Abstract
Aims
There have been no worldwide standardised surveys of prevalence and severity of asthma, rhinoconjunctivitis and eczema in school children for 15 years. The present study aims to provide this information.
Methods
Following the exact International Study of Asthma and Allergies in Childhood (ISAAC) methodology (cross-sectional questionnaire-based survey), Global Asthma Network (GAN) Phase I was carried out between 2015 and 2020 in many centres worldwide.
Results
The study included 157 784 adolescents (13–14 years of age) in 63 centres in 25 countries and 101 777 children (6–7 years of age) in 44 centres in 16 countries. The current prevalence of symptoms, respectively, was 11.0% and 9.1% for asthma, 13.3% and 7.7% for rhinoconjunctivitis and 6.4% and 5.9% for eczema. The prevalence of asthma ever was 10.5% and 7.6%, hay fever ever was 15.2% and 11.1% and eczema ever was 10.6% and 13.4%, respectively. Centres in low or lower middle gross national income countries (LICs or LMICs) had significantly lower prevalence of the three disease symptoms and diagnoses (except for hay fever). In children, the prevalence of asthma and rhinoconjunctivitis symptoms was higher in boys, while the reverse occurred among adolescents. For eczema, while the prevalence among female adolescents was double that of males, there was no sex difference among children. Centre accounted for non-negligible variability in all disease symptoms (10–20%).
Conclusion
The burdens of asthma, rhinoconjunctivitis and eczema vary widely among the limited number of countries studied. Although symptom prevalence is lower in LICs and LMICs, it represents a considerable burden everywhere studied.
Short abstract
There is a substantial global burden of asthma, hay fever and eczema in adolescents and children, representing a major global public health problem. Accessible, affordable, equitable and effective strategies are needed to reduce this burden. https://bit.ly/3nXKkzd
Introduction
Asthma is the most prevalent chronic condition in childhood, causing enormous morbidity and mortality worldwide [1–3]. In the age group of 5–19 years, as calculated in 2019, it caused ∼209 disability-adjusted life years (DALYs) per 100 000, ranking 10th of all diseases, and 0.29 deaths per 100 000, ranking 16th among the non-communicable diseases [4]. When last measured by the International Study of Asthma and Allergy in Childhood (ISAAC), globally 6.9% of adolescents aged 13–14 years had severe asthma symptoms [5]. There are ∼260 million adolescents aged 13–14 years in the world [6]. According to more recent data, asthma prevalence has been relatively stable during the last decades [7], which means that ∼18 million adolescents have severe asthma. Other allergic conditions, such as rhinoconjunctivitis and eczema, do not result directly in death but cause considerable morbidity [8, 9]. As with asthma, prevalence of rhinoconjunctivitis seems to remain stable [10]; thus, ∼15% of adolescents globally have rhinoconjunctivitis. Among those with the condition, a proportion of one in 15 have symptoms severe enough to interfere significantly with their daily activities [10, 11]. These figures indicate that 2.6 million adolescents have severe rhinoconjunctivitis. Unfortunately, no data on the time trends of eczema prevalence worldwide are available at present. But if the trends are also stable, the corresponding figures derived from ISAAC would be that 3.1 million adolescents have severe eczema causing sleep disturbances [12]. Figures in younger children aged 6–7 years would run in parallel [11, 12].
Global Asthma Network (GAN) Phase I aims to offer an updated snapshot of the prevalence and severity of asthma, rhinoconjunctivitis and eczema symptoms from diverse centres around the world, most of which have never been surveyed before.
Material and methods
The objectives and methodology of GAN have been published elsewhere, including response rates, geographical coverage and questionnaire details [13]. Except for a section on asthma management and control, they are identical to those of ISAAC. In summary, GAN is a worldwide cross-sectional study based on written questionnaires distributed in schools. It includes two age groups: compulsory for 13–14-year-olds (adolescents) and optional for 6–7-year-olds (children).
Questionnaires
The definitions of indicators of the three conditions were extracted from the written (or in some instances online) questionnaires completed in school by adolescents or at home by the parents of children. The original questionnaire was in English. Some centres included the optional video questionnaire on asthma in adolescents [14]. Questionnaires, which were validated previously to ISAAC Phase I, were translated into the local languages according to the ISAAC protocol [15].
Definitions
Asthma symptoms and diagnosis
“Current wheeze” was defined as a positive answer to the question “Have you (has your child) had wheezing or whistling in the chest in the past 12 months?” “Severe asthma symptoms” was defined as current wheeze with four or more attacks of wheeze, or more than one night per week sleep disturbance from wheeze, or wheeze affecting speech in the past 12 months. “Asthma ever” was defined as a positive answer to the question “Have you (has your child) ever had asthma?” The scenes of the video questionnaire showed five different situations that represent features of wheezing and severe wheezing. Adolescents were asked whether they had gone through the situations in the scenes during the past 12 months.
Rhinoconjunctivitis symptoms and hay fever diagnosis
“Current rhinoconjunctivitis symptoms” was defined by positive answers to two different questions: “In the past 12 months, have you (has this child) had a problem with sneezing, or a runny or blocked nose when he/she did not have a cold or the flu?” and “In the past 12 months, has this (child's) nose problem been accompanied with itchy-watery eyes?” “Severe rhinoconjunctivitis symptoms” was defined by the response “a lot” to the question “In the past 12 months, how much did this (child's) nose problem interfere with your (his/her) daily activities? (Not at all, a little, a moderate amount, a lot)”. “Hay fever ever” was defined as a positive answer to: “Have you (has your child) ever had hay fever?”
Eczema symptoms and diagnosis
“Current eczema symptoms” was defined as positive answers to the two questions “Have you (has this child) had this itchy rash [defined in a previous question] at any time in the past 12 months?” and “Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes?” “Severe eczema symptoms” was defined as current symptoms being the cause of awakening one or more times per week in “In the past 12 months, how often, on average, have you (has this child) been kept awake at night by this itchy rash? (Never, less than one night per week, one or more nights per week)”. “Eczema ever” was defined as a positive answer to “Have you (has your child) ever had eczema?”
Sample size and power
The study was launched in January 2015 inviting all ISAAC centres to participate. Those centres were familiar with the methods and would allow information to be obtained about time trends. All students of the target age within schools (selected randomly when the number of schools was higher than the needed-to-recruit planned sample) were invited to participate and were selected by grade or by age. A sample size of 3000 was sought in each age group (with a minimum of 1000 deemed acceptable) because this would provide enough power (>90%) to detect (at a significance level of 0.01) prevalence differences of 5% at the expected asthma prevalence, and to allow for testing multiple hypotheses. Additional details of the sample size and power are described elsewhere [13, 16, 17]. High participation rates were sought (response rate >80% for adolescents and 70% for children) and achieved [18]. Centres with a participation rate <50% were excluded.
Data handling and analysis
Data handling from each centre has been described in detail elsewhere [13]. A uniform approach to data processing, checking and analysis was used, using Stata versions 13–15 (Stata Statistical Software, Stata Corp. LLC, College Station, TX, USA). To calculate participation rates, the denominator was the total number of pupils in the target classrooms of the schools chosen to be surveyed in each centre and the numerator was the total number of core symptom questionnaires returned with at least some symptom data in that centre. For prevalence estimations, the number of positive answers to a specific symptom in the centre was divided by the number of completed questionnaires. If apparent inconsistencies were found between responses to a main question and a branched question (one dependent on the response to a main question), these were accepted and not recoded. Centres with major deviations from protocol were excluded from the analysis [19]. Where centres deviated slightly, these were noted and listed in the corresponding tables (as in supplementary table 1 found in [5]).
Income category for each country was calculated from the World Bank classification in June 2020 [20]. Because the number of centres in low income countries (LICs) was very small, the categories of LIC and lower middle income country (LMIC) were merged for analyses (LICs/LMICs). Prevalence variability between centres was expressed as percentiles P10, P50 and P90, together with the ratio between P90 and P10. A Spearman correlation coefficient was used to assess the relationships between centre-level prevalence. Kappa statistics were used to examine the agreement between individual responses to the written and video questionnaire. Multilevel logistic regression was used to estimate how much of the variability of each symptom's prevalence was attributable to centre (cluster) differences, additional to within-centre binomial sampling error. The model included school as a second level. Because the intraclass correlation coefficient was >5% in the null model in all instances, this statistical approach was used to analyse the effect of sex and gross national income (GNI) on the prevalence of symptoms of the three conditions, using centre and school as clusters in a three-level model.
Ethics
All centres were required to attain approval from their local ethics committee. They determined the method of consent as either passive (agreeing by participation) or active (signing a written consent prior to filling in the questionnaire) from parents/caregivers; however, GAN recommended passive consent because active (written) consent could reduce the response rate [21]. Because adolescents should also manifest their own consent, they agreed by participating.
Results
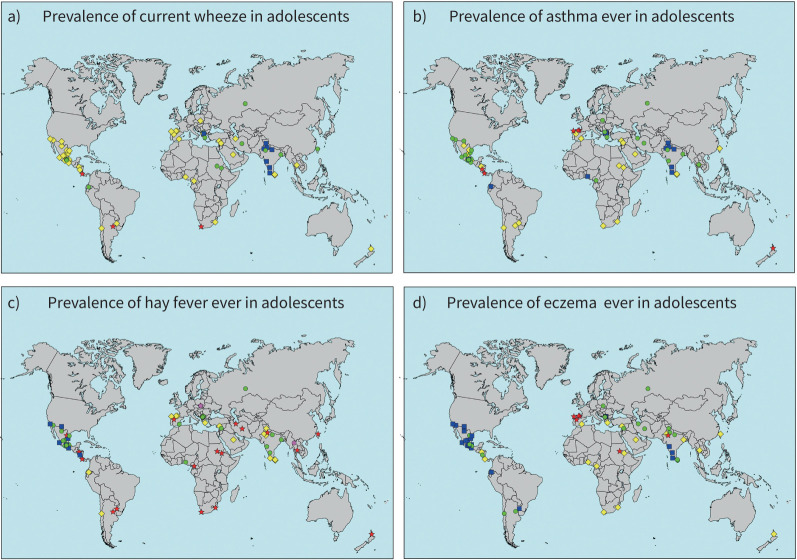
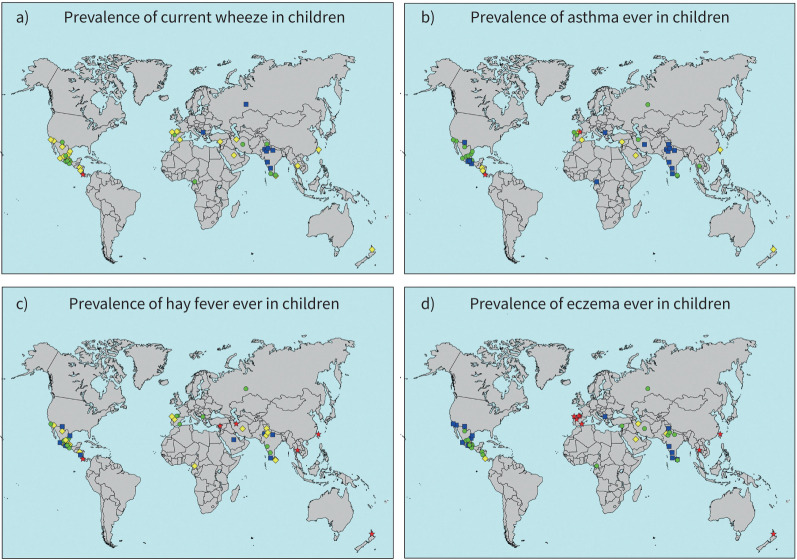
The study included 157 784 adolescents in 63 centres (14 and 26 of which also performed ISAAC Phase I or Phase III, respectively) in 25 countries and 101 777 children in 44 centres (10 and 18 of which also performed ISAAC Phase I or Phase III, respectively) [7] in 16 countries. Data were collected from March 2015 to May 2020 for adolescents and from January 2016 to June 2020 for children (supplementary tables S1–S7). The different periods were due mainly to children of different age groups being in different schools. Table 1 shows the prevalence of the different markers of disease grouped by GNI. Supplementary table S8 shows the prevalence of the different markers of disease grouped by sex. The grouped LICs/LMICs showed a consistent trend in that the prevalence of all symptoms was significantly lower. Maps of the prevalence of the three conditions in each age group are depicted in figures 1 and 2. Centres including both age groups can be derived from supplementary tables S1, S2 and S4–S7.
TABLE 1.
Prevalence of indicators of asthma, rhinoconjunctivitis and eczema in centres grouped by GNI. Global Asthma Network Phase I (2015–2020)
| Age group | GNI | Years | Centres, n | Total, n | Current wheeze | Asthma ever | Severe asthma symptoms | Current rhinoconjunctivitis symptoms | Hay fever ever | Symptoms of severe rhinoconjunctivitis | Current eczema symptoms | Eczema ever | Symptoms of severe eczema |
| 13–14 years | HIC | 2015–20 | 11 | 35 459 | 4720 (13.3) | 6361 (17.6) | 1995 (5.6)# (42.3)¶ | 5483 (15.5) | 6953 (19.6) | 375 (1.0)# (6.8)¶ | 3521 (9.9) | 7364 (20.8) | 389 (1.1)# (11.0)¶ |
| UMIC | 2015–20 | 33 | 77 746 | 9132 (11.7) | 6832 (8.8)*** | 4376 (5.6) (47.9) | 11 056 (14.2) | 10 629 (13.7) | 717 (0.9) (6.5) | 4545 (5.8)*** | 4673 (6.0)**** | 791 (1.0) (17.4) | |
| LIC/LMIC | 2017–19 | 19 | 44 579 | 3587 (8.0)*** | 3400 (7.6)**** | 1911 (4.3) (53.3) | 4379 (9.8)** | 6423 (14.4) | 219 (0.5)*** (5.0) | 1971 (4.4)**** | 4619 (10.4)*** | 351 (0.8) (17.8) | |
| Total | 63 | 157 784 | 17 439 (11.1) | 16 593 (10.5) | 8282 (5.2) (47.5) | 20 918 (13.3) | 24 005 (15.2) | 1311 (0.8) (6.3) | 10 037 (6.4) | 16 656 (10.6) | 1531 (1.0) (15.3) | ||
| 6–7 years | HIC | 2016–19 | 8 | 23 040 | 2680 (11.6) | 3227 (14.0) | 1012 (4.4) (37.7) | 2346 (10.2) | 3501 (15.2) | 163 (0.7) (6.9) | 2334 (10.1) | 8234 (35.7) | 248 (1.1) (10.6) |
| UMIC | 2016–20 | 22 | 49 617 | 4984 (10.0) | 3310 (6.7)*** | 2360 (4.8) (47.3) | 4345 (8.8) | 5444 (11.0) | 334 (0.7) (7.7) | 2799 (5.6)**** | 3719 (6.6)**** | 313 (0.6)* (11.1) | |
| LIC/LMIC | 2017–19 | 14 | 29 120 | 1623 (5.6)**** | 1173 (4.0)**** | 621 (2.1)**** (38.3) | 1132 (3.9)**** | 2343 (8.0) | 86 (0.3)* (6.8) | 909 (3.1)**** | 1720 (5.9)**** | 116 (0.4)**** (12.8) | |
| Total | 44 | 101 777 | 9287 (9.1) | 7710 (7.6) | 3993 (3.9) (43.0) | 7823 (7.7) | 11 288 (11.1) | 583 (0.6) (7.6) | 6042 (5.9) | 13 673 (13.4) | 677 (0.7) (11.2) |
Data presented as n (%), unless otherwise indicated. GNI: gross national income; HIC: high income countries; UMIC: upper middle income countries; LIC/LMIC: low and lower middle income countries. #: total participants denominator; ¶: current symptoms (wheeze, rhinoconjunctivitis or eczema) denominator (see text for definitions). The base for comparisons was the group of HICs. *: p<0.05; **: p<0.01; ***: p<0.005; ****: p<0.001.
FIGURE 1.
Prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in adolescents. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
FIGURE 2.
Prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in children. The symbols indicate prevalence values of <5% (blue squares), 5 to <10% (green circles), 10 to <20% (yellow diamonds) and ≥20% (red stars).
Asthma
The overall prevalence of current wheeze among adolescents was 11.1%. In the multilevel analysis, 12.2% of the variability of the prevalence was attributable to differences between centres. For severe asthma symptoms, the prevalence was 5.2%, and centre accounted for 13.4% of the variability. The prevalence of asthma ever was 10.5%, with variability attributable to centre being 18.3%. For any indicator, variability was high both between countries and between centres within countries (tables 1 and 2, supplementary table S1 and figure 1), although it was higher between countries. Girls had higher prevalence of current wheeze and of severe asthma symptoms, but lower of asthma ever (supplementary table S8).
TABLE 2.
Variation in the prevalence of the different indicators of asthma, rhinoconjunctivitis and eczema among centres
| Percentiles (%) | |||||
| P10 | P50 | P90 | Ratio of P90 to P10 | ||
| 6–7 years | Current wheeze | 2.7 | 10.4 | 14.0 | 5.2 |
| Asthma ever | 1.7 | 6.1 | 15.0 | 8.8 | |
| Severe asthma symptoms | 0.8 | 4.2 | 6.8 | 8.5 | |
| Current rhinoconjunctivitis symptoms | 2.4 | 7.0 | 15.1 | 6.3 | |
| Hay fever ever | 4.5 | 9.5 | 24.6 | 5.5 | |
| Symptoms of severe rhinoconjunctivitis | 0.1 | 0.4 | 1.0 | 11.1 | |
| Current eczema symptoms | 2.3 | 4.8 | 10.2 | 4.4 | |
| Eczema ever | 2.6 | 6.6 | 37.4 | 14.4 | |
| Symptoms of severe eczema | 0.1 | 0.6 | 1.5 | 15.0 | |
| 13–14 years | Current wheeze | 4.6 | 11.4 | 18.9 | 4.1 |
| Asthma ever | 2.4 | 9.2 | 19.1 | 8.0 | |
| Severe asthma symptoms | 1.8 | 5.4 | 9.8 | 5.4 | |
| Current rhinoconjunctivitis symptoms | 7.0 | 12.3 | 21.3 | 3.0 | |
| Hay fever ever | 4.4 | 13.0 | 33.9 | 7.7 | |
| Symptoms of severe rhinoconjunctivitis | 0.2 | 0.6 | 1.6 | 8.0 | |
| Current eczema symptoms | 2.9 | 5.0 | 10.6 | 3.7 | |
| Eczema ever | 2.1 | 7.7 | 18.8 | 9.0 | |
| Symptoms of severe eczema | 0.3 | 0.7 | 1.7 | 5.7 | |
In children, the corresponding figures for current wheeze, severe asthma symptoms and asthma ever were 9.1%, 3.9% and 7.6% (tables 1 and 2, supplementary table S2 and figure 2). Variability was also high (higher between countries), and differences between centres explained 15.1%, 17.6% and 24.1%, respectively, of that variability. For all three asthma indicators, the prevalence was higher in boys (supplementary table S8).
Asthma video questionnaire
The video questionnaire was implemented in 35 centres with a total of 85 669 adolescents. As with the written questionnaire, the prevalence of positive responses to the different scenes was quite variable between countries and between centres within countries (supplementary table S3).
Agreement between written and video questions was reasonable, similarly to ISAAC Phases I and III [5, 14, 17]. Current asthma symptoms had the highest agreement (κ=0.33, 95% CI 0.32–0.34) and coughing at night the lowest (κ=0.22, 95% CI 0.21–0.23).
Hay fever and rhinoconjunctivitis
The prevalence of current rhinoconjunctivitis symptoms, severe rhinoconjunctivitis symptoms and hay fever is shown in tables 1 and 2, supplementary table S4 and figure 1. The overall prevalence of the three indicators was 13.3%, 0.8% and 15.2%, respectively, and was highly variable between countries and between centres within countries. Centre explained 9.9% of the variability of current rhinoconjunctivitis symptoms, 13.6% of that of severe rhinoconjunctivitis symptoms and 22.7% of that of hay fever ever. The prevalence of hay fever indicators was higher among girls than boys (supplementary table S8).
Among children, the corresponding figures for current rhinoconjunctivitis symptoms, severe rhinoconjunctivitis symptoms and hay fever ever were 7.7%, 0.6% and 11.1% (tables 1 and 2, supplementary table S5 and figure 2). There was also considerable variability, with centre explaining a substantial part for current rhinoconjunctivitis symptoms (19.6%), severe rhinoconjunctivitis symptoms (16.1%) and hay fever (25.1%). In contrast to adolescents, in the group of children, boys had a higher prevalence of hay fever indicators than girls (supplementary table S8).
Eczema
Among adolescents, the prevalence of current eczema symptoms, severe eczema symptoms and eczema ever was 6.4%, 1.0% and 10.6%, respectively (tables 1 and 2, supplementary table S6 and figure 1). Variation due to centre tended to be slightly lower than in the other two conditions: 9.6%, 13.0% and 18.6%, respectively, for the three indicators. Adolescent boys had significantly lower prevalence than girls for any of the indicators (supplementary table S8).
The prevalence of eczema indicators among children (tables 1 and 2, supplementary table S7 and figure 2) was 5.9% for current symptoms 5.9%, 0.7% for severe symptoms and 13.4% for eczema ever. Variability was high, as previously, and centre explained 11.8%, 12.6% and 25.8%, respectively, of current eczema symptoms, severe eczema symptoms and eczema ever. The prevalence among boys and girls was similar (supplementary table S8).
Correlations between and within indicators of the three conditions
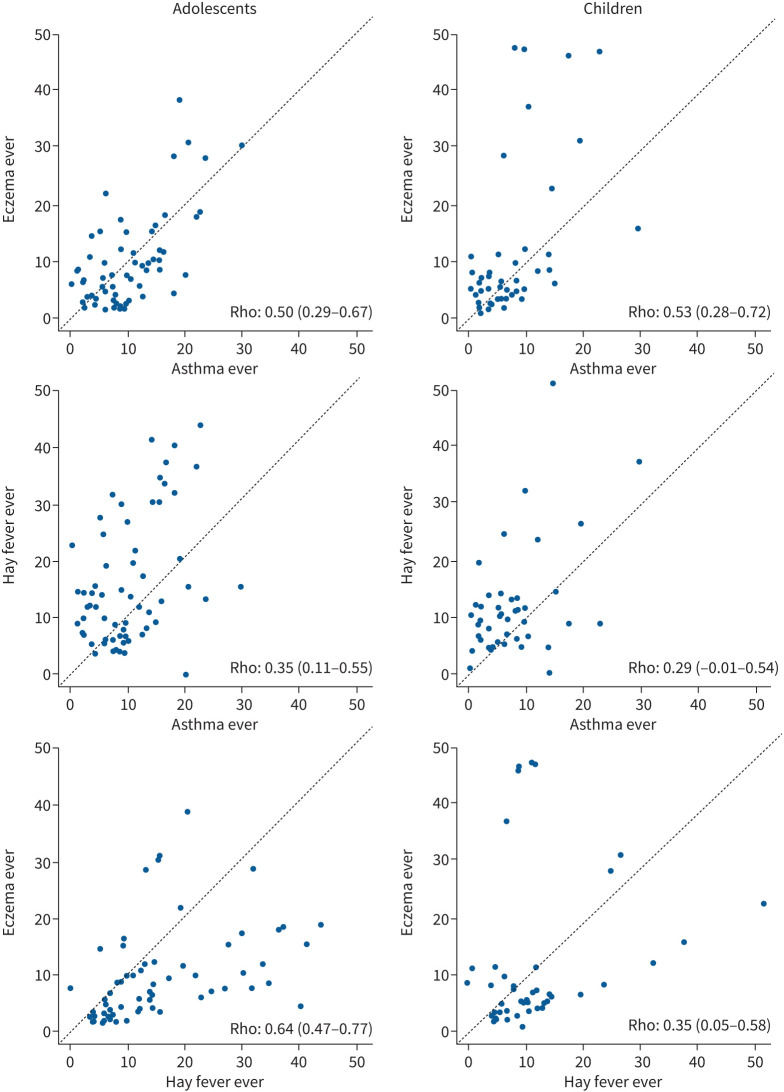
There was moderate to strong correlation between the prevalence of the three different diseases at the centre level, ranging from 0.29 (95% CI 0.23–0.69) between asthma ever and hay fever ever in children (figure 3) to 0.75 (95% CI 0.57–0.92) between current symptoms of asthma and rhinoconjunctivitis in children (supplementary figure S3). A complete rank correlation cross table is included in supplementary table S9.
FIGURE 3.
Rank correlation values and scatter plots of the prevalence of asthma ever, hay fever ever and eczema ever at the centre level in adolescents and children. The dashed line is the normality line. Rank correlation coefficient and 95% confidence intervals are shown in each graph.
Discussion
Although with considerable variations at centre, country and GNI levels, the overall global burden of asthma, rhinoconjunctivitis and eczema remains substantial, with ∼10% of adolescents and children experiencing asthma ever, 15% of adolescents and 11% of children having hay fever ever, and 11% of adolescents and 13% of children having eczema ever. Even though the degree of asthma control is relatively high regardless of income levels, asthma control seems to be a substantial problem across the globe.
The trend found in ISAAC of English-speaking countries and Latin-American countries having relatively higher prevalence of asthma [5, 17, 22, 23] was difficult to ascertain in this study because the number of centres in English-speaking countries was low. As in ISAAC Phase III [5, 24], there was a clear trend of asthma symptoms and severity running in parallel in both age groups. For the complete picture of time trends of asthma symptoms, see the recently published paper by Asher et al. [7].
Prevalence of asthma indicators was lower in the group of LICs/LMICs in both age groups, although this may have been driven by Indian centres, which tended to have the lowest prevalence, consistent with previous ISAAC surveys [5, 17]. Usually in LICs/LMICs, hygiene conditions are poorer and contact with farm animals more frequent, thus individuals are probably more exposed to higher amounts and a greater diversity of bacteria, which is a protective factor for atopy and asthma [25, 26].
Consistent with asthma prevalence is the lower prevalence of rhinoconjunctivitis and severe rhinoconjunctivitis symptoms in LICs/LMICs in both age groups. This was not the case with hay fever ever and might indicate that this concept is more familiar in temperate climates than in tropical countries, including many of the LIC and LMICs in GAN. In contrast to the asthma patterns, India did not seem to be wholly responsible for the low prevalence because the other two countries in this GNI category show similar disease prevalence. To what extent rhinoconjunctivitis is a marker of the atopic condition, which may be lower in less westernised countries, cannot be said but deserves some consideration [27]. The fact that hay fever does not follow the same pattern across countries and does not correlate well with rhinoconjunctivitis symptoms at the centre level may reflect genuine differences in prevalence, but may also be due to diverse diagnostic criteria [28].
The prevalence of eczema indicators was also variable, but substantially higher in high income countries (HICs). A higher prevalence of atopy in HICs might explain this distribution [27]. Furthermore, the different prevalence of non-atopic skin diseases, such as those caused by fungi [29], in the different GNI groups makes the epidemiological context of the diagnosis of eczema diverse.
Prevalence variability attributable to centre accounted for some proportion of the total variability in all three conditions. Risk or protective factors, or even interpretation of questions at the centre and individual level, are probably shared more by centres in the same countries than by centres in different countries. This could explain why the pattern of variability found for all three conditions was lower within than between countries.
The higher prevalence of asthma and rhinoconjunctivitis in male children was reversed in adolescents, a finding that was previously shown in ISAAC and other studies [17, 23, 30]. The reason for this change is not clear although hormonal influences have been suggested [31, 32]. With respect to eczema symptoms, previous ISAAC surveys [12, 33] showed that they were more prevalent in girls than in boys in both age groups although this was strongest in adolescents. We only found a difference among adolescents. This higher prevalence in female adolescents has also been found in prospective cohorts [34] and might again be related to oestrogen and progesterone interacting with skin allergies [31]. The lack of difference between sexes in children might be due to the different geographical distribution of centres in GAN and ISAAC [35].
The strengths of the present study are the ample world coverage, the large numbers of new centres and participants, and the use of the identical, standardised and easy to use ISAAC methodology, which allows both robust cross-sectional inferences as well as meaningful comparisons.
The limitations include the diagnosis of any of the three conditions that may not be perfectly well addressed by a self-administered questionnaire, the lack of a specific translation of “wheezing” in many languages and the perception of questions being different between parents and adolescents. All these circumstances may potentially lead to classification bias. However, questions have been previously validated and the translation and back-translation method of ISAAC and GAN has yielded good results [15].
Incorrect labelling is of special interest in hay fever and eczema: if the proportion of severe symptoms without a diagnosis indicates real and current conditions, their burden would be even higher. When estimating the burden of those diseases globally it might be more appropriate to use severe symptoms than diagnostic labels.
Although the perception of questions between adolescents and their parents may not be the same, the present study avoids comparing results between different age groups and focuses on the differences at centre, country or GNI levels within a specific age group. The main limitation of GAN as compared to ISAAC is the lack of representation of many countries. We are lacking centres from Northern Europe, North America and Australia, which in previous studies have shown the highest prevalence of asthma and atopic diseases [5, 11, 12, 23]. Additionally, we have no information about non-participants, although high response rates help to overcome participation bias. Finally, we cannot say what the impact of GNI on the results would have been if more countries were included in all income groups.
In conclusion, the present study, an updated and unique study on the prevalence of indicators of asthma, rhinoconjunctivitis and eczema, shows the persistence of a considerable burden of those conditions among children and adolescents worldwide. The prevalence of indicators of all three diseases was consistently lower in LICs and LMICs. The wide differences in prevalence, which were higher between countries than within countries, could probably be explained by environmental risk (such as pollution) or protective factors (such as contact with bacteria) that are more similar within countries.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Ranking of centres for the prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in the adolescent group. ERJ-02866-2021.Figure_S1 (852.1KB, jpg)
Supplementary figure S2. Ranking of centres for the prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in the children group. ERJ-02866-2021.Figure_S2 (454.8KB, jpg)
Supplementary table S1. Prevalence of asthma indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S1 (238.5KB, pdf)
Supplementary table S2. Prevalence of asthma indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S2 (206.6KB, pdf)
Supplementary table S3. Prevalence of asthma indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) video-questionnaire by centre (2015-2020). ERJ-02866-2021.Table_S3 (183.5KB, pdf)
Supplementary table S4. Prevalence of rhinoconjunctivitis indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S4 (225.2KB, pdf)
Supplementary table S5. Prevalence of rhinoconjunctivitis indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S5 (195.9KB, pdf)
Supplementary table S6. Prevalence of eczema indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S6 (208.4KB, pdf)
Supplementary table S7. Prevalence of eczema indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S7 (184KB, pdf)
Supplementary table S8. Prevalence of asthma, rhinoconjunctivitis and eczema indicators grouped by sex. GAN Phase I (2015-2020). ERJ-02866-2021.Table_S8 (141.3KB, pdf)
Supplementary table S9. Rank correlations and their 95% CIs between centre prevalence rates of the indicators of the three conditions. GAN Phase I (2015-2020). ERJ-02866-2021.Table_S9 (110.5KB, pdf)
Shareable PDF
Acknowledgements
We are grateful to the children, parents and adults who willingly participated with the help of schools and field workers in GAN Phase I. We thank the children and parents who participated in GAN Phase I, the school staff for their assistance and help with coordination, the principal investigators and their colleagues, and the many funding bodies throughout the world that supported the individual GAN centres. The GAN Global Centre in Auckland was funded by The University of Auckland with additional funding from The International Union Against Tuberculosis and Lung Disease, Boehringer Ingelheim NZ, AstraZeneca Educational Grant. The London Data Centre was supported by a PhD studentship to C.E. Rutter from the UK Medical Research Council (grant number MR/N013638/1) and funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013, ERC grant agreement number 668954). The Murcia Data Centre was supported by the University of Murcia and by Instituto de Salud Carlos III, fund PI17/0170. We thank the National Institute for Health Research (NIHR) Global Health Research Unit on Lung Health and TB in Africa at the Liverpool School of Tropical Medicine – “IMPALA” for helping to make this work possible. In relation to IMPALA (grant reference 16/136/35) specifically: IMPALA was funded by the NIHR using UK aid from the UK Government to support global health research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the UK Department of Health and Social Care. Individual centres involved in GAN Phase I data collection were funded by the following organisations: Costa Rica and Nicaragua partially funded by an unrestricted grant from AstraZeneca for logistic purposes; India (Kottayam, New Delhi, Chandigarh, Bikaner, Jaipur, Lucknow, Pune): GAN Phase I was undertaken by Asthma Bhawan in India which was supported by Cipla Foundation; Mexico: Puerto Vallarta Centro Universitario de la Costa, Universidad de Guadalajara; New Zealand: Auckland Asthma Charitable Trust; Nigeria, Ibadan: funded by NIHR (IMPALA grant reference 16/136/35) using UK aid from the UK Government to support global health research; South Africa: Cape Town, SA Medical Research Council, Allergy Society of South Africa; Syria, Lattakia: The Medical National Syndicate; Spain: Cartagena, Bilbao, Pamplona funded by Instituto de Salud Carlos III (grants PI17/00179, PI17/00694, PI17/00756), Cantabria by Instituto de Investigación Sanitaria Valdecilla (IDIVAL) de Cantabria PRIMVAL 17/01 y 18/01, Salamanca by Gerencia Regional de Salud de la Junta de Castilla y León (grant GRS 1239b/16) and Sociedad Española de Inmunología Clínica, Alergología y Asma Pediátrica, A Coruña by María José Jove foundation.
Global Asthma Network Study Group. Global Asthma Network Steering Group: M.I. Asher, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; K. Bissell, School of Population Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; C-Y. Chiang, International Union Against Tuberculosis and Lung Disease, Paris, France, and Division of Pulmonary Medicine, Dept of Internal Medicine, Wan Fang Hospital, Taipei Medical University, and Division of Pulmonary Medicine, Dept of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; A. El Sony, Epidemiological Laboratory for Public Health and Research, Khartoum, Sudan; E. Ellwood, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; P. Ellwood, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand; L. García-Marcos, Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia, and ARADyAL Allergy Network, Edificio Departamental-LAIB, Murcia, Spain; G.B. Marks, Respiratory and Environmental Epidemiology, University of New South Wales, Sydney, Australia; R. Masekela, Dept of Paediatrics and Child Health, Nelson R. Mandela School of Clinical Medicine, College of Health Sciences, University of KwaZulu Natal, Durban, South Africa; E. Morales, Department of Public Health Sciences, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain; K. Mortimer, Liverpool School of Tropical Medicine, Liverpool, UK; N. Pearce, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK; D.P. Strachan, Population Health Research Institute, St George's, University of London, London, UK.
Global Asthma Network International Data Centres. GAN Global Centre: P. Ellwood, E. Ellwood, M.I. Asher, Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand. Murcia, Spain: L. García-Marcos, Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia, and ARADyAL Allergy Network, Edificio Departamental-LAIB, Murcia, Spain; V. Perez-Fernández, Dept of Paediatrics, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia Spain; E. Morales, Dept of Public Health Sciences, University of Murcia, and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain; A. Martinez-Torres, Paediatric Allergy and Pulmonology Units and Nurse Research Group, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bio-health Research Institute, Edificio Departamental-LAIB, Murcia, Spain. London, UK: D.P. Strachan, Population Health Research Institute, St George's, University of London, London, UK; N. Pearce, S. Robertson and C.E. Rutter, Dept of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK; R.J. Silverwood, Dept of Medical Statistics, London School of Hygiene and Tropical Medicine, and Centre for Longitudinal Studies, UCL Social Research Institute, University College London, London, UK.
Global Asthma Network Principal Investigators. Argentina: H. Badellino, Clinica Regional Del Este, San Francisco; Brazil: M. Urrutia-Pereira, Federal University of Pampa, UNIPAMPA, Uruguaiana; Cameroon: A.E. Ndikum, The University of Yaounde 1, Yaounde; Chile: J. Mallol, University of Santiago de Chile (USACH), South Santiago; Costa Rica: M.E. Soto-Martínez, Hospital Nacional de Niños “Dr. Carlos Saénz Herrera”, Caja Costarricense Seguro Social – Universidad de Costa Rica, San José, Costa Rica; Ecuador: A. Cabrera Aguilar, Respiraclinic, Quito; Greece: K. Douros, National and Kapodistrian University of Athens, Athens; Honduras: S.M. Sosa Ferrari, Instituto Nacional Cardiopulmonar, Tegucigalpa; India: S. Mohammad, Kothari Medical and Research Institute, Bikaner; M. Singh, Postgraduate Institute of Medical Education and Research, Chandigarh; V. Singh (national coordinator), Asthma Bhawan, Jaipur; A.G. Ghoshal, National Allergy Asthma Bronchitis Institute, Kolkata; T.U. Sukumaran, Pushpagiri Institute of Medical Sciences and Research, Thiruvalla, Kottayam; S. Awasthi, King George's Medical University, Lucknow; P.A. Mahesh, JSS Medical College, JSSAHER, Mysuru; S.K. Kabra, All India Institute of Medical Sciences, New Delhi (7); S. Salvi, Chest Research Foundation, Pune; Iran: M. Tavakol, Alborz University of Medical Sciences, Karaj; N. Behniafard, Shahid Sadoughi University of Medical Sciences, Yazd; Kingdom of Saudi Arabia: S.A. Alomary, Ministry of Health, Kingdom of Saudi Arabia; Kosovo: I. Bucaliu-Ismajli, The Principal Center of Family Care, Ferizaj; L. Pajaziti, University Hospital Clinic, Clinic of Dermatology, Prishtina; V. Gashi, American Hospital in Kosovo, Gjilan; X. Kurhasani, UBT College Kosovo, Peja 13–14; B. Gacaferri-Lumezi, University of Prishtina Hasan Prishtina, Peja 6–7; L.N. Ahmetaj (national coordinator), University Hospital (Prishtina); V. Zhjeqi, University of Prishtina, Prizren; México: M.G. Sanchez Coronel, COMPEDIA (Colegio Mexicano de Pediatras Especialistas en Inmunología y Alergia), Aguascalientes; H.L. Moreno Gardea, Hospital Angeles Chihuahua, Chihuahua; G. Ochoa-Lopez, Department of Pediatric Allergology, Ciudad Juárez; R. García-Almaráz, Hospital Infantil de Tamaulipas, Ciudad Victoria; J.A. Sacre Hazouri, Instituto Privado de Alergia, Córdoba; N. Rodriguez-Perez, Instituto de Ciencias y Estudios Superiores de Tamaulipas, Matamoros; J.V. Mérida-Palacio, Centro de Investigacion de Enfermedades Alergicas y Respiratorias, Mexicali; B.E. Del Río Navarro (national coordinator), Service of Allergy and Clinical Immunology, Hospital Infantil de México, México City; L.O. Hernández-Mondragón, CRIT de Michoacán, Michoacán; S.N. González-Díaz, Universidad Autónoma de Nuevo León, Monterrey; R. Garcia-Muñoz, Universidad Regional del Sureste, Oaxaca; Md. Juan Pineda, Universidad de Guadalajara, Puerto Vallarta; Bd. Ramos García, Instituto Mexicano del Seguro Social, San Luis Potosí; A.J. Escalante-Dominguez, Hospital General Tijuana, Isesalud, Tijuana; F.J. Linares-Zapién, Centro De Enfermedades Alergicas Y Asma de Toluca, Toluca Rural; E.M. Navarrete-Rodriguez, Hospital Infantil de México Federico Gómez, Toluca Urban Area; J. Santos Lozano, Medica san Angel, Xalapa; New Zealand: I. Asher, University of Auckland, Auckland; Nicaragua: J.F. Sánchez, Hospital Infantil Manuel de Jesús Rivera, Managua; Nigeria: A.G. Falade, University of Ibadan and University College Hospital, Ibadan; Poland: G. Brożek, Medical University of Silesia, Katowice; Russia: K. Kyzmicheva, Tyumen State Medical University, Tyumen; South Africa: H.J. Zar, SA MRC Unit on Child and Adolescent Health, Cape Town; R. Masekela, University of Kwazulu Natal, Durban; Spain: A. López-Silvarrey Varela, Fundacion Maria Jose Jove, A Coruña; C. González Díaz, Universidad del País Vasco UPV/EHU, Bilbao; A. Bercedo Sanz, Cantabrian Health Service, Valdecilla Research Institute (IDIVAL), Dobra Health Center, Torrelavega, Cantabria; L. García-Marcos (national coordinator), Pediatric Allergy and Pulmonology Units, Virgen de la Arrixaca University Children's Hospital, University of Murcia and IMIB Bioresearch Institute, Murcia; J. Pellegrini Belinchon, Universidad de Salamanca, Salamanca; Sri Lanka: J.C. Ranasinghe, Teaching Hospital Peradeniya, Anuradhapura; S.T. Kudagammana, University of Peradeniya, Peradeniya; Sudan: H. El Sadig, Ministry of Health, Gadarif; M. Nour, Epi-Lab, Khartoum; Syrian Arab Republic: G. Alkhayer, Syrian Private University, Damascus; G. Dib and Y. Mohammad (national coordinator), National Center for Research and Training for Chronic Respiratory Disease and Co-Morbidities, Tishreen University, Lattakia; Taiwan: J. Huang, Department of Pediatrics, Chang Gung Memorial Hospital, New Taipei Municipal TuChen Hospital, and Chang Gung University, Taipei; Thailand: S. Chinratanapisit, Department of Pediatrics, Bhumibol Adulyadej Hospital, Royal Thai Air Force, Bangkok. Global Asthma Network National Co-ordinators not named above: Brazil: D. Solé, Escola Paulista de Medicina, Federal University of São Paulo, São Paulo; Costa Rica: M.E. Soto-Quirós, University of Costa Rica; Kingdom of Saudi Arabia: W.A. Althagafi, Ministry of Health, Kingdom of Saudi Arabia; Sudan: A. El Sony, Epidemiological Laboratory (Epi-Lab) for Public Health, Research and Development, Khartoum; Thailand: P. Vichyanond, Mahidol University, Phutthamonthon.
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.00440-2022
Data sharing: The study protocol including a recommended informed consent form and statistical analysis plan are in the public domain. The GAN Phase I data, including de-identified individual participant data, will be made available on the Global Asthma Network website http://www.globalasthmanetwork.org/ within 12 months of all GAN Phase I analyses being published. Access will require a formal request, a written proposal and a signed data access agreement.
Conflict of interest: C.E. Rutter declares UK Medical Research Council funding for a PhD as part of MRCLID DTP with LSHTM and St Georges, grant number MR/N013638/1, in connection with the present manuscript. All other authors declare no competing interests.
Support statement: This work was supported by the International Union Against Tuberculosis and Lung Disease, Boehringer Ingelheim New Zealand, AstraZeneca Educational Grant, National Institute for Health Research, UK Medical Research Council, European Research Council and Instituto de Salud Carlos III, Spain. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Asher MI, Ellwood P, Gilchrist C, et al. . The Global Asthma Report 2018. Auckland, New Zealand, The Global Asthma Network, 2018. [Google Scholar]
- 2.Meghji J, Mortimer K, Agusti A, et al. . Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet 2021; 397: 928–940. doi: 10.1016/S0140-6736(21)00458-X [DOI] [PubMed] [Google Scholar]
- 3.Mortimer K, Reddel HK, Pitrez PM, et al. Asthma management in low- and middle-income countries: case for change. Eur Respir J 2022; 60: 2103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Burden of Disease 2019. https://vizhub.healthdata.org/gbd-compare/ Date last accessed: November 13, 2021.
- 5.Lai CK, Beasley R, Crane J, et al. . Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009; 64: 476–483. doi: 10.1136/thx.2008.106609 [DOI] [PubMed] [Google Scholar]
- 6.United Nations, Department of Economic and Social Affairs, Population Division . World Population Prospects: The 2019 Revision. 2021. Available from: https://population.un.org/wpp/
- 7.Asher MI, Rutter CE, Bissell K, et al. . Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet 2021; 398: 1569–1580. doi: 10.1016/S0140-6736(21)01450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc 2007; 28: 393–397. doi: 10.2500/aap.2007.28.3013 [DOI] [PubMed] [Google Scholar]
- 9.Silverwood RJ, Mansfield KE, Mulick A, et al. . Atopic eczema in adulthood and mortality: UK population-based cohort study, 1998–2016. J Allergy Clin Immunol 2021; 147: 1753–1763. doi: 10.1016/j.jaci.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strachan DP, Rutter CE, Asher MI, et al. . Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: Global Asthma Network Phase I. Pediatr Allergy Immunol 2022; 33: e13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ait-Khaled N, Pearce N, Anderson HR, et al. . Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy 2009; 64: 123–148. doi: 10.1111/j.1398-9995.2008.01884.x [DOI] [PubMed] [Google Scholar]
- 12.Odhiambo JA, Williams HC, Clayton TO, et al. . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009; 124: 1251–1258. doi: 10.1016/j.jaci.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 13.Ellwood P, Asher MI, Billo NE, et al. . The Global Asthma Network rationale and methods for Phase I global surveillance: prevalence, severity, management and risk factors. Eur Respir J 2017; 49: 1601605. doi: 10.1183/13993003.01605-2016 [DOI] [PubMed] [Google Scholar]
- 14.Crane J, Mallol J, Beasley R, et al. . Agreement between written and video questions for comparing asthma symptoms in ISAAC. Eur Respir J 2003; 21: 455–461. doi: 10.1183/09031936.03.00041403 [DOI] [PubMed] [Google Scholar]
- 15.Ellwood P, Williams H, Ait-Khaled N, et al. . Translation of questions: the International Study of Asthma and Allergies in Childhood (ISAAC) experience. Int J Tuberc Lung Dis 2009; 13: 1174–1182. [PubMed] [Google Scholar]
- 16.Asher MI, Keil U, Anderson HR, et al. . International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491. doi: 10.1183/09031936.95.08030483 [DOI] [PubMed] [Google Scholar]
- 17.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee . Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur Respir J 1998; 12: 315–335. doi: 10.1183/09031936.98.12020315 [DOI] [PubMed] [Google Scholar]
- 18.Ellwood P, Ellwood E, Rutter C, et al. . Global Asthma Network Phase I surveillance: geographical coverage and response rates. J Clin Med 2020; 9: 3688. doi: 10.3390/jcm9113688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellwood P, Asher MI, Beasley R, et al. . The International Study of Asthma and Allergies in Childhood (ISAAC): Phase Three rationale and methods. Int J Tuberc Lung Dis 2005; 9: 10–16. [PubMed] [Google Scholar]
- 20.Hamadeh N, Van Rompaey C, Metreau E. 2020. New World Bank Country Classifications by Income Level: 2021–2022. https://blogs.worldbank.org/opendata/new-world-bank-countryclassifications-income-level-2021-2022 Date last accessed: July 1, 2022.
- 21.Ellwood P, Asher MI, Stewart AW, et al. . The impact of the method of consent on response rates in the ISAAC time trends study. Int J Tuberc Lung Dis 2010; 14: 1059–1065. [PubMed] [Google Scholar]
- 22.The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee . Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet 1998; 351: 1225–1232. doi: 10.1016/S0140-6736(97)07302-9 [DOI] [PubMed] [Google Scholar]
- 23.Asher MI, Montefort S, Bjorksten B, et al. . Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–743. doi: 10.1016/S0140-6736(06)69283-0 [DOI] [PubMed] [Google Scholar]
- 24.Mallol J, Crane J, von Mutius E, et al. . The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr) 2013; 41: 73–85. doi: 10.1016/j.aller.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 25.Ege MJ, Mayer M, Normand AC, et al. . Exposure to environmental microorganisms and childhood asthma. N Engl J Med 2011; 364: 701–709. doi: 10.1056/NEJMoa1007302 [DOI] [PubMed] [Google Scholar]
- 26.Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol 2017; 18: 1076–1083. doi: 10.1038/ni.3829 [DOI] [PubMed] [Google Scholar]
- 27.Weinmayr G, Weiland SK, Bjorksten B, et al. . Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med 2007; 176: 565–574. doi: 10.1164/rccm.200607-994OC [DOI] [PubMed] [Google Scholar]
- 28.Passali D, Cingi C, Staffa P, et al. . The International Study of the Allergic Rhinitis Survey: outcomes from 4 geographical regions. Asia Pac Allergy 2018; 8: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban K, Chu S, Scheufele C, et al. . The global, regional, and national burden of fungal skin diseases in 195 countries and territories: a cross-sectional analysis from the Global Burden of Disease Study 2017. JAAD Int 2021; 2: 22–27. doi: 10.1016/j.jdin.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almqvist C, Worm M, Leynaert B, et al. . Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy 2008; 63: 47–57. [DOI] [PubMed] [Google Scholar]
- 31.Kanda N, Hoashi T, Saeki H. The roles of sex hormones in the course of atopic dermatitis. Int J Mol Sci 2019; 20: 4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei J, Gerlich J, Genuneit J, et al. . Hormonal factors and incident asthma and allergic rhinitis during puberty in girls. Ann Allergy Asthma Immunol 2015; 115: 21–27. doi: 10.1016/j.anai.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 33.Williams H, Robertson C, Stewart A, et al. . Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 1999; 103: 125–138. doi: 10.1016/S0091-6749(99)70536-1 [DOI] [PubMed] [Google Scholar]
- 34.Ballardini N, Kull I, Soderhall C, et al. . Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol 2013; 168: 588–594. doi: 10.1111/bjd.12196 [DOI] [PubMed] [Google Scholar]
- 35.Palmer DJ. Vitamin D and the development of atopic eczema. J Clin Med 2015; 4: 1036–1050. doi: 10.3390/jcm4051036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary figure S1. Ranking of centres for the prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in the adolescent group. ERJ-02866-2021.Figure_S1 (852.1KB, jpg)
Supplementary figure S2. Ranking of centres for the prevalence of a) current wheeze, b) asthma ever, c) hay fever ever and d) eczema ever in the children group. ERJ-02866-2021.Figure_S2 (454.8KB, jpg)
Supplementary table S1. Prevalence of asthma indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S1 (238.5KB, pdf)
Supplementary table S2. Prevalence of asthma indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S2 (206.6KB, pdf)
Supplementary table S3. Prevalence of asthma indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) video-questionnaire by centre (2015-2020). ERJ-02866-2021.Table_S3 (183.5KB, pdf)
Supplementary table S4. Prevalence of rhinoconjunctivitis indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S4 (225.2KB, pdf)
Supplementary table S5. Prevalence of rhinoconjunctivitis indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S5 (195.9KB, pdf)
Supplementary table S6. Prevalence of eczema indicators among adolescents in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S6 (208.4KB, pdf)
Supplementary table S7. Prevalence of eczema indicators among children in the Global Asthma Network Phase I (GAN Phase I) by centre (2015-2020). ERJ-02866-2021.Table_S7 (184KB, pdf)
Supplementary table S8. Prevalence of asthma, rhinoconjunctivitis and eczema indicators grouped by sex. GAN Phase I (2015-2020). ERJ-02866-2021.Table_S8 (141.3KB, pdf)
Supplementary table S9. Rank correlations and their 95% CIs between centre prevalence rates of the indicators of the three conditions. GAN Phase I (2015-2020). ERJ-02866-2021.Table_S9 (110.5KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02866-2021.Shareable (687.9KB, pdf)