Abstract
Purpose
This study aimed to investigate the differences in characteristics, clinical stages, treatment modalities, and survival outcomes in patients with non‐small‐cell lung cancer (NSCLC) based on sex differences using Korean nationwide registry data.
Methods
We analyzed the data of 8650 patients diagnosed with NSCLC between 2014 and 2017, obtained from the Korean Association for Lung Cancer Registry (KALC‐R). The Cox proportional hazard model was used to define the differences in survival based on sex. Propensity score matching was used to adjust for differences between men and women.
Results
Of a total of 10 943 patients, 8650 (79.1%) were diagnosed with NSCLC, of whom 68.7% were men and 31.3% were women. For NSCLC, the median age was higher (69.0 vs. 67.0, p < 0.001) and the proportion of ever‐smokers (84.5% vs. 10.8%, p < 0.001) was higher in men. Adenocarcinoma (55.5% vs. 90.4%, p < 0.001) and stage I NSCLC (26.3% vs. 41.3%, p < 0.001) were more common in women. Survival was significantly lower in men with NSCLC (hazard ratio [HR] 1.493 [95% confidence interval, CI 1.238–1.800], p < 0.001) even after adjusting for meaningful clinical variables, and in the matched cohort (HR 1.339 [1.075–1.667], p = 0.009). Similarly, survival was significantly lower in men with stage IV adenocarcinoma after adjusting for other clinical variables (HR 1.493 [1.238–1.800], p < 0.001) and in the matched cohort (HR 1.339 [1.075–1.667]; p = 0.009).
Conclusions
Male patients with NSCLC had poorer prognosis, not only after variable adjustments for prognostic factors, but also in the matched cohort.
Keywords: epidemiology, Korea, lung cancer, sex, survival
Our current study showed that men had poorer prognosis than women in non‐small‐cell lung cancer despite adjusting for several clinical factors, such as smoking status, performance status, clinical staging, and treatment modalities. These results suggest that it might be necessary to consider sex differences in the prediction of prognosis when performing optimal management in lung cancer.

INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths for both men and women worldwide. 1 To date, incidence rates of lung cancer are known to be higher in males. 2 This sex‐based difference is attributed to tobacco smoking, and genetic and biological differences. 3 However, the incidence of lung cancer has recently increased in women, particularly among nonsmokers. 4 , 5 In addition, non‐small‐cell lung cancer (NSCLC) accounts for a high proportion of lung cancer epidemiology worldwide 6 and the importance of NSCLC is increasing with the various types of target therapy and driver mutations. 7
According to previous studies, men had poorer prognosis for lung cancer compared to women. 8 , 9 However, factors such as age, smoking, clinical staging, and genetic mutation might have influence as confounding factors. 10 Recently, several studies have reported that after adjustment of these confounding factors, men continued to show poor prognosis compared to women. 11 , 12 However, large‐scale studies are sparse in the literature.
The goal of this study was therefore to analyze the characteristics, initial treatment modalities, and clinical stages of lung cancer according to sex, and further investigate the sex‐stratified survival outcomes by adjusting the factors predicted to contribute to the prognosis in NSCLC using a large database constructed through a multicenter nationwide registry in South Korea.
METHODS
Study design and subjects
This study analyzed data from the Korean Association for Lung Cancer Registry (KALC‐R), a multicenter cancer registry. This was set up after the second nationwide survey, and it consists about 10% of the newly diagnosed lung cancer cases in Korea each year. 13 From 2014 to 2017, the KALC‐R registered 8650 patients who were diagnosed with incident NSCLC, of which 11 patients diagnosed with lung cancer before the study period were excluded. Thus, a total of 8650 patients were included (2047, 2098, 2237, and 2268 patients in 2014, 2015, 2016, and 2017, respectively).
The KALC‐R has approximately 80 data fields comprising demographic data. Data on patient age, sex, body mass index (BMI), symptoms, smoking history, performance status, histopathologic type, clinical stage (according to the eighth edition of the TNM International Staging System), initial treatment modality, and results of molecular tests (i.e., epidermal growth factor receptor [EGFR] mutation and anaplastic lymphoma kinase [ALK] aberration) were collected using a standardized protocol. Ever‐smokers were defined as patients with any history of smoking before a lung cancer diagnosis and never‐smokers as those with no smoking history. Performance status was classified based on Eastern Cooperative Oncology Group (ECOG) classification. 14
Statistical analysis
Hazard ratios (HRs) and 95% confidence intervals (CIs) for death in lung cancer according to sex were estimated using Cox proportional hazard regression methods. Propensity score matching was performed to balance differences in baseline characteristics according to sex. Propensity scores were calculated via a logistic regression analysis using baseline covariates such as age, smoking status, performance status, BMI, histopathology, clinical stage, EGFR mutation, and ALK aberrant. Standardized mean differences of <0.1 for a given covariate indicated a relatively small imbalance. 15 Outcomes were compared using Cox regression models with robust standard errors that accounted for the clustering of the matched pairs. On Cox‐proportional hazard assumption, if p was >0.05, the assumption was satisfied, and if p was <0.05, the assumption was not satisfied. The Cox regression plot of this study is presented in Supporting Information Table S1. Adjusted HRs and 95% CIs were estimated for men in relation to women. Overall survival was defined as the time from diagnosis of lung cancer until death from any cause. Patients were followed up until December 31, 2020 or the date of death. Survival was analyzed by the Kaplan–Meier method with log‐rank tests for sex differences, and subgroup analysis of patients with stage IV adenocarcinoma according to EGFR mutation and ALK aberration was also performed.
Data are expressed as the mean ± standard deviation (SD) or median (interquartile range [IQR]). The Mann–Whitney U test was used to compare continuous variables, and the chi‐square or Fisher's exact test was used to compare categorical variables. All p values <0.05 were considered statistically significant. All statistical analyses were performed using SAS software version 9.4 (SAS Institute).
Ethical considerations
The KALC‐R was approved by the Institutional Review Board (IRB) of the National Cancer Center (approval number: NCC 2018–0193). The requirement for informed consent was waived by the IRB due to the retrospective nature of the study. The study was conducted in accordance with the relevant guidelines.
RESULTS
Baseline characteristics
Patients diagnosed with NSCLC were analyzed according to sex (Table 1). Among the 8651 NSCLC patients, 5944 (68.7%) were men and 2706 (31.3%) were women. The median age was significantly higher (69.0 [IQR 62.0–75.0 years] vs. 67.0 [IQR 58.0–75.0 years], p < 0.001) in men. Compared to women, the proportion of ever‐smoker status (84.5% vs. 10.8%, p < 0.001) was significantly higher in men. Respiratory symptoms except for pain were more common in men. Adenocarcinoma was more common in women (55.5% vs. 90.4%, p < 0.001) whereas squamous cell carcinoma was more common in men (43.5% vs. 8.7%, p < 0.001). Early‐stage lung cancer was more prevalent in women (stage I 26.3% vs. 41.3%, p < 0.001). Supporting Information Table S2 shows the baseline characteristics of NSCLC patients after propensity score matching.
TABLE 1.
Baseline characteristics of non‐small‐cell lung cancer patients according to sex from 2014 to 2017
| Total | Male | Female | p value | |
|---|---|---|---|---|
| No. of patients | 8650 | 5944 | 2706 | |
| Age (years) | 69.0 (60.0–75.0) | 69.0 (62.0–75.0) | 67.0 (58.0–75.0) | <0.001 |
| Ever‐smoker | 5316 (61.5%) | 5024 (84.5%) | 292 (10.8%) | <0.001 |
| BMI (kg/m2) | 23.6 (23.2–24.0) | 23.4 (23.0–24.0) | 23.8 (23.2–24.6) | 0.388 |
| Symptoms | 8527 | 5861 | 2666 | |
| Asymptomatic | 1808 (21.2%) | 1075 (18.3%) | 733 (27.5%) | <0.001 |
| Cough | 2766 (32.4%) | 1990 (34.0%) | 776 (29.1%) | <0.001 |
| Sputum | 1676 (19.7%) | 1305 (22.3%) | 371 (13.9%) | <0.001 |
| Dyspnea | 1575 (18.5%) | 1146 (19.6%) | 429 (16.1%) | <0.001 |
| Hoarseness | 136 (1.6%) | 113 (1.9%) | 23 (0.9%) | <0.001 |
| Hemoptysis | 503 (5.9%) | 424 (7.2%) | 79 (3.0%) | <0.001 |
| Weight loss | 512 (6.0%) | 389 (6.6%) | 123 (4.6%) | <0.001 |
| Pain | 1504 (17.6%) | 1072 (17.5%) | 432 (16.2%) | 0.018 |
| Performance status | 6578 | 4490 | 2087 | <0.001 |
| 0–1 | 5796 (88.1%) | 3908 (87.0%) | 1887 (90.4%) | |
| 2–4 | 782 (11.9%) | 582 (13.0%) | 200 (9.6%) | |
| Histopathology | 8213 | 5564 | 2649 | |
| Adenocarcinoma | 5482 (66.7%) | 3086 (55.5%) | 2396 (90.4%) | <0.001 |
| Squamous cell | 2654 (32.3%) | 2423 (43.5%) | 231 (8.7%) | <0.001 |
| Carcinoma | 8524 | 5851 | 2672 | <0.001 |
| Clinical stage | ||||
| Stage I | 2645 (31.0%) | 1541 (26.3%) | 1104 (41.3%) | |
| Stage II | 687 (8.1%) | 525 (9.0%) | 162 (6.1%) | |
| Stage III | 1528 (17.9%) | 1258 (21.5%) | 270 (10.1%) | |
| Stage IV | 3663 (43.0%) | 2527 (43.2%) | 1136 (42.5%) | |
Note: Values are presented as mean ± standard deviation, median (interquartile range), or number (%), unless otherwise indicated.
Abbreviation: BMI, body mass index.
Initial treatment method
In each stage of NSCLC, most of the initial treatment modalities did not have significant differences between sex (Supporting Information Table S3). However, women underwent more surgery for initial treatment in stage I NSCLC (81.5% in men vs. 89.8% in women, p < 0.001), and men received more radiation therapy (7.9% vs. 4.0%, p < 0.001) and chemotherapy (0.9% vs. 0.3%, p = 0.043) compared to women.
Survival according to the sex
In the Kaplan–Meier survival curve, men showed worse survival compared to women with NSCLC (median survival 34.9 vs. 51.1 months in female, p < 0.001) (Figure 1). In all clinical stages of NSCLC, men showed worse survival outcome (median survival 64.8 vs. 76.9 months in stage I, p < 0.001; 48.9 vs. 59.2 months in stage II, p = 0.002; 30.6 vs. 44.7 months in stage III, p < 0.001; 15.5 vs. 26.2 months in stage IV, p < 0.001, respectively), compared to women. According to the Cox proportional hazard model, lung cancer survival was significantly higher for women (HR 1.827 [1.715–1.946], p < 0.001) than for men with NSCLC. In addition, the survival of women was higher (HR 1.542 [1.335–1.781], p < 0.001) than that of men, even after adjusting for several variables (such as age, BMI, performance status, smoking status, clinical stage, initial treatment modality, EGFR mutation, and ALK aberration) affecting prognosis. The same trend of survival was shown in the matched cohort (HR 1.225 [1.025–1.353], p = 0.025) (Table 2 and Supporting Information Figure S1).
FIGURE 1.
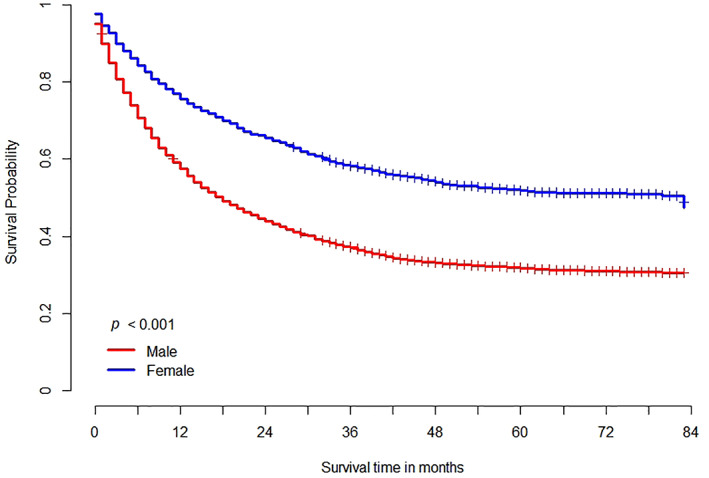
Overall survival in patients with non‐small‐cell lung cancer according to sex
TABLE 2.
Adjusted hazard ratios for death from non‐small‐cell lung cancer in men compared to women according to variables adjustment and matched cohort
| Hazard ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Crude without adjustment | 1.827 | 1.715–1.946 | <0.001 |
| Unmatched but adjusting for all variables a | 1.542 | 1.335–1.781 | <0.001 |
| Propensity score matching (nearest neighbor) | 1.225 | 1.025–1.353 | 0.025 |
Age, BMI, performance status, smoking status, clinical stage, initial treatment methods, histopathology (adenocarcinoma, squamous cell carcinoma), EGFR mutation, ALK aberration.
Subgroup analysis among patients with stage IV adenocarcinoma
Patients diagnosed with stage IV adenocarcinoma were analyzed according to sex (Table 3). Among 2433 patients, 1469 were male. The median age was lower (67.0 [59.0–74.0 years] vs. 69.0 [IQR 58.0–77.0 years], p = 0.036) and the proportion of ever‐smokers was significantly higher (80.1% vs. 8.4%, p < 0.001) among men. In women, EGFR mutation (23.8% vs. 51.1%, p < 0.001) and ALK aberration (6.3% vs. 9.5%, p = 0.005) were higher because EGFR (26.1% vs. 46.7%, p < 0.001) and ALK inhibitors (2.9% vs. 4.8%, p = 0.007) were administered more frequently in women (Supporting Information Table S4). Supporting Information Table S5 shows the baseline characteristics of stage IV adenocarcinoma patients after propensity score matching.
TABLE 3.
Baseline characteristics of stage IV adenocarcinoma patients according to sex during 2014 to 2017
| Total | Male | Female | p value | |
|---|---|---|---|---|
| No. of patients | 2433 | 1469 | 964 | |
| Age (years) | 67.0 (58.0–75.0) | 67.0 (59.0–74.0) | 69.0 (58.0–77.0) | 0.036 |
| Ever‐smoker | 1258 (51.7%) | 1177 (80.1%) | 81 (8.4%) | <0.001 |
| BMI (kg/m2) | 23.0 (22.3–23.9) | 23.2 (22.2–24.3) | 22.8 (21.9–24.2) | 0.636 |
| Symptoms | 2392 | 1445 | 947 | |
| Asymptomatic | 221 (9.2%) | 122 (8.4%) | 99 (10.5%) | 0.099 |
| Cough | 880 (36.8%) | 517 (35.8%) | 363 (38.3%) | 0.216 |
| Sputum | 446 (18.6%) | 287 (19.9%) | 159 (16.8%) | 0.058 |
| Dyspnea | 617 (25.8%) | 368 (25.5%) | 249 (26.3%) | 0.666 |
| Hoarseness | 47 (2.0%) | 35 (2.4%) | 12 (1.3%) | 0.046 |
| Hemoptysis | 104 (4.3%) | 74 (5.1%) | 30 (3.2%) | 0.022 |
| Weight loss | 188 (7.9%) | 116 (8.0%) | 72 (7.6%) | 0.699 |
| Pain | 714 (29.8%) | 455 (31.5%) | 259 (27.3%) | 0.030 |
| Performance status | 1795 | 1099 | 696 | 0.341 |
| 0–1 | 1500 (83.6%) | 916 (83.3%) | 584 (83.9%) | |
| 2–4 | 295 (16.4%) | 183 (16.7%) | 112 (16.1%) | |
| EGFR mutation | 2433 | 1469 | 964 | <0.001 |
| Positive | 842 (34.6%) | 349 (23.8%) | 493 (51.1%) | |
| Negative | 1336 (54.9%) | 955 (65.0%) | 381 (39.5%) | |
| Not tested | 255 (10.5%) | 165 (11.2%) | 90 (9.3%) | |
| ALK | 2433 | 1469 | 964 | 0.005 |
| Positive | 185 (7.6%) | 93 (6.3%) | 92 (9.5%) | |
| Negative | 1599 (65.7%) | 963 (65.6%) | 636 (66.0%) | |
| Not tested | 649 (26.7%) | 413 (28.1%) | 236 (24.5%) |
Note: Values are presented as mean ± standard deviation, median (interquartile range), or number (%), unless otherwise indicated.
Abbreviations: ALK, anaplastic lymphoma kinase; BMI, body mass index; EGFR, epidermal growth factor receptor.
In the Kaplan–Meier survival curve according to sex in stage IV adenocarcinoma, men showed worse survival than women (19.3 vs. 28.6 months, p < 0.001; Figure 2a). In addition, among individuals harboring EGFR mutation (29.2 vs. 33.3 months, p = 0.054; Figure 2b) and ALK aberration (30.8 vs. 40.3 months, p = 0.031; Figure 2c), men showed worse survival compared to women. In the Cox proportional hazard model, survival was significantly higher in women (HR 1.493 [1.238–1.800], p < 0.001) after adjusting for age, BMI, performance status, smoking status, EGFR mutation, and ALK aberration. The same result was seen in the matched cohort (HR 1.339 [1.075–1.667], p = 0.009) (Table 4 and Supporting Information Figure S2).
FIGURE 2.
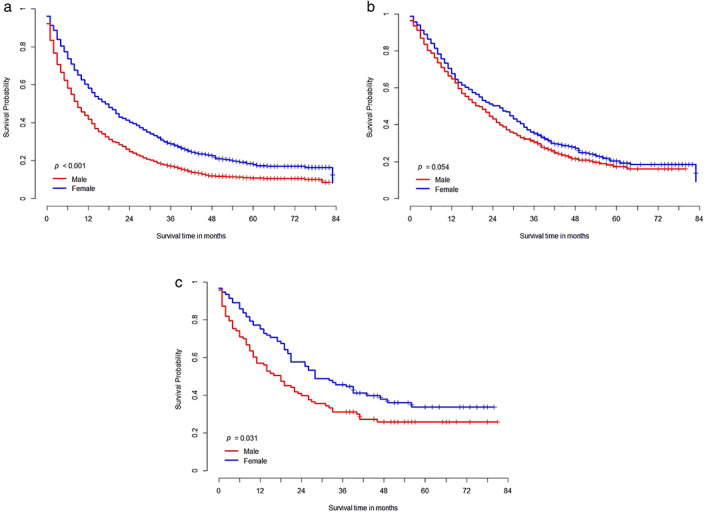
Overall survival in patients with stage IV adenocarcinoma (a), epidermal growth factor receptor mutated stage IV adenocarcinoma (b), and anaplastic lymphoma kinase aberrated stage IV adenocarcinoma (c) according to sex.
TABLE 4.
Adjusted hazard ratios for death from stage IV adenocarcinoma in men compared to women according to variables adjustment and matched cohort
| Hazard ratio | 95% confidence interval | p value | |
|---|---|---|---|
| Crude without adjustment | 1.486 | 1.359–1.626 | <0.001 |
| Unmatched but adjusting for all variables a | 1.493 | 1.238–1.800 | <0.001 |
| Propensity score matching (nearest neighbor) | 1.339 | 1.075–1.667 | 0.009 |
Age, body mass index, performance status, smoking status, epidermal growth factor receptor mutation, anaplastic lymphoma kinase aberration.
DISCUSSION
In our nationwide survey, we analyzed sex differences in patients with NSCLC characteristics, treatment modalities, clinical stages, and survival outcomes. Men had a poorer prognosis than women in the total cohort and stage IV adenocarcinoma. Male lung cancer patients showed higher mortality rates, not only after adjusting for the prognostic factors such as age, smoking status, performance status, clinical stage, and treatment modalities, but also in the matched cohort after propensity score matching.
Recently, an international set of guidelines for Sex and Gender Equity in Research (SAGER) was established by the European Association of Science Editors, which encourages consideration of sex (biological attributes) and gender (shaped by social and cultural circumstances) in research. 16 Conventionally, with a few exceptions, cancers are more common in men than in women, and lung cancer was also considered to be more common in men who were smokers. 17 , 18 However, the lung cancer incidence rate in never‐smoker women has been rapidly increasing recently. 19
Several factors have been proposed as grounds for this sex disparity. To date, biological, physiological, hormonal, and genetic factors have been suggested as the reason for the sex difference. 3 Although lung carcinogenesis is a complicated process, previous studies have shown that tumor‐related gene mutations such as p53 and KRAS were more frequent in women. 20 , 21 Furthermore, a study showed that female sex hormones had a negative impact on smoking metabolism, 22 resulting in an increased risk of lung cancer. Women who received hormone replacement treatment or were on oral contraceptives had an increased risk of lung cancer. 23 In addition, considering that about 80% of women with lung cancer did not meet the criteria for screening, the current system might not be effective for early lung cancer screening in women. 24 With the rise in incidence of lung cancer in women, it is necessary to carry out large‐scale clinical and prognostic studies which may aid in early diagnosis, recognition of tumor characteristics, and appropriate management.
The sex differences in lung cancer demographics in our analysis were similar to those of other studies. 9 , 11 , 12 , 25 , 26 In our current study, the median age at diagnosis and the fact that the median age was lower in women was similar to that of previous studies. 27 , 28 Intrinsic factors such as sex hormones and multiple exogenous exposures might influence the difference in age at the time of diagnosis between the two sexes. 8 However, in our study, the difference in smoking status according to sex was larger than that in previous studies. Contrary to a previous study in which about 70% of women had experienced smoking, 29 our analysis showed only 12.1% of women to be ever‐smokers. This may be the reason for fewer reported cases of squamous cell carcinoma among women in our study, which is closely associated with smoking, compared to that in a previous study. 9 Characteristically, in our study, a higher proportion of women with lung cancer were asymptomatic patients and their performance status was also better. Considering a previous study, which showed a high purchase rate of indemnity private health insurance among young women, 30 there might have been more active screening tests and asymptomatic visits by women with lung cancer. Thus, the proportion of stage I lung cancer was higher in women, which could be associated with better prognosis. However, as our study was not prospective or a randomized controlled trial, it could not verify the rate of screening and the type of insurance. Hence, further studies are needed in the future.
Previous research on differences in NSCLC prognosis based on sex yielded conflicting results; however, the majority of studies found that women had better prognosis than men. 5 , 29 , 31 , 32 According to the previous studies, women had a better outcome regardless of smoking status, clinical staging, histopathology, or treatment modalities. 8 , 26 , 33 In a Swedish nationwide cohort study, men with NSCLC had poorer prognosis even after adjusting for clinical factors such as education, marital status, performance status, comorbidity, and clinical stage. 11 In a United States cohort, women experienced significantly better survival for NSCLC at multiple time frames after controlling covariates like socioeconomic, clinical and comorbidity factors, compared to men. 29 The findings of these studies are in concordance with those of our study. In contrast, a United States‐based study using data from a large single center and the Surveillance, Epidemiology, and End Results (SEER) database showed that sex was not a significant prognostic factor after adjusting for confounding factors. 9 Similarly, an Australian prospective cohort study also demonstrated no significant survival difference according to sex after adjustment. However, this study used a database before tyrosine kinase inhibitors were commonly used and excluded patients under the age of 45 years; these factors might contribute in part to the sex survival rate. In our study, since more than 70% of the patients (higher than that in other studies) had advanced disease (stage III or IV), this might have affected survival according to sex. 12
EGFR mutation is common in women, 34 especially in never‐smoker Asian women. 35 In our study, EGFR mutation was significantly twice as common in women with stage IV adenocarcinoma. However, even after adjusting for both representative target mutations, EGFR mutation and ALK aberration, men with stage IV adenocarcinoma still showed an unfavorable outcome. This may be explained by the findings of a meta‐analysis that reported that the efficacy of EGFR‐tyrosine kinase inhibitor therapy for NSCLC was sex‐dependent, and women had more prolonged overall survival than men. 36 According to a study, among EGFR mutations, EGFR 19 deletion, known to have good response to treatment and better prognosis, was more common in women. 37 This might have affected the difference in survival rates by sex. Our study could not analyze the survival rate according to EGFR mutation type and a response difference for each target agent, therefore further research is needed.
This study had some limitations. First, information such as smoking status and subjective symptoms in the electronic medical record and self‐reported questionnaires might be incomplete. Second, disease‐free survival and progression‐free survival rates could not be analyzed. Third, comorbidities, familial history, socioeconomic status, education level, working status, and reasons for visiting the hospital were not included in our data, thus it was not possible to analyze the effects of these factors on the prognosis of lung cancer according to gender. In addition, the advantage of our analysis is the focus on the prognosis of lung cancer according to sex, even after adjusting for meaningful clinical factors, including EGFR mutation status in stage IV adenocarcinoma. Despite these limitations, to the best of our knowledge this is the first nationwide large‐scale study representing the Korean population to analyze lung cancer mortality according to sex.
To conclude, male patients with lung cancer had a poorer prognosis not only after variable adjustments for prognostic factors but also in the matched cohort. These results suggest that it might be necessary to consider sex differences in the prediction of prognosis when performing optimal management in lung cancer.
AUTHOR CONTRIBUTIONS
Conceptualization: D.S.J., J.W.K., H.R.K., S.Y.S., J.C.L., W.J.J., C.M.C., H.C.K. Methodology: D.S.J., J.W.K., S.G.K., C.M.C., H.C.K. Investigation: D.S.J., J.W.K., S.G.K., H.R.K., S.Y.S., J.C.L., W.J.J., C.M.C., H.C.K. Formal analysis: D.S.J., J.W.K., S.G.K., H.C.K. Resources: H.R.K., S.Y.S., J.C.L., W.J.J., C.M.C., H.C.K. Writing – original draft: D.S.J., J.W.K., H.C.K. Writing – review & editing: D.S.J., J.W.K., H.R.K., S.Y.S., J.C.L., W.J.J., C.M.C., H.C.K. Visualization: D.S.J., J.W.K., S.G.K., H.C.K. Supervision: H.R.K., S.Y.S., J.C.L., W.J.J., C.M.C., H.C.K. Funding acquisition: C.M.C.
CONFLICTS OF INTEREST
The authors confirm that there are no conflicts of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The data used in this study were provided by the Korean Association for Lung Cancer and the Ministry of Health and Welfare, Korea Central Cancer Registry.
Jeon DS, Kim JW, Kim SG, Kim HR, Song SY, Lee JC, et al. Sex differences in the characteristics and survival of patients with non‐small‐cell lung cancer: A retrospective analytical study based on real‐world clinical data of the Korean population. Thorac Cancer. 2022;13(18):2584–2591. 10.1111/1759-7714.14594
Da Som Jeon and Jin Woo Kim contributed equally as co‐first authors.
Funding information Korea Central Cancer Registry; Ministry of Health and Welfare; Korean Association for Lung Cancer
REFERENCES
- 1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elflein J. Incidence rates of lung cancer worldwide as of 2020, by region and gender. Health, Pharma & Medtech›State of Health 2020; Jan 26. https://www.statista.com/statistics/527677/lung-cancer-incidence-rates-worldwide-region-gender/
- 3. Mederos N, Friedlaender A, Peters S, Addeo A. Gender‐specific aspects of epidemiology, molecular genetics and outcome: lung cancer. ESMO Open. 2020;5(Suppl 4):e000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodriguez‐Lara V, Avila‐Costa MR. An overview of lung cancer in women and the impact of estrogen in lung carcinogenesis and lung cancer treatment. Front Med(Lausanne). 2021;17;8:600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sagerup CMT, Småstuen M, Johannesen TB, Helland Å, Brustugun OT. Sex‐specific trends in lung cancer incidence and survival: a population study of 40 118 cases. Thorax. 2011;66(4):301–7. [DOI] [PubMed] [Google Scholar]
- 6. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non‐small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duma N, Santana‐Davila R, Molina JR. Non‐small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–40. [DOI] [PubMed] [Google Scholar]
- 8. Ragavan M, Patel MI. The evolving landscape of sex‐based differences in lung cancer: a distinct disease in women. Eur Respir Rev. 2022;31(163):210100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stabellini N, Bruno DS, Dmukauskas M, Barda AJ, Cao L, Shanahan J, et al. Sex differences in lung cancer treatment and outcomes at a large hybrid academic‐community practice. JTO Clin Res Rep. 2022;3(4):100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi C‐M, Kim HC, Jung CY, Cho DG, Jeon JH, Lee JE, et al. Report of the Korean Association of Lung Cancer Registry (KALC‐R), 2014. Cancer Res Treat. 2019;51(4):1400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, Lambe M. Sex and survival in non‐small cell lung cancer: a nationwide cohort study. PLoS One. 2019;14(6):e0219206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu XQ, Yap ML, Cheng ES, Ngo PJ, Vaneckova P, Karikios D, et al. Evaluating prognostic factors for sex differences in lung cancer survival: findings from a large Australian cohort. J Thorac Oncol. 2022;17(5):688–99. [DOI] [PubMed] [Google Scholar]
- 13. Kim YC, Won YJ. The development of the Korean lung cancer registry (KALC‐R). Tuberc Respir Dis (Seoul). 2019;82(2):91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 15. Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner AD, Oertelt‐Prigione S, Adjei A, Buclin T, Cristina V, Csajka C, et al. Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol. 2019;30(12):1914–24. [DOI] [PubMed] [Google Scholar]
- 18. Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat. 2021;53(2):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat. 2003;21(3):229–39. [DOI] [PubMed] [Google Scholar]
- 21. Matikas A, Mistriotis D, Georgoulias V, Kotsakis A. Targeting KRAS mutated non‐small cell lung cancer: a history of failures and a future of hope for a diverse entity. Crit Rev Oncol Hematol. 2017;110:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Stapelfeld C, Neumann KT, Maser E. Different inhibitory potential of sex hormones on NNK detoxification in vitro: a possible explanation for gender‐specific lung cancer risk. Cancer Lett. 2017;405:120–6. [DOI] [PubMed] [Google Scholar]
- 23. Stapelfeld C, Dammann C, Maser E. Sex‐specificity in lung cancer risk. Int J Cancer. 2020;146(9):2376–82. [DOI] [PubMed] [Google Scholar]
- 24. Vu C, Lin S, Chang C‐F. Gender gaps in care: lung cancer screening criteria in women. Chest. 2019;156(4, Supplement):A407. [Google Scholar]
- 25. Araghi M, Fidler‐Benaoudia M, Arnold M, Rutherford M, Bardot A, Ferlay J, et al. International differences in lung cancer survival by sex, histological type and stage at diagnosis: an ICBP SURVMARK‐2 study. Thorax. 2022;77(4):378–90. [DOI] [PubMed] [Google Scholar]
- 26. Kinoshita FL, Ito Y, Morishima T, Miyashiro I, Nakayama T. Sex differences in lung cancer survival: long‐term trends using population‐based cancer registry data in Osaka. Jpn J Clin Oncol. 2017;47(9):863–9. [DOI] [PubMed] [Google Scholar]
- 27. Pitz MW, Musto G, Navaratnam S. Sex as an independent prognostic factor in a population‐based, non‐small cell lung cancer cohort. Can Respir J. 2013;20:30, 618691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guarga L, Ameijide A, Marcos‐Gragera R, Carulla M, Delgadillo J, Borràs JM, et al. Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain). Sci Rep. 2021;11(1):23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elkbuli A, Byrne MM, Zhao W, Sutherland M, McKenney M, Godinez Y, et al. Gender disparities in lung cancer survival from an enriched Florida population‐based cancer registry. Ann Med Surg. 2020;60:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. You C, Kwon YD. Gender Differences in Factors Affecting Purchase of Indemnity Private Health Insurance and Impact of Indemnity Private Health Insurance on Healthcare Use: Korea Health Panel Survey Data from 2010 to 2016. The Journal of the Korea Contents Association, 20(3):92–105. [Google Scholar]
- 31. Innos K, Padrik P, Valvere V, Aareleid T. Sex differences in cancer survival in Estonia: a population‐based study. BMC Cancer. 2015;15:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barquín M, Calvo V, García‐García F, Nuñez B, Sánchez‐Herrero E, Serna‐Blasco R, et al. Sex is a strong prognostic factor in stage IV non‐small‐cell lung cancer patients and should be considered in survival rate estimation. Cancer Epidemiol. 2020;67:101737. [DOI] [PubMed] [Google Scholar]
- 33. Guerreiro T, Forjaz G, Antunes L, Bastos J, Mayer A, Aguiar P, et al. Lung cancer survival and sex‐specific patterns in Portugal: a population‐based analysis. Pulmonology. 2021;S2531‐0437(21)00190‐2. [DOI] [PubMed] [Google Scholar]
- 34. Baiu I, Titan AL, Martin LW, Wolf A, Backhus L. The role of gender in non‐small cell lung cancer: a narrative review. J Thorac Dis. 2021;13(6):3816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chapman AM, Sun KY, Ruestow P, Cowan DM, Madl AK. Lung cancer mutation profile of EGFR, ALK, and KRAS: meta‐analysis and comparison of never and ever smokers. Lung Cancer. 2016;102:122–34. [DOI] [PubMed] [Google Scholar]
- 36. Xiao J, Zhou L, He B, Chen Q. Impact of sex and smoking on the efficacy of EGFR‐TKIs in terms of overall survival in non‐small‐cell lung cancer: a meta‐analysis. Front Oncol. 2020;10:1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Xie S, He B. Effect of EGFR gene polymorphism on efficacy of chemotherapy combined with targeted therapy for non‐small cell lung cancer in Chinese patients. Am J Cancer Res. 2019;9(3):619–27. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


