Abstract
Purpose
Diminished ovarian reserve (DOR) is associated with compromised fertility that affects approximately 10% of couples. Gene mutations are implicated in the pathogenesis of DOR. Here, we aimed to assess the clinical and genetic characteristics of two sisters with impaired fertility history. The two sisters showed DOR and suffered from recurrent pregnancy loss (RPL) in natural pregnancy and in vitro fertilization-embryo transfer (IVF-ET).
Methods
Whole exome sequencing (WES) was performed for the proband and pathogenic variants detected were validated by Sanger sequencing in all available family members. Minigene assay was performed to evaluate the impact of sequence variants on splicing effect.
Results
Two novel heterozygous variants on the HFM1 gene, c.1978-2A > C and c.2680 + 3_2680 + 4delAT, were observed in the two patients. The genotype of their parents was all heterozygous, while the unaffected sister and brother did not carry the variants. Both variants could produce alternative transcripts compared to wild-type counterparts, which might result in protein dysfunction.
Conclusion
Our results demonstrated that the pathogenic splicing variants in HFM1 are associated with DOR in these two sisters. Mutations in HFM1 may contribute to RPL and poor IVF-ET outcomes because of descending quality and quantity of oocytes. The study enriched the genetic defect spectrum of DOR and understanding of the roles of HFM1 in female reproduction.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-022-02580-3.
Keywords: Diminished ovarian reserve (DOR), Recurrent pregnancy loss (RPL), HFM1, Female infertility, IVF-ET
Introduction
Diminished ovarian reserve is characterized by impaired fertility outcomes and represents a major challenge in reproductive medicine [1]. So far, there is no uniformly accepted definition for DOR. In the ESHRE consensus, anti-mullerian hormone (AMH) and/or antral follicular count (AFC) is rather considered the diagnostic indicators for DOR. Thus, (i) any of the risk factors for poor ovarian responders and/or (ii) an abnormal ovarian reserve test (i.e., AFC < 5–7 follicles or AMH < 0.5–1.1 ng/ml) could be suggested [2]. DOR in advanced age is physiologic but pathologic at younger ages (< 40 years old) [3]. The etiologies of DOR includes genetic, autoimmune, idiopathic, and iatrogenic [4]. Gene mutations and polymorphisms can be associated with DOR, such as FMR1 and GDF9 [4]. Patients with DOR respond poorly to ovarian stimulation and have much lower pregnancy rates in IVF-ET cycles [5]. Moreover, patients with DOR appear to be a high risk in for fetal aneuploidy and may play an important role in unexplained RPL [6, 7].
Whereas many genetic causes of POI are well established, little is known about definitive gene mutations for most patients, especially about etiology of DOR [4]. HFM1 plays an important role in meiosis and is a candidate gene for premature ovarian insufficiency (POI) [8–11]. Although DOR is different from POI, it is possible in some patients that they may share several similar pathogenesis [4]. In this study, we used WES to investigate the genetic cause of poor pregnancy outcomes in a Chinese family. Consequently, two novel splice-site variants in HFM1, i.e., c.1978-2A > C and c.2680 + 3_2680 + 4delAT, in compound heterozygosity were identified in the patients. In vitro minigene assay proved that these variants had deteriorative effects on HFM1 mRNA splicing. HFM1 mutations caused DOR and might further affect the IVF-ET and pregnancy outcomes. To our knowledge, our findings, for the first time, present evidence of mutations in the HFM1 gene as a possible cause of RPL due to DOR.
Materials and methods
Ethical approval.
The study protocol and informed consent forms were approved by The Ethics Committee of Henan Provincial People’s Hospital with the approval number 2020–191. All participants signed informed consent forms during blood sample collection and analysis.
Study participants
The family was recruited from the Henan Provincial People’s Hospital, Zhengzhou, China. The studied sisters had normal uterine cavity and had no history of autoimmune disease, intrauterine infection, pelvic surgery, radiotherapy, or chemotherapy. DOR was diagnosed as described above [3]. RPL was diagnosed based on criteria recommended by ESHRE [12].
DNA extraction and WES
Genomic DNAs were extracted from peripheral blood using the EasyPure Blood Genomic DNA Kit (Beijing, China) following manufacturer’s instructions. The DNA of probands was analyzed through WES using HiSeq2500 sequencing platform (Illumina, San Diego, CA, USA) by the Novogene Bioinformatics Technology Co. Ltd (Tianjin, China). The raw sequencing data included 84,905,048 clean reads, the coverage of target region was 99.5%, and targets covered with at least 20 × depth were 94.2%. The candidate mutations met the following criteria: (i) the sharing by the two sisters; (ii) a low allele frequency (< 0.01) in the public human genome databases of the 1000 Genomes Project and the gnomAD (http://gnomad.broadinstitute.org/, (iii) deleterious variants valued by three predictors, including SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), and MutationTaster (https://www.mutationtaster.org). Splicing effect was scored by varSEAK (https://varseak.bio).
Variant validation and co-segregation analysis
Validation and co-segregation of HFM1 variants in the family were performed through PCR and Sanger sequencing. PCR primers included the following: HFM1 del-F: 5′-GCCTGCCTGTATTCAGTACCTACCA-3′ and HFM1 del-R: 5′-AGACAACTTTGAGGATTTGGGGAAA-3′ for c.2680 + 3_2680 + 4delAT and HFM1 T > G-F: 5′-GCTTGCCAAGAAATACCACTGAATA-3′ and HFM1 T > G-R: 5′-TAGGTATTATGGGAACATCAGGAAG-3′ for c.1978-2A > C. Sequencing reactions were conducted with BigDye Terminator v3.1 (Applied Biosystems, USA). Sequencing was performed on ABI 3130XL (Applied Biosystems, USA).
Construction of hybrid minigenes
A minigene splicing assay was performed to confirm whether the variants influenced splicing products. Amplicons covering HFM1 variant sites c.1978-2A > C and c.2680 + 3_2680 + 4delAT were generated by PCR and cloned into the pIN12 In45-In56 vectors between restriction sites Xba I and Xho I (Fig. S1). PCR and Sanger sequencing was used to confirm the successful construction of plasmids carrying wild-type or mutant insertions. The schematic diagram of the minigene construction is shown in Fig. S1. The vector pIN12 In45-In56 was kindly provided by Dr. Du Jing at Shanghai Institute of Planned Parenthood Research, Shanghai, China.
Plasmid transfection and characterization of minigene expression
HEK293T cells were cultured in complete Dulbecco’s Modified Eagle Medium (DMEM) media. Wild type or mutant minigene plasmids were transfected into HEK293T cells using polyethyleneimine (PEI) transfection reagent (Sigma, USA) following the manufacturer’s instructions. The vector pIN12 In45-In56 was used as control. All cells were harvested 48 h after transfection and the total RNAs were extracted using TRIzol (Thermo Fisher, USA). The total RNAs were reverse-transcribed into cDNA using the PrimeScript RT regent Kit (Takara, Japan). cDNAs were amplified and PCR products were confirmed by agarose electrophoresis. The PCR primers for confirmation of splicing effect included pIN12-132F: 5′-AGTGTGCTGGAATTCGAGCTCACTCT-3′ and pIN12-132R: 5′-CTCCGAACGCCAAGAGCCTAAGCTTA-3′. Notably, amplicon sequences were confirmed by direct Sanger sequencing.
In vitro fertilization-embryo transfer treatment
The proband underwent three controlled ovarian hyperstimulation (COH) cycles. In the first cycle, COH was performed after pituitary downregulation with a gonadotropin-releasing hormone agonist (GnRH-a) 0.1 mL per day (triptorelin acetate, Decapeptyl, Ferring, Germany), beginning from day 21 of the previous menstrual cycle. The GnRH-a dose changed to 0.05 mL per day from COH until the day of human chorionic gonadotropin (hCG) (Livzon Pharmaceutical, China) administration. Multifollicular development was achieved by daily injections of recombinant human FSH (225 IU) (Gonal F; Merck Serono, Italy) and human menopausal gonadotrophin (HMG 75 IU, Livzon Pharmaceutical, China). The second COH cycle was performed with the GnRH antagonist protocol. Ovarian stimulation started on menstrual cycle day (MC) 3 with HMG (300 IU), GnRh-antagonist (0.25 mg/d, Cetrotide, Merck Serono, Italy) was applied on the day 5 of COH until the day of hCG administration. For the third COH cycle, progesterone priming ovary stimulation (PPOS) protocol was applied. Medroxyprogesterone acetate (MPA, 10 mg/d, Shanghai Xinyi Pharmaceutical Co., China) and HMG (300 IU) were performed on MC 3 until the day of hCG administration. Oocyte maturation of all three COH cycles was triggered with 10,000 IU of hCG.
The proband’s sister (II-2) underwent two COH cycles. The first cycle was performed with early-follicular phase GnRH-a long protocol. GnRH-a (3.75 mg triptorelin, Diphereline®, Ipsen, France) was administered on MC 3, and Gonal F (300 IU) was applied 34 days later. For the second COH cycle, only Gn (HMG 300 IU per day) was applied. Oocyte maturation of the two COH cycles was also triggered with 10,000 IU of hCG.
All COH processes were monitored by transvaginal ultrasonography, and blood levels of E2, LH, and P. Transvaginal ultrasound-guided oocyte retrieval was performed 34–36 h after the administration of hCG. Fertilization of all aspirated oocytes was carried out in vitro by conventional insemination.
Endometrial preparation was performed by hormone replacement therapy protocol, specifically, oral estradiol valerate (3 mg twice per day, Progynova, BAYER, Germany) from MC 3 onward. The transfer of day 3 embryos was performed on the fifth day of progesterone (90 mg/day, Crinone gel; Merck Serono and dydrogesterone tablets, 10 mg twice per day, Abbott Healthcare, Netherlands) application.
Results
Clinical findings
The proband (II-3) and her sister (II-2) were diagnosed with DOR (Fig. 1A). Both of the sisters and their spouses had normal karyotypes. Menstrual cycle of the two sisters was 25–28 days. The proband (II-3) and her sister (II-2) were 24 and 27 years old, respectively. Bilateral ovarian sizes for the proband were 19 × 13 mm and 29 × 11 mm, for the proband’s sister were 25 × 14 mm and 25 × 11 mm. Ovarian reserve of two patients (II-3 and II-2) were assessed. Serum follicle-stimulating hormone (FSH) levels of the proband were tested twice in the menstrual phase, showing 22.91 and 18.80 mIU/mL, respectively; AMH was 0.37 ng/mL. Transvaginal ultrasound examination demonstrated decreased ovarian reserve; only one AFC was observed in each ovary. Two FSH measurements of her sister (II-2) showed 8.34 and 6.93 mIU/mL, respectively; AMH was 1.33 ng/mL. Transvaginal ultrasound examination showed four AFC in each ovary. Both proband (II-3) and her sister (II-2) suffered from RPL (Fig. 1A). The proband suffered three pregnancy losses in natural conception; two of which were biochemical, while embryo stopped developing at 7–8 weeks in the remaining one pregnancy. Her elder sister (II-2) experienced two pregnancy losses and one hydatid pregnancy in natural conception. For the two pregnancy losses, one was an abortion at 8 weeks, and one was a biochemical pregnancy loss. Their mother (I-2) had delivered four children and the fertile elder sister (II-1) spontaneously conceived and delivered two healthy girls and one boy; their brother (II-4) had normal semen quality.
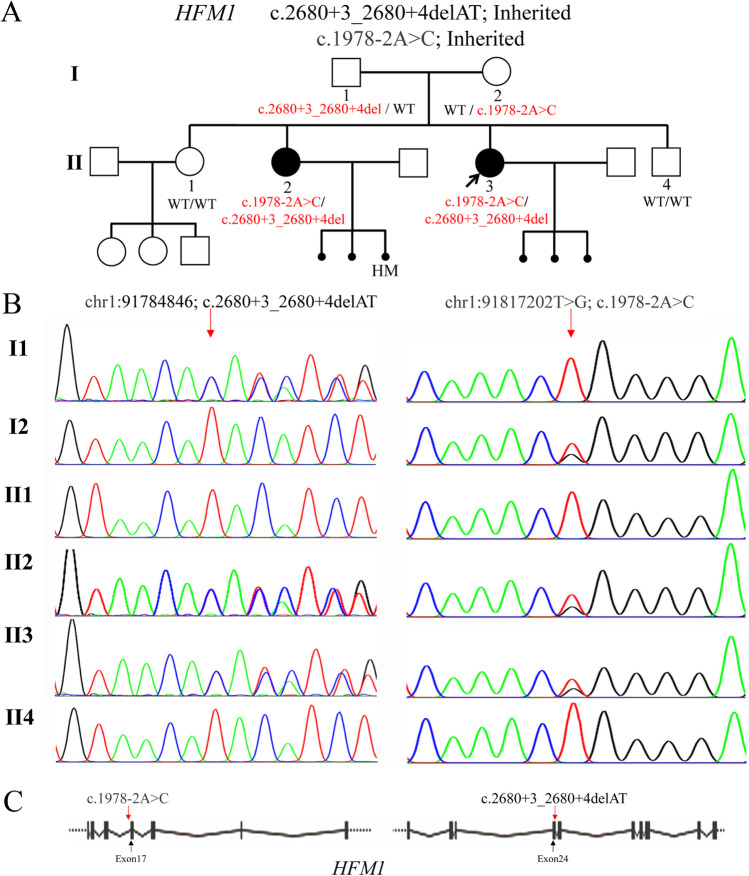
Fig. 1.
Pedigree and characterization of HFM1 gene variants in a Chinese family. A Two family members in the pedigree were diagnosed with DOR and RPL. The solid circle with an arrow indicates the proband. Solid circles indicate the affected family members. Squares and circles indicate males and females, respectively. B The two affected sisters were confirmed to be the compound heterozygosity, c.1978-2A > C and c.2680 + 3_2680 + 4delAT, on the HFM1 gene. Their parents (I1 and I2) were carriers, while both variants were not detected in fertile siblings (II1 and II4). C Localization of the two HFM1 variants identified in this study. HM, hydatidiform mole
Two likely pathogenic splicing variants were detected in the HFM1 gene
Whole-exome sequencing was performed in the proband. After variant filter process, two variants in HFM1 gene, c.2680 + 3_2680 + 4delAT and c.1978-2A > C (NM_001017975.4), were identified in the proband (Fig. 1A). Sanger sequencing revealed that both the proband and her sister (II-2) were compound heterozygotes. Their parents (I-1 and I-2) were heterozygous carriers, while the unaffected sister (II-1) and brother (II4) did not carry the variants (Fig. 1B). The c.1978-2A > C variant, which is rare in the human population, affects the canonical splice site (Fig. 1C). The c.2680 + 3_2680 + 4delAT variant locates in splice donor site of HFM1 intron 17 (Fig. 1C), which is also rare (GnomAD_EAS: 0.00026511; dbSNP: rs760336250). Both variants could be classified as likely pathogenic based on variant classification guidelines of the American College of Medical Genetics and Genomics (ACMG). These results demonstrated that RPL caused by the HFM1 gene might be inherited in autosomal recessive mode.
HFM1 variants caused intron splicing in an alternative mode
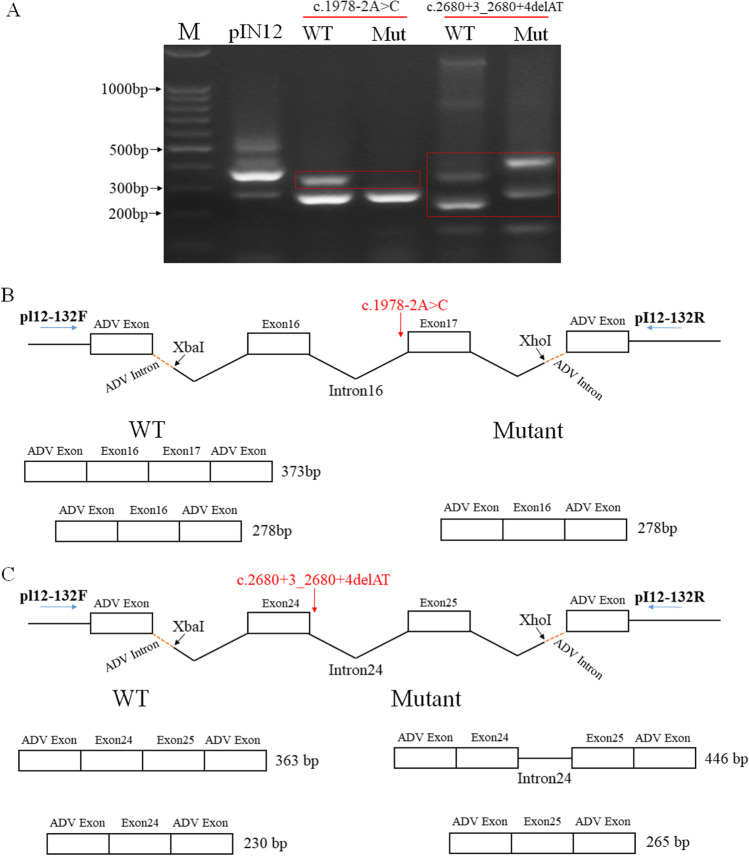
Using splice site effect prediction bioinformatics software varSEAK (https://varseak.bio), both HFM1 variants were predicted to be the loss of function for authentic splice site (class 5, top splicing effect in the varSEAK overall prediction), which might lead to exon skipping. A minigene assay was conducted to validate these prediction results. The vectors carrying wild-type and mutant HFM1 variants were chemically transfected into HEK293T cells as well as the control plasmid pIN12 In45-In56. A band of nearly 390 bp in the control transfection was found (Fig. 2A). For variant c.1978-2A > C, both wild-type and the mutant transcripts produced a small band, representing the splicing skipping of Exon17 (Fig. 2A and B). While wild-type construct tested a normal splice band of about 373 bp containing Exon16 and Exon17, no bands of predicted sizes were found in the mutant one. It suggests that c.1978-2A > C variant may interfere with the mRNA splicing causing abnormal splicing which retained intron16 (intron16: 862 bp, no amplicons due to PCR amplification preference) or degrading of mRNA. For the c.2680 + 3_2680 + 4delAT variant, there were alternative splicing showing two bands in both wild-type and mutant transcripts; however, the sizes were different (Fig. 2A). For the wild-type construct, two bands, 363 bp and 230 bp, represented transcripts comprising Exon24 + Exon25 and Exon24, respectively (Fig. 2A and C). Nonetheless, two bands, 446 bp and 265 bp, showed one transcript retaining intron 24, whereas one transcript comprised Exon25 with skipping of Exon24 (Fig. 2A and C). These findings show that the compound heterozygous HFM1 variants produced alternative transcripts different from the wild-type. A comparison of wild-type and splicing variants sequences identified that both of the variants resulted in amino acid changes (Fig. S2). Sequences of transcripts were supplied in Supplementary data.
Fig. 2.
Splicing effects of HFM1 variants c.1978-2A > C and c.2680 + 3_2680 + 4delAT. A Gel electrophoresis of RT-PCR products obtained from splicing reporter minigene assay for HFM1 mutations. B Splicing alterations in mutation c.1978-2A > C. C Splicing alterations in mutation c.2680 + 3_2680 + 4delAT. pIN12 was used as control vector
HFM1 mutations might be associated with poor IVF-ET outcomes
Both the proband (II-3) and her sister (II-2) attempted IVF-ET treatment because of infertility after pregnancy losses. They refused preimplantation genetic testing for aneuploidy (PGT-A) because of personal reasons. The proband underwent three IVF cycles with different protocols. In the first cycle, three oocytes were retrieved; all of 3 were MII oocytes. There were two normal 2PN zygotes developed to two day 3 embryos (9-cell/grade 2/10% fragments, 8-cell/grade 2/10% fragments). The two embryos were fresh-transferred to the uterus of which the endometrium thickness was 12 mm. The proband got single pregnancy after ET but the embryo stopped developing at 7 weeks. This is the fourth pregnancy loss happened after three pregnancy losses mentioned above. For the second and third cycles, three and three oocytes were retrieved, respectively. All 6 oocytes were MII. There were five normal 2PN zygotes and developed to four embryos (6-cell/grade 2/5% fragments, 10-cell/grade 2/10% fragments, 7-cell/grade 2/10% fragments, 9-cell/grade 2/15% fragments) at day 3. The four embryos were subjected to two frozen-thawed embryo transfer (FET) cycles. Both of the transfers resulted in implantation failure. The proband’s sister underwent two IVF cycles. There were five MII oocytes and two fertilized eggs in the first cycle. On day 3, only one zygote developed to available embryo (12-cell/grade 2/10% fragments). The embryo was transferred to the uterus fresh but failed to implant. For the second cycle, six MII oocytes were obtained. Five of 6 oocytes formed normal 2PN zygotes and developed to five embryos at day 3. She had two day 3 embryos (8-cell/grade 2/0% fragments, 11-cell/grade 2/0% fragments) transferred and the left three (11-cell/grade 2/5% fragments, 8-cell/grade 2/5% fragments, 7-cell/grade 2/10% fragments) were cultured to blastocyst stage. However, the sister did not get pregnant and all three embryos failed to form blastocyst.
Discussion
Diminished ovarian reserve presents clinically as decline in both oocyte quantity and quality. Pathologic DOR affects a significant number of young women with infertility and the etiology remains obscure. Different from physiologic age-related DOR, the etiological diagnosis of idiopathic pathologic DOR may ultimately depend on identification of genetic causes. Herein, we performed a genetic analysis on a family with DOR. We identified two novel compound heterozygous splicing variants of HFM1 gene in two siblings with DOR, history of RPL and poor IVF-ET outcomes. Both of the two HFM1 variants produced alternative transcripts different from the wild-type, suggesting that HFM1 might be a candidate gene for DOR.
HFM1 gene, comprising 39 exons mapped to human chromosome 1q22, encodes helicase for meiosis 1 (HFM1), a ATP-dependent DNA helicase. HFM1 is specifically expressed in germ-line cells [8]. Previous research revealed that HFM1 is implicated in meiotic homologous recombination and synapsis between homologous chromosomes [11]. Recent studies showed that HFM1 also participates in Golgi-associated spindle assembly and division in meiosis [9]. The knockout of Hfm1 in male mice showed that spermatogenesis was blocked at meiotic metaphase [13]. An oocyte-specific Hfm1-cKO mouse model revealed that the Hfm1-cKO mice exhibited decreased follicles in all stages, which was similar to DOR [9].
Mutations in HFM1 is reported to be a cause of premature ovarian failure, which is now commonly named as POI. Hence, HFM1 is also known as POF9 [9]. Previous studies revealed that compound heterozygous HFM1 mutations (c.3470G > A, c.1686-1G > C, c.2651 T > G, c.2206G > A, and c.3929_3930 delinsG) are related to POI inherited in recessive mode [10, 14]. Mutations in gene encoding proteins that regulate meiosis can result in autosomal recessive POI in humans [10]. In present case, the clinical manifestation of the proband was insufficient to diagnose POI but presented a remarkable DOR. The Practice Committee of ASRM has stated that DOR is distinct from POI [1]. However, it is possible in some patients that DOR and POI occur within a spectrum, as they share many etiologies and features [4, 15, 16]. With increasing age, the proband may finally be diagnosed with POI because the AMH value was only 0.37 ng/mL and one follicle in each ovary at her age of 24. Although the DOR was less pronounced in her sister (II-2), only 4 follicles existed in each ovary with an AMH of 1.3 ng/mL at the age of 27 years. The II-2 did not present the apparent DOR may be attributed to incomplete penetrance or the different degrees of expressivity in individuals. We suggest that the compound heterozygous splicing variants of HFM1 gene (c.1978-2A > C and c.2680 + 3_2680 + 4delAT) are the potential cause of DOR in the two sisters.
Patients with DOR underwent IVF will have fewer eggs and fewer embryos than patients with normal ovarian reserve. The pregnancy rates are lower in this category [1]. The two sisters totally underwent five IVF cycles and the average number of oocytes retrieved was 3 for the proband (II-3) and 5.5 for her sister (II-2). Both of the sisters failed to have one successful delivery from IVF-ET treatment. Except for one abortion, the embryos either failed to get implantation or unable to develop blastocyst. This suggested that the fertility was compromised in the two sisters compared with peers. HFM1-deficient mice did show similar phenotypes [9, 17]. The Hfm1-cKO mice were premature infertile, exhibiting basically normal growing follicles but produced four pups per litter compared with seven in control group. Moreover, the Hfm1-cKO mice could undergo successful fertilization but exhibit significantly decreased blastocyst formation rate [9]. These suggested that the variants of HFM1 identified in this study might also be associated with IVF-ET failure in these two sisters.
There is an apparent association between DOR and RPL, specifically the idiopathic RPL. Low AMH and AFC levels could predict higher odds for pregnancy loss [18]. RPL is a highly heterogeneous condition. Different causes can contribute to embryonic development arrest, including aneuploidy and thrombosis. In the idiopathic RPL, it was found that 60–78% of the abortion products were aneuploidies, and the preimplantation genetic screening could reduce the pregnancy loss rate [19, 20]. More importantly, women with DOR have a higher percentage of aneuploid embryos [7]. One study evaluating the euploid rates by preimplantation genetic testing showing that women with DOR had 23% reduced odds of a biopsied blastocyst being euploid compared to women with non-DOR infertility. This phenomenon was evident in women under 35 years old [21]. In this study, both of the sisters had idiopathic RPL history. The findings that aneuploidy rates are higher in women with DOR might also help explain the reason of RPL in the two sisters.
In conclusion, we identified novel splicing HFM1 mutations in two DOR sisters born from a family. Considering the role of HFM1 in meiosis and POI, the compound heterozygous mutations in HMF1 (c.1978-2A > C/c.2680 + 3_2680 + 4delAT) are the possible causes for DOR in the two sisters. The DOR is the potential reason for poor IVF outcomes and RPL. Unfortunately, aneuploidy analysis was not performed on the corresponding aborted products and blastocyst. Thus, we cannot make a definitive conclusion that the aneuploidies were the reason for RPL in the two DOR sisters. Nonetheless, since HFM1 genetic change has been witnessed in only one pedigree, additional studies targeting multiple pedigrees are necessary.
Supplementary information.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the patients for their time and effort in participating in this research. We thank the Home for Researchers editorial team(www.home-for-researchers.com)for the language editing.
Funding
This work was funded by the following: (1) National Natural Science Foundation of China [Grant No. 81801519], (2) Clinical Medicine Foundation of Chinese Medical Association [Grant No.18010380767], (3) University Level Project Research Fund of Anhui University of Science and Technology (fsyyzd2020-01).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Lan Yu and Mingwei Li contributed equally to this work.
References
- 1.Testing and interpreting measures of ovarian reserve a committee opinion. Fertil Steril. 2012;98(6):1407–1415. doi: 10.1016/j.fertnstert.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 2.Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder–a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–1712. doi: 10.1007/s10815-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35(1):17–23. doi: 10.1007/s10815-017-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene AD, Patounakis G, Segars JH. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet. 2014;31(8):935–946. doi: 10.1007/s10815-014-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott RT., Jr Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril. 2001;76(4):666–669. doi: 10.1016/S0015-0282(01)02017-9. [DOI] [PubMed] [Google Scholar]
- 6.Wald KA, Shahine LK, Lamb JD, Marshall LA, Hickok LR. High incidence of diminished ovarian reserve in young unexplained recurrent pregnancy loss patients. Gynecol Endocrinol. 2020;36(12):1079–1081. doi: 10.1080/09513590.2020.1750001. [DOI] [PubMed] [Google Scholar]
- 7.Shahine LK, Marshall L, Lamb JD, Hickok LR. Higher rates of aneuploidy in blastocysts and higher risk of no embryo transfer in recurrent pregnancy loss patients with diminished ovarian reserve undergoing in vitro fertilization. Fertil Steril. 2016;106(5):1124–1128. doi: 10.1016/j.fertnstert.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Miyamoto N, Shouguchi-Miyata J, Ikeda JE. HFM1, the human homologue of yeast Mer3, encodes a putative DNA helicase expressed specifically in germ-line cells. DNA Seq. 2006;17(3):242–246. doi: 10.1080/10425170600805433. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhong C, Yang R, et al. Hfm1 participates in Golgi-associated spindle assembly and division in mouse oocyte meiosis. Cell Death Dis. 2020;11(6):490. doi: 10.1038/s41419-020-2697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Zhang W, Jiang H, Wu BL. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med. 2014;370(10):972–974. doi: 10.1056/NEJMc1310150. [DOI] [PubMed] [Google Scholar]
- 11.Pu D, Wang C, Cao J, et al. Association analysis between HFM1 variation and primary ovarian insufficiency in Chinese women. Clin Genet. 2016;89(5):597–602. doi: 10.1111/cge.12718. [DOI] [PubMed] [Google Scholar]
- 12.Bender Atik R, Christiansen OB, Elson J, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018. 2018(2): hoy004. [DOI] [PMC free article] [PubMed]
- 13.Tang D, Lv M, Gao Y, et al. Novel variants in helicase for meiosis 1 lead to male infertility due to non-obstructive azoospermia. Reprod Biol Endocrinol. 2021;19(1):129. doi: 10.1186/s12958-021-00815-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhe J, Chen S, Chen X, et al. A novel heterozygous splice-altering mutation in HFM1 may be a cause of premature ovarian insufficiency. J Ovarian Res. 2019;12(1):61. doi: 10.1186/s13048-019-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdalla H, Thum MY. An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve. Hum Reprod. 2004;19(4):893–898. doi: 10.1093/humrep/deh141. [DOI] [PubMed] [Google Scholar]
- 16.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 17.Guiraldelli MF, Eyster C, Wilkerson JL, Dresser ME, Pezza RJ. Mouse HFM1/Mer3 is required for crossover formation and complete synapsis of homologous chromosomes during meiosis. PLoS Genet. 2013;9(3):e1003383. doi: 10.1371/journal.pgen.1003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunnewell SJ, Honess ER, Karia AM, Keay SD, Al Wattar BH, Quenby S. Diminished ovarian reserve in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113(4):818–827.e3. doi: 10.1016/j.fertnstert.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Asgari A, Ghahremani S, Saeedi S, Kamrani E. The study of chromosomal abnormalities and heteromorphism in couples with 2 or 3 recurrent abortions in Shahid Beheshti Hospital of Hamedan. Iran J Reprod Med. 2013;11(3):201–208. [PMC free article] [PubMed] [Google Scholar]
- 20.Hodes-Wertz B, Grifo J, Ghadir S, et al. Idiopathic recurrent miscarriage is caused mostly by aneuploid embryos. Fertil Steril. 2012;98(3):675–680. doi: 10.1016/j.fertnstert.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Jaswa EG, McCulloch CE, Simbulan R, Cedars MI, Rosen MP. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril. 2021;115(4):966–973. doi: 10.1016/j.fertnstert.2020.10.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.