Abstract
Background
Acute respiratory failure (ARF) is commonly managed with invasive mechanical ventilation (IMV). The majority of the time that a patient spends on IMV is in the process of weaning. Prediction of the weaning outcome is of paramount importance, as untimely/delayed extubation is associated with a high risk of mortality. Diaphragmatic ultrasonography is a promising tool in the intensive care unit, and its utility in predicting the success of weaning remains understudied.
Methods
In this prospective-observational study, we recruited 54 ARF patients on IMV, along with 50 healthy controls. During a spontaneous breathing trial, all subjects underwent diaphragmatic ultrasonography along with a rapid shallow breathing index (RSBI) assessment.
Results
The mean age was 41.8±17.0 and 37.6±10.5 years among the cases and control group, respectively. Demographic variables were broadly similar in the two groups. The most common cause of ARF was obstructive airway disease. The average duration of IMV was 5.41±2.81 days. Out of 54 subjects, 45 were successfully weaned, while nine patients failed weaning. Age, body mass index, and severity of disease were similar in the successful and failed weaning patients. The sensitivity in predicting successful weaning of percent change in diaphragmatic thickness (Δtdi%) >29.71% was high (93.33%), while specificity was 66.67%. The sensitivity and specificity of mean diaphragmatic thickness (tdi) end-expiratory >0.178 cm was 60.00% and 77.78%, respectively. RSBI at 1 minute of <93.75 had an equally high sensitivity (93.33%) but a lower specificity (22.22%). Similar results were also found for RSBI measured at 5 minutes.
Conclusions
During the weaning assessment, the purpose is to minimize both premature as well as delayed extubation. We found that diaphragmatic ultrasonography, in particular Δtdi%, is better than RSBI in predicting weaning outcomes.
Keywords: artificial respiration, diaphragm, respiratory insufficiency, ultrasound, ventilator weaning
INTRODUCTION
Mechanical ventilation (MV) has been considered a life-saving tool for critically ill patients. Weaning from invasive mechanical ventilation (IMV) is as important as putting the patient on the ventilator. Once the patient is on IMV, weaning constitutes an important aspect of management. It has been estimated that around 42% of the total time that a patient spends on MV is in the process of weaning [1]. Determining the optimal time and mode of weaning is usually an arbitrary clinical decision. Physicians’ predictions for weaning can be inaccurate, with positive (PPV) and negative predictive values (NPV) of only about 50% and 67%, respectively [2]. Choosing the right approach between early extubation and prolonged ventilation is of prime importance to avoid the consequences of either of these, such as nosocomial pneumonia and increased risk of mortality [3].
The rapid shallow breathing index (RSBI) is one of the most extensively studied indices for the weaning assessment. It, therefore, offers the best paradigm for investigating the utility of other parameters. The relationship between respiratory load and respiratory muscle capacity, depicted by RSBI, was found to be a prime determinant of weaning outcomes [4,5]. Most studies have shown high sensitivities ranging from 85% to 100%. Although the PPV of an RSBI >105 breaths/min/L has been consistently reported in the range of 0.75 and above, the extremely high NPV, originally noted at 0.95, has been more difficult to reproduce [6,7].
Diaphragmatic dysfunction is associated with prolonged MV and weaning failure [8,9]. Despite all available diagnostic tools to assess diaphragmatic dysfunction like fluoroscopy and phrenic nerve conduction study, diaphragmatic ultrasonography is the only real-time, non-invasive bedside tool for its functional assessment [10]. Advantages of ultrasonography include safety, avoidance of radiation hazards, short learning curve, high correlation coefficient between and within observers, and availability at the bedside. It carries the advantage of assessing both the structural and functional components of the diaphragm at the bedside. Ultrasonography is similar in accuracy to most other imaging modalities for diaphragm assessment [11]. Ultrasonography can be used to evaluate diaphragm excursions (M mode), diaphragmatic thickness (tdi), and contraction (B mode). The average thickness of the diaphragm is 0.22–0.28 cm in healthy volunteers and 0.13–0.19 cm in a paralyzed diaphragm. A diaphragm thickness less than 0.2 cm, measured at the end of expiration, has been proposed as the cut-off to define diaphragm atrophy [12]. DiNino et al. [13] conducted a study over 63 mechanically-ventilated patients, and diaphragm thickness (tdi) was measured in the zone of apposition of the diaphragm to the rib cage. The percent change in diaphragmatic thickness (Δtdi%) between end-expiration and end-inspiration was calculated during either spontaneous breathing or pressure support weaning trials, and it was found that the combined sensitivity and specificity of Δtdi% ≥30% for extubation success was 88% and 71%, respectively. The PPV and NPV were 91% and 63%, respectively [13]. Recently, decreased diaphragm excursions on M mode have been shown to predict weaning failure with cut-off values of 1.4 cm and 1.2 cm for the right and left hemi-diaphragm, respectively [14,15].
Ultrasonography use to assess diaphragmatic dysfunction, despite its ease, has not yet been frequently used. The use of diaphragmatic ultrasonography as a weaning predictor is evolving [16], since only a few studies have addressed this issue and compared the results to RSBI. There is an urgent need to explore and generate evidence for its use as a weaning predictor.
MATERIALS AND METHODS
This study was an analysis of retrospectively collected data of ARF patients aged 16 to 80 years who were admitted into the intensive care unit (ICU) of a tertiary care institute in north India from May 2018 to December 2019. Inclusion and exclusion criteria are described below. The study protocol was approved by the Biomedical Research Ethics Committee of the institute. Informed consent was obtained. All consenting ICU patients aged 16 to 80 years, with at least 3 days of IMV for acute respiratory failure (ARF), were included in the study. The exclusion criteria included the following: pregnant patients; cases of abdominal, thoracic, or head and neck surgery/trauma; prior history of central nervous system disorder; presence of concomitant pleural effusion/ascites/pneumothorax; or presence of intercostal chest tube drainage. Fifty healthy subjects were assessed by diaphragmatic ultrasonography to calculate normal values for diaphragmatic variables in the Indian population.
Process of Weaning
A detailed history of the patients, including symptoms, demographic profile, and anthropometry, was recorded with all examination findings and investigations. Any history of ventilator-associated pneumonia was noted. After partial or complete resolution of the primary cause of respiratory failure, a decision of spontaneous breath testing (SBT) and liberation from MV was taken. If the patient was conscious, cooperative, alert, and had good cough reflex with stable cardiopulmonary status, an SBT was given (pressure support mode [PS] or t-piece). Once the patient’s breathing pattern was stabilized for at least 2 minutes, the first RSBI was calculated at 1 and 5 minutes. A handheld spirometer (Trueflow, EasyOne; ndd Medizintechnik AG, Zurich, Switzerland) was connected to the patient’s endotracheal tube or tracheostomy tube, and then the patient was made to take 5–6 breaths. Software analyzed the patient’s respiration and gave an average tidal volume and respiratory rate. RSBI was calculated by dividing the respiratory rate by the tidal volume in liters.
Diaphragmatic ultrasonography was performed within 15 minutes of the calculation of the RSBI. It was conducted using a linear 6–13 MHz transducer set to B mode, appropriate for measuring tdi and its change with tidal breathing using the Micromaxx ultrasound machine (Sonosite Inc., Bothell, WA, USA). tdi was measured at end-expiration and end-inspiration in the zone of apposition of the diaphragm with the lateral rib cage (Figure 1). The method of diaphragmatic ultrasonography has been described previously in detail [17]. In brief, the right diaphragm was imaged at the zone of apposition in the mid-axillary line between the 6th and 10th intercostal spaces. Similarly, the left diaphragm was imaged between the 6th and 10th intercostal spaces in the mid to posterior axillary line. The right diaphragm was visualized through the liver window. Visualization of the left diaphragm was facilitated by a more coronal approach and by paralleling the ribs. A total of three readings were recorded, with diaphragmatic contractility calculated with the formula shown below for each breath, and then a mean of all of the recordings was considered.
Figure 1.
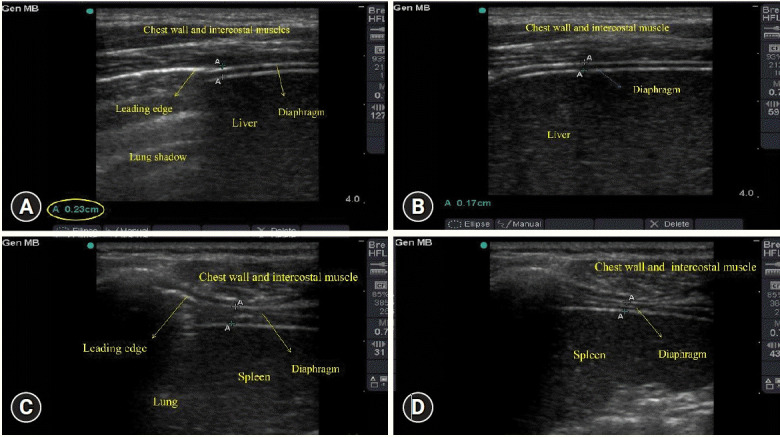
Ultrasonography images of the diaphragm during various times of respiration. (A) Right diaphragm, end-inspiratory phase. (B) Right diaphragm, end-expiratory phase. (C) Left diaphragm, end-inspiratory phase. (D) Left diaphragm, end-expiratory phase.
Successful weaning was defined as spontaneous breathing for more than 48 hours without needing MV or reintubation. All diaphragmatic ultrasonography procedures were done by a single trained operator (MS).
Statistical Analysis
The data was entered into a Microsoft Excel spreadsheet and doubly checked for errors. Appropriate coding was done to facilitate the analysis. Data analysis was done using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Qualitative data were presented as proportions and frequencies. Quantitative data were presented as means, standard deviations, and ranges. The comparison of continuous variables between two groups was made using an independent t-test. The receiver operating characteristic curve was plotted with cut-off points, sensitivity, specificity, PPV, and NPV. A probability value less than 0.05 was considered as the point of statistical significance (P<0.05). The diagnostic accuracies were calculated using the Youden index and compared among various variables using the area under the curve (AUC).
RESULTS
Fifty-four intubated patients were enrolled throughout 1 and a half years (Figure 2), along with 50 healthy subjects. The demographic and clinical profile of the enrolled subjects is shown in Table 1. The study group had more females than did the control group. Of the patients in the study group, 35.2% were active smokers, compared to 30% in the control group. Most of the patients were admitted for acute exacerbation of chronic obstructive pulmonary disease (AECOPD) or a snake bite. Thirty-five patients (64.8%) had hypercapnic respiratory failure, while others had normocapnic respiratory failure. Disease severity was assessed using Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores, calculated within 24 hours of ICU admission. The mean APACHE II and SOFA scores of the studied subjects were 20.04±6.14 and 4.81±1.87, respectively. Patients in the study group had spent 45.11%±13.13% of the total MV days in weaning.
Figure 2.
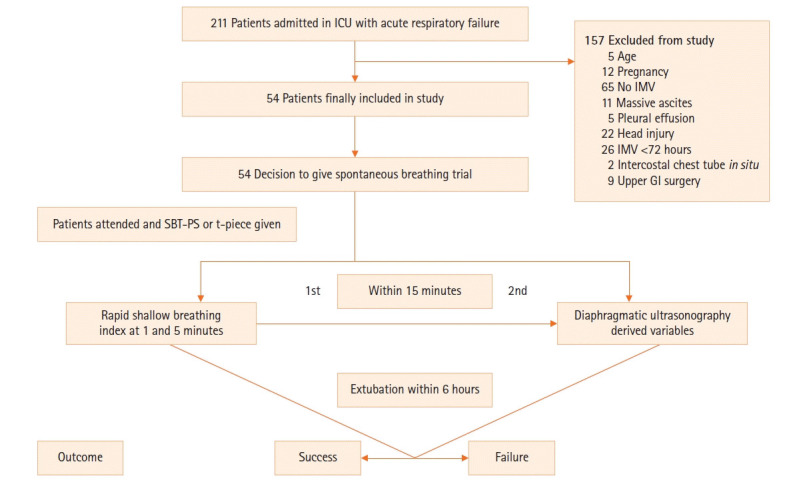
Consort chart of the study participants. ICU: intensive care unit; IMV: invasive mechanical ventilation; GI: gastrointestinal; SBT-PS: spontaneous breathing trial with pressure support.
Table 1.
Demographic and clinical profile of the enrolled cases and controls
| Characteristics | Case (n=54) | Control (n=50) |
|---|---|---|
| Age (yr) | 41.8±17.0 | 37.6±10.5 |
| Male:female (%) | 59:41 | 82:18 |
| BMI (kg/m2) | 23.41±3.56 | 23.31±3.78 |
| Smoking (%) | ||
| Yes | 35 | 30 |
| No | 65 | 70 |
| Diagnosis (%) | ||
| Organophosphorus poisoning | 7.41 | |
| Obstructive airway disease | 37.04 | |
| Snake bite | 20.37 | |
| Trauma | 11.11 | |
| Miscellaneous | 24.07 | |
| Severity of disease | ||
| APACHE II score | 20.04±6.14 | |
| SOFA score | 4.81±1.87 | |
| Duration of ventilation | ||
| IMV duration (day) | 5.41±2.81 | |
| Weaning time (% of IMV duration) | 45.11±13.13 |
Values are presented as mean±standard deviation unless otherwise indicated.
BMI: body mass index; APACHE: Acute Physiology and Chronic Health Evaluation; SOFA: Sequential Organ Failure Assessment; IMV: invasive mechanical ventilation.
Of 54 subjects, 45 were successfully weaned, while nine patients failed weaning. Age, body mass index, and severity of disease (APACHE II and SOFA score) were similar in successful and failed weaning patients. The rates of tracheostomy and mortality were higher in the failed weaning group than in the successful weaning group (P<0.05). Days of MV, ICU stay, hospital length of stay (HLOS), and ventilator-associated pneumonia were higher in patients in the failed weaning group but did not reach statistical significance (Supplementary Table 1).
Variation in tdi during the inspiratory and expiratory phases of respiration in the study and control groups are shown in Supplementary Table 2. The mean tdi and combined Δtdi% of both hemidiaphragms were greater in normal healthy individuals than in the study participants, but neither entity reached statistical significance (P>0.05). The mean RSBI measured at 1 and 5 minutes was 67.21±22.01 and 65.41±20.74, respectively. Mean RSBI values did not differ between the two groups (successful weaning and failed weaning).
The sensitivity of combined Δtdi% of both hemidiaphragms >29.71% was remarkably high (93.33%), while specificity was 66.67%. The sensitivity and specificity of mean end-expiratory tdi >0.178 cm was 60.00% and 77.78%, respectively. RSBI at 1 minute of <93.75 had an equally high sensitivity (93.33%) but a lower specificity (22.22%). Similar results were found for RSBI measured at 5 minutes (Table 2). The AUC was 0.605, 0.689, 0.614, and 0.576 for mean tdi-end expiration, ∆tdi%, RSBI at 1 minute, and RSBI at 5 minutes, respectively (Supplementary Figure 1).
Table 2.
Comparison of the diagnostic accuracy of different weaning parameters in predicting successful weaning
| Parameter | Sensitivity (%) | Specificity (%) | PPV | NPV | Likelihood ratio positive result | Likelihood ratio negative result |
|---|---|---|---|---|---|---|
| Mean ∆tdi% >29.71 | 93.33 | 66.67 | 93.3 | 66.7 | 2.80 | 0.01 |
| Mean tdi end-expiration >0.178 | 60.00 | 77.78 | 93.1 | 28.0 | 2.70 | 0.51 |
| RSBI at 1 minutes <93.75 | 93.33 | 22.22 | 85.7 | 40.0 | 1.20 | 0.30 |
| RSBI at 5 minutes <96.29 | 95.56 | 11.11 | 84.2 | 33.3 | 1.07 | 0.40 |
PPV: positive predictive value; NPV: negative predictive value; ∆tdi%: percent change in diaphragmatic thickness; RSBI: rapid shallow breathing index.
The utility of various predictors (∆tdi%, tdi-end expiration, and RSBI) during SBT-PS or T piece in weaning from MV is shown in Supplementary Table 3. tdi during end-expiration and end-inspiration in the failed weaning group were lower as compared with the successful weaning group, but the difference was not statistically significant (P>0.05) (Supplementary Table 4).
After initial weaning success (remaining off ventilation for over 48 hours), 7 of 45 patients were later reintubated. We combined patients with weaning failure and reintubated patients into a group that was then compared with the remaining patients. Parameters including MV days, ICU days, HLOS, and ventilator-associated pneumonia were significantly higher in patients who either failed weaning or were later reintubated. Tracheostomy and mortality rates remained significantly higher in patients with failed weaning and reintubation (P<0.05) (Table 3). Reasons for weaning failure are shown in Supplementary Table 5.
Table 3.
Outcome comparison between ICU patients with and without weaning failure and reintubation
| Parameter | Patients with weaning failure or reintubation (n=16) | Patients without weaning failure or reintubation (n=38) | P-value |
|---|---|---|---|
| IMV day | 10.25±4.40 | 5.39±2.98 | 0.000 |
| ICU length of stay | 14.12±5.86 | 8.74±5.78 | 0.003 |
| Hospital length of stay | 15.24±6.25 | 11.04±5.92 | 0.023 |
| Tracheostomy | 11 | 3 | 0.000 |
| Mortality | 9 | 0 | 0.000 |
| Ventilator-associated pneumonia | 9 | 7 | 0.014 |
Values are presented as mean±standard deviation.
ICU: intensive care unit; IMV: invasive mechanical ventilation.
DISCUSSION
Despite the increase in the availability and use of MV, it remains a double-edged sword in critical care. Both premature and delayed discontinuation from MV have been found to be associated with increased morbidity [18]. Tools for assessment of the optimal timing of extubation remain limited, despite the evidence accumulated in recent times. It is compounded by the fact that subjective decisions are often wrong [18]. Use of ultrasonography in the ICU is increasing for both diagnostic and therapeutic purposes. Its use in diaphragmatic assessment for weaning, although lucrative, has not been studied extensively.
The current study was done on 54 patients, with 50 controls, who were suffering from ARF in the ICU of a tertiary health care center to assess the use of ultrasonography as a weaning predictor. Of the study participants, 62.96% were given a low-level PS trial as an SBT, while the rest of the patients were tried on t-piece. In this study, we found no difference between clinical outcomes based on the use of the pressure support and t-piece trials as weaning method. The results are consistent with previous studies which have suggested the lack of superiority of one SBT method over the other [19,20]. Similarly to our study, Banerjee and Mehrotra [21] studied the utility of basic diaphragmatic ultrasound parameters to predict weaning outcomes, but all of the parameters failed to outperform RSBI.
The results show that tdi is greater in normal subjects when compared with patients on MV. In a different study, Francis et al. [22] assessed the effect of various modes of MV on tdi using ultrasonography and discovered that patients receiving assist-controlled ventilation showed a mean decline in tdi of 4.7% per day, whereas patients receiving pressure support ventilation demonstrated a mean rise in tdi of 1.5% per day. All our cases were managed using assist-controlled ventilation in the initial stages of illness. Later, all participants were on pressure support ventilation before SBT was initiated.
We found that the sensitivity and specificity of tdi-end expiration using a cut off >1.69 mm in predicting successful weaning were 71.11% and 44.44%, respectively. These findings are similar to the previous study by DiNino et al. [13]. The authors [13] found that tdi-end expiration of ≥1.7 mm is a marker of successful weaning from MV, with a sensitivity and specificity of 90% and 21%, respectively. Δtdi% of ≤29.71 was found to be an indicator of weaning failure. These findings are consistent with the findings of Kawar et al. [23], where they found that a Δtdi% >30% had a sensitivity of 95% and a specificity of 68% for extubation success. Similar results were also demonstrated by Farghaly and Hasan [24] and Alam et al. [25], where tdi-end expiration out-performed RSBI.
The RSBI value at 1 minute of <93.75 had a sensitivity of 93.33% and specificity of 22.33% as a weaning predictor. PPV and NPV were 85.7% and 40%, respectively. Similar findings were also observed for RSBI calculated at 5 minutes. Yang and Tobin et al. [26] demonstrated that RSBI >105, measured during 1 minute of spontaneous breathing, had an extremely high NPV (0.95) for predicting weaning failure. RSBI ≤105 had a high PPV (0.78) for predicting weaning success. A further consideration is the time at which the breathing pattern measurement was taken. In our study, the low specificity of RSBI can be explained due to multiple factors, including weaning on PS mode, post-extubation non-invasive ventilation (NIV) use, using respiratory frequency as a criterion of weaning failure, and selective bias of not extubating patients with RSBI >105. In contrast to the previous studies, RSBI was shown to have high sensitivity as well as specificity in a study by Ferrari et al. [14]. The reason for such outperforming values could have been the liberal SBT criteria used in their study.
Δtdi% has equal sensitivity but high specificity (66.67% vs. 22.22%) compared with RSBI. The AUC for Δtdi% was greater compared with RSBI (0.689 vs. 0.614). AUC for the mean tdi-end expiration was 0.605. We found that AUC for tdi end-expiration was less than that for Δtdi% (0.605 vs. 0.689). Variability of tdi-end expiration among different individuals [27,28] and ventilator-induced diaphragmatic atrophy in some may be responsible for the poor performance of tdi-end expiration as compared to Δtdi% [29]. Pirompanich and Romsaiyut [30] combined the two parameters of tdi and RSBI and demonstrated the superior diagnostic accuracy of the same as compared to the individual two.
Of the study patients, 83.33% were successfully weaned off from the MV. The mortality rate was high in patients with failed weaning as compared to patients with successful weaning (44.44% vs. 11.11%). Patients with failed weaning had a significantly higher requirement of tracheostomy, which is consistent with common knowledge and previous studies.
We used NIV in 31.48% of patients during the post-extubation period, primarily in COPD patients (72.22%). In most patients, NIV was applied within the first 6 hours of extubation. Indications were signs of increased difficulty breathing, evidence of hypercapnic respiratory failure in the post-extubation period, associated coronary artery disease, or physician experience considering high risk. Subjects who continued to remain tachypneic and hypercapnic were reintubated. We did not try high-flow oxygen therapy in any of our extubated patients.
The index study, despite being novel, was not short of limitations, like the small sample size and a larger proportion of AECOPD and snake bite cases, making it difficult for generalization. Addition of a trans-diaphragmatic pressure measurement could have added further value to the study.
Ultrasonography is now ubiquitously present in ICUs, and its use has gained widespread acceptability for various indications. Ultrasonography is advantageous over traditional tools for functional assessments of the diaphragm in being non-invasive, bedside, portable, safe, easy to use, and quick to learn. During the assessment for weaning, minimizing both premature and delayed extubation is of prime importance. We found diaphragmatic ultrasonography is better than RSBI in predicting the weaning outcome, but the same should be validated in a large cohort involving multidisciplinary ICU patients. Training in diaphragmatic ultrasonography is of utmost importance and can be helpful for intensivists and pulmonologists in the day-to-day practice of intensive care and more so in cases of difficult weaning.
KEY MESSAGES
▪ A diaphragmatic ultrasound for the weaning assessment is feasible and provides real-time physiological data.
▪ The percent change in diaphragmatic thickness measurement is superior to the conventional rapid shallow breathing index measurement in predicting weaning success.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: MS, DC. Methodology: LKL, MS, DC. Formal analysis: LKL, MS, PKS, MBG. Data curation: LKL, MS. Software: PKS, MBG. Validation: NA. Investigation: LKL, MS. Writing - original draft preparation: LKL, PKS. Writing - review and editing: MBG, DC, PKS.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.4266/acc.2022.00108.
Comparison demographic and clinical variables between subgroups with successful and failed weaning
Mean and percentage changes in diaphragmatic thickness during inspiration
Comparison of Δtdi%, diaphragmatic end expiratory thickness (tdi-end expiratory and RSBI during different types of weaning trial (PSV mode and T-piece mode)
Comparison of different weaning parameters in patient’s sub-groups of successful and failed weaning
Reasons for failed weaning in study population
Area Under Curve (AUC). (A) AUC of mean expiratory diaphragmatic thickness. (B) AUC of mean percent change in diaphragmatic thickness. (C) AUC of rapid shallow breathing index (RSBI) taken at 1 minutes and (D) at 5 minutes to predict successful weaning in intubated patients. EE: end expiration.
REFERENCES
- 1.Jabaley CS, Groff RF, Sharifpour M, Raikhelkar JK, Blum JM. Modes of mechanical ventilation vary between hospitals and intensive care units within a university healthcare system: a retrospective observational study. BMC Res Notes. 2018;11:425. doi: 10.1186/s13104-018-3534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stroetz RW, Hubmayr RD. Tidal volume maintenance during weaning with pressure support. Am J Respir Crit Care Med. 1995;152:1034–40. doi: 10.1164/ajrccm.152.3.7663780. [DOI] [PubMed] [Google Scholar]
- 3.Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997;155:906–15. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- 4.Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158:378–85. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- 5.Roussos C. Ventilatory muscle fatigue governs breathing frequency. Bull Eur Physiopathol Respir. 1984;20:445–51. [PubMed] [Google Scholar]
- 6.Jacob B, Chatila W, Manthous CA. The unassisted respiratory rate/tidal volume ratio accurately predicts weaning outcome in postoperative patients. Crit Care Med. 1997;25:253–7. doi: 10.1097/00003246-199702000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Mohsenifar Z, Hay A, Hay J, Lewis MI, Koerner SK. Gastric intramural pH as a predictor of success or failure in weaning patients from mechanical ventilation. Ann Intern Med. 1993;119:794–8. doi: 10.7326/0003-4819-119-8-199310150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Houston JG, Morris AD, Grosset DG, Lees KR, McMillan N, Bone I. Ultrasonic evaluation of movement of the diaphragm after acute cerebral infarction. J Neurol Neurosurg Psychiatry. 1995;58:738–41. doi: 10.1136/jnnp.58.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones CD, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med. 2001;20:597–604. doi: 10.7863/jum.2001.20.6.597. [DOI] [PubMed] [Google Scholar]
- 10.Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47:319–29. doi: 10.1002/mus.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SG, Brook MM, Tacy TA. Reliability of two-dimensional echocardiography in the assessment of clinically significant abnormal hemidiaphragm motion in pediatric cardiothoracic patients: comparison with fluoroscopy. Pediatr Crit Care Med. 2006;7:441–4. doi: 10.1097/01.PCC.0000227593.63141.36. [DOI] [PubMed] [Google Scholar]
- 12.Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133:737–43. doi: 10.1378/chest.07-2200. [DOI] [PubMed] [Google Scholar]
- 13.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69:423–7. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J. 2014;6:8. doi: 10.1186/2036-7902-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt K, Agolli A, Patel MH, Garimella R, Devi M, Garcia E, et al. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries (Craiova) 2021;9:e126. doi: 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samanta S, Singh RK, Baronia AK, Poddar B, Azim A, Gurjar M. Diaphragm thickening fraction to predict weaning: a prospective exploratory study. J Intensive Care. 2017;5:62. doi: 10.1186/s40560-017-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boussuges A, Rives S, Finance J, Brégeon F. Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases. 2020;8:2408–24. doi: 10.12998/wjcc.v8.i12.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobin MJ, Yang K. Weaning from mechanical ventilation. Crit Care Clin. 1990;6:725–47. [PubMed] [Google Scholar]
- 19.Cohen JD, Shapiro M, Grozovski E, Lev S, Fisher H, Singer P. Extubation outcome following a spontaneous breathing trial with automatic tube compensation versus continuous positive airway pressure. Crit Care Med. 2006;34:682–6. doi: 10.1097/01.CCM.0000201888.32663.6A. [DOI] [PubMed] [Google Scholar]
- 20.Wilson AM, Gray DM, Thomas JG. Increases in endotracheal tube resistance are unpredictable relative to duration of intubation. Chest. 2009;136:1006–13. doi: 10.1378/chest.08-1938. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee A, Mehrotra G. Comparison of lung ultrasound-based weaning indices with rapid shallow breathing index: are they helpful? Indian J Crit Care Med. 2018;22:435–40. doi: 10.4103/ijccm.IJCCM_331_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis CA, Hoffer JA, Reynolds S. Ultrasonographic evaluation of diaphragm thickness during mechanical ventilation in intensive care patients. Am J Crit Care. 2016;25:e1–8. doi: 10.4037/ajcc2016563. [DOI] [PubMed] [Google Scholar]
- 23.Kawar E, DiNino E, Gartman E, Sethi J, McCool FD. Diaphragm thickening predicts weaning from mechanical ventilation. Am J Respir Crit Care Med. 2012;185:A2723–8. [Google Scholar]
- 24.Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care. 2017;30:37–43. doi: 10.1016/j.aucc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Alam MJ, Roy S, Iktidar MA, Padma FK, Nipun KI, Chowdhury S, et al. Diaphragm ultrasound as a better predictor of successful extubation from mechanical ventilation than rapid shallow breathing index. Acute Crit Care. 2022;37:94–100. doi: 10.4266/acc.2021.01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–50. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 27.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation: impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–8. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 28.Grosu HB, Lee YI, Lee J, Eden E, Eikermann M, Rose KM. Diaphragm muscle thinning in patients who are mechanically ventilated. Chest. 2012;142:1455–60. doi: 10.1378/chest.11-1638. [DOI] [PubMed] [Google Scholar]
- 29.Daley BJ, Garcia-Perez F, Ross SE. Reintubation as an outcome predictor in trauma patients. Chest. 1996;110:1577–80. doi: 10.1378/chest.110.6.1577. [DOI] [PubMed] [Google Scholar]
- 30.Pirompanich P, Romsaiyut S. Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J Intensive Care. 2018;6:6. doi: 10.1186/s40560-018-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison demographic and clinical variables between subgroups with successful and failed weaning
Mean and percentage changes in diaphragmatic thickness during inspiration
Comparison of Δtdi%, diaphragmatic end expiratory thickness (tdi-end expiratory and RSBI during different types of weaning trial (PSV mode and T-piece mode)
Comparison of different weaning parameters in patient’s sub-groups of successful and failed weaning
Reasons for failed weaning in study population
Area Under Curve (AUC). (A) AUC of mean expiratory diaphragmatic thickness. (B) AUC of mean percent change in diaphragmatic thickness. (C) AUC of rapid shallow breathing index (RSBI) taken at 1 minutes and (D) at 5 minutes to predict successful weaning in intubated patients. EE: end expiration.


