Abstract
Objective:
To evaluate the effect of achieving a negative post-induction ANCA assay on the risk of relapse, end stage renal disease (ESRD), and death in ANCA-associated vasculitis (AAV).
Methods:
We emulated a target trial using observational data from the Mass General Brigham AAV cohort comparing patients who achieved vs. did not achieve serologic remission (negative ANCA assay) within 180 days of induction. Outcomes were relapse, ESRD, or death within 5 years, obtained from medical records, the US Renal Data System, and the National Death Index. We placed a “clone” of each patient in both trial arms, censored those deviating from their assigned protocol, and weighted each by the inverse probability of censoring. Outcomes were assessed by pooled logistic regression.
Results:
The study included 506 AAV patients. The mean age was 61 years (SD 18) and the majority were female (58%), White (87%), MPO-ANCA+ (72%) and had renal involvement (68%). Rituximab (59%) or cyclophosphamide (33%) was most often used for induction treatment. Within 5 years, 81 (16%) died, 51 (10%) had ESRD, and 64 (13%) had relapse. Patients treated to a negative ANCA assay within 180 days had hazard ratio (HR) 0.55 (95%CI 0.38 to 0.81) for relapse and HR 0.87 (95%CI 0.61 to 1.25) for the composite of ESRD or death within 5 years.
Conclusions:
In this emulated target trial from a large AAV cohort, achieving serologic remission within 180 days of induction was associated with lower risk of relapse, but no statistically significant difference in ESRD or mortality outcomes.
Keywords: ANCA, titer, vasculitis, outcomes
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a small to medium vessel vasculitis characterized by disease relapses, increased risk of end-stage renal disease, and excess mortality.[1,2] Most AAV patients have circulating ANCA that target proteinase 3 (PR3) or myeloperoxidase (MPO) and are considered pathogenic.[3] ANCA testing has been a central component of AAV diagnosis since the 1980s,[4,5] but the measurement of ANCA titers after treatment has been a controversial practice.
Using contemporary induction strategies, the majority of AAV patients achieve clinical remission.[6] However, only a proportion achieve concurrent serologic remission with negative serum ANCA assay.[7–10] Research on the clinical utility of post-treatment ANCA measurements has generated conflicting findings, perhaps due to heterogeneous methods that have investigated variable patient groups. Some studies focused on patients with “persistently positive” titers, while others investigated those with rising titers or “re-emerging” ANCA after negative testing.[7–16]
Interest in using ANCA as a biomarker for disease activity stems from its potentially pathogenic role in AAV disease and early studies suggesting that rising ANCA titer may predict disease flare and relapse.[17,18] However, a subsequent meta-analysis found that repeat ANCA testing to identify patients with rising or persistent ANCA titers had limited utility for guiding patient management.[14] Despite those findings, there was a resurgence of enthusiasm for repeat ANCA testing after the adoption of rituximab for AAV induction treatment since rituximab depletes circulating precursors to ANCA-producing immune cells and significantly decreases ANCA titers.[6,19] However, recent research, including observational studies and the MAINRITSAN2 randomized clinical trial have suggested that rising ANCA titers may be specific but imperfect predictors of AAV relapse.[20]
In light of these conflicting data, the impact of achieving a serologic remission on later risk of relapse, ESRD, and death remains unknown. To investigate the association of post-induction ANCA titers with key AAV outcomes, we emulated a target trial using observational data to examine the effect of achieving a serologic remission after treatment on the subsequent risks of relapse, ESRD, and death within 5 years.
METHODS
Study Population
We used the Mass General Brigham (MGB) AAV cohort as the data source. The MGB AAV cohort is a retrospective consecutive inception cohort of AAV patients evaluated and treated at a large multi-hospital, healthcare system in the Boston, Massachusetts area. The cohort contains consecutive AAV patients who were diagnosed and received induction treatment between January 1, 2002 and June 30, 2019 identified using a previously described algorithm and confirmed to have AAV by review of electronic health records (EHR).[21] All patients were PR3-ANCA or MPO-ANCA positive; we excluded patients with eosinophilic granulomatosis with polyangiitis. We extracted data on baseline demographics, laboratory testing, and medications from the EHR. The MGB institutional review board approved this study. Consent was waived due to the retrospective nature of the research. Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
ANCA Titers
ANCA testing was performed for clinical purposes by enzyme-linked immunoassay (ELISA) and the assay used varied by calendar time and clinical laboratory. We extracted all available ANCA results from the EHR and classified each test as positive or negative using the associated laboratory reference values. We classified borderline results as positive. We considered a patient to be ANCA-negative if they had a negative ANCA assay (e.g., titer below the assay’s borderline or normal level) result within 180 days of treatment initiation, which we defined as the date of initial immunosuppression prescription for AAV.
Outcomes: End-Stage Renal Disease, Death, and Relapse
The first outcome of interest was relapse (major and minor) within five years of induction treatment (index date). We reviewed the EHR of all patients to identify relapses. We defined relapse as an increase in Birmingham Vasculitis Score Wegener’s Granulomatosis (BVAS/WG) combined with increased immunosuppressive treatment for signs/symptoms of AAV, consistent with prior studies investigating risk factors for AAV relapse.[22] We did not consider an isolated rise in ANCA titer to represent a disease flare.
The second outcome of interest was the composite of ESRD or death within five years of index date. We defined ESRD as (1) requirement of hemodialysis or peritoneal dialysis for >60 days, (2) dialysis until death if the patient died between day 14-60 of follow up, or (3) renal transplant. We obtained data on ESRD and renal transplant from the United States Renal Data System, which is a national registry of ESRD patients, representing an estimated 94% of patients who receive dialysis or kidney transplantation.[23] For ESRD outcome analyses, we excluded four patients who initiated renal replacement therapy >300 days prior to AAV diagnosis for other reasons. Death data were obtained from the National Death Index, a nationwide mortality index run by the Centers for Disease Control.[24] Additionally, we reviewed the EHR of all patients for vital status, ESRD, or renal transplant outcomes not captured in the national databases. We also considered ESRD and death outcomes individually.
Covariates
We extracted demographic and disease-specific features including age at diagnosis, sex, PR3- and MPO-ANCA type, induction treatment, estimated glomerular filtration rate (eGFR), and comorbidities to calculate a Charlson comorbidity index (CCI) from the EHR.[25] We reviewed each patient’s records to determine disease manifestations and baseline BVAS/WG.[26] CCI was missing on 59 patients. There were no other missing covariate data.
Statistical Analysis
We emulated a hypothetical clinical trial comparing the 5-year risks of relapse, ESRD, and death in patients who did vs. did not achieve serologic remission within 180 days of induction treatment. Although the goal of the treating providers may not have been to achieve a specific ANCA level, the emulated target trial assesses the impact of potential treatment strategies and minimizes “immortal time” and other biases associated with retrospective data.[27] Because the exposure of interest (i.e., time to “achieving serologic remission”) was the time duration to reach an exposure level, we adopted a “cloning, censoring, and weighting” approach.[28,29] We created two trial arms, one in which patients achieved a negative ANCA assay within 180 days of induction (“Achieved serologic remission”) and one in which patients’ treatment strategy did not result in serologic remission (“Does not achieve serologic remission.”) We created “clones” of each patient and assigned one duplicate to each trial arm. Censoring of a “clone” occurred when it deviated from the assigned protocol. For example, we censored duplicates assigned to the “serologic remission” group if they did not achieve a negative ANCA assay within 180 days. Similarly, we censored duplicates assigned to the “does not achieve serologic remission” group if their ANCA assay became negative within 180 days. Because censoring may lead to selection bias, we weighted each patient by their inverse probability of censoring. Specifically, the denominator was the probability that a duplicate adhered to the assigned arm determined using a logistic regression model, which consisted of baseline age, sex, ANCA type, induction treatment regimen, BVAS/WG, eGFR, and treatment with plasma exchange. This inverse probability of censoring weighting creates two pseudo-populations where group assignment is independent of prognostic factors for the outcomes of relapse, ESRD or death.
For each analysis, follow up time among those not artificially censored ended at the earliest of: event of interest, end of follow-up at MGB (only for relapse), or 5 years after index date. For the relapse and ESRD outcomes, we accounted for the competing risk of death.[30] We fitted pooled logistic regression models for relapse and the composite outcome of ESRD or death, as well as ESRD and death individually. Because the outcomes were rare, the odds ratios generated from the pooled logistic regressions approximate hazard ratios.[31] We calculated 95% confidence intervals for the estimate of the odds ratios and created cumulative incidence curves for each outcome. We performed several subgroup analyses examining the effect of achieving serologic remission on the risk of each outcome by PR3 or MPO-ANCA+ status, renal involvement, initial induction strategy (rituximab- or cyclophosphamide-based), and use of plasma exchange.
We considered a two-sided p-value <0.05 as the threshold for significance, without adjustment for multiple hypothesis testing. Statistical analysis was performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Sensitivity Analysis
We performed three sensitivity analyses. First, we repeated our main analyses after using a sequential regression method to calculate baseline CCI on the 24 patients missing this baseline data.[32] Second, to test the robustness of the study finding, we repeated the main analysis for all outcomes after extending the grace period (i.e. the time to achieve a serologic remission) from 180 to 365 days. Third, to protect against bias introduced by comparing results from different ANCA testing platforms, we limited the cohort to patients who had ANCA testing performed at Massachusetts General Hospital.
RESULTS
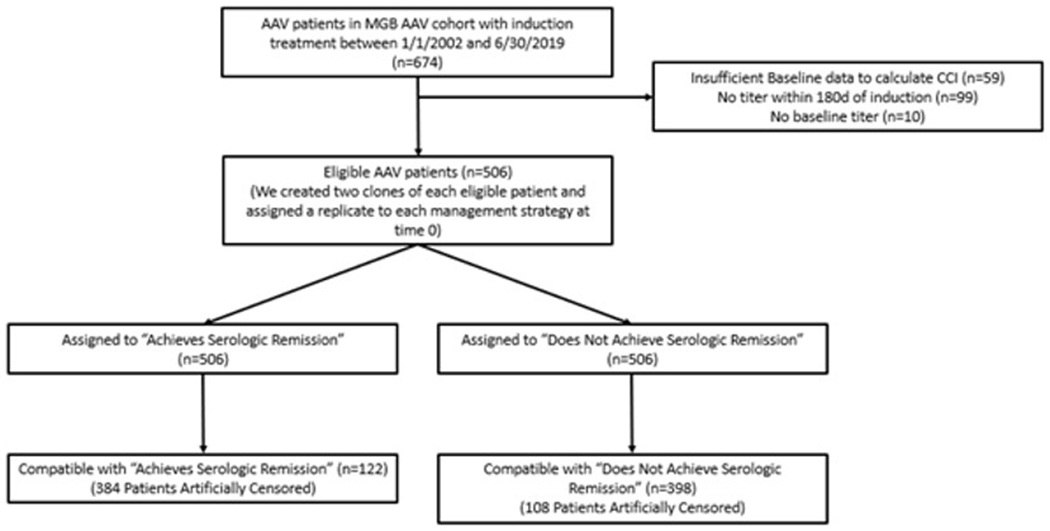
There were 674 patients in the Mass General Brigham AAV cohort screened for inclusion in the target trial. Figure 1 details patient allocation. After excluding patients lacking ANCA titer measurement within 180 days of induction and/or insufficient baseline information to calculate a CCI, we included 506 patients in this analysis (Table 1). The cohort had a mean age of 61 years (SD 18) and was predominately female (293, 58%), white (442, 87%), and MPO-ANCA positive (366, 72%). Overall, 395 (78%) had major organ involvement at baseline. 342 (68%) had renal and 249 (49%) had pulmonary involvement. The mean baseline BVAS/WG score was 5 (SD 2.2). Induction treatment included primarily rituximab in 298 (59%), cyclophosphamide in 166 (33%), or other treatments (e.g., methotrexate) in 42 (8%). Plasma exchange was used in 119 (24%) patients.
Figure 1:
Flow Chart of Eligible Patients and Target Trial Design (180 days)
Table 1:
Baseline Characteristics of Participants (n=506)
| Characteristic | Total (n=506, %) |
|---|---|
| Age (years mean, SD) | 61 (18) |
| Male | 213 (42%) |
| Race | |
| White | 442 (87%) |
| Black | 11 (2%) |
| Asian | 6 (1%) |
| Other | 47 (9%) |
| ANCA status | |
| PR3-ANCA+ | 140 (28%) |
| MPO-ANCA+ | 366 (72%) |
| Organ Involvement | |
| Any major | 395 (78%) |
| Renal | 342 (68%) |
| eGFR (mL/min/1.72m2) | 38.8 (14, 72) |
| Pulmonary | 249 (49%) |
| Head and neck | 213 (42%) |
| Other | 66 (13%) |
| Disease activity at diagnosis (BVAS/WG mean, SD) | 5 (2.2) |
| Charlson Comorbidity Index at diagnosis (CCI mean, SD) | 1.7 (2.3) |
| Induction treatment | |
| Included RTX | 298 (59%) |
| Included CYC | 166 (33%) |
| Included TPE | 119 (24%) |
| Other (no RTX or CYC) | 42 (8%) |
| Follow up | |
| ANCA measurements during follow up* (mean, SD) | 13 (9) |
| ANCA measurements within 180 of induction (mean, SD | 3.5 (1.9) |
within five years of induction or from induction to relapse or last MGB follow up if <5 years
ANCA = antineutrophil cytoplasmic antibody, BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis, CYC = cyclophosphamide, dL = deciliter, eGFR = estimated glomerular filtration rate, mg = milligram, mL = milliliter, mm = millimeter, L = liter, RTX = rituximab, SD = standard deviation
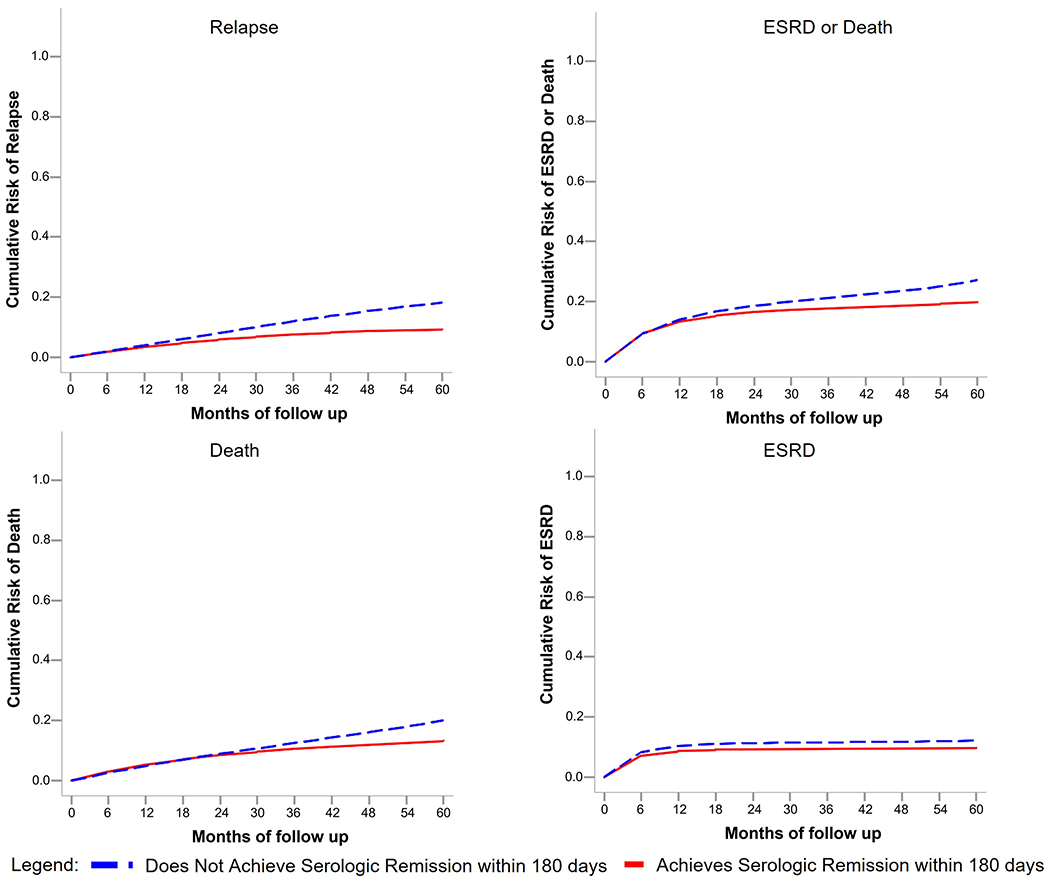
The median follow-up time was 49 months (IQR 20.2-60) for relapse and 60 months (IQR 21.9-60) for the assessment of ESRD and death. The mean number of ANCA measurements performed during the first 180 days after induction was 3.5 (SD 1.9). Additional details of the number of ANCA titer measurements overall and in patients with and without major organ involvement at baseline are provided in Supplemental Tables 1–2. During the five years of follow-up, 81 patients (16%) died, 51 (10%) had ESRD, and 64 (13%) had relapse. Among patients who had each outcome, the median time to death, ESRD, and relapse were 675, 33, and 539 days, respectively. Cumulative incidence curves for each outcome are detailed in Figure 2.
Figure 2:
Cumulative Incidence of Relapse, ESRD or Death by Post-Induction ANCA status
In the target trial analysis for relapse, 122 patients achieved a negative ANCA assay within 180 days of induction and were compatible with the “serologic remission” group, while 398 patients were compatible with the “does not achieve serologic remission” group. Censoring of clones in both trial arms is detailed in Figure 1.
The 5-year cumulative incidence of relapse was 9.4 per 100 patients in the group achieving serologic remission and 18.3 in the group that did not achieve serologic remission within 180 days of induction. The corresponding risk difference was −8.9 (95%CI −17.4 to −0.4) per 100 and the HR was 0.55 (95%CI 0.38 to 0.81) (Table 2, Figure 2). Achieving serologic remission was not significantly associated with decreased risk of death or ESRD. The HR for the composite outcome of death or ESRD within 5 years was 0.87 (95%CI 0.61 to 1.25) for the group that achieved a serologic remission.
Table 2:
The effect of achieving serologic remission on risk of relapse, ESRD, and death using an emulated target trial design (n=506)
| Outcome | Serologic Remission within 180 days | Persistently Positive Titer at 180 days |
|---|---|---|
| Relapse | ||
| Risk over 5 years (95% CI), per 100 | 9.4 (3.4 to 15.4) | 18.3 (9.9 to 26.7) |
| Risk difference over 5 years (95% CI), per 100 | −8.9 (−17.4 to −0.4) | Ref |
| Adjusted HR† (95% CI) | 0.55 (0.38 to 0.81) | 1.0 (Ref) |
| ESRD or Death (composite) * | ||
| Risk over 5 years (95% CI), per 100 | 19.8 (11.2 to 28.6) | 27.1 (17.0 to 37.3) |
| Risk difference over 5 years (95% CI), per 100 | −7.3 (−15.8 to 1.2) | Ref |
| Adjusted HR† (95% CI) | 0.87 (0.61 to 1.25) | 1.0 (Ref) |
| ESRD * | ||
| Risk over 5 years (95% CI), per 100 | 9.7 (3.6 to 15.8) | 12.3 (5.4 to 19.1) |
| Risk difference over 5 years (95% CI), per 100 | −2.5 (−11.0 to 5.9) | Ref |
| Adjusted HR† (95% CI) | 0.93 (0.70 to 1.23) | 1.0 (Ref) |
| Death | ||
| Risk over 5 years (95% CI), per 100 | 13.4 (6.2 to 20.5) | 20.0 (11.3 to 28.8) |
| Risk difference over 5 years (95% CI), per 100 | −6.7 (−15.1 to 1.8) | Ref |
| Adjusted HR† (95% CI) | 0.81 (0.49 to 1.35) | 1.0 (Ref) |
4 patients with ESRD >300d prior to AAV diagnosis were excluded from analyses of ESRD outcomes
adjusted for baseline covariates: age, sex, ANCA type, induction treatment regimen, BVAS/WG, eGFR, and treatment with plasma exchange
ANCA = antineutrophil cytoplasmic antibody, ESRD = end-stage renal disease, HR = hazard ratio, CI = confidence interval
We observed similar results in subgroup analyses stratifying by ANCA type, baseline renal involvement, and induction treatment (Table 3). Achieving serologic remission within 180 days was associated with a statistically significant reduction in the risk of relapse in the MPO-ANCA+ (HR 0.62 95%CI 0.40 to 0.96) and rituximab-treated groups (HR 0.55 95%CI 0.33 to 0.92). Our sensitivity analyses confirmed the robustness of the findings after imputing data for those with missing baseline CCI, extending the time to achieve a serologic remission from 180 days to 365 days, and limiting ANCA testing to a single laboratory (Table 4).
Table 3:
Subgroup Analyses by ANCA Type, Renal Involvement and Induction Treatment Regimen for Relapse, ESRD, and Death
| Outcome | Serologic Remission within 180 days HR (95% CI) | Persistently Positive Titer at 180 days |
|---|---|---|
| Adjusted HR† for Relapse | ||
| PR3-ANCA+ | 0.52 (0.20 to 1.33) | 1.0 (Ref) |
| MPO-ANCA+ | 0.62 (0.40 to 0.96) | 1.0 (Ref) |
| Renal Involvement at Baseline | 0.64 (0.39 to 1.03) | 1.0 (Ref) |
| RTX or RTX/CYC treated | 0.55 (0.33 to 0.92) | 1.0 (Ref) |
| CYC Only treated | 0.47 (0.21 to 1.03) | 1.0 (Ref) |
| TPE Treated | 0.49 (0.10 to 2.49) | 1.0 (Ref) |
| Adjusted HR† for ESRD or Death (composite)* | ||
| PR3-ANCA+ | 0.77 (0.34 to 1.74) | 1.0 (Ref) |
| MPO-ANCA+ | 0.86 (0.58 to 1.28) | 1.0 (Ref) |
| Renal Involvement at Baseline | 0.91 (0.63 to 1.32) | 1.0 (Ref) |
| RTX or RTX/CYC treated | 0.98 (0.61 to 1.59) | 1.0 (Ref) |
| CYC Only treated | 0.95 (0.55 to 1.64) | 1.0 (Ref) |
| TPE Treated | 0.95 (0.64 to 1.40) | 1.0 (Ref) |
| Adjusted HR† for ESRD* | ||
| PR3-ANCA+ | 0.55 (0.20 to 1.53) | 1.0 (Ref) |
| MPO-ANCA+ | 1.03 (0.75 to 1.41) | 1.0 (Ref) |
| Renal Involvement at Baseline | 1.00 (0.74 to 1.34) | 1.0 (Ref) |
| RTX or RTX/CYC treated | 0.80 (0.53 to 1.21) | 1.0 (Ref) |
| CYC Only treated | 1.28 (0.87 to 1.90) | 1.0 (Ref) |
| TPE Treated | 0.91 (0.58 to 1.44) | 1.0 (Ref) |
| Adjusted HR† for Death | ||
| PR3-ANCA+ | 1.04 (0.35 to 3.14) | 1.0 (Ref) |
| MPO-ANCA+ | 0.73 (0.41 to 1.31) | 1.0 (Ref) |
| Renal Involvement at Baseline | 0.88 (0.49 to 1.57) | 1.0 (Ref) |
| RTX or RTX/CYC treated | 1.04 (0.55 to 1.96) | 1.0 (Ref) |
| CYC Only treated | 0.73 (0.27 to 1.96) | 1.0 (Ref) |
| TPE Treated | 1.18 (0.69 to 2.02) | 1.0 (Ref) |
4 patients with ESRD >300d prior to AAV diagnosis were excluded from analyses of ESRD outcomes
adjusted for baseline covariates: age, sex, ANCA type, induction treatment regimen, BVAS/WG, eGFR, and treatment with plasma exchange
ANCA = antineutrophil cytoplasmic antibody, CYC = cyclophosphamide, ESRD = end-stage renal disease, HR = hazard ratio, MPO = myeloperoxidase, PR3 = proteinase-3, RTX = rituximab, TPE = plasma exchange
Table 4:
Sensitivity analyses examining target trial outcomes of ESRD, relapse, and death within 5 years
| Sensitivity Analysis 1: Imputation of Missing Baseline Data (n=530)* | ||
|---|---|---|
| Serologic Remission within 180 Days HR (95%CI) | Persistently Positive Titer at 180 Days | |
| All Patients with imputed baseline data (n=530)* | ||
| Relapse | 0.62 (0.43 to 0.89) | 1.0 (Ref) |
| ESRD or Death* | 0.85 (0.61 to 1.19) | 1.0 (Ref) |
| ESRD* | 0.85 (0.67 to 1.06) | 1.0 (Ref) |
| Death | 0.79 (0.48 to 1.29) | 1.0 (Ref) |
| Sensitivity Analysis 2: Extending Grace Period to 365 Days (n=506)* | ||
| Serologic Remission within 365 Days HR (95%CI) | Persistently Positive Titer at 180 Days | |
| All Patients with complete data (n=506)* | ||
| Relapse | 0.73 (0.54 to 0.99) | 1.0 (Ref) |
| ESRD or Death* | 0.94 (0.68 to 1.28) | 1.0 (Ref) |
| ESRD* | 0.96 (0.72 to 1.28) | 1.0 (Ref) |
| Death | 0.73 (0.46 to 1.17) | 1.0 (Ref) |
| Sensitivity Analysis 3: ANCA testing performed at Massachusetts General Hospital | ||
| Serologic Remission within 180 Days HR (95%CI) | Persistently Positive Titer at 180 Days | |
| All Patients with ANCA performed at MGH (n=453)* | ||
| Relapse | 0.61 (0.40 to 0.93) | 1.0 (Ref) |
| ESRD or Death* | 0.95 (0.65 to 1.39) | 1.0 (Ref) |
| ESRD* | 1.00 (0.73 to 1.38) | 1.0 (Ref) |
| Death | 0.90 (0.53 to 1.51) | 1.0 (Ref) |
4 patients with ESRD >300d prior to AAV diagnosis were excluded from analyses of ESRD outcomes
ANCA = antineutrophil cytoplasmic antibody, CYC = cyclophosphamide, ESRD = end-stage renal disease, MPO = myeloperoxidase, PR3 = proteinase-3, RTX = rituximab, TPE = plasma exchange
DISCUSSION
In this target trial emulation study using observational data from a large cohort of AAV patients, achieving serologic remission (negative ANCA assay) within 180 days of induction was associated with decreased risk of relapse, but was not associated with statistically significant reduction in the risk of ESRD or death within 5 years. We observed similar results when stratifying by ANCA type and induction treatment strategy. These findings suggest that achieving a negative ANCA assay during and after induction may result in fewer subsequent disease relapses.
Our study investigates an ongoing controversy in AAV care that has led to varying ANCA testing practices following diagnosis. Previous studies have yielded conflicting results, in part due to significant heterogeneity of study designs investigating the association of relapses with rise in ANCA titer[11–14], reemergence of ANCA titer[10], ANCA persistence[7–9,14,16], or a combination[20]. Many studies were also conducted prior to the introduction of rituximab, which renewed enthusiasm for ANCA as a clinical biomarker, since rituximab targets the B-cell lineage that ultimately produces ANCA.[19] Recent investigations have suggested that the utility of ANCA titers as a marker of relapse risk may vary by ANCA subtype and specific disease manifestations.[13,16,33]
We expand on these studies using a contemporary cohort of newly diagnosed patients undergoing remission induction, many with rituximab, and applying methods to address immortal time bias and confounding. We focused on the impact of achieving serologic remission (negative ANCA assay) within six months of remission induction. This is an important timepoint in AAV care that typically marks the end of “remission induction” and a transition from induction immunosuppression to maintenance therapy. Achieving a negative ANCA assay at this time may have prognostic significance and inform the choice and intensity of maintenance therapy or subsequent monitoring by identifying patients with favorable AAV treatment response and low risk of subsequent relapse. Our findings remained rather consistent across subgroups stratified by ANCA titer and induction treatment, in contrast to previous studies. Additional studies are needed to evaluate the association of rising ANCA titers with relevant outcomes using similar methodologies to address potential confounding and immortal time bias.
Significant basic and translational research has demonstrated the importance of ANCA for AAV disease pathogenesis. ANCA have been shown to bind to autoantigens and activate neutrophils, leading to microvascular injury.[2] In light of the recognition of the effect that ANCA have on immune cells in animal models and in vitro studies, the inconsistent association between ANCA levels and disease activity remains incompletely understood. Two recent studies suggest that post-translational modification of ANCA immunoglobulins may correlate with differences in disease activity. Espy and colleagues demonstrated that sialylation of PR3-ANCA increased in patients with inactive disease[34] whereas Lardinois and colleagues demonstrated that glycosylation of the Fc segment of IgG was reduced in PR3-ANCA+ patients with active disease.[35] Our findings indicate that, at least in some patients, persistent ANCA beyond remission induction are pathogenic given their effects on relapse risk. However, it is also known that not all patients with a persistent ANCA titer will experience a relapse. More detailed examination of ANCA expression, including post-translational modification, may offer further insights into disease risk in AAV.
Strengths of our study include the use of a large AAV cohort and the assessment of the clinically meaningful outcomes of relapse, ESRD, and death. There has been minimal prior research on the association between ANCA titers and renal and mortality outcomes.[36] We obtained outcome data from comprehensive sources including electronic health record review, the US Renal Data System, and the National Death Index. Another strength was the inclusion of a majority of MPO-ANCA+ patients. Prior literature on the utility of ANCA titers to predict disease flares and outcomes have focused primarily on patients with granulomatosis with polyangiitis who are often PR3-ANCA+.[14] The use of an emulated target trial design with cloning, censoring, and weighting was also a strength of our study. This approach allowed for assessment of the impact of treatment to serologic remission using observational data without the cost of a prospective clinical trial. This technique also leveraged a rich observational dataset while minimizing the effects of immortal time bias, baseline confounding, and selection bias in the weighting step.[37]
Our study has certain potential limitations. First, we relied on observational data from a single healthcare system, which may limit the generalizability of the results. However, the Mass General Brigham system includes community and tertiary care hospitals, primary care, and other specialty clinics throughout many sites in the New England area. Second, we adjusted our analysis for patient baseline factors, but the possibility of residual confounding remains. Third, because we used ANCA test results from multiple reference laboratories and information about the specific assay used was not always available, we were unable to examine if ELISA type impacted the observed associations and directly compare baseline ANCA values between assays.[13] Fourth, we specified a 180 day “grace period” for patients to achieve serologic remission in our target trial, but this window may miss differences between patients who have serologic response early or late within that time period. Fifth, we assessed for relapse outcomes using clinical notes and defined a relapse as intensification of therapy with rise in BVAS/WG score. Although these criteria are agnostic to ANCA titer, the treating providers were not blinded to ANCA results and we cannot account for differences in subsequent treatment and monitoring. Additionally, the relapse rate that we observed was lower than reported in some AAV clinical trials.[6,38,39] This is likely multifactorial, including the MPO-ANCA predominance of our cohort, which has a lower risk of flare[40], as well as the enrollment of patients with relapse into some clinical trials and therefore selection for patients at higher risk of relapse, or other factors. Further prospective studies investigating the effect of achieving serologic remission using structured assessment of disease activity are needed. Finally, we observed an association between achieving serologic remission with decreased risk of relapse and a trend towards decreased ESRD or mortality that did not reach statistical significance. It is possible that our study was underpowered to detect differences in ESRD and death outcomes, which may be long-term consequences of recurrent disease activity. However, our study represents one of the largest published AAV cohorts and was relatively enriched for these outcomes with 23% of subjects experiencing ESRD or death during follow up. Alternatively, significant morbidity and mortality in AAV patients may be less related to disease activity in the modern treatment era. Although we adjusted for induction immunosuppression in our analyses, the induction regimen was not randomly selected and was instead chosen at the discretion of the treating physician based on clinical and other patient factors. Therefore, our findings regarding the prognostic significance of post-induction ANCA titers should not be used to guide clinical management decisions regarding the choice or intensity of induction immunosuppression or subsequent treatment. This represents an important avenue for future prospective research.
In conclusion, we found that achieving serologic remission (negative ANCA assay) during the first 180 days after induction was associated with a decreased risk of relapse within 5 years. We did not observe a statistically significant difference in the risk of ESRD or death within 5 years comparing patients who achieved serologic remission to those who did not achieve serologic remission. We observed similar results after stratifying by ANCA type and induction treatment strategy. These findings suggest that achieving a serologic remission within 180 days of induction is associated with a decreased risk of AAV relapse but may have lower impact on ESRD and mortality outcomes. Further studies are needed to investigate how post-induction ANCA titers and other disease biomarkers may guide AAV management strategies.
Supplementary Material
KEY MESSAGES.
What is already known about this subject?
Anti-neutrophil cytoplasmic antibodies (ANCA) in ANCA-associated vasculitis (AAV) are useful for establishing a diagnosis of AAV and are considered pathogenic.
The utility of post-induction ANCA titers to inform management or expected outcomes in ANCA-associated vasculitis (AAV) remains controversial.
What does this study add?
Using a large cohort of patients with AAV, we performed an emulated target trial comparing patients achieving serologic remission (e.g., negative ANCA assay) to patients who did not achieve serologic remission within 180 days.
Patients who achieved serologic remission within 6 months of induction treatment had lower risk of relapse by 5 years, but no statistically significant improvement in ESRD or mortality outcomes.
How might this impact on clinical practice or future developments?
Treatment of AAV patients to serologic remission may reduce the risk of subsequent disease relapse.
Future prospective studies should determine the utility of serial ANCA measurements to guide ANCA treatment decisions.
Funding:
GCM is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32AR055855). ZSW is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR073334 and R03AR078938), the Rheumatology Research Foundation (K Supplement), and a COVID-19 Junior Investigator Award from the Massachusetts General Hospital Department of Medicine.
Competing interests:
ZSW has performed consultancy for Viela Bio/Horizon, MedPace, Zenas Biopharma, and Sanofi/Principia. ZSW has received grant support from BMS and Sanofi/Principia for unrelated work.
Footnotes
Disclaimer: The funders had no role in the decision to publish or in the preparation of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic healthcare centres or the National Institutes of Health.
Patient and public involvement: Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Mass General Brigham Institutional Review Board, protocol number 2016P000633.
Data availability statement:
Data available upon reasonable request and with appropriate institutional review board approval.
REFERENCES
- 1.Tan JA, Dehghan N, Chen W, et al. Mortality in ANCA-associated vasculitis: a meta-analysis of observational studies. Ann Rheum Dis 2017;76:1566–74. doi: 10.1136/annrheumdis-2016-210942 [DOI] [PubMed] [Google Scholar]
- 2.Kitching AR, Anders HJ, Basu N, et al. ANCA-associated vasculitis. Nature Reviews Disease Primers 2020;6. doi: 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 3.Nakazawa D, Masuda S, Tomaru U, et al. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nature Reviews Rheumatology 2019;15:91–101.doi: 10.1038/s41584-018-0145-y [DOI] [PubMed] [Google Scholar]
- 4.Van Der Woude FJ, Lobatto S, Permin H, et al. Autoantibodies Against Neutrophils and Monocytes: Tool for Diagnosis and Marker of Disease Activity in Wegener’S Granulomatosis. The Lancet 1985;325:425–9. doi: 10.1016/S0140-6736(85)91147-X [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Liu S, Merkel PA, et al. Diagnostic performance of antineutrophil cytoplasmic antibody tests for idiopathic vasculitides: Metaanalysis with a focus on antimyeloperoxidase antibodies. Journal of Rheumatology 2001;28:1584–90. [PubMed] [Google Scholar]
- 6.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. doi: 10.1056/NEJMoa0909905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClure ME, Wason J, Gopaluni S, et al. Evaluation of PR3-ANCA Status After Rituximab for ANCA-Associated Vasculitis. J Clin Rheumatol 2019;25:217–23. doi: 10.1097/RHU.0000000000001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders JSF, Huitma MG, Kallenberg CGM, et al. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology 2006;45:724–9. doi: 10.1093/rheumatology/kei272 [DOI] [PubMed] [Google Scholar]
- 9.Thai LH, Charles P, Resche-Rigon M, et al. Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener’s) relapses? Results of a retrospective study on 126 patients. Autoimmunity Reviews 2014;13:313–8. doi: 10.1016/j.autrev.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 10.Terrier B, Saadoun D, Sène D, et al. Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Annals of the Rheumatic Diseases 2009;68:1564–71. doi: 10.1136/ard.2008.094714 [DOI] [PubMed] [Google Scholar]
- 11.Boomsma MM, Stegeman CA, Van Der Leij MJ, et al. Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: A prospective study. Arthritis and Rheumatism 2000;43:2025–33. doi: [DOI] [PubMed] [Google Scholar]
- 12.Kemna MJ, Damoiseaux J, Austen J, et al. ANCA as a predictor of relapse: Useful in patients with renal involvement but not in patients with nonrenal disease. Journal of the American Society of Nephrology 2015;26:537–42. doi: 10.1681/ASN.2013111233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fussner LA, Hummel AM, Schroeder DR, et al. Factors Determining the Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3. Arthritis and Rheumatology 2016;68:1700–10. doi: 10.1002/art.39637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomasson G, Grayson PC, Mahr AD, et al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis-a meta-analysis. Rheumatology 2012;51:100–9. doi: 10.1093/rheumatology/ker280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles P, Perrodeau É, Samson M, et al. Long-Term Rituximab Use to Maintain Remission of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Trial. Ann Intern Med 2020;173:179–87. doi: 10.7326/M19-3827 [DOI] [PubMed] [Google Scholar]
- 16.van Dam LS, Dirikgil E, Bredewold EW, et al. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transplant 2021;36:1408–17. doi: 10.1093/ndt/gfaa066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen Tervaert JW, Van der Woude FJ, Fauci AS, et al. Association between active Wegener’s granulomatosis and anticytoplasmic antibodies. Archives of Internal Medicine 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461 [DOI] [PubMed] [Google Scholar]
- 18.Hamour S, Salama AD, Pusey CD. Management of ANCA-associated vasculitis: Current trends and future prospects. Ther Clin Risk Manag 2010;6:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortazar FB, Pendergraft WF, Wenger J, et al. Effect of Continuous B Cell Depletion With Rituximab on Pathogenic Autoantibodies and Total IgG Levels in Antineutrophil Cytoplasmic Antibody—Associated Vasculitis. Arthritis and Rheumatology 2017;69:1045–53. doi: 10.1002/art.40032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charles P, Terrier B, Perrodeau É, et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: Results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Annals of the Rheumatic Diseases 2018;77:1144–50. doi: 10.1136/annrheumdis-2017-212878 [DOI] [PubMed] [Google Scholar]
- 21.Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222–7. doi: 10.1136/ard.2006.054593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh M, Flossmann O, Berden A, et al. Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis and Rheumatism 2012;64:542–8. doi: 10.1002/art.33361 [DOI] [PubMed] [Google Scholar]
- 23.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020;75:A6–7. doi: 10.1053/j.ajkd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 24.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major U.S. mortality databases. Annals of Epidemiology 2002;12:462–8. doi: 10.1016/S1047-2797(01)00285-X [DOI] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26.Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. Arthritis and Rheumatism 2001;44:912–20. doi: [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Sauer BC, Hernández-Díaz S, et al. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. Journal of Clinical Epidemiology 2016;79:70–5. doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernán MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American Journal of Epidemiology 2016;183:758–64. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyu H, Yoshida K, Zhao SS, et al. Delayed Denosumab Injections and Fracture Risk Among Patients With Osteoporosis: A Population-Based Cohort Study. Ann Intern Med 2020;173:516–26. doi: 10.7326/M20-0882 [DOI] [PubMed] [Google Scholar]
- 30.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd Edition. Cary, NC: : SAS Press; 2010. [Google Scholar]
- 31.Thompson WA. On the treatment of grouped observations in life studies. Biometrics 1977;33:463–70. [PubMed] [Google Scholar]
- 32.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: : John Wiley & Sons; 1987. [Google Scholar]
- 33.Thompson GE, Fussner LA, Hummel AM, et al. Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3 in ANCA-Associated Vasculitis. Frontiers in Immunology 2020;11. doi: 10.3389/fimmu.2020.02053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Espy C, Morelle W, Kavian N, et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis and Rheumatism 2011;63:2105–15. doi: 10.1002/art.30362 [DOI] [PubMed] [Google Scholar]
- 35.Lardinois OM, Deterding LJ, Hess JJ, et al. Immunoglobulins G from patients with ANCA-associated vasculitis are atypically glycosylated in both the Fc and Fab regions and the relation to disease activity. PLoS One 2019;14:e0213215. doi: 10.1371/journal.pone.0213215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen CL, Tian YC, Wu HH, et al. High anti-neutrophil cytoplasmic antibody titers are associated with the requirement of permanent dialysis in patients with myeloperoxidase-ANCA-associated vasculitis. Journal of the Formosan Medical Association 2019;118:1408–15. doi: 10.1016/j.jfma.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 37.Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ 2018;360:k182. doi: 10.1136/bmj.k182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones RB, Willem Cohen Tervaert J, Hauser T, et al. Rituximab versus Cyclophosphamide in ANCA-Associated Renal Vasculitis. 2010. www.vasculitis.org. [DOI] [PubMed]
- 39.Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003;349:36–44. doi: 10.1056/NEJMoa020286 [DOI] [PubMed] [Google Scholar]
- 40.Lionaki S, Blyth ER, Hogan SL, et al. Classification of antineutrophil cytoplasmic autoantibody vasculitides: The role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis and Rheumatism 2012;64:3452–62. doi: 10.1002/art.34562 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request and with appropriate institutional review board approval.


