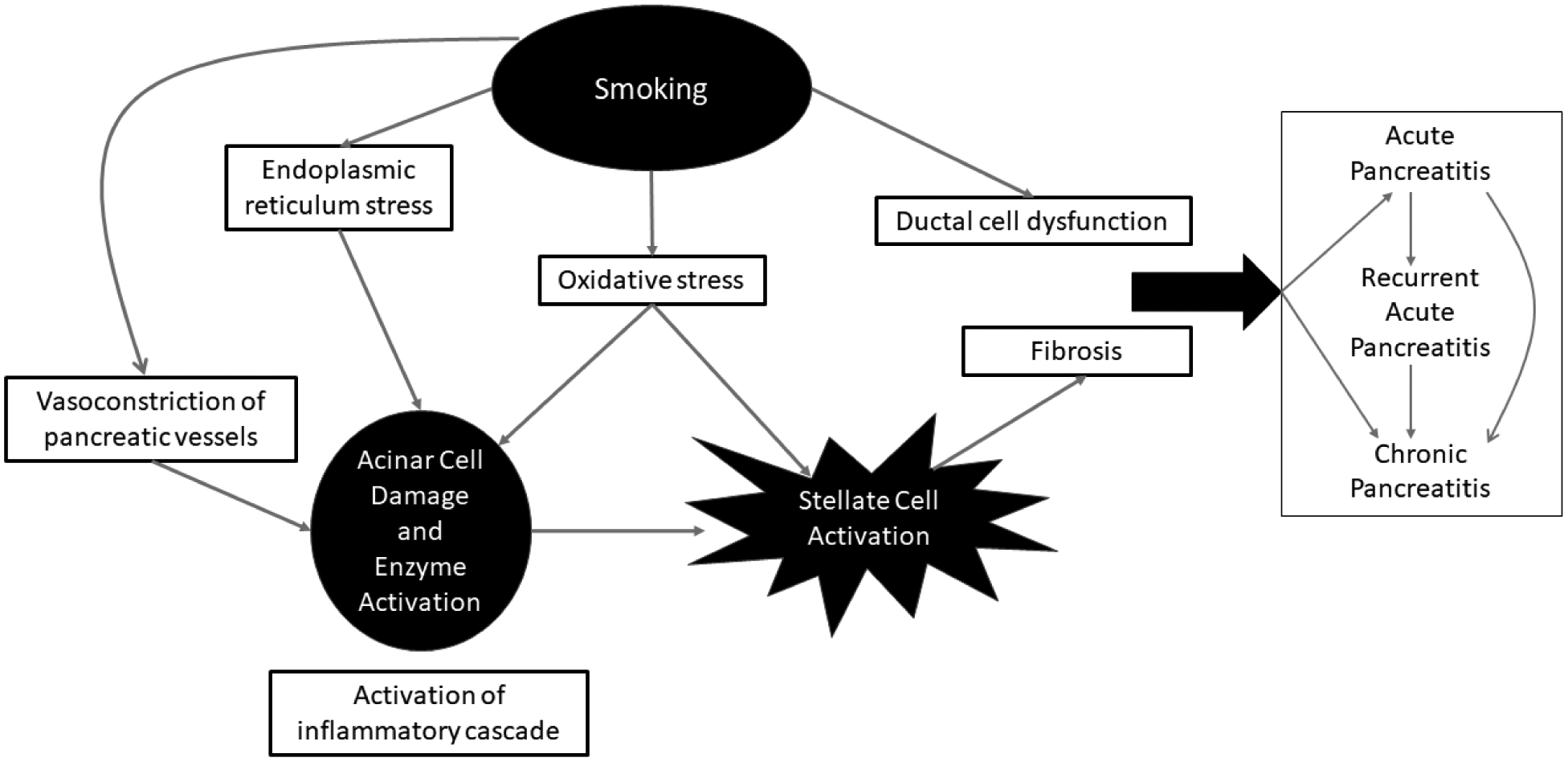
Conceptual framework of cigarette smoking and its contribution to the pathogenesis and disease progression of chronic pancreatitis
Another pathogenic mechanism of smoking involves the activation of the inflammatory cascade and necro-inflammatory response, which ultimately results in fibrosis. Numerous studies have demonstrated how smoking damages pancreatic acinar cells, activating pancreatic stellate cells and thereby inducing fibrosis.13–19 In a murine model, aryl hydrocarbon receptor ligands found in cigarette smoke increased levels of interleukin 22, which then upregulated pancreatic stellate cell extracellular matrix gene expression to promote fibrosis.15 Smoking was also found to affect acinar cells by inhibiting X-box binding protein 1 (XBP1s) formation, thus leading to endoplasmic reticulum stress and eventually cell death.16 In an autopsy study, smokers without CP were found to have significantly higher levels of total and intralobular fibrosis in the pancreas than non-smokers.17 In regards to the decreased blood flow to the pancreas observed in CP, studies have found significantly higher levels of endothelin-1, a hormone related to vasoconstriction, in both the blood and pancreatic tissue in smokers with CP compared to non-smokers with CP.20, 21 Thus, smoking may also compromise the vascular supply of the pancreas, with resultant ischemia damaging the pancreas.
Oxidative stress may represent an additional mechanism of smoking induced pancreatic injury. In one study, smokers with CP had significantly higher levels of serum interleukin-6 (IL-6), a pro-inflammatory cytokine, than non-smokers with CP.19 Furthermore, these patients also had higher levels of copper/zinc superoxide dismutase activity and glutathione peroxidase activity in pancreatic fluid, further implicating smoking as a contributor to local oxidative stress of the pancreas and resultant systemic inflammation.19
Smoking and Progression from Acute to Chronic Pancreatitis
Pancreatic disease progression encompasses progression from acute pancreatitis (AP) to recurrent acute pancreatitis (RAP) to CP, as well as the advancement of CP in regards to morphology, endocrine insufficiency, and exocrine insufficiency. In population-based studies, patients’ smoking status has typically been categorized as current smoker, former smoker, and no history of smoking (i.e., never).22–26 Quantification of smoking exposure remains variable with some studies reporting the number of pack-years and others reporting the number of cigarettes or packs of cigarettes/day.27 While self-reported smoking history may suffer from recall bias, it is generally considered reliable for assessment of long-term exposure, although accurately capturing a dose-dependent response to smoking exposure may prove challenging.28, 29 Nevertheless, it remains unclear as to how prospectively collected smoking history compares to retrospectively ascertained smoking history. Biomarkers, such as cotinine or DNA methylation, represent an objective form of quantifying smoking exposure, but have not been measured in epidemiologic studies examining acute or chronic pancreatitis.30 It is important to consider these limitations while interpreting the following epidemiological studies.
Patients can progress over time from having AP to CP, while many patients will present with CP without a prior documented episode of pancreatitis.22, 31, 32 In examining 669 patients who presented with AP, the Dutch Pancreatitis Study Group reported that current smoking was an independent risk factor for development of CP (OR: 2.9, 95% CI, 1.4–5.9) when excluding recurrent acute pancreatitis (RAP) as a covariate.22
Numerous studies have identified smoking as an independent risk factor for CP (Table). A large study from Denmark found a dose-dependent relationship of smoking history with development of CP independent of alcohol.23 Smokers who had ≥ 60 pack-years had the greatest risk (HR: 4.1, 95% CI, 1.9–9.7) with the risk decreasing with fewer pack-years; presence of recurrent pancreatitis was not recorded in this study. Similarly, the NAPS2 study demonstrated an independent, dose-dependent relationship of smoking and CP in men, women, and ever-drinkers, with CP subjects having a significantly higher amount of smoking and duration of smoking compared to those with RAP.33 A sub-group analysis also found that smoking was independently associated with idiopathic CP, including ever-smokers (OR: 1.65, 95% CI, 1.08–2.52), current smokers (OR: 1.8, 95% CI, 1.10–3.05) and smoking ≥ 1 pack per day (OR: 1.87, 95% CI: 1.10–3.12).25 A meta-analysis including 4 prospective studies with CP populations found a relative risk (RR) of 1.93 (95% CI, 1.60–2.32) for current smokers, RR of 1.30 (95% CI, 1.08–1.57) for former smokers, and RR of 1.59 (95% CI, 1.39–1.82) for ever-smokers.27 Further analysis revealed a RR of 1.10 (95% CI, 0.99–1.21) per 10 pack-years. In summary, there is compelling evidence that cigarette smoking is independently associated with the progression from acute to chronic pancreatitis in a dose-dependent manner.
Table.
Selected studies depicting the negative effects of smoking on disease progression in chronic pancreatitis
| Smoking contributes to progression | |
|---|---|
| Progression from acute pancreatitis to chronic pancreatitis |
Ahmed et al. 2016
Tolstrup et al. 2009 Coté et al, 2011 Rebours et al, 201266 |
| Morphologic progression of chronic pancreatitis |
Maisonneuve et al, 2005
Lee et al, 2016 Hirota et al, 2014 Talamini et al, 2007 |
| Exocrine pancreatic insufficiency |
Olesen et al, 2019
Luaces-Reguiera et al, 2014 Ru et al, 2021 |
| Pancreatic endocrine insufficiency (ie, diabetes) |
Maisonneuve et al, 2005
Hirota et al, 2014 Olesen et al, 2019 |
Smoking and Disease Progression of Chronic Pancreatitis
Several studies have linked accelerated disease progression with smoking in CP. In an international multicenter cohort of 934 patients, smokers showed earlier age of diagnosis as compared to non-smokers (mean difference of 4.7 years) and greater prevalence of calcifications at the time of diagnosis (OR: 2.0, 95% CI, 1.1–3.8).34 Current smoking was also associated with the development of calcifications after initial diagnosis (HR: 4.9, 95% CI, 2.3–10.5) and strikingly, by the age of 60, 80% of smokers had calcifications compared to 40% of non-smokers. Similarly, in a Korean population, smoking was found to be an independent risk factor for progression of calcifications for both patients who smoked < 1 pack per day (OR: 6.1, 95% CI, 1.1–34.6) and ≥ 1 pack per day (OR: 36.6, 95% CI, 3.8–353.3).35 A nationwide survey of Japan found that smoking, and not alcohol, was independently associated with calcifications (OR: 2.0, 95% CI, 1.5–2.8) in patients with CP.36 Finally, a prospective study from Italy compared the development of calcifications by smoking cohorts, finding that calcifications developed in 57.8% of patients who had never smoked, 59.5% of patients who stopped smoking at the time of diagnosis, and 79.1% of patients who continued smoking (p<0.001).37 The odds of developing calcifications followed a dose-dependent relationship with patients smoking > 1 pack per day having the greatest risk (OR: 1.79, 95% CI, 1.1–2.9).
Exocrine pancreatic insufficiency (EPI) is frequently encountered in CP and can develop due to loss of functioning acini, ductal dysfunction, and/or duct obstruction.1 Numerous studies have linked smoking with development of EPI in CP. In a Scandinavian cohort of 1071 subjects with CP, smoking was associated with both exocrine and endocrine insufficiency (OR: 1.42, 95% CI, 1.0–2.0).7 In a study from Spain which used a 13C-mixed triglyceride breath test to assess exocrine function, smoking was the only independent risk factor for development of EPI (OR: 2.5, 95% CI, 1.2–5.2).38 Lastly, a large study from China focusing on CP patients with known genetic factors such as SPINK1, PRSS1, CTRC, and CFTR mutations also identified smoking as an independent risk factor for development of steatorrhea (HR: 1.56, 95% CI, 1.1–2.3).39
The development of diabetes represents one of the later complications of CP, typically occurring 10–20 years after diagnosis.1 One multicenter cohort study found that CP patients who smoked had a greater risk (HR: 2.3, 95% CI, 1.2–4.2) of developing diabetes during the course of follow-up.34 The aforementioned survey study from Japan also found smoking to be independently associated with diabetes, independent of a history of heavy alcohol consumption.36 Whether smoking affects the risk of diabetes by means of insulin secretion, insulin signaling, insulin sensitivity or by beta cell loss remains to be investigated.40 In a US cohort, men who smoked ≥ 25 cigarettes/day carried a greater risk of developing diabetes (RR: 1.94, 95% CI, 1.25–3.03), which was also seen in a Chinese cohort, where men who smoked ≥ 20 cigarettes/day had a greater risk (HR: 1.28, 95% CI, 1.04–1.57) of developing diabetes than non-smoking men.41, 42 Further investigation is needed to delineate how smoking contributes to the development of diabetes.
Chronic pancreatitis is a known risk factor for pancreatic cancer with the risk of cancer varying based on etiology.43, 44 At the same time, smoking is also well-established as increasing the risk of pancreatic cancer in a dose-dependent manner with a relative risk of at least 1.5 in most studies.45, 46 It follows that smoking further increases the risk of pancreatic cancer in patients with CP. One study found that patients with CP who smoked had a significantly increased risk (OR: 20.3, 95% CI, 2.7–158) of developing pancreatic cancer during follow-up.47 In a landmark study involving patients with hereditary pancreatitis, smoking doubled the risk (OR: 2.1, 95% CI, 0.7–6.1) of pancreatic cancer and was associated with earlier development of cancer by nearly 20 years although it remains important to note that in a follow-up study of 217 patients with PRSS1 mutations, only five patients developed pancreatic cancer, two of which were smokers.48, 49 In a large multicenter cohort study involving 2,015 patients with CP, however, only increasing age, and not smoking, was associated with an increased risk of pancreatic cancer.44 The primary limitation of these studies, however, remain the relatively low number of patients who do develop pancreatic cancer within these cohorts and larger studies with long-term follow-up are ideally needed to elucidate the potential additive effect of smoking on the risk of pancreatic cancer in CP.
Smoking and Patient-Centered Outcomes in Chronic Pancreatitis
Incorporating patient-centered outcomes in assessing patients with CP remains critical in understanding the holistic impact of the disease yet are often not assessed as part of standard of care. A large Scandinavian/Baltic cohort of individuals with CP found that smoking was associated with the presence of pain.50 Furthermore, smokers reported higher rates of constant pain, as opposed to intermittent pain, which has been shown to have a greater reduction of quality of life.51–53 Part of these associations may reflect that some people smoke to alleviate pain although current smoking has been associated with a negative impact in both physical and mental quality of life.6, 52 Similarly, a cross-sectional study from the U.S. found smoking to be independently associated with worse quality of life in CP patients.54 While pain and quality of life represent important patient-centered outcomes, further qualitative research is needed to further understand the effect of smoking in this patient population.
Smoking Cessation in Chronic Pancreatitis
Given the consequences of smoking in CP, smoking cessation represents a critical target for potential therapy in this population, particularly given the lack of treatment options for reversing the disease course.55 Smoking cessation, however, remains challenging for any population of patients, including those with CP. Smoking cessation strategies generally consist of a combination of behavioral and pharmacotherapies as only 3–6% of unaided smokers will remain abstinent when attempting smoking cessation.56, 57 A single-center prospective study utilizing these strategies in a CP population, found that out of 27 subjects enrolled in a smoking cessation program, none had quit smoking at 18 months, illustrating the immense challenge in these patients.58 Notably, among study participants, there was a high rate of unemployment and disability, which mirror the factors associated with high smoking prevalence in the general population and represent important barriers to smoking cessation within the CP population. Similarly, a large prospective study from Italy found that among 592 smokers, 37 (3.7%) quit smoking within 5 years of diagnosis.37 More encouragingly, a retrospective cohort study demonstrated that in 127 smokers, 56 (44.1%) were compliant with recommendations to stop smoking, with higher rates seen when recommendations were made by gastroenterologists or pancreas specialists.59 Birth cohort analyses of the NAPS2 data have demonstrated that tobacco policies have had an impact in changing smoking practices in male CP patients, but prevalence of smoking among female CP patient have not decreased over time.6
There are no prospective data to determine whether smoking cessation alters the natural history of CP. In a cohort of patients with alcoholic CP who received pancreatic surgery for chronic pain, post-operative smoking cessation was independently associated with narcotic cessation at 6 months post-surgery and was the only independent risk factor for narcotic cessation at 12 months post-surgery.60 Furthermore, smoking cessation was also associated with improved quality of life at 6 and 12 months post-procedure, which point towards the potential short-term benefits of smoking cessation, particularly in patients undergoing pancreatic surgery where other benefits such as decreased perioperative complications should be considered. Further investigation is also warranted to determine how smoking cessation affects the efficacy of other treatments, such as pancreatic endotherapy.
Future Directions
Smoking has deleterious effects on the pancreas along all aspects of the pancreatitis spectrum. Additional research is needed to further improve our understanding of the effects of smoking and importantly, help patients across the pancreatitis spectrum quit smoking. Further studies are needed to elucidate the pathways by which smoking contributes to the pathogenesis of CP and progression of AP to CP, which may help identify potential therapeutic targets. In our clinical experience, smoking cessation is extraordinarily challenging in patients with pancreatitis, and novel strategies are greatly needed. Once smoking cessation is achieved prospective studies will be helpful to understand how this affects the course of the disease. Non-pharmacological treatments such as disease-specific and culturally-tailored educational interventions and mindfulness represent alternative approaches that may improve smoking cessation rates within this patient population.61, 62 In line with this, with the growing use of e-cigarettes, also known as vaping, as a smoking cessation tool, much remains to be seen regarding its efficacy within this population along with its safety profile.63 The effect of second-hand smoke, while challenging to measure, also warrants further investigation given how it may even affect children presenting with pancreatitis.64 Lastly, additional research is needed to understand the effects of marijuana in CP. Marijuana is becoming increasingly available due to varying degrees of legalization in the United States and worldwide, and is commonly considered for non-opioid analgesia. In fact, one small study has demonstrated opioid sparing effects in CP.65 However, additional studies are needed to understand if the potential benefits are isolated to symptom relief or if there is also disruption of the pathophysiological mechanisms of CP.
Conclusions
In summary, a high proportion of patients with CP actively smoke or have a prior history of smoking. Smoking likely contributes to the development of CP and is independently associated with the progression of AP to CP. Furthermore, it acts as a disease accelerant in CP and adversely effects patient-centered outcomes such as pain and quality of life. Smoking cessation, therefore, remains a prime target for the management of this disease, but novel treatments and approaches are needed to reduce the contribution of smoking-related pancreatic injury to the progression of pancreatitis.
Acknowledgements:
Research reported in this publication was supported by the National Cancer Institute (NCI) and National Institute of Diabetes And Digestive and Kidney Diseases (NIDDK) under award numbers related to The Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer U01DK108306 (DY), Department of Defense PR182623 (DY) and U01DK108327 (DC, PH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beyer G, Habtezion A, Werner J, et al. Chronic pancreatitis. Lancet 2020;396:499–512. [DOI] [PubMed] [Google Scholar]
- 2.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45–55. [DOI] [PubMed] [Google Scholar]
- 3.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022;162:621–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machicado JD, Dudekula A, Tang G, et al. Period prevalence of chronic pancreatitis diagnosis from 2001–2013 in the commercially insured population of the United States. Pancreatology 2019;19:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389:1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon CY, Feldman R, Althouse A, et al. Lifetime smoking history and cohort-based smoking prevalence in chronic pancreatitis. Pancreatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen SS, Nøjgaard C, Poulsen JL, et al. Chronic Pancreatitis Is Characterized by Distinct Complication Clusters That Associate With Etiological Risk Factors. Am J Gastroenterol 2019;114:656–664. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen JE, Sheridan JT, Polk W, et al. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J Biol Chem 2014;289:7671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegyi P, Wilschanski M, Muallem S, et al. CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis. Rev Physiol Biochem Pharmacol 2016;170:37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadiyala V, Lee LS, Banks PA, et al. Cigarette smoking impairs pancreatic duct cell bicarbonate secretion. Jop 2013;14: 31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raju SV, Jackson PL, Courville CA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 2013;188:1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wittel UA, Singh AP, Henley BJ, et al. Cigarette smoke-induced differential expression of the genes involved in exocrine function of the rat pancreas. Pancreas 2006;33:364–70. [DOI] [PubMed] [Google Scholar]
- 13.Luaces-Regueira M, Castineira-Alvarino M, Castro-Manzanares M, et al. Pathophysiological Events Associated With Pancreatitis in Response to Tobacco: An In Vitro Comparative Study With Ethanol in Primary Acinar Cell Culture. Pancreas 2018;47:1304–1311. [DOI] [PubMed] [Google Scholar]
- 14.Lee AT, Xu Z, Pothula SP, et al. Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol Clin Exp Res 2015;39:2123–33. [DOI] [PubMed] [Google Scholar]
- 15.Xue J, Zhao Q, Sharma V, et al. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology 2016;151:1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lugea A, Gerloff A, Su HY, et al. The Combination of Alcohol and Cigarette Smoke Induces Endoplasmic Reticulum Stress and Cell Death in Pancreatic Acinar Cells. Gastroenterology 2017;153:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Geenen EJ, Smits MM, Schreuder TC, et al. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol 2011;106:1161–6; quiz 1167. [DOI] [PubMed] [Google Scholar]
- 18.Sliwinska-Mosson M, Jelen M, Milnerowicz H. Somatostatin expression in the pancreatic cells of smoking and non-smoking chronic pancreatitis patients with or without diabetes. Pancreatology 2016;16:225–30. [DOI] [PubMed] [Google Scholar]
- 19.Sliwinska-Mosson M, Milnerowicz H, Jablonowska M, et al. The effect of smoking on expression of IL-6 and antioxidants in pancreatic fluids and tissues in patients with chronic pancreatitis. Pancreatology 2012;12:295–304. [DOI] [PubMed] [Google Scholar]
- 20.Śliwińska-Mossoń M, Milnerowicz S, Nabzdyk S, et al. The effect of smoking on endothelin-1 in patients with chronic pancreatitis. Appl Immunohistochem Mol Morphol 2015;23:288–96. [DOI] [PubMed] [Google Scholar]
- 21.Sliwińska-Mossoń M, Sciskalska M, Karczewska-Górska P, et al. The effect of endothelin-1 on pancreatic diseases in patients who smoke. Adv Clin Exp Med 2013;22:745–52. [PubMed] [Google Scholar]
- 22.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol 2016;14:738–46. [DOI] [PubMed] [Google Scholar]
- 23.Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med 2009;169:603–9. [DOI] [PubMed] [Google Scholar]
- 24.Andriulli A, Botteri E, Almasio PL, et al. Smoking as a cofactor for causation of chronic pancreatitis: a meta-analysis. Pancreas 2010;39:1205–10. [DOI] [PubMed] [Google Scholar]
- 25.Coté GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011;9:266–73; quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Setiawan VW, Pandol SJ, Porcel J, et al. Prospective Study of Alcohol Drinking, Smoking, and Pancreatitis: The Multiethnic Cohort. Pancreas 2016;45:819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aune D, Mahamat-Saleh Y, Norat T, et al. Tobacco smoking and the risk of pancreatitis: A systematic review and meta-analysis of prospective studies. Pancreatology 2019;19:1009–1022. [DOI] [PubMed] [Google Scholar]
- 28.Brigham J, Lessov-Schlaggar CN, Javitz HS, et al. Validity of recall of tobacco use in two prospective cohorts. Am J Epidemiol 2010;172:828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soulakova JN, Hartman AM, Liu B, et al. Reliability of adult self-reported smoking history: data from the tobacco use supplement to the current population survey 2002–2003 cohort. Nicotine Tob Res 2012;14:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattes W, Yang X, Orr MS, et al. Biomarkers of tobacco smoke exposure. Adv Clin Chem 2014;67:1–45. [DOI] [PubMed] [Google Scholar]
- 31.Sankaran SJ, Xiao AY, Wu LM, et al. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015;149:1490–1500.e1. [DOI] [PubMed] [Google Scholar]
- 32.Hori Y, Vege SS, Chari ST, et al. Classic chronic pancreatitis is associated with prior acute pancreatitis in only 50% of patients in a large single-institution study. Pancreatology 2019;19:224–229. [DOI] [PubMed] [Google Scholar]
- 33.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005;54:510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JW, Kim HG, Lee DW, et al. Association between Smoking and the Progression of Computed Tomography Findings in Chronic Pancreatitis. Gut Liver 2016;10:464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology 2014;14:490–6. [DOI] [PubMed] [Google Scholar]
- 37.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas 2007;35:320–6. [DOI] [PubMed] [Google Scholar]
- 38.Luaces-Regueira M, Iglesias-García J, Lindkvist B, et al. Smoking as a risk factor for complications in chronic pancreatitis. Pancreas 2014;43:275–80. [DOI] [PubMed] [Google Scholar]
- 39.Ru N, Xu XN, Cao Y, et al. The Impacts of Genetic and Environmental Factors on the Progression of Chronic Pancreatitis. Clin Gastroenterol Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 40.Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res 2017;184:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rimm EB, Chan J, Stampfer MJ, et al. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. Bmj 1995;310:555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi L, Shu XO, Li H, et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS One 2013;8:e77919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24:349–58. [DOI] [PubMed] [Google Scholar]
- 44.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med 1993;328:1433–7. [DOI] [PubMed] [Google Scholar]
- 45.Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol 2009;170:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosetti C, Lucenteforte E, Silverman DT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol 2012;23:1880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci 1999;44:1303–11. [DOI] [PubMed] [Google Scholar]
- 48.Lowenfels AB, Maisonneuve P, Whitcomb DC, et al. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. Jama 2001;286:169–70. [DOI] [PubMed] [Google Scholar]
- 49.Shelton CA, Umapathy C, Stello K, et al. Hereditary Pancreatitis in the United States: Survival and Rates of Pancreatic Cancer. Am J Gastroenterol 2018;113:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tjora E, Dimcevski G, Haas SL, et al. Patient reported exposure to smoking and alcohol abuse are associated with pain and other complications in patients with chronic pancreatitis. Pancreatology 2020;20:844–851. [DOI] [PubMed] [Google Scholar]
- 51.Olesen SS, Kuhlmann L, Novovic S, et al. Association of multiple patient and disease characteristics with the presence and type of pain in chronic pancreatitis. J Gastroenterol Hepatol 2020;35:326–333. [DOI] [PubMed] [Google Scholar]
- 52.Machicado JD, Amann ST, Anderson MA, et al. Quality of Life in Chronic Pancreatitis is Determined by Constant Pain, Disability/Unemployment, Current Smoking, and Associated Co-Morbidities. Am J Gastroenterol 2017;112:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han S, Patel B, Min M, et al. Quality of life comparison between smokers and non-smokers with chronic pancreatitis. Pancreatology 2018;18:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gardner TB, Adler DG, Forsmark CE, et al. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol 2020;115:322–339. [DOI] [PubMed] [Google Scholar]
- 56.Stead LF, Koilpillai P, Fanshawe TR, et al. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016;3:Cd008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Babb S, Malarcher A, Schauer G, et al. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65:1457–1464. [DOI] [PubMed] [Google Scholar]
- 58.Han S, Kheder J, Bocelli L, et al. Smoking Cessation in a Chronic Pancreatitis Population. Pancreas 2016;45:1303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Srivoleti P, Yang AL, Jin DX, et al. Provider type influences adherence to lifestyle changes in chronic pancreatitis. Pancreatology 2021;21:42–45. [DOI] [PubMed] [Google Scholar]
- 60.Bordacahar B, Couvelard A, Vullierme MP, et al. Predicting the efficacy of surgery for pain relief in patients with alcoholic chronic pancreatitis. Surgery 2018;164:1064–1070. [DOI] [PubMed] [Google Scholar]
- 61.Cox LS, Nollen NL, Mayo MS, et al. Effect of Varenicline Added to Counseling on Smoking Cessation Among African American Daily Smokers: The Kick It at Swope IV Randomized Clinical Trial. Jama 2022;327:2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend 2011;119:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajek P, Phillips-Waller A, Przulj D, et al. A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med 2019;380:629–637. [DOI] [PubMed] [Google Scholar]
- 64.Ballengee CR, Brooks P, Leong T, et al. Effects of Second-Hand Smoke on Pancreatitis in Children. Pancreas 2019;48:706–710. [DOI] [PubMed] [Google Scholar]
- 65.Barlowe TS, Koliani-Pace JL, Smith KD, et al. Effects of Medical Cannabis on Use of Opioids and Hospital Visits by Patients With Painful Chronic Pancreatitis. Clin Gastroenterol Hepatol 2019;17:2608–2609.e1. [DOI] [PubMed] [Google Scholar]
- 66.Rebours V, Vullierme MP, Hentic O, et al. Smoking and the course of recurrent acute and chronic alcoholic pancreatitis: a dose-dependent relationship. Pancreas 2012;41:1219–24. [DOI] [PubMed] [Google Scholar]


