Abstract
Aims
Asthma, hay fever and eczema are three common chronic conditions. There have been no recent multi-country data on the burden of these three conditions in adults; the aims of this study are to fill this evidence gap.
Methods
The Global Asthma Network Phase I is a multi-country cross-sectional population-based study using the same core methodology as the International Study of Asthma and Allergies in Childhood Phase III. It provides data on the burden of asthma, hay fever and eczema in children and adolescents, and, for the first time, in their parents/guardians.
Results
Data were available from 193 912 adults (104 061 female; mean±sd age 38±7.5 years) in 43 centres in 17 countries. The overall prevalence (range) of symptoms was 6.6% (0.9–32.7%) for current wheeze, 4.4% (0.9–29.0%) for asthma ever, 14.4% (2.8–45.7%) for hay fever ever and 9.9% (1.6–29.5%) for eczema ever. Centre prevalence varied considerably both between countries and within countries. There was a moderate correlation between hay fever ever and asthma ever, and between eczema ever and hay fever ever at the centre level. There were moderate to strong correlations between indicators of the burden of disease reported in adults and the two younger age groups.
Conclusion
We found evidence for a substantial burden of asthma, hay fever ever and eczema ever in the countries examined, highlighting the major public health importance of these diseases. Prevention strategies and equitable access to effective and affordable treatments for these three conditions would help mitigate the avoidable morbidity they cause.
Short abstract
There is a substantial global burden of asthma, hay fever and eczema in adults, which represents a major global public health problem. Accessible, affordable, equitable and effective strategies are needed to reduce this burden across the life-course. https://bit.ly/3uBTQfl
Introduction
Asthma, hay fever and eczema are three common chronic conditions that typically start in childhood and often continue across the life-course [1]. All three conditions cause considerable morbidity globally, especially when basic effective treatments are unavailable [2, 3]. Asthma is an important cause of avoidable mortality [4].
The International Study of Asthma and Allergies in Childhood (ISAAC) investigated the symptom prevalence and determinants of asthma, rhinoconjunctivitis and eczema in schoolchildren on two previous occasions (ISAAC Phase I in 1993–5 and ISAAC Phase III in 2001–3) [5–16]. The work of ISAAC has been continued by the Global Asthma Network (GAN) through ISAAC centres and new centres that are interested in GAN Phase I. This study is a multi-country population-based cross-sectional study designed to assess the three conditions and their severity, management and risk factors in 13–14-year-old adolescents, 6–7-year-old children and their parents/guardians using the same methods as ISAAC Phase III [17].
There has been no large survey on the prevalence of asthma in adults since the World Heath Organization implemented the World Health Survey (WHS) in 2002 and 2003 [18], and no such surveys for hay fever ever or eczema ever. In this paper we report data on the prevalence of asthma symptoms, hay fever ever and eczema ever in adults in GAN Phase I. We compare their global patterns, and contrast them with those observed in children in the same populations.
Methods
The GAN methodology has previously been published [17, 19], and will only be briefly summarised here.
Participants
The adult participants were the parents (or guardians) of children and adolescents in GAN Phase I [19]. Cluster sampling was applied to randomly select at least 10 schools from a geographically defined sampling frame. All schools were included if there were <10 schools in the sampling frame. The compulsory age group in GAN Phase I was adolescents, who self-completed written questionnaires at school. Additional inclusion of 6–7-year-olds was optional. Schools could choose to offer parents/guardians the option to complete similar questionnaires on their own health (the adult group), and the linkage between adults and children and adolescents was documented.
Questionnaires
Questionnaires for adults were developed by building on questionnaires used in ISAAC and the European Community Respiratory Health Survey [20, 21]. The original questionnaire was in English, with translation to local languages and back-translation to English completed using a specific methodology common to ISAAC and GAN [17].
Definitions
“Current wheeze” in asthma was defined as a positive answer to the question “Have you had wheezing or whistling in the chest in the past 12 months?” People with “severe asthma symptoms” were defined as those with current wheeze who, in the past 12 months, reported having had four or more attacks of wheeze, or more than one night per week sleep disturbance from wheeze, or wheeze affecting speech. “Asthma ever” was defined as a positive answer to the question “Have you ever had asthma?” “Hay fever ever” was defined as a positive answer to the question “Have you ever had hay fever?” “Eczema ever” was defined as a positive answer to the question “Have you ever had eczema?”
Sample size and study power
Sample sizes of at least 1000 and preferably 3000 were sought for the adolescents and children within each centre [18]. We aimed for a participation rate of >80% for the adolescents and >70% for the children [22]. The actual response rate was 90% for adolescents and 79% for children [23]. We were unable to calculate a conventional response rate for the adults because some schoolchildren have only one parent or guardian and the number of adults who received the questionnaire was unknown. The estimated median participation rate of adults, using a “per child” approach [22], was 82.9% (range 30.2–100%); the median for four centres could not be calculated owing to insufficient information.
Data handling and analysis
All centres submitted their datasets and a Centre Report documenting the methodology used to the GAN Global Centre in Auckland (New Zealand) [17]. A first quality control check was performed together with a careful review of the Centre Report for adherence to protocol. Depending on the language used locally, the dataset was then sent to one of the two GAN data centres, in Murcia, Spain (Spanish- and Portuguese-speaking centres) or London, UK (all other languages), for a standardised and coordinated data check. Centres reported in this analysis are any centres that were included in the analysis of data from children and adolescents [19] that also collected data from adults. For prevalence estimations, the number of positive answers to a specific symptom in the centre was divided by the number of completed questionnaires.
Global national income (GNI) category for each country was calculated using cut-off points provided by the World Bank in June 2020 [24], and countries were classified as high income countries (HICs), upper middle income countries (UMICs) or low income/lower middle income countries (LICs/LMICs). A Spearman correlation coefficient was used to estimate the correlation between symptoms of different conditions and between age groups using centre-level data. The correlation was defined as strong if the correlation coefficient was ≥0.7, moderate if ≥0.4 but <0.7 and weak if <0.4. Multilevel log-binomial regression was used to estimate how much of the variability of each symptom's prevalence was dependent on centre-level variation, additional to within-centre binomial sampling error. Because the intraclass correlation coefficient was >5% (ICC>0.05) in the null model in all instances, multilevel models fitting centre as a random effect were used to estimate the effect of sex and GNI in the prevalence of symptoms of the three conditions. A uniform approach to data processing, checking and analysis was used, using Stata versions 13–15 (Stata Corp LLC, College Station, TX, USA).
Centre funding and ethics
All centres in GAN Phase I obtained their own funding and applied for ethics approval from their local ethics committee before starting the study.
Role of the funding source
The funding sources had no role in study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the paper for publication.
Results
Data were collected from 193 912 adults (104 061 female, mean±sd age 38±7.5 years, 5.0% >50 years old, 16.8% current smokers, 47.2% parents of adolescents) in 43 centres (including 12 ISAAC Phase I and 19 ISAAC Phase III centres) in 17 countries between 2015 and 2020 (supplementary tables S1 and S2). The prevalence of current wheeze was highest (10.6%, 95% CI 10.2–10.9%) among participants from HICs, followed by 8.4% (8.2–8.6%) among participants from UMICs and 3.6% (3.5–3.8%) among participants from LICs/LMICs (table 1). Similar trends across GNI categories were noted for asthma ever, severe asthma symptoms and hay fever ever, with the exception of eczema ever which was lowest in UMICs.
TABLE 1.
Prevalence of asthma, hay fever ever and eczema ever in centres grouped by GNI
| GNI | Years | Centres, n | Total, n | Current wheeze | Asthma ever | Severe asthma symptoms# | Severe asthma symptoms¶ (population denominator) | Eczema ever | Hay fever ever |
| HIC | 2015–2020 | 6 | 30 556 | 3231 (10.6%) | 3106 (10.2%) | 1179 (36.5%) | 1179 (3.9%) | 5081 (16.6%) | 9453 (30.9%) |
| UMIC | 2015–2020 | 25 | 74 897 | 6299 (8.4%) | 3502 (4.7%) | 2669 (42.4%) | 2669 (3.6%) | 5377 (7.2%) | 9736 (13.0%) |
| LIC/LMIC | 2017–2019 | 12 | 88 459 | 3208 (3.6%) | 1926 (2.2%) | 1161 (36.2%) | 1161 (1.3%) | 8791 (9.9%) | 8695 (9.8%) |
| Total | 43 | 193 912 | 12 738 (6.6%) | 8534 (4.4%) | 5009 (39.3%) | 5009 (2.6%) | 19 249 (9.9%) | 27 884 (14.4%) |
Data presented as n (%), unless otherwise indicated. Chi-squared p-values for comparison between GNI categories: current wheeze <0.0001, asthma ever <0.0001, severe asthma symptoms <0.0001, severe asthma symptoms (population denominator) <0.0001, eczema ever <0.0001, hay fever ever <0.0001. GNI: gross national income; HIC: high income country; UMIC: upper middle income country: LIC/LMIC: low income country/lower middle income country. #: current wheeze denominator; ¶: total participants denominator.
Asthma
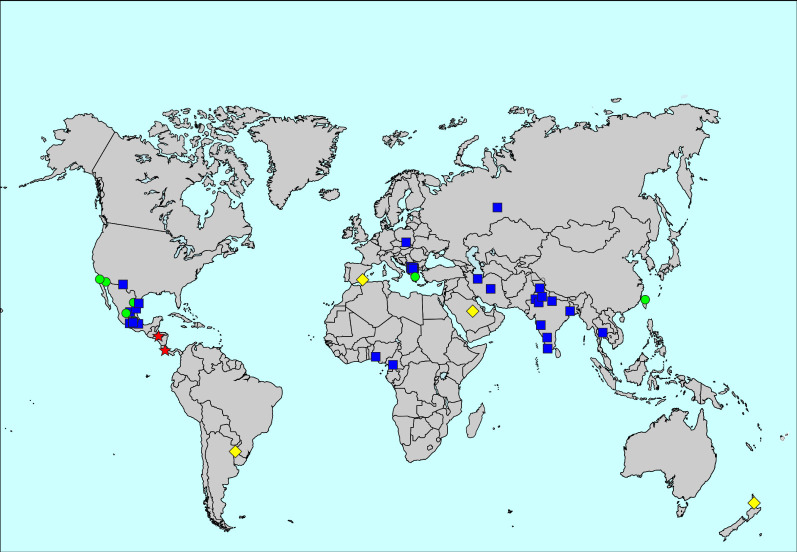
The prevalence of asthma ever was 4.4%, ranging from 0.9% in Gjilan and Ferizaj, Kosovo, to 29.0% in Costa Rica. Heterogeneity was high both between countries and between centres within countries (figure 1, supplementary table S1 and supplementary figures S1 and S2). Centre-level variation explained 21.8% of the variability of the prevalence in the multilevel analysis. Women had a higher prevalence of asthma ever than men (4.8% versus 3.9%; adjusted relative risk (aRR) for men 0.85, 95% CI 0.82–0.89).
FIGURE 1.
Map of the prevalence of asthma ever. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
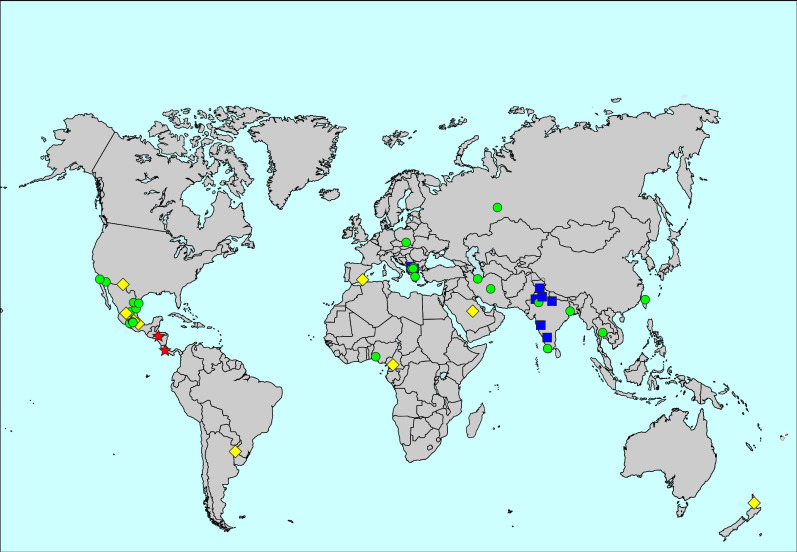
The overall prevalence of current wheeze was 6.6%, ranging from 0.9% in New Delhi, India, to 32.7% in Tegucigalpa, Honduras. Heterogeneity of the prevalence of current wheeze was high (figure 2, supplementary table S1 and supplementary figures S1 and S2). Centre-level variation explained 13.1% of the variability of the prevalence of current wheeze in the multilevel analysis. Women had a higher prevalence of current wheeze than men (6.8% versus 6.2%; aRR for men 0.97, 95% CI 0.94–1.00).
FIGURE 2.
Map of the prevalence of current wheeze. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
The prevalence of severe asthma symptoms was 2.6%, ranging from 0.2% in Bikaner, India, to 20.9% in Tegucigalpa, Honduras. The prevalence of severe asthma symptoms among those who reported current wheeze was 39.3%, ranging from 15.0% in Bikaner, India, to 63.9% in Tegucigalpa, Honduras (supplementary table S1 and supplementary figures S1 and S2). Centre-level variation accounted for 17.0% of the variability of the prevalence of severe asthma symptoms. Women had a higher prevalence of severe asthma symptoms than men (2.9% versus 2.2%; aRR for men 0.83, 95% CI 0.82–0.90).
Hay fever
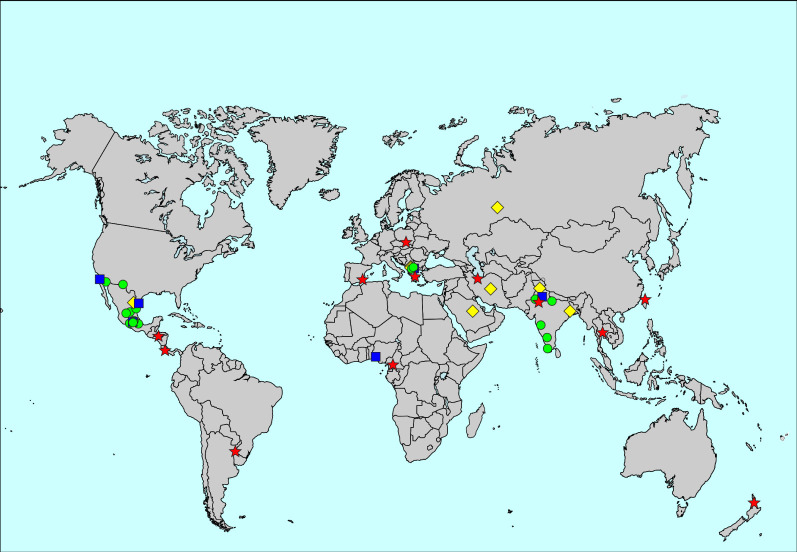
The prevalence of hay fever ever was 14.4%, ranging from 2.8% in Ibadan, Nigeria, to 45.7% in Bangkok, Thailand (figure 3, supplementary table S1 and supplementary figures S1 and S2). Centre-level variation explained 21.8% of the variability of the prevalence of hay fever ever. The prevalence of hay fever ever was higher in women than men (14.7% versus 14.0%; aRR for men 0.92, 95% CI 0.90–0.93).
FIGURE 3.
Map of the prevalence of hay fever ever. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
Eczema
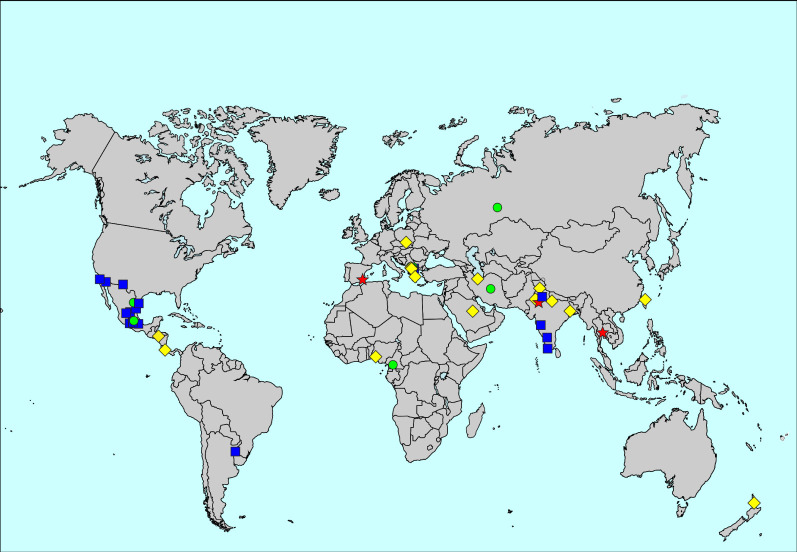
The prevalence of eczema ever was 9.9%, ranging from 1.6% in Tijuana, Mexico, to 29.5% in Bangkok, Thailand (figure 4, supplementary table S1 and supplementary figures S1 and S2). Centre-level variation explained 19.6% of the variability of the prevalence of eczema ever. The prevalence of eczema ever was higher in women than men (10.0% versus 9.9%; aRR for men 0.90, 95% CI 0.88–0.93).
FIGURE 4.
Map of the prevalence of eczema ever. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
Correlations of prevalence between the three conditions
There was moderate correlation between the prevalence of hay fever ever and asthma ever (Rho 0.54, 95% CI 0.32–0.76), and between eczema ever and hay fever ever (Rho 0.66, 95% CI 0.48–0.83), but no significant correlation between asthma ever and eczema ever (Rho 0.13, 95% CI −0.19–0.45) at the centre level (figure 5). The correlation between the prevalence of hay fever ever and asthma ever and between the prevalence of eczema ever and hay fever ever remained after stratification by sex (supplementary figure S3).
FIGURE 5.
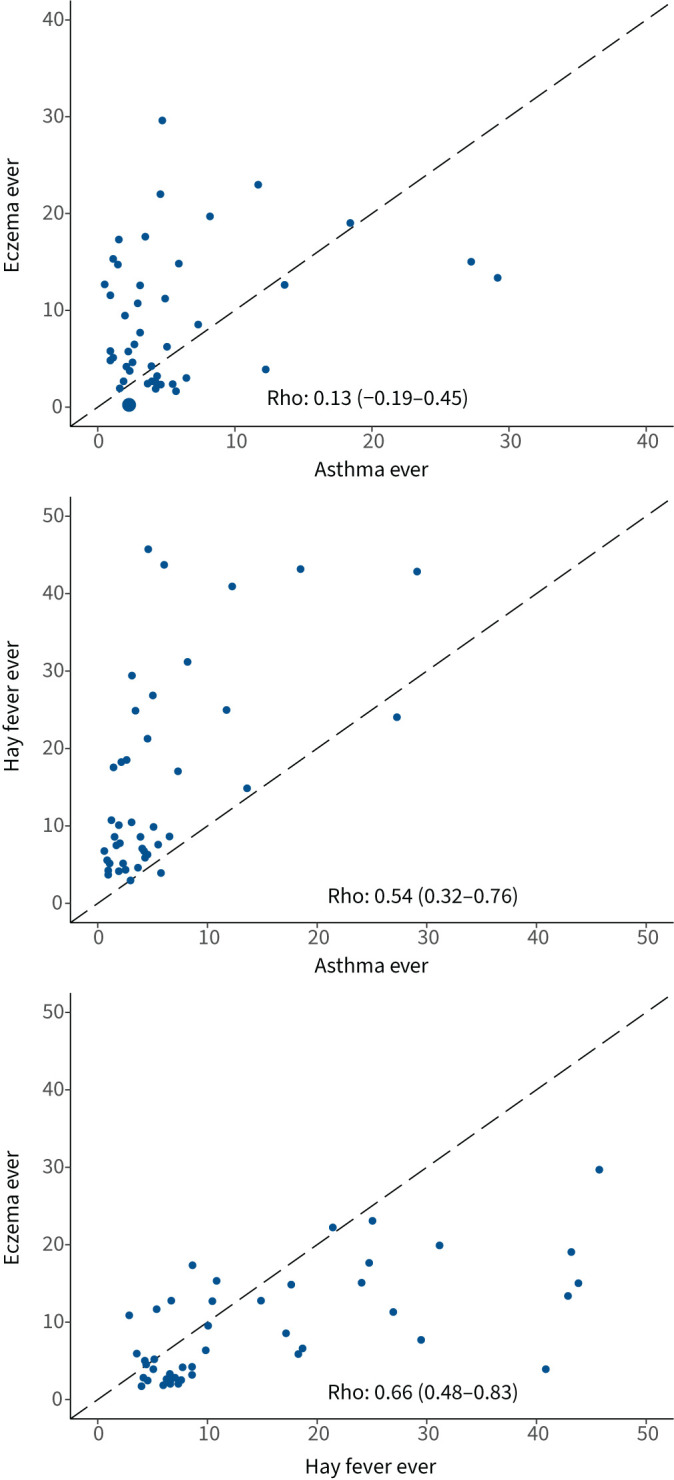
Rank correlation values and scatter plots of prevalence of the three conditions at the centre level. The dashed line is the identity line. Rank correlation coefficient (Rho) and 95% CI are shown in each graph.
Relationship between age groups
There was strong correlation between the prevalence of asthma ever in adults versus adolescents (Rho 0.87, 95% CI 0.79–0.95), between asthma ever in adults versus children (Rho 0.83, 95% CI 0.66–1.00), between current wheeze in adults versus adolescents (Rho 0.81, 95% CI 0.68–0.94), between severe asthma symptoms in adults versus adolescents (Rho 0.79, 95% CI 0.67–0.92), between severe asthma symptoms in adults versus children (Rho 0.82, 95% CI 0.65–0.98) between hay fever ever in adults versus adolescents (Rho 0.75, 95% CI 0.57–0.92), between eczema ever in adults versus adolescents (Rho 0.87, 95% CI 0.78–0.95) and between eczema ever in adults versus children (Rho 0.71, 95% CI 0.51–0.91). There was moderate correlation between current wheeze in adults versus children, and between hay fever ever in adults versus children (supplementary figure S4).
Discussion
The major findings of GAN Phase I were as follows: 1) the overall prevalence of symptoms of current wheeze, asthma ever, hay fever ever and eczema ever was 6.6%, 4.4%, 14.4% and 9.9%, respectively; 2) centre prevalence varied considerably both between countries and within countries; 3) the burden of all three conditions was higher in female participants and in higher income countries; 4) there was a moderate correlation between hay fever ever and asthma ever, and between eczema ever and hay fever ever at the centre level; 5) there were moderate to strong correlations between the prevalence of asthma symptoms, hay fever ever and eczema ever reported in adults and the two younger age groups.
A multi-country survey on the prevalence of asthma in adults was conducted by the European Community Respiratory Health Survey (ECRHS) in the 1990s [25]. The questions used in the ECRHS were “Have you had wheezing or whistling in your chest at any time in the last 12 months?” and “Have you had an attack of asthma in the last 12 months?” [26]. The ECRHS reported large geographical variations in the prevalence of asthma [26]. The median prevalence of current asthma was 4.5% (range 2.0–11.9%) in ECRHS stage one and 5.2% (range 1.2–13.0%) in ECRHS stage two [27]. Women had a higher prevalence of current asthma than men and the prevalence of wheeze was negatively associated with age. The ECRHS concluded that the geographical variations in the prevalence of asthma were most likely due to environmental factors [7]. The WHS enrolled 308 218 adults aged ≥18 years from 64 countries [18]. The WHS defined current wheeze symptoms as a positive response to “during the last 12 months, have you experienced any of the following: 1) attacks of wheezing or whistling breathing? (yes/no); or 2) attacks of wheezing that came on after you stopped exercising or some other physical activity? (yes/no)”. The WHS reported that global prevalence of current wheeze symptoms was 9.2%, ranging from 2.4% in Vietnam to 24.0% in Brazil. The prevalence of current wheeze symptoms increased with age, was higher among men than women, more common in smokers than non-smokers, and was relatively high in HICs and LICs and relatively low in middle income countries [18]. In our survey, the prevalence of asthma ever varied markedly between centres, as did the prevalence of current wheeze symptoms, with less variability within countries than seen in children and adolescents. Current severe asthma symptoms were commonly reported (range 15.0–63.9%) among participants reporting wheeze in the past 12 months across all centres, suggesting a concerning level of poor asthma control [28]. There was a clear association between GNI category and the prevalence of current wheeze, which was highest in HICs and lowest in LICs/LMICs, similar to the pattern seen of a lower prevalence of current wheeze symptoms in children and adolescents in LICs/LMICs [19]. Asthma symptoms were more common in female participants, as seen in the adolescent group and other studies of older children [5, 29] and the ECRHS. The WHS reported that men were more likely to report current wheeze symptoms, perhaps because its study population was older (34% aged >50 years), and 30% were smokers, thus may have had wheeze due to chronic obstructive pulmonary disease. Whether the difference between our findings and those of WHS with regards to GNI was attributable to differences in definitions (e.g. of symptoms), study population, different prevalence of environmental risk factors and genetic backgrounds requires further investigation.
The ECRHS reported that the median prevalence of nasal allergy and hay fever was 20.9% (range 9.5–40.9%) [27]. Subjects with perennial rhinitis were more likely to have current asthma. In our study, the prevalence of hay fever ever was 14.4% but was variable (from 2.8% in Ibadan, Nigeria, to 45.7% in Bangkok, Thailand) with less variability within countries. This was also seen in children and adolescents, as was an association between hay fever and GNI categories, with the greatest burden seen in HICs [19]. Consistent with the ECRHS, our study shows moderate correlation between the prevalence of hay fever ever and asthma ever.
The overall prevalence of eczema ever was 9.9% but this varied from 1.6% in Tijuana, Mexico, to 29.5% in Bangkok, Thailand, with less variability within countries. We did find an association between eczema ever and GNI categories, with the greatest burden seen in HICs, which was also seen in children and adolescents in GAN Phase I [19]. A difference between sexes (more prevalent in females) was found in previous ISAAC surveys, the adolescents in GAN Phase I and other cohort studies [8, 19, 30, 31] and we found the same in this study of adults. No significant correlation between the prevalence of asthma ever and eczema ever in adults was identified. Because eczema tends to occur in the early stage of life and decreases with age [32], whether this was in part due to recall bias was unknown.
There was considerable variation in the prevalence of all three conditions in adults, which was partly accounted for by centre-level variation. We speculate that the difference in the prevalence of risk factors may have contributed to the difference in observed prevalence between centres and countries; risk factors associated with the three conditions collected as part of GAN Phase I will be analysed and reported separately, which should provide more insight into this issue. We found moderate to strong correlations between the prevalence of asthma symptoms, hay fever ever and eczema ever reported in adults and the two younger age groups, likely indicating that parents/guardians and the two younger age groups have similar environmental and genetic risk factors of the three conditions.
The strengths and weaknesses of the ISAAC and GAN Phase I methodology have been discussed in depth previously [5] and have been summarised elsewhere [17, 19]. We acknowledge the limitations of the small number of GAN Phase I centres versus ISAAC Phase I and III, the self-selection of centres potentially limiting representativeness, challenges of inferring clinical diagnoses from self-reporting via questionnaires (e.g. risk of recall bias and lack of direct physician diagnoses), and the difficulties around the translation of concepts such as “wheezing” into different languages. Furthermore, we did not collect information on current symptoms of hay fever and eczema, but only information of hay fever ever and eczema ever, which may not represent the current prevalence of hay fever and eczema because both conditions may remit during adolescence. Moreover, it is possible that parents of children with hay fever or eczema may be more aware of these two conditions and more likely to report having hay fever ever or eczema ever than parents of children with no hay fever or eczema; such potential recall bias may affect the correlation of the prevalence of hay fever ever and eczema ever between children and adults. There was difficulty in obtaining a high response rate for some centres [23]. The correlation analysis is an ecological analysis (at centre level) and these correlations may not hold at the individual level. Key additional strengths are the linkages between the child, adolescent and adult participants that have enabled additional analyses, including exploring the relationship between symptoms reported by the different age groups. However, recruiting the parents of the child and adolescent participants will have led to a degree of selection bias and the included adult population may not be fully representative of the local population in terms of factors including age and socioeconomic status.
In conclusion, the present study offers a unique picture of current symptoms related to asthma, and lifetime history of asthma, hay fever and eczema. Our findings in adults were largely consistent with our findings in children and adolescents (particularly) [19] and the burden of the three conditions seems to correlate across the three age groups. Further studies are needed to confirm whether findings from one group may be cautiously extrapolated to the others.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Ranking of centres for the symptom prevalences of current wheeze, asthma ever, hay fever ever and eczema ever. ERJ-02865-2021.Figure_S1 (9.1KB, pdf)
Supplementary figure S2. Ranking of centres for the symptom prevalences of current wheeze, asthma ever, hay fever ever and eczema ever by sex (males on left). ERJ-02865-2021.Figure_S2 (13.1KB, pdf)
Supplementary figure S3. Rank correlation values and scatter plots of prevalence of the three conditions at the centre level by sex (males on left). The dashed line is the identity line. Rank correlation coefficient and 95% CI is shown in each graph. ERJ-02865-2021.Figure_S3 (7.7KB, pdf)
Supplementary figure S4. Rank correlation comparing centre prevalence (%) of reporting current wheeze, asthma ever, severe asthma symptoms, hay fever ever and eczema ever between the three age groups (children, adolescents, adults) included in GAN Phase I. The dashed line is the identity line. Rank correlation coefficient and 95% CI is shown in each graph. ERJ-02865-2021.Figure_S4 (17.3KB, pdf)
Supplementary table S1. Demographic summary by centre. ERJ-02865-2021.Table_S1 (92.6KB, pdf)
Supplementary table S2. Symptom prevalence of asthma, hay fever and eczema by centre. ERJ-02865-2021.Table_S2 (126.6KB, pdf)
Shareable PDF
Acknowledgements
We are grateful to the children, adolescents and adults who willingly participated with the help of schools and field workers in GAN Phase I. We thank the children, adolescents and parents who participated in GAN Phase I; the school staff for their assistance and help with coordination; the principal investigators and their colleagues; and the many funding bodies throughout the world that supported the individual GAN centres. The GAN Global Centre in Auckland was funded by The University of Auckland with additional funding from the International Union Against Tuberculosis and Lung Disease, Boehringer Ingelheim NZ and an AstraZeneca Educational Grant. The London Data Centre was supported by a PhD studentship to C.E. Rutter from the UK Medical Research Council (grant number MR/N013638/1) and funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013, ERC grant agreement number 668954). The Murcia Data Centre was supported by the University of Murcia and by Instituto de Salud Carlos III, fund PI17/0170. M. Lesosky was supported in part by the Academy of Medical Sciences Newton Advanced Fellowship (NAF\R2\180681). We thank the National Institute for Health Research (NIHR) Global Health Research Unit on Lung Health and TB in Africa at the Liverpool School of Tropical Medicine – “IMPALA” for helping to make this work possible (grant reference 16/136/35); IMPALA was commissioned by the NIHR Global Health Research using UK aid from the UK Government. The views expressed in this publication are those of the authors and not necessarily those of any of the funders. Individual centres involved in GAN Phase I data collection were funded by the following organisations: Brazil, Uruguaiana, funded by Dr Marilyn Urrutia Pereira; Cameroon, Yaounde, funded by Elvis Ndikum (95%) and by family and friends (5%); Costa Rica: partially funded by an unrestricted grant from AstraZeneca for logistic purposes; India (Bikaner, Chandigarh, Jaipur, Kolkata, Kottayam, Lucknow, Mysuru, New Delhi, Pune), GAN Phase I was undertaken by Asthma Bhawan in India which was supported by Cipla Foundation; Iran, Karaj, Alborz University of Medical Sciences; Kosovo, Gjakova, Municipality of Gjakova and the Directorate for Health and Education; Mexico, Puerto Vallarta, Centro Universitario de la Costa, Universidad de Guadalajara; New Zealand, Auckland, Auckland Asthma Charitable Trust; Nigeria, Ibadan, funded by the NIHR (IMPALA, grant reference 16/136/35) using UK aid from the UK Government to support global health research; Poland, Katowice, funded by the Medical University of Silesia.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00440-2022
Global Asthma Network Study Group. Global Asthma Network Steering Group: M.I. Asher, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; K. Bissell, School of Population Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; C-Y. Chiang, International Union Against Tuberculosis and Lung Disease, Paris, France, and Division of Pulmonary Medicine, Dept of Internal Medicine, Wan Fang Hospital, Taipei Medical University, and Division of Pulmonary Medicine, Dept of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; A. El Sony, Epidemiological Laboratory for Public Health and Research, Khartoum, Sudan; E. Ellwood, P. Ellwood, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; L. García-Marcos, Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia, and ARADyAL Allergy Network, Edificio Departamental-LAIB, Murcia, Spain; G.B. Marks, Respiratory and Environmental Epidemiology, University of New South Wales, Sydney, Australia; R. Masekela, Dept of Paediatrics and Child Health, Nelson R. Mandela School of Clinical Medicine, College of Health Sciences, University of KwaZulu Natal, Durban, South Africa; E. Morales, Department of Public Health Sciences, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain; K. Mortimer, Liverpool School of Tropical Medicine, Liverpool, UK; N. Pearce, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK; D.P. Strachan, Population Health Research Institute, St George's, University of London, London, UK.
Global Asthma Network International Data Centres. GAN Global Centre: P. Ellwood, E. Ellwood, M.I. Asher, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand. Murcia, Spain: L. García-Marcos, Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia, and ARADyAL Allergy Network, Edificio Departamental-LAIB, Murcia, Spain; V. Perez-Fernández, Dept of Paediatrics, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia Spain; E. Morales, Dept of Public Health Sciences, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain; A. Martinez-Torres, Paediatric Allergy and Pulmonology Units and Nurse Research Group, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain. London, UK: D.P. Strachan, Population Health Research Institute, St George's, University of London, London, UK; N. Pearce, S. Robertson and C.E. Rutter, Dept of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK; R.J. Silverwood, Dept of Medical Statistics, London School of Hygiene and Tropical Medicine, and Centre for Longitudinal Studies, UCL Social Research Institute, University College London, London, UK.
Global Asthma Network Adult Principal Investigators. Brazil: M. Urrutia Pereira, Federal University of Pampa, UNIPAMPA (Uruguaiana); Cameroon: G.A. Ajeagah, The University of Yaounde 1 (Yaounde); Costa Rica: M.E. Soto-Martínez, Hospital Nacional de Niños “Dr. Carlos Saénz Herrera”, Caja Costarricense Seguro Social – Universidad de Costa Rica, San José, Costa Rica; Greece: K. Priftis, National and Kapodistrian University of Athens, Athens; Honduras: J. Sanchez, Instituto Nacional Cardiopulmonar, Tegucigalpa; India: S.K. Kochar, Sardar Patel Medical College, Bikaner; M. Singh, Postgraduate Institute of Medical Education and Research, Chandigarh; N. Singh, Asthma Bhawan, Jaipur; N. Sit, National Allergy Asthma Bronchitis Institute, Kolkata; T.U. Sukumaran, Pushpagiri Institute of Medical Sciences and Research, Thiruvalla, Kottayam; S. Awasthi, King George's Medical University, Lucknow; P.A. Mahesh, JSS Medical College, JSSAHER, Mysuru; S. Sinha, All India Institute of Medical Sciences, New Delhi; M. Barne, Chest Research Foundation, Pune; Iran: M. Tavakol, Alborz University of Medical Sciences, Karaj; N. Behniafard, Shahid Sadoughi University of Medical Sciences, Yazd; Kingdom of Saudi Arabia: S.A. Alomary, Ministry of Health, Kingdom of Saudi Arabia; Kosovo: I. Bucaliu-Ismajli, The Principal Center of Family Care, Ferizaj; L. Hana-Lleshi, General Hospital “Isa Grezda” Gjakova, Kosovo; V. Gashi, American Hospital in Kosovo, Gjilan; X. Kurhasani, UBT College Kosovo, Peja; B. Gacaferri-Lumezi, University of Prishtina Hasan Prishtina, Peja 6–7; L.N. Ahmetaj (national coordinator), University Hospital (Prishtina); V. Lokaj-Berisha, University of Prishtina, Prizren; México: M.G. Sanchez Coronel, COMPEDIA (Colegio Mexicano de Pediatras Especialistas en Inmunología y Alergia), Aguascalientes; G. Ochoa-Lopez, Department of Pediatric Allergology, Ciudad Juárez; R. García-Almaráz, Hospital Infantil de Tamaulipas, Ciudad Victoria; J.A. Sacre Hazouri, Instituto Privado de Alergia, Córdoba; M.d.J. Ambriz-Moreno, Hospital General de Matamoros Tamaulipas, Mexico “Dr. Alfredo Pumarejo Lafaurie”, Matamoros; J.V. Mérida-Palacio, Centro de Investigacion de Enfermedades Alergicas y Respiratorias, Mexicali; O.J. Saucedo-Ramirez, Hospital Angeles Pedregal, Mexico City North; L.O. Hernández-Mondragón, CRIT de Michoacán, Michoacán); A. Arias-Cruz, Hospital Universitario, Monterrey; C.A. Jiménez González, Universidad Autonoma of San Luis Potosí, San Luis Potosí; A.J. Escalante-Dominguez, Hospital General Tijuana, Isesalud, Tijuana; F.J. Linares-Zapién, Centro De Enfermedades Alergicas Y Asma de Toluca, Toluca Rural Area; E.M. Navarrete-Rodriguez, Hospital Infantil de México Federico Gómez, Toluca Urban Area; New Zealand: I. Asher, University of Auckland, Auckland; Nigeria: A.G. Falade, University of Ibadan and University College Hospital, Ibadan; Poland: G. Brożek, Medical University of Silesia, Katowice; Russia: K. Kyzmicheva, Tyumen State Medical University, Tyumen; Spain: L. García-Marcos (national coordinator), Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia, Cartagena; Taiwan: K. Yeh, Chang Gung Memorial Hospital, Taipei; Thailand: S. Chinratanapisit, Department of Pediatrics, Bhumibol Adulyadej Hospital, Royal Thai Air Force, Bangkok. Global Asthma Network National Co-ordinators not named above: Brazil: D. Solé, Escola Paulista de Medicina, Federal University of São Paulo; Costa Rica: M.E. Soto-Quirós, University of Costa Rica; India: V. Singh, Asthma Bhawan; Kingdom of Saudi Arabia: W.A. Althagafi, Ministry of Health; México: B.E. Del Río Navarro, Service of Allergy and Clinical Immunology, Hospital Infantil de México, México City; Thailand: P. Vichyanond, Mahidol University, Phutthamonthon.
Data sharing: The study protocol including a recommended informed consent form and statistical analysis plan are in the public domain (http://globalasthmanetwork.org/surveillance/surveillance.php). The GAN Phase I data, including de-identified individual participant data, will be password protected and made available on the Global Asthma Network website http://www.globalasthmanetwork.org/ within 12 months of all GAN Phase I analyses being published. Access for non-GAN researchers will require a formal request to the GAN steering group for consideration, by submission of a written proposal and, on acceptance, a signed data access agreement.
Author contributions: The following individual contributions were made. Conceptualisation: M.I. Asher, K. Bissell, C-Y. Chiang, A. El Sony, P. Ellwood, L. García-Marcos, G.B. Marks, N. Pearce and D.P. Strachan; data curation: E. Ellwood, P. Ellwood, L. García-Marcos, E. Morales, V. Perez-Fernandez, C.E. Rutter, S. Robertson, R.J. Silverwood and M. Lesosky; verification of the underlying data: C.E. Rutter, N. Pearce, V. Perez-Fernandez and D.P. Strachan; formal analysis: M. Lesosky, N. Pearce, C.E. Rutter and D.P. Strachan; investigation: M.I. Asher; methodology: M.I. Asher, C-Y. Chiang, P. Ellwood, L. García-Marcos, N. Pearce, C.E. Rutter, D.P. Strachan and R.J. Silverwood; project administration: M.I. Asher, E. Ellwood and P. Ellwood; resources: M.I. Asher; supervision: L. García-Marcos, N. Pearce, D.P. Strachan and R.J. Silverwood; validation: P. Ellwood; visualisation: E. Ellwood, P. Ellwood and C.E. Rutter; writing original draft: K. Mortimer and C-Y. Chiang; manuscript review/editing: M.I. Asher, G.B. Marks, A. Martínez-Torres, S. Robertson, C.E. Rutter, K. Bissell, A. El Sony, E. Ellwood, P. Ellwood, L. García-Marcos, E. Morales, V. Perez-Fernandez, N. Pearce, D.P. Strachan, R.J. Silverwood, M. Lesosky and the Global Asthma Network Phase I Study Group; the latter contributed original data to the analyses. All authors shared responsibility for the decision to submit the manuscript.
Conflict of interest: The authors declare that they have no conflict of interest.
Support statement: This work was supported by the International Union Against Tuberculosis and Lung Disease, Boehringer Ingelheim New Zealand, AstraZeneca Educational Grant, National Institute for Health Research, UK Medical Research Council, European Research Council and Instituto de Salud Carlos III, Spain.
References
- 1.Meghji J, Mortimer K, Agusti A, et al. . Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021; 397: 928–940. doi: 10.1016/S0140-6736(21)00458-X [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, et al. . Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mortier K, Reddel HK, Pitrez PM, et al. Asthma management in low- and middle-income countries: case for change. Eur Resp J 2022; 60: 2103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asher I, Bissell K, Chiang C-Y, et al. . Calling time on asthma deaths in tropical regions: how much longer must people wait for essential medicines? Lancet Respir Med 2019; 7: 13–15. doi: 10.1016/S2213-2600(18)30513-7 [DOI] [PubMed] [Google Scholar]
- 5.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee . Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998; 351: 1225–1232. doi: 10.1016/S0140-6736(97)07302-9 [DOI] [PubMed] [Google Scholar]
- 6.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee . Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J 1998; 12: 315–335. doi: 10.1183/09031936.98.12020315 [DOI] [PubMed] [Google Scholar]
- 7.Strachan D, Sibbald B, Weiland S, et al. . Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allergy Immunol 1997; 8: 161–168. doi: 10.1111/j.1399-3038.1997.tb00156.x [DOI] [PubMed] [Google Scholar]
- 8.Williams H, Robertson C, Stewart A, et al. . Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol 1999; 103: 125–138. doi: 10.1016/S0091-6749(99)70536-1 [DOI] [PubMed] [Google Scholar]
- 9.Ellwood P, Asher MI, Björkstén B, et al. . Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: an ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur Respir J 2001; 17: 436–443. doi: 10.1183/09031936.01.17304360 [DOI] [PubMed] [Google Scholar]
- 10.Foliaki S, Nielsen SK, Björkstén B, et al. . Antibiotic sales and the prevalence of symptoms of asthma, rhinitis, and eczema: the International Study of Asthma and Allergies in Childhood (ISAAC). Int J Epidemiol 2004; 33: 558–563. doi: 10.1093/ije/dyh031 [DOI] [PubMed] [Google Scholar]
- 11.Weiland SK, Hüsing A, Strachan DP, et al. . Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup Environ Med 2004; 61: 609–615. doi: 10.1136/oem.2002.006809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burr ML, Emberlin JC, Treu R, et al. . Pollen counts in relation to the prevalence of allergic rhinoconjunctivitis, asthma and atopic eczema in the International Study of Asthma and Allergies in Childhood (ISAAC). Clin Exp Allergy 2003; 33: 1675–1680. doi: 10.1111/j.1365-2222.2003.01816.x [DOI] [PubMed] [Google Scholar]
- 13.Asher MI, Montefort S, Björkstén B, et al. . Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–743. doi: 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 14.Pearce N, Aït-Khaled N, Beasley R, et al. . Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007; 62: 758–766. doi: 10.1136/thx.2006.070169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CKW, Beasley R, Crane J, et al. . Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009; 64: 476–483. doi: 10.1136/thx.2008.106609 [DOI] [PubMed] [Google Scholar]
- 16.Ellwood P, Williams H, Aït-Khaled N, et al. . Translation of questions: the International Study of Asthma and Allergies in Childhood (ISAAC) experience. Int J Tuberc Lung Dis 2009; 13: 1174–1182. [PubMed] [Google Scholar]
- 17.Ellwood P, Asher MI, Billo NE, et al. . The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J 2017; 49: 1601605. doi: 10.1183/13993003.01605-2016 [DOI] [PubMed] [Google Scholar]
- 18.Sembajwe G, Cifuentes M, Tak SW, et al. . National income, self-reported wheezing and asthma diagnosis from the World Health Survey. Eur Respir J 2010; 35: 279–286. doi: 10.1183/09031936.00027509 [DOI] [PubMed] [Google Scholar]
- 19.García-Marcos L, Asher MI, Pearce N, et al. . The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J 2022; 60: 2102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The European Community Respiratory Health Survey II Steering Committee . The European Community Respiratory Health Survey II. Eur Respir J 2002; 20: 1071–1079. doi: 10.1183/09031936.02.00046802 [DOI] [PubMed] [Google Scholar]
- 21.Ellwood P, Asher MI, Ellwood E, et al. http://globalasthmanetwork.org/surveillance/manual/Global_Asthma_Network_Manual.pdf The Global Asthma Network Manual for Global Surveillance: Prevalence, Severity and Risk Factors; August 2015. Date last accessed: March 11, 2022.
- 22.Ellwood P, Ellwood E, Rutter C, et al. . Global Asthma Network Phase I surveillance: geographical coverage and response rates. J Clin Med 2020; 9: 3688. doi: 10.3390/jcm9113688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asher MI, Rutter CE, Bissell K, et al. . Worldwide trends in the burden of asthma symptoms in school children: Global Asthma Network Phase I cross-sectional studies. Lancet 2021; 398: 1569–1580. doi: 10.1016/S0140-6736(21)01450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serajuddin U, Hamadeh N. New World Bank Country Classifications by Income Level: 2020–2021. https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021. Date last accessed: May 10, 2021.
- 25.Burney P, Luczynska C, Chinn S, et al. . The European Community Respiratory Health Survey. Eur Respir J 1994; 7: 954–960. doi: 10.1183/09031936.94.07050954 [DOI] [PubMed] [Google Scholar]
- 26.European Community Respiratory Health Survey . Variations in the prevalence of respiratory symptoms, self-reported asthma attacks, and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1996; 9: 687–695. doi: 10.1183/09031936.96.09040687 [DOI] [PubMed] [Google Scholar]
- 27.Janson C, Anto J, Burney P, et al. . The European Community Respiratory Health Survey: what are the main results so far? Eur Respir J 2001; 18: 598–611. doi: 10.1183/09031936.01.00205801 [DOI] [PubMed] [Google Scholar]
- 28.Chiang C-Y, Bissell K, Macé C, et al. . The Asthma Drug Facility and the future management of asthma. Int J Tuberc Lung Dis 2022; in press [https://doi.org/10.5588/ijtld.22.0034]. [DOI] [PubMed] [Google Scholar]
- 29.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008; 63: 47–57. [DOI] [PubMed] [Google Scholar]
- 30.Odhiambo JA, Williams HC, Clayton TO, et al. . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009; 124: 1251–1258. doi: 10.1016/j.jaci.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 31.Ballardini N, Kull I, Söderhäll C, et al. . Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol 2013; 168: 588–594. doi: 10.1111/bjd.12196 [DOI] [PubMed] [Google Scholar]
- 32.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol 2018; 120: 131–137. doi: 10.1016/j.anai.2017.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Ranking of centres for the symptom prevalences of current wheeze, asthma ever, hay fever ever and eczema ever. ERJ-02865-2021.Figure_S1 (9.1KB, pdf)
Supplementary figure S2. Ranking of centres for the symptom prevalences of current wheeze, asthma ever, hay fever ever and eczema ever by sex (males on left). ERJ-02865-2021.Figure_S2 (13.1KB, pdf)
Supplementary figure S3. Rank correlation values and scatter plots of prevalence of the three conditions at the centre level by sex (males on left). The dashed line is the identity line. Rank correlation coefficient and 95% CI is shown in each graph. ERJ-02865-2021.Figure_S3 (7.7KB, pdf)
Supplementary figure S4. Rank correlation comparing centre prevalence (%) of reporting current wheeze, asthma ever, severe asthma symptoms, hay fever ever and eczema ever between the three age groups (children, adolescents, adults) included in GAN Phase I. The dashed line is the identity line. Rank correlation coefficient and 95% CI is shown in each graph. ERJ-02865-2021.Figure_S4 (17.3KB, pdf)
Supplementary table S1. Demographic summary by centre. ERJ-02865-2021.Table_S1 (92.6KB, pdf)
Supplementary table S2. Symptom prevalence of asthma, hay fever and eczema by centre. ERJ-02865-2021.Table_S2 (126.6KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02865-2021.Shareable (688.6KB, pdf)