Abstract
Purpose
To identify specific likelihoods that an embryo will be classified as appropriate for transfer after preimplantation genetic testing for detection of a monogenic disorder (PGT-M), with or without preimplantation genetic testing for aneuploidy (PGT-A), separated by inheritance pattern.
Methods
Retrospective chart review of 181 selected PGT-M cycles performed at CooperGenomics in 2018 or 2019. For each cycle, the following main outcome data was collected: the number of embryos classified as affected with monogenic disease, the number detected to be chromosomally abnormal, the number that were recombinant, the number that had no result, and if applicable, the number which were aneuploid.
Results
There were significantly fewer embryos appropriate to consider for transfer when PGT-A was included for autosomal recessive and X-linked disorders. There were also fewer for autosomal dominant disorders, though this was not statistically significant. When PGT-A was not included, 45.8% of autosomal dominant, 69% of autosomal recessive, and 47.8% of X-linked embryos were appropriate to consider for transfer. When PGT-A analysis was included, 29% of autosomal dominant, 41% of autosomal recessive, and 22% of X-linked embryos were appropriate to consider for transfer. 96.8% of women elect to include PGT-A when pursuing PGT-M.
Conclusion
This study resulted in specific likelihoods that an embryo would be found appropriate for clinicians and patients to consider for transfer based on the inheritance pattern of the monogenic disease being tested for and whether aneuploidy analysis was included.
Keywords: Preimplantation genetic testing, Monogenic, Single gene disorder, Aneuploidy, Inheritance pattern, Embryo
Introduction
Preimplantation genetic testing (PGT), completed in conjunction with in vitro fertilization (IVF), is a revolutionary way to screen embryos for genetic and chromosomal disorders prior to uterine transfer for achievement of pregnancy [1–10]. The ability of PGT to increase the odds of successful pregnancy, and live-born children free of familial disease, is life-changing for many individuals. However, identifying an appropriate embryo, achieving pregnancy, and delivering a healthy baby may take multiple egg retrievals and, ultimately, successful transfer and live birth is not guaranteed [11]. Counseling patients regarding expectations prior to beginning IVF-PGT is important. Numerous research studies have been done on the rates of successful implantation, ongoing pregnancy, spontaneous abortion, and live birth when PGT identifies an embryo to be transferred [2, 7, 9, 12]; however, there has been little research done on the chance of identifying an embryo suitable for transfer.
PGT may be pursued for a variety of referral reasons; at least 20% of all current PGT cases are for identification of monogenic disorder(s) (PGT-M) [1]. PGT-M may be utilized by patients who have a personal or family history of a genetic disorder that they do not want to transmit to offspring. Additionally, PGT-M is increasingly being performed after a reproductive risk is identified for a couple via expanded carrier screening [11].
In order to improve the odds of successful implantation per transfer, many individuals undergoing IVF elect to pursue PGT-A, which assesses for aneuploidy in embryos [1]. PGT-A has been shown to increase pregnancy rates, particularly in older women with multiple embryos available for biopsy, by removing aneuploidy embryos from the transfer pool [13–17]. It is well known that the rate of aneuploidy increases with maternal age [1–3, 5, 12–15, 18–20]; but aneuploidy is frequently detected in embryos produced by women of all ages. A study of embryos in which PGT-A was performed concurrently with PGT-M found that approximately one-third of embryos produced by women undergoing PGT-A are found to be aneuploid [21]. PGT-A can detect complete aneuploidy as well as mosaic aneuploidy, in which two or more chromosomally distinct cell lines are identified. The clinical utility of PGT-A has been called into question [7, 12–18, 21, 22], especially when used for younger women, or when considering that embryos may be identified as mosaic. Embryos with mosaic PGT-A results are less likely to implant after transfer and are more likely to miscarry [8, 22, 23], but about 20–40% of embryos with mosaic PGT-A results that are transferred lead to the birth of apparently healthy infants [7]. Uniform inclusion of PGT-A has been suggested but is not currently recommended [16, 17]. Because of the potential to incur difficult decision-making in the event of a mosaic result, pre-test counseling about all types of outcomes is important during consent for inclusion of PGT-A.
The purpose of this study was to retrospectively examine the frequency at which embryos are deemed appropriate for transfer when patients utilize in vitro fertilization with preimplantation genetic testing technologies for monogenic disease (IVF-PGT-M), with or without PGT-A analysis. It is important to determine how often embryos are found to be unaffected and euploid, so that providers may better inform their patients during the consent process for IVF-PGT. This study will provide outcomes that may be discussed with patients considering PGT-M, giving them more accurate expectations of the PGT process. We hypothesize that the number of embryos appropriate for transfer will differ across inheritance patterns and between individuals who pursue PGT-M alone and those who pursue PGT-M with PGT-A.
Materials and methods
This is a retrospective chart review, quantitatively analyzing the mean number of embryos appropriate for transfer following PGT. Results belonging to patients who had PGT-M performed at CooperGenomics in 2018 or 2019 were reviewed. CooperGenomics is a reproductive genetics laboratory that provides PGT and genetic counseling. All data was collected remotely, and data analysis was performed at Case Western Reserve University (CWRU) in Cleveland, Ohio. This study was approved by CWRU’s Institutional Review Board on November 25, 2019.
Patients whose primary indication for pursuing PGT was for detection of monogenic disease in 2018 or 2019 were included. These patients may or may not have elected to include aneuploidy analysis. Patients who pursued PGT-A only or PGT for structural rearrangements (PGT-SR) were not included. As trophectoderm biopsy is the current gold standard for embryo sampling [6, 18], only samples obtained through this method were included. Additional exclusion criteria include patients who opted out of research, patients who had PGT performed for > 1 disease, patients whose report indicated either member of the reproductive couple was affected with the autosomal recessive disorder being tested for (as it would modify the chance for an affected embryo), and those who had PGT-A analysis performed through a different laboratory. Additionally, if a patient had more than one IVF-PGT cycle performed at CooperGenomics in 2018 or 2019, only their first cycle was included.
Of the available 1700 IVF-PGT-M cycles in the study time range, 181 were included. Researcher ES identified which cycles would be included based on classification of two variables: inheritance pattern of monogenic disease and whether PGT-A analysis was performed. Six categorical groupings were made: (a) PGT-M with PGT-A, autosomal dominant; (b) PGT-M with PGT-A, autosomal recessive; (c) PGT-M with PGT-A, X-linked; (d) PGT-M alone, autosomal dominant; (e) PGT-M alone, autosomal recessive; and (f) PGT-M alone, X-linked. Cycles were selected for inclusion beginning from January 2019 and proceeded chronologically until approximately forty cycles in a group was reached. Forty cycles was selected based on recommendation from the department’s statistic specialist. When all 2019 cycles were exhausted, 2018 cycles were reviewed in reverse chronological order, beginning with December 2018. All 2018 cycles were reviewed, though the goal of forty cycles was not reached in three groups (d, e, and f).
CooperGenomics’ classification of results was the guide used to determine if an embryo was or was not appropriate to consider for transfer in this data set. For autosomal dominant disorders, embryos homozygous for the wild-type allele were classified as appropriate. For autosomal recessive disorders, embryos homozygous for the wild-type allele and heterozygous carriers were classified as appropriate. For X-linked disorders, unaffected males and non-carrier females were classified as appropriate; for Fragile X syndrome, only non-carrier and intermediate allele carriers were classified as appropriate (premutations and full mutations are not considered appropriate for transfer). Embryos are classified as unsuitable to consider for transfer if they are affected with monogenic disease, aneuploid or mosaic, if status cannot be determined due to recombination involving the gene variant, if they are identified as chromosomally abnormal (“chromosomally abnormal” is a PGT-M result, and thus is unique from an aneuploid result from PGT-A), or if no result is obtained for either PGT-M or PGT-A analysis due to insufficient quality/quantity of DNA (if additional testing was performed in these no result cases, that data was not accessible for this study).
Classification of ploidy via next-generation sequencing at CooperGenomics characterizes embryos as euploid if they show < 20% aneuploidy and aneuploid if > 80% aneuploidy. Mosaic result interpretation is based on percentage of abnormal cells detected, under the presumption of a 5-cell biopsy. Low-level mosaic embryo samples have 20–40% abnormal cells, and high-level mosaics have 40–80% abnormal cells. Inclusion of mosaic reporting is per IVF clinic preference (83.5% opted in). For ordering providers who seek euploid/aneuploid classification alone (opt-out), embryos that are < 40% aneuploid are reported as euploid, and those with > 40% aneuploidy or > 20% aneuploidy involving chromosomes 13, 14, 15, 18, 21, X, or Y are reported as aneuploid. Aneuploidy involving ≥ 3 chromosomes is reported as complex abnormal.
When patients elect to include PGT-A, one of three testing options is selected. Simultaneous testing indicates that all biopsied samples will be analyzed by both PGT-M and PGT-A. Serial testing indicates that only embryos which are deemed appropriate by the former analysis proceed to the latter analysis; patients may elect to do serial testing with either PGT-M or PGT-A first. Due to the serial testing method, results regarding affectedness/ploidy status of embryos which did not proceed to the subsequent analysis could not be included in this study.
The following data was collected from the detailed results obtained for each of the 181 cycles included in this study: the monogenic disease being tested and its inheritance pattern, age of the female patient, total number of embryos biopsied, number of embryos classified as appropriate for transfer, number affected by monogenic disease, number that were chromosomally abnormal (identified by PGT-M analysis via karyomapping, indicative of haploidy or triploidy), number that were recombinant, number that had no PGT-M result, whether PGT-A analysis was included and, if applicable, the number that were aneuploid, number that had “no result” on PGT-A analysis, number that were mosaic, and the level of mosaicism. One woman, age 43 years, utilized an egg donor, age 39 years (0.55% of the sample); the age of the donor was used in determining mean maternal age.
SPSS version 26 was utilized for analysis of data. Descriptive statistics were used to quantify the frequency in which each reason that an embryo was deemed inappropriate for transfer occurred. The percentage of embryos appropriate for each cycle was calculated, and these percentages were averaged for each of the six groups. Three independent samples t-tests were performed, one for each inheritance pattern, using these means to determine if there is a significant difference in the number of embryos appropriate to consider for transfer when PGT-A is included versus when it is not. Three one-tailed t-tests were performed to determine if our observed rate of affectedness was consistent with the expected Mendelian rate of affectedness for each inheritance pattern. A chi-square analysis was used to determine if individuals preferentially select a certain type of testing (simultaneous, serial with PGT-M first, serial with PGT-A first) when including PGT-A analysis.
Results
Of the 1700 cycles meeting criteria, 1646 women chose to include PGT-A with PGT-M (96.8%). Detailed results for the 54 cycles belonging to women who opted not to pursue PGT-A, and an additional 127 cycles belonging to women who did include PGT-A analysis, were obtained, for a total of 181 cycles included. A t-test comparing the mean oocyte ages (PGT-M alone: 33.29 years, PGT-M with PGT-A: 33.98 years) was performed and found that there was not a statistically significant difference in mean oocyte age between the two groups (p-value = 0.26). The number of embryos biopsied in each cycle ranged between 1 and 20 embryos, with a mean of 5.46 embryos biopsied, and the total number of embryos biopsied was 991. Three subgroups based on inheritance pattern exist in each of the two groups: autosomal dominant (AD), autosomal recessive (AR), and X-linked (X). The number of cycles, number of embryos, and mean maternal age for each of the six groups can be seen in Table 1.
Table 1.
Cycle characteristics for patients undergoing PGT-M only or PGT-M with PGT-A
| PGT-M only | PGT-M with PGT-A | |||||
|---|---|---|---|---|---|---|
| Autosomal dominant | Autosomal recessive | X-linked | Autosomal dominant | Autosomal recessive | X-linked | |
| Number of cycles | 21 | 24 | 9 | 43 | 42 | 42 |
| Mean age of patient (years) | 32.43 | 34.63 | 31.78 | 33.23 | 34.00 | 34.74 |
| Number of embryos biopsied | 120 | 100 | 46 | 272 | 273 | 180 |
| Number of transferable embryos | 55 | 69 | 22 | 80 | 112 | 40 |
| Mean number of transferable embryos per cycle | 2.62 | 2.88 | 2.44 | 1.88 | 2.66 | 0.95 |
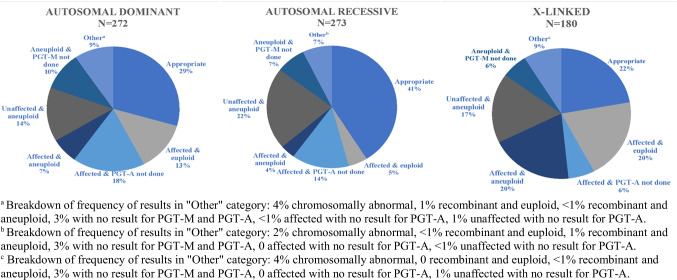
Of the 127 women (725 embryos) who pursued both PGT-M and PGT-A analysis in our 181-patient sample, a similar number completed simultaneous testing (39.4%) as serial testing with PGT-M first (40.9%), with fewer completing serial testing with PGT-A first (19.7%); see Fig. 1. The chi-square analysis (X2(4) > = 8.008, p = 0.091) did not prove an association between inheritance pattern and type of testing pursued. Two hundred forty-nine embryos underwent simultaneous testing, and 63 were identified as both affected by monogenic disease and aneuploid (25.3%). Three hundred eighteen embryos underwent serial PGT-M first testing, and 98 were affected with monogenic disease and PGT-A not performed (30.8%). One hundred fifty-six embryos underwent serial PGT-A first testing, and 58 were aneuploid and PGT-M not performed (37.2%); see Fig. 2 for the distribution of these embryos across inheritance patterns.
Fig. 1.
Type of testing chosen by women who elect to include PGT-A analysis. Data reported as number of women who elected simultaneous testing (dark gray), serial testing with PGT-M first (medium gray), and serial testing with PGT-A first (light gray). The last set of bars represents the total number of women in the group, with the percentage of the total reported above the bars
Fig. 2.
Distribution of types of results across inheritance patterns for women who elected to include PGT-A analysis. N represents number of embryos. Data are presented as the percentage of embryos in the corresponding inheritance pattern which were classified as each result
The total number of embryos sampled in each inheritance pattern group for women who did PGT-M only can be seen in Table 1. For AD-PGT-M alone, 55 embryos were appropriate to consider for transfer (45.8%). For AR-PGT-M alone, 69 embryos were appropriate to consider for transfer (69%). For X-linked-PGT-M alone, 22 embryos were appropriate to consider for transfer (47.8%). The mean number of transferrable embryos in each group can be seen in Table 1. The reasons in which any PGT-M only embryo, regardless of inheritance pattern, would be inappropriate for transfer is most often because it is affected by disease (37.6%), but also includes chromosomally abnormal (1.9%), recombinant (1.5%), and no result (4.5%). The frequency that embryos were affected with disease was not statistically different from expected Mendelian rates of affectedness, p-values 0.856, 0.473, and 0.242, respectively.
For women who elected to include PGT-A, 80 embryos (29.4%) in the AD group were appropriate, 112 embryos (41.0%) in the AR group were appropriate, and 40 embryos (22.2%) in the X-linked group were appropriate; see Fig. 2. An independent samples t-test does not suggest a statistically significant difference between AD: 29.4% and X-linked: 22.2% (p-value: 0.069). Inheritance pattern and type of testing chosen was not found to have a statistical association. The “affected & euploid” and “unaffected & aneuploid” frequencies are similar for AD (13% and 14%, respectively) and X-linked (20% and 17%, respectively), yet are discordant for AR (5% and 22%, respectively). The “other” category is similar (9%, 7%, 9%, respectively) for all groups.
Of 991 embryos in the data set, PGT-M analysis identified 27 embryos to be chromosomally abnormal (2.7%) via karyomapping, indicative of a triploid or haploid embryo. Of these, 17 had PGT-A analysis, which showed 8 triploid, 5 haploid, 1 -X mosaic, 2 aneuploid with ≥ 3 findings (complex abnormal), 1 with partial monosomy, and 1 euploid. Fourteen of the 991 total embryos were recombinant (1.4%). Forty-two of the 991 total embryos had “no result” (4.2%). Twelve belonged to women in which only PGT-M analysis was elected, 22 had no result for both PGT-M and PGT-A, and 8 had no result for PGT-A while a PGT-M result was able to be reported.
Across inheritance patterns, a similar number of cycles involved embryos which originated from a clinic that opted into receiving mosaic information (AD 81.4%, AR 81.0%, and X 88.1%), indicating that inheritance pattern is not associated with opting into receiving mosaic information. An estimated 570 embryos belonged to women who opted into receiving mosaic information. Sixty-seven of these embryos had a mosaic result (11.75%). Of the 67 mosaic embryos, 28.4% were low-level mosaic, 53.7% were high-level mosaics, and 17.9% had 3 or more mosaic findings so were reported as abnormal.
Three independent samples t-tests were utilized, one for each inheritance pattern. The percentage of embryos deemed appropriate for each cycle was calculated, and those percentages were averaged to give a mean (M) value for each of the six groups. For the AD groups, the 43 cycles pertaining to PGT-M with PGT-A analysis (M = 33.14, SD = 26.415) compared to the 21 cycles pertaining to PGT-M analysis alone (M = 46.92, SD = 31.814) indicated fewer embryos appropriate for transfer, t(62) = 1.830, p = 0.072. This p-value does not demonstrate statistical significance. For the AR groups, the 42 cycles pertaining to PGT-M with PGT-A analysis (M = 42.07, SD = 29.525) compared to the 24 cycles pertaining to PGT-M analysis alone (M = 65.19, SD = 31.582) demonstrated statistical significance; fewer embryos were appropriate for transfer, t(64) = 2.984, p = 0.004. For the X-linked groups, the 43 cycles pertaining to PGT-M with PGT-A analysis (M = 22.77, SD = 28.318) compared to the 9 cycles pertaining to PGT-M analysis alone (M = 45.79, SD = 21.226) demonstrated statistical significance; fewer embryos were appropriate for transfer, t(49) = 2.298, p = 0.026.
The most common autosomal dominant, autosomal recessive, and X-linked disorders screened for were breast and ovarian cancer susceptibility (n = 14), cystic fibrosis (n = 21), and fragile X syndrome (n = 27), respectively. Table 2 shows the other disorders tested for by more than one woman in the study. The “Other” categories comprise the genetic conditions which were screened for by only one woman.
Table 2.
Genetic conditions for which PGT-M was indicated
| Autosomal Dominant (n = 64 charts) | Autosomal recessive (n = 66 charts) | X-linked (n = 51 charts) | |||
|---|---|---|---|---|---|
| Breast/ovarian cancer susceptibility (BRCA1, BRCA2, PALB2, BARD1, FANCC) | 14 | Cystic fibrosis | 21 | Fragile X syndrome | 27 |
| Other cancer susceptibility (RET, VHL, MLH1, CDH1, APC, TP53) | 10 | Hemoglobinopathies and thalassemias (HBB and HBA1/HBA2) | 12 | Duchenne-Becker muscular dystrophy | 7 |
| Neurofibromatosis type 1 | 7 | Spinal muscular atrophy | 4 | Hemophilia | 6 |
| Huntington disease | 6 | Autosomal recessive polycystic kidney disease | 4 | Other | 11 |
| Autosomal dominant polycystic kidney disease | 3 | Nonsyndromic hearing loss | 2 | ||
| Facioscapulohumeral muscular dystrophy | 3 | Gaucher disease | 2 | ||
| Osteogenesis imperfecta | 3 | Phenylketonuria | 2 | ||
| Myotonic dystrophy | 2 | Tay Sachs disease | 2 | ||
| Chromosomal deletions/duplications | 2 | Meckel syndrome | 2 | ||
| Other | 14 | Other | 15 | ||
Discussion
Our data show that including PGT-A analysis results in significantly fewer embryos appropriate for transfer in autosomal recessive and X-linked conditions and is suggestive for the same outcome in autosomal dominant conditions. It is expected that completing two genetic analyses would result in fewer appropriate embryos than completing one analysis, for any inheritance pattern, but it is plausible that the difference is less significant for the autosomal dominant and X-linked groups than for the autosomal recessive group. In a recessive disease, one would expect 75% of outcomes to be suitable for transfer via PGT-M, compared to 50% for a dominant condition. This would mean that embryos tested for a recessive condition have a greater likelihood to even proceed to PGT-A. Future research should retrospectively examine a similar population and determine how maternal age impacts the frequency of embryos appropriate for transfer, i.e., at what age does the risk of aneuploidy surpass the risk of affectedness by monogenic disease? In our study, more embryos in the autosomal recessive group are aneuploid and unaffected by monogenic disease than are affected by monogenic disease and euploid. PGT-A analysis reduces the pool of embryos suitable for transfer in all groups, though may have the greatest impact on when testing for an autosomal recessive condition as for all ages of women the embryo may be at a greater risk of aneuploidy than of monogenic disease. For autosomal dominant and X-linked conditions, a future study could explore at which age aneuploidy becomes more likely than affectedness.
Our study explored only the likelihood that an embryo would be appropriate for transfer. Therefore, a future study should obtain pregnancy outcome data to determine ongoing pregnancy rates and live birth rates for embryos in which PGT-M was performed. A recently published study found that including PGT-M with PGT-A decreases the number of embryos appropriate for transfer compared to PGT-A only, but found that ongoing pregnancy rates per embryo transfer were consistent in both groups [24]. A future study could expand on these researchers’ findings to examine and report ongoing pregnancy rates for each inheritance pattern when both PGT-A and PGT-M are performed.
Including PGT-A analysis results in fewer embryos appropriate for transfer, but not including PGT-A analysis means that the ploidy status is unknown. Some studies suggest including PGT-A analysis as standard clinical practice [25, 26], though the clinical utility is dependent on maternal age. We did not incorporate oocyte age in our data analysis beyond the mean in each group; this should be performed in a future study. 96.8% of patients in our study elected to include PGT-A analysis. Data regarding the country of residence for patients in this study was not collected; some healthcare systems do not offer PGT-A analysis due to its controversy regarding clinical utility and medical necessity. Similarly, many insurance companies cover PGT-M but will not cover PGT-A, so insurance coverage may impact this decision. Because the cost of IVF-PGT is dependent on the number of samples analyzed, insurance policies and socioeconomic status could also impact the type of testing (serial or simultaneous) chosen by those who do include PGT-A. A future study should collect demographic data and explore patients’ decision-making process regarding the inclusion of PGT-A and type of testing.
Explanations for abnormal results extend beyond PGT-M or PGT-A abnormal. PGT-M is based on linkage analysis, so if recombination occurs near the loci of interest, the affectedness may not be able to be determined. Additionally, issues in interpretation of results may be due to insufficient DNA quality or concentration. Embryos that fall into this category are classified as “no result.” These results are not representative of the genetic and chromosomal status of the embryo, as insufficient quantity/quality of DNA may be caused by several technical factors. Embryos with no results may be rebiopsied for another attempt at analysis. As PGT-M and PGT-A testing is performed using separate platforms, it is possible that results are available for one status and not the other. The “other” sections in Fig. 2 includes embryos found to be chromosomally abnormal via PGT-M, recombinant, or “no result.” When PGT-A was performed with PGT-M, 7% of AR embryos had an “other” result, while 9% of AD and X-linked embryos had an “other” result. These values could be reviewed with patients alongside the expected rate of affectedness and expected rate of aneuploidy to calculate a more individualized likelihood of finding a suitable embryo for transfer.
Limitations
This study is not generalizable to embryos tested outside of CooperGenomics. The portion of patients who elected to do PGT-M only in this study is not representative of the entire sample, as these patients were hand-selected for inclusion based on the research question. Most women elected to include PGT-A analysis. The goal of forty cycles was unable to be met for any of the three PGT-M only categorical groups; these small sample sizes, especially with only 9 cycles in X-linked-PGT-M only, may play a role in the p-values that do not reach statistical significance. Demographic data such as maternal BMI and infertility diagnoses was not collected. The cycles analyzed in this study are representative of embryos created by each patient’s fertility clinic; differences in euploidy rates, given who performed each biopsy, are unable to be accounted for [27].
When a patient’s clinic opts out of receiving mosaic information, embryo samples with < 40% abnormal cells are reported as euploid. However, if mosaic information is reported, embryo samples that have 20–40% abnormal cells are classified as low-level mosaic and unsuitable for transfer. Both patients who opted into receiving mosaic information, and those who opted out, were included in this study.
Due to the nature of serial testing, some results were not able to be obtained. For example, if an embryo belonging to a patient who elected serial testing with PGT-A first was found to be aneuploidy, PGT-M was not performed. Because the “charts” in this study were complete cycles, rather than individual embryos, these embryos were included in the study as they were part of cycles in which a risk for monogenic disease was presented and PGT-M was intended to be performed. The authors understand that not having PGT-M results for these embryos is a limitation of the study.
Until recently, a limitation of next-generation sequencing for PGT-A was that haploidy or 69,XXX triploid karyotypes could not be identified. Embryos which were identified as chromosomally abnormal via PGT-M analysis, but not haploid or triploid via PGT-A analysis (5 embryos), are triploid or haploid. Recent advances in technology allow PGT-A testing to identify haploidy and 69,XXX triploid karyotypes, so if these embryos were sampled now, result interpretation may be different.
Conclusion
CooperGenomics has an abundance of internal data about the rate of aneuploidy for patients who undergo PGT-A; however, this is one of the first studies exploring the rate of affectedness for PGT-M patients. Our research expands beyond anecdotal evidence and allows for the likelihood of having at least one embryo deemed appropriate to consider for transfer to be determined, beyond standard Mendelian calculations. These preliminary estimates should be confirmed with a larger dataset to allow for them to be utilized in pre-test counseling for couples pursuing IVF-PGT. Additionally, this research determined the differences in the frequency of available embryos in the PGT-M alone group versus the PGT-M with PGT-A group and provides an average number of transferable embryos in each of these groups for each inheritance pattern. Multiple egg retrieval cycles may be necessary to identify an embryo that is euploid and/or unaffected by monogenic disease. Informed consent and discussion of potential outcomes is critical when counseling for preimplantation genetic testing, though this process is not yet standardized. The results of this study provide additional data points that, if replicated in a larger study, may be integrated into the informed consent process and can be a tool to help manage patient expectations of outcomes before initiating a cycle start.
Acknowledgements
This work was conducted by researcher ES to fulfill Master’s degree requirements for Case Western Reserve University’s Genetic Counseling Training Program in Cleveland, Ohio. The authors would like to thank Kevin Cavanagh, PhD, for his contributions to the statistical analyses performed in this study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simpson JL, Kuliev A, Rechitsky S. Overview of preimplantation genetic diagnosis (PGD): historical perspective and future direction [Internet]. Humana Press, New York, NY; 2019 [cited 2019 Feb 26]. p. 23–43. Available from: 10.1007/978-1-4939-8889-1_2 [DOI] [PubMed]
- 2.Won SY, Kim H, Lee WS, Kim JW, Shim SH. Pre-implantation genetic diagnosis and pre-implantation genetic screening: two years experience at a single center. Obstet Gynecol Sci [Internet] 2018 [cited 2019 Feb 3];61(1):95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29372155 [DOI] [PMC free article] [PubMed]
- 3.Brezina PR, Anchan R, Kearns WG. Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J Assist Reprod Genet [Internet] 2016 [cited 2019 Mar 11];33(7):823–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27299602 [DOI] [PMC free article] [PubMed]
- 4.Zimmerman RS, Eccles J, Jalas C, Treff NR, Scott RT. Molecular testing for preimplantation genetic diagnosis of single gene disorders [Internet]. Humana Press, New York, NY; 2019 [cited 2019 Feb 26]. p. 61–71. Available from: 10.1007/978-1-4939-8889-1_4 [DOI] [PubMed]
- 5.Treff NR, Zimmerman RS. Advances in preimplantation genetic testing for monogenic disease and aneuploidy. Annu Rev Genomics Hum Genet [Internet] 2017;18(1):189–200. doi: 10.1146/annurev-genom-091416-035508. [DOI] [PubMed] [Google Scholar]
- 6.Geraedts J. Healthy children without fear: reproductive options for patients or couples carrying inherited diseases. EMBO Rep [Internet] 2017 [cited 2019 Mar 11];18(5):666–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28396574 [DOI] [PMC free article] [PubMed]
- 7.Masbou AK, Friedenthal JB, McCulloh DH, McCaffrey C, Fino ME, Grifo JA, et al. A comparison of pregnancy outcomes in patients undergoing donor egg single embryo transfers with and without preimplantation genetic testing. Reprod Sci [Internet] 2018 [cited 2019 Mar 11];193371911882047. Available from: 10.1177/1933719118820474 [DOI] [PubMed]
- 8.Kim TG, Neblett MF, Shandley LM, Omurtag K, Hipp HS, Kawwass JF. National mosaic embryo transfer practices: a survey. Am J Obstet Gynecol [Internet] 2018 [cited 2019 Mar 11];219(6):602.e1–602.e7. Available from: https://www.sciencedirect.com/science/article/pii/S0002937818308226?via%3Dihub [DOI] [PubMed]
- 9.Fragouli E, Alfarawati S, Spath K, Babariya D, Tarozzi N, Borini A, et al. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid–aneuploid blastocysts. Hum Genet [Internet] 2017;136(7):805–19. doi: 10.1007/s00439-017-1797-4. [DOI] [PubMed] [Google Scholar]
- 10.Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, et al. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril [Internet] 2018 [cited 2019 Feb 3];109(1):77–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29191449 [DOI] [PubMed]
- 11.Vaz-de-Macedo C, Harper J. A closer look at expanded carrier screening from a PGD perspective. Hum Reprod [Internet] 2017 [cited 2019 Jun 17];32(10):1951–6. Available from: http://academic.oup.com/humrep/article/32/10/1951/4097721/A-closer-look-at-expanded-carrier-screening-from-a [DOI] [PubMed]
- 12.Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M, Wells D, Gordon T, Stock-Myer S, Willman S, STAR Study Group Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir VA, Darmon SK, Albertini DF, Barad DH, Gleicher N. Effectiveness of in vitro fertilization with preimplantation genetic screening: a reanalysis of United States assisted reproductive technology data 2011–2012. Fertil Steril [Internet] 2016 [cited 2019 Mar 13];106(1):75–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26952783 [DOI] [PubMed]
- 15.Murphy LA, Seidler EA, Vaughan DA, Resetkova N, Penzias AS, Toth TL, et al. To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A). Hum Reprod [Internet] 2019 [cited 2019 Mar 11];34(2):268–75. Available from: https://academic.oup.com/humrep/article/34/2/268/5219186 [DOI] [PubMed]
- 16.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Electronic address: ASRM@asrm.org; Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018 Mar;109(3):429–436. 10.1016/j.fertnstert.2018.01.002 [DOI] [PubMed]
- 17.Goldman KN, Nazem T, Berkeley A, Palter S, Grifo JA. Preimplantation genetic diagnosis (PGD) for monogenic disorders: the value of concurrent aneuploidy screening. J Genet Couns [Internet] 2016;25(6):1327–37. doi: 10.1007/s10897-016-9975-4. [DOI] [PubMed] [Google Scholar]
- 18.Brezina PR, Kutteh WH, Bailey AP, Ke RW. Preimplantation genetic screening (PGS) is an excellent tool, but not perfect: a guide to counseling patients considering PGS. Fertil Steril [Internet] 2016 [cited 2019 Mar 11];105(1):49–50. Available from: https://www.sciencedirect.com/science/article/pii/S0015028215020063?via%3Dihub [DOI] [PubMed]
- 19.Dahdouh EM, Balayla J, García-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril [Internet] 2015 [cited 2019 Mar 13];104(6):1503–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26385405 [DOI] [PubMed]
- 20.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril [Internet] 2014 [cited 2019 Mar 13];101(3):656–663.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24355045 [DOI] [PubMed]
- 21.Toft CLF, Ingerslev HJ, Kesmodel US, et al. A systematic review on concurrent aneuploidy screening and preimplantation genetic testing for hereditary disorders: what is the prevalence of aneuploidy and is there a clinical effect from aneuploidy screening? Acta Obstet Gynecol Scand. 2020;99:696–706. doi: 10.1111/aogs.13823. [DOI] [PubMed] [Google Scholar]
- 22.Brezina PR, Kutteh WH. Clinical applications of preimplantation genetic testing. BMJ [Internet] 2015 [cited 2019 Mar 18];350:g7611. Available from: https://www.bmj.com/content/350/bmj.g7611 [DOI] [PubMed]
- 23.Viotti M, Victor AR, Barnes FL, Zouves CG, Besser AG, Grifo JA, Cheng EH, Lee MS, Horcajadas JA, Corti L, Fiorentino F, Spinella F, Minasi MG, Greco E, Munné S. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil Steril. 2021;115(5):1212–1224. doi: 10.1016/j.fertnstert.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Insogna IG, Lanes A, Dobson L, Ginsburg ES, Racowsky C, Yanushpolsky E. Blastocyst conversion rate and ploidy in patients with structural rearrangements. J Assist Reprod Genet. 2021 doi: 10.1007/s10815-021-02131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalfoglou AL, Scott J, Hudson K. PGD patients’ and providers’ attitudes to the use and regulation of preimplantation genetic diagnosis. Reprod Biomed Online [Internet] 2005 [cited 2019 Mar 18];11(4):486–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16274615 [DOI] [PubMed]
- 26.McGowan ML, Burant CJ, Moran R, Farrell R. Patient education and informed consent for preimplantation genetic diagnosis: health literacy for genetics and assisted reproductive technology. Genet Med [Internet] 2009 [cited 2019 Mar 18];11(9):640–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19652605 [DOI] [PMC free article] [PubMed]
- 27.Munné S, Alikani M, Ribustello L, Colls P, Martínez-Ortiz PA, McCulloh DH, Referring Physician Group Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32(4):743–749. doi: 10.1093/humrep/dex031. [DOI] [PubMed] [Google Scholar]