Abstract
Background
Real‐world application of osimertinib with antiangiogenic agents in non‐small cell lung cancer (NSCLC) is common, but the efficacy data are rarely reported.
Methods
To obtain an objective efficacy report of different real‐world treatment models of osimertinib and antiangiogenic agents.
Results
A total of 54 patients with NSCLC were enrolled into the study. Twelve (22.2%) who received a combination of antiangiogenic agents, when there was a trend of osimertinib resistance but did not reach imageology progressive disease (PD), were assigned to Group A, with a median overall survival (OS) and progression‐free survival (PFS) of 48.0 (95% CI, not reached) and 21.0 (95% CI: 16.7–25.3) months, respectively. Thirty (55.6%) who received a combination of antiangiogenic agents when there was imageology PD during treatment with osimertinib were assigned to Group B, with a median OS and PFS of 31.8 (95% CI: 26.6–37.1) and 9.2 (95% CI: 5.9–12.6) months, respectively. Twelve (22.2%) who received a combination of antiangiogenic agents at the initial treatment with osimertinib were assigned to Group C, with a median OS and PFS of 28.5 (95% CI: 15.2–41.8) and 15.3 (95% CI: 7.9–22.7) months, respectively. Patients in Group A achieved a significant prolonged median PFS (p < 0.001) compared with Groups B and C. Absence of epidermal growth factor receptor (EGFR) T790M mutations (p = 0.043; hazard ratio [HR] = 2.124, 95% CI: 1.023–4.413) and no previous antiangiogenic agent application (p = 0.012; HR = 0.362, 95% CI: 0.163–0.863) were the independent prognostic factors of OS.
Conclusion
The well‐timed action to combine antiangiogenic agents was when there was a trend of osimertinib resistance. The absence of EGFR T790M mutations and previous use of antiangiogenic agents were poor prognostic factors.
Keywords: antiangiogenic agents, non‐small cell lung cancer, osimertinib, resistance
The real‐world application of osimertinib with antiangiogenic agents in NSCLC includes three models; combining antiangiogenic agents at the beginning (Group C), at resistance (Group A), or at PD (Group B) during osimertinib treatment, in which, the survival of Group A achieved a trend of prolongation.

The antiangiogenic agents mainly included two types of three drugs; the tyrosine kinase inhibitors anlotinib and apatinib, and monoclonal antibody of bevacizumab, and there was no difference in OS.
INTRODUCTION
Non‐small cell lung cancer (NSCLC) accounts for about 85% of all lung cancer histological subtypes, of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma are the most common subtypes. 1 Epidermal growth factor receptor (EGFR) belongs to the ERBB family of cell‐surface receptor tyrosine kinases, also called HER1 or ERBB1, located on the short arm of the seventh human chromosome and contains 28 exons. 2 EGFR sensitive mutations, including three mutations in exon 18 (G719A, G719C, G719S), 19 deletions in exon 19, S768I mutation in exon 20, and two mutations in exon 21 (L858R, L861Q), are found in about 10%–20% Caucasian LUAD patients 3 , 4 and about 50%–60% East Asia LUAD patients. 5 , 6 , 7 Deletions in exon 19 and the point mutation of L858R are the most prevalent EGFR kinase domain mutations and approximately up to 90%, and are termed “classical” activating mutations, which can cause continuous tyrosine kinase activation. 8
Since the first‐generation EGFR tyrosine kinase inhibitor (TKI) gefitinib was approved for the treatment of NSCLC in 2003, therapies targeting EGFR signaling have experienced a prosperous development, and have developed to third and fourth generation9, 10 to date. Osimertinib is one of the third‐generation EGFR‐TKIs that targets both EGFR‐sensitive and resistant (EGFR T790M) mutations, 11 showing favorable efficacy over cytotoxic chemotherapy in NSCLC with EGFR mutation, 12 and also showing favorable efficacy over the first‐generation EGFR‐TKIs. 13 , 14 However, drug resistance ultimately occurs and osimertinib is no exception, which poses a significant challenge due to the paucity of available post‐osimertinib treatment options to date.
To put off, or overcome, the resistance to treatment with osimertinib, many osimertinib‐based combination therapies 15 are being investigated in which treatment with osimertinib with antiangiogenic agents is highly regarded. Several angiogenic inhibitors have been approved to treat cancer in China, and four different antiangiogenic agents are often used in patients with NSCLC, including bevacizumab, which inhibits vascular endothelial growth factor, 16 rh‐endostatin which represses cell cycle control and antiapoptosis genes in proliferating endothelial cells, 17 small molecular TKIs of anlotinib and apatinib, which target vascular endothelial growth factor receptor and other related molecules 18 , 19 which block tumor angiogenesis.
Some prospective studies have explored the efficacy of osimertinib plus bevacizumab 20 , 21 and suggested that this regimen might not work synergistically. However, the real‐world applications of osimertinib and antiangiogenic agents are much more diverse and complex than well designed clinical trials, and we know little about this data. Therefore, in our present retrospective study, we comprehensively report on the patients with NSCLC treated with osimertinib and antiangiogenic agents, and summarize the different real‐world treatment models, to obtain an objective efficacy and safety report of different treatment models.
METHODS
Patient selection and data collection
We reviewed the real‐world records of patients with NSCLC treated with osimertinib plus antiangiogenic agents from November 1, 2015, to September 30, 2021, in the database of the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China. The treatment line of combination therapy was not limited. The antiangiogenic agents included bevacizumab, anlotinib, and apatinib, and rh‐endostain was excluded.
The inclusion criteria were as follows: (1) Pathological or cytological diagnosis of NSCLC. (2) A confirmed staging of III or IV. (3) Patients must be treated with osimertinib with one of the antiangiogenic agents simultaneously until disease progression or intolerable toxicities. (4) Patients must have operable EGFR driver mutations before the initial treatment with EGFR‐TKIs. The need for informed patient consent was waived because of the retrospective nature of the study.
In this study, we retrospectively collected the clinical characteristics of age, sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), pathological type, detailed TNM classification, detailed metastatic information, prior treatment history (systematic anticancer therapy, radiotherapy, lung cancer surgery), detailed osimertinib based therapy, treatment lines, driver gene variations of EGFR, tumor response, safety, progression, and death. Extrapulmonary metastatic organs were defined as distant metastases except for lung and pleura. All data were obtained by follow‐up visits, telephone, and electronic medical records. This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences.
Patient grouping and tumor response evaluation
Group A was defined as patients who received a combination of antiangiogenic agents when there was a trend of resistance during the treatment with osimertinib but did not reach imageology progressive disease (PD). Group B was defined as patients who received a combination of antiangiogenic agents when there was imageology PD during the treatment with osimertinib. Group C was defined as patients who received a combination of antiangiogenic agents at the initial treatment with osimertinib.
Progressive‐free survival (PFS) was calculated as the time from the initial osimertinib (Group A and Group B) or the initial osimertinib with the antiangiogenic agent (Group C) to imageology PD or death. Overall survival (OS) was calculated as the time from treatment with the initial osimertinib (Group A and Group B) or the initial osimertinib with the antiangiogenic agent (Group C) to death from any cause. Important to note was that we additionally set PFS2 for Group B as an index to evaluate the progression‐free survival time from the start of treatment with osimertinib plus an antiangiogenic agent to the imageology PD or death.
Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1. The objective response rate (ORR) was defined as the percentage of patients having achieved complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as the percentage of patients having achieved CR, PR, or stable disease (SD).
Adverse events
Safety data was collected and assessed throughout the records of the electronic medical system, follow‐up visits, and telephone. Adverse events (AEs) were graded according to the U.S. National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0).
Statistical analysis
The primary endpoint was OS, the secondary endpoints included PFS, ORR, DCR, and safety data. Efficacy and safety data were evaluated in all enrolled patients who received at least one dose of osimertinib plus antiangiogenic agents. Survival was calculated by Kaplan–Meier method, Cox proportional hazard models were used to evaluate prognostic factors for OS and PFS by univariate and multivariate analysis. IBM SPSS software (22.0), RStudio (4.0), and GraphPad Prism (8.0) were used for the statistical analysis. Continuous data are expressed as median with an interquartile range as appropriate. Categorical data are expressed in numbers and percentages. The safety data were analyzed by descriptive statistical analyses. A two‐sided p‐value of <0.05 was considered statistically significant.
RESULTS
Patients and treatment
Between November 1, 2015, and September 30, 2021, a total of 54 consecutively enrolled patients with locally advanced or metastatic NSCLC received osimertinib with antiangiogenic agent treatment (Figure 1), and the median follow‐up time was 26.1 months (range, 2.1–61.1). Baseline demographic and clinical characteristics are listed in Table 1. Of all the enrolled patients, 12 (22.2%) were assigned to Group A, 30 (55.6%) were assigned to Group B, and 12 (22.2%) were assigned to Group C. With regard to the types of different antiangiogenic agents, there were 23 (42.6%), 22 (40.7%), and nine (16.7%) patients, respectively who were treated with bevacizumab, anlotinib, and apatinib.
FIGURE 1.
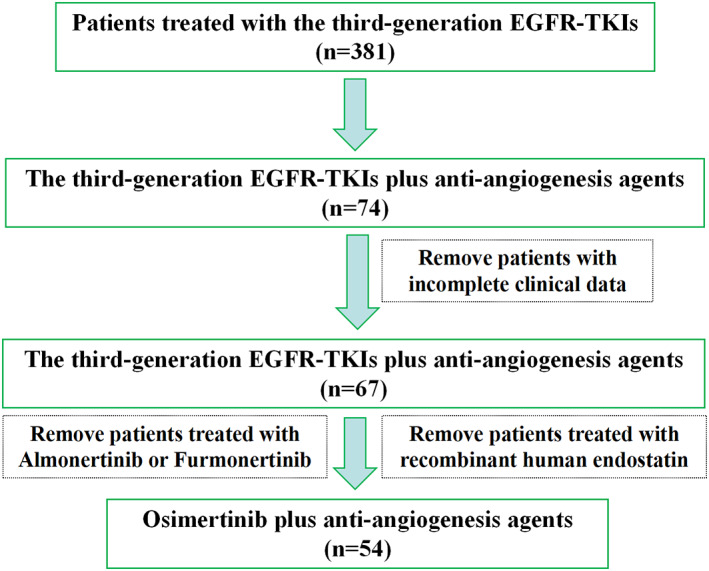
Patient flow of the study. EGFR‐TKIs, epidermal growth factor receptor‐tyrosine kinase inhibitors
TABLE 1.
Baseline patient characteristics
| Characteristic | Patients (n = 54) |
|---|---|
| Groups | |
| Group A | 12 (22.2) |
| Group B | 30 (55.6) |
| Group C | 12 (22.2) |
| Median age, (year) | 59 (range, 27–75) |
| Pathological type | |
| Lung adenocarcinoma | 51 (94.4) |
| NSCLC ‐ not otherwise specified | 3 (5.6) |
| Sex | |
| Male | 24 (44.4) |
| Female | 30 (55.6) |
| Smoking history | |
| Yes | 17 (31.5) |
| No | 37 (68.5) |
| ECOG PS | |
| 0 | 9 (16.7) |
| 1 | 40 (74.1) |
| 2 | 5 (9.3) |
| Stage | |
| III | 2 (3.7) |
| IVA | 13 (24.1) |
| IVB | 39 (72.2) |
| Brain metastases | |
| Yes | 24 (44.4) |
| No | 30 (55.6) |
| Hepatic metastases | |
| Yes | 6 (11.1) |
| No | 48 (88.9) |
| Bone metastases | |
| Yes | 24 (44.4) |
| No | 30 (55.6) |
| EGFR status | |
| EGFR 19del mutation | 21 (38.9) |
| EGFR L858R mutation | 29 (53.7) |
| EGFR 20ins mutation | 1 (1.9) |
| Not accessible | 3 (5.6) |
| EGFR T790M before osimertinib | |
| Positive | 28 (51.9) |
| Negative | 17 (31.5) |
| Not accessible | 9 (16.7) |
| Treatment line | |
| Median number (range) | 3 (1–7) |
| ≤3 | 35 (64.8) |
| >3 | 19 (35.2) |
| Previous first or second generation EGFR‐TKIs | |
| Yes | 50 (92.6) |
| No | 4 (7.3) |
| Previous chemotherapy treatment | |
| Yes | 31 (57.4) |
| No | 23 (42.6) |
| Previous antiangiogenic treatment | |
| Yes | 15 (27.8) |
| No | 39 (72.2) |
| Previous lung cancer operation | |
| Yes | 14 (25.9) |
| No | 40 (74.1) |
| Therapeutic regimens | |
| Osimertinib plus anlotinib | 22 (40.7) |
| Osimertinib plus apatinib | 9 (16.7) |
| Osimertinib plus bevacizumab | 23 (42.6) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR‐TKIs, epidermal growth factor receptor‐tyrosine kinase inhibitors; NSCLC, non‐small cell lung cancer.
The median diagnosed age of these patients was 59 years (range: 27–75). The pathological type of most patients was LUAD (94.4%), the remaining three were the other not specified type of NSCLC (5.6%). There were more female patients (55.6%) than male patients (44.4%), and over half of the patients (68.5%) denied smoking histories. The vast majority of patients (90.8%) had an ECOG PS of 0–1 score, and 5 (9.3%) were graded with two scores. All patients were restaged before treatment with osimertinib, and 96.3% of patients were staged as IVA (24.1%) or IVB (72.2%). At the initial diagnosis, apart from three (5.6%) patients with unknown EGFR mutations, the remainder were recorded with specific EGFR mutations, EGFR 19 exon deletions and EGFR 21 exon L858R point mutations accounted for 38.9 and 53.7%, respectively. There was one patient (1.9%) with EGFR 20 exon insert mutation. Before treatment with osimertinib, 51.9% of patients had positive mutations of EGFR T790M, and 31.5% were negative. Osimertinib application in our cohort was relatively late, the median treatment line was 3 (range, 1–7), and only three patients were treated with osimertinib in the first‐line (Table S1). Fifty patients (92.6%) had previously received treatment with first‐ or second‐generation EGFR‐TKIs, and 31 (57.4%) and 15 (27.8%) patients had previously been treated with chemotherapy and antiangiogenic agents, respectively.
Efficacy
In Group A (Table 2), a total of eight patients had evaluable lesions during treatment with osimertinib alone, of which four patients, respectively achieved PR and SD, and finally achieved an ORR of 50.0%, and a DCR of 100% (Figure 2). Nine patients had evaluable lesions during treatment with osimertinib and antiangiogenic agents, of which nine patients achieved SD, and finally achieved an ORR of 0%, and a DCR of 100% (Figure 2). The median PFS and median OS of Group A were 21.0 (95% CI: 16.7–25.3) months and 48.0 (95% CI: not reach) months (Figure 3a, b).
TABLE 2.
The efficacy of different groups
| Groups | PR (no. of patients with evaluable lesions) | SD (no. of patients with evaluable lesions), % | PD (no. of patients with evaluable lesions), % | PFS (months, 95% CI) (FAS) | OS (months, 95%CI) (FAS) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Osimertinib | Osimertinib plus antiangiogenic agents | Osimertinib | Osimertinib plus antiangiogenic agents | Osimertinib | Osimertinib plus antiangiogenic agents | ||||
| Group A (n = 12) | 4 (8), 50 | 0 (9), 0 | 4 (8), 50 | 9 (9), 100 | 0 (8), 0 | 0 (9), 0 | 21.0 (16.7–25.3) | 48.0 (NR) | |
| Group B (n = 30) | 9 (19), 47.4 | 3 (19), 15.8 | 8 (19), 42.1 | 13 (19), 68.4 | 2 (19), 22.2 | 3 (19), 15.8 | 9.2 (5.9–12.6) | 5.0* (4.5–5.5) | 31.8 (26.6–37.1) |
| Group C (n = 12) | / | 2 (5), 40 | / | 3 (5), 60 | / | 0 (5), 0 | 15.3 (7.9–22.7) | 28.5 (15.2–41.8) | |
Abbreviations: CI, confidence interval; FAS, full analysis set; NR, not reached; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Note: *PFS2 of patients in Group B.
FIGURE 2.
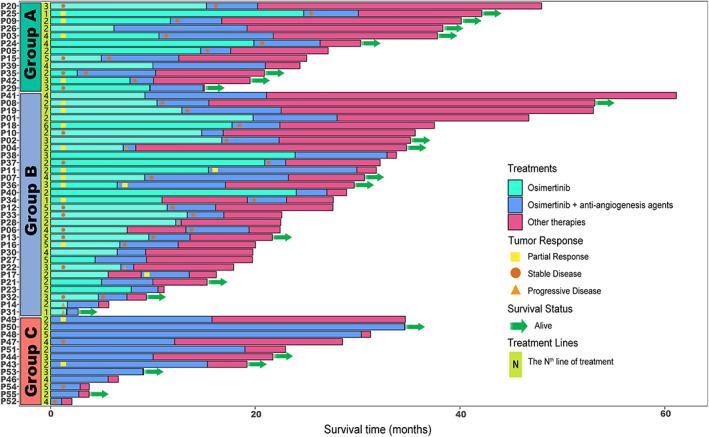
Swimmer plots of the survival status of each patient with NSCLC from the initial treatment with osimertinib. NSCLC, non‐small cell lung cancer
FIGURE 3.
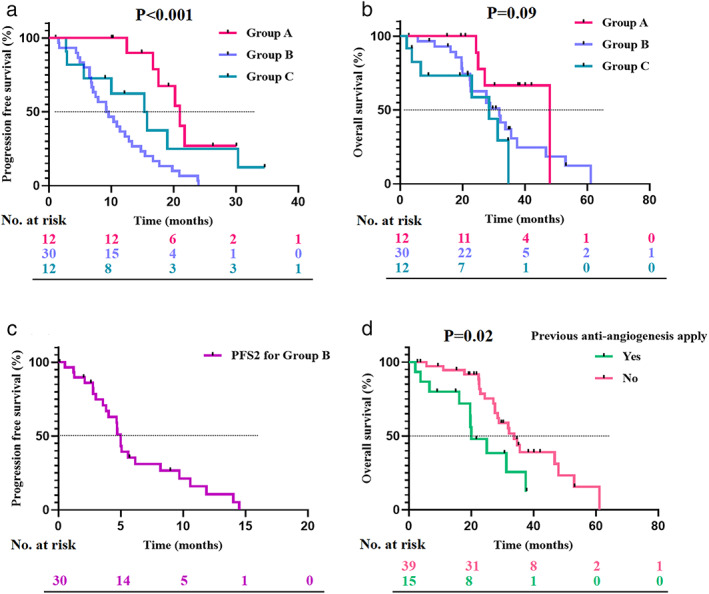
A Kaplan–Meier curve for PFS and OS of patients with NSCLC. (a) A Kaplan–Meier curve for PFS according to Group A, Group B, and Group C. (b) A Kaplan–Meier curve for OS according to Group A, Group B, and Group C. (c) A Kaplan–Meier curve for PFS2 of Group B. (d) A Kaplan–Meier curve for OS according to the previous application of antiangiogenic agents. PFS, progression‐free survival; OS, overall survival; NSCLC, non‐small cell lung cancer
In Group B (Table 2), there was a total of 19 patients, respectively with evaluable lesions during treatment with osimertinib monotherapy and osimertinib plus antiangiogenic agents. Nine and eight patients, respectively achieved PR and SD during osimertinib monotherapy, with an ORR of 47.4% and a DCR of 89.5% (Figure 2). Three and 13 patients achieved PR and SD during combination treatment with osimertinib and antiangiogenic agents, with an ORR of 15.8% and a DCR of 84.2% (Figure 2). The median PFS of Group B was 9.2 (95% CI: 5.9–12.6) months (Figure 3a), after progression, and patients continued treatment with osimertinib combined with antiangiogenic agents, with a median PFS 2 of 5.0 (95% CI: 4.5–5.5) months (Figure 3c). In addition, the median OS of Group B was 31.8 (95% CI: 26.6–37.1) months (Figure 3b).
For Group C (Table 2), a total of five patients with evaluable lesions, of which two and three patients achieved PR and SD, respectively and finally achieved an ORR of 40% and a DCR of 100% (Figure 2). The median PFS and median OS of Group C were 15.3 (95% CI: 7.9–22.7) months and 28.5 (95% CI: 15.2–41.8) months, respectively (Figure 3a, b).
Patients in Group A achieved a significant longer median PFS (p < 0.001) and a trend of longer median OS (p = 0.09) than Groups B and C (Figure 3a, b).
Subgroup analysis
We next performed subgroup analysis for OS in all the enrolled patients. In the full analysis set (FAS), there were 32 patient recorded deaths, with a median OS of 31.8 (95% CI: 26.7–37.0) months (Supplementary Figure 1). Previous application of antiangiogenic agents was significantly correlated with inferior OS (p = 0.02; hazard ratio [HR] = 0.41, 95% CI: 0.19–0.89), with a median OS of 20.0 (95% CI: 11.9–28.1) months, while others achieved a median OS of 33.8 (95% CI: 29.3–38.3) months (Figure 3d). Patients with positive EGFR T790M mutations before osimertinib exhibited a trend of longer median OS than others (37.5 vs. 27.6 months), with a marginal difference (p = 0.08; HR = 1.90, 95% CI: 0.93–3.90) (Figure 4).
FIGURE 4.
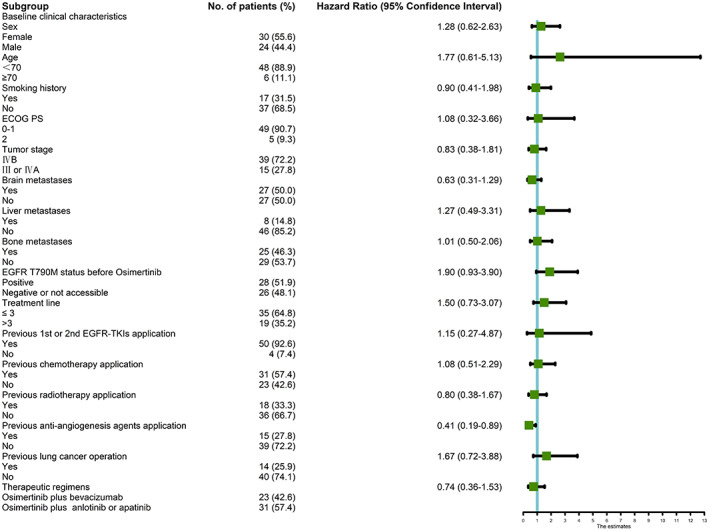
A forest‐graph of the association between different clinical characteristics and OS of all the enrolled patients in our cohort. OS, overall survival; EGFR‐TKIs, epidermal growth factor receptor‐tyrosine kinase inhibitors
There were no statistically significant differences in other variables, such as sex (female vs. male), age (<70 years vs. ≥70 years), smoking history (Yes vs. No), ECOG PS (0–1 vs. 2), tumor stage (IVB vs. III or IVA), brain metastases (Yes vs. No), liver metastases (Yes vs. No), bone metastases (Yes vs. No), EGFR status (19del vs. L858R or 20 exon ins or not accessible), previous first‐ or second‐generation EGFR‐TKIs application (Yes vs. No), previous chemotherapy (Yes vs. no), previous radiotherapy (Yes vs. No), and previous lung cancer operations (Yes vs. No) (p > 0.05) (Figure 4). With regard to the effect of different antiangiogenic agents (bevacizumab vs. anlotinib or apatinib) on the median OS, there was no significant statistical differences in FAS (Supplementary Figure 2A). In Group A (Supplementary Figure 2B), patients who received osimertinib with anlotinib or apatinib achieved a trend of prolonged median OS compared with those who received osimertinib with bevacizumab (48.0 vs. 26.1 months; p = 0.02, HR = 0.13, 95% CI: 0.01–1.14), but this phenomenon was not seen in Group B (Supplementary Figure 2C) and Group C (Supplementary Figure 2D).
Multivariate Cox regression analysis results (Table S2) showed that the absence of EGFR T790M mutations (p = 0.043; HR = 2.124, 95% CI: 1.023–4.413) and no previous antiangiogenic agent application (p = 0.012; HR = 0.362, 95% CI: 0.163–0.863) were the independent prognostic factors of OS.
Safety
Treatment‐related adverse events (TRAE) during osimertinib mono or combination therapy are listed in Table S3. The overall incidence of TRAE was 57.4% (n = 31), and the most common TRAEs among patients included diarrhea (20.4%), rash (16.7%), positive urinary protein (14.8%), and oral mucositis (13.0%). Grade 3–4 TRAE patients were 7.4% (n = 4), two patients had venous thrombosis (3.7%), one patient had severely decreased appetite (1.9%), and another patient had grade 3 thrombocytopenia.
DISCUSSION
This study provides an objective report on the efficacy and safety results of osimertinib plus antiangiogenic agents in patients with locally advanced or metastatic NSCLC, and the three treatment models of Groups A, B, and C that defined can also objectively reflect the real‐world practice habit of treatment with osimertinib in China. To the best of our knowledge, this is the first study that includes all common types of antiangiogenic agents on the basis of osimertinib, and is also the first study to compare the survival benefits of different treatment models; however, the ultimate goal is to focus on and reflect the most real clinical application.
In our study, patients labeled as Group C who were directly treated with osimertinib plus antiangiogenic agents achieved a median PFS and median OS of 15.3 and 28.5 months, respectively. A retrospective single‐arm study 22 reported the clinical outcomes of osimertinib plus anlotinib in previously treated EGFR T790M‐positive NSCLC patients and the median PFS and median OS were 15.5 and 23.8 months, respectively. The two studies obtained a similar median PFS, but the median OS was better in our study cohort. Different treatment therapies after osimertinib might be one of the possible reasons for this.
Another phase II study explored the efficacy of patients with advanced LUAD that progressed with prior EGFR‐TKIs and acquired EGFR T790M mutation and were treated with osimertinib plus bevacizumab or osimertinib alone, and there was no significant difference in survival between the two arms (p > 0.05), the median PFS and median OS were 9.4 months and not reached, respectively in the combination arm, 20 which was shorter than our study and the above‐mentioned retrospective study. Different types of antiangiogenic agents might be one of the important reasons because patients in this phase II study 20 were all treated with bevacizumab. However, we explored the survival differences between bevacizumab and small molecule antiangiogenic agents (anlotinib and apatinib) in our study cohort, and there was no significant difference in Group C.
Patients in Group A who received osimertinib combined with antiangiogenic agents after a trend of osimertinib resistance achieved a median PFS and median OS of 21.0 and 48.0 months, respectively. In addition, patients who received osimertinib plus anlotinib or apatinib in Group A achieved a trend of longer median OS than with osimertinib plus bevacizumab. In the AURA3 trial, 12 patients with EGFR T790M mutations in the second‐line setting achieved a median PFS of 10.1 months after treatment with osimertinib. In another phase III trial of FLAURA, 14 patients with EGFR mutations treated with osimertinib in the first‐line setting achieved a median PFS of 18.9 months. In our cohort, all patients who received osimertinib were all beyond first‐line except one person; however, the median PFS was not only longer than the PFS of the AURA3 trial but also longer than the FLAURA trial. Furthermore, through the comparative results of PFS among Group A (21.0 months, 95% CI: 16.7–25.3) and Group B (9.2 months, 95% CI: 5.9–12.6), it was again confirmed that the combination of antiangiogenic agents when an osimertinib resistance trend happened could achieve a significant PFS benefit. However, the sample size was small, and the multivariate Cox regression analysis achieved a negative result for the OS benefits of Group A, and these conclusions still need to be further verified by larger samples.
Patients from Group B all progressed after treatment with osimertinib, then combined with antiangiogenic agents, the median PFS was 9.2 months, which was comparable with the PFS results (10.1 months) of the AURA3 trial. 12 The ORR, DCR, and median PFS2 of combination therapy were 15.8%, 84.2%, and 5.0 months (95% CI: 4.5–5.5), respectively, which was also comparable with a retrospective study, 23 in which the efficacy of apatinib with osimertinib after the progression of osimertinib in EGFR positive LUAD patients was explored, and the ORR, DCR, and median PFS of combination therapy were 12.8%, 79.5%, and 4 months, respectively.
As is known to all, antiangiogenic agents are usually an excellent partner for other mechanisms of antitumor drugs, such as cytotoxic agents, immune checkpoint inhibitors, and first‐ or second‐generation EGFR‐TKIs. 24 , 25 , 26 , 27 However, there is relatively little data on osimertinib with antiangiogenic agents, and no definite answer to the timing of antiangiogenic combination. In our study, we compared the survival differences of three different models of antiangiogenic combination. The results demonstrated that Group A was a better treatment model than Groups B and C, showing a prolonged median PFS with a statistically significant difference (p < 0.001) and a trend of longer median OS (p = 0.09). These results enlightened us that when there are some signs of osimertinib resistance, immediate combination with other mechanisms of drugs, such as antiangiogenic agents, may be a good choice for the disease control of patients with NSCLC, but this view needs further verification.
Subgroup univariate analysis of our study showed that previous use of antiangiogenic agents (20.0 vs. 33.8 months, p = 0.02) was significantly associated with inferior median OS, and the multivariate analysis further confirmed the survival affection of previous use of antiangiogenic agents (HR = 0.362, 95% CI: 0.163–0.863; p = 0.012). It is easy to understand that antiangiogenic therapy also faces the problem of drug resistance, 28 and once drug resistance occurs, will compromise its efficacy.
In addition, the multivariate analysis results showed that EGFR T790M mutation status was another independent prognostic factor of OS (HR = 2.124, 95% CI: 1.023–4.413; p = 0.043), and although the univariate analysis did not reach a statistical difference (HR = 1.90, 95% CI: 0.93–3.90), patients with positive T790M had a better OS. The key point is that most patients (92.6%) in our study cohort had previously received first‐ or second‐generation EGFR‐TKIs, and for them, osimertinib worked by irreversibly binding to EGFR proteins expressed by acquired EGFR T790M mutation, 29 and explained why patients with T790M mutation achieved a trend of better OS.
The overall incidence of TRAEs in our study cohort was 57.4%, grade 3–4 TRAE was 7.4%, and both were lower than other similar studies, 20 , 23 the possible reason for this was that our study was retrospective, which would lead to the underestimation of the incidence of adverse events.
Several limitations can be seen in our study. First, this was a single‐arm study, and owing to the fact this protocol is rarely performed in a clinical setting, we had to enroll a heterogeneous patient population using a variety of antiangiogenic agents with osimertinib, and therefore confounding factors cannot be completely avoided. Therefore, the overall efficacy results of treatment with osimertinib and antiangiogenic agents should be carefully decoded, and the positive results need further identification by prospective case–control studies. Second, based on the characteristic that this was a real‐world retrospective study, memory bias of toxicities inevitably happened, but the focal point of our study was to explore the feasibility and profits of different treatment models of treatment with osimertinib and antiangiogenic agents, instead of focusing on safety like phase I trials. Third, the sample of our cohort was small, which might have caused selection bias of enrolled patients and a variation in results, so we adopted the method of continuously including patients in chronological order, and verified the results with univariate and multivariate methods simultaneously to avoid these biases as much as possible. Of course, these results need to be verified by further prospective studies.
In conclusion, the combination of osimertinib and antiangiogenic agents is a feasible treatment regimen for patients with NSCLC and EGFR mutations under certain circumstances, and a well‐timed action is to combine antiangiogenic agents with osimertinib in the early stage of resistance. In addition, the absence of EGFR T790M mutations and previous use of antiangiogenic agents are poor prognostic factors for patients treated with osimertinib and antiangiogenic agents.
CONFLICT OF INTEREST
The authors have declared that no competing interest exists.
Supporting information
Supplementary Figure 1 A Kaplan–Meier curve for OS of all the enrolled NSCLC patients in our cohort. OS, overall survival; NSCLC, non‐small cell lung cancer.
Supplementary Figure 2 A Kaplan–Meier curve for OS classified by different antiangiogenic agents; (A) A Kaplan–Meier curve for OS classified by antiangiogenic agents in FAS; (B) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group A; (C) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group B; (D) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group C. OS, overall survival; FAS, full analysis set.
Supplementary Tables S1 Patient characteristics for each individual.
Supplementary Tables S2. Cox multivariate regression analysis results of overall survival.
Supplementary Tables S3. Treatment related adverse events.
Feng Y, Huang L, Zhu H, Tang L, Hu X, Shi Y. The exploration of three different treatment models of osimertinib plus antiangiogenic agents in non‐small cell lung cancer: A real‐world study. Thorac Cancer. 2022;13(18):2641–2649. 10.1111/1759-7714.14603
Contributor Information
Xingsheng Hu, Email: huxingsheng66@163.com.
Yuankai Shi, Email: syuankaipumc@126.com.
REFERENCES
- 1. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. 10.4065/83.5.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar A, Petri ET, Halmos B, Boggon TJ. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26:1742–51. 10.1200/JCO.2007.12.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–67. 10.1056/NEJMoa0904554 [DOI] [PubMed] [Google Scholar]
- 4. Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non‐small‐cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–65. 10.1200/JCO.2005.08.043 [DOI] [PubMed] [Google Scholar]
- 5. Shi Y, Li J, Zhang S, Wang M, Yang S, Li N, et al. Molecular epidemiology of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology ‐ mainland China subset analysis of the PIONEER study. PLoS One. 2015;10:e0143515. 10.1371/journal.pone.0143515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 7. Chou TY, Chiu CH, Li LH, Hsiao CY, Tzen CY, Chang KT, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non‐small cell lung cancer. Clin Cancer Res. 2005;11:3750–7. 10.1158/1078-0432.CCR-04-1981 [DOI] [PubMed] [Google Scholar]
- 8. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81. 10.1038/nrc2088 [DOI] [PubMed] [Google Scholar]
- 9. Schalm SS, Dineen T, Lim SM, Park CW, Hsieh J, Woessner R, et al. 1296P BLU‐945, a highly potent and selective 4th generation EGFR TKI for the treatment of EGFR T790M/C797S resistant NSCLC. Ann Oncol. 2020;31:S839. 10.1016/j.annonc.2020.08.1610 [DOI] [Google Scholar]
- 10. Zhong Q, Tao Y, Chen H, Zhou Y, Huang L, Han X, et al. The changing landscape of anti‐lung cancer drug clinical trials in mainland China from 2005 to 2020. Lancet Reg Health West Pac. 2021;11:100151. 10.1016/j.lanwpc.2021.100151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yver A. Osimertinib (AZD9291)‐a science‐driven, collaborative approach to rapid drug design and development. Ann Oncol. 2016;27:1165–70. 10.1016/j.lanwpc.2021.100151 [DOI] [PubMed] [Google Scholar]
- 12. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum‐Pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:629–40. 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho BC, Chewaskulyong B, Lee KH, Dechaphunkul A, Sriuranpong V, Imamura F, et al. Osimertinib versus standard of care EGFR TKI as first‐line treatment in patients with EGFRm advanced NSCLC: FLAURA Asian subset. J Thorac Oncol. 2019;14:99–106. 10.1016/j.jtho.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 14. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113–25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 15. Tanaka K, Asahina H, Kishimoto J, Miyata Y, Uchida T, Watanabe K, et al. Osimertinib versus osimertinib plus chemotherapy for non‐small cell lung cancer with EGFR (T790M)‐associated resistance to initial EGFR inhibitor treatment: An open‐label, randomised phase 2 clinical trial. Eur J Cancer. 2021;149:14–22. 10.1016/j.ejca.2021.02.019 [DOI] [PubMed] [Google Scholar]
- 16. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. 10.1016/j.ctrv.2020.102017 [DOI] [PubMed] [Google Scholar]
- 17. Shichiri M, Hirata Y. Antiangiogenesis signals by endostatin. FASEB J. 2001;15:1044–53. 10.1096/fj.99-1083com [DOI] [PubMed] [Google Scholar]
- 18. Shen G, Zheng F, Ren D, du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi‐targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tian S, Quan H, Xie C, Guo H, Lü F, Xu Y, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor‐2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–80. 10.1111/j.1349-7006.2011.01939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akamatsu H, Toi Y, Hayashi H, Fujimoto D, Tachihara M, Furuya N, et al. Efficacy of Osimertinib plus bevacizumab vs Osimertinib in patients with EGFR T790M‐mutated non‐small cell lung cancer previously treated with epidermal growth factor receptor‐tyrosine kinase inhibitor: West Japan oncology group 8715L phase 2 randomized clinical trial. JAMA Oncol. 2021;7:386–94. 10.1001/jamaoncol.2020.6758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soo RA, Han JY, Dafni U, Cho BC, Yeo CM, Nadal E, et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second‐line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European thoracic oncology platform (ETOP 10‐16) BOOSTER trial. Ann Oncol. 2022;33:181–92. 10.1016/j.annonc.2021.11.010 [DOI] [PubMed] [Google Scholar]
- 22. Zhou B, Gong Q, Li B, Qie HL, Li W, Jiang HT, et al. Clinical outcomes and safety of osimertinib plus anlotinib for patients with previously treated EGFR T790M‐positive NSCLC: a retrospective study. J Clin Pharm Ther. 2022;47:643–51. 10.1111/jcpt.13591 [DOI] [PubMed] [Google Scholar]
- 23. Yang X, Xia Y, Xu L, Liang L, Zhuo M, Wu M, et al. Efficacy and safety of combination treatment with Apatinib and Osimertinib after Osimertinib resistance in epidermal growth factor receptor‐mutant non‐small cell lung carcinoma‐a retrospective analysis of a multicenter clinical study. Front Mol Biosci. 2021;8:639892. 10.3389/fmolb.2021.639892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double‐blind, placebo‐controlled, multicenter, phase III study of first‐line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2015;33:2197–204. 10.1200/JCO.2014.59.4424 [DOI] [PubMed] [Google Scholar]
- 25. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 26. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR‐positive advanced non‐squamous non‐small‐cell lung cancer (NEJ026): interim analysis of an open‐label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–35. 10.1016/S1470-2045(19)30035-X [DOI] [PubMed] [Google Scholar]
- 27. Ichihara E, Hotta K, Nogami N, Kuyama S, Kishino D, Fujii M, et al. Phase II trial of gefitinib in combination with bevacizumab as first‐line therapy for advanced non‐small cell lung cancer with activating EGFR gene mutations: the Okayama lung cancer study group trial 1001. J Thorac Oncol. 2015;10:486–91. 10.1097/JTO.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 28. Giuliano S, Pagès G. Mechanisms of resistance to anti‐angiogenesis therapies. Biochimie. 2013;95:1110–9. 10.1016/j.biochi.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 29. Bollinger MK, Agnew AS, Mascara GP. Osimertinib: a third‐generation tyrosine kinase inhibitor for treatment of epidermal growth factor receptor‐mutated non‐small cell lung cancer with the acquired Thr790Met mutation. J Oncol Pharm Pract. 2018;24:379–88. 10.1177/1078155217712401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 A Kaplan–Meier curve for OS of all the enrolled NSCLC patients in our cohort. OS, overall survival; NSCLC, non‐small cell lung cancer.
Supplementary Figure 2 A Kaplan–Meier curve for OS classified by different antiangiogenic agents; (A) A Kaplan–Meier curve for OS classified by antiangiogenic agents in FAS; (B) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group A; (C) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group B; (D) A Kaplan–Meier curve for OS classified by antiangiogenic agents in Group C. OS, overall survival; FAS, full analysis set.
Supplementary Tables S1 Patient characteristics for each individual.
Supplementary Tables S2. Cox multivariate regression analysis results of overall survival.
Supplementary Tables S3. Treatment related adverse events.


