Abstract
Background
Fibulin‐4, namely, EFEMP2, is an essential matricellular protein associated with a variety of malignancies. The aim of this study was to explore the role of fibulin‐4 in the progression of esophageal squamous cell carcinoma (ESCC), as well as its effect on ESCC sensitivity to apatinib treatment.
Methods
The expression of fibulin‐4 in ESCC tissues and cell lines was detected. Stably transfected ESCC cells were established by transducing lentiviral vectors for silencing or overexpressing the fibulin‐4 gene into ESCC cells, and a subcutaneous xenograft tumor model of ESCC in mice was successfully established. IHC, RT–qPCR and western blotting were used to detect the expression of related genes and proteins. The CCK8 assay, EdU cell proliferation assay, wound healing assay, transwell assay and flow cytometry were used to evaluate the proliferation, invasion, migration and apoptosis of ESCC cells. After mice were sacrificed, the transplanted tumors were resected, and their volumes were measured.
Results
The expression of fibulin‐4 was significantly increased in both ESCC tissues and cell lines, and the high expression was closely related to the poor clinicopathological features. Downregulation of fibulin‐4 inhibited the proliferation, invasion and migration of ESCC cells in vitro and in vivo. Meanwhile, fibulin‐4 knockdown inhibited autophagy of tumor cells by activating the Akt–mTOR signaling pathway and significantly promoted apatinib‐induced apoptosis of ESCC cells.
Conclusion
Our study showed that fibulin‐4 is an oncogene that can promote ESCC progression and inhibit apoptosis. Downregulation of fibulin‐4 enhances the sensitivity of ESCC cells to apatinib by inhibiting cellular protective autophagy through activating the Akt–mTOR signaling pathway.
Keywords: Akt–mTOR signaling pathway, apatinib, autophagy, esophageal squamous cell carcinoma, fibulin‐4
We examined the expression and function of fibulin‐4 in the development of ESCC for the first time and further explored its role and mechanism in the autophagy of ESCC cells as well as the effect of autophagy on the sensitivity of ESCC tumor cells to apatinib. We found, for the first time, that fibulin‐4 was highly expressed in ESCC, and the high expression of fibulin‐4 was closely related to the poor clinicopathological features. Upregulation of fibulin‐4 promoted the proliferation, invasion and migration of ESCC cells but inhibited their apoptosis. Highly expressed fibulin‐4 also promoted autophagy in ESCC cells through the Akt–mTOR signaling pathway, while silencing fibulin‐4 inhibited autophagy and promoted the sensitivity of ESCC cells to apatinib, thereby promoting apatinib‐induced apoptosis. We predicted that fibulin‐4 could be used as a specific biomarker to predict poor prognosis of ESCC and provided a basis for its innovative application in anti‐ESCC treatment strategies.
INTRODUCTION
Esophageal cancer is the seventh leading cause of cancer and the sixth leading cause of cancer‐related death worldwide. China accounts for more than half of all new cases and deaths from esophageal cancer worldwide, and it is estimated that China will have approximately 346 000 new cases and 323 000 deaths by 2022. 1 Esophageal squamous cell carcinoma (ESCC) is the main pathological subtype in China, accounting for more than 90% of all esophageal cancer cases. 2 Although remarkable progress has been made in the treatment of esophageal cancer, the 5‐year relative survival rate of patients with esophageal cancer is still very low, only approximately 20%. 3 In recent years, targeted drugs, as new therapeutic agents, have become a safe and effective treatment strategy for esophageal cancer. 4 Among them, apatinib, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor 2 (VEGFR‐2), has been proven to play an important role in improving treatment outcomes in patients with advanced ESCC. 5 , 6 , 7 , 8 , 9 However, most reports about apatinib in ESCC treatment lack relevant mechanistic studies. Its adverse reactions, optimal dose and effective combination with chemotherapy still need to be further explored.
In recent years, the role of autophagy in tumor progression and treatment resistance has attracted increasing attention. 10 , 11 Autophagy is a process of self‐digestion by lysosomal‐mediated degradation of cellular components, such as defective organelles and misfolded protein aggregates. Autophagy specifically removes abnormal proteins from cells, engulfs damaged organelles, and maintains cell homeostasis. 12 , 13 , 14 Increasing evidence indicates that autophagy‐degraded proteins and organelles can be used as renewable energy and raw materials to participate in tumorigenesis and anticancer drug sensitivity or resistance. 11 Autophagy is also called type II programmed cell death, and there is a complex interaction between autophagy and apoptosis. A recent study showed that apatinib induces autophagy and apoptosis in ESCC cells. Inhibition of autophagy by the autophagy inhibitor chloroquine (CQ) promoted apatinib‐induced apoptosis of ESCC cells through the IRE‐1α‐Akt–mTOR signaling pathway, and CQ combined with apatinib significantly enhanced the sensitivity of ESCC cells to paclitaxel. 15
Fibulin‐4, also known as epidermal growth factor‐containing fibulin‐like extracellular matrix protein 2 (EFEMP2), is a member of the fibulin family. It is commonly found in organs and tissues rich in elastic fibers and is essential for the structural stability of the extracellular matrix, the formation of elastic fibers and the development of connective tissues. Abnormal regulation of fibulin‐4 is closely associated with a series of tumors. 16 , 17 , 18 , 19 , 20 , 21 However, the function of fibulin‐4 in ESCC has not been reported thus far. A previous study has found that fibulin‐4 promotes metastasis and invasion of osteosarcoma also through the PI3K‐Akt–mTOR pathway. 19 As a conserved serine/threonine protein kinase, mTOR is known to be the confluence of upstream pathways that regulate cell growth, survival, proliferation, motility and autophagy. mTOR kinase is easily controlled and driven by oncogenic nodes in many important pathways including the Akt–mTOR signaling pathway. 22 Therefore, for the first time, this study aimed to investigate the function of fibulin‐4 in the malignant biological behavior and tumor progression of ESCC. In addition, the role and mechanism of fibulin‐4 in regulating autophagy in ESCC cells and affecting the sensitivity of ESCC cells to apatinib were explored through the Akt–mTOR signaling pathway.
METHODS
Tumor tissue samples
In total, 125 ESCC tissue samples (including tumor and normal adjacent tissues) were collected from Shandong Provincial Hospital affiliated to Shandong First Medical University from 2010 to 2017. All ESCC patients were diagnosed according to the eighth edition of the tumor‐node‐metastasis (TNM) classification for esophageal cancer as recommended by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC). The inclusion criteria were as follows: (i) underwent surgery, and the postoperative pathology was ESCC; (ii) no preoperative radiotherapy or chemotherapy was performed; and (iii) the pathological margin was negative.
Cell lines
Four ESCC cell lines (Eca109, KYSE30, KYSE150 and KYSE510) were purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. All cells were cultured in RPMI‐1640 medium (Kibbutz Beit HaEmek BI) containing 10% fetal bovine serum (FBS) in a 37°C, 5% CO2 incubator with no more than 15 passages.
Immunohistochemistry (IHC)
The tissue was fixed with formalin, embedded in paraffin, and then cut into 5 μm sections. After dewaxing, rehydration and antigen retrieval, sections were stained with the SP method. All sections were treated with antifibulin‐4 primary antibody (Abcam) (1:3000) at 4°C overnight, labeled with secondary antibody for 30 min at 37°C, and stained with DAB and hematoxylin. Brown granules in cells were positive for fibulin‐4 expression. The proportion score of positively stained cells was 0–4: 0 (0%–5%), 1 (6%–25%), 2 (26%–50%), 3 (51%–75%), and 4 (76%–100%). The intensity score of positive staining was 0–3: 0 (negative), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The total staining score was calculated by multiplying the proportion score of positively stained cells by the intensity score of positive staining and was defined as 0–7 for low expression and 8–12 for high expression. Two pathologists scored each section independently. If there was a difference in scores, an agreement was reached through discussion.
Lentiviral transfection
Eca109 and KYSE30 cells at exponential phase were cultured in 24‐well plates for 24 h, and the lentivirus titer was determined. The lentivirus vectors with small hairpin RNA (shRNA) and cDNA targeting the fibulin‐4 gene and corresponding negative control lentivirus vectors (Yijin Biotech) were transduced into Eca109 and KYSE30 cells, respectively. Infection quantity was determined by multiplicity of infection (MOI), with a value of 50 for RNA interference (RNAi) and 100 for overexpression. The stably transfected cell lines were screened with 5 μg/ml puromycin.
Real‐time quantitative reverse transcription polymerase chain reaction (qRT–PCR)
Total RNA was extracted from cells by RNAiso Plus (TaKaRa) and reverse transcribed into complementary DNA (cDNA) by an RT reagent Kit (TaKaRa). qPCR was performed using SYBR Green Realtime PCR Master Mix (TaKaRa) on a LightCycler 480 System. β‐actin was used as an internal reference gene. Specific forward and reverse primers were as follows: fibulin‐4, GAGTGTCTGACCATCCCTGAG (forward primer) and GCGTGTAGGTTGATGAC (reverse primer); β‐actin, CTACCTCATGAGATCCTCACCCCGA (forward primer) and TTCTCCTTAATGCGCGCT (reverse primer); both were purchased from TaKaRa. The two‐step PCR procedure was run to generate a standard curve. The relative expression levels of target genes were analyzed by the 2−ΔΔCt method.
Western blot
Tissues and cells were lysed with RIPA lysis buffer containing PMSF to extract proteins. Protein samples were separated by SDS–PAGE and transferred to PVDF membranes. After sealing with 5% BSA for 1 h, the membrane was incubated with primary antibodies, including anti‐fibulin‐4, anti‐LC3B‐I/II (Abcam), anti‐P62, anti‐Beclin‐1, anti‐Akt1/2/3, anti‐mTOR, anti‐p‐Akt1, anti‐p‐mTOR, and anti‐GAPDH (Huabio), overnight at 4°C. The membrane was then incubated with the corresponding secondary antibody at room temperature for 1 h, and developed by enhanced chemiluminescence (ECL). The gray values of the protein bands were measured by ImageJ (National Institutes of Health), and the relative expression of target proteins was analyzed by GAPDH as an internal reference.
EdU cell proliferation assay
In total, 5000 cells transfected with lentiviral vectors at exponential phase were inoculated in 96‐well plates and cultured for 24 h. It was later marked by EdU labeling, cell immobilization, Apollo and DNA staining, and images were acquired under an inverted fluorescence microscope.
CCK‐8 analysis
In total, 5000 cells transfected with lentiviral vectors at exponential phase were inoculated in 96‐well plates for 24, 48, 72 and 96 h. The cells were then treated with CCK‐8 for 1 h at 37°C, and the absorbance of each well was measured at 450 nm using a Thermo Scientific Varioskan Flash microplate reader.
Wound healing assay
When the confluency of cells transfected with lentiviral vectors reached 90%–95%, one linear scratch was formed on the monolayer cells using the tip of a pipette. The monolayer cells were cultured in serum‐free medium for 24 h, and cell migration was measured under an optical microscope. ImageJ was used to measure the wound area at the same location. Cell mobility was calculated as follows: cell mobility (%) = (initial wound area − wound area 24 h later)/initial wound area × 100%.
Cell invasion and migration assay
The 24‐well transwell chamber consisted of an upper and lower compartment separated by a polycarbonate membrane with an 8 μm aperture. Polycarbonate membranes coated with Matrigel matrix (Corning) were used to examine cell invasion, and uncoated membranes were used to examine cell migration. The solution of cells transfected with lentiviral vectors at exponential phase was added to the top compartment of the transwell chamber, and medium containing 20% FBS was added to the bottom of the chamber. After 12 h of culture at 37°C and 5% CO2, the cells passing through the polycarbonate membrane were fixed with 4% paraformaldehyde and stained with crystal violet. Then, the number of stained cells migrating or invading across the polycarbonate membrane into the lower compartment was calculated under an inverted microscope.
Detection of apoptosis by flow cytometry
Apoptosis was detected by flow cytometry using a PE annexin‐V apoptosis detection kit I (BD Biosciences). The cells to be tested were digested by trypsin to prepare a cell suspension. After double staining with P‐phycoglobin (PE) and 7‐aminoninomycetin D (7‐AAD), the samples were analyzed by flow cytometry and FlowJo.
ESCC xenograft model
Thirty‐six female BALB/C mice aged 4–6 weeks and weighing 18–22 g were randomly divided into a downregulated fibulin‐4 group and a negative control group. They were reared in a specific pathogen‐free (SPF) environment at 20%–26°C and 40%–60% humidity. Then, the Eca109 cell suspension transfected with the fibulin‐4 shRNA lentivirus vector was subcutaneously inoculated into the right axilla of mice. Tumor size was measured every 5 days when the xenograft tumors grew to 100 mm3 in mice. All mice were sacrificed 4 weeks after inoculation. The subcutaneous tumor was completely removed, and its volume was measured. The volume was calculated as follows: V (mm3) = maximum diameter (mm) × minimum diameter2 (mm2) × 1/2.
Statistical analysis
GraphPad Prism 8 was used for statistical analysis. The data are expressed as the means ± standard deviation (SD). Significant differences between two groups were analyzed by Student's t test. Significant differences among three or more groups were analyzed by one‐way ANOVA. The correlation between the expression of fibulin‐4 and clinical features was verified by the chi‐square test. p < 0.05 was considered statistically significant.
RESULTS
Expression of fibulin‐4 in ESCC tissue
IHC and western blot results showed that the expression of fibulin‐4 in ESCC tissues was significantly higher than that in normal esophageal tissues (Figure 1a,b). There was no significant difference in the expression of fibulin‐4 between patients aged ≤60 years and those aged >60 years (p > 0.05). There was no significant difference between male and female patients (p > 0.05). The expression of fibulin‐4 was not related to the pathological classification of ESCC, such as medullary type, mushroom umbrella type, ulcer type and narrow type (p > 0.05). However, it was closely correlated with TNM clinical staging and lymph node metastases (p < 0.05) (Table 1).
FIGURE 1.
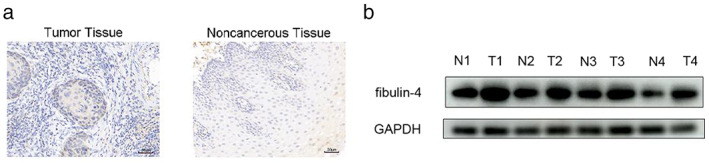
Expression of fibulin‐4 in esophageal squamous cell carcinoma (ESCC) samples. (a) Fibulin‐4 expression in ESCC and noncancerous tissue samples detected by immunohistochemistry. (b) Fibulin‐4 expression in four pairs of representative ESCC and noncancerous tissue samples detected by western blot.
TABLE 1.
Relationship between the expression of fibulin‐4 and the clinicopathological features of patients with ESCC
| Clinical pathological features | N | Fibulin‐4 | χ 2 | p‐value | |
|---|---|---|---|---|---|
| High(%) | Low(%) | ||||
| Age | 1.593 | 0.207 | |||
| ≤60 | 52 | 37 (71.2) | 15 (28.8) | ||
| >60 | 73 | 59 (80.8) | 14 (19.2) | ||
| Gender | 1.305 | 0.253 | |||
| Male | 76 | 61 (80.3) | 15 (19.7) | ||
| Female | 49 | 35 (71.4) | 14 (28.6) | ||
| TNM clinical staging | 15.623 | 0.000 | |||
| Stage I and II | 59 | 36 (61.0) | 23 (39.0) | ||
| Stage III and IV | 66 | 60 (90.9) | 6 (9.1) | ||
| Pathological classification | 4.507 | 0.212 | |||
| Medullary type | 34 | 22 (64.7) | 12 (35.3) | ||
| Mushroom umbrella type | 33 | 27 (81.8) | 6 (18.2) | ||
| Ulcer type | 28 | 24 (85.7) | 4 (14.3) | ||
| Narrow type | 30 | 23 (76.7) | 7 (23.3) | ||
| Lymph node metastases | 11.744 | 0.001 | |||
| Negative | 60 | 38 (63.3) | 22 (36.7) | ||
| Positive | 65 | 58 (89.2) | 7 (10.8) | ||
Note: The expression of fibulin‐4 in ESCC was not related to the age, sex and pathological classification of the patients (p > 0.05), but closely correlated with TNM clinical staging and lymph node metastases (p < 0.05).
Fibulin‐4 promotes proliferation, invasion and migration but inhibits apoptosis of ESCC cells
The expression of fibulin‐4 in the Eca109, KYSE30, KYSE150 and KYSE510 cell lines was detected by qRT–PCR. As the expression of fibulin‐4 in the Eca109 and KYSE30 cell lines was higher than that in the KYSE150 and KYSE510 cell lines, we selected the Eca109 and KYSE30 cell lines for further experiments (Figure 2a). First, we transduced shRNA or cDNA lentiviral vectors targeting fibulin‐4 into Eca109 and KYSE30 cells, and their transfection efficiency was detected by Western blot (Figure 2b). Then, the results of CCK‐8 assay and EdU cell proliferation assay showed that, compared with cells in the corresponding blank and negative control groups, silencing of fibulin‐4 significantly decreased the proliferation of Eca109 and KYSE30 cells, while overexpression of fibulin‐4 significantly increased the proliferation of Eca109 and KYSE30 cells (Figure 2c,d). Subsequently, the results of the wound healing test showed that, compared with cells in the corresponding blank and negative control groups, silencing of fibulin‐4 significantly delayed wound healing, while overexpression of fibulin‐4 significantly promoted wound healing (Figure 2e,f). The results of the transwell assay showed that, compared with cells in the corresponding blank and negative control groups, silencing of fibulin‐4 significantly reduced cell penetration across the membrane both in invasion and migration, while overexpression of fibulin‐4 significantly enhanced the transmembrane ability of cells either in invasion or migration (Figure 3a,b). Finally, the results of flow cytometry showed that, compared with cells in the corresponding blank and negative control groups, silencing of fibulin‐4 significantly increased the apoptosis of Eca109 and KYSE30 cells, while overexpression of fibulin‐4 significantly reduced the apoptosis of Eca109 and KYSE30 cells (Figure 3c,d).
FIGURE 2.
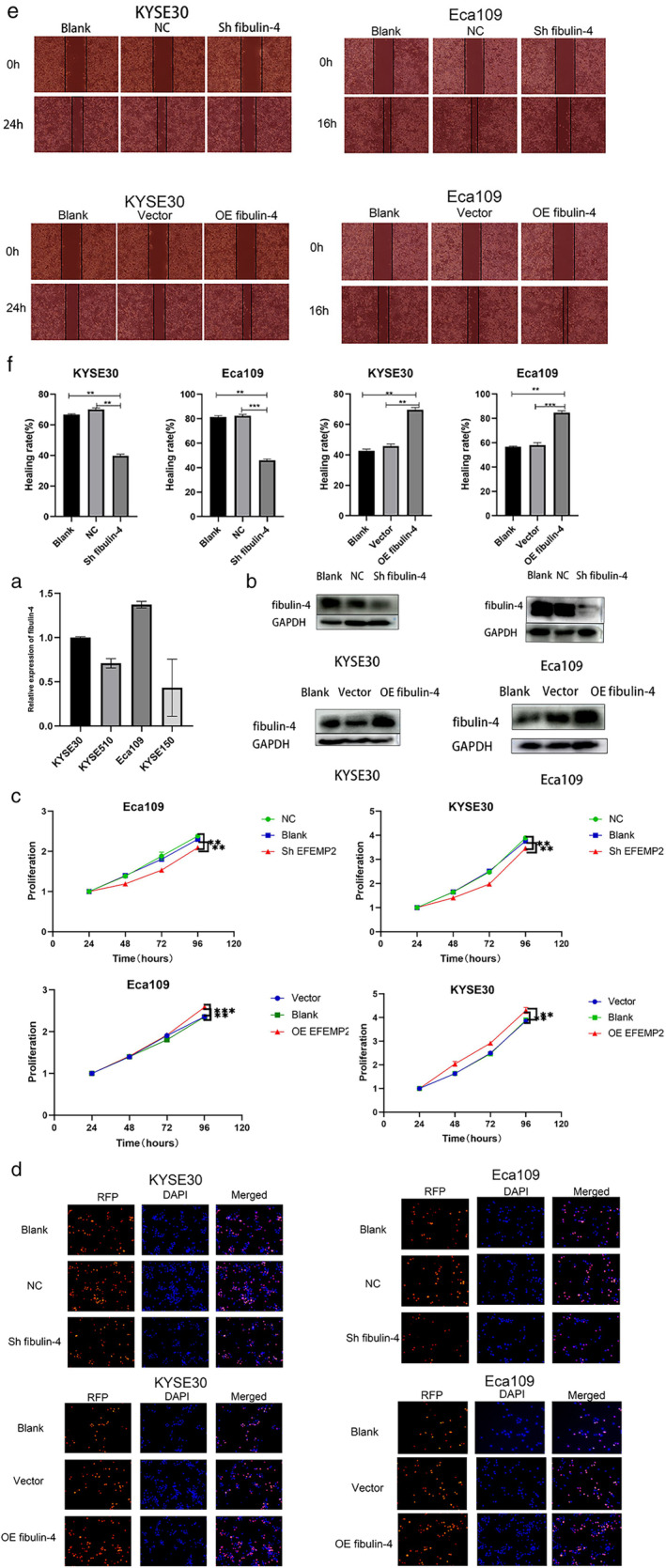
Fibulin‐4 promotes proliferation and migration in esophageal squamous cell carcinoma (ESCC) cells. (a) Fibulin‐4 expression in the KYSE30, KYSE510, Eca109, and KYSE150 cell lines was detected by qRT–PCR. (b) Transfection efficiency of the fibulin‐4 silencing lentivirus or overexpression lentivirus was detected by western blot. (c) Eca109 and KYSE30 cells treated or left untreated were seeded at 5000 cells/well (96‐well plate). OD values were measured after 24, 48, 72 and 96 h. Proliferation was calculated as OD (24, 48, 72 and 96 h)/OD (24 h). (d) Eca109 and KYSE30 cells treated or left untreated were seeded at 5000 cells/well (96‐well plate). Cells were later marked by EdU, immobilized, Apollo stained, and stained with DNA. Images were acquired under an inverted fluorescence microscope. (e) Cells were cultured in FBS‐free medium for 24 h after the “wound” was made, and images were acquired with an optical microscope. (f) The migration rate of the cells was calculated from three independent experiments. The error bars indicate the means ± SD averaged from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NC: Negative control of silencing lentiviral vector; vector: Negative control of overexpression lentiviral vector.
FIGURE 3.
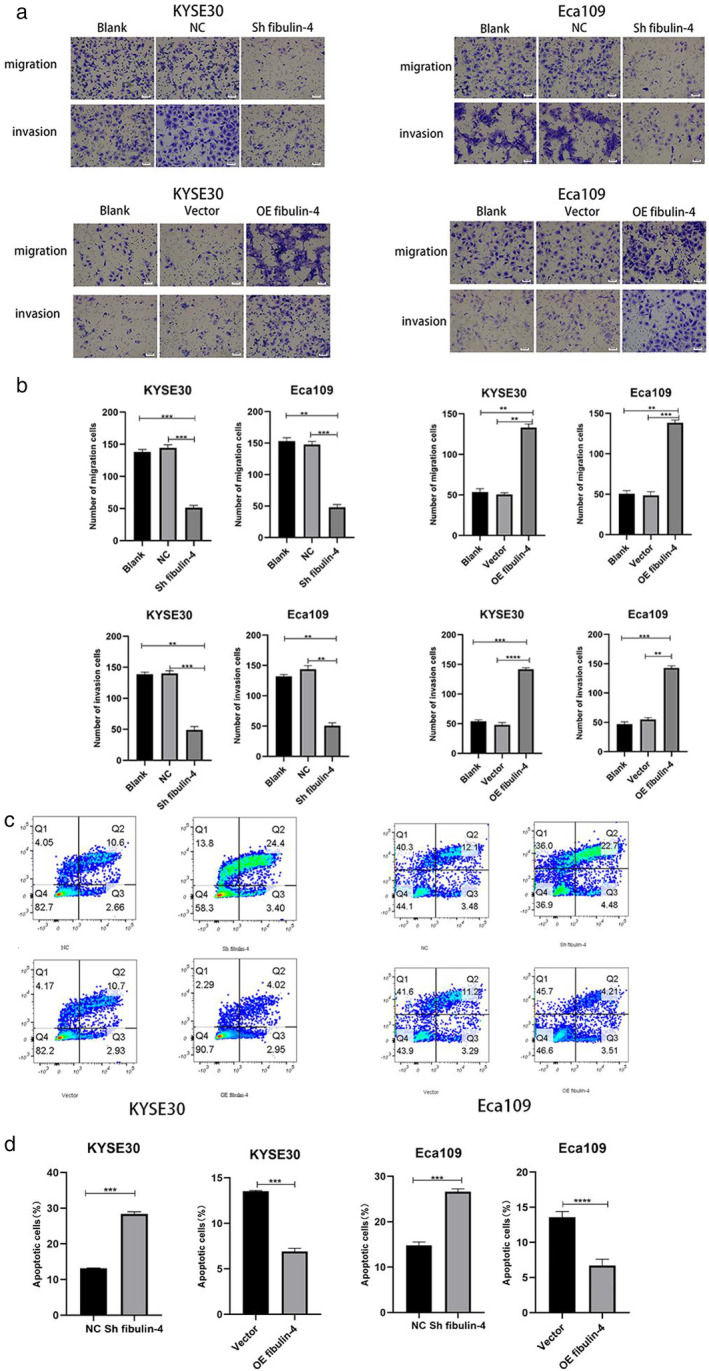
Fibulin‐4 promotes invasion and migration but inhibits apoptosis in esophageal squamous cell carcinoma (ESCC) cells. (a) Eca109 or KYSE30 cells that migrated or invaded across the membrane were fixed and stained. (b) Cells that passed through the membrane were counted in three random fields. (c) Eca109 and KYSE30 cells treated or left untreated were analyzed by flow cytometry. The percentage of PE‐ and/or 7‐AAD‐positive cells is included in the panels. (d) The results are expressed as the percentages (mean standard deviation [SD] of three repeated experiments) of apoptotic/necrotic cells. The error bars indicate the means ± SD averaged from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. NC: Negative control of silencing lentiviral vector; vector: Negative control of overexpression lentiviral vector.
Fibulin‐4 promotes autophagy in ESCC cells
To further evaluate the role of fibrin‐4 in autophagy, we detected the levels of autophagy‐related proteins in Eca109 and KYSE30 cells using western blotting. Compared with cells in the corresponding blank and negative control groups, the expression level of the autophagy substrate p62 in the fibulin‐4‐silenced cells was significantly increased, and the expression levels of the autophagy‐related proteins Beclin‐1 and LC3B‐II were significantly decreased, suggesting that the level of autophagy was significantly reduced (Figure 4a–d). In contrast, the expression level of p62 in fibulin‐4‐overexpressing cells was significantly decreased, and the expression levels of Beclin‐1 and LC3B‐II were significantly increased, suggesting that the level of autophagy was significantly increased (Figure 4e–h). After 3‐MA (autophagy inhibitor) (Selleck Chemicals) was added to Eca109 cells with fibulin‐4 overexpression, the expression level of p62 was significantly increased, and the expression levels of Beclin‐1 and LC3B‐II were significantly decreased, indicating that autophagy was effectively inhibited (Figure 4i,j).
FIGURE 4.
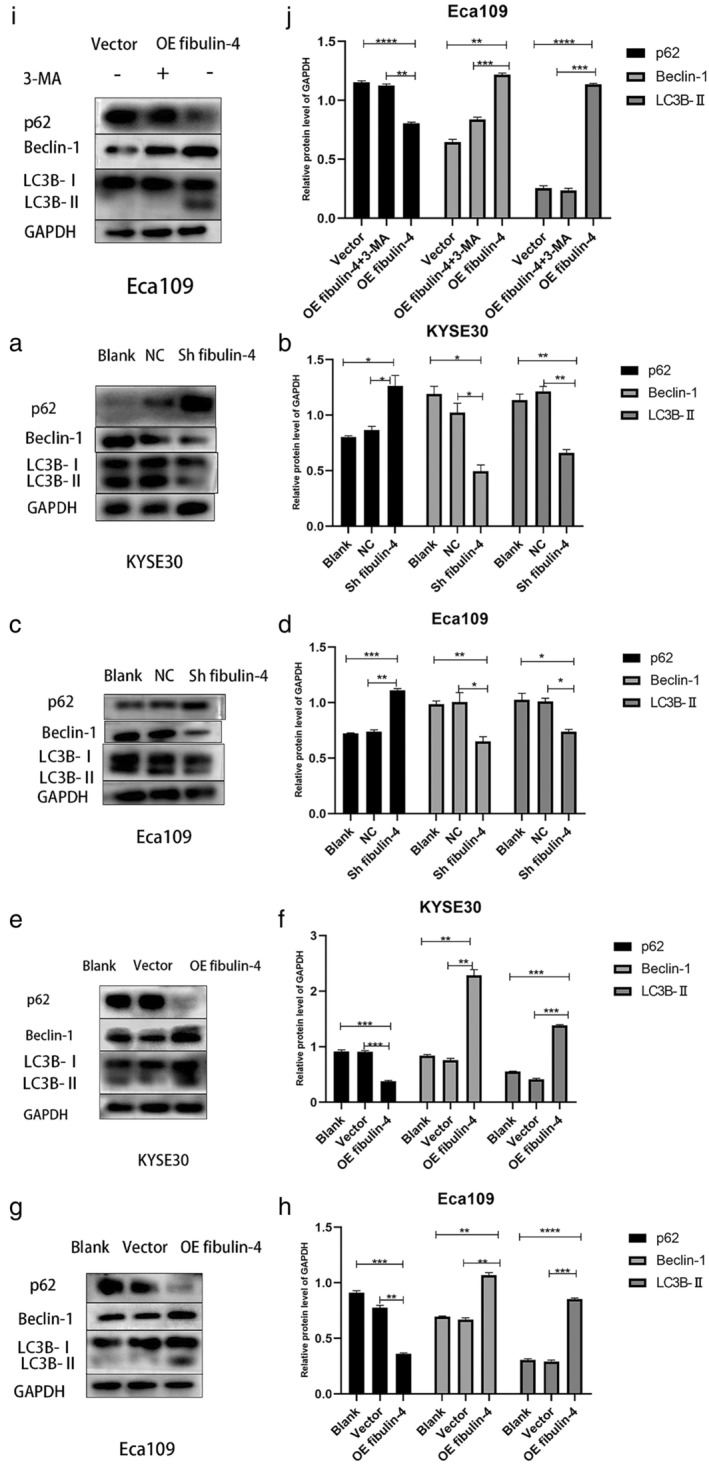
Fibulin‐4 regulates autophagy in esophageal squamous cell carcinoma (ESCC) cells. (a, b, c, d, e, f, g, h) Markers of autophagy were detected by western blot. Quantitative analysis of autophagy‐related protein expression with normalization to GAPDH. (i, j) 3‐MA (autophagy inhibitor) was added to Eca109 cells with fibulin‐4 overexpression. Markers of autophagy were detected by western blot. Quantitative analysis of autophagy‐related protein expression with normalization to GAPDH. The error bars indicate the means ± SD averaged from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Fibulin‐4 promotes autophagy by inhibiting the Akt–mTOR signaling pathway in ESCC cells
To clarify the regulatory mechanism of fibulin‐4 on autophagy, western blotting was used to detect changes in the Akt–mTOR signaling pathway in Eca109 cells. Compared with cells in the blank and negative control groups, the expression levels of p‐Akt and p‐mTOR, as well as the ratios of p‐Akt/Akt and p‐mTOR/mTOR in the fibulin‐4‐silenced cells, were significantly increased, indicating that the Akt–mTOR signaling pathway was activated. At the same time, the expression level of p62 was significantly increased in those cells, and the expression levels of Beclin‐1 and LC3B‐II were significantly decreased, suggesting that autophagy was significantly decreased upon activation of the Akt–mTOR signaling pathway. After the fibulin‐4‐silenced cells were treated with triciribine (Akt inhibitor) (Beyotime), the expression levels of p‐Akt and p‐mTOR, along with the ratios of p‐Akt/Akt and p‐mTOR/mTOR, were significantly decreased, and this was accompanied by a decreased level of p62 and an increased level of Beclin‐1 and LC3B‐II (Figure 5a–d), indicating that fibulin‐4 knockdown inhibited ESCC cell autophagy by phosphorylating and activating the Akt–mTOR signaling pathway.
FIGURE 5.
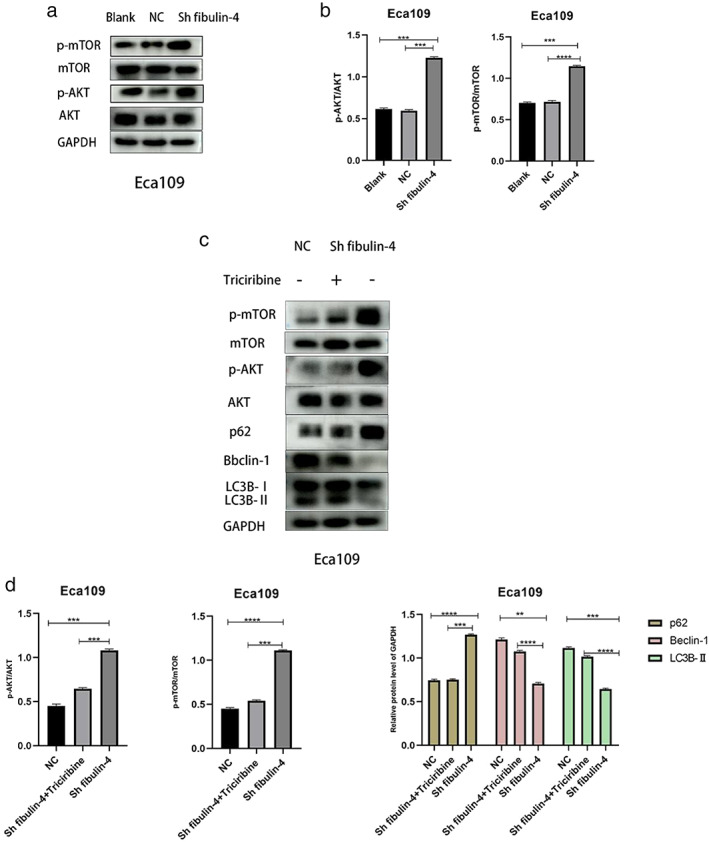
Downregulation of fibulin‐4 inhibits autophagy by activating the AKT–mTOR signaling pathway in esophageal squamous cell carcinoma (ESCC) cells. (a, b) The effects of fibulin‐4 silencing on AKT and mTOR phosphorylation in Eca109 cells were detected by western blot. The phosphorylation levels of AKT and mTOR were compared with the overall levels. (c, d) Triciribine (AKT inhibitor) was added to fibulin‐4‐silenced Eca109 cells. Western blot was used to detect AKT, mTOR and markers of autophagy. The phosphorylation levels of AKT and mTOR were compared with the overall levels. Quantitative analysis of autophagy‐related protein expression with normalization to GAPDH. The data are presented as the means ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Apatinib is more effective in ESCC cells with low fibulin‐4 expression
To investigate whether the effect of apatinib (Hengrui Pharmaceuticals) on ESCC is affected by the level of autophagy, apoptosis induced by apatinib was detected by flow cytometry in Eca109 and KYSE30 cells. The results showed that the apoptosis rate induced by apatinib in fibulin‐4‐silenced cells was significantly higher than that in fibulin‐4‐overexpressing cells (Figure 6a–c).
FIGURE 6.
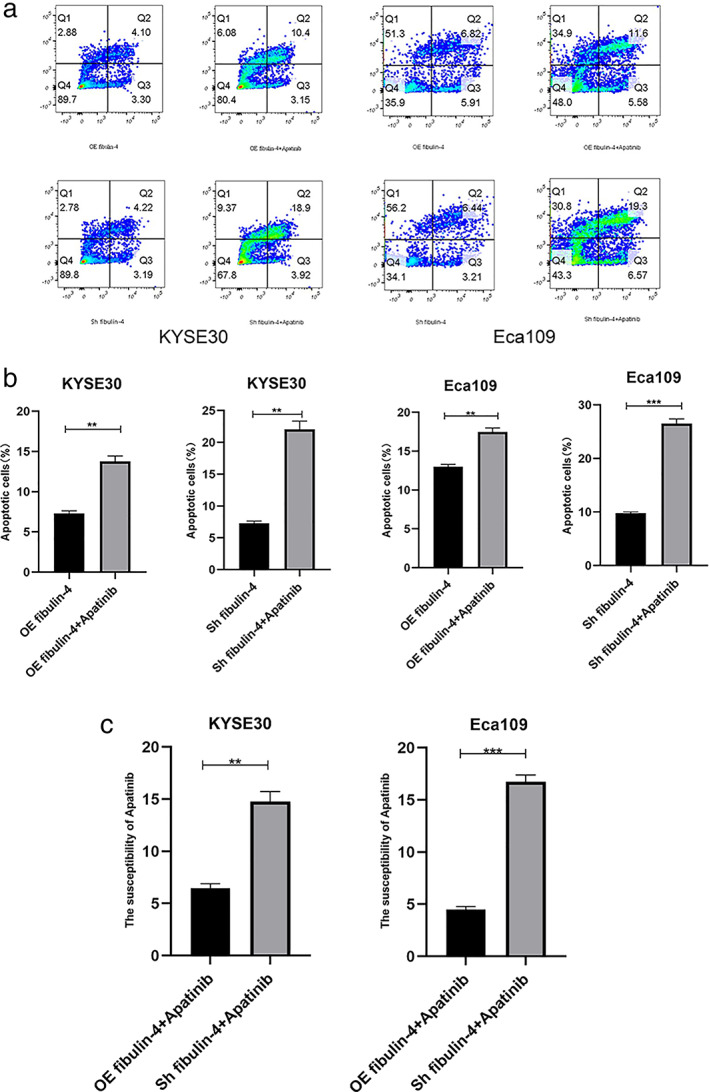
Apatinib is more effective in esophageal squamous cell carcinoma (ESCC) cells with low fibulin‐4 expression. (a) Eca109 and KYSE30 cells treated with apatinib or left untreated were analyzed by flow cytometry. The percentage of PE‐ and/or 7‐AAD‐positive cells is included in the panels. (b) The results are expressed as the percentages (mean standard deviation [SD] of three repeated experiments) of apoptotic/necrotic cells. (c) Effects of apatinib‐induced apoptosis. The error bars indicate the means ± SD averaged from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Downregulation of fibulin‐4 inhibits the growth of xenograft tumors
To further evaluate the effect of fibulin‐4 downregulation on ESCC tumor proliferation in vivo, Eca109 cells transfected with fibulin‐4 shRNA or negative control were subcutaneously inoculated into nude mice to establish xenograft models. Compared with tumors formed by negative control cells, both the volume and growth rate of tumors formed by fibulin‐4‐silenced cells were significantly decreased (Figure 7a,b).
FIGURE 7.
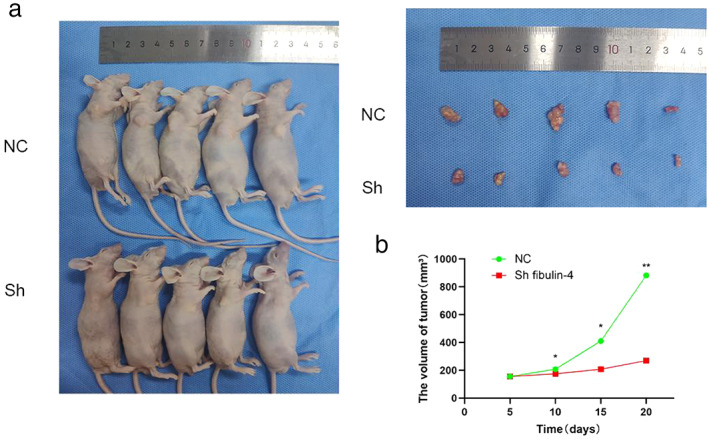
Downregulation of fibulin‐4 inhibits the growth of ESCC xenograft tumors in mice. (a) Nude mice were executed after 20 days since being subcutaneously inoculated. (b) The results are expressed as tumor volume (mean ± SD). *p < 0.05, **p < 0.01.
DISCUSSION
ESCC is a serious life‐threatening malignant tumor with high morbidity, high mortality and early metastasis. Due to the high aggressiveness of the tumor and the limited efficacy of traditional antitumor drugs, the treatment of patients with advanced ESCC remains unsatisfactory. 3 , 23 In this study, we examined the expression and function of fibulin‐4 in the development of ESCC for the first time and further explored its role and mechanism in the autophagy of ESCC cells as well as the effect of autophagy on the sensitivity of ESCC tumor cells to apatinib.
As a member of the fibulin family, fibulin‐4 plays an irreplaceable role in cell morphological maintenance, growth and elasticity in many human organs. Abnormal regulation of fibulin‐4 is associated with a series of tumors. In cervical cancer, 16 ovarian cancer, 17 glioma 18 and osteosarcoma, 19 the high expression of fibulin‐4 is closely related to tumor development and poor prognosis. However, in endometrial cancer 20 and lung cancer, 21 fibulin‐4 has been shown to inhibit tumor invasion and metastasis. These conflicting findings reflect the different roles of fibulin‐4 in different tumor environments. Thus far, the role of fibulin‐4 in the pathogenesis and development of ESCC has not been reported. In this study, we found, for the first time, that fibulin‐4 was highly expressed in ESCC tissues, and its high expression was closely related to clinical tumor stage and lymph node metastasis. We further examined the expression of fibulin‐4 in ESCC cells and investigated whether downregulation or upregulation of fibulin‐4 could affect tumor growth or malignant behavior. The results showed that downregulation of fibulin‐4 significantly suppressed the proliferation, invasion and migration of ESCC cells but promoted their apoptosis in vitro. In addition, we established a xenograft model of ESCC in nude mice and found that downregulation of fibulin‐4 significantly inhibited the growth of ESCC tumors in vivo. Therefore, we speculated that fibulin‐4 could be used as a specific biomarker to predict poor prognosis of ESCC.
Interestingly, we found that the level of autophagy in ESCC cells also changed. Is the effect of fibulin‐4 on the malignant behavior of ESCC mentioned above directly related to changes in autophagy levels? Autophagy is the main regulator of p62 levels. One consequence of the inhibition of autophagy in tumor cells is the continuous increase in p62 levels, which may affect tumor progression and drug resistance in some cases. 24 , 25 A recent study pointed to a symbiotic collaboration between tumor cells and adipocytes in metabolism, in which p62 deficiency triggers a general shutdown of energy utilization pathways in adipose tissue by inhibiting mTORC1. This provides more nutrients for fatty acid oxidation in tumor cells to support their aggressive malignant phenotypes. 26 In our study, when fibulin‐4 was upregulated in ESCC cells, we found that the expression of p62 was significantly decreased, while the expression of the autophagy‐related proteins Beclin‐1 and LC3B‐II was significantly increased, indicating that the autophagy level of tumor cells was enhanced. Subsequently, the autophagy inhibitor 3‐MA was used to verify whether the changes in the autophagy level of ESCC cells were caused by the regulation of fibulin‐4. When 3‐MA was added to ESCC cells with fibulin‐4 overexpression, we found that the elevated autophagy level was effectively inhibited. These results showed that the high expression of fibulin‐4 was significantly correlated with the increased level of autophagy in ESCC cells and speculated that autophagy was involved in the process of fibulin‐4 affecting the malignant biological behavior of ESCC.
Autophagy both promotes tumors and enhances the resistance of tumor cells to chemotherapy drugs. The role of autophagy in acquired resistance to targeted therapies in tumor cells has been a focus of recent studies. 10 , 11 , 27 , 28 Currently, targeted therapy for esophageal cancer involves a variety of drugs, but most of them are still in the stage of clinical or basic research and lack ideal therapeutic targets. Fortunately, apatinib, an anti‐VEGFR‐2 drug, has made significant progress in a series of clinical trials in the treatment of esophageal cancer. 8 , 9 , 29 , 30 Camrelizumab in combination with apatinib and chemotherapy has shown encouraging efficacy and safety in first‐line therapy for patients with unresectable locally advanced ESCC. 8 One recent study showed that apatinib inhibits the proliferation of ESCC cells and induces their apoptosis and also activates endoplasmic reticulum stress, which triggers protective autophagy. Inhibition of autophagy by CQ can enhance apatinib‐induced apoptosis through the IRE‐1α‐Akt–mTOR signaling pathway, thus confirming that inhibition of autophagy can promote the therapeutic effect of apatinib on ESCC. 15 Importantly, in our study, we aimed to explore the potential therapeutic effects and related molecular mechanisms of fibulin‐4 on ESCC and to elucidate the sensitization of fibulin‐4 on apatinib‐treated ESCC cells. Our results confirmed that apatinib‐induced apoptosis was significantly increased in ESCC cells with low fibulin‐4 expression and further speculated that silencing fibulin‐4 may significantly promote apatinib‐induced apoptosis of ESCC cells by inhibiting autophagy through activation of the Akt–mTOR signaling pathway.
Autophagy is finely regulated by autophagy‐related genes, which involve multiple signaling pathways, among which the AMPK and mTOR signaling pathways are the core regulatory pathways. AMPK promotes autophagy, while mTOR inhibits autophagy. In addition, there is a complex interaction between autophagy and apoptosis, which can be activated by multiple stress stimuli, share multiple regulatory molecules, and even coordinate and transform with each other. 31 , 32 Since many signaling pathways involved in autophagy regulation converge at mTOR, we focused on the role of the Akt–mTOR signaling pathway in this study. 15 , 33 , 34 By binding to different chaperone proteins, mTOR forms two complexes: mTORC1 and mTORC2. mTORC1 inhibits autophagy by directly phosphorylating ULK1 and disrupting the interaction between ULK1 and AMPK. mTORC1 can also indirectly inhibit the stability of ULK1 through phosphorylated AMBRA1, leading to autophagy inhibition. 35 , 36 In our study, downregulation of fibulin‐4 led to significant increases in the levels of p‐Akt and p‐mTOR, the ratios of p‐Akt/Akt and p‐mTOR/mTOR and the level of p62, but the levels of Beclin‐1 and LC3B‐II were significantly reduced, suggesting that low expression of fibulin‐4 induced Akt–mTOR pathway activation along with decreased autophagy levels. To further verify whether fibulin‐4 affects autophagy through the Akt–mTOR signaling pathway, we treated fibulin‐4‐silenced cells with the Akt inhibitor triciribine and analyzed the activities of Akt and mTOR in these ESCC cells. The results showed that, in fibulin‐4‐silenced cells, triciribine significantly reduced the levels of p‐Akt and p‐mTOR, the ratios of p‐Akt/Akt and p‐mTOR/mTOR, and the level of p62, while the levels of Beclin‐1 and LC3B‐II were significantly increased. These data suggested that downregulation of fibulin‐4 inhibits autophagy in ESCC cells through phosphorylation and activation of the Akt–mTOR signaling pathway, thus significantly promoting apatinib‐induced apoptosis of ESCC cells. Further comprehensive and in‐depth research on the interaction mechanism between autophagy and apoptosis will bring a breakthrough in the understanding and treatment of ESCC.
In conclusion, in this study, we found, for the first time, that fibulin‐4 was highly expressed in ESCC, and the high expression of fibulin‐4 was closely related to the poor clinicopathological features. Upregulation of fibulin‐4 promoted the proliferation, invasion and migration of ESCC cells but inhibited their apoptosis. Highly expressed fibulin‐4 also promoted autophagy in ESCC cells through the Akt–mTOR signaling pathway, while silencing fibulin‐4 inhibited autophagy and promoted the sensitivity of ESCC cells to apatinib, thereby promoting apatinib‐induced apoptosis. We therefore predicted that fibulin‐4 could be used as a specific biomarker to predict poor prognosis of ESCC and provided a basis for its innovative application in anti‐ESCC treatment strategies.
AUTHOR CONTRIBUTIONS
Xiangyu Chen, Xiangyan Liu and Xiaopeng He designed the study. Xiangyu Chen, Jianyu Wang performed the experiment. Liang Song, Yang Yu, Mo Shi, Wenpeng Jiang contributed to analysis and manuscript preparation. All authors contributed to the writing and revision of the article and approved the submitted version.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
Chen X, Wang J, Song L, Yu Y, Shi M, Jiang W, et al. Downregulation of fibulin‐4 inhibits autophagy and promotes the sensitivity of esophageal squamous cell carcinoma cells to apatinib by activating the Akt‐mTOR signaling pathway. Thorac Cancer. 2022;13(18):2592–2605. 10.1111/1759-7714.14595
Funding information Natural Science Foundation of Shandong Province, Grant/Award Number: ZR2020MH244; Shandong Medicine and Health Technology Development Project, Grant/Award Number: 2018WS282
REFERENCES
- 1. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135(5):584–90. 10.1097/CM9.0000000000002108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 4. Yang YM, Hong P, Xu WW, He QY, Li B. Advances in targeted therapy for esophageal cancer. Signal Transduct Target Ther. 2020;5(1):229. 10.1038/s41392-020-00323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yanwei L, Feng H, Ren P, Yue J, Zhang W, Tang P, et al. Safety and efficacy of Apatinib monotherapy for Unresectable, metastatic esophageal cancer: a single‐arm, open‐label, phase II study. Oncologist. 2020;25(10):e1464–72. 10.1634/theoncologist.2020-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu G, Wang Y, Wang C, He Y. Clinical efficacy and safety of apatinib as maintenance treatment in patients with advanced esophageal squamous cell carcinoma. Expert Rev Clin Pharmacol. 2020;13(12):1423–30. 10.1080/17512433.2020.1844004 [DOI] [PubMed] [Google Scholar]
- 7. Chu L, Chen Y, Liu Q, Liang F, Wang S, Liu Q, et al. A phase II study of Apatinib in patients with chemotherapy‐refractory esophageal squamous cell carcinoma (ESO‐Shanghai 11). Oncologist. 2021;26(6):e925–35. 10.1002/onco.13668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first‐line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond). 2020;40(12):711–20. 10.1002/cac2.12119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng X, Wu T, Hong Y, Fan Q, Ren Z, Guo Y, et al. Camrelizumab plus apatinib as second‐line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): a single‐arm, open‐label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7(3):245–53. 10.1016/S2468-1253(21)00378-2 [DOI] [PubMed] [Google Scholar]
- 10. Li YY, Lam SK, Zheng CY, Ho JC. The effect of tumor microenvironment on autophagy and sensitivity to targeted therapy in EGFR‐mutated lung adenocarcinoma. J Cancer. 2015;6(4):382–6. 10.7150/jca.11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu M, Zhang P. EGFR‐mediated autophagy in tumourigenesis and therapeutic resistance. Cancer Lett. 2020;469:207–16. 10.1016/j.canlet.2019.10.030 [DOI] [PubMed] [Google Scholar]
- 12. Liu H, He Z, Simon HU. Protective role of autophagy and autophagy‐related protein 5 in early tumorigenesis. J Mol Med (Berl). 2015;93(2):159–64. 10.1007/s00109-014-1241-3 [DOI] [PubMed] [Google Scholar]
- 13. Folkerts H, Hilgendorf S, Vellenga E, Bremer E, Wiersma VR. The multifaceted role of autophagy in cancer and the microenvironment. Med Res Rev. 2019;39(2):517–60. 10.1002/med.21531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xia H, Green DR, Zou W. Autophagy in tumour immunity and therapy. Nat Rev Cancer. 2021;21(5):281–97. 10.1038/s41568-021-00344-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang YM, Xu X, Tang J, Sun ZY, Fu YJ, Zhao XJ, et al. Apatinib induces endoplasmic reticulum stress‐mediated apoptosis and autophagy and potentiates cell sensitivity to paclitaxel via the IRE‐1α‐AKT‐mTOR pathway in esophageal squamous cell carcinoma. Cell Biosci. 2021;11(1):124. 10.1186/s13578-021-00640-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Zhang J, Liu X, Fang R, Zhao Y, Ma D. Overexpression of fibulin‐4 is associated with tumor progression and poor prognosis in patients with cervical carcinoma. Oncol Rep. 2014;31(6):2601–10. 10.3892/or.2014.3139 [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Liu Z, Fang S, Fang R, Liu X, Zhao Y, et al. Fibulin‐4 is associated with tumor progression and a poor prognosis in ovarian carcinomas. BMC Cancer. 2015;15:91. 10.1186/s12885-015-1100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Chen Q, Chen Z, Tian D, Xu H, Cai Q, et al. EFEMP2 is upregulated in gliomas and promotes glioma cell proliferation and invasion. Int J Clin Exp Pathol. 2015;8(9):10385–93. [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang D, Wang S, Chen J, Liu H, Lu J, Jiang H, et al. Fibulin‐4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int J Oncol. 2017;50(5):1513–30. 10.3892/ijo.2017.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang T, Wang M, Fang S, Wang Q, Fang R, Chen J. Fibulin‐4 is associated with prognosis of endometrial cancer patients and inhibits cancer cell invasion and metastasis via Wnt/β‐catenin signaling pathway. Oncotarget. 2017;8(12):18991–9012. 10.18632/oncotarget.15086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song L, Li XX, Liu XY, Wang Z, Yu Y, Shi M, et al. EFEMP2 suppresses the invasion of lung cancer cells by inhibiting epithelial‐mesenchymal transition (EMT) and Down‐regulating MMPs. Onco Targets Ther. 2020;13:1375–96. 10.2147/OTT.S236111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B. 2021;11(11):3379–92. 10.1016/j.apsb.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov. 2019;9(9):1167–81. 10.1158/2159-8290.CD-19-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moscat J, Karin M, Diaz‐Meco MT. p62 in cancer: signaling adaptor beyond autophagy. Cell. 2016;167(3):606–9. 10.1016/j.cell.2016.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang J, Duran A, Reina‐Campos M, Valencia T, Castilla EA, Müller TD, et al. Adipocyte p62/SQSTM1 suppresses tumorigenesis through opposite regulations of metabolism in adipose tissue and tumor. Cancer Cell. 2018;33(4):770–784.e6. 10.1016/j.ccell.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu X, Shi S, Wang H, Yu X, Wang Q, Jiang S, et al. Blocking autophagy improves the anti‐tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci Rep. 2017;7(1):4559. 10.1038/s41598-017-04258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Suo H, Zhou S, Hou Z, Bu M, Liu X, et al. Afatinib induces pro‐survival autophagy and increases sensitivity to apoptosis in stem‐like HNSCC cells. Cell Death Dis. 2021;12(8):728. 10.1038/s41419-021-04011-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan Z, Yao ZH, Yao SN, Wang HY, Chu JF, Song M, et al. Camrelizumab plus apatinib successfully treated a patient with advanced esophageal squamous cell carcinoma. Immunotherapy. 2020;12(16):1161–6. 10.2217/imt-2020-0197 [DOI] [PubMed] [Google Scholar]
- 30. Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, et al. Camrelizumab combined with chemotherapy followed by Camrelizumab plus Apatinib as first‐line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res. 2021;27(11):3069–78. 10.1158/1078-0432.CCR-20-4691 [DOI] [PubMed] [Google Scholar]
- 31. Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21(7):387–92. 10.1016/j.tcb.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su M, Mei Y, Sinha S. Role of the crosstalk between autophagy and apoptosis in cancer. J Oncol. 2013;2013:102735. 10.1155/2013/102735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Zhang H. Regulation of autophagy by mTOR signaling pathway. Adv Exp Med Biol. 2019;1206:67–83. 10.1007/978-981-15-0602-4_3 [DOI] [PubMed] [Google Scholar]
- 34. Xu J, Deng Y, Wang Y, Sun X, Chen S, Fu G. SPAG5‐AS1 inhibited autophagy and aggravated apoptosis of podocytes via SPAG5/Akt/mTOR pathway. Cell Prolif. 2020;53(2):e12738. 10.1111/cpr.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang RC, Wei Y, An Z, Zou Z, Xiao G, Bhagat G, et al. Akt‐mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science. 2012;338(6109):956–9. 10.1126/science.1225967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25–32. 10.1172/JCI73939 [DOI] [PMC free article] [PubMed] [Google Scholar]


