Abstract
Background:
There is a shortage of clinical studies examining the efficacy of Nicotinamide Adenine Dinucleotide and Enkephalinase infusions (IV1114589NAD) in treating Substance Use Disorder (SUD).
Objective:
This study aims to provide evidence that IV1114589NAD infusions significantly attenuate substance craving behavior.
Methods:
The study cohort consisted of addicted poly-drug, mixed gender, multi-ethnic individuals resistant to standard treatment. The investigation utilized Likert-Scales to assess behavioral outcomes.
Results:
Using Wilcoxon signed-rank tests and sign tests, our team detected significant results by comparing baseline to post outcome scores after IV1114589NAD injections: craving scores (P=1.063E-9); anxiety (P=5.487E-7); and depression (P=1.763E-4). A significant reduction in cravings, anxiety, and depression followed a dose-dependent linear trend. Linear trend analyses showed a significant relationship between NAD infusions and decreasing scores for cravings (P=0.015), anxiety (P=0.003), and depression (P=8.74E-5). A urine analysis was conducted on a subset of 40 patients midway through the study to assess relapse; 100% of the urine samples analyzed failed to detect illicit substance use.
Discussion:
The opioid crisis in America has claimed close to 800,000 lives since 2004; daily deaths are estimated to stand at 127, and in 2021, over 107,000 deaths were due to overdose. There is an urgency to find safe, side-effect-free solutions. Current interventions, such as Naltrexone implants, are invasive and may interfere with dopamine homeostasis leading to an anti-reward phenomenon. Larger randomized double-blinded placebo-controlled studies are needed to elucidate further the significance of the results presented in this study. The current pilot study provides useful preliminary data regarding the effectiveness of IV1114589NAD infusions in SUD treatment.
Conclusion:
This pilot study provides significant evidence that NAD infusions are beneficial in the treatment of SUD. This investigation serves as a rationale to extend these findings onto future research investigating the use of NAD/NADH as a stand-alone treatment, especially in patients showing high genetic risk as measured in the Genetic Addiction Risk Severity (GARS) test. Utilizing GARS will help provide a real personalized therapeutic approach to treat Reward Deficiency Syndrome (RDS).
Keywords: Nicotinamide adenine dinucleotide (ND+) infusions, cravings, anxiety depression, dopamine homeostasis, reward deficiency syndrome (RDS), Medication Assistant Treatment (MAT)
1. INTRODUCTION
1.1. Understanding Reward Deficiency and Required Dopaminergic-Homeostasis
This article of original research proposes a unique combination of coupling the compound Nicotinamide adenine dinucleotide (NAD+) with known Enkephalinase Inhibitors (EI), including DL-Phenylalanine, [1] to help detoxify and treat individuals diagnosed with reward deficiency as manifested by Substance Use Disorder (SUD). This article describes intravenous therapy of a specialized cocktail consisting of B vitamins, NAD and DLPA, and other amino acids (IV1114589NAD).
It is widely acknowledged that in both food and drug-addicted individuals, there is dopamine resistance because of an association with the DRD2 gene A1 allele [2–4]. Evidence is emerging for utilizing a natural, non-addicting, safe, putative D2 agonist as a means to recover from reward deficiency syndrome (RDS) for patients addicted to psychoactive chemicals [4]. Employing quantitative electroencephalography (qEEG) as an imaging tool, we show the impact of Synaptamine Complex Variant KB220™ containing DLPA as a putative activator of the mesolimbic system [3–5]. For the first time, it has been shown that the KB220z variant, given via intravenous administration, decreases or “normalizes” aberrant electrophysiological parameters of the reward circuitry site [6]. For that published pilot study [6], it has been found that the qEEG’s of a heroin abuser and an alcoholic demonstrate abnormalities (widespread alpha and theta activity respectively) during protracted abstinence; however, their qEEG’s were significantly normalized by the administration of 1 intravenous dose of Synaptamine Complex Variant KB220Z™. Specifically, both patients were genotyped for a variety of neurotransmitter reward genes to ascertain the extent of putative dopaminergic risk alleles they carry that may predispose them to heroin or alcohol dependence, respectively. The tested genes included the dopamine transporter (DAT1, locus symbol SLC6A3), DRD2 TaqIA (rs1800497), dopamine D4 receptor exon 3 VNTR (DRD4), monoamine oxidase A upstream VNTR (MAOA-uVNTR), serotonin transporterlinked polymorphic region (5HTTLPR, locus symbol SLC6A4), and COMT val158 met SNP (rs4680). It has been maintained that these are case studies, and it would be unlikely for all individuals to carry the relevant putative risk alleles. Based on our previous research and qEEG studies, we cautiously suggest that long-term activation of dopaminergic receptors (i.e, DRD2 receptors) will result in their proliferation and lead to an increased “dopamine sensitivity” and a greater sense of happiness, particularly in carriers of the DRD2 A1 allele [7].
Moreover, this initial work is supported by a clinical trial on Synaptamine Complex Variant KB220™ utilizing intravenous administration in more than 600 alcoholic patients, resulting in significant reductions in RDS behaviors [8]. It is noteworthy that Substance Use Disorders (SUD) are quite heritable. Reward genes play a major role in the hypodopaminergic functioning observed in SUDs. We evaluated the natural dopaminergic agonist, KB220 intravenous (IV), and its oral variants, to enhance dopaminergic function in SUD. Our pilot experiment revealed a significant reduction of chronic symptoms, measured by the Chronic Abstinence Symptom Severity (CASS) Scale. The combined group (IV and oral) did significantly better than the oral-only group over the first week and a 30-day follow-up period. Following this, the combination was given to 129 subjects, and three factors were measured: Emotion, Somatic, and Impaired Cognition. Each had eigenvalues larger than one, and they were extracted for baseline CASS-Revised (CASS-R) variables. Paired sample t-tests for pre and post-treatment scales showed significant reductions (p = .00001) from pre-treatment to post-treatment. The values were t = 19.1 for Emotion, t = 16.1 for Somatic, and t = 14.9 for Impaired Cognition. A two-year follow-up of 23 subjects who experienced KB220IV therapy, which includes at least five IV treatments over seven days and oral treatments for 30+ days, revealed that 21 (91%) of the subjects were sober at six months and 19 (82%) had no relapse. 19 (82%) subjects were sober at one year, and 18 (78%) had no relapse. 21 (91%) subjects were sober two-years post-treatment, and 16 (70%) had no relapse. We await additional research and advise caution in interpreting these encouraging results. It is also confirmed by the growing number of oral studies, encompassing at least 38 on the Complex KB220Z™ [9].
Additionally, the powerful effects of KB220, as evidenced by more recent neuroimaging studies, have clearly shown the importance of pro-dopamine regulation along with the Brain Reward Cascade (BRC) (Fig. 1). There is evidence that modifications in synchronous neural activity between brain regions involved in reward and other cognitive functions may significantly contribute to substance-related disorders. Our previous work by Febo et al. [10] provided the first evidence demonstrating that the pro-dopaminergic nutraceutical KB220Z significantly increases (above placebo) the functional connectivity between reward and cognitive brain regions in rats. The following regions affected included: the nucleus accumbens, anterior cingulate gyrus, anterior thalamic nuclei, hippocampus, prelimbic and infralimbic loci. In addition, significant functional connectivity, increased brain connectivity volume recruitment (potentially neuroplasticity), and dopaminergic functionality were found across the brain reward circuitry. Most importantly, increases in functional connectivity were specific to these regions and were not broadly dispersed across the brain. Though these initial findings have been observed in drug naïve rodents, this robust yet selective response suggests clinical relevance for addicted individuals at risk for relapse, who show reductions in functional connectivity after protracted withdrawal.
Fig. (1).
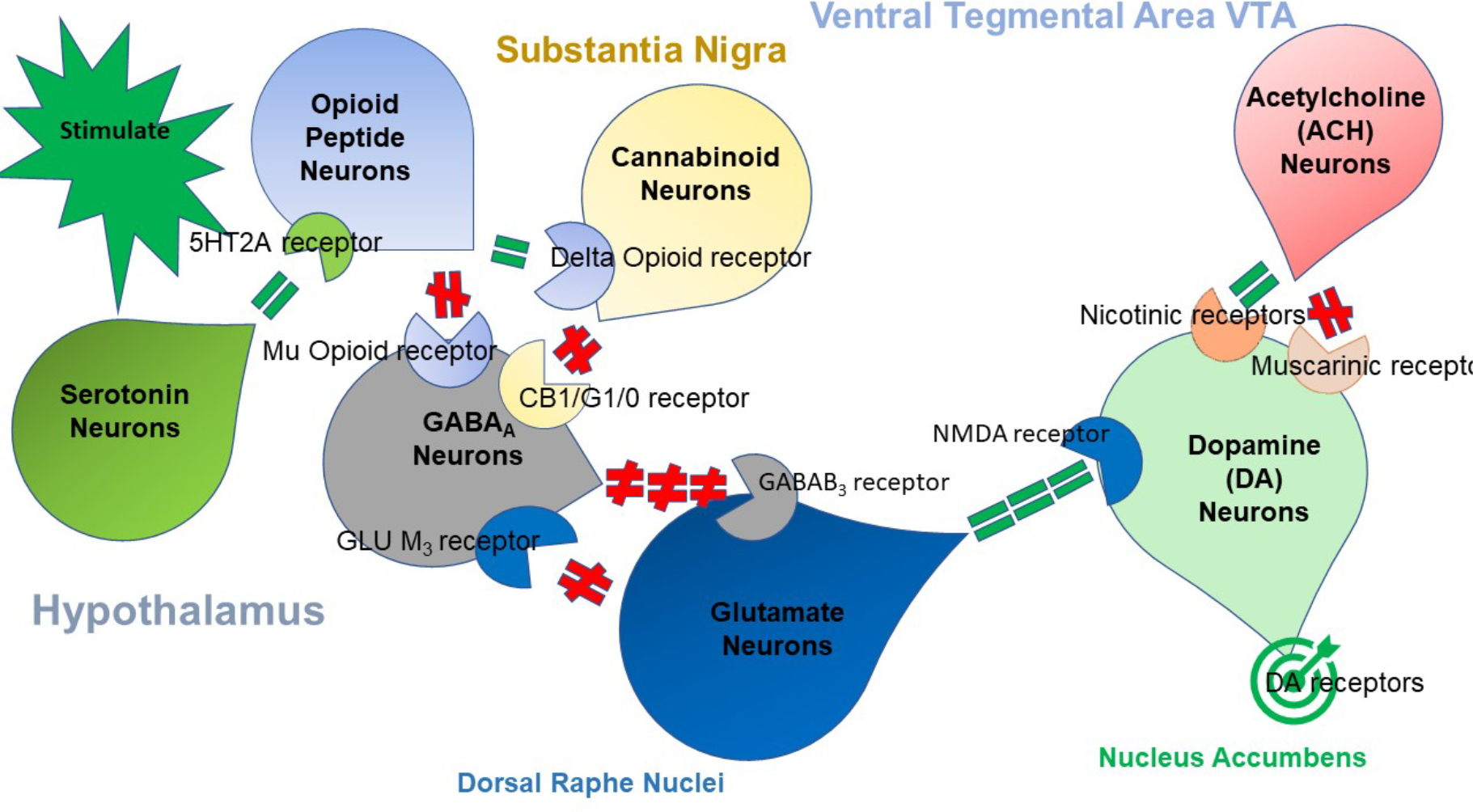
The brain reward cascade. “Reprinted from Publication Author(s), Gold MS, Baron D, Bowirrat A, Blum K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome? J Neurol Sci. 2020 Nov 15;418:117137. doi: 10.1016/j.jns.2020.117137. Epub 2020 Sep 15. PMID: 32957037; PMCID: PMC7490287.with permission.
Furthermore, our laboratory also showed the effect of KB220Z™ on reward circuitry for ten heroin addicts undergoing protracted abstinence (on average 16.9 months). In a placebo-controlled, randomized crossover study of KB220Z, five subjects participated in a triple-blinded experiment where the subject, the person evaluating the response to treatment, and the person administering the treatment were all blinded to the treatment that any individual subject was receiving. Additionally, nine subjects were genotyped utilizing the Genetic Addiction Risk Score (GARS) test. Previously, Blum’s group [11] preliminarily reported that KB220Z induced an increase in BOLD activation in caudate-accumbens-dopaminergic pathways relative to placebo, following a one-hour acute administration. Moreover, KB220Z also decreased resting-state activity in the putamen of abstinent heroin addicts. For the second phase of this pilot study, we noted that three brain regions were significantly activated from the resting-state by KB220Z relative to placebo (p < 0.05) for all 10 abstinent heroin-dependent subjects. Enhanced functional connectivity was observed in a putative network, which includes the medial frontal gyrus, nucleus accumbens, posterior cingulate, occipital cortical areas, cerebellum, and dorsal anterior cingulate.
Future studies are necessary, utilizing both functional magnetic resonance imaging and positron emission tomography scanning, to ascertain the acute and chronic effects of oral and or intravenous KB220™ on numbers of D2 receptors and the direct interactions at the nucleus accumbens. Verification of these results in large, populationbased, case-controlled experiments is needed. These studies would offer important information that could eventually lead to significant improvement in recovery for individuals with RDS and dopamine deficiency, as a consequence of multiple neurotransmitter signal transduction breakdowns in the brain reward cascade (Fig. 1).
Fig. (1) illustrates the interaction of at least seven major neurotransmitter pathways involved in the Brain Reward Cascade (BRC). In the hypothalamus, environmental stimulation can cause the release of serotonin. After binding to the relevant receptors, for example, 5HT-2a receptors, this would activate (green equal sign) the release of opioid peptides from opioid peptide neurons, which are also in the hypothalamus. Subsequently, the opioid peptides can have two distinct effects, possibly via two different opioid receptors. One that inhibits (red hash sign) through the mu-opioid receptor (possibly via enkephalin) and projects to the substania nigra to GABAA neurons. Another stimulates (green equal sign) cannabinoid neurons (e.g., 2-archydonoglcerol and anandamide) through beta–endorphin-linked delta receptors, which in turn, results in inhibition of GABAA neurons at the substania nigra. Activated cannabinoids, primarily 2-archydonoglcerol, can indirectly cause disinhibition (red hash sign) of GABAA neurons in the substania nigra by activating G1/0 coupled to CB1 receptors. Glutamate neurons within the dorsal raphe nuclei (DRN) can also indirectly disinhibit GABAA neurons in the substania nigra through activation of GLU M3 receptors (red hash sign). GABAA neurons, when stimulated, will powerfully (red hash signs) inhibit VTA glutaminergic drive via GABAB 3 neurons. It is also possible that stimulation of ACH neurons at the Nucleus Accumbens can stimulate both muscarinic (red hash) or nicotinic (green hash). Finally, glutamate neurons in the VTA will project to dopamine neurons through NMDA receptors (green equal sign) to preferentially release dopamine at the nucleus accumbens (NAc), shown as a bullseye, which indicates a euphoria or “wanting” response. The result is that when dopamine release is low, unhappiness or endorphin Deficiency follows. At the same time, general (usual) happiness depends on the dopamine homeostatic tonic set point.
These results, along with other quantitative electroencephalogy (qEEG) study results, suggest a putative [12] anti-craving/anti-relapse role of KB220Z in psychostimulant addiction by direct or indirect dopaminergic interaction. Recently, Willuhn et al., [13] reported that cocaine use, as well as non-substance-related addictive behaviors, increase as dopaminergic function is decreased. Chronic cocaine exposure has been associated with reductions in D2/D3 receptors and lower activation of cues in the occipital cortex and cerebellum, which was found in a PET study by Park et al. [14] Therefore, treatment strategies, such as dopamine agonist therapy that conserves dopamine function, could be an interesting approach to prevention of relapse in psychoactive drug and behavioral addictions.
1.2. Enkephalinase Inhibition (EI) and Addiction
There are a number of compounds that have been shown to inhibit the degradation of enkephalins [14]. Enkephalinase Inhibitors (EIs) appear to be promising as therapeutic agents due to their analgesic properties, which are accomplished by increasing enkephalins. Endogenous EIs include peptides like opiorphin and spinorphin. Endogenous and synthetic inhibitors of enkephalindegrading enzymes have been studied in vivo utilizing standard animal models. The potential EI targets seem to be APN (Aminopeptidase N), NEP (Neutral endopeptidase), and DPP-III (Dipeptidyl peptidase). EIs possess a distinct advantage in that they lack the side effects of opioids. Along these lines, it is important to understand the regulation, synthesis, and expression of receptors, particularly the catabolism (role of proteolytic enzymes) of brain endorphins and especially enkephalins [15, 16]. It is currently known that these enkephalinase inhibitors prevent the degradation of enkephalins, whereby these compounds produce naloxone reversible analgesia and potentiate the analgesia produced by enkephalins and acupuncture [17–20]. D-phenylalanine has proven to be beneficial in many human patients with chronic, intractable pain [21, 22]. Blum’s group [2] proposed that enkephalinase inhibitors may be effective in a number of human “endorphin deficiency diseases” such as depression, schizophrenia, convulsive disorders, and arthritis. Cheng et al., [22] revealed that the D-amino acids (DAA), D-phenylalanine, and D-leucine, produce naloxone reversible analgesia; electroacupuncture (EA) also produces analgesia, which is blocked by naloxone. Combining the two treatments produces an additive effect with larger analgesia than that produced by either treatment given alone. This combined effect is also blocked by naloxone. Moreover, only 62% of the mice show EA analgesia, and 53% show D-amino acid (DAA) analgesia; 80% of the animals show marked analgesia with both EA plus DAA treatment.
In addition, EIs may alleviate other conditions associated with decreased endorphin levels, such as alcohol/opiate withdrawal symptoms [23]. In the Blum et al. [24] review article, they point out that the consensus of the literature supports the concept that brain neurotransmitters and second messengers are involved in the net release of dopamine in the mesolimbic region, especially the nucleus accumbens (NAc). Furthermore, the release of neuronal dopamine is directly linked to motivation, anti-stress, incentive salience (wanting), and well-being. The role of dopamine in terms of alcohol withdrawal symptomology, cocaine-craving behavior, dopamine-condensation products (TIQs), and, more recently, the genetic aspects of drug-seeking and pro-dopamine regulation, provide compelling evidence of the relevant molecular neurological correlates of dopaminergic/endorphinergic mechanisms in reward circuitry due to genetic polymorphisms and epigenetic insults.
Certainly, in the face of the American opioid epidemic, the clinical consensus is to treat opioid use disorder (OUD) with life-long opioid substitution therapy. However, it has been suggested that a paradigm shift involving novel modalities, such as targeting the endorphinergic system linked to dopamine release at the NAc, leading to the induction of “dopamine homeostasis [25].” Utilizing the known gene-environment interaction theorem P = G +E, Blum et al. [26] previously provided a clear rationale for the adoption of genetic risk testing coupled with endorphinergic/dopamine regulation to address dysfunction across the brain reward circuitry.
The goal of altering resting-state, functional connectivity may require a gentle “neurotransmitter fix” via enkephalinase inhibition (e.g. D-phenylalanine) to overcome self-induction of acute dopamine release via psychoactive substance misuse, resulting in chronic dopamine downregulation. As subsets of reward deficiency, we are poised to provide novel, genetically guided therapy for endorphinergic, opioidergic, and dopaminergic deficiencies and related syndromes, utilizing “Precision Addiction Management”.
In terms of the therapeutic benefits of enkephalinase inhibition, there have been a series of articles that have focused on the role of endorphins in alcoholism. [27] One of these articles revealed that alcohol intake significantly reduces Leuenkephalin synthesis in brain circuits. [28] In a related article, it was also found that alcohol intake in genetically-bred ethanol-preferring or ethanol-averse mice was found to be an inverse function of the amount of brain methionine-enkephalin (METENK) present. [29] Simply, the lower amount of brain methionine-enkephalin, the higher the intake of alcohol in C57/Bl mice (low METENK), and the lower intake of alcohol in DBA mice (high METENK). Using these studies as a rationale, Blum et al., [30] performed the first pharmacogenetic engineering experiment employing D-Phenylalanine, the enkephalinase inhibitor, to convert ethanol-preferring C57/Bl mice to behave like ethanol-averse DBA mice. These authors were able to significantly attenuate both volitional and forced ethanol intake respectively by acute and chronic treatment with D-phenylalanine. Since D-phenylalanine, through its enkephalinase inhibitory activity, raises brain enkephalin levels, it was shown that 18 days of treatment with D-phenylalanine significantly attenuated excessive alcohol intake in C57/BL to the same or even lower levels than its counter-part DBA mice. This suggests that alcohol intake can be regulated by alteration of endogenous brain opioid peptides.
1.3. NAD+ and Addiction
The significance of NAD+ in addictive disorders arises from the work of Paul O’ Hollaren [31], who revealed to have successfully utilized IV NAD+ for the treatment and prevention of over 104 cases of addiction to alcohol, among other drugs of abuse, including opium extract, heroin, morphine, dihydromorphine, meperidine, codeine, cocaine, amphetamines, barbiturates, and tranquillizers. For his retrospective case series, IV NAD+ was given at a dose of 500–1000 mg, which was added to 300 cc normal saline daily for 4 days, twice per week for a month. This was followed by a maintenance dose twice per month until addiction was ameliorated, with limited toxic effects. NAD+ is likely to represent an economical and holistic approach for the estimated millions of addicts worldwide; it may be an effective adjunct to psychotherapy, by ameliorating symptoms of physical addiction through a variety of mechanisms. While there have been animal studies showing the benefits of NAD with cocaine [32], there is a paucity of human studies on drugs of abuse. Nicotinamide phosphoribosyltransferase (NAMPT) is an important rate-limiting enzyme found throughout the body that converts the intracellular pool of nicotinamide adenine dinucleotide (NAD) into nicotinamide mononucleotide (NMN). A study published in Experimental Neurology by the Cen group demonstrated that NAMPT contributes to cocaine reward through sirtuin 1 (SIRT1) signaling in the ventral tegmental area. Thus, targeting the NAMPT/SIRT1 signaling pathway may provide a promising therapeutic strategy against cocaine addiction. [32] Additionally, Nicotinamide (NAM) is a small molecule that can oppose cellular adaptations observed following cocaine exposure in the rodent self-administration and reinstatement model of addiction. Furthermore, NAM has utility against symptoms of withdrawal and vulnerability to relapse to cocaine use; this has been suggested by case studies and anecdotal reports [33]. In fact, chronic NAM administered throughout extinction dose-dependently attenuated cue-primed reinstatement in male rats, but not female rats [33].
Nicotinamide adenine dinucleotide (NAD+) and its reduced form, NADH, both respond to cellular energy demands and redox states [34]. Other studies have shown that NAD+ is important for maintaining energy homeostasis in the brain, as well as calcium transport and mitochondrial respiration affecting dopamine neuronal release in the brain reward circuit [35].
While there is little evidence directly published on NAD/NADH, RDS, and associated drug and non-drug addictions, there is some rationale to utilize this substance based on metabolic effects. However, albeit emerging newer positive evidence regarding NAD and SUD, as an off-label modality. [36] The above brief historical information not only provides a rationale to incorporate NAD into clinical practice to treat SUD, but it begs for additional studies to support its potential benefits for this incredibly vulnerable population. The following is a detailed original executed pilot demonstration to assist in future larger required experiments in human and animal self-administration models.
1.4. Glycine
The work related to the possible role of the molecule glycine in alcoholism was initiated with the published works of Blum et al. [37], published in Science in 1972. They found both glycine and its precursor serine significantly enhanced the sleeping time (loss of the righting reflex) that was induced by ethanol in mice. The observed synergistic effect between ethanol and the amino acids is probably not related to an alteration of ethanol metabolism, but rather to an interaction of these compounds in the central nervous system. Moreover, Blum’s group also reported on the antialcohol intoxication effects of these compounds. Specifically, both glycine and serine significantly reduced acute alcoholic intoxication in mice. [38] The enhancement of the soporific effects of ethanol in mice was confirmed in 1995 by Williams et al. [39] A Pubmed search using the words “Glycine and Drug Abuse” on November 26, (th) 2020, resulted in 416 citations. Many of these articles involved glycine receptors and glycine transporters. In terms of the benefit of glycine as an antialcohol compound, there have been mixed results. The most recent study by Serrita et al., [40] utilized glycine as an agonist of the glycine B coagonist site of the NMDA receptor on alcohol consumption and cravings. In this study, Glycine showed no benefit over placebo in the reduction of heavy drinking days or craving for alcohol over a 12-week treatment period.
However, there is important data related to the interaction of ethanol, glycine, and dopamine. Specifically, previous research has shown that strychnine-sensitive glycine receptors in the nucleus accumbens [NAc] are involved in regulating dopamine release and in mediating the reinforcing effects of alcohol. One noteworthy finding is that alcohol-induced dopamine release is blocked by local treatment with the glycine receptor antagonist strychnine (20 μM) or furosemide (100 μM or 1 mM [41]. There are also studies showing that Anandamide (AEA) and delta9-tetrahydrocannabinol (THC) are endogenous and exogenous ligands, respectively, for cannabinoid receptors in pharmacologically relevant concentrations. Utilization of these peptides per se administered into animals and humans was not followed by any side effects. No complaints from the subjects were noted. These results may suggest a safe and efficient use of Ala-Gln as a source of free glutamine in parenteral nutrition. Certainly, glutamine per se or its interaction at specific loci such as glutaminergic drive at the VTA, regulates dopamine release [42].
In contrast, the glycine transporter 1 inhibitor Org 25935 demonstrated no benefit over placebo in preventing alcohol relapse [43]. Despite this, other work showed that Org 25935 decreased alcohol intake and alcohol preference [44]. Other studies show that NMDA/glycine (glycine(B)) site antagonists attenuate wet dog shakes (withdrawal) and the development of addiction, induced by chronic morphine administration in rats [45].
The presence of GlyRs has been described in higher regions, such as the hippocampus and nucleus accumbens, with a prevalence of α2/α3 subunits. Burgos et al. [46], detailed the following aspects of alcohol effects on GlyRs: (1) direct interaction of alcohol with amino acids in the extracellular or transmembrane domains, and indirect mechanisms through the activation of signal transduction pathways; (2) analysis of α2 and α3 subunits having different sensitivities to alcohol, which allows the identification of structural requirements for alcohol modulation present in the intracellular domain and C-terminal region; (3) Genetically modified knock-in mice for α1 GlyRs that have an impaired interaction with G proteins and demonstrate reduced alcohol sensitivity without changes in glycinergic transmission; and (4) GlyRs as potential therapeutic targets. Of particular interest is the work of Michino et al., [47], showing that a divergent glycine in extracellular loop 1 [EL1] is critical to the dopamine D3 receptor [D3R] over the dopamine D2 receptor [D2R] subtype selectivity. It should be noted that DRD3 genotypes are very relevant in African-Americans compared to Caucasians with regard to binge drinking [48].
Finally, the mesocorticolimbic dopamine system, originating in the ventral tegmental area (VTA), is regulated by GABA-mediated synaptic inhibition. Accumulating evidence indicates that long-term potentiation of GABAergic synapses (LTPGABA) in VTA dopamine neurons plays an important role in the actions of drugs of abuse, including alcohol. It has been shown that a single infusion of glycine into the VTA of rats strongly reduces ethanol intake for 24 hours [49]. In follow-up studies, Gian & Ye [50] reported 10 μM glycine prevented trains of high-frequency stimulation (HFS) from producing LTPGABA, which was rescued by the glycine receptor (GlyR) antagonist strychnine. The authors suggested that the blockade of LTPGABA by glycine is probably resulting from suppressing glutamate release by activating the GlyRs on the glutamatergic terminals. This effect of glycine may contribute to the reduction in ethanol intake induced by intra-VTA glycine observed in vivo.
1.5. Alanylglutamine Dipeptide
A Pubmed search using the search term “Alanylglutamine-dipeptide and addiction or drug abuse” on November 26th, 2020 retrieved no results. However, its incorporation into the IV1114589NADASE infusion is predicated upon the potential release of glutamine in vivo. Using biotechnological and chemical methods, the stable and highly soluble peptide L-alanyl-L-glutamine (Ala-Gln) can be synthesized in substantial yields. Studies in experimental dogs and rats demonstrate the effective utilization of intravenously supplied Ala-Gln as well as the rapid provision of free glutamine for maintenance of the muscle-free, intracellular glutamine pool in catabolic conditions.
Subsequent studies in healthy volunteers provide strong evidence that the infused Ala-Gln is rapidly eliminated from plasma (t1/2:3.8 minutes), associated with a prompt equimolar increase in the concentrations of free glutamine and alanine. Continuous infusion and bolus injection of the peptide was not associated with any side effects, and no complaints by the subjects were noted. These results may indicate a safe and efficient use of Ala-Gln as a source of free glutamine in parental nutrition [51].
2. METHODS & MATERIALS
The primary objective of the present investigation is to provide some additional clinical evidence to show that IV1114589NADASE infusions significantly attenuate substance craving behavior and concomitant psychiatric burden sequalae in poly-drug abusers attending an out-patient chemical dependency program. IV1114589NAD [NAD] infusions are displayed in Table 1.
Table 1.
Detail of Infusions per session.
| - | Infusion #1 | Infusion #2 | Infusion #3 | Infusion #4 | Infusion #5 | Infusion #6 | Infusion #7 |
|---|---|---|---|---|---|---|---|
| Hydration Multi-Vitamin Immune Support with Amino Acids | X | X | X | X | - | - | - |
| Hydration Multi-Vitamin Immune Support | - | - | - | - | X | X | X |
| Alpha Lipoic Acid | X | X | - | - | - | - | - |
| Glutathione | - | - | X | X | X | X | X |
| NAD + | X | X | X | X | - | - | - |
| NAD + with Amino Acids | - | - | - | - | X | X | X |
The study cohort (n=50) as a subgroup consisted of highly addicted, poly-drug, mixed-gender, and ethnicity individuals previously resistant to standard treatment with a failed range of treatment attempts. The incorporation of the NAD infusions involved detoxification, in-patient residence, and out-patients. Table 1 – provides the demographics of this cohort. Table 2. represents a summary status of the demographics.
Table 2.
Demographics of the Fifty patients in this cohort.
| Gender | 29 Male (58%), 21 Female (42%) |
|---|---|
| Age (years) | 34.48 ± 7.46 (range 21 to 61 years) |
| Drug of Choice (DOC) | 3 Air Duster (6%), 8 Benzodiazepines (16%), 1 Cocaine (2%), 1 Crack (2%), 16 Ethanol (32%), 16 Heroin (32%), 4 Marijuana (8%), 38 Methamphetamine (76%), 5 Opiates (10%), and 1 Suboxone (2%); one substance: 22 (44%), 2 substances: 17 (34%), 3 substances: 8 (16%), and 4 substances: 3 (6%) |
| Days since Last Use | 21.76 ± 15.28 (range 1 to 80 days) |
| Level of Care | 11 Detox (22%), 4 IOP (8%), 12 PHP (24%), and 23 Residential (46%) |
| No. Treatment Facilities | one facility: 32 (64%), 2 facilities: 6 (12%), 3 facilities: 5 (10%), 4 facilities: 2 (4%), 5 facilities: 1 (2%), 7 facilities: 1 (2%), 8 facilities: 2 (4%), and 10 facilities: 1 (2%) |
| Longest Sobriety (years) | 1.18 ± 1.41 (range 4 days to 7 years) |
| Years of Use | 16.80 ± 6.83 (range 5 to 33 years) |
| Random UA Screening | 40 negative UASO (80%) |
| Ethnicity | 2 African American (4%), 1 Arabic (2%), 42 Caucasian (84%), 1 Hispanic (2%), 4 Native American (8%) |
Scrutiny of this data reveals that the various drugs of choice in these 50 patients include: opiates (heroin), methamphetamine, cocaine, ethanol, benzodiazepines, marijuana, and spray cans of air duster. The study consisted of twenty-one (n=21) females and twenty-nine (n=29) males. The age of the subjects ranged from 21 to 61 years, where the average for females was 34.7 ± 8.9 years and males were 34.3 ± 6.4. The study participants consisted of 84% Caucasians, 4% African-Americans, 2% Hispanic, 2% Arabic, and 8% Native Americans. The years of use ranged from 5 to 33, with an average of 16.80 ± 6.83. The number of treatment facilities attended by these 50 patients ranged from the first time to as high as 10, with an average of 2.1 ± 2.1. In these 50 patients, the range of last use of any drug of abuse is self-reported from one day to 80 days, with an average of 21.76 ± 15.28. The longest sobriety of these patients ranged from 4 days to 7 years, with an average sobriety rate of 1.18 ± 1.41 years. In this cohort, the years of use varied from 5 to 33 years, with an average of 16.80 ± 6.83. Each patient included in this study received a minimum of 7 infusions for a duration of four weeks, with one to two infusions per week. The data on a number of variables included: craving scores, anxiety, depression, and sleep. While there are existing validated scales to measure the various behavioral-related levels, it has been decided to utilize the Likert scale [1–10] for self-reported responses, accomplished via a counselor-to-patient structured interview. It is to be noted that each of the two treatment centers used self-reported Likert scales to obtain the data on 50 patients. Throughout these 7 infusions of NAD, each of the subjects included in this study agreed, as per treatment policy, to be urine screened for the presence or absence of non-prescribed psychoactive drugs. The first and last urine samples on these subjects were analyzed and presented herein.
2.1. Statistical Analysis
In terms of statistical analysis, we utilized the Fisher Exact test to evaluate the extent of utilizing NAD infusions (10 per patient over 5 weeks), comparing self-reported pre-baseline Likert scores and post-infusion scores. We also employed the Mantel–Haensel test for linear trends showing a potential consistent relationship between the effect of these NAD infusions on craving anxiety, depression, and sleep. P-value is the level of marginal significance within a statistical hypothesis test, representing the probability of the occurrence of a given event. In terms of P values indicating E means exponential to the power of 10.
3. RESULTS
Using Wilcoxon signed-rank tests and sign tests, it has been found the following significance comparing the baseline scores to post outcome scores after IV1114589NAD infusions; craving scores (P=1.063E-9); anxiety (P=5.487E-7); depression (P=1.763E-4), sleep outcome was found to be non-significant. The significant reductions in cravings, anxiety, and depression followed a linear trend whereby with cravings (pre 5.90, post 0.22); anxiety (pre 4.66, post 1.54); and depression (pre 2.58, post 0.88). Linear trend analyses showed a significant association of NAD infusions with decreasing scores of cravings (P=0.015), anxiety (P=0.003), and depression (P=8.74E-5) (Figs. 2–5).
Fig. (2).
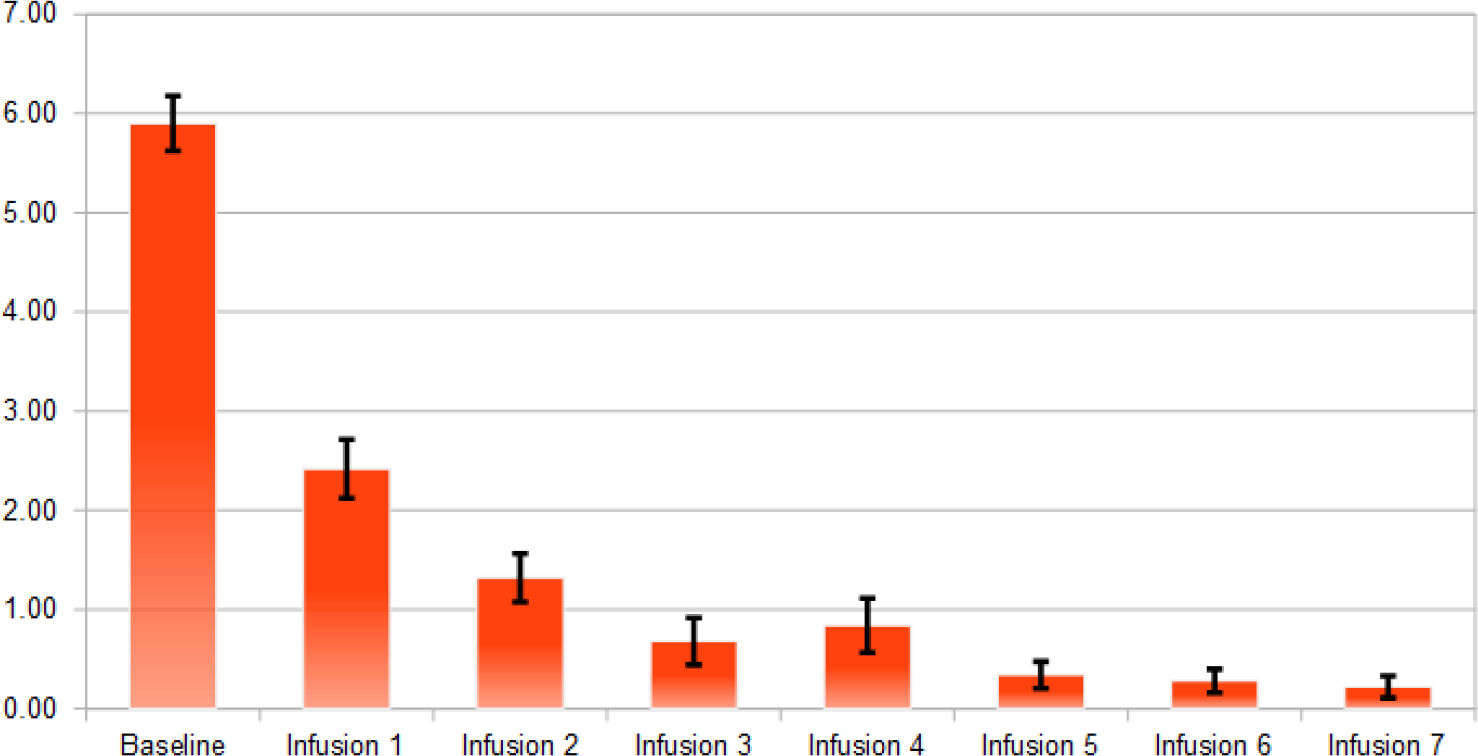
Average of cravings scores after each NAD infusion (n=50).
Fig. (5).
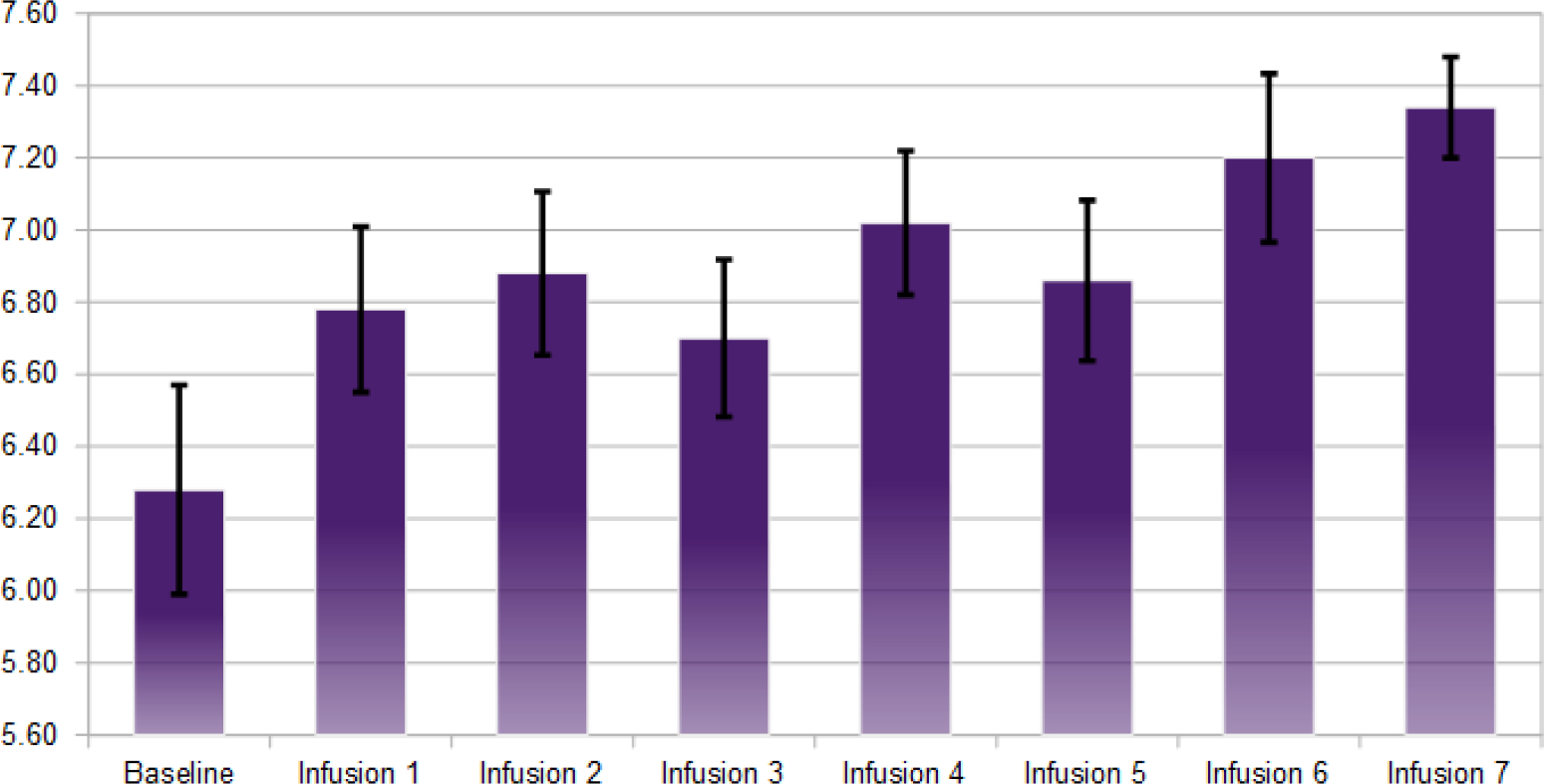
Average hours of uninterrupted sleep after each NAD infusion (n=50).
The significant reductions in cravings, anxiety, and depression followed a linear regression (Fig. 6) with a) cravings (pre 5.90, post 0.22); b) anxiety (pre 4.66, post 1.54); and c) depression (pre 2.58, post 0.88).
Fig. (6).
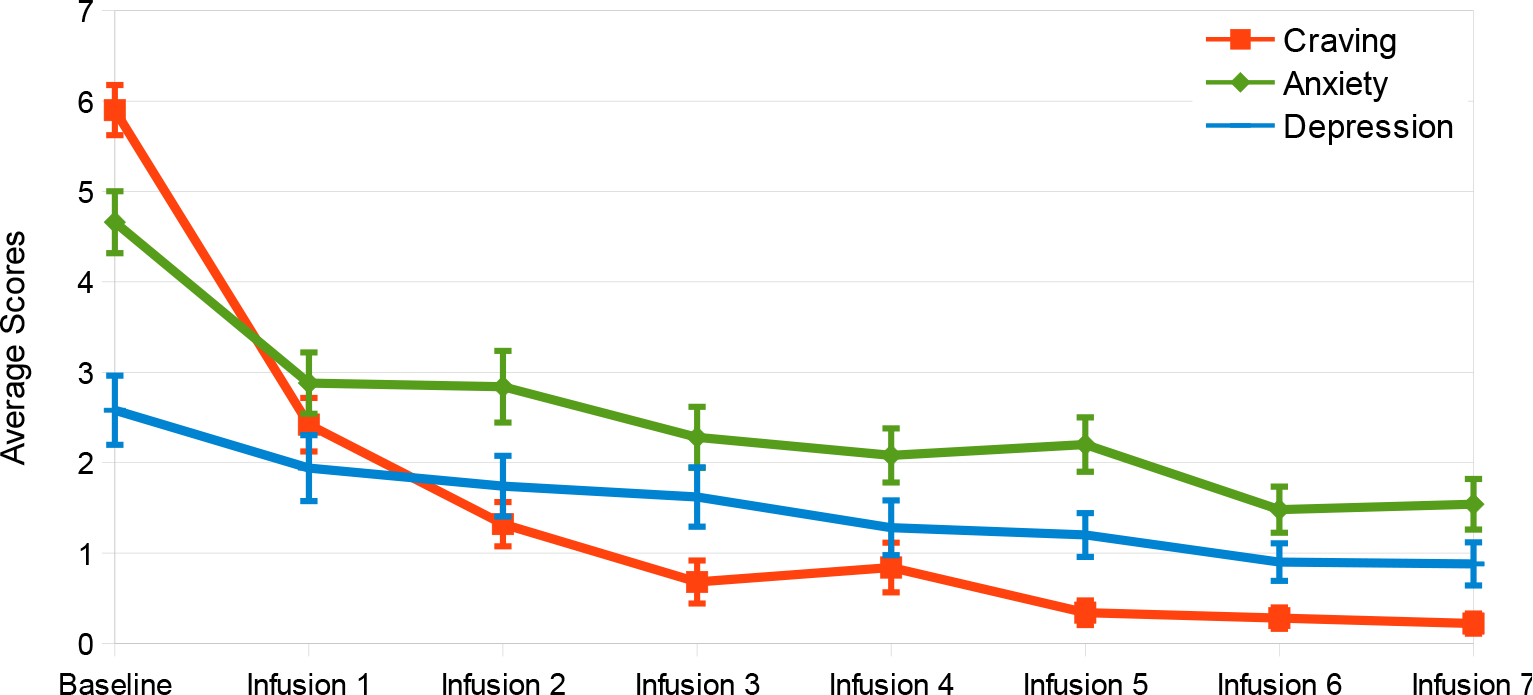
Averages of outcome measures after each NAD infusion (n=50).
The results of urine drug screens. A subset of 40 out of 50 patients during the infusion period was provided by each treatment center. The urine analysis was performed by Southwest Medical Laboratory, which is a CLIO-certified toxicological reference site. The panel included: 6-Acetylmorphine, Amphetamine, Barbiturate, Benzodiazepine, Buprenorphine, Cocaine- Fentanyl- Methadone, Methamphetamine- Opiate, Oxycodone, THC, and Tramadol. 100% of those patients tested midway into the infusion period tested negative. Therefore, 80 percent (40/50) of all subjects of the total of fifty cases were tested (Table 3).
Table 3.
Urine analysis results.
| - | Gender | Age | DOC | Last Use | Level of Care | # of Treatment Facilities | Longest Sobriety | Years of Use | Random UA Screening | Ethnicity |
|---|---|---|---|---|---|---|---|---|---|---|
| - | Gender | Age | DOC | Last Use | Level of Care | # of Treatment Facilities | Longest Sobriety | Years of Use | Random UA Screening | Ethnicity |
| Patient 1 | Female | 42 | Meth, Benzos | 22 Days | PHP | 1 (st) Time | 1 Year | 20 Years | Negative | Native American |
| Patient 2 | Female | 35 | Meth, ETOH, Duster | 15 Days | Residential | 3 | 9 Months | 19 Years | Negative | Caucasian |
| Patient 3 | Female | 27 | Meth | 19 Days | PHP | 1 (st) Time | 7 Months | 11 Years | Negative | Caucasian |
| Patient 4 | Female | 41 | ETOH | 7 Days | Detox | 1 (st) Time | 6 Months | 20 Years | Negative | Caucasian |
| Patient 5 | Female | 45 | Meth, Marijuana | 24 Days | PHP | 1 (st) Time | 24 Days | 23 Years | Negative | Caucasian |
| Patient 6 | Female | 45 | Meth, Duster, ETOH, Marijuana | 27 Days | PHP | 4 | 1.5 Years | 33 Years | Negative | Caucasian |
| Patient 7 | Female | 29 | Meth, Opiates, Benzos | 4 Days | Detox | 1 (st) Time | 5 Years | 9 Years | ------------ | Caucasian |
| Patient 8 | Female | 28 | Suboxone | 12 Days | Residential | 1 (st) Time | 32 Days | 5 Years | Negative | Caucasian |
| Patient 9 | Female | 38 | Meth | 4 Days | Detox | 1 (st) Time | 4 Days | 5 Years | Negative | Caucasian |
| Patient 10 | Female | 32 | Meth, Opiates, Benzos | 22 Days | Residential | 1 (st) Time | 9 Months | 19 Years | Negative | Caucasian |
| Patient 11 | Female | 34 | Meth, Heroin, ETOH | 23 Days | Residential | 1 (st) Time | 4 Years | 11 Years | Negative | Caucasian |
| Patient 12 | Female | 32 | Meth, Heroin, ETOH | 30 Days | Residential | 1 (st) Time | 30 Days | 27 Years | Negative | Native American |
| Patient 13 | Female | 21 | Meth, Benzos | 10 Days | PHP | 1 (st) Time | 28 Days | 5 Years | Negative | Hispanic |
| Patient 14 | Female | 61 | Opiates, Meth | 22 Days | Residential | 3 | 90 Days | 20 Years | Negative | Caucasian |
| Patient 15 | Female | 29 | Meth | 1 Day | Detox | 1 | 1 Year | 11 Years | Negative | Caucasian |
| Patient 16 | Female | 28 | Meth, ETOH | 46 Days | PHP | 7 | 46 Days | 13 Years | Negative | Caucasian |
| Patient 17 | Female | 36 | Cocaine, Meth | 30 Days | PHP | 1 (st) Time | 30 Days | 16 Years | Negative | Caucasian |
| Patient 18 | Female | 30 | Heroin | 21 Days | Residential | 2 | 2 Years | 11 Years | Negative | Caucasian |
| Patient 19 | Female | 29 | Meth, Benzos, ETOH, Marijuana | 17 Days | Residential | 1 (st) Time | 17 Months | 16 Years | Negative | Native American |
| Patient 20 | Female | 41 | Heroin | 80 Days | IOP | 2 | 80 Days | 30 Years | Negative | Caucasian |
| Patient 21 | Female | 26 | Meth, Heroin | 17 Days | Residential | 1 (st) Time | 17 Days | 9 Years | Negative | Caucasian |
| Patient 22 | Male | 42 | ETOH, Benzos | 22 Days | Residential | 1 (st) Time | 1 Year | 26 years | Negative | Caucasian |
| Patient 23 | Male | 33 | Meth, ETOH | 26 Days | Residential | 2 | 2 Years | 21 Years | Negative | Caucasian |
| Patient 24 | Male | 42 | ETOH | 28 Days | Residential | 10 | 2 Years | 26 Years | Negative | Caucasian |
| Patient 25 | Male | 34 | Meth | 19 Days | Residential | 3 | 2 ½ Years | 17 Years | Negative | Caucasian |
| Patient 26 | Male | 35 | Heroin, Opiates | 23 Days | Residential | 2 | 2 Years | 13 Years | Negative | Caucasian |
| Patient 27 | Male | 43 | Meth, Heroin | 23 Days | Residential | 1 (st) Time | 7 Years | 20 Years | Negative | Caucasian |
| Patient 28 | Male | 40 | Meth | 19 Days | Residential | 1 (st) Time | 17 Months | 20 Years | Negative | Caucasian |
| Patient 29 | Male | 36 | Meth, ETOH | 28 Days | Residential | 3 | 5 Months | 23 Years | Negative | Caucasian |
| Patient 30 | Male | 32 | Meth, ETOH | 22 Days | Residential | 1 (st) Time | 1 Year | 16 Years | Negative | Caucasian |
| Patient 31 | Male | 28 | Meth, Heroin | 13 Days | Residential | 2 | 100 Days | 12 Years | Negative | Caucasian |
| Patient 32 | Male | 23 | Heroin, Meth, Crack | 50 Days | IOP | 5 | 1 Year | 7 Years | Negative | Caucasian |
| Patient 33 | Male | 31 | Meth | 1 Day | Detox | 1 (st) Time | 30 Days | 15 Years | Negative | Caucasian |
| Patient 34 | Male | 30 | ETOH, Marijuana | 30 Days | Residential | 1 (st) Time | 30 Days | 15 Years | ----------- | Caucasian |
| Patient 35 | Male | 37 | Meth | 20 Days | PHP | 3 | 3 Years | 25 Years | Negative | African American |
| Patient 36 | Male | 30 | Heroin, Meth, Duster, ETOH | 30 Days | PHP | 4 | 2 Years | 15 Years | Negative | Caucasian |
| Patient 37 | Male | 31 | Meth, Heroin, Benzos | 23 Days | Residential | 1 (st) Time | 9 Months | 16 Years | Negative | Caucasian |
| Patient 38 | Male | 32 | Meth | 20 Days | PHP | 1 (st) Time | 1 Year | 16 Years | Negative | Caucasian |
| Patient 39 | Male | 41 | Meth | 29 Days | PHP | 1 (st) Time | 4 Years | 21 Years | Negative | Caucasian |
| Patient 40 | Male | 26 | Meth, Heroin | 1 Day | Detox | 1 (st) Time | 6 Months | 10 Years | ----------- | African American |
| Patient 41 | Male | 42 | Meth | 1 Day | Detox | 2 | 8 Months | 24 Years | ----------- | Caucasian |
| Patient 42 | Male | 29 | Meth | 1 Day | Detox | 1 (st) Time | 5 Months | 15 Years | ----------- | Caucasian |
| Patient 43 | Male | 40 | Meth | 57 Days | IOP | 1 (st) Time | 57 Days | 26 Years | ----------- | Native American |
| Patient 44 | Male | 24 | Heroin, Meth | 41 Days | IOP | 1 (st) Time | 41 Days | 10 Years | Negative | Caucasian |
| Patient 45 | Male | 30 | Heroin, Opiates, Benzos | 29 Days | Residential | 1 (st) Time | 29 Days | 18 Years | Negative | Arabic |
| Patient 46 | Male | 32 | Heroin | 22 Days | Residential | 1 (st) Time | 22 Days | 20 Years | Negative | Caucasian |
| Patient 47 | Male | 46 | Meth | 5 Days | Detox | 1 (st) Time | 2 Years | 28 Years | ----------- | Caucasian |
| Patient 48 | Male | 45 | ETOH | 5 Days | Detox | 8 | 1 ½ Years | 8 Years | ----------- | Caucasian |
| Patient 49 | Male | 28 | Meth, ETOH | 40 Days | PHP | 1 (st) Time | 9 Months | 14 Years | ----------- | Caucasian |
| Patient 50 | Male | 33 | Heroin | 7 Days | Detox | 8 | 1 ½ Years | 10 Years | ----------- | Caucasian |
4. DISCUSSION
The present study demonstrates a method to treat and detoxify patients with Substance Use Disorder (SUD), utilizing a series of Nicotinamide adenine dinucleotide (NAD+) and Enkephalinase (IV1114589NADASE) infusions in subjects attending chemical dependency programs. A PUBMED search using the term “NAD and addiction” resulted in fifty-one articles and using the term “NAD and Substance Abuse” resulted in three hundred and seventy-one. However, there is a paucity of clinical studies on humans with SUD and the incorporation of NAD infusions as developed in this current cohort. The earliest known published clinical study on NAD for addiction was authored by Paul O’ Hollaren in 1961 [30]. Since then, many treatment programs have adopted its use [30–36, 52–76].
The primary objective of the present investigation is to provide some additional clinical evidence to show that NAD+ and other amino acids, including D-phenylalanine [77], glycine, ananylglutamine dipeptide, and Myers cocktail (B complex) infusions, significantly attenuate substance-craving behavior and concomitant psychiatric burden sequalae in poly-drug abusers attending both in-patient and out-patient level of care in a number of chemical dependency programs in Orange County.
Importantly, urine analysis of a standard panel of illicit drugs of abuse [78, 79] was utilized during NAD infusions. A subset of 40 patients was tested midway during the infusions resulting in 100% of these patients testing negative. The remaining ten subjects were not urine screened.
To reiterate, after obtaining the baseline measurements, each subject was followed throughout the experiment of five weeks and ten infusions. Using the statistical analyses based on Wilcoxon signed-rank tests and sign tests P, we found the following significance comparing the baseline scores to the post outcome scores after NAD infusions: craving scores (P=1.063E-9); anxiety (P=5.487E-7); depression (P=1.763E-4), with sleep levels showing a trend to a higher number of hours slept post infusions [80], though this trend was found to be non-significant (Figs. 2–5).
4.1. Limitations
In the face of the current opioid crisis in the US, killing close to 800,000 people since 2004 and accruing an estimated death rate daily of 127, there is a pressing need to find a safe solution free of side effects [81]. We are cognizant that, for example, Naltrexone pellets have heuristic value but may interfere with dopamine homeostasis and induce anti-reward benefits [82]. This pilot demonstration study with a small N requires future larger, randomized, double-blinded, placebo-controlled studies enabling a clearer interpretation. However, it does contribute to the emerging literature concerning NAD efficacy in SUD. One other limitation relates to the fact that many studies show that NAD alters dopaminergic signaling. [83–85], there is a paucity of neuroimaging reports related to addictive behaviors and reward processing [86].
CONCLUSION
These robust pilot results demonstrate the significant positive effects of NAD, amino-acid, and enkephalinase inhibition infusions in treatmentresistant probands showing reward deficiency. This provides the rationale to extend these seemingly clinically relevant findings in extended future investigations utilizing NAD/NADH alone coupled with the GARS test as a DNA guided precision-matched pro-dopamine regulator to help induce required “dopamine homeostasis”.
Fig. (3).
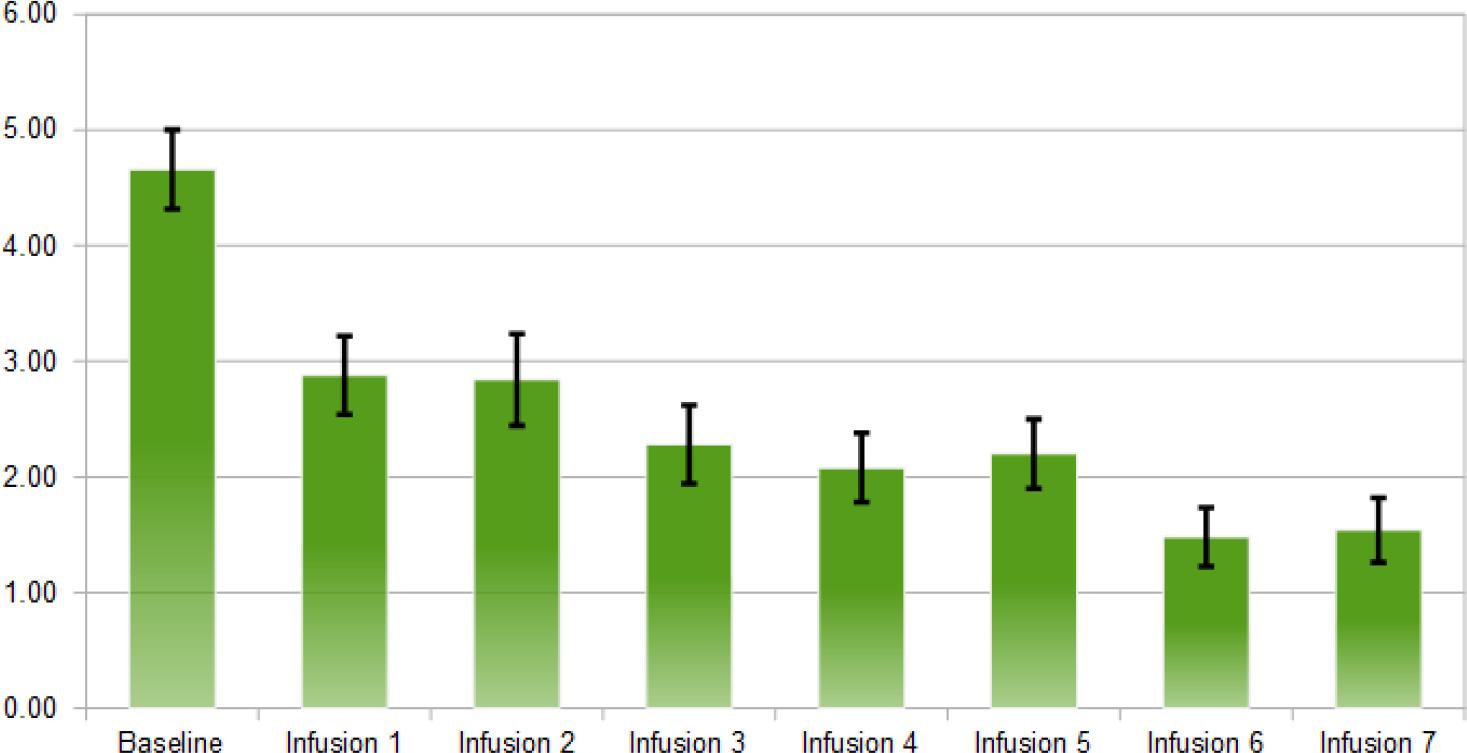
Average of anxiety scores after each NAD infusion (n=50).
Fig. (4).
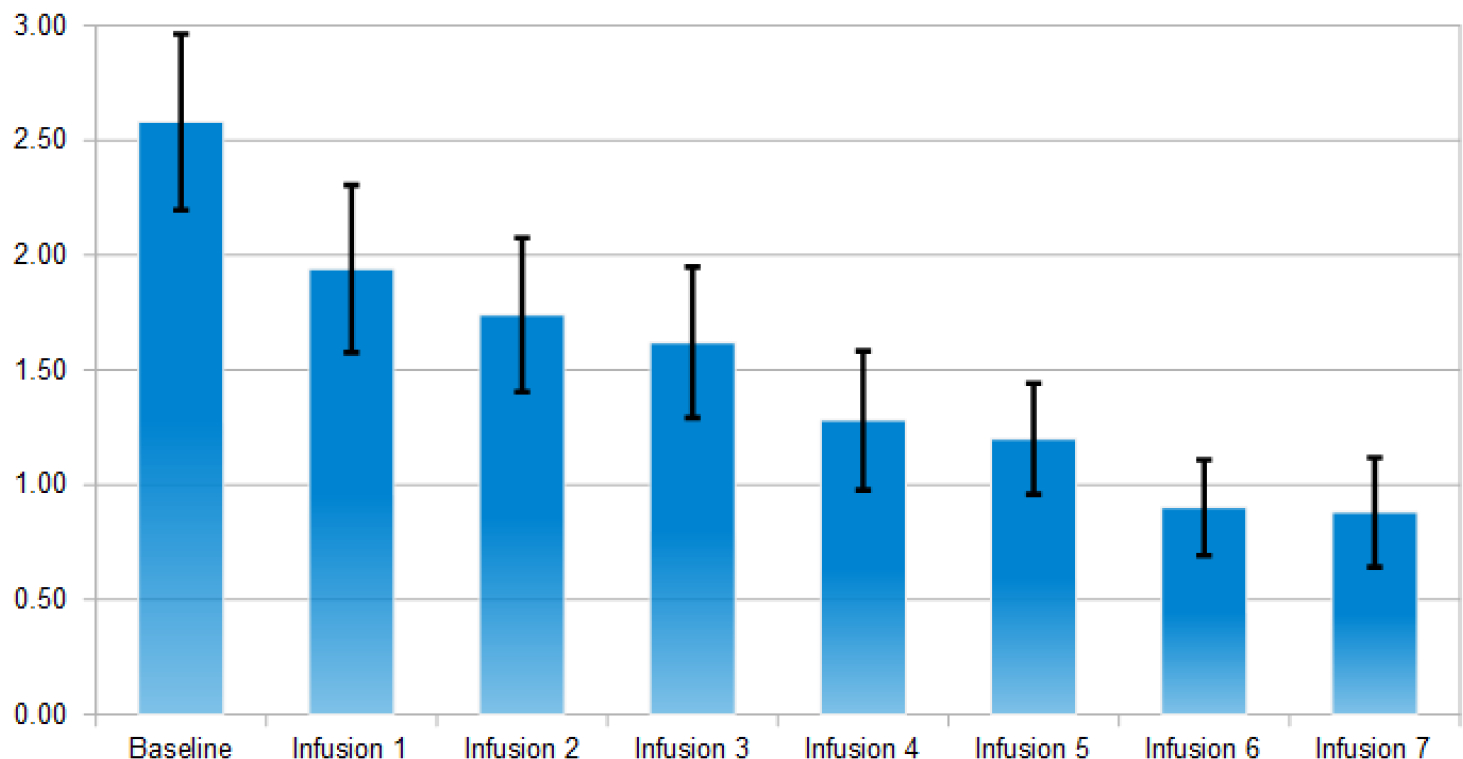
Average of depression scores after each NAD infusion (n=50).
ACKNOWLEDGEMENTS
The authors appreciate the expert edits by Margaret A. Madigan and our expert clinical team.
FUNDING
Dr. Blum and Marjorie Gondre-Lewis (Howard University) are NIH recipients of R41 MD012318/MD/NIMHD NIH HHS/United States.
Footnotes
CONFLICT OF INTEREST
It is to be noted that KB is the inventor of amino-acid enkephalinase therapy and, as such, holds a number of the USA and foreign patents issued and pending.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The use of intravenous administration of amino acids for the potential treatment of RDS was approved by the Path Foundation NY IRB along with approved consent forms. The IRB approval was in the form of exemption status that has been utilized in a number of studies. All subjects entered into the study meeting inclusion criteria, and all subjects signed an informed consent statement. The PATH Research Foundation approved the study (NIH registration # 00002334) for general research utilizing variants of the Pro-dopamine regulation in the infusion form. In addition, the IRB of Western University Health Sciences also approved a study related to GARS testing in RDS candidates with pharmaceutical treatment in 2021.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans used were in accordance with the approval of the Path Foundation NY IRB and with the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Subjects not only signed written consent to participate and allow publication but also agreed to volunteer for a feasibility study. For patient protection, the data will conform to standard HIPPA practices mandated by law. A distribution center provided NAD infusions to treatment facilities, involving the 50 subsets of patients derived from two programs located in the Orange County area, and their associated treatment facilities have now performed approximately 1,000 infusions on 900 patients without any serious side effects, pointing to the safety of this procedure.
STANDARDS OF REPORTING
STROBE guidelines were followed.
DISCLAIMER: The above article has been published, as is, ahead-of-print, to provide early visibility but is not the final version. Major publication processes like copyediting, proofing, typesetting and further review are still to be done and may lead to changes in the final published version, if it is eventually published. All legal disclaimers that apply to the final published article also apply to this ahead-of-print version.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available within the article.
REFERENCES
- [1].Oates JA, Gillespie L, Udenfriend S, Sjoerdsma A. Decarboxylase inhibition and blood pressure reduction by alpha-methyl-3,4-dihydroxy-DL-phenylalanine. Science 1960; 131(3417): 1890–1. 10.1126/science.131.3417.1890 [DOI] [PubMed] [Google Scholar]
- [2].Febo M, Blum K, Badgaiyan RD, et al. Dopamine homeostasis: Brain functional connectivity in reward deficiency syndrome. Front Biosci 2017; 22(4): 669–91. 10.2741/4509 [DOI] [PubMed] [Google Scholar]
- [3].Blum K, Febo M, Badgaiyan RD, et al. Neuronutrient amino-acid therapy protects against reward deficiency syndrome: Dopaminergic key to homeostasis and neuroplasticity. Curr Pharm Des 2016; 22(38): 5837–54. 10.2174/1381612822666160719111346 [DOI] [PubMed] [Google Scholar]
- [4].Blum K, Gold MS, Jacobs W, et al. Neurogenetics of acute and chronic opiate/opioid abstinence: treating symptoms and the cause. Front Biosci 2017; 22(8): 1247–88. 10.2741/4544 [DOI] [PubMed] [Google Scholar]
- [5].Blum K, Febo M, Fried L, et al. Hypothesizing that neuropharmacological and neuroimaging studies of glutaminergic-dopaminergic optimization complex (KB220Z) are associated with “dopamine homeostasis” in reward deficiency syndrome (RDS). Subst Use Misuse 2017; 52(4): 535–47. 10.1080/10826084.2016.1244551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miller DK, Bowirrat A, Manka M, et al. Acute intravenous synaptamine complex variant KB220™ “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: Part 1, Pilot Study With 2 Case Reports. Postgrad Med 2010; 122(6): 188–213. 10.3810/pgm.2010.11.2236 [DOI] [PubMed] [Google Scholar]
- [7].Blum K, Chen TJH, Downs BW, et al. Synaptamine (SG8839) An amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of reward deficiency syndrome (RDS). Trends Appl Sci Res 2007; 2(2): 132–8. 10.3923/tasr.2007.132.138 [DOI] [Google Scholar]
- [8].Miller M, Chen AL, Stokes SD, et al. Early intervention of intravenous KB220IV--neuroadaptagen amino-acid therapy (NAAT) improves behavioral outcomes in a residential addiction treatment program: a pilot study. J Psychoactive Drugs 2012; 44(5): 398–409. 10.1080/02791072.2012.737727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blum K, Modestino EJ, Gondre-Lewis MC, et al. Pro-dopamine regulator (KB220) a fifty year sojourn to combat reward deficiency syndrome (RDS): evidence based bibliography (Annotated). CPQ Neurol Psychol 2018; 1(2) https://www.cientperiodique.com/journal/fulltext/CPQNP/1/2/13 [PMC free article] [PubMed] [Google Scholar]
- [10].Febo M, Blum K, Badgaiyan RD, et al. Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z. PLoS One 2017; 12(4): e0174774. 10.1371/journal.pone.0174774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blum K, Liu Y, Wang W, et al. rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med 2015; 127(2): 232–41. 10.1080/00325481.2015.994879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blum K, Chen TJ, Morse S, et al. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and poly-drug abusers utilizing putative dopamine D₂ agonist therapy: part 2. Postgrad Med 2010; 122(6): 214–26. 10.3810/pgm.2010.11.2237 [DOI] [PubMed] [Google Scholar]
- [13].Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci 2014; 17(5): 704–9. 10.1038/nn.3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park K, Volkow ND, Pan Y, Du C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci 2013; 33(40): 15827–36. 10.1523/JNEUROSCI.1935-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thanawala V, Kadam VJ, Ghosh R. Enkephalinase inhibitors: potential agents for the management of pain. Curr Drug Targets 2008; 9(10): 887–94. 10.2174/138945008785909356 [DOI] [PubMed] [Google Scholar]
- [16].Ramírez-Sánchez M, Prieto I, Segarra AB, Martínez-Cañamero M, Banegas I, de Gasparo M. Enkephalinase regulation. Vitam Horm 2019; 111: 105–29. 10.1016/bs.vh.2019.05.007 [DOI] [PubMed] [Google Scholar]
- [17].Ehrenpreis S Analgesic properties of enkephalinase inhibitors: animal and human studies. Prog Clin Biol Res 1985; 192: 363–70. [PubMed] [Google Scholar]
- [18].Ehrenpreis S Pharmacology of enkephalinase inhibitors: animal and human studies. Acupunct Electrother Res 1985; 10(3): 203–8. 10.3727/036012985816714478 [DOI] [PubMed] [Google Scholar]
- [19].Pomeranz B Do endorphins mediate acupuncture analgesia? Adv Biochem Psychopharmacol 1978; 18: 351–9. [PubMed] [Google Scholar]
- [20].Cheng RS, Pomeranz B. Electroacupuncture analgesia could be mediated by at least two pain-relieving mechanisms; endorphin and non-endorphin systems. Life Sci 1979; 25(23): 1957–62. 10.1016/0024-3205(79)90598-8 [DOI] [PubMed] [Google Scholar]
- [21].Kitade T, Odahara Y, Shinohara S, et al. Studies on the enhanced effect of acupuncture analgesia and acupuncture anesthesia by D-phenylalanine (2nd report)--schedule of administration and clinical effects in low back pain and tooth extraction. Acupunct Electrother Res 1990; 15(2): 121–35. 10.3727/036012990816358252 [DOI] [PubMed] [Google Scholar]
- [22].Cheng RSS, Pomeranz B. A combined treatment with D-amino acids and electroacupuncture produces a greater analgesia than either treatment alone; naloxone reverses these effects. Pain 1980; 8(2): 231–6. 10.1016/0304-3959(88)90010-3 [DOI] [PubMed] [Google Scholar]
- [23].Blum K, Topel H. Opioid peptides and alcoholism: genetic deficiency and chemical management. Funct Neurol 1986; 1(1): 71–83. [PubMed] [Google Scholar]
- [24].Blum K, Baron D, McLaughlin T, Gold MS. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS). J Neurol Sci 2020; 411: 116733. 10.1016/j.jns.2020.116733 [DOI] [PubMed] [Google Scholar]
- [25].Blum K, Modestino EJ, Gondré-Lewis M, et al. Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic. J Syst Integr Neurosci 2017; 3(6) 10.15761/JSIN.1000183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blum K, Chen ALC, Thanos PK, et al. Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence. Front Biosci (Elite Ed) 2018; 10(1): 175–96. 10.2741/e816 [DOI] [PubMed] [Google Scholar]
- [27].Trachtenberg MC, Blum K. Alcohol and opioid peptides: neuropharmacological rationale for physical craving of alcohol. Am J Drug Alcohol Abuse 1987; 13(3): 365–72. 10.3109/00952998709001520 [DOI] [PubMed] [Google Scholar]
- [28].Blum K, Briggs AH, Elston SF, DeLallo L, Sheridan PJ, Sar M. Reduced leucine-enkephalin--like immunoreactive substance in hamster basal ganglia after long-term ethanol exposure. Science 1982; 216(4553): 1425–7. 10.1126/science.7089531 [DOI] [PubMed] [Google Scholar]
- [29].Blum K, Elston SF, DeLallo L, Briggs AH, Wallace JE. Ethanol acceptance as a function of genotype amounts of brain [Met]enkephalin. Proc Natl Acad Sci USA 1983; 80(21): 6510–2. 10.1073/pnas.80.21.6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blum K, Briggs AH, Trachtenberg MC, Delallo L, Wallace JE. Enkephalinase inhibition: regulation of ethanol intake in genetically predisposed mice. Alcohol 1987; 4(6): 449–56. 10.1016/0741-8329(87)90084-X [DOI] [PubMed] [Google Scholar]
- [31].O’Hollaren P Diphosphopyridine nucleotide in the prevention, diagnosis and treatment of drug addiction. A preliminary report. West J Surg, Obstet Gynecol 1961; 69: 213–5. [PubMed] [Google Scholar]
- [32].Singh S, William M, Chu XP. Nicotinamide phosphoribosyltransferase contributes to cocaine addiction through sirtuin 1. Int J Physiol Pathophysiol Pharmacol 2019; 11(6): 318–20. [PMC free article] [PubMed] [Google Scholar]
- [33].Witt EA, Reissner KJ. The effects of nicotinamide on reinstatement to cocaine seeking in male and female Sprague Dawley rats. Psychopharmacology (Berl) 2020; 237(3): 669–80. 10.1007/s00213-019-05404-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev 2010; 31(2): 194–223. 10.1210/er.2009-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hirrlinger J, Dringen R. The cytosolic redox state of astrocytes: Maintenance, regulation and functional implications for metabolite trafficking. Brain Res Brain Res Rev 2010; 63(1–2): 177–88. 10.1016/j.brainresrev.2009.10.003 [DOI] [PubMed] [Google Scholar]
- [36].Braidy N, Villalva MD, van Eeden S. Sobriety and satiety: Is NAD+ the answer?. Antioxidants (Basel) 2020; 9(5): 425. 10.3390/antiox9050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blum K, Wallace JE, Geller I. Synergy of ethanol and putative neurotransmitters: glycine and serine. Science 1972; 176(4032): 292–4. 10.1126/science.176.4032.292 [DOI] [PubMed] [Google Scholar]
- [38].Blum K, Wallace JE, Friedman RN. Reduction of acute alcoholic intoxication by alpha amino acids: glycine and serine. Life Sci 1974; 14(3): 557–65. 10.1016/0024-3205(74)90370-1 [DOI] [PubMed] [Google Scholar]
- [39].Williams KL, Ferko AP, Barbieri EJ, DiGregorio GJ. Glycine enhances the central depressant properties of ethanol in mice. Pharmacol Biochem Behav 1995; 50(2): 199–205. 10.1016/0091-3057(94)00288-T [DOI] [PubMed] [Google Scholar]
- [40].Serrita J, Ralevski E, Yoon G, Petrakis I. A pilot randomized, placebo-controlled trial of glycine for treatment of schizophrenia and alcohol dependence. J Dual Diagn 2019; 15(1): 46–55. 10.1080/15504263.2018.1549764 [DOI] [PubMed] [Google Scholar]
- [41].Adermark L, Clarke RB, Olsson T, Hansson E, Söderpalm B, Ericson M. Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol 2011; 16(1): 43–54. 10.1111/j.1369-1600.2010.00206.x [DOI] [PubMed] [Google Scholar]
- [42].Hejazi N, Zhou C, Oz M, Sun H, Ye JH, Zhang L. Delta9-tetrahydrocannabinol and endogenous cannabinoid anandamide directly potentiate the function of glycine receptors. Mol Pharmacol 2006; 69(3): 991–7. 10.1124/mol.105.019174 [DOI] [PubMed] [Google Scholar]
- [43].de Bejczy A, Nations KR, Szegedi A, Schoemaker J, Ruwe F, Söderpalm B. Efficacy and safety of the glycine transporter-1 inhibitor org 25935 for the prevention of relapse in alcohol-dependent patients: a randomized, double-blind, placebo-controlled trial. Alcohol Clin Exp Res 2014; 38(9): 2427–35. 10.1111/acer.12501 [DOI] [PubMed] [Google Scholar]
- [44].Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B. The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol 2007; 42(1): 11–8. 10.1093/alcalc/agl085 [DOI] [PubMed] [Google Scholar]
- [45].Kotlińska J Attenuation of morphine dependence and withdrawal by glycine B site antagonists in rats. Pharmacol Biochem Behav 2001; 68(1): 157–61. 10.1016/S0091-3057(00)00443-3 [DOI] [PubMed] [Google Scholar]
- [46].Burgos CF, Muñoz B, Guzman L, Aguayo LG. Ethanol effects on glycinergic transmission: From molecular pharmacology to behavior responses. Pharmacol Res 2015; 101: 18–29. 10.1016/j.phrs.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Michino M, Donthamsetti P, Beuming T, et al. A single glycine in extracellular loop 1 is the critical determinant for pharmacological specificity of dopamine D2 and D3 receptors. Mol Pharmacol 2013; 84(6): 854–64. 10.1124/mol.113.087833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhou H, Rentsch CT, Cheng Z, et al. Association of OPRM1 functional coding variant with opioid use disorder: A genome-wide association study. JAMA Psychiatry 2020; 77(10): 1072–80. 10.1001/jamapsychiatry.2020.1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Guan YZ, Ye JH. Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology 2010; 35(9): 1841–9. 10.1038/npp.2010.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Minami H, Morse EL, Adibi SA. Characteristics and mechanism of glutamine-dipeptide absorption in human intestine. Gastroenterol 1992; 103(1): 3–11. 10.1016/0016-5085(92)91088-L [DOI] [PubMed] [Google Scholar]
- [51].Blum K, Badgaiyan RD, Braverman ER, et al. Hypothesizing that, a pro-dopamine regulator (KB220Z) should optimize, but not hyper-activate the activity of trace amine-associated receptor 1 (TAAR-1) and induce anti-craving of psychostimulants in the long-term. J Reward Defic Syndr Addict Sci 2016; 2(1): 14–21. 10.17756/jrdsas.2016-023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Becker-Krail DD, Parekh PK, Ketchesin KD, et al. Circadian transcription factor NPAS2 and the NAD+ - dependent deacetylase SIRT1 interact in the mouse nucleus accumbens and regulate reward. Eur J Neurosci 2022; 55(3): 675–93. 10.1111/ejn.15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].French SW. Chronic alcohol binging injures the liver and other organs by reducing NAD⁺ levels required for sirtuin’s deacetylase activity. Exp Mol Pathol 2016; 100(2): 303–6. 10.1016/j.yexmp.2016.02.004 [DOI] [PubMed] [Google Scholar]
- [54].Simplicio JA, do Vale GT, Gonzaga NA, et al. Reactive oxygen species derived from NAD(P)H oxidase play a role on ethanol-induced hypertension and endothelial dysfunction in rat resistance arteries. J Physiol Biochem 2017; 73(1): 5–16. 10.1007/s13105-016-0519-z [DOI] [PubMed] [Google Scholar]
- [55].Huang S, Zhang B, Chen Y, et al. Poly(ADP-Ribose) polymerase inhibitor PJ34 attenuated hepatic triglyceride accumulation in alcoholic fatty liver disease in mice. J Pharmacol Exp Ther 2018; 364(3): 452–61. 10.1124/jpet.117.243105 [DOI] [PubMed] [Google Scholar]
- [56].Xiong X, Yu J, Fan R, et al. NAMPT overexpression alleviates alcohol-induced hepatic steatosis in mice. PLoS One 2019; 14(2): e0212523. 10.1371/journal.pone.0212523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol 1997; 38: 601–28. 10.1016/S1054-3589(08)61001-7 [DOI] [PubMed] [Google Scholar]
- [58].Li Q, Xie G, Zhang W, et al. Dietary nicotinic acid supplementation ameliorates chronic alcohol-induced fatty liver in rats. Alcohol Clin Exp Res 2014; 38(7): 1982–92. 10.1111/acer.12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Luo G, Huang B, Qiu X, et al. Resveratrol attenuates excessive ethanol exposure induced insulin resistance in rats via improving NAD+ /NADH ratio. Mol Nutr Food Res 2017; 61(11): 1700087. 10.1002/mnfr.201700087 [DOI] [PubMed] [Google Scholar]
- [60].Cederbaum AI. Microsomal generation of reactive oxygen species and their possible role in alcohol hepatotoxicity. Alcohol Alcohol Suppl 1991; 1: 291–6. [PubMed] [Google Scholar]
- [61].Smith JW, Johnson LC, Burdick JA. Sleep, psychological and clinical changes during alcohol withdrawal in NAD-treated alcoholics. Q J Stud Alcohol 1971; 32(4): 982–94. 10.15288/qjsa.1971.32.982 [DOI] [PubMed] [Google Scholar]
- [62].Dou X, Shen C, Wang Z, Li S, Zhang X, Song Z. Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice. J Nutr Biochem 2013; 24(8): 1520–8. 10.1016/j.jnutbio.2012.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dicker E, Cederbaum AI. Increased NADH-dependent production of reactive oxygen intermediates by microsomes after chronic ethanol consumption: comparisons with NADPH. Arch Biochem Biophys 1992; 293(2): 274–80. 10.1016/0003-9861(92)90395-D [DOI] [PubMed] [Google Scholar]
- [64].Rappaport M NAD effects on the biochemistry and psychological performance of alcoholics under ethanol stress. Q J Stud Alcohol 1969; 30(3): 570–84. 10.15288/qjsa.1969.30.570 [DOI] [PubMed] [Google Scholar]
- [65].Rashba-Step J, Turro NJ, Cederbaum AI. Increased NADPH- and NADH-dependent production of superoxide and hydroxyl radical by microsomes after chronic ethanol treatment. Arch Biochem Biophys 1993; 300(1): 401–8. 10.1006/abbi.1993.1054 [DOI] [PubMed] [Google Scholar]
- [66].You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 2008; 294(4): G892–8. 10.1152/ajpgi.00575.2007 [DOI] [PubMed] [Google Scholar]
- [67].Badawy AA, Evans M. The mechanism of the antagonism by naloxone of acute alcohol intoxication. Br J Pharmacol 1981; 74(3): 514–6. 10.1111/j.1476-5381.1981.tb10458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kosenko EA, Kaminsky YG. A comparison between effects of chronic ethanol consumption, ethanol withdrawal and fasting in ethanol-fed rats on the free cytosolic NADP+/NADPH ratio and NADPH-regenerating enzyme activities in the liver. Int J Biochem 1985; 17(8): 895–902. 10.1016/0020-711X(85)90173-9 [DOI] [PubMed] [Google Scholar]
- [69].Kukiełka E, Cederbaum AI. The effect of chronic ethanol consumption on NADH- and NADPH-dependent generation of reactive oxygen intermediates by isolated rat liver nuclei. Alcohol Alcohol 1992; 27(3): 233–9. [PubMed] [Google Scholar]
- [70].Rodrigo R, Egaña E. Alcohol:NAD oxidoreductase in brain of rats from a colony fed dilute ethanol for many generations. J Neurochem 1975; 25(5): 645–7. 10.1111/j.1471-4159.1975.tb04382.x [DOI] [PubMed] [Google Scholar]
- [71].Xiao Y, Phelp P, Wang Q, et al. Cardioprotecive properties of known agents in rat ischemia-reperfusion model under clinically relevant conditions: Only the NAD precursor nicotinamide riboside reduces infarct size in presence of fentanyl, midazolam and cangrelor, but not propofol. Front Cardiovasc Med 2021; 8: 712478. 10.3389/fcvm.2021.712478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 1986; 29(3): 307–13. [PubMed] [Google Scholar]
- [73].Requardt RP, Wilhelm F, Rillich J, Winkler U, Hirrlinger J. The biphasic NAD(P)H fluorescence response of astrocytes to dopamine reflects the metabolic actions of oxidative phosphorylation and glycolysis. J Neurochem 2010; 115(2): 483–92. 10.1111/j.1471-4159.2010.06940.x [DOI] [PubMed] [Google Scholar]
- [74].Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun 2010; 393(1): 66–72. 10.1016/j.bbrc.2010.01.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hageman GJ, Stierum RH, van Herwijnen MH, van der Veer MS, Kleinjans JC. Nicotinic acid supplementation: effects on niacin status, cytogenetic damage, and poly(ADP-ribosylation) in lymphocytes of smokers. Nutr Cancer 1998; 32(2): 113–20. 10.1080/01635589809514728 [DOI] [PubMed] [Google Scholar]
- [76].Brodie MJ, Czapinski P, Pazdera L, et al. A phase 2 randomized controlled trial of the efficacy and safety of cannabidivarin as add-on therapy in participants with inadequately controlled focal seizures. Cannabis Cannabinoid Res 2021; 6(6): 528–36. 10.1089/can.2020.0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Russell AL, McCarty MF. DL-phenylalanine markedly potentiates opiate analgesia - an example of nutrient/pharmaceutical up-regulation of the endogenous analgesia system. Med Hypotheses 2000; 55(4): 283–8. 10.1054/mehy.1999.1031 [DOI] [PubMed] [Google Scholar]
- [78].Blum K, Han D, Modestino EJ, et al. A systematic, intensive statistical investigation of data from the Comprehensive Analysis of Reported Drugs (CARD) for compliance and illicit opioid abstinence in substance addiction treatment with buprenorphine/naloxone. Subst Use Misuse 2018; 53(2): 220–9. 10.1080/10826084.2017.1400064 [DOI] [PubMed] [Google Scholar]
- [79].Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One 2014; 9(9): e104275. 10.1371/journal.pone.0104275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Blum K, Oscar-Berman M, Badgaiyan RD, Khurshid KA, Gold MS. Dopaminergic neurogenetics of sleep disorders in Reward Deficiency Syndrome (RDS). J Sleep Disord Ther 2014; 3(2): 126. 10.4172/2167-0277.1000e126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Doctor JN, Sullivan MD. Knowledge translation and the opioid crisis. Am J Public Health 2022; 112(S1): S15–7. 10.2105/AJPH.2021.306670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Blum K, Chen TJ, Bailey J, et al. Can the chronic administration of the combination of buprenorphine and naloxone block dopaminergic activity causing anti-reward and relapse potential? Mol Neurobiol 2011; 44(3): 250–68. 10.1007/s12035-011-8206-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Saleh EM, Hamdy GM, Hassan RE. Neuroprotective effect of sodium alginate against chromium-induced brain damage in rats. PLoS One 2022; 17(4): e0266898. 10.1371/journal.pone.0266898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, Hirrlinger J. Ca²⁺ signals of astrocytes are modulated by the NAD⁺/NADH redox state. J Neurochem 2012; 120(6): 1014–25. 10.1111/j.1471-4159.2012.07645.x [DOI] [PubMed] [Google Scholar]
- [85].Wallius E, Tohka J, Hirvonen J, Hietala J, Ruotsalainen U. Evaluation of the automatic threedimensional delineation of caudate and putamen for PET receptor occupancy studies. Nucl Med Commun 2008; 29(1): 53–65. 10.1097/MNM.0b013e3282f1bba0 [DOI] [PubMed] [Google Scholar]
- [86].Zhu XH, Lee BY, Tuite P, et al. Quantitative assessment of occipital metabolic and energetic changes in Parkinson’s patients, using in vivo31P MRS-based metabolic imaging at 7T. Metabolites 2021; 11(3): 145. 10.3390/metabo11030145 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available within the article.


