Abstract
Background
In sub-Saharan Africa, co-morbidity with malaria, schistosomiasis, and soil transmitted helminths (STH) is common among young children. The current study investigated malaria, urinary schistosomiasis and their co-infection and anemia among school-age children in an endemic community, Nakolo in the Kassena-Nankana East District of northern Ghana.
Methods
A cross-sectional survey of 336 school-age children, 5–16 years was undertaken. Urine samples were examined for Schistosoma haematobium ova using microscopy. Finger prick blood samples were examined for Plasmodium parasites using microscopy and haemoglobin concentration measured with HemoCue Hb301 photometer.
Results
The mean age was 10.52 (Standard deviation: ±2.27; range: 5–16 years), of which 50.6% (170/336) were males. The overall prevalence of urinary schistosomiasis and Plasmodium (P.) falciparum was 12.8% (43/336) and 37.8% (127/336), respectively with 6.0% (20/336) coinfection. Participants with only P. falciparum infection had 17.8% (19/107) of moderate anemia whilst 21.7% (5/23) of children infected with only S. haematobium had moderate anemia and 4.3% (1/23) had severe anemia. 5.0 % (1/20) of moderate anemia was observed in concurrent infections of P. falciparum and S. haematobium. Use of open water bodies was associated with increased risk of S. haematobium infection (OR = 1.21; 95% CI = [1.06–1.39]; p = 0.001), with females being at reduced risk (OR = 0.93; 95%CI = [0.87–0.99]; p = 0.005). Absence of self-reported haematuria had 0.81 times reduced odds of S. haematobium infection (OR = 0.81; 95%CI = [0.74–0.87]; p < 0.001).
Conclusion
This study has revealed that urinary schistosomiasis remains prevalent in Kassena-Nankana East district and suggests that urinary schistosomiasis may contribute to moderate anemia among school-age children as compared to asymptomatic malaria infection. These findings call for an evaluation of the annual mass drug administration of Praziquantel among in-school children to ascertain its impact on urinary schistosomiasis prevalence across the district.
Keywords: Urogenital schistosomiasis, Schistosoma haematobium, Malaria, Anemia, Northern Ghana
Urogenital schistosomiasis; Schistosoma haematobium; Malaria; Anemia; Northern Ghana.
1. Introduction
Malaria and schistosomiasis (bilharzia) are responsible for childhood morbidity and mortality in sub-Saharan Africa (sSA) [1]. Schistosomiasis is an acute-on-chronic parasitic worm infection caused by blood flukes (trematodes) of the genus Schistosoma [2]. It is estimated that at least 90% of people requiring treatment for schistosomiasis reside in sSA [3]. Schistosomiasis presents either as urogenital or intestinal disease. Urogenital schistosomiasis is caused by Schistosoma haematobium whilst the intestinal form is due to infection with a variety of species including S. mansoni, S. japonicum, S. mekongi, S. guineensis or S. intercalatum [4]. S. haematobium infection may lead to urological complications among school children such as lesions of the urinary tract, calcified bladder, deformity of the ureter and hydronephrosis [5, 6], anemia, and growth retardation among young children [7].
Schistosomiasis and malaria are often co-endemic in most parts of sSA with huge socioeconomic cost [8, 9]. The concurrence of Plasmodium falciparum malaria with other parasitic infections such as soil transmitted helminths and Schistosome infections are common across sSA [10, 11, 12, 13]. Such a concurrence of parasitic infections often results in anemia among school-age children with serious consequences on their health and significant reduction in school attendance [8, 14]. Ghana has been implementing mass drug administration (MDA) campaigns for school age children (SAC) for schistosomiasis since 2008 [15]. Notwithstanding the paucity of data on a national evaluation of the program, previous assessments from various endemic communities across the countries show that the MDA for SAC had a significant impact on schistosome prevalence, particularly prevalence of S. haematobium, bringing prevalence rates down to <15% [16]. Notably, MDA for SAC with Praziquantel in the Asuogyaman District of Eastern Region of Ghana is reported to have contributed in reducing the prevalence of urinary schistosomiasis to about 10.4%. However, uptake of Praziquantel is highly variable and important foci of high prevalence (>19%) remain [16, 17, 18], raising the question of expanding the program to include pre-school children, community-based treatment of out of school children and adults [15]. Also, previous evidence associates specific risk factors with the likelihood of increased schistosome infection. Key among these factors include age, sex, socio-economic status (though not universal), access and contact with infested water bodies for swimming, bathing and fishing [19].
Prior to the introduction of annual in-school MDA of Praziquantel against schistosomiasis, the overall prevalence of urinary schistosomiasis (S. haematobium) was estimated as 18.9% in the Kassena-Nankana Districts of northern Ghana [4], where malaria transmission is high and co-endemic with schistosomiasis. It is expected that the scale up of MDAs nearly two decades ago following the landmark World Health Assembly in 2001 (WHA 54.19) for control of schistosomiasis and soil-transmitted helminthiasis [20], and the general decline in malaria transmission across sSA [21], within this period will reduce the burden of the co-morbidity imposed by these parasites, particularly anemia among school children. However, there is little to no post-MDA intervention data to show the effect of these intervention activities on the prevalence of schistosome(s) single infection, and P. falciparum coinfection and the parasitic attributable burden of anemia in this northern Ghanaian population. Intermittent preventive treatment of malaria and deworming reduced the prevalence of anemia among children in this region [22] Therefore, the current study sought to determine the prevalence of urinary schistosomiasis among school-age children and to explore P. falciparum malaria co-infection and assess demographic and behavioral risk factors for urinary infection among SAC in northern Ghana.
2. Materials and methods
2.1. Ethical considerations
Ethical approval for use of human participants in this study was obtained from the Institutional Review Board of the Navrongo Health Research Centre. Written parental informed consent was obtained from parents (or guardians) of children 5–16 years attending District Assembly (D/A) and English and Arabic (E/A) primary schools in Nakolo. Additional assent was obtained from children 10–16 years. Permission for the study was obtained from the District Education Office as well as Heads of the two schools. The survey was conducted with the support of the school “Health-Teachers”. Children who tested positive for schistosomiasis and/or malaria were facilitated to receive the recommended single dose of praziquantel (40 mg/kg body weight) for urinary schistosomiasis or advised to visit the nearest Health Centre for treatment of malaria as per Ghana Health Service protocols when they develop fever. As per current malaria treatment guidelines in Ghana, asymptomatic infections are not eligible for treatment.
2.2. Study design and population
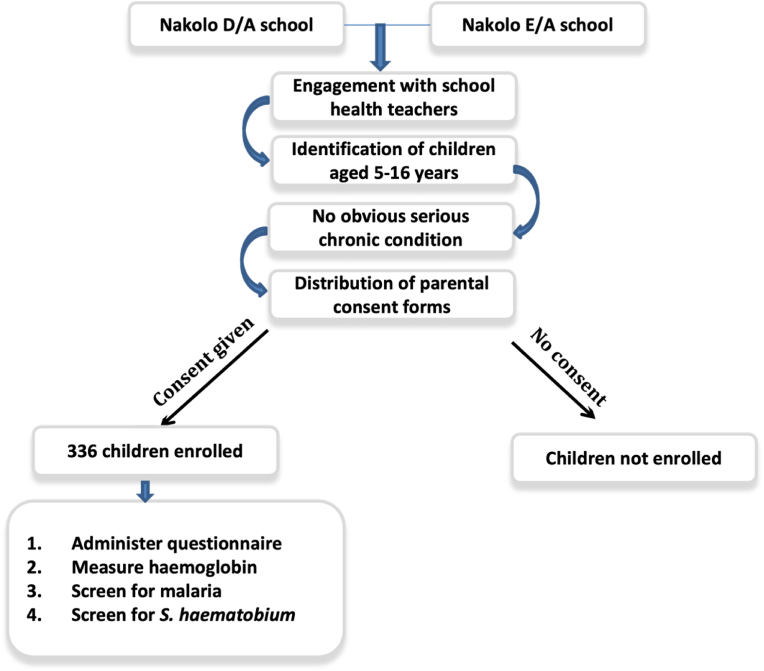
A cross sectional survey of school children attending two schools located in Nakolo was conducted between September and October 2018. Figure 1 below shows the study recruitment procedure and enrolment criteria. A total of 336 children (males = 170, females = 166), 5–16 years of age, were recruited. Children in this age category who were apparently healthy, had no serious chronic condition, were willing to participate and had their parent/guardians contacted for informed consent for a malaria blood smear, capillary blood for Hb measurement, and a urine sample collection were eligible for study participation. Children were sampled by convenience and of the total number of school children screened, 71.1% (239/336) came from the D/A primary school and 28.9% (97/336) were children attending the E/A primary school.
Figure 1.
A flowchart of study recruitment procedure and enrolment criteria.
The District Assembly (D/A) primary school in Nakolo is a government public school under the management of the Kassena-Nankana West District (KNWD) Assembly located in Paga near Ghana's border with Burkina-Faso. Nakolo English and Arabic (E/A) primary school is a faith-based public school under the joint management of the Muslim mission and KNWD. These sister schools located next to each other in the Nakolo community.
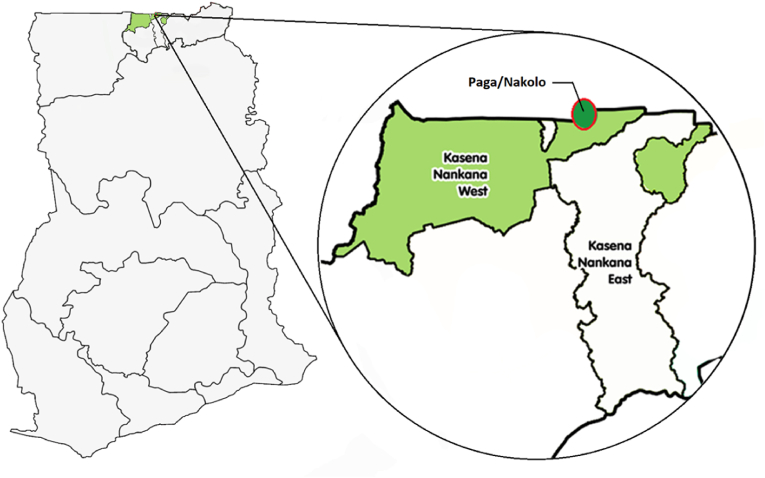
The study site, Nakolo, is a farming community in the KNWD of the Upper East Region of Ghana. The KNWD is located approximately between latitude 10.97o North and longitude 01.10o West (Figure 2). The main occupation of the inhabitants of Nakolo is subsistence farming and rearing of ruminants. Nakolo is one of several communities that form the framework for the Navrongo Health and Demographic Surveillance System [23]. Though rainfall in this area is variable, the average annual rainfall in the district is about 850 mm and occurs almost entirely between the months of June–October [24]. There is one major dam, the Tono irrigation dam, and several smaller dams and dug-outs dotted around the study area, which provide water for limited vegetable cultivation and for animals during the long dry season. Clinic attendance records had shown cases of urinary schistosomiasis in this region but there is little to no data on the current prevalence of the infection. Previous studies had shown that Bulinus globosus and B. truncates were prevalent in this area and are the intermediate host snails of S. haematobium with human prevalence of 3.8% in school-age children [4]. It was assumed that following almost two decades of mass drug administration against the infection in school-age children in the area, there will be at least 15% decrease in S. haematobium infection among children of school-age. Giving an estimated prevalence of 3.8%, it was estimated that a minimum sample size of 351 was sufficient to give a power of 80% to detect infection with alpha error margin of 5%.
Figure 2.
A map of Ghana showing location of the Kassena-Nankana East and West Districts within the Upper East Region: Expanded district map showing Nakolo community highlighted with a circle.
2.3. Urine analysis for S. haematobium ova
Urine samples collected from participants in schools between 10:00 am and 2:00 pm were transported to the Navrongo Health Research laboratory and analysed using the microscopy method [25]. The samples were allowed to rest on the bench for approximately 30 min. The urine in each bottle was then carefully drawn off and discarded, leaving about 10 mL in each bottle. The content of each bottle was then resuspended by gently shaking and the sediments transferred into a 20 mL centrifuge tube. The tubes were centrifuged at 1000 rpm for 5 min. The supernatant was discarded, and the residue put on a clean glass slide, covered with a cover slip and examined for S. haematobium ova (Supplementary Figure S1) using 10x objective lens of the microscope. Intensity of infection was estimated by counting the number of eggs per 10 mL of urine.
2.4. Examination of blood smear for malaria infection
Thin and thick blood films were prepared from a finger prick. The thin film was fixed with methanol for a few seconds. Both blood films were then stained with 10% Giemsa stain for 15 min for malaria parasite identification and quantification. The stained blood smears were rinsed with running tap water for about 10 s and allowed to air dry. Malaria parasite species identification was performed by examining the thin film. Malaria parasite density was scored as parasites (trophozoites or gametocytes) per 200 white blood cells (WBCs) on the thick film. 200 high power fields of the thick films were examined at x100 magnification to assign a negative result. Parasite counts were converted to parasites/μL assuming 8000 W BCs/μL of blood.
2.5. Determination of haemoglobin concentration
Haemoglobin (Hb) was determined in the field using a portal HemoCue Hb301 photometer (HemoCue AB, Angelholm, Sweden). Blood drops from finger prick were collected to fill the microcuvette and inserted into the photometer as per manufacturer's instructions. Haemoglobin concentration was measured in g/dL. Haemoglobin concentration was grouped based on the severity of anemia as Hb < 7.0 g/dL (severe anemia), 7.0–11.0 g/dL (moderate anemia) and ≥11.0 g/dL (normal).
2.6. Questionnaire administration
A semi-structured questionnaire was designed, pre-tested and updated. Data on water contact activities (bathing, washing of clothes and swimming in streams and the like) were collected using the semi-structured questionnaire. The questionnaire was also used to capture school enrolment status and demographic information. The children were also asked if they have ever seen blood in their urine. All data forms were checked for completeness and any omissions corrected in the field before being entered into an EpiData version 3.1.
2.7. Statistical analysis
Data analysis was done using the open source statistical software R (version 3.4.1) [26]. Difference between means was analysed using the Wilcoxon signed ranked test with continuity correction since the data was not normally distributed. In order to assess the association of childhood activities, demographic factors and clinical symptoms with risk of schistosomiasis single infections and co-infection with malaria, a multi-variable logistic regression was implemented with a Poisson error distribution. Estimates were considered statistically significant at ∝ <0.05.
3. Results
3.1. Demographic and clinical characteristics
The study screened a total of 336 participants with mean age of 10.52 (Standard deviation: 2.27; range: 5–16 years), of which 50.6% were males. The overall prevalence of urinary schistosomiasis and P. falciparum malaria were 12.8% and 37.8%, respectively. About 6.0% of participants were coinfected with both S. haematobium and P. falciparum parasites. There was a statistically significant difference between the mean age of male (10.8) and female (10.2) participants (p-value = 0.029). The mean haemoglobin level of female was (12.18 ± 1.33) g/dL and male was (12.07 ± 1.06) g/dL. The difference was however not statistically significant (p = 0.39). Table 1 shows the prevalence of anemia based on WHO cutoffs for anemia definitions [27]. The overall prevalence of moderate anemia (Hb < 11.0 g/dL) among study participants (infected and non-infected) was 14.0% (47/336). Out of the total study participants who were only infected with P. falciparum (n = 107), 17.8% (19/107) had moderate anemia and none had severe anemia, 21.7% (5/23) of children infected with only S. haematobium had moderate anemia, and 4.3% (1/23) had severe anemia. Of the children who had concurrent P. falciparum and S. haematobium infections none had severe anemia and only 5.0% had moderate anemia (Table 1).
Table 1.
Prevalence of anemia in children with malaria, urinary schistosomiasis or concurrent infections.
| Disease group | Mean Age (SD)∗ | No. of children | Haemoglobin (Hb) concentration (g/dL) |
||
|---|---|---|---|---|---|
| Hb < 7.0 n∗∗ (%) | Hb < 11.0 n∗∗ (%) | Hb ≥ 11.0 n∗∗ (%) | |||
| Malaria | 10.85 (2.15) | 107 | 0 (0.0) | 19 (17.8) | 88 (82.2) |
| Schistosomiasis | 10.50 (2.84) | 23 | 1 (4.3) | 5 (21.7) | 16 (69.7) |
| Co-Infected | 10.40 (2.52) | 20 | 0 (0.00) | 1 (5.00) | 19 (95.00) |
SD = Standard Deviation. Mean age is recoded in completed years.
n = Number of children.
3.2. Intensity of S. haematobium infection
The overall mean S. haematobium egg count was 25 (range: 1 to 203 eggs)/10 ml of urine. The mean egg count for females (24 eggs/10 ml) was lower than males (26 eggs/10 ml), but the difference was not statistically significant (p = 0.930). The intensity of infection was highest in children less than 10 years with mean egg count of 34 eggs/10 ml of urine.
3.3. Comparison of hemoglobin levels between co-infections, single infections and non-infected children
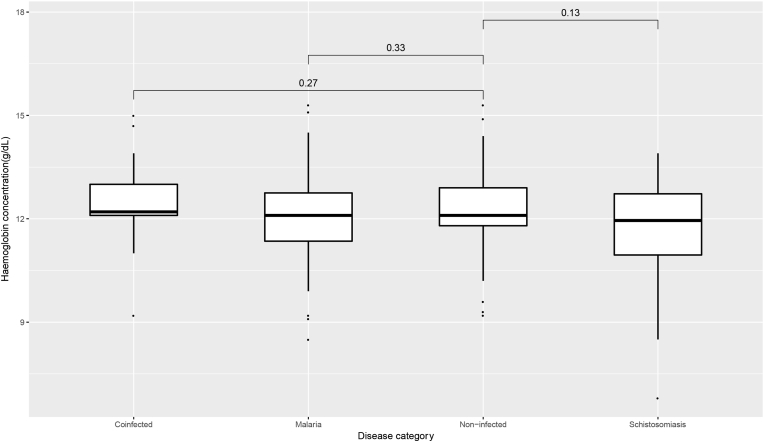
The median haemoglobin of children who were infected with only P. falciparum or S. haematobium was (12.13 ± 1.25) g/dL and (11.64 ± 1.68) g/dL respectively whilst that for non-infected and concurrently infected children was (12.27 ± 1.09) g/dL and (12.48 ± 1.27) g/dL respectively. Despite the comparatively lower median Hb concentration of children with single S. haematobium infection compared to non-infected children, a pairwise comparison of the median hemoglobin concentrations between the two groups was not statistically significant (P = 0.13). Likewise, the comparison of medians between malaria only (p = 0.33) or coinfected (p = 0.27) groups with the non-infected group (Figure 3) was not statistically significant.
Figure 3.
Median haemoglobin concentration in non-infected, malaria infected, Schistosoma infected and co-infected children from the KNED. Coinfected: Children infected with both P. falciparum and S. haematobium; Malaria: Children infected with only P. falciparum; Non-infected: Children not infected by both P. falciparum and S. haematobium; Schistosomiasis: Children infected with only S. haematobium. Pairwise estimates of differences in means are Wilcoxon p-values.
3.4. Risk factors for urinary schistosomiasis infections
Table 2 shows the association of childhood activities, demographic, and clinical factors with the risk of S. haematobium infection. It was observed that compared to males, female children had a significant reduced risk of urinary schistosomiasis in this region of northern Ghana (OR = 0.93; 95%CI = [0.87–0.99]; p = 0.005). In addition, the use of open water bodies such as dams, dug outs, and streams was found to be associated with a significant increased risk of urinary schistosomiasis infection (OR = 1.21; 95% CI = [1.06–1.39]; p = 0.001). Furthermore, the frequency of these activities was found to significantly increase the risk of infections. Absence from school was associated with 1.05 times increased risk of urinary schistosomiasis but this was not statistically significant (OR = 1.05; 95%CI = [0.98–1.13]; p = 0.14). Location was observed as marginally significant in the risk of urinary schistosomiasis. Children within the catchment area of the E/A school were found to have a reduced risk of urinary schistosomiasis (OR = 0.92; 95%CI = [0.85–0.99]; p = 0.046) than those of D/A primary school. Self-reported absence of haematuria had 0.81 times reduced risk of S. haematobium infection (OR = 0.81; 95%CI = [0.74–0.87]; p < 0.001) among the children [Table 2].
Table 2.
Clinical, Demographic and childhood activities and associated risk of urinary schistosomiasis infection.
| Characteristic | OR | LCI | UCI | P-value |
|---|---|---|---|---|
| Sex (Female) | 0.93 | 0.87 | 0.99 | 0.005 |
| Use of open water bodies1 | 1.21 | 1.06 | 1.39 | 0.001 |
| Frequency of activities/day | ||||
| 1 to 2 times | 1.24 | 1.12 | 1.36 | <0.001 |
| Between 3 to 5 times | 1.10 | 1.01 | 1.20 | 0.027 |
| Greater than 5 times | 1.22 | 1.06 | 1.40 | 0.005 |
| Absent from school | 1.05 | 0.98 | 1.13 | 0.14 |
| Location (E/A) | 0.92 | 0.85 | 0.99 | 0.046 |
| No blood in urine2 | 0.81 | 0.74 | 0.87 | <0.001 |
Open water bodies included streams, dam, dugouts/ponds and rice paddies. Activities include bathing, washing of clothes, utensils etc; OR, odds ratio; LCI and UCI is lower, and upper 95% confidence intervals respectively.
children who did not report seeing blood after passing urine.
4. Discussion
In the current study, the prevalence of S. haematobium single infection and co-infection with P. falciparum among school age children was surveyed. We observed the prevalence of S. haematobium infection to be 12.8%, which is a lower rate of infection compared to previous estimates of 18.9% within the study communities in the northern part of the Kassena-Nankana District by Anto et al in 2014 [28]. Despite over a decade of annual mass drug administration (MDA) with Praziquantel targeted at mainly in-school children, our findings suggest persistence of high focal urinary schistosomiasis in the phase of this intervention program. This may be attributed to several factors, key among these being poor coverage of MDA target group below the WHO benchmark of 75%–100% of school-age children at risk of morbidity to have been achieved by 2010 [29], and/or sub-optimal treatments due to reduced Praziquantel efficacy either as a result of resistance or under dosing resulting from poor weight estimates by school health teachers. The poor coverage may be due to absenteeism in schools in the area and programme challenges such as irregular drug supply. Indeed, our data shows that absenteeism in Nakolo D/A and E/A primary schools were 53.9% and 46.4% respectively during the period of sampling. Therefore, a significant proportion of children in the study area are unlikely to have received the MDA treatment year in year out. Similar surveys in other parts of Ghana, have shown that average uptake of Praziquantel targeted at school age children (SAC) can go as low as 41% but varies to as high as 85% [16]. Therefore, others have argued for an expansion of the MDA for SAC to include adults, pre-school children and school-aged children who are out of school using community-based distribution, though this has been difficult to implement due to logistic challenges including inadequate supply of drugs.
In addition, interventions that include Water, Sanitation and Hygiene (WASH) have been shown to be highly effective in reducing exposure to, and transmission of eggs and larvae for Schistosomiasis [30, 31]. Therefore, in order to drive down the prevalence of urinary schistosomiasis in these endemic communities, it may be necessary to augment MDA chemotherapy with WASH and other interventions such as health promotion to achieve prevention of reinfection [30].
The study area is malaria endemic with perennial P. falciparum malaria transmission and significant seasonal fluctuation in intensity. The high prevalence (37.8%) of P. falciparum malaria observed in this study was no surprise but the 6.0% prevalence of concurrent infection by both parasites is of public health concern since these asymptomatic carriers act as reservoirs that continue to fuel malaria transmission and urinary schistosomiasis transmission by contaminating fresh water bodies with their excreta that contain schistosome eggs. Similar studies in peri-urban communities in Greater Accra, where malaria transmission is much lower reported 12.9% prevalence of P. falciparum mono-infections and 4.7% S. haematobium mono infection [32]. The prevalence of co-infection with both parasites was under 1%. In Ghana, malaria transmission is heterogenous, with parasite prevalence reflective of the three varying ecological zones, with the lowest prevalence along the Coastal areas [33]. Therefore, these conflicting reports may reflect the influence of the varying transmission dynamics of these two parasites, with transmission intensity being higher in the Kassena-Nankana Districts compared to Accra [34]. In addition, because malaria transmission is seasonal across Ghana, the potential for co-infection with both parasites may differ markedly between surveys depending on the season of the school survey. Nonetheless, such local foci of parasite co-infection when unidentified, may undermine intervention efforts at controlling the two parasitic diseases of topmost public health importance in Ghana. Identifying such local ‘hotspots’ of high prevalence for endemic pathogens such as these is critical for targeting of limited resources to interrupt transmission.
Though previous reports have shown that children who are concurrently infected with malaria and urinary schistosomiasis were found to have lower haemoglobin (Hb) concentration compared to those with single infections and uninfected, we did not observe any significant differences in the mean Hb concentration between children co-infected with S. haematobium and P. falciparum and uninfected children (Figure 2). Rather, children who were infected with only S. haematobium had lower Hb concentrations compared to those diagnosed with malaria or concurrent infections of S. haematobium and P. falciparum. 21.7% of children who had urinary schistosomiasis alone had moderate to severe anemia, suggesting that chronic urinary schistosomiasis may be an important cause of childhood anemia as compared to asymptomatic malaria in this population. Indeed, soil-transmitted helminth (STH) studies, in the middle-belt of Ghana showed that children with low-intensity hookworm infections had reduced risk of anemia [35], and successful treatment of hookworm infection was associated with improved haemoglobin levels in children [36]. Coinfections with malaria was a significant predictor of hookworm infection but not anemia in these studies conducted in the middle belt of Ghana [37]. These contrasting findings are indicative of the gross nature of anemia as a phenotype, which could have other underlining courses other than parasitic infections. Indeed, the current study was conducted at the time of harvest, when food is abundant and children in these settings generally show improved wellbeing which could potentially ameliorate the effects of these infections on Hb levels in the comparison groups. In addition, we found no significant correlation between S. haematobium egg counts/intensity and Hb levels, though a study in Ethiopia by Deribew et al. [8] found a significant correlation between egg counts and Hb concentration. The current study also shows age variation in the intensity of S. haematobium infection with children less than 10 years having the higher mean egg counts. Exposure to contaminated water alone cannot explain these findings, which have also been reported elsewhere, and that slow acquisition of immunity may play role [38]. In tandem with several studies that could not establish any significant difference between the intensity of infection among females and males [39, 40, 41], we found higher intensity of infection among males than females but the difference was not statistically significant.
We explored risk factors for urinary schistosomiasis and observed that female children had a reduced risk of S. haematobium infection than males (Table 2). This is consistent with previous studies in northern Ghana and other schistosomiasis endemic areas, which showed that the risk of schistosomiasis was higher in males than in females [42, 43, 44]. It is plausible that differences in gender roles due to cultural norms may result in relatively higher frequency of exposure to infection for male than females. It was not surprising that childhood activities such as use of streams, dams, dugouts and other open water bodies for various activities including bathing, recreational activities such as swimming significantly increased the risk of urinary schistosomiasis (Table 2). These findings are corroborated by several studies that have reported proximity to cercariae infested fresh water bodies as risk factor for school-age children [39, 43, 45, 46, 47]. As observed in other studies conducted in this area [44], the present study shows that children who were involved in activities that frequently exposed them to these open water bodies had an increased individual risk of urinary schistosomiasis infection (Table 2). Interestingly, our data also shows that there was increased risk of urinary schistosomiasis infection among children with more school absence, though not statistically significant. However, location significantly influenced individual risk of infection. Children who attended the E/A school were less likely to have urinary schistosomiasis compared to those attending the D/A school. Risk of schistosome infection may vary depending on the number and type of open waters within a particular locality. The current study area has a non-uniform distribution of stagnant open waters some of which often dry up during the long dry season. Thus, affecting the risk and distribution within the present study area. It was observed that self-reported absence of haematuria significantly reduced the risk of children from S. haematobium infection, which is biologically plausible because of the ability of S. haematobium eggs to penetrate the urinary tract and cause damage resulting in blood in urine when urinating [1, 14]. Therefore, haematuria remains a key symptom for diagnosis of urinary schistosomiasis in rural settings where laboratory diagnosis is not possible.
5. Conclusion
This study has revealed that urinary schistosomiasis remains prevalent in Kassena-Nankana districts with significant variation in the risk of infection among school-age children depending on locality. The findings suggest that chronic urinary schistosomiasis alone may contribute to moderate anemia among school-age children as compared to asymptomatic malaria infection. These findings call for an evaluation of the annual mass drug administration of Praziquantel among in-school children to ascertain its impact on urinary schistosomiasis prevalence across the district. Also, the current data points to an urgent need for the District Health Management Team in this area to focus attention on out of school children and scale up of water, sanitation and hygiene activities and health education in order to interrupt transmission of schistosomiasis in this area where co-morbidity with P. falciparum is common.
6. Limitations of the study
The current study is limited in scope and therefore, the level of urinary schistosomiasis reported could be an understatement of the actual prevalence among school-age children. A well-designed randomized study of school age children within the Kassena-Nankana districts is required to uncover the actual prevalence of urinary schistosomiasis and to estimate co-infection with Plasmodium spp in the study population. Several studies have shown improved sensitivity of the 10 ml urine filtration method for detection of S. haematobium ova when multiple urine samples or slides are used. However, due to limited funding (self-funded), two consecutive samples were examined before declaring a participant negative. Therefore, S. haematobium egg density estimates might have been adversely affected by the limited number of urine replicates processed in this study. In addition, for logistic reasons the study sample size was small and aimed at being representative of children 5–16 years in schools sampled rather than the community. Indeed, the small sample size might have limited the power of the current study to detect any correlation of these parasitic infections (either mono- or co-infections) with anemia. Notwithstanding these limitations, our findings provide a reappraisal of the prevalence of urinary schistosomiasis, and co-morbidity with malaria in the district for evidence-based decision making and calls for study design evaluation of these intervention programmes.
Declarations
Author contribution statement
Sylvester Dassah: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gideon K. Asiamah: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Valentine Harun: Performed the experiments; Wrote the paper.
Kwaku Appiah-Kubi: Conceived and designed the experiments; Wrote the paper.
Abraham Oduro: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Victor Asoala: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lucas Amenga-Etego: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are appreciative of the enthusiasm with which pupils of Nakolo D/A and E/A primary schools participated in this study and the support of the staff. To Gideon Akunjaab, we are grateful for your support and contributions towards the development of this idea. We are also grateful to the staff of the Navrongo Health Research Centre laboratory for contributing in diverse ways to support this project. To Miss Genita Ami-soo Mbabila, thank you for helping in the study participants recruitment process and sample collection and processing. To Miss Dorcas Asabre, we say thank you for your support in our field activities.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sousa-Figueiredo J.C., Gamboa D., Pedro J.M., Fançony C., Langa A.J., Magalhães R.J.S., et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance System in northern Angola. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Key Facts Schistosomiasis | Epidemiological Situation, World Health Organization, Geneva,. In: WHO [Internet]. 17 Apr 2019 [cited 4 Jun 2019]. Available: http://www.who.int/schistosomiasis/epidemiology/en/.
- 4.Anto F. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in rural northern Ghana. J. Bacteriol. Parasitol. 2013;4 [Google Scholar]
- 5.Shiff C., Veltri R., Naples J., Quartey J., Otchere J., Anyan W., et al. Ultrasound verification of bladder damage is associated with known biomarkers of bladder cancer in adults chronically infected with Schistosoma haematobium in Ghana. Trans. R. Soc. Trop. Med. Hyg. 2006;100:847–854. doi: 10.1016/j.trstmh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Forsyth D.M., MacDonald G. Urological Complications of Endemic Schistosomiasis in School-Children: Part 1. Usagara School. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1965;59:171–178. doi: 10.1016/0035-9203(65)90078-7. [DOI] [PubMed] [Google Scholar]
- 7.King C.H., Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chron. Illness. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 8.Deribew K., Tekeste Z., Petros B. Urinary schistosomiasis and malaria associated anemia in Ethiopia. Asian Pac. J. Trop. Biomed. 2013;3:307–310. doi: 10.1016/S2221-1691(13)60068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briand V., Watier L., Hesran J.-Y.L., Garcia A., Cot M. Coinfection with plasmodium falciparum and schistosoma haematobium: protective effect of schistosomiasis on malaria in SENEGALESE children? Am. J. Trop. Med. Hyg. 2005;72:702–707. [PubMed] [Google Scholar]
- 10.Kinung’hi S.M., Magnussen P., Kaatano G.M., Kishamawe C., Vennervald B.J. Malaria and helminth Co-infections in school and preschool children: a cross-sectional study in magu district, north-western Tanzania. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mboera L.E.G., Senkoro K.P., Rumisha S.F., Mayala B.K., Shayo E.H., Mlozi M.R.S. Plasmodium falciparum and helminth coinfections among schoolchildren in relation to agro-ecosystems in Mvomero District, Tanzania. Acta Trop. 2011;120:95–102. doi: 10.1016/j.actatropica.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Brooker S.J., Pullan R.L., Gitonga C.W., Ashton R.A., Kolaczinski J.H., Kabatereine N.B., et al. Plasmodium–helminth coinfection and its sources of heterogeneity across East Africa. J. Infect. Dis. 2012;205:841–852. doi: 10.1093/infdis/jir844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diallo T.O., Remoue F., Gaayeb L., Schacht A.-M., Charrier N., Clerck D.D., et al. Schistosomiasis coinfection in children influences acquired immune response against Plasmodium falciparum malaria antigens. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman J.F., Kanzaria H.K., McGarvey S.T. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham L.J., Campbell S.J., Armoo S., Koukounari A., Watson V., Selormey P., et al. Assessing expanded community wide treatment for schistosomiasis: baseline infection status and self-reported risk factors in three communities from the Greater Accra region, Ghana. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0007973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abass Q., Bedzo J.Y., Manortey S. Impact of mass drug administration on prevalence of schistosomiasis in eight riverine communities in the asuogyaman district of the eastern region, Ghana. Int. J. Trop. Dis. Health. 2020:18–32. [Google Scholar]
- 17.Aboagye I.F., Edoh D. Investigation of the risk of infection of urinary schistosomiasis at mahem and galilea communities in the greater Accra region of Ghana. West Afr. J. Appl. Ecol. 2009;15 [Google Scholar]
- 18.Aryeetey M.E., Wagatsuma Y., Yeboah G., Asante M., Mensah G., Nkrumah F.K., et al. Urinary schistosomiasis in southern Ghana: 1. Prevalence and morbidity assessment in three (defined) rural areas drained by the Densu river. Parasitol. Int. 2000;49:155–163. doi: 10.1016/s1383-5769(00)00044-1. [DOI] [PubMed] [Google Scholar]
- 19.Lima e Costa M.F., Magalhães M.H., Rocha R.S., Antunes C.M., Katz N. Water-contact patterns and socioeconomic variables in the epidemiology of schistosomiasis mansoni in an endemic area in Brazil. Bull. World Health Organ. 1987;65:57–66. [PMC free article] [PubMed] [Google Scholar]
- 20.Lo N.C., Addiss D.G., Hotez P.J., King C.H., Stothard J.R., Evans D.S., et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect. Dis. 2017;17:e64–e69. doi: 10.1016/S1473-3099(16)30535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber B.E., Rajahram G.S., Grigg M.J., William T., Anstey N.M. World Malaria Report: time to acknowledge Plasmodium knowlesi malaria. Malar. J. 2017;16:135. doi: 10.1186/s12936-017-1787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Opoku E.C., Olsen A., Browne E., Hodgson A., Awoonor-Williams J.K., Yelifari L., et al. Impact of combined intermittent preventive treatment of malaria and helminths on anaemia, sustained attention, and recall in Northern Ghanaian schoolchildren. Glob. Health Action. 2016;9 doi: 10.3402/gha.v9.32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oduro A.R., Wak G., Azongo D., Debpuur C., Wontuo P., Kondayire F., et al. Profile of the Navrongo health and demographic surveillance System. Int. J. Epidemiol. 2012;41:968–976. doi: 10.1093/ije/dys111. [DOI] [PubMed] [Google Scholar]
- 24.Koram K.A., Owusu-Agyei S., Fryauff D.J., Anto F., Atuguba F., Hodgson A., et al. Seasonal profiles of malaria infection, anaemia, and bednet use among age groups and communities in northern Ghana. Trop. Med. Int. Health. 2003;8:793–802. doi: 10.1046/j.1365-3156.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 25.Okanla E.O., Agba B.N., Awotunde J.O. Schistosoma haematobium: prevalence and socio-economic factors among students in Cape Coast Ghana. Afr. J. Biomed. Res. 2003;6 [Google Scholar]
- 26.R Core T. R . R Foundation for Statistical Computing; Vienna, Austria: 2017. A Language and Environment for Statistical Computing.https://www.R-project.org/ Available: [Google Scholar]
- 27.WHO. WHO | Archived: Iron Deficiency Anaemia: Assessment, Prevention and Control. In: WHO [Internet]. [cited 3 Jun 2019]. Available: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/WHO_NHD_01.3/en/.
- 28.Francis Anto V.A. Childhood activities and schistosomiasis infection in the kassena-nankana district of northern Ghana. J. Infect. Dis. 2014;2 [Google Scholar]
- 29.WHO WHO Convenes Experts to Sustain Progress against Soil-Transmitted Helminthiases and Schistosomiasis. In: WHO [Internet]. [cited 6 Jun 2019]. Available: http://www.who.int/neglected_diseases/news/WHO_convenes_experts_to_sustain_progre/en/.
- 30.Campbell S.J., Savage G.B., Gray D.J., Atkinson J.-A.M., Magalhães R.J.S., Nery S.V., et al. Water, sanitation, and hygiene (WASH): a critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Neglected Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosinski K.C., Adjei M.N., Bosompem K.M., Crocker J.J., Durant J.L., Osabutey D., et al. Effective control of schistosoma haematobium infection in a Ghanaian community following installation of a water recreation area. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyarko R., Torpey K., Ankomah A. Schistosoma haematobium, Plasmodium falciparum infection and anaemia in children in Accra, Ghana. Tropical Diseases. Travel Medicine and Vaccines. 2018;4:3. doi: 10.1186/s40794-018-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awine T., Malm K., Bart-Plange C., Silal S.P. Towards malaria control and elimination in Ghana: challenges and decision making tools to guide planning. Glob. Health Action. 2017;10 doi: 10.1080/16549716.2017.1381471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mensah-Brown H.E., Abugri J., Asante K.P., Dwomoh D., Dosoo D., Atuguba F., et al. Assessing the impact of differences in malaria transmission intensity on clinical and haematological indices in children with malaria. Malar. J. 2017;16:96. doi: 10.1186/s12936-017-1745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humphries D., Mosites E., Otchere J., Twum W.A., Woo L., Jones-Sanpei H., et al. Epidemiology of hookworm infection in kintampo North municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am. J. Trop. Med. Hyg. 2011;84:792–800. doi: 10.4269/ajtmh.2011.11-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humphries D., Nguyen S., Kumar S., Quagraine J.E., Otchere J., Harrison L.M., et al. Effectiveness of albendazole for hookworm varies widely by community and correlates with nutritional factors: a cross-sectional study of school-age children in Ghana. Am. J. Trop. Med. Hyg. 2017;96:347–354. doi: 10.4269/ajtmh.16-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adu-Gyasi D., Asante K.P., Frempong M.T., Gyasi D.K., Iddrisu L.F., Ankrah L., et al. Epidemiology of soil transmitted Helminth infections in the middle-belt of Ghana, Africa. Parasite Epidemiology and Control. 2018;3 doi: 10.1016/j.parepi.2018.e00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tukahebwa E.M., Magnussen P., Madsen H., Kabatereine N.B., Nuwaha F., Wilson S., et al. A very high infection intensity of schistosoma mansoni in a Ugandan lake victoria fishing community is required for association with highly prevalent organ related morbidity. PLoS Neglected Trop. Dis. 2013;7:e2268. doi: 10.1371/journal.pntd.0002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alebie G., Erko B., Aemero M., Petros B. Vol. 7. Parasites & Vectors; 2014. p. 15. (Epidemiological Study on Schistosoma Mansoni Infection in Sanja Area, Amhara Region, Ethiopia). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amuta E., Houmsou R. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pacific Journal of Tropical Medicine. 2014;7:34–39. doi: 10.1016/S1995-7645(13)60188-1. [DOI] [PubMed] [Google Scholar]
- 41.Morenikeji O.A., Idowu B.A. Studies on the prevalence of urinary schistosomiasis in ogun state, south-western Nigeria. W. Afr. J. Med. 2011;30:62–65–65. doi: 10.4314/wajm.v30i1.69921. [DOI] [PubMed] [Google Scholar]
- 42.Chipeta M.G., Ngwira B., Kazembe L.N. Analysis of schistosomiasis haematobium infection prevalence and intensity in chikhwawa, Malawi: an application of a two Part Model. PLoS Neglected Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Waleedi A.A., El-Nimr N.A., Hasab A.A., Bassiouny H.K., Al-Shibani L.A. Urinary schistosomiasis among schoolchildren in Yemen: prevalence, risk factors, and the effect of a chemotherapeutic intervention. J. Egypt. Publ. Health Assoc. 2013;88:130. doi: 10.1097/01.EPX.0000441277.96615.96. [DOI] [PubMed] [Google Scholar]
- 44.Anto F. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in rural northern Ghana. J. Bacteriol. Parasitol. 2013;4 [Google Scholar]
- 45.Sady H., Al-Mekhlafi H.M., Mahdy M.A.K., Lim Y.A.L., Mahmud R., Surin J. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Neglected Trop. Dis. 2013;7:e2377. doi: 10.1371/journal.pntd.0002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senghor B., Diallo A., Sylla S.N., Doucouré S., Ndiath M.O., Gaayeb L., et al. Vol. 7. Parasites & Vectors; 2014. p. 5. (Prevalence and Intensity of Urinary Schistosomiasis Among School Children in the District of Niakhar, Region of Fatick, Senegal). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bogoch, Andrews J.R., Ephraim R.K.D., Utzinger J. Simple questionnaire and urine reagent strips compared to microscopy for the diagnosis of Schistosoma haematobium in a community in northern Ghana. Trop. Med. Int. Health. 2012;17:1217–1221. doi: 10.1111/j.1365-3156.2012.03054.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.