Abstract
Background
Premature ovarian insufficiency (POI) occurs in women before the age of 40 years, accompanied by amenorrhea, hypoestrogenism, hypergonadotropinism, and infertility. The pathology of POI is complex and the molecular genetic mechanisms are poorly understood. Bone morphogenetic protein 15 (BMP15) plays a crucial role in oocyte maturation and follicular development through the activation of granulosa cells. Dysfunction of BMP15 causes ovarian dysgenesis and is related to POI. Identifying pathogenic variants contributes to revealing genetic mechanisms and making clinical diagnoses of POI.
Methods
The study involved two sisters diagnosed with POI. Whole-exome sequencing (WES) was performed to identify causative genes. Sanger sequencing was used to validate the mutations in patients with POI and members of the family with no clinical signs or symptoms. The effect of the novel mutations on the BMP15 structure was analyzed by PSIPRED. By over-expressing wild-type (WT) or mutant BMP15 plasmids in vitro, a functional study of the BMP15 mutant was conducted by real-time qPCR and western blotting. Through cocultivation with HEK293T cells, the effects of secreted BMP15 WT and variants on granulosa cell proliferation and apoptosis were detected through a cell counting kit-8 assay and flow cytometric analysis.
Results
We identified biallelic variants in BMP15, c.791G > A (p. R264Q) and c.1076C > T (p. P359L), in two siblings with POI. Both sisters carried the same biallelic variants, while the other female members of their family carried only one of them. Structural prediction showed that the variants have not affected the secondary structure of BMP15 but may change the conformation of water molecules around protein surfaces and thermal stability of BMP15. Real-time qPCR showed no significant difference in mRNA levels among WT and the two variants. Western blotting indicated a reduction in BMP15 expression with the c.791G > A and c.1076C > T variants compared to WT. Moreover, mutants 791G > A and 1076C > T impaired the function of secreted BMP15 in promoting granulosa cell proliferation and suppressing cell apoptosis caused by reactive oxygen species.
Conclusions
This study identified novel biallelic variants, c.791G > A and c.1076C > T, of BMP15 in two siblings with POI. Both missense variants reduced the level of the BMP15 protein and impaired the function of BMP15 in promoting granulosa cell proliferation in vitro. Taken together, our findings provide a novel molecular genetic basis and potential pathogenesis of BMP15 variants in POI.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-022-02574-1.
Keywords: Premature ovarian insufficiency, BMP15, Biallelic variants, WES
Introduction
Premature ovarian insufficiency (POI) occurs in women before the age of 40 years (1,2). It is diagnostic of amenorrhea, hypoestrogenism, hypergonadotropinism, and infertility. [1, 3] The underlying mechanisms of most cases have not been identified. The known pathogenic causes include genetic defects (X chromosomal number variations, translocations, and genetic mutations), autoimmune diseases, iatrogenic, and environmental factors (viral infections and toxins). [2, 4] Among them, genetic defects are substantial causes of POI. Most POI cases are heterogeneous idiopathic, [2, 3] and the underlying mechanisms are largely unclear.
Over the past few decades, numerous candidate genes of POI have emerged, but few were shown causative with functional validation. These candidate genes are involved in primordial germ cell migration and proliferation (NANOS3), cell death (PGRMC1 and FMR1), oocyte-specific transcription (FIGLA and NOBOX), folliculogenesis (NR5A1, WT1, and FOXL2), transforming growth factor-β superfamily (BMP15 and GDF9), and hormone reception (FSHR, AMH, and AMHR2). [5, 6]
BMP15 is an oocyte-specific growth/differentiation factor (GDF) that stimulates follicle formation and granulosa cell growth. [7] It is located on chromosome X and expressed in oocytes in the early stages of follicle formation. [7] Dysfunction of BMP15 causes ovarian dysgenesis-2 (ODG2) and premature ovarian failure-4 (POF4). [8, 9] As members of TGF-β superfamily, BMP15 and GDF9 may form heterodimers. [10, 11] The GDF9/BMP15 heterodimer potently activates granulosa cells via both the SMAD2/3 and SMAD1/5/8 pathways. [12] Over the past few decades, there have been several variants of BMP15 identified in POI patients, including H81R, G199R, A180T, F194S, C357Y, and V136L. However, the known pathogenic variants are limited for the genetic diagnosis and prognosis of POI.
Here, we identified the novel biallelic variants of BMP15, c.791G > A (p.R264Q) and c.1076C > T (p.P359L), in two siblings with POI. Both variants expressed lower BMP15 protein levels than the wild-type (WT). The biallelic variants impaired BMP15 function. Overall, our findings provide a genetic basis for the mutation of BMP15 in POI. Mutations c.791G > A and c.1076C > T are most likely pathogenic in BMP15-dependent POI.
Material and methods
Patients
Two sisters with POI were recruited for the study. Family members of the patients were additionally enrolled. Written informed consent for blood sampling and genetic investigations was approved by the ethical review board of West China Second University Hospital, Sichuan University. Informed consent was obtained from each subject in our study.
Whole-exome sequencing (WES) and Sanger sequencing
Genomic DNA was extracted from peripheral blood samples of the two patients using a whole blood DNA purification kit (QIAGEN, Germany). Whole-exome sequencing (WES) was performed for the two POI patients. In brief, 1 µg of genomic DNA was used for exon capture by the Agilent SureSelect Human All Exon V6 Kit (Agilent Technologies, Santa Clara, CA) and sequenced on the Illumina HiSeq X system following the manufacturer’s instructions. The average sequencing depth on target was 108.11, and the ratio of target fraction covered at a minimum depth of 10 × was 95%. Reads were mapped to the reference genome (UCSC GRCh37/hg19) using the Burrows-Wheeler Aligner (BWA, http://biobwa.sourceforge.net) to obtain the original mapping and duplicates were marked and removed using Picard (http://broadinstitute.github.io/picard/index.html). ANNOVAR was performed for nucleotide and amino acid annotations. Population frequencies and mutation references were analyzed through public resources, including HGMD, 1000 Genomes database, dbSNP, ExAC, ClinVar, and OMIM. After filtering, the retained nonsynonymous SNVs were submitted to PolyPhen-2, SIFT, MutationTaster, and CADD for functional prediction.
Candidate pathogenic variants in the family members were validated by Sanger sequencing. The DNA of the family members was extracted from the oral epithelium or peripheral blood. Polymerase chain reaction (PCR) amplification was performed using the PCR System (Bio-Rad, USA). The BMP15 gene was amplified using the following primers: forward 5′-3′ CGCCATCATCTCCAACTAACTC, and reverse R 5′-3′ GACTCAGCAATCATACCCTCATACTC.
DNA sequencing of PCR products was conducted on an ABI 377A DNA Sequencer (Applied Biosystems, USA).
Cell culture and transfection
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) high glucose medium (Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) and 100 IU/mL penicillin/streptomycin (P/S, Gibco, USA) at 37 °C and 5% CO2. Human granulosa KGN cells were cultured in DMEM/F12 medium (Gibco, USA) supplemented with 10% FBS and 100 IU/mL P/S 5% at 37 °C and 5% CO2. Cells were transfected with Lipofectamine® 2000 (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions.
Plasmid construction
The cDNAs of wild-type (WT) and mutant (c.791G > A, c.1076C > T) BMP15 were synthesized and separately inserted into the pcDNA3.1-Flag plasmid.
RNA extraction and real-time qPCR
RNA was extracted from cells using RNAiso Plus (cat# 9109, TaKaRa, Japan) and reverse transcribed using the PrimeScript™ RT reagent Kit (cat# RR047A, TaKaRa, Japan) according to the manufacturer’s instructions. RT-qPCR was performed on a real-time PCR system. The primer sequences for BMP15 were as follows: forward 5′-3′ CGCCATCATCTCCAACTAACTC, and reverse 5′-3′ AGCGTTAGACATCAGGGAAGGT. The relative levels of mRNA expression were normalized to the GAPDH level and analyzed by the ∆∆CT method.
Western blots
Cells were collected and lysed with RIPA buffer (plus cocktail) on ice. Protein quantitation was performed by bicinchoninic acid assay (BCA Protein Assay, Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. A total of 60 µg of denatured proteins were subjected to SDS-PAGE and transferred to PVDF membranes (Millipore, USA). The BMP15 and GAPDH proteins were blotted on a ChemiDoc Touch Imaging System (Bio-Rad, USA). Anti-Flag (1:2000, cat# 66008–2-Ig, rabbit) antibody was from Proteintech (China). Anti-GFP (1:2500, cat# ab290, rabbit) and anti-GAPDH (1:5000, cat# ab8245, mouse) antibody was from Abcam (USA). Goat anti-rabbit (1:5000, cat# 32230) or anti-mouse (1:5000, cat# 6120) IgG secondary antibody-HRP was purchased from Thermo Fisher Scientific (USA).
Cell viability assay
A cell counting kit-8 (CCK-8) was used to measure cellular viability. HEK293T (200,000) cells were plated into 6-well plates and transfected for 24 h. Then, the medium (containing secreted BMP15) was collected and filtered with 0.45-μm filters (Millipore, USA). A total of 2000 KGN cells were seeded in 96-well plates for the cell proliferation assay. Subsequently, KGN cells were cultured with a conditioned medium (containing DMEM/F12 medium plus 50% harvested HEK293T cell medium). Ten microliters of CCK-8 solution was added to each well every 12 h and treated for 1 h. The absorbance at 450 nm was measured with a microplate reader.
Flow cytometric analysis
Apoptotic cells were determined using Annexin V-FITC/propidium iodide (PI) (Beyotime, China). HEK293T (200,000) cells were plated into 6-well plates and transfected for 24 h. Then, the medium (containing secreted BMP15) was collected and filtered with 0.45-μm filters. Subsequently, KGN cells were seeded in 6-well plates and cultured with a conditioned medium (containing DMEM/F12 medium plus 50% harvested HEK293T cell medium). An appropriate concentration of H2O2 was added to the cells based on the IC50 (half-maximal inhibitory concentration). After staining with Annexin V-FITC/PI, apoptotic cells were detected by flow cytometry.
Statistical analysis
Protein expression levels were calculated by ImageJ software (USA). All statistical analyses were performed with GraphPad Prism 9 software (USA). Each experiment was repeated in biological triplicates. Comparisons were performed with one-way ANOVA. Error bars were presented as the mean ± SD. p value < 0.05 was considered statistically significant.
Results
Identification of the POI phenotype in two sisters
Two sister patients presented with amenorrhea and infertility, with normal 46,XX karyotypes. They had no clinical history of autoimmunity diseases, surgery, or chemotherapy. The older sister (II-3) had menarche at the age of 14 and started amenorrhea 6 months after menarche. She was diagnosed with POI at age 31. The younger sister (II-5) had menarche at the age of 15 and has an irregular menstrual cycle (15–90 days). She was diagnosed with POI at age 21 with symptoms of amenorrhea for 3 years. Both of them have withdrawal bleeding following estrogen-progesterone therapy. Hormonal assays showed elevated FSH and decreased AMH levels in both patients (Table 1). B-Mode ultrasonography showed bilateral ovarian volume reduction and antral follicle loss in both patients’ ovaries (II-3: the left ovary size was 1.6 × 1.2 × 1.1 cm, and the right ovary size was 2.0 × 1.1 × 1.7 cm; II-5: the left ovary size was 2.2 × 1.7 × 2.1 cm, and the right ovary size was 2.2 × 1.1 × 2.0 cm) (Fig. S-1).
Table 1.
Clinical, hormonal, and ultrasonography characterization of two patients with POI
| Patients | II-3 | II-5 |
|---|---|---|
| Age at evaluation | 35 years | 31 years |
| Age at amenorrhea | 15 years | 21 years |
| Age at menarche | 14 years | 15 years |
| Age at POI diagnosed | 31 years | 21 years |
| FSH (mUI/ml) | 35.3 | 62.1 |
| E2 (pmol/l) | 16.8 | 26.6 |
| AMH (pmol/l) | < 0.06 | 0.47 |
| LH (mUI/m) | 13.5 | 49.8 |
| Androstenedione (nmol/l) | 1.28 | 2.71 |
| Prolactin (mUI/l) | 11.6 | 7.8 |
| T (nmol/l) | 0.22 | 0.08 |
Compound heterozygosis mutations of c.791G > A and c.1076C > Twere detected in the two patients
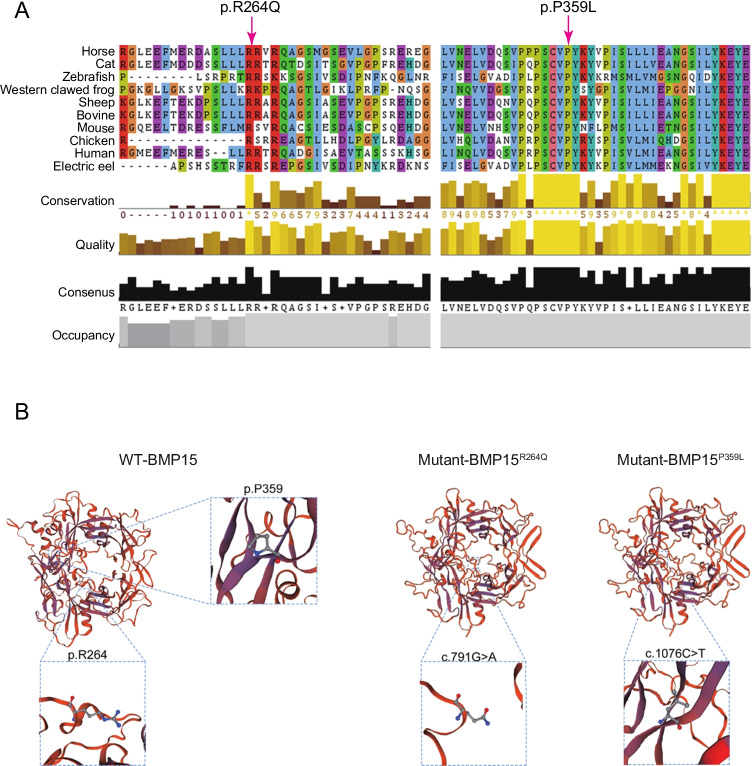
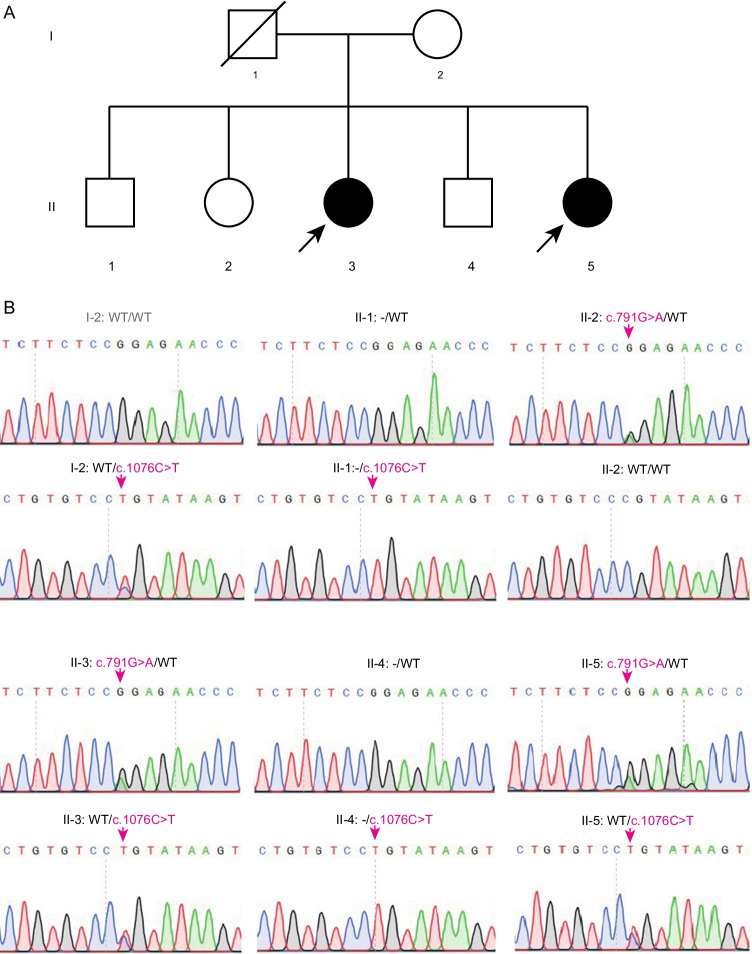
To explore the genetic causes of POI in this family, genomic DNA was extracted from the peripheral blood leukocytes of the two sisters and their family members. Whole-exome capture and sequencing were performed using the Agilent SureSelect whole-exome capture and Illumina sequencing technology following the standard procedures. Read alignment and variant calling were performed with BWA-MEM software (Burrows-Wheeler Aligner, http://bio-bwa.sourceforge.net), using the default parameters and with the human genome assembly hg19 (GRCh37) as the reference. Variants were identified by using the GATK (Genome Analysis Toolkit, https://software.broadinstitute.org/gatk/, Broad Institute, Cambridge, MA, USA) HaplotypeCaller and then the GATK Variant Recalibrator. The variants were functionally annotated using ANNOVAR (http://annovar.openbioinformatics.org/). The candidate mutations met the following criteria: (i) mutations carried by both sisters; (ii) a low allele frequency in the public human genome databases of the 1000 Genomes Project and the gnomAD (http://gnomad.broadinstitute.org/, Broad Institute, Cambridge, MA, USA); (iii) deleterious variants valued by three predictors, including SIFT (http://sift.jcvi.org/, J. Craig Venter Institute, La Jolla, CA, USA), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/, Havard University, Cambridge, MA, USA), and MutationTaster (https://www.mutationtaster.org). There were 79 causative variants that predicted damage function and 32 mutations linked to the POI phenotype in two patients. Through filtering candidate variants in WES data by analyzing of clinical phenotype or inheritance pattern, we found two compound heterozygous mutations of c.791G > A and c.1076C > T in BMP15, a known causative gene of POI, in both patients (Table 2, Table S1-2). Moreover, these two sites are highly conserved among species (Fig. 2A). To validate the two identified variants by WES, Sanger sequencing was processed among the family members. As shown in Fig. 1B, two patients (II-3, II-5) both carried two heterozygosis mutations (c.791G > A and c.1076C > T). Their mother (I-2) carried only one heterozygosis mutation, c.1076C > T. Their father passed away a few years ago and his data was not available. The two brothers (II-1, II-4) carried the same mutation as their mother. Another sister (II-2) carried the heterozygosis mutation c.791G > A. Therefore, we hypothesized that these two variants of BMP15 might be the genetic cause of POI in these two sibling sisters (II-3, II-5).
Table 2.
Biallelic BMP15 mutation identified in two patients affected by POI
| Gene | BMP15 | BMP15 |
|---|---|---|
| Variant coordinates* | ChrX:50,659,219, G > A | ChrX: 50,659,504, C > T |
| Transcript | NM_005448 | NM_005448 |
| Base change | c.791G > A | c.1076C > T |
| AA change | p. R264Q | p. P359L |
| Mutation type | Missense, heterozygous | Missense, heterozygous |
| Inheritance | X-linked recessive | X-linked recessive |
| Allele frequencies in human population | ||
| Allele frequency in EXAC_ALL | 1.17E-05 | 1.14E-05 |
| Allele frequency in EXAC_EAS | 0 | 0 |
| Allele frequency in gnomAD_ALL | 1.133E-05 | 5.64E-06 |
| Allele frequency in gnomAD_EAS | 0 | 0 |
| Allele frequency in 1000g_ALL | - | - |
| Allele frequency in 1000g_EAS | - | - |
| Functional prediction | ||
| PolyPhen-2 | Probably damaging | Probably damaging |
| SIFT | Deleterious | Deleterious |
| MutationTaster | Polymorphism | Disease causing |
| M-CAP | Probably damaging | Probably damaging |
*Variant coordinates are based on the human genome assembly GRCh37/h19
EAS, East Asian
Fig. 2.
The amino acids R264 and P359 are highly conserved among species. The BMP15 sequences of various species in the database were blast searched. A The left arrow represents the conserved 264th amino acid Arg. The right arrow represents the conserved 359th amino acid Pro. B The secondary structure among BMP15 wild-type (WT) and mutations c.791G > A and c.1076C > T was predicted by PSIPRED
Fig. 1.
The biallelic variants in BMP15 were identified in two POI siblings. A Pedigree. I and II refer to the two successive generations. Squares represent males, circles represent females, solid symbols represent members with variants, and hollow symbols represent unaffected members. Slash indicates that the family member has passed away and the data is not available. Black arrows indicated the probands. B Sanger sequencing verification of the family carrying the variants c.791G > A and c.1076C > T
Mutations c.791G > A and c.1076C > T impaired BMP15 protein expression
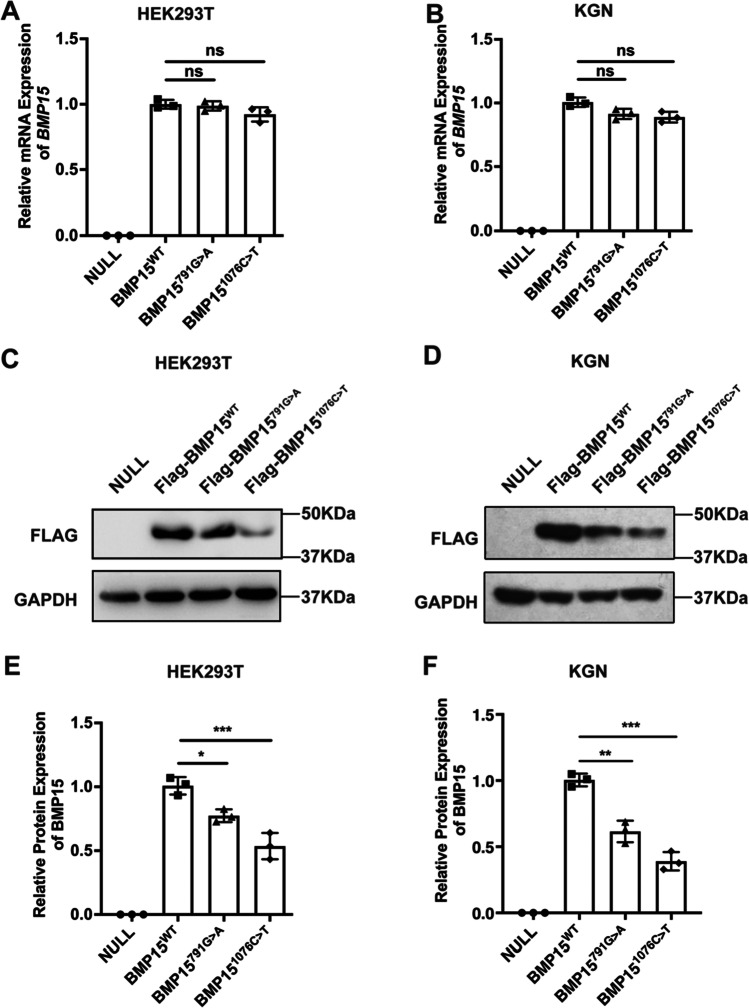
Next, we predicted the secondary structure among BMP15 wild-type (WT) and mutants c.791G > A and c.1076C > T by PSIPRED. The results suggested that both variants have no influence on the secondary structure of BMP15. However, c.791G > A could change Arg to Gln and c.1076C > T could change Pro to Leu (Fig. 2). The conformation of water molecules around polar and nonpolar protein surfaces determines the function and interaction of proteins. [13] The p. R264Q change from basic Arg to polar Gln might affect the conformation of water molecules around BMP15 and its function. Moreover, Pro has an essential contribution to the thermal stability of the protein. The p. P359L change may reduce the protein stability of BMP15. To further identify the effect of mutations on BMP15 expression, we transfected BMP15WT, BMP15791G>A, or BMP151076C>T vectors into HEK293T cells, followed by the detection of mRNA and protein expression. There was no significant difference in the transfection efficiency among BMP15 WT and variant plasmids (Fig. S-2). The results showed no significant difference in BMP15 mRNA levels among WT and two variants (Fig. 3A). Western blotting revealed a reduction in BMP15 protein levels in cells transfected with BMP15791G>A and BMP151076C>T compared to cells transfected with BMP15 WT (Fig. 3B–C). These results indicated that the mutations c.791G > A and c.1076C > T could impair BMP15 protein expression.
Fig. 3.
Mutations c.791G > A and c.1076C > T impaired BMP15 expression. A ~ B After over-expressing BMP15 WT or mutants in HEK293T or KGN cells, the transcription levels were measured by RT-qPCR. C ~ D The empty vector, flag-tagged BMP15WT, BMP15791G>A, and BMP151076C>T plasmids were transfected in HEK293T or KGN cells; the protein expression levels were detected by WB. E ~ F The protein expression levels were measured by ImageJ. Statistical significance was determined with one-way ANOVA. Error bars = mean ± SD, n = 3. *p < 0.05; ***p < 0.001; ns, not statistically significant
BMP15 variants failed to promote proliferation and inhibit apoptosis of granulosa cells
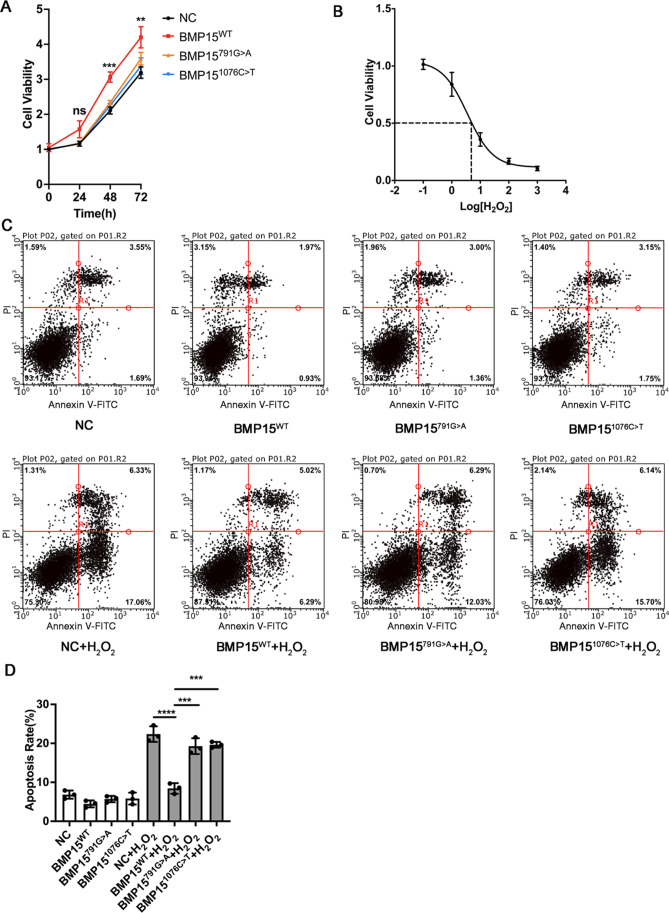
BMP15 is a secreted protein and interacts with TGF-β receptors on the surface of granulosa cells, activating downstream SMAD signaling. [14] To further verify the function of variants in vitro, we expressed WT and variants of BMP15 in HEK293T cells and collected the medium (containing secreted BMP15). Then, the immortalized human granulosa KGN cells were cultured with a conditioned medium (containing DMEM/F12 medium plus 50% harvested HEK293T cell medium). The CCK-8 assay showed that cultivation with conditioned medium from BMP15WT-expressing HEK293T cells promoted the proliferation of KGN cells, while expressing variants had little influence on proliferation (Fig. 4A). To explore the contribution of BMP15 to resisting ROS (reactive oxygen species)-induced cell death, we treated KGN cells with a gradient concentration of H2O2. The results showed that the IC50 of H2O2 on KGN cells was approximately 3.19 µM. Therefore, we cultured KGN cells with conditioned medium (containing DMEM/F12 medium plus 50% harvested HEK293T cell medium). Then, KGN cells were treated with 3.19 µM H2O2 for 48 h. Flow cytometric analysis indicated that BMP15 inhibited the apoptosis induced by H2O2, while BMP151076C>T did not suppress apoptosis, and BMP15791G>A was less effective than WT BMP15 on KGN cells. The results suggested that BMP15 promoted granulosa cell proliferation and suppressed apoptosis induced by ROS, while mutants 791G > A and 1076C > T impaired the function of BMP15 on proliferation and apoptosis.
Fig. 4.
The variants of BMP15 failed to promote proliferation and inhibit apoptosis of KGN cells. A Cell proliferation assay. HEK293T cells were transfected with BMP15WT, BMP15791G>A, and BMP151076C>T plasmids. Then, the medium (containing secreted BMP15) was collected. KGN cells were cultured with DMEM/F12 plus 50% HEK293T cell medium. KGN cell proliferation was measured by CCK-8 assay every 24 h. B IC50 of H2O2. KGN cells were treated with a gradient concentration of H2O2. Cell viability was detected by CCK-8 assay. The IC50 of H2O2 on KGN cells was approximately 3.19 µM. C Apoptosis analysis. HEK293T cells were transfected with BMP15WT, BMP15791G>A, and BMP151076C>T plasmids. Then, the medium (containing secreted BMP15) was collected. KGN cells were cultured with DMEM/F12 plus 50% HEK293T cell medium. After treatment with 3.19 µM H2O2 for 24 h, the apoptotic KGN cells were stained with Annexin V-FITC/PI and detected by flow cytometry. The lower left quadrants (Annexin V−/PI−) represent living cells. The lower right quadrants (Annexin V+/PI−) represent early apoptotic cells. The upper right quadrants (Annexin V+/PI+) represent late apoptotic/necrotic cells. The upper left quadrants (Annexin V−/PI+) represent dead cells. D Statistical analysis of apoptotic cells. Statistical significance was determined with one-way ANOVA. Error bars are presented as the mean ± SD, n = 3. *p < 0.05; **p < 0.01; ***p < 0.001; ns, not statistically significant
Discussion
A wide spectrum of pathogenic causes may lead to the occurrence and development of POI, such as genetic defects and autoimmune, iatrogenic, and environmental factors. [2, 4] In this study, we identified the novel biallelic variants c.791G > A (p. R264Q) and c.1076C > T (p. P359L) in two siblings. Both patients carried the two heterozygous mutations, while their fertile mother and another sister carried a single mutation of one allele. Therefore, the biallelic variants of BMP15 might be pathogenic mechanisms of the two sister patients. These missense mutations led to a partial reduction in BMP15 protein expression and impaired the function of suppressing ROS-induced granulosa cell death.
BMP15 promotes oocyte development and maturation, and suppresses granulosa cell apoptosis. [7] There have been several naturally occurring mutations of BMP15 in POI cases thus far. Two variants (H81R, G199R) have been described in a cohort of patients with POI. [15] A c.151_152delGA deletion and a c.189_198delAGGGCATTCAinsTG deletion/insertion yielded a human “knockout-like” effect of BMP15. [16] A homozygous missense mutation, c. G1070A (p. C357Y) of BMP15, has been found in a proband with POI and was highly conserved among species and predicted to be disorder-causing. [17] Recently, a BMP15 mutation, c.406 G > C (p. V136L) identified in POI patients, caused a reduction in the activity of the mature protein. [18] However, the function of most variants has not been verified by experiments. This study found novel biallelic mutations (c.791G > A and c.1076C > T) of the BMP15 gene in two sister patients with POI. Functional studies revealed that either of the two mutations could reduce BMP15 expression and cause impaired BMP15 function. In vitro, the variants of secreted BMP15 failed to promote the proliferation and suppress the apoptosis of KGN granulosa cells.
In this study, both patients carried two mutations c.791G > A and c.1076C > T of BMP15, and they displayed phenotypes of POI and infertility. Their mother, brothers, and sister all carried mutation c.1076C > T, and they are fertile. These results suggested that biallelic mutations impaired the function of BMP15 and then led to POI. A single mutation of one allele of BMP15 is insufficient to ultimately impair BMP15 functions. The conformation of water molecules around polar and nonpolar protein surfaces determines the function and interaction of proteins. [13] We predict that c.791G > A would change basic Arg to polar Gln, which might affect the conformation of water molecules around BMP15 and its function. In addition, mutation of c.1076C > T leads to an amino acid change from Pro to Leu. Since Pro has an important contribution to the stability of the protein, this change may reduce the stability of BMP15. BMP15 and GDF9 can form dimers. [10, 11] Thus, we hypothesize that the variant could affect the interaction of BMP15 with GDF9, thus impairing the activation of the PI3K/Akt and Smad2/3 pathways. Further studies are needed to verify this hypothesis.
In conclusion, this study identified novel biallelic variants in BMP15 in two siblings with POI, thus expanding the limited known spectrum of BMP15 gene mutations. The in vitro experiments elucidated the pathogenicity of these mutations in BMP15 expression. Therefore, our findings provide genetic information for POI diagnosis and prognosis.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file2 Identification and analysis of all variants of II-3 (XLSX 11929 KB)
Supplementary file3 Identification and analysis of all variants of II-5 (XLSX 12521 KB)
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jankowska K. Premature ovarian failure. Prz Menopauzalny. 2017;16:51–56. doi: 10.5114/pm.2017.68592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 3.Ghahremani-Nasab M, Ghanbari E, Jahanbani Y, Mehdizadeh A, Yousefi M. Premature ovarian failure and tissue engineering. J Cell Physiol. 2020;235:4217–4226. doi: 10.1002/jcp.29376. [DOI] [PubMed] [Google Scholar]
- 4.Lakhal B, Laissue P, Braham R, Elghezal H, Saad A, Fellous M, Veitia RA. A novel BMP15 variant, potentially affecting the signal peptide, in a familial case of premature ovarian failure. Clin Endocrinol (Oxf) 2009;71:752–753. doi: 10.1111/j.1365-2265.2009.03571.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiao X, Ke H, Qin Y, Chen ZJ. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol Metab. 2018;29:795–807. doi: 10.1016/j.tem.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Belli M, Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam Horm. 2018;107:317–348. doi: 10.1016/bs.vh.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. 2014;20:869–883. doi: 10.1093/humupd/dmu036. [DOI] [PubMed] [Google Scholar]
- 8.Rossetti R, Di Pasquale E, Marozzi A, Bione S, Toniolo D, Grammatico P, Nelson LM, Beck-Peccoz P, Persani L. BMP15 mutations associated with primary ovarian insufficiency cause a defective production of bioactive protein. Hum Mutat. 2009;30:804–810. doi: 10.1002/humu.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 11.Mottershead DG, Harrison CA, Mueller TD, Stanton PG, Gilchrist RB, McNatty KP. Growth differentiation factor 9:bone morphogenetic protein 15 (GDF9:BMP15) synergism and protein heterodimerization. Proc Natl Acad Sci U S A. 2013;110:E2257. doi: 10.1073/pnas.1303459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottershead DG, Sugimura S, Al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J, Mueller TD, Harrison CA, Gilchrist RB. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-beta family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem. 2015;290:24007–24020. doi: 10.1074/jbc.M115.671487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao B, Jimenez-Angeles F, Nguyen TD, Olvera de la Cruz M. Water follows polar and nonpolar protein surface domains. Proc Natl Acad Sci U S A. 2019;116:19274–19281. doi: 10.1073/pnas.1910225116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35:1741–1750. doi: 10.1007/s10815-018-1268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiotiu D, Alvaro Mercadal B, Imbert R, Verbist J, Demeestere I, De Leener A, Englert Y, Vassart G, Costagliola S, Delbaere A. Variants of the BMP15 gene in a cohort of patients with premature ovarian failure. Hum Reprod. 2010;25:1581–1587. doi: 10.1093/humrep/deq073. [DOI] [PubMed] [Google Scholar]
- 16.Mayer A, Fouquet B, Pugeat M, Misrahi M. BMP15 "knockout-like" effect in familial premature ovarian insufficiency with persistent ovarian reserve. Clin Genet. 2017;92:208–212. doi: 10.1111/cge.12970. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Wang J, Wang X, Li L, Pan H, Chen B, Zhu Y, Li T, Cao Y, Wang B. A novel homozygous mutation of bone morphogenetic protein 15 identified in a consanguineous marriage family with primary ovarian insufficiency. Reprod Biomed Online. 2018;36:206–209. doi: 10.1016/j.rbmo.2017.10.104. [DOI] [PubMed] [Google Scholar]
- 18.Ferrarini E, De Marco G, Orsolini F, Gianetti E, Benelli E, Fruzzetti F, Simoncini T, Agretti P, Tonacchera M. Characterization of a novel mutation V136L in bone morphogenetic protein 15 identified in a woman affected by POI. J Ovarian Res. 2021;14:85. doi: 10.1186/s13048-021-00836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file2 Identification and analysis of all variants of II-3 (XLSX 11929 KB)
Supplementary file3 Identification and analysis of all variants of II-5 (XLSX 12521 KB)