Abstract
Background:
Sodium-glucose co-transporter-2 inhibitors (SGLT2i) are foundational therapy in patients with heart failure with reduced ejection fraction (HFrEF), yet underlying mechanisms of benefit are not well defined. We sought to investigate the relationships between SGLT2i treatment, changes in metabolic pathways, and outcomes using targeted metabolomics.
Methods:
Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE-HF) was a placebo-controlled trial of dapagliflozin in HFrEF. We performed targeted mass spectrometry-based profiling of 63 metabolites (45 acylcarnitines [markers of fatty acid oxidation], 15 amino acids, and 3 conventional metabolites) in plasma samples at randomization and 12 weeks. Using mixed models, we identified principal components analysis (PCA)-defined metabolite clusters that changed differentially with treatment, and also examined the relationship between change in metabolite clusters with change in Kansas City Cardiomyopathy Questionnaire (KCCQ) Scores and N-terminal pro-B-type natriuretic peptide (NT-proBNP). Models were adjusted for relevant clinical covariates, and nominal p<0.05 with FDR-adjusted p-value<0.10 were used to determine statistical significance.
Results:
Among the 234 DEFINE-HF participants with targeted metabolomic data, the mean age was 62.0±11.1 years, 25% were women, 38% were Black, and mean ejection fraction was 27±8%. Dapagliflozin increased ketone-related and short/medium-chain acylcarnitine PCA metabolite clusters compared with placebo (nominal p=0.01, FDR-adjusted p-value=0.08 for both clusters). However, ketosis (ß-hydroxybutyrate levels > 500 μM), was infrequently achieved (3 [2.5%] in dapagliflozin arm vs. 1 [0.9%] in placebo arm), and supraphysiologic levels were not observed. Conversely, increases in long-chain acylcarnitine, long-chain dicarboxylacylcarnitine, and aromatic amino acid metabolite clusters were associated with decreases in KCCQ scores (i.e. worse quality of life) and increases in NT-proBNP levels, without interaction by treatment group.
Conclusions:
In this study of targeted metabolomics in a placebo-controlled trial of SGLT2i in HFrEF, we observed effects of dapagliflozin on key metabolic pathways, supporting a role for altered ketone and fatty acid biology with SGLT2i in patients with HFrEF. Reassuringly, only physiologic levels of ketosis were observed. Additionally, we identified several metabolic biomarkers associated with adverse HFrEF outcomes.
Keywords: SGLT2, heart failure, ketone bodies, metabolomics, acylcarnitines
INTRODUCTION
Robust evidence has established sodium-glucose co-transporter-2 inhibition (SGLT2i) as foundational therapy in heart failure with reduced ejection fraction (HFrEF).1-3 Despite extensive studies to date, the underlying mechanisms of benefit remain incompletely understood.4-6 Reductions in plasma volume, interstitial fluid, inflammation, body weight, endothelial dysfunction, glycemia, blood pressure, oxidative stress, fibrosis, anemia, intraglomerular hypertension, and sympathetic nervous system activity (among other mechanisms) have all been posited to contribute to the beneficial treatment effects.6 However, with metabolic inflexibility and increasing reliance on alternative fuels observed in HF,7 several studies have also highlighted the relevance of alterations in systemic and myocardial metabolism induced by SGLT2i. In preclinical and clinical models of diabetes (with limited pre-clinical data in HF), SGLT2 inhibition has been associated with a shift away from glucose metabolism toward fatty acid and ketone body fuel utilization,8-12 though these changes in fuel selection have not been uniformly observed.13, 14 The limited understanding of SGLT2i-mediated metabolic effects relates, in part, to the lack of studies in clinical HFrEF with relevant health-related outcomes that also account for placebo effects.
Metabolomic profiling can provide insight into the relationship between SGLT2i treatment, changes in metabolic pathways, disease pathobiology, and outcomes in HFrEF.15 Specifically, understanding the metabolic signature of SGLT2i allows inference into metabolic mechanisms linking treatment to cardiovascular benefit. While a previous metabolomics approach among patients with diabetes in a single-arm SGLT2i study yielded some important insights,16 such a study in clinical HFrEF with a placebo control and relevant endpoints has not been performed. In the Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction (DEFINE-HF) trial, dapagliflozin improved the proportion of patients experiencing clinically meaningful improvements in HF-related health status and N-terminal pro-b-type natriuretic peptide (NT-proBNP).3 We performed a targeted metabolomics evaluation in DEFINE-HF to understand 1) metabolite changes associated with dapagliflozin treatment versus placebo; and 2) whether baseline metabolite levels and change in metabolites are associated with change in HF-related health status and NT-proBNP.
METHODS
The data that support the findings of this study may be made available upon reasonable request with the proper ethical oversight and approval from the DEFINE-HF steering committee and the study sponsor.
Study design
Briefly, DEFINE-HF was an investigator-initiated, randomized, double-blind, placebo-controlled, multi-center trial that enrolled patients with HFrEF, defined as an established diagnosis of HF for at least 16 weeks, left ventricular ejection fraction ≤40%, New York Heart Association class II-III symptoms, elevated natriuretic peptides (NT-proBNP ≥400 pg/mL, or BNP ≥100 pg/ml for patients in sinus rhythm, with higher thresholds for those in atrial fibrillation), and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73m2.3 Patients were randomized to treatment with dapagliflozin 10 mg daily or matching placebo in addition to guideline-directed standard of care therapy for 12 weeks. The trial was conducted across 26 sites in the United States. The primary objectives of the trial were to evaluate the effects of dapagliflozin on HF disease-specific biomarkers (NT-proBNP) and health status (symptoms, function, and quality of life), as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ). Institutional review boards approved the DEFINE-HF study for all sites, and all patients provided informed consent. The metabolomics substudy was approved by the Institutional Review Boards at Saint Luke’s Mid America Heart Institute and Duke University. For the present analyses, we included all participants with available baseline and follow-up blood samples, preferentially selecting the 12-week visit samples. Of the original cohort of 263 participants, 234 (89%) met the inclusion criteria: 222 participants provided 12-week visit samples, while 12 participants provided 6-week visit samples.
Metabolomic profiling
Quantitative metabolomic profiling of 63 total metabolites (15 amino acids, 45 acylcarnitines, and three conventional metabolites) was performed using methods previously described (Table S1).17-20 Frozen plasma samples were thawed and protein precipitated with methanol. Following precipitation, the supernatants were dried and esterified with hot acidic methanol (acylcarnitines) or n-butanol (amino acids). Metabolite assays were performed using tandem flow injection mass spectrometry (MS) with a Xevo TQD instrument (Waters Corporation, Milford, MA). Metabolite quantification was achieved by the addition of stable isotope labeled internal standards. Three conventional metabolites (nonesterified fatty acids, total ketone bodies, and ß-hydroxybutyrate) were quantified on a Beckman-Coulter DxC600 clinical chemistry analyzer using previously described methods.20 The reagents used were obtained from Wako (Richmond, VA). Coefficients of variation for assays have been previously reported.20
We replaced metabolites reported as undetectable with half of the minimum observed for a given metabolite. No metabolite was reported as undetectable in >25% of samples (a criterion we have previously used for inclusion),19 and therefore all 63 metabolites were include in subsequent analyses. Forty-eight (48) metabolites were above the limits of detection in all samples; percentages of samples below the lower limits for detection for the remaining 15 metabolites is shown in Table S2. To assess for quality control, distributions of each metabolite were first examined for concordance with expected distributions based on previous assays, potential drift over time during assay, and unusual outliers (with no concerns identified).
Statistical analysis
Baseline characteristics of the study cohort, stratified by treatment arm, were described using means±standard deviation, medians and interquartile ranges, or percentages as appropriate. We employed Welch’s two sample t-test or the Wilcoxon rank sum test for continuous variables, and chi-squared tests or Fisher’s exact test for categorical variables.
For the targeted metabolomics analysis, we first performed dimensionality reduction on all available metabolomics data from the baseline visit using principal components analysis (PCA) with varimax rotation.20 Briefly, PCA is an unsupervised technique that algorithmically creates a set of orthogonal factors that explain the variance observed in metabolite levels in the dataset. These factors are weighted linear combinations of levels of correlated metabolites; we retained all factors for analysis that had an eigenvalue > 1 (Kaiser criterion). After performing PCA on the baseline data, we projected the resulting factor weights onto scaled data from both baseline and later timepoints. We considered metabolites with absolute value of factor load > 0.4 to be of primary importance, and these were used to describe each factor (Table S3), as we have done previously.17, 18, 21 Because each factor is composed of different weightings from each metabolite (Table S3), it is possible that several factors could be described by the same biological pathway.21, 22
To identify factor levels that changed differentially between dapagliflozin and placebo, we fit a linear regression model of change in factor between baseline and subsequent timepoint (either 6 or 12 weeks) regressed on 1) randomized treatment group and baseline factor level, and 2) additionally adjusted for age, race/ethnicity, sex, eGFR, and type 2 diabetes mellitus. Among the factors associated with treatment in adjusted analyses, we further assessed for longitudinal differences (baseline and 12-week levels) in metabolite levels loaded on the factor by performing paired t-test for analyses stratified by treatment received and a two-sample t-test when assessing differences between treatment arms. To assess predictors of ketone body levels, we performed multivariate linear regression of change in beta-hydroxybutyrate (outcome) on candidate predictor variables from Table 1 with biologic plausibility, adjusted for baseline beta-hydroxybutyrate levels and treatment arm. To select variables that have the greatest clinical relevance for predicting change in ketone bodies, predictor variables were chosen using Least Absolute Shrinkage and Selection Operator (LASSO) selection techniques. If the candidate covariates had a value of variance inflation factor > 8 (indicating strong correlation between a given predictor variable and other predictor variables), we removed one of the highly correlated predictors.
Next, we assessed the relationship between baseline factor levels and change in endpoints (log-transformed NT-proBNP, KCCQ-OSS, and KCCQ-CSS) using multivariable linear regression adjusted for age, race/ethnicity, sex, eGFR, type 2 diabetes mellitus, and the corresponding baseline values of the respective endpoint. Finally, to understand the relationship between change in factors and change in endpoints, we performed linear regression adjusting for baseline factor, baseline endpoint, and the same covariates above. In these models, we assessed for interactions by treatment*change in factor. A heatmap cluster correlation matrix was also constructed to depict the correlations between changes in individual metabolites and changes in endpoints.
For each of these metabolomic analyses, we tested all retained PCA factors for significance in the described models, considering factors with nominal p<0.05 and false discovery rate (FDR) adjusted p<0.10 to determine statistical significance, as employed previously.16 We then identified the heavily loaded individual metabolites in each significant factor and tested these using analogous models, using a 2-tailed α of 0.05 to determine statistical significance in these descriptive analyses. Analyses were performed using R Studio v4.1.2.
RESULTS
Baseline characteristics
Baseline characteristics of the patients in the original DEFINE-HF study population (N=263), DEFINE-HF biomarker subset study (N=234), and those excluded due to lack of available sample (N=29) are displayed in Tables S4 and S5. Those excluded from the present analyses were younger, had been hospitalized with HF more recently, less likely to be taking hydralazine, and had greater proportion of New York Heart Association class III symptoms (p<0.05 for all comparisons). Among the 234 participants included in the present study (Table 1), 121 were randomized to dapagliflozin and 113 to placebo. The average age was 62±11 years, 25% were women, and 38% were Black. The average EF was significantly reduced (27±8%), while the average heart rate (72±12 beats per minute) and systolic blood pressure (124±20 mmHg) fell within normal limits. The median [25th-75th percentile] NT-proBNP level was elevated (1,132 [616, 2,193] pg/mL). The average eGFR was mildly diminished (68±21 ml/min/m2), and hemoglobin A1c was elevated (7.1±1.8%). Baseline characteristics were balanced between randomized groups. In general, baseline fasting levels of the 63 measured metabolites were similar between study arms (Table S6).
Table 1.
Baseline characteristics of patients in the biomarker subset by treatment groups
| Characteristic | Dapagliflozin N = 121 |
Placebo N = 113 |
p-value |
|---|---|---|---|
| Demographics | |||
| Age, y | 62.7 (10.6) | 61.4 (11.5) | 0.36 |
| Male | 89 (73.6%) | 87 (77.0%) | 0.54 |
| Race | 0.68 | ||
| White | 71 (58.7%) | 60 (53.1%) | |
| African American | 43 (35.5%) | 45 (39.8%) | |
| Other | 7 (5.8%) | 8 (7.1%) | |
| Medical history | |||
| Duration of heart failure, y | 6.7 (5.5) | 7.5 (7.0) | 0.34 |
| Previous hospitalization for heart failure | 94 (77.7%) | 93 (82.3%) | 0.38 |
| Time since last hospitalization for heart failure, y | 1.4 (2.3) | 2.0 (3.3) | 0.12 |
| Missing | 27 | 20 | |
| Ejection fraction, % | 27 (8) | 26 (8) | 0.13 |
| Ischemic heart disease | 65 (53.7%) | 58 (51.3%) | 0.71 |
| Type 2 diabetes mellitus | 78 (64.5%) | 71 (62.8%) | 0.80 |
| Atrial Fibrillation | 53 (43.8%) | 46 (40.7%) | 0.63 |
| ICD | 80 (66.1%) | 63 (55.8%) | 0.10 |
| CRT | 0.057 | ||
| No | 42 (52.5%) | 43 (68.3%) | |
| Yes | 38 (47.5%) | 20 (31.8%) | |
| Missing | 41 | 50 | |
| Baseline HF/CV medications | |||
| ACEI/ARB | 64 (52.9%) | 65 (57.5%) | 0.48 |
| Angiotensin receptor neprilysin inhibitor | 42 (34.7%) | 30 (26.6%) | 0.18 |
| ß-blocker | 120 (99.2%) | 107 (94.7%) | 0.059 |
| Hydralazine | 19 (15.7%) | 26 (23.0%) | 0.16 |
| Long-acting nitrates | 41 (33.9%) | 39 (34.5%) | 0.92 |
| MRA | 69 (57.0%) | 74 (65.5%) | 0.18 |
| Loop diuretics | 105 (86.8%) | 94 (83.2%) | 0.44 |
| Digoxin | 24 (19.8%) | 20 (17.7%) | 0.68 |
| Lipid-lowering agents | 100 (82.6%) | 91 (80.5%) | 0.68 |
| Anticoagulant agent | 51 (42.2%) | 40 (35.4%) | 0.29 |
| Glucose lowering medications among patients with type 2 diabetes mellitus | |||
| Insulin | 40 (51.3%) | 36 (50.7%) | 0.94 |
| GLP-1RA | 3 (3.9%) | 1 (1.4%) | 0.62 |
| DPP4-inhibitor | 11 (14.1%) | 8 (11.3%) | 0.60 |
| Sulfonylurea | 19 (24.4%) | 13 (18.3%) | 0.37 |
| Metformin | 29 (37.2%) | 27 (38.0%) | 0.91 |
| Physical exam | |||
| Body mass index (median Q1, Q3) | 31.6 (27.5, 35.9) | 30.5 (27.6, 36.3) | 0.81 |
| Missing | 3 | 1 | |
| Heart rate3 | 72 (12) | 72 (13) | 0.80 |
| Missing | 4 | 8 | |
| Systolic blood pressure3 | 124 (19) | 125 (21) | 0.50 |
| Missing | 0 | 1 | |
| Baseline laboratory studies | |||
| NT-proBNP, pg/mL (median Q1, Q3) | 1,104 (668, 2,424) | 1,136 (551, 2,026) | 0.42 |
| Missing | 0 | 2 | |
| BNP, pg/mL (median Q1, Q3) | 279 (158, 577) | 242 (142, 553) | 0.44 |
| Missing | 0 | 2 | |
| eGFR, mL/min/1.73m^2 | 67 (21) | 70 (22) | 0.21 |
| Urine albumin/creatinine ratio, mg/g (median Q1, Q3) | 21 (7, 89) | 25 (7, 106) | 0.91 |
| Missing | 12 | 12 | |
| Hemoglobin A1c, % | 7.1 (1.8) | 7.1 (1.7) | >0.99 |
| Missing | 1 | 0 | |
| Hemoglobin, g/dL | 13.5 (1.9) | 13.3 (1.7) | 0.36 |
| Missing | 1 | 3 | |
| Functional measures | |||
| NYHA Class | 0.62 | ||
| Class II | 84 (69.4%) | 75 (66.37%) | |
| Class III | 37 (31.6%) | 38 (34.63%) | |
| KCCQ-OSS | 67 (22) | 67 (22) | 0.90 |
| KCCQ-CSS | 70 (22) | 69 (22) | 0.74 |
| 6-minute walk distance, meters (median Q1, Q3) | 306 (235, 369) | 305 (227, 378) | 0.93 |
| Missing | 1 | 0 | |
Data presented as mean (standard deviation), median (interquartile range), or n (%).
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CRT, cardiac resynchronization therapy; CSS, Clinical Summary Score; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter-defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; MRA, mineralocorticoid antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Effect of dapagliflozin compared with placebo on targeted metabolites
PCA resulted in 13 factors reflecting clustering of metabolites in shared biologic pathways (Table S3). For example, PCA factor 1 was heavily loaded with medium-chain carbon length acylcarnitines reflecting mitochondrial fatty acid β-oxidation. The treatment effect of dapagliflozin compared with placebo on PCA metabolite factors is shown in Table 2. After adjusting for age, race/ethnicity, sex, eGFR, type 2 diabetes mellitus, and baseline PCA factor level, two factors, the ketone-related metabolites and short-chain acylcarnitines (factor 6) and the medium-chain acylcarnitines (factor 7), changed differentially with dapagliflozin versus placebo during follow-up (nominal p-value=0.01, FDR-adjusted p-value=0.08 for both). These treatment effects did not differ by background use of mineralocorticoid antagonists or angiotensin-receptor/neprilysin inhibitors (Table S7). Dapagliflozin did not differentially alter the levels of PCA metabolite factors characterized by long-chain acylcarnitines (LCAC) or branched chain amino acids.
Table 2.
Effect of dapagliflozin compared with placebo on principal components analysis defined metabolite factors
| Factors | Annotations for factors | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| Nominal P value |
FDR corrected P value |
Nominal P value |
FDR corrected P value |
||
| Factor 1 | Medium-chain acylcarnitines | 0.96 | 0.96 | 0.83 | 0.98 |
| Factor 2 | Branched-chain and aromatic amino acids | 0.25 | 0.83 | 0.31 | 0.95 |
| Factor 3 | Long-chain acylcarnitines | 0.05 | 0.22 | 0.07 | 0.31 |
| Factor 4 | Long-chain dicarboxyl acylcarnitines | 0.80 | 0.96 | 0.95 | 0.98 |
| Factor 5 | Miscellaneous amino acids | 0.35 | 0.83 | 0.39 | 0.95 |
| Factor 6 | Ketone-related metabolites and short-chain acylcarnitines | 0.02 | 0.11 | 0.01 | 0.08 |
| Factor 7 | Medium-chain acylcarnitines | 0.01 | 0.09 | 0.01 | 0.08 |
| Factor 8 | Miscellaneous amino acids | 0.71 | 0.96 | 0.92 | 0.98 |
| Factor 9 | Long-chain acylcarnitines | 0.54 | 0.96 | 0.56 | 0.98 |
| Factor 10 | Miscellaneous amino acids | 0.93 | 0.96 | 0.98 | 0.98 |
| Factor 11 | Branched-chain amino acids and short-chain acylcarnitines | 0.86 | 0.96 | 0.96 | 0.98 |
| Factor 12 | Miscellaneous amino acids | 0.38 | 0.83 | 0.44 | 0.95 |
| Factor 13 | Short-chain dicarboxyl acylcarnitines | 0.82 | 0.96 | 0.82 | 0.98 |
Model 1: randomized treatment group and baseline factor level
Model 2: additionally adjusted for age, race/ethnicity, sex, eGFR, and type 2 diabetes mellitus
FDR-corrected p values <0.10 were considered statistically significant.
FDR, false discovery rate.
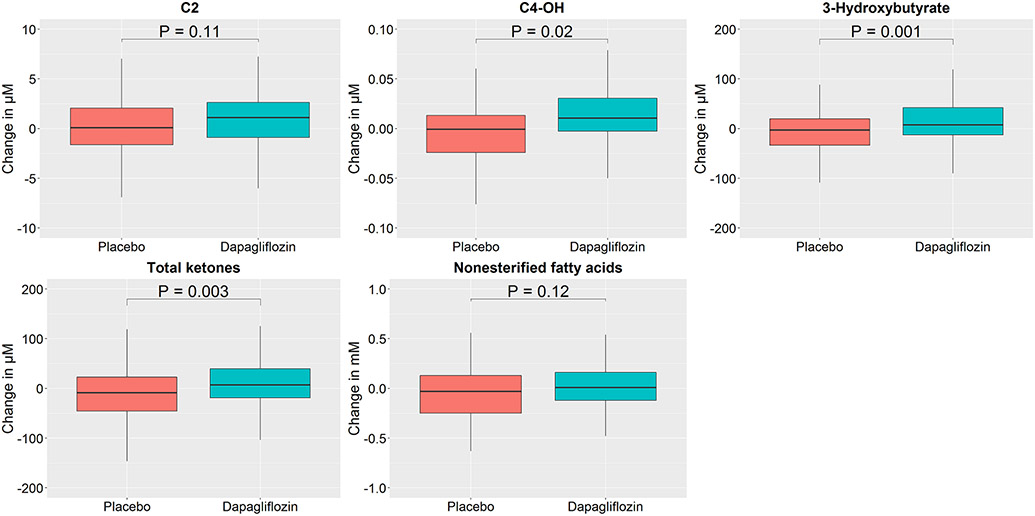
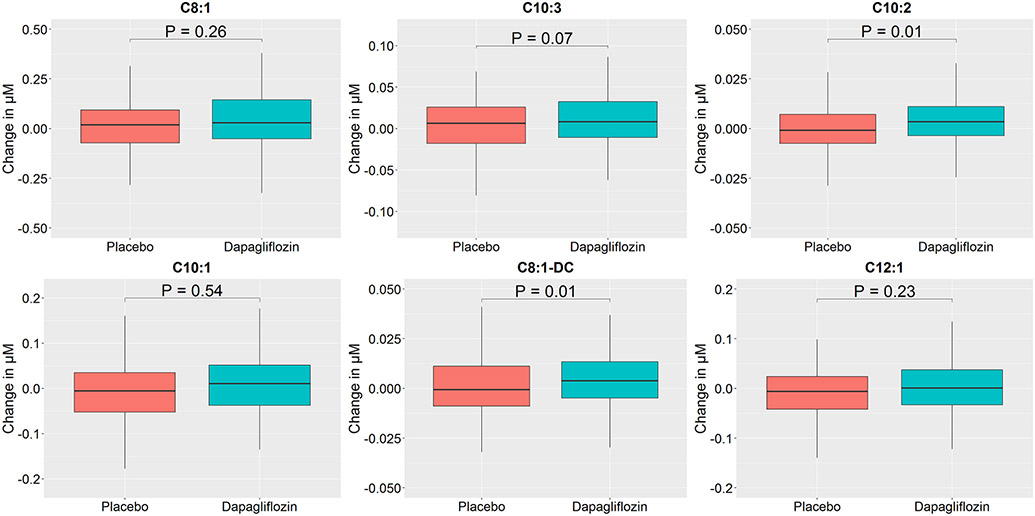
We then focused on these two PCA metabolite factors (6 and 7) and assessed individual metabolites within each factor to identify which changed most with dapagliflozin versus placebo and to assess directionality using absolute concentrations (Figures 1 and 2; Tables S8 and S9). In the dapagliflozin group, several individual metabolites within factors 6 and 7 including C2, ketone related metabolites (C4-OH, ß-hydroxybutyrate, total ketone bodies), and medium chain acylcarnitines (C8:1, C10:3, C10:2, and C8:1-DC) increased over 12 weeks, whereas they were unchanged in the placebo arm (Table S8). When comparing changes in these metabolites at 12 weeks between study arms, there were significant differences in ketone-related metabolites levels (C4-OH, ß-hydroxybutyrate, total ketone bodies) and some medium chain acylcarnitines (C10:2 and C8:1-DC) (Table S9).
Figure 1: Box-and-whisker plots of changes in metabolite levels heavily loaded on factor 6 by treatment group.
Changes in individual metabolites heavily loaded on factor 6 are detailed using boxplots, stratified by treatment group. Outliers not depicted. All metabolite units are in μM, aside from non-esterified fatty acids (mmol/L). P-value shown for difference between groups. C2, Acetyl carnitine; C4-OH, 3-hydroxy-butyryl carnitine.
Figure 2: Box-and-whisker plots of changes in metabolite levels heavily loaded on factor 7 by treatment group.
Changes in individual metabolites heavily loaded on factor 7 are detailed using boxplots, stratified by treatment group. Outliers not depicted. All metabolite units are in μM. P-value shown for difference between groups. C8:1, Octenoyl carnitine; C10-3, Decatrienoyl carnitine; C10-2, Decadienoyl carnitine; C10:1, Decenoyl carnitine; C8:1-DC, Octenedioyl carnitine; C12:1, Lauroyl carnitine.
While ketone body levels increased with SGLT2i, ketosis (ß-hydroxybutyrate levels > 500 μM), was infrequently achieved (3 [2.5%] in dapagliflozin arm vs. 1 [0.9%] in placebo arm), and the maximum level achieved in each group was 1009 μM (dapagliflozin) and 645 μM (placebo). Clinical predictor variables associated with the change in ketone body levels are shown in Table S10. LASSO selection resulted in treatment arm, baseline beta-hydroxybutyrate, and lipid lowering agents as predictors of level of change in beta-hydroxybutyrate levels.
Association of metabolite clusters with natriuretic peptide levels and quality of life
The relationships between baseline metabolite clusters with change in NT-proBNP, KCCQ clinical summary score (CSS), and KCCQ overall summary score (OSS) are displayed in Table S11. No PCA metabolite factors at baseline were associated with change in these endpoints. However, changes in metabolite clusters were associated with changes in several endpoints (Table 3 and Figure S1). Specifically, changes in LCAC/dicarboxylated LCAC (factors 3 and 4) were positively associated with change in NT-proBNP, while changes in proline and histidine (factor 10) were negatively associated with changes in NT-proBNP (Table S12). Further, changes in aromatic amino acids, LCAC, and dicarboxylated LCAC were negatively associated with change in KCCQ scores (factors 2-4) (Tables S13 and S14). There were no significant interactions by treatment group of the association between changes in metabolite clusters with these outcomes (FDR corrected p-value>0.3 for all comparisons).
Table 3.
Association of changes in metabolite clusters with changes in log NT-proBNP, KCCQ-OSS, and KCCQ-CSS
| Changes in factor |
Factor description | log NT-proBNP | KCCQ-OSS | KCCQ-CSS | |||
|---|---|---|---|---|---|---|---|
| Nominal P value |
FDR corrected P value |
Nominal P value |
FDR corrected P value |
Nominal P value |
FDR corrected P value |
||
| Factor 1 | Medium-chain acylcarnitines | 0.18 | 0.38 | 0.11 | 0.25 | 0.10 | 0.21 |
| Factor 2 | Branched-chain and aromatic amino acids | 0.31 | 0.51 | 0.003 | 0.01 | 0.004 | 0.02 |
| Factor 3 | Long-chain acylcarnitines | 0.01 | 0.06 | <0.001 | <0.001 | <0.001 | <0.001 |
| Factor 4 | Long-chain dicarboxyl acylcarnitines | <0.001 | <0.001 | 0.001 | 0.01 | <0.001 | <0.001 |
| Factor 5 | Miscellaneous amino acids | 0.40 | 0.58 | 0.71 | 0.71 | 0.75 | 0.75 |
| Factor 6 | Ketone-related metabolites and short-chain acylcarnitines | 0.08 | 0.25 | 0.14 | 0.26 | 0.33 | 0.48 |
| Factor 7 | Medium-chain acylcarnitines | 0.53 | 0.63 | 0.49 | 0.57 | 0.07 | 0.21 |
| Factor 8 | Miscellaneous amino acids | 0.12 | 0.32 | 0.48 | 0.57 | 0.49 | 0.58 |
| Factor 9 | Long-chain acylcarnitines | 0.53 | 0.63 | 0.08 | 0.25 | 0.10 | 0.21 |
| Factor 10 | Miscellaneous amino acids | 0.002 | 0.01 | 0.57 | 0.62 | 0.24 | 0.40 |
| Factor 11 | Branched-chain amino acids and short-chain acylcarnitines | 0.31 | 0.51 | 0.11 | 0.25 | 0.19 | 0.35 |
| Factor 12 | Miscellaneous amino acids | 0.63 | 0.68 | 0.45 | 0.57 | 0.56 | 0.60 |
| Factor 13 | Short-chain dicarboxyl acylcarnitines | 0.92 | 0.92 | 0.48 | 0.57 | 0.43 | 0.56 |
Model adjusted for baseline factor level, baseline value of the respective outcome, age, race/ethnicity, sex, eGFR, and type 2 diabetes mellitus.
FDR corrected p-values <0.10 were considered statistically significant.
eGFR, estimated glomerular filtration rate; FDR, false discovery rate; KCCQ, Kansas City Cardiomyopathy Questionnaire; CSS, clinical summary score; OSS, overall summary score; NT-proBNP, N-terminal pro B-type natriuretic peptide.
DISCUSSION
The mechanisms underlying the striking clinical benefits of SGLT2i in HFrEF remain incompletely understood. Given that these agents inhibit renal tubular glucose reabsorption and thereby generate caloric loss, substantial interest remains in understanding changes in metabolic pathways that may underpin the treatment effect. Herein, we leveraged targeted metabolomic assays to delineate circulating profiles of numerous metabolites and shed insight into energy homeostasis and metabolism.23
In this study of targeted metabolomics in DEFINE-HF, we observed several findings that identify metabolic pathways altered with SGLT2i. First, dapagliflozin increased short/medium chain acylcarnitines and ketone-related metabolites compared with placebo, supporting a growing appreciation of altered ketone and fatty acid biology with SGLT2i in HFrEF. Specifically, the increases in ketone body levels were very modest, and only a few participants actually achieved ketosis (ß-hydroxybutyrate levels > 500 μM). Further, only physiological levels of ketosis were observed, and the maximum reported ß-hydroxybutyrate level in the dapagliflozin arm (1009 μM) fell well within the range of levels that, for comparison, are observed with the ketogenic diet.24 In addition, increases in long-chain acylcarnitines, long-chain dicarboxylacylcarnitines, and aromatic amino acids were independently associated with worse quality of life and higher NT-proBNP, irrespective of treatment arm. Collectively, these findings support the effects of SGLT2i on mitochondrial function, highlight potential mechanisms of SGLT2i benefit through metabolic reprogramming, and identify biomarkers associated with adverse clinical features in HFrEF.
Dapagliflozin increased ketone-related metabolites and several short/medium chain acylcarnitines compared with placebo. There has been substantial interest in the role of ketosis in potentially mediating SGLT2i benefit in HFrEF, as speculated in the “thrifty substrate hypothesis”.5-7, 9, 12, 25-27 This hypothesis describes how augmentation of ketone body oxidation with SGLT2i may improve oxygen use and mechanical efficiency within mitochondria, and further that these changes cooperate synergistically with SGLT2i-induced hemoconcentration to improve oxygen delivery to tissue beds.9 Ketone body oxidation is increased three-fold in the failing heart, contributing to 16% of adenosine triphosphate production, and this metabolic switch has been proven adaptative.28-30 Indeed, exogenous ketone bodies augment cardiac function across the spectrum of cardiovascular health.31, 32 Yet, only one placebo-controlled study has investigated the effects of SGLT2i on circulating ketone body levels in HFrEF, which did not detect a significant effect when adjusting for changes observed with placebo, which was likely related to that study’s limited power given that the magnitude of effect was similar to what was observed in DEFINE-HF.33 SGLT2i increase ketone body levels through several pathways, including stimulation of lipolysis, increase in ketone body generation by the liver or perhaps extra-hepatic organs (both of which may, at least in part, be triggered by alterations in the circulating glucagon:insulin ratio), and decreases in urinary ketone body clearance.26, 34 While we observed statistically significant increases in circulating ketone body levels (including C4-OH, a marker of ketone, though also fatty acid, oxidation),35 the absolute increases were modest (50 μM for ß-hydroxybutyrate), particularly when compared with the responses observed in previous studies of individuals with diabetes.26 Further, very few patients in the dapagliflozin arm (2.5%) achieved ketosis, which is of clinical relevance, and consistent with the observation that SGLT2i in HFrEF have, thus far, not been associated with euglycemic diabetic ketoacidosis in clinical trials.36 Regardless, ketone body cardiac oxidation is unregulated and proportional to serum concentrations, suggesting that even small increases may augment cardiac oxidation.28, 37 Additionally, the relevance of SGLT2i-induced ketosis to clinical benefit may not be completely captured by circulating levels, as SGLT2i also upregulate enzymatic machinery fundamental to ketone body oxidation.8 Ketone bodies also have pleiotropic effects that include histone deacetylation (attenuating inflammation and oxidative stress), augmenting endothelial function, and upregulating mitochondrial biogenesis,7, 25 and it remains unclear whether these effects are dose-dependent.
Likewise, the increases observed in short/medium chain acylcarnitines is also of interest. Acylcarnitines are byproducts of fatty acid oxidation. SGLT2i reduce glucose utilization and oxidation,8, 26 and some studies demonstrate a shift in fuel selection to fatty acids in HF and diabetes.8, 10, 11, 16 While inference into the specific mechanism responsible for the increase in these acylcarnitines is limited with plasma metabolomics, the observed increase in short- and medium-chain acylcarnitines, without an increase in LCAC, could suggest an overall increase in fatty acid oxidation.15 Since the failing heart is characterized by metabolic inflexibility and decreased fatty acid oxidation,38 our findings may support a role for SGLT2i in metabolic reprogramming. Of note, given the analysis of circulating metabolites, these results could reflect mitochondrial processes in the myocardium but also other muscle beds including skeletal muscle, a key tissue affected by the failing heart and integrally tied to cardiac metabolism and substrate availability.
It is noteworthy that preclinical studies in HFrEF and diabetes have demonstrated that SGLT2i-related shifts in fuel utilization toward fatty acid and ketone oxidation have been associated with improved energetics.8, 12, 13, 39 In addition, SGLT2i improve left ventricular volumes and systolic and diastolic function, which may relate to the observed enhancement in energetics and changes in fuel selection.40, 41 Understanding the relevance of these shifts in fuel selection to myocardial energetics in clinical models is under further exploration.42
The relationships between LCAC, dicarboxylated LCAC, and aromatic amino acids with adverse HF related features adds to a growing literature on this topic. LCACs are highly prognostic in HFrEF, associate with impaired exercise capacity, and even decrease with mechanical circulatory support.23 Accumulation of LCAC may reflect greater degrees of mitochondrial dysfunction in HFrEF, though it may also potentiate disease worsening by predisposing to arrhythmias, promoting cellular stress and mitochondrial inflammation, and interfering with insulin resistance.19, 23, 43, 44 The association of dicarboxylated LCAC may reflect dysregulated fatty acid oxidation in the endoplasmic reticulum and peroxisomes.44 Likewise, the prognostic nature of aromatic amino acids in HF might reflect increased muscle breakdown or liver dysfunction resulting in plasma accumulation.44, 45 Our findings in a clinical trial population extend previous literature to show the interplay of LCAC, dicarboxylated LCAC, and aromatic amino acids on HF-related health status and NT-proBNP, two features closely linked to clinical events in HFrEF. Interestingly, these associations also did not differ by whether participants were randomized to SGLT2i treatment or placebo, suggesting the validity of the predictive value of these metabolites in the modern treatment era.
Overall, dapagliflozin increased two metabolite clusters that were distinct from those clusters associated with HF-related health status and NT-proBNP. Therefore, the link between the beneficial effects of dapagliflozin and these endpoints may not be mediated by change in these pathways as reflected by plasma concentrations of metabolites. However, a few points are critical to consider in assessing these relationships. First, while the metabolite changes induced by SGLT2i do not relate to endpoints studied in DEFINE-HF, this does not rule out the possibility of their relationship to event-based endpoints (i.e. HF hospitalization, cardiovascular mortality) given only modest correlation between biomarkers and events that has been generally observed.46 Second, changes at the plasma level provide a window in systemic physiology but may not reflect tissue level biology. Third, we assayed several, but not all, relevant biological pathways in this analysis.
As such, there are some limitations of the current study. First, as discussed, the plasma metabolome provides snapshots of metabolic physiology and reflects both central and peripheral processes. Concomitant flux studies with use of stable isotope tracers or tissue-based metabolomic assessments could enhance interpretation of our findings. In addition, untargeted platforms detail greater numbers of plasma metabolites, though are limited in interpretability and often are less quantitative.15 Strengths of our study include the relatively large sample size for metabolomic analysis, measurements at baseline and 12 weeks, placebo control, characterization of a significant number of relevant metabolites, and inclusion of several relevant HF endpoints.
In conclusion, leveraging targeted metabolomics in a placebo-controlled SGLT2i trial in HFrEF, dapagliflozin increased ketone-related metabolites and short/medium-chain acylcarnitines compared with placebo. However, ketosis (ß-hydroxybutyrate levels > 500 μM) was rarely achieved in either arm, and supraphysiological levels of ketones were not observed. In addition, increases in LCAC, long-chain dicarboxylacylcarnitines, and aromatic amino acids were independently associated with worse quality of life and higher NT-proBNP. These findings add to the growing body of literature implicating changes in mitochondrial function and metabolic reprogramming as potential pathway of SGLT2i benefit and provide robust support for specific metabolites that can serve as biomarkers of impaired HF-related health status.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Among participants with heart failure with reduced ejection fraction, dapagliflozin increased metabolite clusters enriched with ketone-related metabolites and short/medium-chain acylcarnitines compared with placebo.
Our findings suggest SGLT2i may have effects on key metabolic pathways and add to growing appreciation of altered ketone and fatty acid pathways with SGLT2i.
We identified several metabolite clusters that associate with adverse HFrEF endpoints, including natriuretic peptides and HF-related health status.
What are the clinical implications?
While SGLT2i have been linked to euglycemic ketoacidosis in diabetes, our findings support the notion that increases in ketone bodies are very mild with no patients achieving unsafe ketone body levels in HFrEF.
Strategies to reverse the impaired fatty acid oxidation profile of HF patients may ameliorate disease severity.
ACKNOWLEDGEMENTS
We thank the DEFINE-HF trial participants for their participation.
SOURCES OF FUNDING
DEFINE-HF was an investigator-initiated trial funded by AstraZeneca and conducted by Saint Luke’s Mid America Heart Institute independent of the funding source. The DEFINE-HF metabolomics sub-study was funded by the Duke Health Scholars award (to S.H.S.) and support from NIH grant P30DK124723 (to C.B.N.).
DISCLOSURES
Dr. Selvaraj: currently or recently has received related research support from the Doris Duke Charitable Foundation (Physician Scientist Fellowship Award 2020061), the Measey Foundation (Physician Scientist Fellowship), Institute for Translational Medicine and Therapeutics (Junior Investigator Preliminary/Feasibility Grant Program award and Translational Biomedical Imaging Center award) and the American Society of Nuclear Cardiology (Institute for the Advancement of Nuclear Cardiology award). Dr. Newgard: member of the Eli Lilly Global Diabetes Advisory Board. Dr. Inzucchi: Advisor/Consultant: Astra Zeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, Pfizer, VTV Therapeutics, Abbott, Esperion. Lectures: Astra Zeneca, Boehringer Ingelheim. Dr. McGuire: Research Support for Clinical Trials Leadership from Boehringer Ingelheim, Sanofi, Merck & Co, Pfizer, AstraZeneca, Novo Nordisk, Esperion, Lilly USA, Lexicon, CSL Behring; honoraria for consultancy from Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Metavant, Sanofi, Afimmune, CSL Behring, Bayer; Dr. Scirica: institutional research grants to Brigham and Women’s Hospital from Better Therapeutics, Merck, NovoNordisk, and Pfizer. Consulting fees from Allergan, Boehringer Ingelheim, Better Therapeutics, Elsevier Practice Update Cardiology, Esperion, Hamni, Lexicon, NovoNordisk, and equity in Health [at] Scale and Doximity. Dr. Lanfear: is a consultant for Abbott Laboratories, Amgen, Cytokinetics, Illumina, Janssen, Martin Pharmaceuticals, Otsuka, Vicardia, and DCRI (Novartis) and has participated in research with Amgen, AstraZeneca, Bayer, Critical Diagnostics, Lilly, Janssen and Somalogic. Dr. Javaheri: received grant support from AstraZeneca and serves on the scientific advisory board of Mobius Scientific. Dr. Mentz: Research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Kosiborod: Grant/Research Support; Company Relationship; Astra Zeneca, Boehringer Ingelheim. Honoraria; Company Relationship; Astra Zeneca, Boehringer Ingelheim, Novo Nordisk. Consultant; Company Relationship; Alnylam, Amgen, Applied Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi, Vifor Pharma. Dr. Shah: Sponsored Research Agreement with Astra Zeneca, Lilly Inc. and Verily Inc. The other authors report no disclosures.
ACRONYMS AND ABBREVIATIONS
- DEFINE-HF
Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction
- eGFR
estimated glomerular filtration rate
- HFrEF
heart failure with reduced ejection fraction
- LCAC
long chain acylcarnitines
- KCCQ-CSS
Kansas City Cardiomyopathy Questionnaire Clinical Summary Score
- KCCQ-OSS
Kansas City Cardiomyopathy Questionnaire Overall Summary Score
- PCA
principal components analysis
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
Footnotes
ClinicalTrials.gov Unique Identifier: NCT 02653482.
REFERENCES
- 1.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424 [DOI] [PubMed] [Google Scholar]
- 3.Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The define-hf trial. Circulation. 2019;140:1463–1476 [DOI] [PubMed] [Google Scholar]
- 4.Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: A review. JAMA. 2020;324:488–504 [DOI] [PubMed] [Google Scholar]
- 5.Verma S, McMurray JJV. Sglt2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia. 2018;61:2108–2117 [DOI] [PubMed] [Google Scholar]
- 6.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: Jacc state-of-the-art review. J Am Coll Cardiol. 2020;75:422–434 [DOI] [PubMed] [Google Scholar]
- 7.Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Ishikawa K, Watanabe S, Picatoste B, Flores E, Garcia-Ropero A, Sanz J, Hajjar RJ, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73:1931–1944 [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Mark M, Mayoux E. Cv protection in the empa-reg outcome trial: A "thrifty substrate" hypothesis. Diabetes Care. 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195 [DOI] [PubMed] [Google Scholar]
- 12.Yurista SR, Sillje HHW, Oberdorf-Maass SU, Schouten EM, Pavez Giani MG, Hillebrands JL, van Goor H, van Veldhuisen DJ, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in nondiabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21:862–873 [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E, et al. Empagliflozin increases cardiac energy production in diabetes: Novel translational insights into the heart failure benefits of sglt2 inhibitors. JACC Basic Transl Sci. 2018;3:575–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdurrachim D, Teo XQ, Woo CC, Chan WX, Lalic J, Lam CSP, Lee PTH. Empagliflozin reduces myocardial ketone utilization while preserving glucose utilization in diabetic hypertensive heart disease: A hyperpolarized (13) c magnetic resonance spectroscopy study. Diabetes Obes Metab. 2019;21:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ussher JR, Elmariah S, Gerszten RE, Dyck JR. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J Am Coll Cardiol. 2016;68:2850–2870 [DOI] [PubMed] [Google Scholar]
- 16.Kappel BA, Lehrke M, Schutt K, Artati A, Adamski J, Lebherz C, Marx N. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation. 2017;136:969–972 [DOI] [PubMed] [Google Scholar]
- 17.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214 [DOI] [PubMed] [Google Scholar]
- 18.Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–850 e841 [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Anstrom K, Ilkayeva O, Muehlbauer MJ, Bain JR, McNulty S, Newgard CB, Kraus WE, Hernandez A, Felker GM, et al. Sildenafil treatment in heart failure with preserved ejection fraction: Targeted metabolomic profiling in the relax trial. JAMA Cardiol. 2017;2:896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SH, Hauser ER, Bain JR, Muehlbauer MJ, Haynes C, Stevens RD, Wenner BR, Dowdy ZE, Granger CB, Ginsburg GS, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol. 2009;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: Evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc. 2016;5:e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad T, Kelly JP, McGarrah RW, Hellkamp AS, Fiuzat M, Testani JM, Wang TS, Verma A, Samsky MD, Donahue MP, et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol. 2016;67:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj S, Margulies KB, Dugyala S, Schubert E, Tierney A, Arany Z, Pryma DA, Shah SH, Rame JE, Kelly DP, et al. Comparison of exogenous ketone administration versus dietary carbohydrate restriction on myocardial glucose suppression: A crossover clinical trial. J Nucl Med. 2022;63:770–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yurista SR, Chong CR, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease: Jacc state-of-the-art review. J Am Coll Cardiol. 2021;77:1660–1669 [DOI] [PubMed] [Google Scholar]
- 26.Saucedo-Orozco H, Voorrips SN, Yurista SR, de Boer RA, Westenbrink BD. Sglt2 inhibitors and ketone metabolism in heart failure. J Lipid Atheroscler. 2022;11:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taegtmeyer H Failing heart and starving brain: Ketone bodies to the rescue. Circulation. 2016;134:265–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science. 2020;370:364–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedi KC Jr., Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frokiaer J, Eiskjaer H, Jespersen NR, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvaraj S, Hu R, Vidula MK, Dugyala S, Tierney A, Ky B, Margulies KB, Shah SH, Kelly DP, Bravo PE. Acute echocardiographic effects of exogenous ketone administration in healthy participants. J Am Soc Echocardiogr. 2022;35:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietschner R, Kolwelter J, Bosch A, Striepe K, Jung S, Kannenkeril D, Ott C, Schiffer M, Achenbach S, Schmieder RE. Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. 2021;20:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opie LH, Owen P. Effects of increased mechanical work by isolated perfused rat heart during production or uptake of ketone bodies. Assessment of mitochondrial oxidized to reduced free nicotinamide-adenine dinucleotide ratios and oxaloacetate concentrations. Biochem J. 1975;148:403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carley AN, Maurya SK, Fasano M, Wang Y, Selzman CH, Drakos SG, Lewandowski ED. Short-chain fatty acids outpace ketone oxidation in the failing heart. Circulation. 2021;143:1797–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavka L, Bencak Ferko U, Pitz N, Trpkovski Z, Lainscak M. Sodium-glucose cotransporter 2 inhibitor-induced euglycaemic diabetic ketoacidosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8:2631–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94:2837–2842 [DOI] [PubMed] [Google Scholar]
- 39.Abdurrachim D, Manders E, Nicolay K, Mayoux E, Prompers JJ. Single dose of empagliflozin increases in vivo cardiac energy status in diabetic db/db mice. Cardiovasc Res. 2018;114:1843–1844 [DOI] [PubMed] [Google Scholar]
- 40.Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel-Perez F, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–255 [DOI] [PubMed] [Google Scholar]
- 41.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, Garcia-Ropero A, Ishikawa K, Watanabe S, Picatoste B, Vargas-Delgado AP, Flores-Umanzor EJ, Sanz J, et al. Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: A multimodality study. JACC Cardiovasc Imaging. 2021;14:393–407 [DOI] [PubMed] [Google Scholar]
- 42.Hundertmark MJ, Agbaje OF, Coleman R, George JT, Grempler R, Holman RR, Lamlum H, Lee J, Milton JE, Niessen HG, et al. Design and rationale of the empa-vision trial: Investigating the metabolic effects of empagliflozin in patients with heart failure. ESC Heart Fail. 2021;8:2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz M, Labarthe F, Fortier A, Bouchard B, Thompson Legault J, Bolduc V, Rigal O, Chen J, Ducharme A, Crawford PA, et al. Circulating acylcarnitine profile in human heart failure: A surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am J Physiol Heart Circ Physiol. 2017;313:H768–H781 [DOI] [PubMed] [Google Scholar]
- 45.Cheng ML, Wang CH, Shiao MS, Liu MH, Huang YY, Huang CY, Mao CT, Lin JF, Ho HY, Yang NI. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: Diagnostic and prognostic value of metabolomics. J Am Coll Cardiol. 2015;65:1509–1520 [DOI] [PubMed] [Google Scholar]
- 46.Vaduganathan M, Claggett B, Packer M, McMurray JJV, Rouleau JL, Zile MR, Swedberg K, Solomon SD. Natriuretic peptides as biomarkers of treatment response in clinical trials of heart failure. JACC Heart Fail. 2018;6:564–569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.