Abstract
The aims of this study were to analyze prospectively and comparatively the peri-implant bone crest levels, bone density, stability and success rate of implants with different surface treatments in human edentulous mandibles. Twenty edentulous patients were selected. Four different implants were placed between the mental foramen. Four groups were evaluated: (1) laser-modified surface (LASER), (2) surface modified by laser with deposition of apatites (LASER + HA), (3) surface modified by double acid etching (ACID, Implacil De Bortoli) and (4) surface modified by sandblasting and acid etching (SLActive®, Straumann). Clinical, radiographic, resonance frequency and tomographic analyses were used. After 4 months, mandibular fixed implant prostheses were installed. Clinical and radiographic analyses were performed at times T0 (immediately after implant placement), T1 (15 days), T2 (30 days), T3 (60 days), T4 (90 days), T5 (120 days), T6 (180 days) and T7 (360 days), post-implant placement. The resonance frequency analysis (RFA) was measured at T0, T4, T6 and T7. The tomographic analysis was performed at T0, T4 and T7. In the radiographic bone density analysis, a statistical difference was found between the SLActive® and LASER + HA groups at T4 (p < 0.05). Statistical differences were observed in RFA at T4 (90 days), between the SLActive® and LASER groups (p < 0.05) and between the SLActive® and LASER + HA groups (p < 0.05). At T6 and T7, statistical differences were found between the SLActive® group and all other implant surfaces (p < 0.01). The experimental surfaces analyzed showed encouraging positive outcomes compared to those of the SLActive® surface. Long-term follow-up should be performed to confirm these results.
Keywords: Dental implants, Imaging, Lasers, Resonance frequency analysis, Surface
Introduction
The treatment with dental implants has become more and more predictable, based on the osseointegration phenomenon, which involves the establishment of a direct relation of the implant with the alveolar bone, without the interposition of connective tissue [1–3].
Potential candidates for implant rehabilitation with completely edentulous jaws may be interested in receiving a fixed prosthesis as opposed to a removable overdenture. During the treatment planning for implant fixed rehabilitation, several factors should be considered, such as location and number of implants to be placed, restorative design including material, opposing arch and occlusal pattern. Also, guidelines for the treatment of edentulous patients with implants should include consistent clinical and radiographic evaluation criteria for an accurate outcome assessment. Fixed implant restorations are totally implant supported, with no transference of load to denture-bearing areas, thus avoiding the possibility of further resorption associated with tissue-bone prostheses [4].
The occlusion of choice is a controversial topic in conventional complete denture treatments [5, 6]. In the present study, the balanced schemes were performed because, among other factors, they improved the patient’s satisfaction over time [7].
The failure rates and loss of implants have been attributed to poor-quality bone beds which, consequently, increase the possibility of anchorage loss [8]. The primary determinants of stability are the level of primary bone contact, which is mainly influenced by trabecular bone quality, as well as implant length, geometry and surface, at the time of implant placement [9].The physical and chemical properties of the implant surfaces can increase the amount of bone–implant contact and thus interfere in the biological responses and subsequent repair at the bone–implant interface [10–12].
Surface modifications can be created using different methods; subtractive methods use acid treatments with or without sandblasting with titanium oxide (TiO2) or aluminum oxide (Al2O3) and laser radiation [13, 14]. Additive methods include coatings with titanium plasma spray (TPS) and hydroxyapatite (HA), as well as coatings with HA and other calcium phosphates [11, 14]. Studies evaluating the topographic and physico-chemical modifications of dental implant surfaces in vivo and in vitro are numerous [2, 10–13, 15–18].
Several methods have been proposed to evaluate implant stability, such as insertion and removal torque [9, 13], percussion sound (Perio-Test®) [19] and RFA [20, 21].
To evaluate the success rate of osseointegration of different implant surfaces in humans, resonance frequency and cone beam computed tomography (CBCT), the two most frequently used methods of analysis, are used [2]. RFA consists of measuring the stability of the implant using a device via ultrasound (Ostell Mentor®) [22]; however, the use of CBCT allows a three-dimensional evaluation around the bone–implant region. Therefore, it is possible to calculate peri-implant bone loss using CBCT [2, 23, 24]. These methods can be performed at any time during the dental implant therapy, because they present neither invasive nor destructive characteristics [20, 21].
When considering the importance of implant surface treatment to promote osseointegration in shorter periods of time, this prospective and comparative clinical study in humans is relevant. The aims of this study were to analyze prospectively and comparatively the peri-implant bone crest levels, bone density, stability, and success rate of implants with different surface treatments in human edentulous mandibles.
Materials and Methods
This study was approved by the Research Ethics Committee of the Dentistry Course of the University of Araraquara (UNIARA, SP, Brazil), protocol #580.879, (Certificate of Presentation for Ethical Appreciation: 24020613.8.0000.5383) and was conducted at the Dental Clinic of UNIARA. All patients were informed of the study protocol and agreed to sign the consent form prior to being enrolled in the study. All patients were treated according to the principles embodied in the World Medical Association Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2013 (World Medical Association) [25].
Patient Inclusion Criteria
Twenty patients who had no systemic involvement were selected. They were nonsmokers, totally edentulous on the upper and lower jaws, and showed bone availability verified by digital panoramic radiography. Digital panoramic radiography was used because it is a low-cost imaging examination with less biological risk. Also, it is considered an acceptable method to assess the available bone height in the mandible [26, 27]. A minimal bone height (measured from the bone crest to the mandibular base in the region between the mental foramen) of 15 mm was a requisite.
Patient Exclusion Criteria
Patients who had any systemic, oral or parafunctional habits.
Surgical Procedure
Prior to the surgical procedure, 1 g of amoxicillin (Generic Drug—EMS®—São Paulo, SP, Brazil) and 4 mg of dexamethasone (Generic Drug—EMS®—São Paulo, SP, Brazil) were administered to prevent infection and for modulation of postoperative edema, respectively.
After intraoral antisepsis with 0.12% chlorhexidine gluconate and extraoral with 2% chlorhexidine gluconate, the patient was subjected to anesthetic block of the mental nerves and local infiltration anesthesia of the surrounding tissues. Next, an incision was made on the alveolar ridge, from the first molar on one side to the first molar on the contralateral side followed by a relaxing incision in the central region (to promote a larger flap amplitude). Subsequently, mucoperiosteal detachment was performed to locate the mental foramen bilaterally.
Regularization of the alveolar ridge was performed with a straight handpiece in low rotation tipped with a maxicut drill under intense irrigation with sterile physiological saline solution (0.9%). The drilling techniques for the placement of the dental implants followed the guidelines recommended by the manufacturers Implacil De Bortoli (São Paulo, SP, Brazil) (implants with surface modified by double acid etching surfaces [ACID, commercially available; fluted implant], surface modified by laser beam [LASER; fluted implant], surface modified by laser beam with deposition of apatites by the biomimetic method [LASER + HA; fluted implant] and Straumann [implants with surface modified by sandblasting and acid etching [SLActive®; non-fluted implant]). The drilling techniques were performed under intense irrigation with sterile physiological saline solution (0.9%). The study was carried out with the aim of evaluating the effects of laser treatment with apatite deposition by the biomimetic method (LASER + HA) and the SLActive® (Straumann) surface.
Four implants (4 mm in diameter and 10 mm in length, Cone Morse connection) were distributed between the mental foramen with one implant of each surface per patient. The implants were manually installed using a torque wrench, with a 40 N.cm torque, following the guidelines recommended by both manufacturers at the bone crest level. The distribution of the implants was assigned by drawing lots. In addition, 4 cover screws were installed on these implants. The flap was repositioned and sutured with nylon 4.0 (Nylon 4.0, Ethicon, Johnson, São José dos Campos, SP, Brazil).
The postoperative drug protocol included antibiotic therapy (amoxicillin 500 mg—Generic drug—EMS®—São Paulo, SP, Brazil), a non-steroidal anti-inflammatory drug (nimesulide 100 mg—Generic drug—MEDLEY® Group—Campinas, SP, Brazil), an analgesic (dipyrone sodium 500 mg—Generic drug—MEDLEY Group®—Campinas, SP, Brazil) and mouthwash with chlorhexidine gluconate 0.12%. All patients received standardized instructions to perform oral hygiene after the surgical procedure.
After a 3-month period (T4) following implant placement, the implant sites were reopened to provide proper keratinized soft tissue around the implants, and the healing caps were placed. Before the placement of the healing caps, clinical and resonance frequency analyses were performed. After the placement of the healing caps, radiographic and tomographic analyses were performed.
Prosthetic Phase
The rehabilitation procedures for implant-supported prostheses started fifteen days after the placement of the healing screws. The prostheses were installed 4 months after the placement of the dental implants. Intermediate abutments were installed for screw-retained implant-supported prostheses, microunit type, in the implants of Groups 1 (LASER), 2 (LASER + HA) and 3 (ACID, Implacil de Bortoli) with a torque of 20 N.cm and, straight multiband abutment, for Group 4 (Straumann, Villeret, Switzerland) with a torque of 35 N.cm according to the manufacturer's indication. After the installation of the abutments, the transfer impression was carried out, using addition silicone (Virtual, Ivoclar Vivadent, 3 M, Barueri, SP, Brazil) and the inter-occlusal registers with the multifunctional guide using Pattern resin (GC, Alsip, IL, USA).
After 7 days of molding, the metallic infrastructure tests were confirmed, and their adaptation was confirmed by digital periapical radiographs (Gendex, Dentisply Int., Chicago, IL, USA). In addition, the occlusion of the wax plate on the metallic infrastructure was evaluated. One week later, the mandibular fixed implant prosthesis was installed with 10 N.cm screw tightening. Final occlusal adjustments were performed, and sealing screws were placed.
In order to prepare the mandibular fixed implant prosthesis, the healing screws were removed (after a 15-day healing), the intermediate abutments were installed and, later, the transferors were placed on their respective abutments. Following the adjustment of the multifunctional guide to obtain the intermaxillary relations and its union with the transferents using acrylic resin, addition silicone at light density was injected into the guide for carrying out the transfer impression. After the construction of the metallic infrastructure, the clinical test was checked to verify its adaptation. Subsequently, the clinical test of the occlusion of the wax teeth was performed on the metallic infrastructure. The final installation of the protocol-type prosthesis was performed after its acrylization, 4 months after implant placement.
Clinical Analysis
The following parameters were evaluated at T0-T7: pain or paresthesia in the implanted area, infection or peri-implant suppuration, implant mobility and radiolucency at the bone–implant interface.
Resonance Frequency Analysis (RFA)
The stability of the implants was evaluated using the Osstell® (Integration Diagnostics AB, Göteborg, Sweden) noninvasive device that allows clinical measurement of stability of an implant through the evaluation of the resonance frequency at T0, T4, T6 and T7.
For the measurement of each implant the SmartPeg (transducer) was coupled to the implant platform using a torque of 4 to 8 N.cm. Four measurements were then taken with the measuring probe in the mesio-distal, disto-mesial, bucco-lingual and linguo-buccal directions. These measurements were taken in triplicate, and the mean was recorded. These values were transferred to a specific software (Osstell™ data manager) for later statistical analysis. A significance level of 5% was adopted. The values of the measurement, the date and time of the measurement, the type of transducer, the serial number, the calibration value and the length and diameter of the implant were recorded.
Digital Periapical Radiographic Analysis
For the radiographic evaluation of the implants during the different time periods of analysis, radiographic measurements of the implant region (one X-ray for each two implants) were taken by the parallelism technique using the digital radiographic sensor (Digital Sensor, Digora Optime Soredex, Fin). In the image acquisition, no filter or manipulation of the image was applied.
The standardization of radiographs was obtained by using the same exposure time, X-ray apparatus geometric position and acrylic resin device. The radiographic positioner was stabilized in the patient's mouth by the application of acrylic resin (Pattern Resin, GC, Tokyo, Japan) at the base and in the tooth of the total superior denture antagonist to the implant region, allowing the reproducibility of X-rays. Therefore, two acrylic resin fins were used for each patient (one fin for each two implants). All X-rays were performed with the same X-ray apparatus following the same exposure parameters, with 70 kvp, 7 mA and 0,32 s, at T0-T7.
Measurement of bone height to evaluate possible (peri-implant) marginal bone loss was taken at the distal and mesial end of each implant using the uppermost portion of the implant platform as a reference point until bone bilaterally contacted the implant body (i.e., the highest point of the marginal bone crest) [26]. This was done using the latest version of the image analysis software, ImageJ. These analyses were performed by an experienced investigator and were blinded and calibrated (ABSS). The values obtained were tabulated and stored for further statistical analysis. Also, the analyses were repeated 3 times.
Tomographic Analysis
In order to measure the level of the peri-implant bone crest in relation to the implant platform bilaterally (marginal bone loss), CBCT scans (40 s, 0.25 voxel, I-cat, Imaging Sciences International, PA, USA) were performed at T0, T4, T6 and T7.
Bone height measurements were taken on the buccal and lingual regions of each implant using the uppermost portion of the implant platform as a reference point until the bone bilaterally contacted the implant body (the uppermost point of the marginal bone crest) [22], in standardized cuts in the central region of each implant.
Figure 1 presents a flowchart depicting the workflow of the placed implants (LASER, LASER + HA, ACID and SLActive®), the installation of the mandibular fixed implant prostheses and the analysis performed (clinical, radiographic, RFA and tomographic) at each time of evaluation (T0-T7).
Fig. 1.
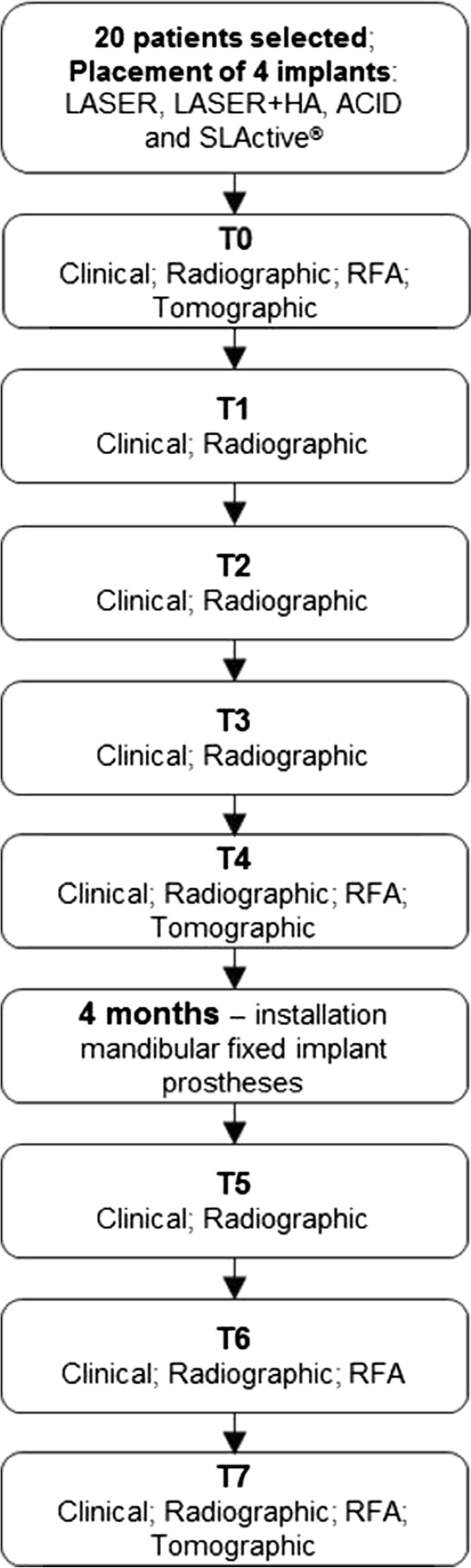
Study flowchart depicting the workflow of the placed implants
Statistical Analysis
D'Agostino & Pearson normality test was employed to evaluate the distribution of data. The quantitative data of the radiographic (bone loss and bone density), frequency of resonance (Osstell®) and tomography (bone density and linear loss) analyses were evaluated.
Kruskal–Wallis statistical test complemented by the Dunn test (nonparametric data analysis) was used to evaluate radiographic changes regarding bone density and resorption around the implants.
The ANOVA test complemented by Tukey's post-test (parametric data) was used to evaluate resonance frequency analysis off all installed implants in the different periods of evaluation.
GraphPad Prism 6 software (GraphPad Prism software, Inc., La Jolla, San Diego, CA, USA) was used to visualize the data and perform the statistical analysis. All data were expressed as mean ± standard error of the mean (SEM). All tests were applied at the 95% confidence level, adopting the significance level of 5%. Statistical analysis was performed comparing the 4 different surfaces in each of the evaluated periods.
Results
Twenty patients were initially included; however, one of them reported not having adapted to the use of the prosthesis and refused to continue the treatment. Consequently, he was excluded. The mean age of the 19 participants was 82.8 years, ranging 54–84, 11 were females, and 8 were males.
Considering the clinical and radiographic parameters used in the present study, it was possible to verify the absence of pain or paresthesia, absence of infection or suppuration, absence of implant mobility and radiolucency at the bone–implant interface in the 7-time period evaluated (T0-T7). A total of seventy-six implants were placed, and only one was lost due to lack of osseointegration being replaced without impairing the treatment.
In the analysis of the radiographic bone density, a statistically significant difference was found between the SLActive® and LASER + HA groups at T4 (p < 0.05) (Fig. 2). Statistical differences were observed in AFR at T4 (90 days), between the SLActive® and LASER groups (p < 0.05) and between the SLActive® and LASER + HA groups (p < 0.01). At T6 and T7, statistically significant differences were found between the SLActive® group and the other implant surfaces (p < 0.001) (Fig. 3).
Fig. 2.

Digital periapical radiographic bone density analysis. Quantification of the radiographic findings obtained at T0–T7 after implant placement for the 4 evaluated implant surfaces (*p < 0.05)
Fig. 3.

Resonance frequency analysis. Quantification of the RFA findings obtained at T0, T4, T6 and T7 after implant placement for the 4 evaluated implant surfaces (*p < 0.05; **p < 0.01; ***p < 0.001)
There were no statistically significant differences among the different groups and periods when considering the digital periapical radiographic analyses of linear bone loss (Fig. 4) and the tomographic analyses of bone density (Fig. 5) and linear bone loss (Fig. 6).
Fig. 4.
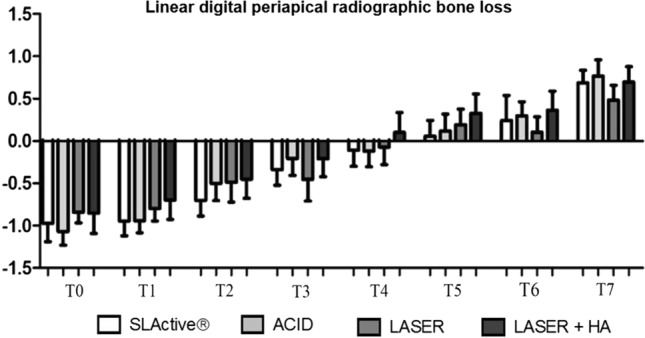
Linear digital periapical radiographic bone loss analysis. Quantification of the radiographic findings obtained at T0–T7 after implant placement for the 4 evaluated implant surfaces
Fig. 5.

Mesio-distal (top) and bucco-lingual (bottom) tomography density analysis. Quantification of the tomographic findings obtained at T0, T4, T6 and T7 after implant placement for the 4 evaluated implant surfaces
Fig. 6.

Linear tomographic bone loss analysis. Quantification of the tomographic findings obtained at T0, T4, T6 and T7 after implant placement for the 4 evaluated implant surfaces
Discussion
The physico-chemical and morphological properties of dental implant surfaces are essential in the early stages of osseointegration [2, 28, 29]. Considerable advances have been made in understanding the bone–implant surface interaction to increase the biological response around dental implants and in the development of new bioactive implant surfaces [3, 11, 13, 28–32].
Several studies have been developed, aiming to increase the clinical predictability and acceleration of bone healing using new technologies for physico-chemical and morphological modifications of the implant surfaces [11–13, 29–34]. To obtain these results, the implant surfaces are irradiated by laser beam followed by the deposition of calcium phosphates (CP; apatites) using the biomimetic method. It has been shown that there is an improvement in both layer stability and physico-chemical interaction between the coatings and the implant surface [12, 31].
Preclinical studies in rabbits have shown histologically that laser beam-modified surfaces and the addition of CP favored bone–implant interaction and increased bone formation around the implant coils. These surfaces demonstrated excellent biological behavior, favoring the osseointegration of dental implants when compared to conventional implants of machined surfaces modified by double acid etch [11, 29, 32].
Since these new surfaces were highly favorable to the osseointegration process in our preclinical studies, the objective of the present study was to evaluate the clinical behavior of these surfaces in total edentulous patients and to compare them with the most widespread, characterized and studied implant surface currently available in the market called SLActive® (Straumann). To this end, nineteen total edentulous patients were included, who randomly received 4 different implant surfaces distributed between the mental foramen in the mandible, as described: (1) laser-modified surface (LASER), (2) surface modified by laser with deposition of apatites (LASER + HA), (3) surface modified by double acid etching (ACID, Implacil de Bortoli) and (4) surface modified by sandblasting and acid etching (SLActive®, Straumann). All implants placed had the same lengths and diameters in order to reduce the variability among the groups.
After a 4-month healing period, the implants were reopened similarly to the beginning of the procedures for making the final prosthesis. Clinical, radiographic, tomographic and resonance frequency analyses were performed, and the patients were followed up for 1 year after the installation of the mandibular fixed implant prosthesis. Our results demonstrated that the surfaces developed in our laboratory were as efficient as the surfaces that are commercially available on the market. Of the 19 patients included and a total of 76 implants placed, there was only one implant lost, a clinical success rate above 98%.
To evaluate the progression of implant stability, RFA was performed in the present study at T0, T4, T6 and T7 after implant placement for the 4 evaluated implant surfaces by the implant stability coefficient (ISQ) [20]. The ISQ value is influenced by the stability of the implant inserted in the bone, implant geometry (length and width), bone density and osseointegration. Previous studies have shown that ISQ values ranging from 57 to 82 (mean value 67) represent great implant stability and a complete osseointegration process [20, 21, 35].
All the implants evaluated had ISQ values above 70 or higher in all the periods evaluated (Fig. 3), demonstrating high implant stability for all studied surfaces. However, the SLActive® surface demonstrated a statistically significant difference when compared to the other surfaces at T4, T6 and T7 post-implantation times. Although statistically significant, these differences do not influence the final clinical result since all surfaces presented satisfactory ISQ values for obtaining the osseointegration.
In the present study, both digital periapical radiographs and concomitant computed tomography scans were used to increase the accuracy of the analyses. Thus, evaluations of linear bone loss and periapical density were measured at different periods of the study (1 year after the installation of the final prosthesis).
Several longitudinal studies using radiographic images show that bone crest loss around implants is 0.9 to 1.6 mm during the first year of implant function. After the first year, there is an annual bone crest loss between 0.05 and 0.13 mm [36–40]. These findings were so consistent that measurements of 1.0 mm during the first year and 0.1 mm for all subsequent years became a criterion for the success of dental implants in general [39]. The results of the radiographic analyses did not show statistically significant differences in relation to the studied surfaces. As expected, a bone crest loss below 1 mm was evidenced for the different surfaces after one year of follow-up (Figs. 4 and 6). These results agree with previous studies and systematic reviews where linear bone loss is below 1 mm at the 12-month follow-up [36–40].
To increase the accuracy of radiographic measurements, the X-ray incidence angle was standardized using an individually created acrylic resin guide for occlusal records in all patients during all X-rays. This allowed an adequate measurement of the bone loss around the implants and the measurement of the radiographic density longitudinally. The radiographic results did not show significant differences among the groups, which demonstrate the reliability of the tested surfaces, since the SLActive® surface (positive control) is widely diffused as one of the best implant surfaces currently developed with a high degree of clinical success and reliability [41–43]. In addition, this study demonstrates the effectiveness and high predictability of the surfaces tested, especially the surfaces developed by our research group (LASER and LASER + HA), which were developed and tested in the last 10 years, evidenced by in vitro studies, preclinical and now, with this clinical study [11–13, 29–34].
It is important to highlight the limitations of the present study. The radiographic density changes can imply in either bone thickness or bone density alterations. The observed changes cannot be automatically allocated to either phenomena. CBCT scans have a high radiation burden and artifacts involved in imaging implants (e.g., scatter and blooming). Also, CBCT scans are not the ideal imaging examination, but it was a complement to observe the peri-implant bone tissue under a different aspect (bucco-lingual), since we performed the two-dimensional analysis using standardized periapical radiographs [44, 45]. Despite the above-mentioned limitations, it was possible to evaluate in a precise and reproducible way the images obtained through both the radiographic and the CBCT scans. More, the comparisons of the images over time were taken with accurate registration of follow-up images in order to standardize the measurements and evaluate the differences following a well-established methodology [46].
The inclusion and exclusion criteria of the selected patients in the present study were established to minimize possible bias that could interfere with the analyses performed. We sought to obtain a homogeneous and representative population of edentulous patients without systemic involvement and with adequate bone height (minimum of 15 mm) to receive 4 implants between the mental foramen and subsequent fixed implant-supported prostheses. Also, we were careful to perform new full dentures for all patients. From our point of view, the results obtained in the study showed no implications related to the selected patients.
Future studies are necessary to evaluate the effectiveness of the tested surfaces (LASER and LASER + HA) in periods shorter than 90 days, to verify the possible acceleration of the osseointegration process of the developed surfaces. Also, single (isolated) prosthetic implant rehabilitation should be considered to better understand the biological and biomechanical behavior of the studied surfaces.
In conclusion, within the limitations of this study, the experimental surfaces analyzed showed encouraging positive outcomes compared to those of the SLActive® surface. Long-term follow-up should be performed to confirm these results.
Acknowledgements
The authors gratefully acknowledge financial support from the São Paulo Research Foundation (FAPESP; 2014/05626-8). The authors are thankful to Implacil De Bortoli (São Paulo, SP, Brazil) for providing their implants and abutments.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Research Ethics Committee of the Dentistry Course of the University of Araraquara (UNIARA, SP, Brazil), protocol #580.879, (Certificate of Presentation for Ethical Appreciation: 24020613.8.0000.5383).
Patient Consent
All patients were informed of the study protocol and agreed to sign the consent form prior to being enrolled in the study. All patients were treated according to the principles embodied in the World Medical Association Helsinki Declaration of 1975 for biomedical research involving human subjects, as revised in 2013 (World Medical Association).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fernando P. S. Guastaldi and Thallita P. Queiroz have contributed equally to this study.
References
- 1.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20:172–184. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hamdan SH, Al-Hamdan K, Junker R, et al. Effect of implant surface properties on peri-implant bone healing: implant stability and microcomputed tomographic analysis. Int J Oral Maxillofac Implants. 2012;27:77–83. [PubMed] [Google Scholar]
- 3.Insua A, Monje A, Wang HL, et al. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J Biomed Mater Res A. 2017;105:2075–2089. doi: 10.1002/jbm.a.36060. [DOI] [PubMed] [Google Scholar]
- 4.Bedrossian E, Sullivan RM, Fortin Y, et al. Fixed-prosthetic implant restoration of the edentulous maxilla: a systematic pretreatment evaluation method. J Oral Maxillofac Surg. 2008;66:112–122. doi: 10.1016/j.joms.2007.06.687. [DOI] [PubMed] [Google Scholar]
- 5.Zhao K, Mai QQ, Wang XD, et al. Occlusal designs on masticatory ability and patient satisfaction with complete denture: a systematic review. J Dent. 2013;41:1036–1042. doi: 10.1016/j.jdent.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Peck CC. Biomechanics of occlusion–implications for oral rehabilitation. J Oral Rehabil. 2016;43:205–214. doi: 10.1111/joor.12345. [DOI] [PubMed] [Google Scholar]
- 7.Moradpoor H, Salari F, Ebadian B, et al. Patient satisfaction with occlusal scheme of conventional complete dentures: a randomised clinical trial (Part II) J Oral Rehabil. 2018;45:702–709. doi: 10.1111/joor.12660. [DOI] [PubMed] [Google Scholar]
- 8.Troiano G, Lo Russo L, Canullo L, et al. Early and late implant failure of submerged versus non-submerged implant healing: A systematic review, meta-analysis and trial sequential analysis. J Clin Periodontol. 2018;45:613–623. doi: 10.1111/jcpe.12890. [DOI] [PubMed] [Google Scholar]
- 9.Falco A, Berardini M, Trisi P (2018) Correlation between implant geometry, implant surface, insertion torque, and primary stability: in vitro biomechanical analysis. Int J Oral Maxillofac Implants 33:824–830 [DOI] [PubMed]
- 10.Guastaldi FP, Yoo D, Marin C, et al (2003) Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int J Biomater 1–6 [DOI] [PMC free article] [PubMed]
- 11.Queiroz TP, Souza FA, Guastaldi AC, et al. Commercially pure titanium implants with surfaces modified by laser beam with and without chemical deposition of apatite. Biomechanical and topographical analysis in rabbits. Clin Oral Implants Res. 2013;24:896–903. doi: 10.1111/j.1600-0501.2012.02471.x. [DOI] [PubMed] [Google Scholar]
- 12.Souza FA, Queiroz TP, Guastaldi AC, et al. Comparative in vivo study of commercially pure Ti implants with surfaces modified by laser with and without silicate deposition: biomechanical and scanning electron microscopy analysis. J Biomed Mater Res B. 2013;101:76–84. doi: 10.1002/jbm.b.32818. [DOI] [PubMed] [Google Scholar]
- 13.Faeda RS, Tavares HS, Sartori R, et al. Biological performance of chemical hydroxyapatite coating associated with implant surface modification by laser beam: biomechanical study in rabbit tibias. J Oral Maxillofac Surg. 2009;67:1706–1715. doi: 10.1016/j.joms.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Mistry S, Roy S, Jyoti Maitra N, et al. Safety and efficacy of additive and subtractive surface modification of Ti6Al4V endosseous implant in goat bone. J Mech Behav Biomed Mater. 2016;57:69–87. doi: 10.1016/j.jmbbm.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Braga FJC, Marques RFC, Almeida Filho E, et al. Surface modification of Ti dental implants by Nd:YVO4 laser irradiation. Appl Surf Sci. 2007;253:9203–9208. doi: 10.1016/j.apsusc.2007.05.048. [DOI] [Google Scholar]
- 16.Faeda RS, Spin-Neto R, Marcantonio E, et al. Laser ablation in titanium implants followed by biomimetic hydroxyapatite coating: Histomorphometric study in rabbits. Microsc Res Tech. 2012;75:940–948. doi: 10.1002/jemt.22018. [DOI] [PubMed] [Google Scholar]
- 17.Sisti KE, de Andrés MC, Johnston D, et al. Skeletal stem cell and bone implant interactions are enhanced by LASER titanium modification. Biochem Biophys Res Commun. 2016;473:719–725. doi: 10.1016/j.bbrc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Bressel TAB, de Queiroz JDF, Gomes Moreira SM, et al. Laser-modified titanium surfaces enhance the osteogenic differentiation of human mesenchymal stem cells. Stem Cell Res Ther. 2017;8:269. doi: 10.1186/s13287-017-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida KP, Delgado-Ruiz R, Carneiro LG, et al. Influence of drilling speed on stability of tapered dental implants: an ex vivo experimental study. Int J Oral Maxillofac Implants. 2016;31:795–798. doi: 10.11607/jomi.4485. [DOI] [PubMed] [Google Scholar]
- 20.Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol. 2000;2008(47):51–66. doi: 10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 21.Sennerby L, Andersson P, Verrocchi D, et al. One-year outcomes of Neoss bimodal implants. A prospective clinical, radiographic, and RFA study. Clin Implant Dent R. 2012;14:313–320. doi: 10.1111/j.1708-8208.2010.00273.x. [DOI] [PubMed] [Google Scholar]
- 22.Gabay E, Cohen O, Machtei EE. A novel device for resonance frequency assessment of one-piece implants. Int J Oral Maxillofac Implants. 2012;27:523–527. [PubMed] [Google Scholar]
- 23.Schouten C, Meijer G, Beucken J, et al. The quantitative assessment of peri-implant bone responses using histomorphometry and micro-computed tomography. Biomaterials. 2009;30:4539–4549. doi: 10.1016/j.biomaterials.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Hsu JT, Huang HL, Chang CH, et al. Relationship of three-dimensional bone-to-implant contact to primary implant stability and peri-implant bone strain in immediate loading: microcomputed tomographic and in vitro analyses. Int J Oral Maxillofac Implants. 2013;28:367–374. doi: 10.11607/jomi.2407. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez L, Nizamaldin Y, Combescure C, et al. Accuracy of vertical height measurements on direct digital panoramic radiographs using posterior mandibular implants and metal balls as reference objects. Dentomaxillofac Radiol. 2013;42:20110429. doi: 10.1259/dmfr.20110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vazquez L, Saulacic N, Belser U, et al. Efficacy of panoramic radiographs in the preoperative planning of posterior mandibular implants: a prospective clinical study of 1527 consecutively treated patients. Clin Oral Implants Res. 2008;19:81–85. doi: 10.1111/j.1600-0501.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 28.Smeets R, Stadlinger B, Schwarz F, et al (2016) Impact of dental implant surface modifications on osseointegration. Biomed Res Int 6285620 [DOI] [PMC free article] [PubMed]
- 29.Queiroz TP, de Molon RS, Souza FA, et al. In vivo evaluation of cp Ti implants with modified surfaces by laser beam with and without hydroxyapatite chemical deposition and without and with thermal treatment: topographic characterization and histomorphometric analysis in rabbits. Clin Oral Investig. 2017;21:685–699. doi: 10.1007/s00784-016-1936-7. [DOI] [PubMed] [Google Scholar]
- 30.Sisti KE, de Rossi R, Antoniolli AM, et al. Surface and biomechanical study of titanium implants modified by laser with and without hydroxyapatite coating, in rabbits. J Oral Implantol. 2012;38:231–237. doi: 10.1563/AAID-JOI-D-10-00030. [DOI] [PubMed] [Google Scholar]
- 31.Sisti KE, Piattelli A, Guastaldi AC, et al. Nondecalcified histologic study of bone response to titanium implants topographically modified by laser with and without hydroxyapatite coating. Int J Periodont Restor Dent. 2013;33:689–696. doi: 10.11607/prd.1151. [DOI] [PubMed] [Google Scholar]
- 32.Souza FA, Queiroz TP, Sonoda CK, et al. Histometric analysis and topographic characterization of cp Ti implants with surfaces modified by laser with and without silica deposition. J Biomed Mater Res B. 2014;102:1677–1688. doi: 10.1002/jbm.b.33139. [DOI] [PubMed] [Google Scholar]
- 33.Sisti KE, Garcia IR, Jr, Guastaldi AC, et al. Analysis of titanium surface irradiated with laser, with and without deposited of durapatite. Acta Circ Bras. 2006;21:57–62. doi: 10.1590/S0102-86502006001000013. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira NT, Guastaldi FP, Perrotti V, et al. Biomedical Ti-Mo alloys with surface machined and modified by laser beam: biomechanical, histological, and histometric analysis in rabbits. Clin Implant Dent R. 2013;15:427–437. doi: 10.1111/j.1708-8208.2011.00354.x. [DOI] [PubMed] [Google Scholar]
- 35.Queiroz TP, Aguiar SC, Margonar R, et al. Clinical study on survival rate of short implants placed in the posterior mandibular region: resonance frequency analysis. Clin Oral Implants Res. 2015;26:1036–1042. doi: 10.1111/clr.12394. [DOI] [PubMed] [Google Scholar]
- 36.Pontes AE, Ribeiro FS, da Silva VC, et al. Clinical and radiographic changes around dental implants inserted in different levels in relation to the crestal bone, under different restoration protocols, in the dog model. J Periodontol. 2008;79:486–494. doi: 10.1902/jop.2008.070145. [DOI] [PubMed] [Google Scholar]
- 37.Laurell L, Lundgren D. Marginal bone level changes at dental implants after 5 years in function: a meta-analysis. Clin Implant Dent Res. 2011;13:19–28. doi: 10.1111/j.1708-8208.2009.00182.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Formoso N, Rilo B, Mora MJ, et al. Radiographic evaluation of marginal bone maintenance around tissue level implant and bone level implant: a randomised controlled trial. A 1-year follow-up. J Oral Rehabil. 2012;39:830–837. doi: 10.1111/j.1365-2842.2012.02343.x. [DOI] [PubMed] [Google Scholar]
- 39.Kinaia BM, Shah M, Neely AL, et al. Crestal bone level changes around immediately placed implants: a systematic review and meta-analyses with at least 12 months' follow-up after functional loading. J Periodontol. 2014;85:1537–1548. doi: 10.1902/jop.2014.130722. [DOI] [PubMed] [Google Scholar]
- 40.Aimetti M, Ferrarotti F, Mariani GM, et al. Soft tissue and crestal bone changes around implants with platform-switched abutments placed nonsubmerged at subcrestal position: a 2-year clinical and radiographic evaluation. Int J Oral Maxillofac Implants. 2015;30:1369–1377. doi: 10.11607/jomi.4017. [DOI] [PubMed] [Google Scholar]
- 41.Filippi A, Higginbottom FL, Lambrecht T, et al. A prospective noninterventional study to document implant success and survival of the Straumann Bone Level SLActive dental implant in daily dental practice. Quintessence Int. 2013;44:499–512. doi: 10.3290/j.qi.a29611. [DOI] [PubMed] [Google Scholar]
- 42.Roccuzzo M, Bonino L, Dalmasso P, et al. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res. 2014;25:1105–1112. doi: 10.1111/clr.12227. [DOI] [PubMed] [Google Scholar]
- 43.van Velzen FJ, Ofec R, Schulten EA, et al. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: a prospective cohort study in 177 fully and partially edentulous patients. Clin Oral Implants Res. 2015;26:1121–1128. doi: 10.1111/clr.12499. [DOI] [PubMed] [Google Scholar]
- 44.Liedke GS, Spin-Neto R, da Silveira HED, et al. Factors affecting the possibility to detect buccal bone condition around dental implants using cone beam computed tomography. Clin Oral Implants Res. 2017;28:1082–1088. doi: 10.1111/clr.12921. [DOI] [PubMed] [Google Scholar]
- 45.Liedke GS, Spin-Neto R, da Silveira HED, et al. Accuracy of detecting and measuring buccal bone thickness adjacent to titanium dental implants-a cone beam computed tomography in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;126:432–438. doi: 10.1016/j.oooo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Schropp L, Stavropoulos A, Spin-Neto R, et al. Implant image quality in dental radiographs recorded using a customized imaging guide or a standard film holder. Clin Oral Implants Res. 2012;23:55–59. doi: 10.1111/j.1600-0501.2011.02180.x. [DOI] [PubMed] [Google Scholar]


